Water - Specific Volume vs. Temperature (original) (raw)
Specific volume is the inverse of density, or the ratio of the volume to the mass of a substance:
v = V / m
= 1 / ρ (1)
where
v = specific volume, units typically (cm3/g) or (ft3/lb)
V = volume, units typically (cm3) or (ft3)
m = mass, units typically (g) or (lb)
ρ = density, units typically (g/cm3) or (lb/ft3)
Online Water specific volume Calculator
The calculator below can be used to calculate the liquid water specific volume at given temperatures.
The output specific volume is given as cm3/g, ft3/lb, gal(US liq)/lb and ft3/ sl.
Temperatur must be within the ranges 0-370 °C, 32-700 °F, 273-645 K and 492-1160 °R
The specific volume of water depends on temperature as shown below:
See Water and Heavy Water - thermodynamic properties.
See also Water Boiling points at high pressure, Boiling points at vacuum pressure, Density, specific weight and thermal expansion coefficient, Dynamic and kinematic viscosity, Enthalpy and entropy, Heat of vaporization, Ionization Constant, pKw , of normal and heavy water, Melting points at high pressure, Saturation pressure, Specific gravity and Specific heat (heat capacity) for online calculatores, and similar figures and tables as shown below.
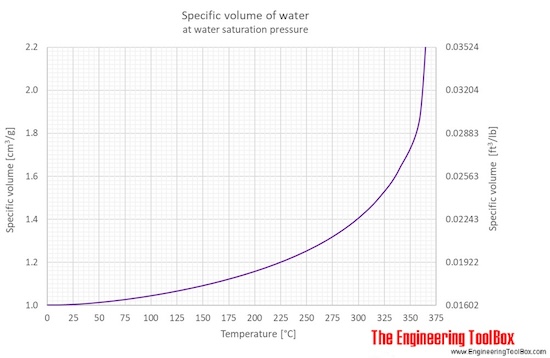
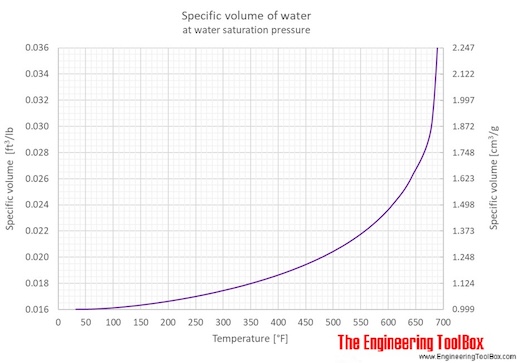
Water specific volume at temperatures given in degree Celcius:
Water - Specific Volume vs. Temperature
| Temperature | Specific volume (0-100°C at 1 atm, >100 °C at saturation pressure) | ||
|---|---|---|---|
| (°C) | (cm3/g) | (ft3/lb) | (gal(US liq)/lb) |
| 0.1 | 1.00015 | 0.016021 | 0.11981 |
| 1 | 1.00010 | 0.016020 | 0.11981 |
| 4 | 1.00003 | 0.016019 | 0.11982 |
| 10 | 1.00030 | 0.016023 | 0.11979 |
| 15 | 1.00090 | 0.016033 | 0.11972 |
| 20 | 1.00180 | 0.016047 | 0.11961 |
| 25 | 1.00296 | 0.016066 | 0.11947 |
| 30 | 1.00437 | 0.016088 | 0.11931 |
| 35 | 1.00600 | 0.016115 | 0.11911 |
| 40 | 1.00785 | 0.016144 | 0.11889 |
| 45 | 1.00989 | 0.016177 | 0.11865 |
| 50 | 1.01210 | 0.016212 | 0.11839 |
| 55 | 1.01452 | 0.016251 | 0.11811 |
| 60 | 1.01709 | 0.016292 | 0.11781 |
| 65 | 1.01984 | 0.016336 | 0.11750 |
| 70 | 1.02275 | 0.016383 | 0.11716 |
| 75 | 1.02581 | 0.016432 | 0.11681 |
| 80 | 1.02903 | 0.016483 | 0.11645 |
| 85 | 1.03241 | 0.016538 | 0.11607 |
| 90 | 1.03594 | 0.016594 | 0.11567 |
| 95 | 1.03962 | 0.016653 | 0.11526 |
| 100 | 1.04346 | 0.016715 | 0.11484 |
| 110 | 1.05158 | 0.016845 | 0.11395 |
| 120 | 1.06032 | 0.016985 | 0.11301 |
| 140 | 1.07976 | 0.017296 | 0.11097 |
| 160 | 1.10199 | 0.017652 | 0.10874 |
| 180 | 1.12740 | 0.018059 | 0.10629 |
| 200 | 1.15652 | 0.018526 | 0.10361 |
| 220 | 1.19016 | 0.019065 | 0.10068 |
| 240 | 1.22945 | 0.019694 | 0.09746 |
| 260 | 1.27611 | 0.020441 | 0.09390 |
| 280 | 1.33284 | 0.021350 | 0.08990 |
| 300 | 1.40422 | 0.022493 | 0.08533 |
| 320 | 1.49905 | 0.024012 | 0.07994 |
| 340 | 1.63755 | 0.026231 | 0.07317 |
| 360 | 1.89541 | 0.030362 | 0.06322 |
| 373.946 | 3.10559 | 0.049747 | 0.03858 |
Water specific volume at temperatures given in degree Fahrenheit:
Water - Specific Volume vs. Temperature
| Temperature | Specific volume (0-212°F at 1 atm, >212 °F at saturation pressure) | |||
|---|---|---|---|---|
| (°F) | (ft3/lb) | (gal(US liq)/lb) | (ft3/sl) | (cm3/g) |
| 32.2 | 0.01602 | 0.1198 | 0.5155 | 1.0002 |
| 34 | 0.01602 | 0.1198 | 0.5154 | 1.0001 |
| 39.2 | 0.01602 | 0.1198 | 0.5154 | 1.0000 |
| 40 | 0.01602 | 0.1198 | 0.5154 | 1.0000 |
| 50 | 0.01602 | 0.1199 | 0.5155 | 1.0003 |
| 60 | 0.01603 | 0.1199 | 0.5159 | 1.0010 |
| 70 | 0.01605 | 0.1201 | 0.5164 | 1.0020 |
| 80 | 0.01607 | 0.1202 | 0.5171 | 1.0034 |
| 90 | 0.01610 | 0.1204 | 0.5180 | 1.0050 |
| 100 | 0.01613 | 0.1207 | 0.5190 | 1.0070 |
| 110 | 0.01617 | 0.1209 | 0.5201 | 1.0092 |
| 120 | 0.01620 | 0.1212 | 0.5214 | 1.0116 |
| 130 | 0.01625 | 0.1215 | 0.5227 | 1.0143 |
| 140 | 0.01629 | 0.1219 | 0.5242 | 1.0171 |
| 150 | 0.01634 | 0.1222 | 0.5258 | 1.0202 |
| 160 | 0.01639 | 0.1226 | 0.5274 | 1.0234 |
| 170 | 0.01645 | 0.1230 | 0.5292 | 1.0269 |
| 180 | 0.01651 | 0.1235 | 0.5311 | 1.0305 |
| 190 | 0.01657 | 0.1239 | 0.5331 | 1.0343 |
| 200 | 0.01663 | 0.1244 | 0.5351 | 1.0384 |
| 212 | 0.01671 | 0.1250 | 0.5378 | 1.0435 |
| 220 | 0.01677 | 0.1255 | 0.5396 | 1.0471 |
| 240 | 0.01693 | 0.1266 | 0.5446 | 1.0567 |
| 260 | 0.01710 | 0.1279 | 0.5501 | 1.0675 |
| 280 | 0.01728 | 0.1292 | 0.5558 | 1.0785 |
| 300 | 0.01748 | 0.1307 | 0.5623 | 1.0911 |
| 350 | 0.01799 | 0.1346 | 0.5789 | 1.1232 |
| 400 | 0.01861 | 0.1392 | 0.5987 | 1.1616 |
| 450 | 0.01943 | 0.1453 | 0.6251 | 1.2129 |
| 500 | 0.02044 | 0.1529 | 0.6577 | 1.2761 |
| 550 | 0.02177 | 0.1629 | 0.7004 | 1.3591 |
| 600 | 0.02357 | 0.1763 | 0.7583 | 1.4713 |
| 625 | 0.02491 | 0.1864 | 0.8015 | 1.5552 |
| 650 | 0.02683 | 0.2007 | 0.8631 | 1.6747 |
| 675 | 0.02964 | 0.2217 | 0.9535 | 1.8501 |
| 700 | 0.03380 | 0.2528 | 1.0875 | 2.1101 |
See also Properties of Water - Imperial Units and Water - Thermodynamic Properties - SI units
Related Documents
Densities of Aqueous Solutions of Organic Acids
Changes in density of aqueous solutions with changes in concentration at 20°C. Density of acetic acid, citric acid, formic acid, D-lactic acid, oxalic acid and trichloroacetic acid in water is plotted as function of wt%, mol/kg water and mol/l solution.
Heavy Water - Thermophysical Properties
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and more.
Hydrocarbons - Physical Data
Molweight, melting and boiling point, density, flash point and autoignition temperature, as well as number of carbon and hydrogen atoms in each molecule for 200 different hydrocarbons.
Ice and Water - Melting Points vs. Pressure
Online calculator, figures and tables with melting points of ice to water at pressures ranging from 0 to 29000 psia (0 to 2000 bara). Temperature given as °C, °F, K and °R.
Organic Sulfur Compounds - Densities
Liquid density of different kinds of organic sulfur compounds with varying carbon number (20°C/68°F). Comparison of thiols, sulfides, disulfides and thiophenes.
Water - Properties at Gas-Liquid Equilibrium Conditions
Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, Prandtl number, thermal diffusivity, entropy and enthalpy).
Water - Specific Gravity vs. Temperature
Figures and tables showing specific gravity of liquid water in the range of 32 to 700 °F or 0 to 370°C, using water density at four different temperatures as reference.



