Heavy Water - Thermophysical Properties (original) (raw)
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and more.
Heavy water ( deuterium oxide , 2H2O , D2O ) is a form of water that contains a larger than normal amount of the hydrogen isotope deuterium (= heavy hydrogen = 2H = D), rather than the common hydrogen-1 isotope (1H = H = protium) that makes up most of the hydrogen in normal water.
Thermodynamic properties of heavy water - D2O:
- Boiling temperature (at 101.325 kPa): 101.40 oC = 214.52 °F
- Bulk modulus elasticity (at 25°C): 2.10×109 Pa or N/m2
- Critical density: 0.356 g/cm3 = 0.691 slug/ft3 = 3.457 lbm/gal(US)
- Critical pressure : 213.88 atm = 220.98 bar = 21.671 MPa (MN/m2) = 3143 psi (=lbf/in2)
- Critical temperature : 370.697 oC = 699.255 °F
- Ionization constant, pKw (at 25°C): 14.951
- Latent heat of evaporation (at 101.4°C): 41.521 KJ/mol = 2073.20 kJ/kg = 891.32 Btu(IT)/lb
- Latent heat of fusion: 6.132 kJ/mol = 306.2 kJ/kg = 131.64 Btu(IT)/lb
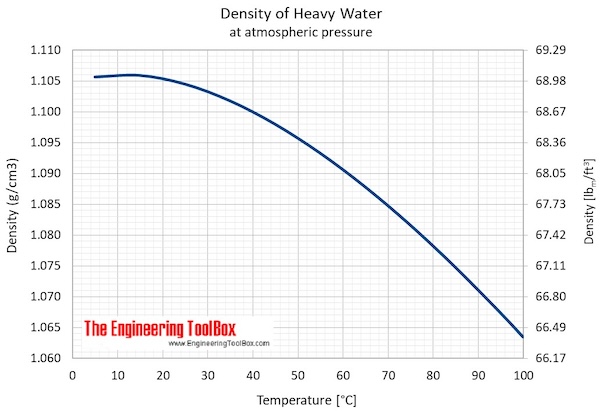
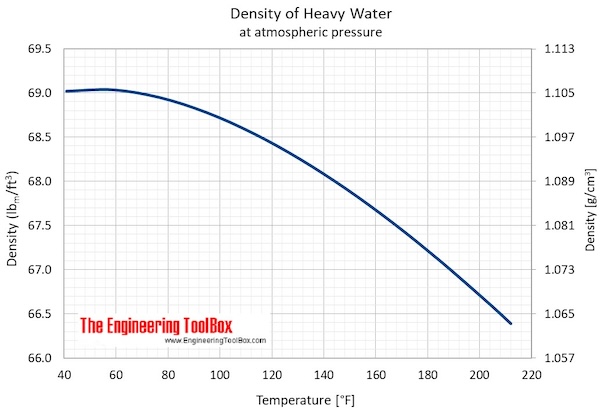
- Maximum density (at 11.23 oC): 1105.9 kg/m3 = 2.1460 slug/ft3 = 10.74048 lbm/gal(US)
- Melting temperature (at 101.325 kPa): 3.81 oC = 38.86 °F
- Molar mass: 20.02751 g/mol
- pD (~pH) (at 25°C): 7.43
- Specific heat (Cp) water (at 20°C): 4.219 kJ/kgK = 1.008 Btu(IT)/(lbm °F) or kcal/(kg K)
- Specific weight (at 11.23 oC): 10.8452 kN/m3 = 69.0391 lbf/ft3
- Surface tension (at 25°C): 71.87 dyn/cm
- Triple point pressure: 0.00652 atm = 0.00661 bar = 661 Pa = 0.0959 psi (=lbf/in2)
- Triple point temperature: 3.82 °C = 38.88 °F
- Vapor pressure (at 25°C): 20.6 mmHg = 0.027 atm = 0.028 bar = 2750 Pa = 0.398 psi
- Viscosity (at 20°C): 1.251 cP or mPa s
Ionization Constant, pKw , of normal and heavy water with varying temperature.
See also more about atmospheric pressure, and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure,
as well as Thermophysical properties of: Acetone, Acetylene, Air, Ammonia, Argon, Benzene, Butane, Carbon dioxide, Carbon monoxide, Ethane, Ethanol, Ethylene, Helium, Hydrogen, Hydrogen sulfide, Methane, Methanol, Nitrogen, Oxygen, Pentane, Propane, Toluene and Water.
My Short List
Related Topics
Densities of solids, liquids and gases. Definitions and convertion calculators.
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more.
Design of steam & condensate systems with properties, capacities, sizing of pipe lines, system configuration and more.
Work, heat and energy systems.
Design of hot and cold water service and utility systems with properties, capacities, sizing of pipe lines and more.
Related Documents
Acetone - Thermophysical Properties
Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid. Phase diagram included.
Air Properties - Density, Viscosity, Heat Capacity, Thermal Conductivity, and more
Thermal properties of air, including density, viscosity, thermal conductivity, specific heat and more at different temperatures and pressures. Comprehensive reference with formulas, tables, and charts to support engineering calculations.
Heat Capacity
The amount of heat required to change the temperature of a substance by one degree.
Ice and Water - Melting Points vs. Pressure
Online calculator, figures and tables with melting points of ice to water at pressures ranging from 0 to 29000 psia (0 to 2000 bara). Temperature given as °C, °F, K and °R.
Methanol - Thermophysical Properties
Chemical, physical and thermal properties of methanol, CH3OH (also called carbinol, wood alcohol, hydroxy methyl and methyl alcohol). Phase diagram included.
Water - Properties at Gas-Liquid Equilibrium Conditions
Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, Prandtl number, thermal diffusivity, entropy and enthalpy).