Water Properties: Vaporization Heat vs. Temperature - Charts and Calculator (original) (raw)
The (latent) heat of vaporization (∆Hvap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance, to transform a given quantity of the substance into a gas .
The enthalpy of vaporization is a function of the pressure at which that transformation takes place. The heat of vaporization diminishes with increasing temperature and it vanishes completely at a certain point called the critical temperature (Critical temperature for water: 373.946 °C or 705.103 °F, Critical pressure: 220.6 bar = 22.06 MPa = 3200 psi).
Online Water Heat of Vaporization Calculator
The calculator below can be used to calculate the liquid water heat of vaporization at vapor pressure at given temperatures.
The output heat is given as kJ/mol, kJ/kg, kWh/kg, cal/g, Btu(IT)/mol and Btu(IT)/lbm.
Note! Temperature must be within the ranges 0-370 °C, 32-700 °F, 273-645 K and 492-1160 °R to get valid values.
For vapor pressure - check tables below.
See Water and Heavy Water - for thermodynamic properties.
See also Water Boiling points at high pressure, Boiling points at vacuum pressure, Density, specific weight and thermal expansion coefficient, Dynamic and kinematic viscosity, Enthalpy and entropy, Ionization Constant, pKw , of normal and heavy water, Melting points at high pressure, Saturation pressure, Specific gravity, Specific heat (heat capacity) and Specific volume for online calculatores, and similar figures and tables as shown below.

Heat of vaporization for liquid water at saturation pressure at temperatures from 0 to 374 °C:
Water - Heat of Vaporization vs. Temperature
| Temperature | Vapor pressure | Heat of vaporization, ∆Hvap | |||
|---|---|---|---|---|---|
| (°C) | (kPa) (100*bar) | (J/mol) | (kJ/kg) | (Wh/kg) | (Btu(IT)/lbm ) |
| 0.01 | 0.61165 | 45054 | 2500.9 | 694.69 | 1075.2 |
| 2 | 0.70599 | 44970 | 2496.2 | 693.39 | 1073.2 |
| 4 | 0.81355 | 44883 | 2491.4 | 692.06 | 1071.1 |
| 10 | 1.2282 | 44627 | 2477.2 | 688.11 | 1065.0 |
| 14 | 1.5990 | 44456 | 2467.7 | 685.47 | 1060.9 |
| 18 | 2.0647 | 44287 | 2458.3 | 682.86 | 1056.9 |
| 20 | 2.3393 | 44200 | 2453.5 | 681.53 | 1054.8 |
| 25 | 3.1699 | 43988 | 2441.7 | 678.25 | 1049.7 |
| 30 | 4.2470 | 43774 | 2429.8 | 674.94 | 1044.6 |
| 34 | 5.3251 | 43602 | 2420.3 | 672.31 | 1040.5 |
| 40 | 7.3849 | 43345 | 2406.0 | 668.33 | 1034.4 |
| 44 | 9.1124 | 43172 | 2396.4 | 665.67 | 1030.3 |
| 50 | 12.352 | 42911 | 2381.9 | 661.64 | 1024.0 |
| 54 | 15.022 | 42738 | 2372.3 | 658.97 | 1019.9 |
| 60 | 19.946 | 42475 | 2357.7 | 654.92 | 1013.6 |
| 70 | 31.201 | 42030 | 2333.0 | 648.06 | 1003.0 |
| 80 | 47.414 | 41579 | 2308.0 | 641.11 | 992.26 |
| 90 | 70.182 | 41120 | 2282.5 | 634.03 | 981.30 |
| 96 | 87.771 | 40839 | 2266.9 | 629.69 | 974.59 |
| 100 | 101.42 | 40650 | 2256.4 | 626.78 | 970.08 |
| 110 | 143.38 | 40167 | 2229.6 | 619.33 | 958.56 |
| 120 | 198.67 | 39671 | 2202.1 | 611.69 | 946.73 |
| 140 | 361.54 | 38630 | 2144.3 | 595.64 | 921.88 |
| 160 | 618.23 | 37508 | 2082.0 | 578.33 | 895.10 |
| 180 | 1002.8 | 36286 | 2014.2 | 559.50 | 865.95 |
| 200 | 1554.9 | 34944 | 1939.7 | 538.81 | 833.92 |
| 220 | 2319.6 | 33462 | 1857.4 | 515.94 | 798.54 |
| 240 | 3346.9 | 31804 | 1765.4 | 490.39 | 758.99 |
| 260 | 4692.3 | 29934 | 1661.6 | 461.56 | 714.36 |
| 280 | 6416.6 | 27798 | 1543.0 | 428.61 | 663.37 |
| 300 | 8587.9 | 25304 | 1404.6 | 390.17 | 603.87 |
| 320 | 11284 | 22310 | 1238.4 | 344.00 | 532.42 |
| 340 | 14601 | 18507 | 1027.3 | 285.36 | 441.66 |
| 360 | 18666 | 12967 | 719.8 | 199.9 | 309.5 |
| 373.946 | 22064 | 0 | 0.0 | 0.0 | 0.0 |
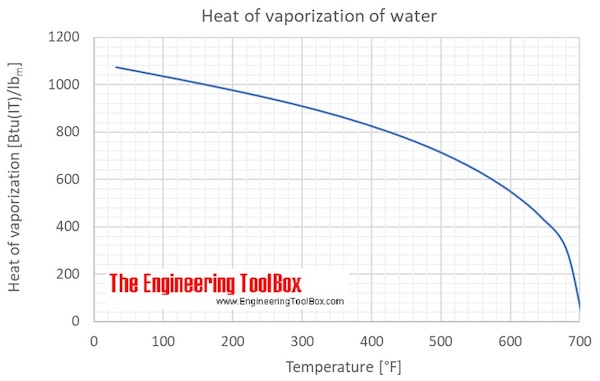
Heat of vaporization for liquid water at saturation pressure at temperatures from 0 to 705 °F:
Water - Heat of Vaporization vs. Temperature
| Temperature | Vapor pressure | Heat of vaporization, ∆Hvap | |||
|---|---|---|---|---|---|
| (°F) | (psi) | (Btu(IT)/mol) | (Btu(IT)/lbm ) | (cal/g) | (kJ/kg) |
| 32.2 | 0.0891 | 42.70 | 1075.2 | 597.33 | 2500.9 |
| 40 | 0.1219 | 42.52 | 1070.7 | 594.82 | 2490.4 |
| 50 | 0.1783 | 42.30 | 1065.0 | 591.67 | 2477.2 |
| 60 | 0.2564 | 42.07 | 1059.4 | 588.54 | 2464.1 |
| 70 | 0.3632 | 41.85 | 1053.7 | 585.39 | 2450.9 |
| 80 | 0.5073 | 41.62 | 1048.0 | 582.25 | 2437.7 |
| 90 | 0.6990 | 41.40 | 1042.4 | 579.09 | 2424.5 |
| 100 | 0.9506 | 41.17 | 1036.7 | 575.92 | 2411.3 |
| 110 | 1.277 | 40.95 | 1030.9 | 572.74 | 2398.0 |
| 120 | 1.695 | 40.72 | 1025.2 | 569.55 | 2384.6 |
| 130 | 2.226 | 40.49 | 1019.4 | 566.34 | 2371.2 |
| 140 | 2.893 | 40.26 | 1013.6 | 563.13 | 2357.7 |
| 150 | 3.723 | 40.02 | 1007.7 | 559.86 | 2344.0 |
| 160 | 4.747 | 39.79 | 1001.8 | 556.58 | 2330.3 |
| 170 | 6.000 | 39.55 | 995.87 | 553.26 | 2316.4 |
| 180 | 7.519 | 39.31 | 989.85 | 549.92 | 2302.4 |
| 190 | 9.350 | 39.07 | 983.76 | 546.54 | 2288.2 |
| 200 | 11.54 | 38.83 | 977.60 | 543.11 | 2273.9 |
| 210 | 14.14 | 38.58 | 971.35 | 539.64 | 2259.4 |
| 212 | 14.71 | 38.53 | 970.08 | 538.93 | 2256.4 |
| 220 | 17.20 | 38.33 | 965.02 | 536.12 | 2244.6 |
| 240 | 24.99 | 37.81 | 952.05 | 528.92 | 2214.5 |
| 260 | 35.45 | 37.28 | 938.64 | 521.46 | 2183.3 |
| 280 | 49.22 | 36.73 | 924.71 | 513.73 | 2150.9 |
| 300 | 66.6 | 36.15 | 910.21 | 505.67 | 2117.1 |
| 350 | 135 | 34.59 | 870.97 | 483.87 | 2025.9 |
| 400 | 247 | 32.82 | 826.41 | 459.12 | 1922.2 |
| 450 | 422 | 30.78 | 774.93 | 430.51 | 1802.5 |
| 500 | 680 | 28.37 | 714.36 | 396.87 | 1661.6 |
| 550 | 1044 | 25.48 | 641.56 | 356.42 | 1492.3 |
| 600 | 1541 | 21.93 | 552.09 | 306.72 | 1284.2 |
| 625 | 1849 | 19.41 | 488.64 | 271.46 | 1136.6 |
| 650 | 2205 | 16.95 | 426.81 | 237.11 | 992.8 |
| 675 | 2615 | 13.47 | 339.22 | 188.46 | 789.0 |
| 705.103 | 3196 | 0.00 | 0.00 | 0.00 | 0.0 |
Related Documents
Ice and Water - Melting Points vs. Pressure
Online calculator, figures and tables with melting points of ice to water at pressures ranging from 0 to 29000 psia (0 to 2000 bara). Temperature given as °C, °F, K and °R.
Seawater - Properties
Seawater properties like density, saturation pressure, specific heat, electrical conductivity and absolute viscosity.
Water - Properties at Gas-Liquid Equilibrium Conditions
Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, Prandtl number, thermal diffusivity, entropy and enthalpy).
Water - Specific Gravity vs. Temperature
Figures and tables showing specific gravity of liquid water in the range of 32 to 700 °F or 0 to 370°C, using water density at four different temperatures as reference.

