The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease (original) (raw)
Abstract
Metabolic dysfunction-associated fatty liver disease (MAFLD) affects over one-fourth of the global adult population and is the leading cause of liver disease worldwide. To address this, the Asian Pacific Association for the Study of the Liver (APASL) has created clinical practice guidelines focused on MAFLD. The guidelines cover various aspects of the disease, such as its epidemiology, diagnosis, screening, assessment, and treatment. The guidelines aim to advance clinical practice, knowledge, and research on MAFLD, particularly in special groups. The guidelines are designed to advance clinical practice, to provide evidence-based recommendations to assist healthcare stakeholders in decision-making and to improve patient care and disease awareness. The guidelines take into account the burden of clinical management for the healthcare sector.
Introduction
The Asia–Pacific region is the largest global landmass with more than half of the world's population. Approximately half of the global burden of liver disease, mainly cirrhosis and hepatocellular carcinoma (HCC) and liver-related mortality occurs in this region [1]. While there has been substantial improvements in the prevention and treatment of viral hepatitis, the prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD) is expected to increase in the future due to the rising burden of metabolic disorders and the consumption of energy dense, nutrient-poor foods, a sedentary lifestyle and reduced physical activity, both in high and low resources settings. A person living with MAFLD plays a vital role in their disease management, as the cornerstone of treatment is adherence to a healthy diet and being physically active. In this context, the development of effective strategies for managing MAFLD is paramount for reducing liver-related morbidity and mortality [2].
This document is the clinical practice guidelines of the Asian Pacific Association for the Study of the Liver (APASL) on MAFLD. These guidelines provide a broad framework for its management, encompassing aspects such as diagnosis, screening, treatment, surveillance, and detection of related co-morbid diseases. They are also designed to offer practical insights for healthcare professionals treating adult patients with MAFLD, with particular reference to special groups whenever necessary. The statements in this document adhere to the Grading of Recommendation Assessment, Development, and Evaluation approach as outlined in Table 1. The ultimate goal is to enhance patient care and raise awareness about MAFLD, while also aiding stakeholders in making informed decisions based on evidence-based data.
Table 1 Evidence grade used for the APASL Clinical Practice Guidelines on MAFLD (adapted from the GRADE system) [3]
Epidemiology
The prevalence of MAFLD in the Asia–Pacific region ranges from 28 to 40%, largely reflecting that observed globally [4,5,6]. A recent meta-analysis provides more specific prevalence rates for different regions as follows: South Asia 34% (23 − 47%), South-East Asia 33% (19 − 51%), East Asia 30% (26 − 34%), and the Pacific region 28% (25 − 32%) [6]. The escalating prevalence of MAFLD in the aging population, currently impacting 120 million elderly people is anticipated to place a significant burden on the healthcare system in the near future [7]. the global steatohepatitis prevalence is 5.27%, with around 4.49% in the Asia Pacific. Additionally, the rising prevalence among young people is very concerning as the related health burden will be experienced across the lifespan.
At a country level, a study in China which included 75,570 participants undergoing regular health check-up visits, reported an overall MAFLD prevalence of 37%, with a higher rate in men (46%) than women (24%) [8]. The study also found that the prevalence increased with advancing age [8]. Another investigation from an urban Chinese population found an MAFLD prevalence of 26% as determined by ultrasonography [9]. Likewise, in a study of 6146 participants from New Delhi, India, approximately half of the study population was found to have MAFLD [10]. Another study which included 2782 participants showed that about one-third of the population of Bangladesh is affected by MAFLD [11]. A nationwide study from South Korea, which utilized ultrasonography and magnetic resonance elastography to investigate hepatic steatosis and fibrosis, reported MAFLD prevalence of 34% [12]. Of these patients, 3% exhibited advanced fibrosis. In a Japanese cohort of 2254 patients who underwent transient elastography during regular check-up visits, 35% were diagnosed with MAFLD. Of these, 9% had an increased risk for progressive liver disease as determined by the FibroScan-AST (FAST) score [13]. A separate investigation conducted in North Eastern Iran of 4242 patients reported an MAFLD prevalence of 23% [14]. A multicenter study from Turkey, representative of the general population, revealed a higher prevalence of MAFLD at 46% [15]. Notably, this cohort exhibited a more dysfunctional metabolic profile, with prevalence rates of obesity, type 2 diabetes mellitus (T2DM), metabolic syndrome, dyslipidemia, and hypertension of 43%, 25%, 52%, 92%, and 32%, respectively. Another Turkish study which included 424 biopsy-proven MAFLD patients, found that 16% of patients had evidence of advanced fibrosis [16]. Similarly, MAFLD prevalence in Australia has increased from 33 to 39% between 2003 and 2018[17]. Another study from Australia of 722 participants from randomly selected households showed that the unadjusted prevalence was 47% [18].
Considering the collective data from investigations conducted in the Asia–Pacific, it is evident that patients with MAFLD from this region exhibit impaired metabolic profiles and an elevated risk for progressive liver disease, notwithstanding variations attributable to discrepancies in study methodologies and ethnic backgrounds. However, the epidemiological patterns in Asian countries largely align with global trends [6].
Definition and diagnosis of MAFLD
To address issues with the former nonalcoholic fatty liver disease (NAFLD) term, APASL was the first society to endorse the MAFLD definition [19]. MAFLD is a shift towards a diagnosis of inclusion based on the presence of metabolic dysfunction, the key driver of the disease [20, 21].
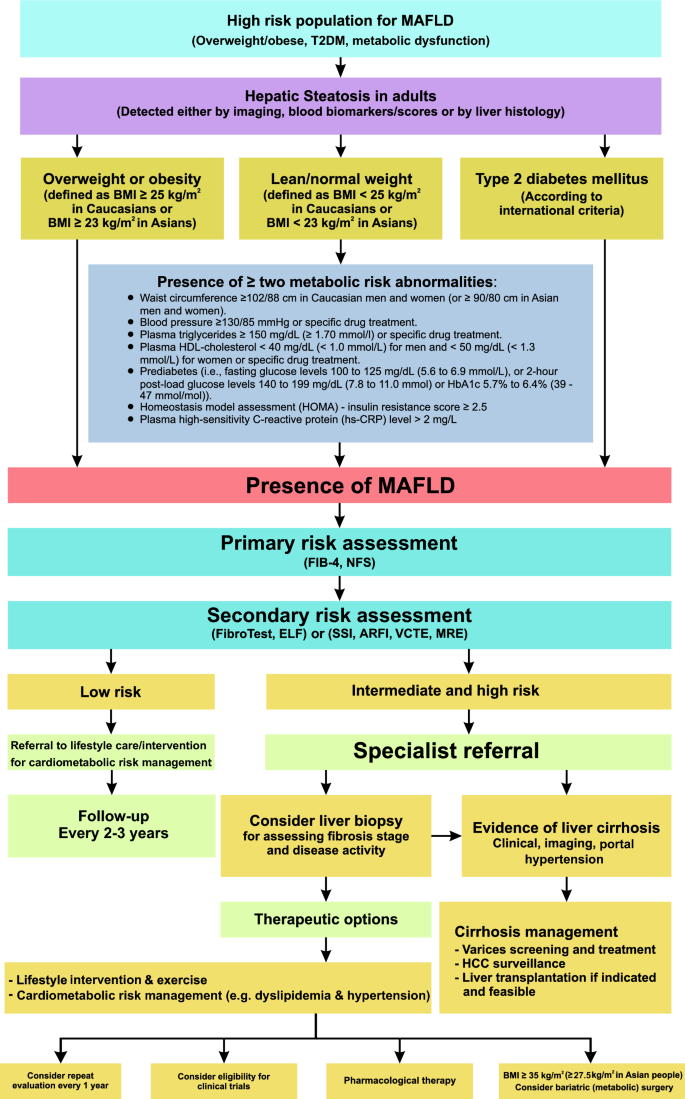
The diagnosis of MAFLD is based on the detection of liver steatosis (liver histology, non-invasive biomarkers or imaging) together with the presence of at least one of three criteria that include overweight or obesity, T2DM, or clinical evidence of metabolic dysfunction in lean subjects (Fig. 1).
Fig. 1
Recommended algorithm to diagnose, evaluate, and monitor disease severity in suspected patients with MAFLD and management approach for confirmed cases. HDL-cholesterol, high-density lipoprotein cholesterol; FIB-4, Fibrosis-4 index; NFS, MAFLD fibrosis score; ELF, enhanced liver fibrosis; SSI, supersonic shear imaging; ARFI, acoustic radiation force impulse; VCTE, vibration-controlled transient elastography; MRE, magnetic resonance elastography
Recent data confirm the superior utility of MAFLD for identifying patients at high risk for hepatic and extra-hepatic complications [22], as well as those who would benefit from genetic testing, including patients with concomitant other liver diseases. As MAFLD segregates patients into three homogenous groups (diabetic, overweight/obese and lean with evidence of metabolic dysfunction) with different baseline characteristics, clinical trajectories and outcomes, it represents an advance for the field in how we should think of liver disease related to systemic metabolic dysregulation [23]. Additionally, MAFLD can guide efforts towards a more inclusive, equitable, and patient-centered approach.
Risk factors for MAFLD
The pathogenesis of MAFLD is complex and likely influenced by the dynamic interactions between various risk factors such as central obesity, T2DM, dyslipidemia, and genetic risk factors.[24] These risk factors can be divided into modifiable and non-modifiable categories (Table 2).
Table 2 Risk factors for MAFLD
The presence of obesity is associated with MAFLD, and the prevalence of MAFLD increases as body mass index (BMI) increases [9, 25, 26]. In addition to BMI, growing evidence suggests that central obesity plays a major role in the development and progression of MAFLD [27]. Central obesity should be considered when assessing risk factors in Asian patients with MAFLD [28] as they are more likely to have high central fat deposition despite a lower BMI [24]. It was reported that T2DM is a major risk factor for the development and progression of MAFLD [26], and the global prevalence of MAFLD among patients with T2DM is approximately 65% [29]. Dyslipidemia also affects the development of MAFLD [26].
While overweight/obesity is associated with the onset and progression of MAFLD, weight gain without reaching overweight status plays a crucial role in metabolic disease pathogenesis and in MAFLD [30]. According to a systematic review of 93 studies from 24 countries and areas around the world, among individuals with MAFLD, 19% were classified as lean, while 41% were classified as non-obese with no differences in histological severity between lean and obese patients. Remarkably, about one-third of patients with normal BMI and MAFLD meet the criteria for metabolic syndrome [31].
A low level of physical activity is reported to increase the risk of MAFLD [32, 33]. Similarly, sarcopenia and MAFLD share many common pathophysiologic mechanisms, thus they are associated in a bidirectional manner [34, 35]. The risk of MAFLD increases in the presence of sarcopenia, and sarcopenia is associated with significant fibrosis [36,37,38]. Recently, it has been suggested that myosteatosis and muscle quality can play an important role in the progression of early-stage MAFLD [39, 40], even among the non-obese population [41]. A high-caloric or high-fructose diet is another risk factor for MAFLD, regardless of obesity [42, 43]. Recent studies indicate that gut microbiota and its metabolites play a critical role in the onset of MAFLD [44, 45]. In addition, obstructive sleep apnea, hypothyroidism, polycystic ovary syndrome, and hypogonadism are associated with the development of MAFLD [46,47,48,49].
As for non-modifiable risk factors, a study of 73,566 ethnic Chinese reported that male sex and older age were risk factors for MAFLD [25]. Genetic factors contribute to the development and progression of MAFLD. For example, gene variants such as PNPLA3, TM6SF2 and MBOAT7 are associated with the full spectrum of MAFLD [50,51,52]. Growing evidence also suggests that epigenetic factors are implicated in MAFLD pathogenesis. Likewise, the length of telomeres in liver cells shortens as fibrosis stage advances in patients with MAFLD [53, 54].
Natural history of MAFLD
The progression of MAFLD is complex and not entirely clear due to it being part of a multisystem disorder with hepatic and extrahepatic complications. In general, the natural history of MAFLD is slowly progressive, starting with metabolic dysfunction-associated fatty liver (Simple steatosis) characterized by lipid accumulation in liver cells. It is estimated that within 7 years, about 44% of patients, especially those with genetic predisposition, will develop lipotoxicity leading to steatohepatitis-(Metabolic dysfunction-associated steatohepatitis—MASH) [55]. However, observational data also indicate that MASH patients may regress to steatosis spontaneously (around 7% within 7 years) although it is uncertain if this is true regression, is due to variability in liver biopsy, or due to improvements in systemic metabolic dysregulation. Patients with pure fatty infiltration without inflammation or fibrosis have a relatively low risk of disease progression [56].
At the MASH stage, characterized by the activation of inflammatory cascades in various cell types (including Kupffer cells and stellate cells), there is excessive intercellular matrix deposition. This leads to early-stage liver fibrosis occurring in about a quarter of patients over approximately 3.4 years [57]. The average progression rate by an additional one fibrosis stage in patients with steatosis and steatohepatitis is 14.3 years and 7.1 years, respectively [[58](/article/10.1007/s12072-024-10774-3#ref-CR58 "Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015. https://doi.org/10.1016/j.cgh.2014.04.014
")\]. Approximately 16% of patients with low-grade fibrosis (< F3) will progress to advanced fibrosis \[[6](/article/10.1007/s12072-024-10774-3#ref-CR6 "Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–1347")\]. However, around 13.2% of patients may experience regression, reducing by one fibrosis stage \[[59](/article/10.1007/s12072-024-10774-3#ref-CR59 "Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun. 2018;2(2):199–210")\].Approximately 2.6% of people with MAFLD-related cirrhosis will develop HCC annually [60]. A systematic review showed that among 470,404 MAFLD patients, the incidence rate of HCC was 0.03/100 person-years, and 3.78/100 person-years in the pre-cirrhotic and cirrhotic stages, respectively [61]. Additionally, data show that 35–47% of HCC cases among patients with MAFLD may develop in the absence of cirrhosis [62, 63].
Assessment for MAFLD
MAFLD affects a large proportion of the general population, but only a small yet significant proportion develop advanced liver fibrosis (≥ F3 stage) [64]. Patients with advanced liver fibrosis are at increased risk of liver-related events and mortality [65, 66]. However, patients with MAFLD, even in the presence of advanced liver fibrosis, are often asymptomatic and may present for the first time with hepatic decompensation, thereby missing the opportunity for preventive intervention [67]. Widespread screening for MAFLD in the general population followed by treatment is currently not justified until further evidence emerges. Therefore, active case findings among high-risk groups are more appropriate.
Ultrasonography, the most common test for the diagnosis of hepatic steatosis can be utilised for screening. There is a high prevalence of MAFLD among patients with T2DM who are at increased risk of steatohepatitis and for advanced liver fibrosis [68, 69]. Furthermore, T2DM has been identified as an independent risk factor for hepatic decompensation and HCC among patients with MAFLD [70]. Therefore, patients with metabolic comorbidities generally and those with T2DM particularly, represent an important target population for identification of MAFLD and advanced liver fibrosis. In this context, assessment of the liver can be included as part of the assessment for target organ damage in patients with T2DM. This can be achieved with simple blood tests (i.e., serum ALT and AST levels and platelet count) and blood-based biomarkers and scores.
Recommended pathway for referral from primary care to tertiary hospital
Clear referral pathways aid in risk-stratifying patients who are most in need of specialist assessment, from those with mild disease who can be managed in primary care.
Elevated serum alanine aminotransferase (ALT) and aspartate transaminase (AST) levels should prompt evaluation for the cause of liver injury. However, normal serum ALT and AST levels do not exclude underlying liver disease. Patients with MAFLD and steatohepatitis and/or advanced liver fibrosis or cirrhosis can have normal serum ALT and AST levels [71,72,73]. Current risk-stratification algorithms use non-invasive tests (NIT’s) in a sequential fashion to identify patients with advanced liver fibrosis (F3-4) who are present in 2-5% of the primary care population [12, 74, [75](/article/10.1007/s12072-024-10774-3#ref-CR75 "Mahady SE, Macaskill P, Craig JC, Wong GL, Chu WC, Chan HL, et al. Diagnostic accuracy of noninvasive fibrosis scores in a population of individuals with a low prevalence of fibrosis. Clin Gastroenterol Hepatol. 2017. https://doi.org/10.1016/j.cgh.2017.02.031
")\]. Recent studies have shown that significant liver fibrosis (F2-4) is associated with an increased risk of liver-related death compared to no fibrosis. This population is currently the target of ongoing clinical trials.Currently, there is no standardised cutoff established for diagnostic accuracy for advanced fibrosis; the recommended cutoffs to rule in advanced hepatic fibrosis using NITs are shown in Table 3. Fibrosis-4 Index (FIB-4) is recommended as the first-line test for fibrosis assessment in general practice. Although it has modest accuracy with a meta-analysis of 37 studies (_n_=5735) finding a summary area under the curve (AUC) of 0.76, its appeal relates to its availability and low-cost.[76] A cut-off of 1.3 has a sensitivity of 74% (95% CI 72–76%) and a negative predictive value (NPV) of 95–97% for excluding advanced fibrosis, highlighting its role in identifying patients who can remain in primary care. These patients should be monitored with a repeat FIB-4 in 2-3 years; patients with an FIB-4 persistently <1.3 are at very low risk of developing cirrhosis and HCC.[77] A cut-off of 2.67 is 94% (95% CI 93–94%) specific for advanced fibrosis and patients above this threshold should be referred for specialist assessment.[76] It is important to recognize that the positive predictive value (PPV) of a FIB-4 >2.67 in primary care is only 24–40%, highlighting the need for other investigations to confirm a diagnosis of advanced fibrosis. Approximately 30% of patients will have an indeterminate FIB-4 between 1.3 and 2.67 and will benefit from further testing.[78, 79]. There are limitations of FIB-4 in screening for advanced liver fibrosis, particularly in individuals with diabetes. Additionally, the FIB-4 score is affected by age, making it less reliable in patients under 35 or over 65 years old. Although using age-specific thresholds raising the cut-off for participants ≥65 years old from 1.3 to 2.0 (while high-risk thresholds remain the same) was suggested, it might lead to an undesirable reduction in sensitivity (from 84 to 36%). Nevertheless, considering the widespread occurrence of MAFLD in the general population, it is reasonable to use a cutoff of <1.3, along with other clinical measures, to rule out most individuals with advanced fibrosis. The potential coexistence of MAFLD with other liver diseases, including viral hepatitis and alcohol-related liver disease, should be considered, particularly in patients with persistently elevated liver enzymes or higher FIB-4 levels.
Table 3 Recommended cutoff to rule in advanced hepatic fibrosis
Second-line testing of patients who fall within indeterminate FIB-4 ranges can be performed using elastography or direct serum tests according to local availability and cost. Vibration-controlled transient elastography (VCTE) or Fibroscan® accurately excludes advanced fibrosis, with a large multicentre study from Asia demonstrating a liver stiffness measurement (LSM) threshold of 8 kPa being 96% sensitive with an NPV of 99.7% [80]. XL probes are available to determine LSM in overweight or obese patients. Subjects with an indeterminate FIB-4 and a subsequent LSM of < 8 kPa can be managed in primary care and have a rate of risk of future liver-related events that is similar to patients with a FIB-4 < 1.3 [81]. Two-dimensional shear wave elastography (2D-SWE) using the Aixplorer® platform provides similar accuracy to VCTE when used in a sequential fashion in MAFLD patients with an indeterminate FIB-4 result [82]. In a study of 577 patients, a threshold of 8 kPa using 2D-SWE provided a false negative rate of 8% for the presence of advanced fibrosis, however, the rate of invalid scans was relatively high (23%) [82]. The most accurate non-invasive method to quantify liver fibrosis is MR elastography [83], but this has limited accessibility and requires additional technical hardware beyond a standard MR scan and is not recommended as a first-line approach for risk stratification in a patient with MAFLD [84].
A strategy of using the Enhanced Liver Fibrosis (ELF) test (which is a serum test that measures 3 molecules that are directly involved in liver matrix metabolism: (1) hyaluronic acid (HA), (2) procollagen III amino-terminal peptide (PIIINP), and (3) tissue inhibitor of matrix metalloproteinase 1 (TIMP-1)) for those with an indeterminate FIB-4, has a low false negative rate (8%) for predicting an elevated LSM (>8 kPa) when using an ELF cut-off of 9.8.[78] This strategy is also associated with a reduced referral rate of 14% [78]. A study based in primary care in the UK confirmed the effectiveness of the sequential use of FIB-4 followed by ELF, demonstrating a four-fold increase in the diagnosis of advanced fibrosis and cirrhosis and an 81% reduction in unnecessary referrals [85]. Other direct serum tests include Hepascore (a serum fibrosis model that uses bilirubin, α2-macroglobulin, hyaluronic acid, and γ-glutamyl transpeptidase) and Fibrometer (which includes indirect blood markers (AST, urea, platelets, prothrombin time) and direct (hyaluronate, alpha2-macroglobulin)) tests which have similar accuracy to VCTE for predicting liver-related events in patients with MAFLD [86]. In a study of 938 MAFLD patients, sequential use of FIB-4 followed by Hepascore or Fibrometer had diagnostic accuracies of 80–83% and 100% specificity for both approaches, but only 50–59% sensitivity for the diagnosis of advanced fibrosis [87]. A PRO‐C3‐based score (ADAPT) accurately identifies patients with MAFLD and advanced fibrosis [88, 89].
Implementing referral pathways in primary care needs to be combined with educational strategies to increase disease awareness and understanding of the prevalence, natural history and assessment of patients with MAFLD.[90] Computer decision support systems consisting of automatic liver fibrosis risk calculators and electronic reminders can aid in increasing referrals.[91] New combinations of novel NIT’s are likely to be more accurate and can result in fewer inappropriate referrals, however, they will need to be of low cost and easily available to enable widespread implementation. With effective pharmacotherapy likely available in the near future, referral pathways will need to be altered to identify ‘At Risk MASH’ (MASH and significant fibrosis, F2/F3 stage), the patient group targeted in current clinical trials.
Assessment of disease severity
Hepatic steatosis is traditionally detected by conventional ultrasonography, although this is limited by its non-quantitative nature, inter-operator variability and lower sensitivity for mild steatosis [92]. Controlled attenuation parameter (CAP), performed via VCTE, quantifies ultrasound attenuation radiofrequency which correlates with the histological degree of steatosis. Based on a meta-analysis of biopsy-based studies, optimal cut-offs of 248, 268 and 280 dB/m were reported for histological mild, moderate and severe steatosis respectively, achieving area under the curves of 0.82–0.89 [93]. While CAP measurements are extensively used due to their point-of-care applicability and easy reproducibility, variabilities in quantification still exist especially in patients with obesity and other metabolic risk factors and the association between CAP measurements and clinical outcomes are not well established. To optimize diagnostic performance, the obesity-specific XL probe has been developed [94], while an interquartile range of 40 dB/m is employed as a criterion for the original M probe [95]. Magnetic resonance (MR)-proton density fat fraction (PDFF) is the most accurate non-invasive method for steatosis quantification but has limited accessibility [96]. Additionally, blood biomarkers and scores, such as the fatty liver index (FLI), are considered suitable for epidemiological studies to detect hepatic steatosis in adults.
Histological steatohepatitis, characterized by hepatocyte injury and ballooning, is associated with an increased rate of disease progression and liver-related complications [[58](/article/10.1007/s12072-024-10774-3#ref-CR58 "Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015. https://doi.org/10.1016/j.cgh.2014.04.014
")\]. Serum ALT alone poorly predicts the presence of steatohepatitis \[[97](/article/10.1007/s12072-024-10774-3#ref-CR97 "Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013;33(9):1398–1405")\]. The FAST score, which combines LSM and CAP from VCTE and serum AST, was found in a meta-analysis to achieve a sensitivity and specificity of 89% for identifying patients with steatohepatitis, increased inflammatory activity and significant fibrosis \[[98](/article/10.1007/s12072-024-10774-3#ref-CR98 "Ravaioli F, Dajti E, Mantovani A, Newsome PN, Targher G, Colecchia A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: a systematic review and meta-analysis. Gut. 2023;72(7):1399–1409")\]. No other serum or ultrasound-based single biomarker or multimarker score was able to accurately predict steatohepatitis \[[99](/article/10.1007/s12072-024-10774-3#ref-CR99 "Vali Y, Lee J, Boursier J, Petta S, Wonders K, Tiniakos D, et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol. 2023;8(8):714–725"), [100](/article/10.1007/s12072-024-10774-3#ref-CR100 "Wong VW-S, Anstee QM, Nitze LM, Geerts A, George J, Nolasco V, et al. FibroScan-aspartate aminotransferase (FAST) score for monitoring histological improvement in non-alcoholic steatohepatitis activity during semaglutide treatment: post-hoc analysis of a randomised, double-blind, placebo-controlled, phase 2b trial. EClinicalMedicine. 2023;66:102310")\]. The combined assessment of a ≥ 30% decline in MRI-PDFF and a ≥ 17 U/L decrease in ALT are associated with an increased probability of steatohepatitis resolution (adjusted odds ratio 7.32), although this finding has yet to be validated in real-world practice \[[101](/article/10.1007/s12072-024-10774-3#ref-CR101 "Huang DQ, Sharpton SR, Amangurbanova M, Tamaki N, Sirlin CB, Loomba R. Clinical utility of combined MRI-PDFF and ALT response in predicting histologic response in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2023;21(10):2682-2685.e2684")\].Non-invasive tests are predictive of clinical outcomes. The abovementioned FIB-4 and LSM combination are independent predictors of liver-related events [81, 102], and on their own had a similar predictability as histologically-assessed liver fibrosis [65]. Non-invasive tests can hence be considered as alternatives to liver biopsy for disease prognostication.
Who should undergo liver biopsy?
Liver biopsy is the best method to evaluate hepatic architectural distortion and the complex relationships between fibrosis, inflammation, and cellular injury. While liver biopsy is not routinely recommended for the evaluation of MAFLD patients, it remains required for MAFLD evaluation in some circumstances. This includes patients with atypical presentations where histology can be an aid to diagnosis, especially in individuals with equivocal or discordant non-invasive test results. Patients enrolled in late phase clinical trials require liver biopsy as regulatory authorities require the resolution of steatohepatitis and/or improvement in fibrosis as endpoints. These endpoints can only be evaluated by liver biopsy [103]. Lastly, liver biopsy results are used as reference standards to validate non-invasive biomarkers.
There are limitations to liver biopsy including sampling error, interobserver variability, and cost, as well as some rare complications [104]. It is worth noting that staging and classification systems are heavily reliant on the assessment of fibrosis and inflammation. However, liver tissue sampling by a biopsy is one snapshot of a miniscule portion of the liver in an otherwise long-term, fluctuating chronic liver disease that spans decades. Liver biopsy does not consider changes in the inflammatory state, i.e. chronic or sporadic relapse, as is found in many chronic liver diseases [105] and this may also be the case with MAFLD.
Pathological recommendations: standardisation of assessment and reporting
If percutaneous liver biopsy is performed, a 14 or 16-gauge needle is recommended since there is no data to suggest a higher complication rate as compared to the use of a smaller needle [106]. Suction biopsy should be avoided since it can cause fragmentation of cirrhotic liver tissue. An optimum biopsy should be 1.5–2.5 cm in length and when possible, the right lobe of the liver is preferred since the left lobe is thinner with more fibrous septa closer to the liver capsule. Once obtained, fresh tissue should be immersed in 10% neutral buffered formalin immediately and left for fixation for at least 6 h, but less than 72 h.
The minimum required staining includes hematoxylin and eosin (H&E; for detection of morphological features including lobular inflammation, ballooning, and steatosis) and Masson trichrome (for detection of fibrosis); alternatively, Picrosirius red or Mallory’s stain can be used for detection of fibrosis.
Pathological reporting systems
The common system for evaluation of fibrosis in MAFLD is the Brunt score [107]. The NAFLD activity score (NAS) is composed of the unweighted sum of semiquantitative scores for steatosis, lobular inflammation and hepatocellular ballooning. Although a high NAS grade closely links to disease progression, grading should be used in conjunction with the NASH CRN fibrosis staging system.
The fatty liver inhibition of progression (FLIP) algorithm or SAF (steatosis, activity, and fibrosis) scoring system also provides a detailed histological assessment (Table 4). [108] [109]
Table 4 Comparisons of grading and staging of histological lesions in MAFLD
Overall, the NAS and SAF score share many similarities, but they are not interchangeable, and both the NASH CRN and SAF score show improved interobserver variability and has been validated clinically. Integration of both might be of added value. However, a recent study has shown that current reporting systems remain suboptimal for end-point determination in clinical trials [110, 111]. Digital image analysis and artificial intelligence (AI) show strong correlations with fibrosis but their performance is lower when assessing inflammation. More data will be generated in this field over the coming years.
Extrahepatic manifestations of MAFLD
MAFLD is a multi-system disease [112,113,114]. Existing evidence has confirmed that MAFLD is associated with dysfunction in multiple organ systems, including cardiovascular disease (CVD), chronic kidney disease (CKD), T2DM and extrahepatic cancers [115,116,117,118,119,120,121,122,123,124]. In addition, MAFLD is associated with sarcopenia, chronic obstructive pulmonary disease, SARS-Co-V2 infection and related morbidity, ischemic stroke and cognitive dysfunction [115, 125,126,127,128,129]. In one study, compared to non-MAFLD, MAFLD was associated with increased risk of 10 of the 24 examined cancers, including those of the uterus, gallbladder, liver, kidney, thyroid, oesophagus, pancreas, bladder, breast, colorectum and anal canal [130].
Many studies have shown that MAFLD is an independent risk factor for CKD, and its severity is associated with a ~ 1.3-fold higher risk of having CKD [115, 116, 131,132,133]. Similar to CKD, CVD is an important outcome event in the MAFLD population. Previous studies indicate that patients with MAFLD have 1.5 times the risk of fatal and non-fatal CVD events compared to patients without MAFLD. This result was also confirmed in a recent meta-analysis of seven cohort studies [120, 124, 134, 135].
The molecular mechanisms for these multi-system effects are complex. Proposed mechanisms include genetic predisposition, shared environmental risk factors and interacting metabolic diseases, the gut-liver axis, bile acids, endotoxins and adipokines in the setting of a dysmetabolic milieu. In concert to varying degrees, these factors promote MAFLD progression [136,137,138,139,140].
Management
Setting up integrated, multi-disciplinary care for MAFLD patients
The complex and bidirectional relationships between MAFLD and other conditions associated with metabolic dysfunction suggests that the implementation of integrated, multidisciplinary models for the delivery of standardized care can strike the right balance between cost-effectiveness, improved clinical outcomes, and patient satisfaction [24, 141, 142].
Implementing multidisciplinary models of care, comprising a hepatologist, a cardiologist, a diabetologist, a nephrologist and a medically supervised diet and exercise program, has been reported to be effective in improving liver and cardiometabolic-related health parameters, liver function, and weight loss [143,144,145]. Another study showed that a model tailored for MAFLD patients “ICHANGE”, coordinated by hepatologists but engaging other relevant subspecialties not only facilitated enhanced quality of care, patient compliance with treatment, and better alignment between healthcare providers and patients, but also added value in terms of cost-time efficacy and fewer hospital visits [146].
However, there are challenges in sustainably implementing care models, particularly with the diverse resourcing and health settings reflected in APASL countries. These include travelling long distances for a consultation to tertiary centres, developing one-stop coordinated consultations with various subspecialists, and having well-coordinated referrals without longer wait times. Further, training and collaboration between primary and secondary care and upskilling general practitioners for timely triage, screening, and referrals, as well as the need for constant capacity building to cater to the increasing burden of patients with increasing awareness is another issue [142]. Additionally, the high prevalence of the disease poses a challenge to the widespread application of these programs, requiring prioritization of those at greatest risk (i.e., MASH with significant fibrosis). Leveraging existing multidisciplinary care models, including in low-resource settings such as at diabetes, CKD, TB and HIV clinics, for managing MAFLD is a possible approach. Additionally, nurses and potentially nurse-led clinics play a critical role in providing critical primary care level services, which can be expanded to enhance care coverage.
Hence, while the adoption of integrated, multidisciplinary care models can streamline the delivery of standardized care to patients, the structure of the models and the composition of practitioners involved in organizing the care may vary depending on the context and healthcare system, particularly in resource-constrained settings. Therefore, it is crucial to consider the target population, settings, and subspecialties involved in multidisciplinary care, as well as the dynamics of integrating and coordinating services between primary, secondary, and tertiary care. Optimally done, this ensures an effective, cost-efficient, time-efficient, patient-centred approach, while also promoting health system sustainability [147, 148].
Lifestyle management recommendations
Lifestyle interventions, including diet and physical activity, are the main treatments for MAFLD. Overweight or obese MAFLD patients should be advised to lose 5–10% of body weight. A weight reduction of > 5% reduces liver steatosis, 7–10% leads to MASH resolution, and > 10% improves liver fibrosis [149]. Frequent self-weighing (at least weekly), reduced-calorie diets, and increased physical activity are associated with better long-term weight management.
Practical recommendations for dietary management
The dietary plan should be individualized while considering cultural background and social context. Dietary calorie restriction results in weight reduction and improvement of serum liver enzymes, hepatic steatosis, inflammation, and fibrosis [149,150,151,152]. MAFLD patients should be advised to consume 1200–1800 kcal a day or 500–750 kcal less daily for weight loss.
A diet comprising added sugar (fructose, high-fructose corn syrup, and sucrose), such as sugar-sweetened beverages, excess saturated fat (for instance, palm oil), as well as ultra-processed foods (UPF), should be avoided [151, 153]. UPF is a potential risk factor for MAFLD, obesity, and metabolic syndrome [154].
The Mediterranean-type dietary pattern is the most evidence-based for MAFLD [155,156,157,158,159]. This diet is characterized by a reduced intake of refined carbohydrates, sugars, and processed foods while increasing consumption of monounsaturated and omega-3 fatty acids. A Mediterranean-style diet is associated with a reduced risk of T2DM, CVD, liver fat, and fibrosis in patients with MAFLD [160,161,162]. In addition, this dietary pattern is associated with a reduced risk for HCC [163]. A diet style tailored to local eating habits may improve adherence. However, this needs further validation in future studies.
Intermittent fasting and time-restricted feeding have recently gained popularity. Intermittent fasting involves alternating feeding days with fasting days, while time-restricted feeding involves restricting eating to an 8 h or less period each day within a 24 h cycle. Both dietary approaches lead to significant weight reduction and metabolic improvements in overweight and obese subjects. Evidence regarding the efficacy of these dietary strategies on MAFLD is limited. However, a meta-analysis suggested, with moderate- to high-quality evidence, that intermittent fasting and time-restricted feeding improve hepatic inflammation, steatosis, and stiffness, as well as promoting weight loss in adults with MAFLD [164].
The ketogenic diet is a high-fat, low-carbohydrate (less than 20–50 g/day) dietary pattern. Ketogenic diets reduce body weight and liver steatosis but not fibrosis as suggested by previous small randomized-controlled trials [165, 166]. At this time, ketogenic diets should only be followed after recommendation and support from a health care professional or dietitian- and is likely only suitable and sustainable in the short term. Further studies are required to assess the effects of ketogenic diets on liver-related outcomes and the sustainability of this diet approach in MAFLD.
Coffee consumption (three or more cups per day), regardless of caffeine content, is considered to be beneficial. Epidemiological and meta-analyses have demonstrated that coffee consumption is associated with a lower risk of MAFLD and decreased liver fibrosis in patients with MAFLD [167, 168]. However, a caveat is that all these studies are non-randomised, epidemiological reports that compare coffee drinkers to non-coffee drinkers and are therefore potentially subject to unmeasured confounding.
Practical recommendations for exercise management
Physical activity is an integral component of the multidisciplinary care of patients with MAFLD and should be evaluated (e.g., via the simple Physical Activity Vital Sign [169]) and prioritised [170, 171]. While a combination of diet and structured exercise training has synergistic benefits for MAFLD [172, 173], regular exercise alone elicits broad hepatic and cardiometabolic benefits irrespective of weight loss and can improve health-related quality of life [170]. Regular exercise improves insulin sensitivity, reduces inflammation, and alters substrate metabolism in the muscle, liver and adipose tissue, which affects hepatic free fatty acid flux [174,175,176,177,178].
A total of 150–240 min per week of moderate-to-vigorous-intensity aerobic exercise is recommended for reducing hepatic steatosis by 2–4% (absolute reduction), equating to a clinically meaningful ~ 30% relative reduction in liver fat [33, 133, 179]; however as little as 135 min per week may be effective [170]. Brisk walking, cycling, and jogging are modalities commonly reported to elicit a benefit. This volume of aerobic exercise is likely to reduce visceral adiposity, increase cardiorespiratory fitness, reduce LDL-cholesterol and improve vascular health in people with MAFLD, although large-scale randomised controlled trials with long-term follow-up are lacking [170].
While intensity-dependent benefits are not apparent for hepatic steatosis [180,181,182], emerging evidence demonstrates that high-intensity interval training (HIIT) involving one or more bursts of high-intensity exercise interspersed with lower-intensity recovery periods is equally effective under the supervision of an exercise professional [181] [183].
There are limited data to inform the efficacy of exercise for the histological features of MAFLD given the challenges associated with repeated liver biopsy. Pilot data has indicated a one-stage regression in liver fibrosis and hepatocyte ballooning in 58% and 67% of MAFLD participants, respectively, with 12 weeks of moderate-vigorous aerobic exercise on 3–5 days per week [184]. Histological improvements were more strongly associated with improvements in cardiorespiratory fitness than weight loss [184]. Moreover, moderate-intensity aerobic exercise led to improvements in serum- and imaging-based surrogates of liver fibro-inflammation and histological activity [185, 186].
The benefits of resistance training for MAFLD are less clear with equivocal findings for efficacy on hepatic steatosis, likely due to the heterogeneity of training methodologies [170]. However, given the interrelationship between sarcopenia and MAFLD [187], and the profound benefits of resistance training on lean mass, bone mass, blood pressure and glycaemic control [[188](/article/10.1007/s12072-024-10774-3#ref-CR188 "El-Kotob R, Ponzano M, Chaput JP, Janssen I, Kho ME, Poitras VJ, et al. Resistance training and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020. https://doi.org/10.1139/apnm-2020-0245
"), [189](/article/10.1007/s12072-024-10774-3#ref-CR189 "Abou Sawan S, Nunes EA, Lim C, McKendry J, Phillips SM. The health benefits of resistance exercise: beyond hypertrophy and big weights. Exercise Sports Movement. 2023;1(1):e00001")\], resistance exercise is likely beneficial for many individuals with MAFLD and should be recommended in addition to aerobic exercise. A meta-analysis showed that resistance exercise improves MAFLD with less energy consumption \[[190](/article/10.1007/s12072-024-10774-3#ref-CR190 "Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66(1):142–152")\]. While data is lacking, resistance training on 2–3 days per week should be prioritised in MAFLD patients with co-existing sarcopenia, T2DM, low functional capacity and/or those reducing body weight substantially via pharmacological approaches to minimise losses of lean and bone mass. There are no evidence-supported recommendations regarding the intensity, frequency, number of sets and repetitions of resistance exercise required for hepatic benefit \[[170](/article/10.1007/s12072-024-10774-3#ref-CR170 "Keating SE, Sabag A, Hallsworth K, Hickman IJ, Macdonald GA, Stine JG, et al. Exercise in the management of metabolic-associated fatty liver disease (MAFLD) in adults: a position statement from exercise and sport science Australia. Sports Med. 2023;53(12):2347–2371")\].It is prudent to acknowledge that most MAFLD patients will have low initial cardiorespiratory fitness and varying degrees of cardiovascular dysfunction and therefore a stepped approach to achieving exercise recommendations is required. Referral to an appropriately qualified exercise professional is recommended to provide tailored prescriptions, cognisant of individual capability and preferences, which address common barriers to exercise in MAFLD (e.g., time, access to equipment/facilities, musculoskeletal limitations, low exercise-related self-efficacy) [191,192,193].
Metabolic surgery and endoscopic bariatric and metabolic therapies for MAFLD
Metabolic surgery and Endoscopic Bariatric and Metabolic Therapies (EBMT) are highly effective in the management of morbid obesity. While they have not been tested specifically for the treatment of MAFLD, multiple studies have demonstrated efficacy in improving the features of MAFLD [194,195,196].
Metabolic surgery, which includes sleeve gastrectomy, Roux-en-Y gastric bypass, adjustable gastric banding or biliopancreatic diversion, is an accepted standard of care for morbid obesity. These therapies are effective in inducing sustained weight loss by up to 30%, and also improve metabolic dysfunction and adverse clinical outcomes from T2DM, and cardiovascular disease and reduce overall mortality [197]. Within the Asian context, the ethnicity-adjusted BMI risk thresholds for obesity should be used and should also take into account metabolic comorbidity risk [198]. In the Asian population, a BMI ≥ 25 kg/m2 suggests clinical obesity, and individuals with a BMI ≥ 27.5 kg/m2 should be considered for metabolic and bariatric surgery.
Specific to MAFLD, several meta-analyses have shown concordant observations that bariatric surgery results in the resolution of steatosis, ballooning degeneration and inflammation in up to 50% of patients; about 24% demonstrate improvement in liver fibrosis [196, 199, 200]. Interestingly, pooled analysis shows that Asian MAFLD patients, respond better to metabolic surgery in terms of improvement of liver enzymes, steatosis and fibrosis compared to non-Asian patients [196]. Randomized controlled studies with bariatric surgery have demonstrated up to 3.6-fold higher efficacy in the resolution of biopsy-proven steatohepatitis without worsening of liver fibrosis compared to standard medical therapy [201]; these benefits can be sustained for up to 5 years [202]. Large cohort studies have also shown a reduction in the 10-year cumulative incidence of major adverse liver outcomes from 9.6 to 2.3% compared to a matched non-surgical cohort [203].
While the efficacy of bariatric surgery for MAFLD is acknowledged, it is worth noting that the evidence for patients with advanced fibrosis undergoing bariatric surgery is still low. Liver fibrosis can progress in a subset of these patients and monitoring is recommended [200]. A further note of caution is that while bariatric surgery is relatively safe in compensated cirrhosis, adverse events and mortality can be as high as 18% in decompensated cirrhosis, highlighting the need to screen for portal hypertension [204, 205].
Endoscopic bariatric and metabolic therapies are a rapidly evolving field, where non-surgical endoscopic procedures are used to replicate the outcomes of bariatric surgery. Some of the techniques include intragastric balloons, endoscopic sleeve gastroplasty; primary obesity surgery endoluminal (POSE) plication device, aspiration therapy, and transpyloric shuttle. These broad-ranging techniques were analysed in a meta-analysis of 18 high-quality studies (including 5 from Asia) and showed promising efficacy for weight loss, improvement in liver enzymes, steatosis, steatohepatitis and fibrosis, albeit not all used gold standard liver biopsies [194, 195]. While EBMT theoretically has lower morbidity compared to bariatric surgery, the relative sustained efficacy for MAFLD needs to be determined and the risk of potential adverse events such as ulcer bleeding, leaks and peritonitis requires further study.
Pharmacological treatment
Current potential therapies
Recent studies have focused on the potential for repurposing approved agents for alleviating steatosis, inflammation and fibrosis in MAFLD. Among these, evidence on the efficacy of anti-diabetic medications is rapidly accumulating.
The beneficial effects of pioglitazone on hepatic histology have been reported in patients with steatohepatitis, with and without T2DM [206, 207]. A double-blind randomized trial showed that a 24-weeks pioglitazone treatment was well-tolerated and effective in improving liver histology and reducing liver steatosis in Asian patients with MASH [208]. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have been widely used for the treatment of T2DM and cardiovascular disease, with some evidence of benefit for MAFLD [209, 210]. The beneficial effects of SGLT2i’s such as dapagliflozin, empagliflozin and canagliflozin on liver fat content has been reported as well as improvement of serum transaminases and non-invasive scores for fibrosis [209,210,211,212,213,214]. There remains a paucity of data on the efficacy of these agents for the improvement of liver inflammation and fibrosis. Recent reports demonstrate that licogliflozin led to improvement in surrogate markers of liver fibrosis assessed by the ELF score and serum PIIINP levels [215]. Similarly, empagliflozin led to significant reductions in LSM, but not other non-invasive scores of fibrosis [216]. These reports lack histological evidence for improvements in steatohepatitis or fibrosis. Trials of ipragliflozin and tofogliflozin included paired liver biopsy to demonstrate histological benefits in MAFLD patients [216, 217], nevertheless, the relatively small sample sizes mean that the level of evidence is not strong. In addition, most of these studies were carried out among T2DM patients with MAFLD, with few investigating SGLT2 inhibitors in non-diabetic patients with MAFLD [218]. Metformin does not improve hepatic histology in patients with MAFLD [219,220,221,222]. However, metformin improves insulin resistance [219, 221, 222] and reduces the risk of HCC in patients with MAFLD, although available studies have not been prospective or randomized [223, 224]. A non-randomized interventional cohort study reported that among oral antidiabetic drugs for patients with T2DM accompanied by MAFLD, SGLT2 inhibitors are preferable considering the improvement of MAFLD and the reduction of incident adverse liver-related outcomes when compared to thiazolidinediones, DPP4 inhibitors, and sulfonylureas. This retrospective study was conducted using South Korea’s National Health Insurance Database. It followed 80,178 patients over 219,941 person-years follow-up and demonstrated improvement of MAFLD in 4,102 cases. SGLT2 inhibitors had a higher likelihood of improving MAFLD and significantly lower rates of adverse liver-related outcomes such as liver-related hospitalizations, death, liver transplants, and HCC compared to other oral antidiabetic drugs [225].
Vitamin E has been reported to be effective in improving hepatic histology in patients with steatohepatitis [226,227,228,229]. However, several studies have failed to demonstrate its beneficial effects and level 1 evidence is thus lacking [216, 221, 230, 231]. Recently, a propensity score matching analysis demonstrated that vitamin E decreases the risk of death or transplant and hepatic decompensation in MASH patients with bridging fibrosis or cirrhosis [217]. The development of prostate cancer and hemorrhagic stroke is a possible concern with vitamin E [218].
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) might have beneficial effects for MAFLD [232], with some reports from observational studies showing their use to be associated with milder liver fibrosis [233] and a reduced risk of liver-related events. However, the benefits of ACEIs or ARBs for MAFLD patients with early-stage fibrosis need further study.
One international phase II placebo-controlled randomized comparative trial investigated the therapeutic effects of low-dose aspirin on MAFLD without cirrhosis. Patients were randomly assigned in a 1:1 ratio to a low-dose aspirin group (81 mg once daily) and a placebo group, and treated for six months. The primary endpoint was a change in liver fat content based on MRS at six-month marks. The change in liver fat content was 3.6% in the placebo group, whereas it was − 6.6% in the low-dose aspirin group (difference between groups: − 10.2%, 95% confidence interval [CI] − 27.7 to − 2.6, p = 0.009). [234]
Statins have shown potential benefits on liver function tests in patients with MAFLD [235, 236], but their efficacy on steatosis or fibrosis is uncertain. Recently, ezetimibe with rosuvastatin treatment reduced liver fat as assessed by MRI-PDFF, but not fibrosis [237]. Future studies with larger sample sizes and robust endpoints are required, ideally based on plausible mechanistic data.
Pipeline of new drug treatments
A number of new drugs have shown promise in phase 2b or registration phase 3 studies. With the approval of Resmetirom by the FDA in March 2024, it is anticipated that more pharmacotherapies may be available for the treatment of MASH in the foreseeable future. Resmetirom, a selective thyroid hormone receptor-beta agonist, demonstrated positive results in two phase 3 studies. In the MAESTRO-NASH study, patients with biopsy-proven at-risk MASH were randomized to placebo (n = 318), resmetirom 80 mg (n = 316) or resmetirom 100 mg (n = 321) [238]. The study reached both interim histological endpoints at week 52. MASH resolution with no worsening of fibrosis was achieved in 26% and 30% in the resmetirom 80 mg and 100 mg arms, respectively, compared with 10% in the placebo arm. Fibrosis improvement without worsening of MASH occurred in 24% in the 80 mg arm, 26% in the 100 mg arm, and 14% in the placebo arm. In the accompanying MAESTRO-NAFLD-1 study based entirely on noninvasive tests, resmetirom again demonstrated superiority in reducing hepatic fat, liver stiffness, serum LDL-cholesterol, apolipoprotein B and triglycerides [239]. Resmetirom is well tolerated with a mild increase in diarrhea and nausea. No cardiovascular toxicity has been reported. Prescribing resmetirom necessitates thorough patient assessment by a specialist and should be overseen within a multidisciplinary context. Resmetirom can be considered in patients with stage 2 or stage 3 fibrosis or those with histological evidence of steatohepatitis, using several non-invasive criteria or liver biopsy, depending on their availability in individual practice settings. It is recommended that patients in the early stages of MAFLD not be treated, as also patients likely to have established cirrhosis. The benefits of resmetirom in this population are still being assessed in a phase 3 cirrhosis trial. Patients meeting treatment criteria should receive weight-based dosing, with 80 mg for patients weighing less than 100 kg and 100 mg for those weighing over 100 kg. There are still unanswered questions regarding the duration of treatment and the criteria for stopping treatment due to lack of effectiveness. Ongoing analysis of emerging data, including real-world evidence, is likely to provide further guidance in the future, especially concerning monitoring therapeutic response and clinical outcomes.
One of the biggest breakthroughs in the management of obesity and T2DM is the introduction of glucagon-like peptide-1 receptor agonists (GLP-1RA). This class of drugs reduces appetite and slows gastric emptying, resulting in weight loss of 5–15% [240]. In clinical trials with long-term follow-up, GLP-1RAs also reduced major adverse cardiovascular events and mortality. In a phase 2b study in patients with MASH, semaglutide at a dose of 0.4 mg daily given subcutaneously for 72 weeks achieved MASH resolution with no worsening of fibrosis in 59% of patients, compared with 17% in the placebo group, though differences in fibrosis improvement did not reach statistical significance [241]. In a subsequent study in patients with compensated MASH-related cirrhosis, semaglutide at a dose of 2.4 mg weekly failed to increase the rate of either MASH resolution or fibrosis improvement [242]. This suggests that the weight loss effect of GLP-1RAs, though potent, may be too late for patients with advanced liver disease. Currently, dual and triple incretin agonists involving glucose-dependent insulinotropic polypeptide and glucagon receptor are under development. The newer drugs have demonstrated superiority in reducing body weight and glycated hemoglobin compared to GLP-1RA alone, but their effects on MASH and liver fibrosis remain to be proven [243, 244]. Tirzepatide is a single molecule that combines glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonism; it demonstrated positive effects on both MASH resolution and fibrosis improvement in a relatively short phase 2 study [243]. These promising results need to be replicated in phase 3 studies, which are underway. The need for subcutaneous injections and gastrointestinal side effects (most notably nausea and vomiting and the potential increased risk of pancreatitis) are the main liability of this class of drugs, with treatment cessation required in around 10% of patients. Survodutide is another GLP-1/glucagon dual agonist that demonstrated histological improvement of MASH in a phase 2 randomized trial [245].
Lanifibranor (a pan-PPAR agonist) [246] and fibroblast growth factor-21 analogues (e.g., efruxifermin and pegozafermin) have also shown promising results in early-stage clinical trials, while phase 3 studies are underway [247, 248].
Monitoring progress and response to treatment
There is currently no agreement on the most effective approach for monitoring patients with MAFLD and their response to pharmacotherapy [249]. Given that the severity of fibrosis is the principle determinant of both liver-related outcomes and mortality, and as patients with MAFLD are expected to progress by an average of 0.12 (range: 0.07 − 0.18) fibrosis stages per year [[58](/article/10.1007/s12072-024-10774-3#ref-CR58 "Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015. https://doi.org/10.1016/j.cgh.2014.04.014
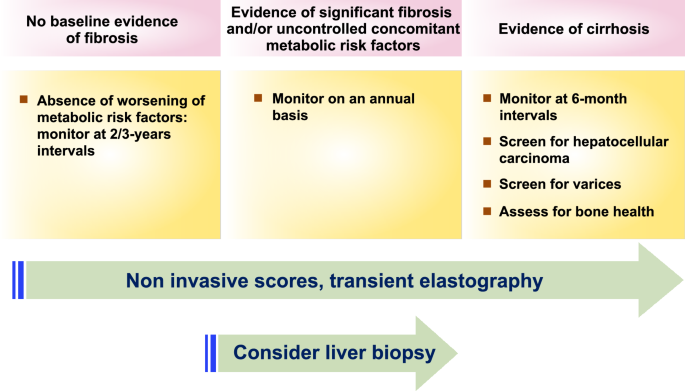
"), [250](/article/10.1007/s12072-024-10774-3#ref-CR250 "Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1611-1625.e1612")\], the following algorithm is recommended (Fig. [2](/article/10.1007/s12072-024-10774-3#Fig2)).Fig. 2
Monitoring protocol for patients with MAFLD in clinical practice
Interval of follow-up
- Patients without fibrosis should be monitored at 2–3 years intervals if there has been no worsening of concomitant metabolic risk factors.
- Patients with fibrosis or evidence of uncontrolled concomitant metabolic risk factors should be monitored on an annunal basis, particularly with the emergence of new drugs.
- For selected patients at high risk of fibrosis progression, monitoring might involve baseline liver biopsy assessment, unless they already have established cirrhosis, where MELD scoring may be required.
- Patients with cirrhosis should undergo monitoring at 6-month intervals, including surveillance for HCC and even clinically significant portal hypertension (please see the next sections for details).
Method of follow-up
Monitoring for fibrosis progression in clinical settings can rely on a combination of noninvasive scores (such as FIB-4) and liver stiffness measurement, although this approach requires further validation.
Patient-reported outcomes
A person living with MAFLD plays a vital role in their disease management as the cornerstone of treatment is adherence to a healthy diet and being physically active [24, 156, 251]. Despite the high prevalence of MAFLD, it has a lower level of awareness among the general population compared to that of other metabolic diseases. A global study of patients with MAFLD reported an impairment in quality of life [252]. The impairment of health status is more pronounced in patients with advanced liver disease [253, 254]. This calls for strategic efforts to integrate prevention and management with patient involvement at the core.
Systematic reviews of studies of people with chronic liver diseases including MAFLD reveal several unmet needs which could help them better cope with their chronic illness and improve their quality of life. Affected communities have highlighted the need for high-quality education and health promotion information to better understand and manage their disease, and the need for support services [253]. For the initiation and maintenance of lifestyle change in patients with MAFLD and for patient empowerment, there is evidence to support the use of digital technology for providing their screening results together with advice on lifestyle changes [255, 256]. Recommendations for empowering patients with MAFLD are provided in supplementary Table 1.
MAFLD-related cirrhosis
With the prevalence of MAFLD increasing worldwide, a considerable proportion will progress to cirrhosis and other associated complications [257]. In a Medicare data analysis of over 10 million patients, the cumulative risk of progression to cirrhosis was 39%, and from compensated to decompensated cirrhosis it was 45%, over 8 years of follow-up [258].
Medical history and physical examination can identify patients with or at risk for cirrhosis. The diagnostic criteria for MAFLD cirrhosis is patients with cirrhosis who do not exhibit typical histology but have past or present evidence of metabolic risk factors that meet the criteria for diagnosing MAFLD. These patients can be diagnosed as having MAFLD cirrhosis if they in addition, meet at least one of the following additional criteria. a) documentation of steatosis on a previous liver biopsy; b) historical documentation of steatosis by hepatic imaging [24, 259].
Portal hypertension assessment in patients with MAFLD
In patients with MAFLD, the presence of portal hypertension is not always aligned with liver disease progression and is recognised to occur in people with MAFLD but without cirrhosis [260, 261]. Therefore, early detection of the possible complications of portal hypertension, such as the presence of esophageal as well as gastric varices is important. Additional clinical features that are surrogate markers of clinically significant portal hypertension (CSPH) include the presence of intraabdominal varices by imaging, and ascites.
Hepatic venous pressure gradient (HVPG) measurement is the gold standard for portal hypertension assessment. However, this procedure might not be feasible in the same session as esophagogastroduodenoscopy for variceal screening and is not widely accessible in many APASL countries [263,264,264]. Endoscopic ultrasound (EUS)-guided portal pressure gradient has recently been studied for more accurate portal hypertension assessment, especially in the presence of varices. This can be performed together with other procedures such as endoscopic band ligation, EUS-guided liver biopsy, and EUS-guided cyanoacrylate injection of large gastroesophageal varices [266,267,267]. However, considerable technical expertise is required to establish these evolving procedures. The combination of baseline liver stiffness measurement and platelet count (Baveno-VII criteria) correlates with CSPH and could be used to define CSPH. The recently proposed novel prediction model, the LS-spleen diameter to platelet ratio score (LSPS), uses TE values and the spleen diameter to platelet ratio, which also reflects CSPH [268]. Additionally, the ANTICIPATE model predicts CSPH in patients with viral and alcohol-related cACLD using two parameters: platelet count and LSM. The ANTICIPATE–NASH model is an adapted version for MAFLD patients, incorporating BMI. This model could be a valuable clinical tool for assessing the risk of liver-related events in MAFLD patients [269].
Screening for varices in MAFLD cirrhosis
There is insufficient data to support the utilization of any serological markers such as platelet count alone or the enhanced liver fibrosis panel to exclude CSPH and eliminate the need for endoscopic assessment in detecting varices that require treatment. LSM is recommended for determining thresholds for non-invasive screening of varices in patients with MAFLD-related cirrhosis. LSM less than 9 kPa, in the absence of other known clinical signs, rules out compensated advanced chronic liver disease (cACLD). LSM ≥ 15 kPa is suggestive of cACLD [270]. An LSM ≥ 25 kPa indicates CSPH and a CAP less than 220 dB/m, measured using the XL probe identifies patients with MAFLD-related cACLD who are at high risk for decompensation [270]. The threshold of 21 kPa was confirmed independently to be associated with a higher occurrence of hepatic decompensation. Not only baseline LSM but also change in LSM is associated with the risk of liver-related events and mortality [271].
In patients with MAFLD-related cirrhosis, although HVPG ≥ 10 mmHg remains strongly associated with the presence of clinical signs of portal hypertension, these signs can also be present in a small proportion of patients with HVPG values < 10 mmHg. About 40–50% of patients with MAFLD belong to the “gray zone” of LSM 15–25 kPa, in which a precise estimation of the risk of CSPH is not possible. In this instance, spleen stiffness measurement (SSM) can be used to reduce the proportion of patients in the indeterminate group [272, 273]. About 40–60% of patients in the Baveno VII diagnostic algorithm remain in the grey zone. The addition of SSM (40 kPa) to the model significantly reduced the grey zone to 7–15%, maintaining adequate negative and positive predictive values [274].
Non-selective beta blocker(s) (NSBBs) such as propranolol, nadolol, or carvedilol should be considered as a treatment to prevent decompensation in patients with CSPH. Carvedilol is the preferred NSBB for compensated cirrhosis because it effectively reduces HVPG, has greater benefits in preventing decompensation, is better tolerated, and has been proven to improve survival compared to no active therapy in compensated patients with CSPH [275]. The decision to use NSBBs should be based on clinical indicators, regardless of the ability to measure HVPG. Patients with compensated cirrhosis who are taking NSBBs to prevent decompensation do not require a screening endoscopy to detect varices, as endoscopy will not change their management.
Individuals with compensated cirrhosis who cannot initiate NSBB use (due to contraindication or intolerance) to prevent decompensation should undergo an endoscopy for variceal screening if their LSM is 20 kPa or higher, or if their platelet count is 150 × 10^9/L or lower. For patients unable to use NSBBs and requiring an endoscopy based on the Baveno VI criteria (LSM by TE ≥ 20 kPa or platelet count ≤ 150 × 10^9/L), spleen stiffness measurement by TE of 40 kPa or lower can identify those at low risk of high-risk varices, potentially making endoscopy unnecessary. Patients with cACLD who are on NSBB therapy and show no clear signs of CSPH (LSM < 25 kPa), should be considered for repeat endoscopy, preferably after 1–2 years. If no varices are detected, NSBB therapy can be stopped. In cACLD patients with high-risk varices who cannot use NSBBs due to contraindications or intolerance, endoscopic band ligation is recommended to prevent the first occurrence of variceal bleeding.
Screening for HCC
MAFLD is a major etiology for HCC in the Asia–Pacific region with substantial morbidity and mortality; its prevalence is expected to further increase in the coming decades. [2, 24, 277,278,279,280,281,281] In addition, MAFLD is associated with the onset/recurrence of HCC and poor prognosis in patients with viral hepatitis [283,284,285,286,287,288,289,290,290].
When HCC is diagnosed at an early stage, there are existing acceptable and curative therapies, for example, surgical resection, local ablation, or liver transplantation [291]. Therefore screening can reduce HCC-specific mortality and is recommended [292, 293].
The key concern about surveillance/screening for HCC is the threshold (annual incidence of HCC), with the aim of reaching cost-effectiveness. Based on cost-effectiveness analyses, when the incidence of HCC is > 1 or 2%/year, it is cost-effective to perform HCC screening/surveillance [295,296,297,297]. In contrast, when the incidence of HCC is < 0.2%/year, the cost-effectiveness is poor and screening is not recommended [295,296,297,297].
For MAFLD-related HCC, the threshold for screening is recommended at 1%/year [296]. Thus, for MAFLD-related cirrhosis subjects (annual incidence of HCC: 1–1.5%/year), screening is cost-effective and recommended [296,297,298,298]. In contrast, for MAFLD-related non-cirrhosis patients, the annual incidence of HCC is 0.08–0.63 per 1,000 person-years [298]. Thus, screening of this low-risk population is not recommended.
Notably, recent epidemiologic data reveals that about 35–47% of MAFLD-related HCCs develop in people without cirrhosis [299, 300]. Accordingly, if the screening/surveillance is done only in patients with cirrhosis, HCC in subjects without cirrhosis would likely be diagnosed at a late stage with resultant poor outcomes. Nevertheless, if all subjects without cirrhosis with MAFLD are screened, then the cost-effectiveness would be quite low. To resolve this issue, more sensitive and reliable biomarkers of risk, or scoring systems for surveillance that improve cost-effectiveness is required [299,300,301,301]. In this way, patients with non-cirrhotic MAFLD but at higher risk of developing HCC can be identified and placed on surveillance. Commonly used screening tests (non-invasive tumor biomarkers such as AFP, AFP-L3, PIVKA-II; and liver imaging such as by ultrasonography) have low- intermediate sensitivity/specificity [292, 293], so their specific role in non-cirrhotic MAFLD is yet to be clarified. Alternatively, big data or the development of an AI-based prediction model may help in the identification of high-risk populations for HCC surveillance [299,300,301,302,302].
Management of MAFLD-related HCC
Control of metabolic dysfunction plays an important role in the management of patients with HCC [303]. A high BMI is one of the criteria for MAFLD diagnosis [304]. In Western populations, a high BMI is linked to higher HCC-related mortality [305]. However, there appears to be no relationship between a high BMI and HCC-related mortality in Asian patients based on meta-analysis and cohort studies [307,308,308]. Sarcopenia may explain this inconsistency, as it is highly prevalent in patients with HCC [310,311,312,312] and is a prognostic factor in Asian patients with HCC [307, 314,315,316,317,318,319,320,321,321].
Physical activity is linked to improved survival in HCC patients [322]. A recent meta-analysis revealed that combining resistance with aerobic exercise reduces serious adverse events [323].
T2DM is a criterion for MAFLD diagnosis. Metformin has been demonstrated to significantly reduce the risk of HCC in MAFLD patients with an HbA1c level above 7.0% [223]. Additionally, a meta-analysis demonstrated that metformin prolonged the survival of HCC patients with T2DM after curative HCC treatment [224]. Therefore, metformin, along with lifestyle intervention, may be a beneficial treatment for MAFLD-related HCC patients with T2DM.
Nutritional therapy focusing on protein intake and correcting amino acid imbalance might be beneficial for the management of patients with MAFLD-related HCC. The administration of branched-chain amino acids is useful for the management of adverse events from the treatment of HCC. Theoretically, this includes improvements in body composition and nitrogen balance, liver cell regeneration, protein and albumin synthesis and immune function [324].
Liver transplantation is the treatment of choice for patients with early HCC whose liver function does not allow for surgical resection. It is also recommended for patients with a locally advanced disease if their tumors can be reduced in size, number, and alpha-fetoprotein (AFP) production to acceptable levels through locoregional therapy. Immunotherapy is currently the first-line treatment for unresectable HCC not amenable to locoregional therapy. However, its anti-tumor effect has been reported to be limited for MAFLD-related HCC [325, 326]. Lenvatinib is a multi-kinase inhibitor and effectively inhibits the tumor vasculature, resulting in anti-tumor effects. Lenvatinib is used for the treatment of unresectable HCC and treatment response and prognosis is reported to be better in HCC patients with MAFLD than non-MAFLD [327]. In other studies, lenvatinib is associated with a significant survival benefit compared to immunotherapy in patients with MAFLD-related HCC [326, 328].
MAFLD is associated with various extra-hepatic complications and since these events could affect the prognosis and quality of life of patients, those with MAFLD-related HCC need to be managed with attention not only to the liver but also to systemic complications.
Liver transplantation for MAFLD
MAFLD has emerged as a leading indication for liver transplantation globally for both decompensated cirrhosis and for HCC, which introduces some unique challenges [329]. These patients are often older that those with other indications for liver transplantation and commonly have significant underlying comorbidities that increase the short and long-term risks of transplantation [330]. The over-representation of obesity, T2DM, hypertension, cardiovascular disease, peripheral and cerebro-vascular disease, CKD [330, 331], and sarcopenia [332] all increase the risk and complexities of liver transplantation. Even if successfully transplanted, the long-term risks from cardiovascular disease, CKD and malignancy often remain [333]. Despite these added risks, well-selected patients with MAFLD-related HCC appear to have posttransplant survival equivalent to other patient groups[330], however those with decompensated MAFLD cirrhosis, particular with CKD requiring simultaneous liver and kidney transplantation, may have significantly inferior outcomes [334, 335].
Patient selection for transplantation requires a detailed assessment of end-organ disease that may preclude liver transplantation. As cardiac complications are a leading cause of morbidity and mortality in liver transplant recipients and MAFLD patients are at particular risk, a comprehensive cardiovascular assessment is essential to identify risks, to allow for pretransplant optimisation of cardiac status, and to potentially exclude some candidates. Pretransplant assessment in MAFLD patients should be standardised and usually should incorporate electrocardiography, transthoracic and stress echocardiography, and in some patients, coronary CT angiography, invasive coronary angiography and cardiology consultation [336]. Even after MAFLD patients are placed on a transplant waiting list, challenges remain as they are less likely to receive a transplant and have higher waitlist mortality than non-MAFLD patients [337].
Donor selection for liver transplantation is impacted by the increasing rates of MAFLD and other metabolic diseases in the community that are present in both living and deceased donors. Moderate to marked steatosis in particular poses a significant risk of primary nonfunction, early allograft dysfunction and biliary strictures [338]. Potential living donors with obesity and steatosis may be able to be utilised with good outcomes particularly if subject to short-term weight loss interventions [339] but may be at risk for perioperative wound complications [340], and post-donation metabolic syndrome [341]. Steatotic deceased donor grafts are frequently discarded but may be able to be used in well-selected recipients and optimal peritransplant conditions [342]. Machine-based perfusion strategies may broaden the acceptability and utilisation of steatotic grafts in the future [343]. Patients transplanted for MAFLD may be adversely impacted by the presence of donor metabolic risk factors, particularly diabetes [344].
Patients transplanted for MAFLD are at risk of recurrent graft steatosis and complications of metabolic syndrome. Immunosuppressive medications may compound the risk for metabolic complications, and steroid minimisation or avoidance, and minimisation of calcineurin inhibitor dosing should be considered [345]. Multidisciplinary management with a focus on weight control (including consideration for bariatric surgery and its timing), optimal T2DM, hypertension and lipid management, and monitoring for complications is essential in transplant recipients to ensure good long-term outcomes of liver transplantation [346].
Management of the patient with MAFLD cirrhosis beyond normal cirrhosis care, and management of MAFLD-related hepatic decompensation
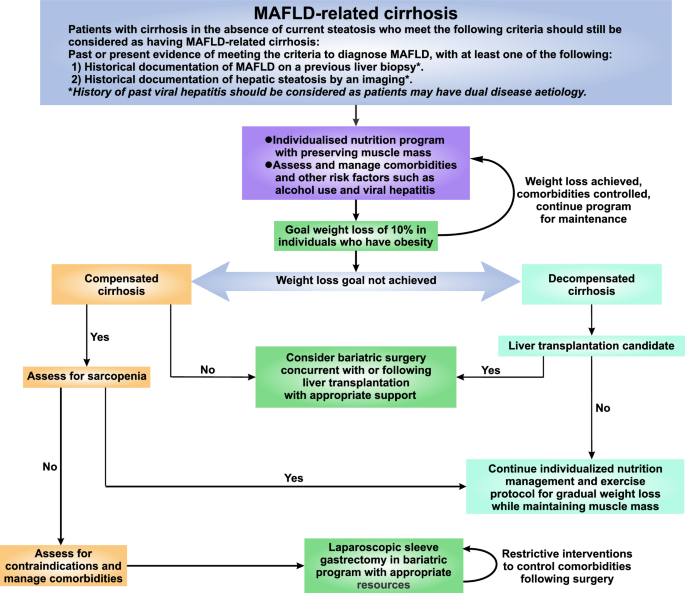
While standard cirrhosis care continues to apply in patients with MAFLD-associated cirrhosis, peculiarities distinct from other liver etiologies need to be considered (Fig. 3). Unlike other liver disease etiologies, as a multi-system disease, MAFLD is commonly intertwined with multiple metabolic comorbidities that may impact the progression of disease and its complications [347]. Optimization of these comorbidities is integral to manage MAFLD-related cirrhosis effectively [348].
Fig. 3
Recommended algorithm to diagnose and manage patients with MAFLD-related cirrhosis
Lifestyle interventions, although recognised as the cornerstone of MAFLD management, can be challenging to institute and sustain long term, particularly in patients with MAFLD-related cirrhosis [349]. The optimal target for weight loss among these patient is unclear, however taking into consideration the high prevalence of sarcopenia and sarcopenic obesity associated with MAFLD-related cirrhosis, the management approach should emphasise weight loss with fat mass reduction while maintaining muscle mass and strength [350]. This includes specific dietary and physical activity considerations that are personalized for each individual and delivered ideally by a multidisciplinary team through a dedicated structured program [348].
While a Mediterranean-type dietary pattern is beneficial for non-cirrhotic MAFLD, less data is available for those with MAFLD-related cirrhosis. In general, dietary advice adhering to the tenets of the Mediterranean diet macronutrient composition, with emphasis on adequate protein consumption (1.2–1.5 g/kg per day) and avoidance of prolonged fasting periods should be emphasised [351]. Alcohol abstinence should be instituted for MAFLD-related cirrhosis.
With regard to physical activity, exercise prescription to target 150 min of moderate or 75 min of vigorous intensity physical activity per week should be considered [352]. Resistance training should be incorporated in the exercise regime, which helps counteract sarcopenia [353].
Additionally, 5–10% weight loss via lifestyle interventions has been reported to improve portal hypertension in patients with cirrhosis [354]. This may take on more significance in MAFLD-related cirrhosis, where MAFLD patients decompensate at lower portal pressures compared to other etiologies [355]. Admittedly, this may be challenging to achieve in patients with possible anorexia, reduced aerobic capacity, frailty and the physical limitations of cirrhosis. The development of sarcopenia should be monitored for and prevented with high protein energy supplements.
There is limited data about bariatric surgery in the context of MAFLD-related cirrhosis. Compared to non-cirrhotics undergoing bariatric surgery, a twofold and 21-fold increased mortality risk is reported in compensated and decompensated cirrhosis, respectively [356]. Nonetheless, bariatric surgery (usually sleeve gastrectomy) can be considered in carefully selected patients with compensated cirrhosis, ideally in high-volume centres and taking into account liver status, clinically significant portal hypertension, comorbidities and surgical factors [357, 358].
Special groups
Pediatric MAFLD
The definition of MAFLD acknowledges that the disease is a spectrum across the life span. Consequently, children with fatty liver who have metabolic dysfunction also meet the MAFLD diagnostic criteria [359]. Children with fatty liver should however be assessed for other diseases such as inherited metabolic disorders according to accepted clinical guidance. Timely diagnosis and specific treatment are essential for these other conditions. This is particularly crucial for children under the age of five or who do not exhibit typical features of metabolic dysfunction [361,362,362]. The understanding of paediatric MAFLD is currently limited, and additional research is necessary to confirm its natural history, genetic associations, assessment, and treatment modalities.
Hormone imbalance in women
The prevalence of MAFLD is higher in males, but this sex difference gradually diminishes after menopause. A possible reason is the hormone balance between estradiol and androgen [363]. Pre-menopausal patients with polycystic ovary syndrome (PCOS) have a higher incidence of MAFLD, and those with gestational eclampsia, T2DM, and pregnancy-associated hypertension also have an increased likelihood of developing MAFLD in later life. Strategies to reduce this impact during the peri-menopausal period should be considered.
Lean MAFLD
Individuals of lean/normal weight can have MAFLD, and the subgroup is often referred to as lean MAFLD [364]. The definition of "lean" varies between Asia and the West. In Asia, it is defined as a BMI less than 23 kg/m2, while in the West it is defined as less than 25 kg/m2 (Table 5).
Table 5 Working definition of overweight/obesity and central obesity for Asian adults
Current evidence indicates that the prevalence of lean MAFLD is approximately 5% in the general population [365, 366]. The pathogenesis of lean MAFLD is not fully understood, and besides metabolic dysfunction, genetics and other factors such as metabolic flexibility and adaptability play crucial roles [28, 368,369,369]. It is generally accepted that although the BMI is within the healthy range, the clinical outcomes and prognosis are similar to those in the overweight/obese patient. Likewise, management is similar to that of the overweight/obese patients with MAFLD. The amount of weight reduction needed to achieve remission is however less than that for non-obese patients. Five percent body weight loss induces MAFLD remission in both obese and lean patients with MAFLD [370]. Therefore, lifestyle intervention with regular exercise is effective in treating MAFLD and in improving overall fitness and metabolic co-morbidities irrespective of baseline BMI. A 3–5% weight reduction may be sufficient in lean MAFLD [370].
Dual etiology liver disease
The positive diagnostic criteria for MAFLD have allowed for the identification of the coexistence of MAFLD with other liver diseases. Dual etiology liver disease is commonly encountered in clinical practice particularly in Asia where concurrent chronic hepatitis B, chronic hepatitis C, and alcohol-related liver disease is common.
Interaction between MAFLD and viral hepatitis
Bi-directional interactions between HBV infection and MAFLD
Patients with both hepatitis B virus (HBV) infection and MAFLD are not uncommon in the Asia–Pacific regions. The prevalence of MAFLD in CHB patients ranges from 14 to 59% [284, 371, 372]. Most studies in animals or in humans report an inverse association between hepatic steatosis and HBV replication. Decreased serum HBV-related antigens and HBV DNA are also observed in HBV cloned mice fed a high-fat diet [373]. A meta-analysis of 54 studies including 28,648 HBV-infected patients demonstrated that MAFLD was negatively associated with serum HBV DNA levels and HBeAg-seropositivity [374].
CHB patients with MASH have accelerated progression of liver disease compared to patients with either CHB alone or MASH alone. CHB patients with concurrent hepatic steatosis also have more severe hepatic inflammation, advanced fibrosis, liver-related complications and mortality [375, 376], especially among those with T2DM [377]. However, MAFLD is associated with a significantly lower risk of HCC and a higher chance of hepatitis B surface antigen (HBsAg) seroclearance than those without MAFLD [378, 379]. Several studies report that hepatic steatosis confers a lower risk of HCC among untreated CHB patients [286, 380, 381]. Notably, a recent study observed that the presence of hepatic steatosis is associated with a lower risk of HCC, whereas the burden of metabolic dysfunction aggravates the risk of HCC in untreated CHB patients [286]. A large cohort study from Taiwan demonstrated that MAFLD is associated with increased risks of cirrhosis and HCC only in antiviral-treated CHB patients [382].
The lower prevalence and incidence of MAFLD among CHB patients than those without HBV infection is observed both in cross-sectional [383] and prospective studies [384]. The presence of hepatic steatosis in CHB patients is mainly correlated with metabolic dysfunction, but not HBV infection [385]. The impact of CHB on lipid and glucose metabolism is still unclear. The reduced risk of steatosis and the influence of glucose and lipid metabolism may be through the action of the HBx and PreS1 proteins [386].
For management, antiviral therapy for HBV control and lifestyle modification for weight reduction remain the cornerstone of treatment in patients with combined CHB and MAFLD [387]. Since T2DM and the burden of metabolic derangement are significant risk factors for long-term liver complications [286, 290], it is crucial to control metabolic dysregulation to reduce the risk of HCC and MAFLD-related complications [388]. Notably, increased body weight, insulin resistance and metabolic derangement are observed in CHB patients switching from Tenofovir disoproxil fumarate to tenofovir alafenamide. However, the mechanisms underlying this observation remain unclear and merit further investigation [389, 390]. Remarkably, weight loss and elimination of MAFLD among obese patients receiving bariatric surgery can induce HBV reactivation [391]. Long-term care of CHB patients with MAFLD under antiviral therapy or rapid weight reduction necessitates the evaluation of cardiometabolic risks and potential HBV reactivation risk, respectively.
Bi-directional interactions between HCV and MAFLD
Hepatic steatosis is a common manifestation of CHC. It has been reported in 30–45% of patients in large cohorts by imaging [392, 393] and in 50–70% by histology[394, 395], The coexistence of CHC and MAFLD is estimated to be approximately 9–38% [396].
Hepatic steatosis is considered a risk factor for fibrosis progression and HCC in CHC patients. The impact of MAFLD on liver-related outcomes is maintained after successful anti-HCV treatment [382]. Studies have shown that among patients who achieve SVR after antiviral therapy, those with steatosis exhibit a higher degree of liver fibrosis compared to those without [397, 398]. Likewise, hepatic steatosis and MAFLD are associated with the development of HCC in patients after HCV eradication [305, 399]. This suggests that even after clearance of the HCV, the presence of MAFLD can exacerbate liver damage.
Post-viral clearance, there is a phenomenon of lipid rebound, which may increase the risk of hepatic steatosis and atherosclerosis. A recent systematic review has shown that half of the studies reported increased hepatic steatosis whereas one-third of studies observed hepatic steatosis after direct-acting antiviral agents (DAAs) treatment [400]. Profound dyslipidemia that might occur after HCV eradication is associated with a risk of cardio-cerebrovascular disease [401]. Additionally, the presence of HCV infection increases the incidence of new-onset DM and hypertension [402]. As cardiovascular complications are the leading causes of mortality in MAFLD, in post-SVR hepatitis C patients, more attention should be paid to concurrent MAFLD and it is essential to monitor their serum lipid levels and to treat hyperlipidemia.
DAA therapy for HCV eradication and lifestyle modification for weight reduction remain the cornerstone of management in patients with combined CHC and MAFLD [403]. Use of metformin for patients with T2DM and statins for those with hyperlipidemia might be beneficial for reducing the risk of HCC after SVR [403]. Taken collectively, Holistic and multidisciplinary care of CHC patients beyond the surveillance for HCC is warranted to mitigate the consequences of concomitant metabolic traits after virus eradication [398].
Bi-directional interactions between human immunodeficiency virus (HIV) and MAFLD
The burden of MAFLD is high among people living with HIV infection.[404] Among them, those with MAFLD have a higher risk of significant liver disease and atherosclerosis compared to those without. However, there is no significant difference in HIV viral load between the two groups [405].
Bi-directional interactions between MAFLD and alcohol-related liver disease (ALD)
There is a substantial overlap between alcohol-related liver disease (ALD) and MAFLD, with some studies showing a shared mechanism between the two entities [406]. In Asia, a Japanese study found the prevalence of metabolic syndrome to be higher in very heavy drinkers (> 60 g/day) when compared to non-drinkers[407], while a Korean study found that even very light alcohol consumption (0.1 – 5 g/day) is associated with metabolic syndrome [408].
The harmful amount of alcohol for MAFLD is not well-defined. The coexistence of ALD and MAFLD can worsen disease progression by interacting with different pathological mechanisms. A study involving 8,345 individuals with hepatic steatosis with a mean follow-up of 11.1 years found that even a modest alcohol intake of 10–19 g/day doubled the risk for advanced liver disease [409]. Similarly, population-level data suggests that just one standard drink per day can increase disease and mortality rates [410].
For management, it is important to assess patients with ALD for coexisting MAFLD and vice versa. While assessing metabolic risk factors in ALD is relatively straightforward, detecting and quantifying alcohol consumption can be challenging and requires clinical awareness. The use of standardized self-reporting methods like the Alcohol Disorders Use Identification Testing-Consumption (AUDIT-C) is recommended to identify drinking habits, but their accuracy can be limited by underreporting. Using objective biomarkers like serum phosphatidylethanol may help accurately quantify alcohol intake, though further research is needed to validate their diagnostic performance in different populations.
Current evidence does not support a safe level of alcohol consumption for MAFLD [19]. Therefore, all MAFLD patients should be advised to abstain from drinking, along with other lifestyle recommendations such as weight loss, exercise, and dietary control.
Public health issues in MAFLD: Defining preventive strategies for MAFLD
With the growing prevalence of MAFLD and the challenge of screening for MAFLD in the general population, preventive strategies accompanied by approaches for the identification of high-risk populations is paramount.
The recognition that MAFLD as part of the wider problem of metabolic dysfunction should encourage more concerted efforts to tackle the universal root causes, instead of each speciality working within its own silo. For this, a framework that shifts from treatment of disease only, to inclusion of health promotion at the individual and population level to improve metabolic health is recommended. The diagnosis of MAFLD can be an opportunity for targeted intervention for improving the metabolic health of the individual and for a better quality of life [411].
To prevent MAFLD, a collaborative approach involving government, healthcare leaders, patient representatives, and community advocates is required [390]. The development of global and national strategies to underpin this approach is critical. Short-term plans to raise awareness of the disease and its complications among different communities, identifying at-risk persons and educating them on how to prevent disease occurrence (or progression) through lifestyle change are crucial first steps. Long-term frameworks to decrease disease burden at global, continental, and national levels can be done through global, continental, and national taskforces. These taskforces can help formulate a global strategy including key elements for intervention that can be tailored to continental and national realities. Our recommended preventive actions for MAFLD are presented in supplementary Table 2.
Scope of artificial intelligence in MAFLD
The role of artificial intelligence (AI) as an emerging technology is rapidly gaining traction. In healthcare, the scope of AI is diverse and expansive, providing groundbreaking advancements in diagnosis, prognosis, and personalized treatment options. This ranges from predictive analytics through data mining of various large datasets, to management systems to robotic surgery and personalized medicine [412].
Disease progression or populations at risk can be predicted by analysing patient data, including genetic, metabolic, and lifestyle factors. Applications of AI have proven to enhance diagnostics with a higher degree of accuracy in comparison to traditional methods [413]. This predictive power in future might be crucial for identifying patients at risk of developing severe subtype like MASH or advanced fibrosis [414].
Use of AI technology in MAFLD can help reduce the requirement for liver biopsy by advancing non-invasive technologies and overcoming the limitations of misclassification which can result in patients missing out on the chance to participate in clinical trials or being able to access therapy. For example, a recent study using an AI-based algorithm (qFibrosis) was found to identify subtle histological features that distinguish stage 1 (F1) from stage 2 (F2) in patients with MASH and to serve as an assistive tool for in-house pathologists [415].
However, harnessing the potential of AI necessitates collaborative endeavours encompassing fields as diverse as healthcare, technology, and ethics. As we continue to make progress, it is crucial to prioritize the responsible and ethical integration of AI into healthcare. Our goal should be to utilize these powerful tools to improve patient care and outcomes in MAFLD. Further investigation should prioritize the incorporation of AI tools into clinical practice, confirming their efficacy in practical environments, and tackling ethical and logistical challenges.
Conclusion
This APASL clinical practice guideline provides recommendations for the assessment and management of patients and specific populations affected by MAFLD. This disease is increasingly prevalent in the Asia–Pacific region, while viral hepatitis and alcohol-related liver disease are common causes of dual etiology liver disease. MAFLD is one of the primary causes of advanced chronic liver disease and HCC, but is also associated with various systemic complications such as T2DM, CVD, CKD, and extrahepatic malignancies. Advanced liver fibrosis is the primary determinant of all MAFLD complications, and liver biopsy remains the gold standard for its diagnosis. Nonetheless, there are various non-invasive methods, including biomarkers and imaging tests available (and increasingly utilized) for fibrosis assessment. Patients with cirrhosis should undergo surveillance for varices and for HCC. Lifestyle intervention remains the foundation for best-practice management. Exciting for the field, the first drug specific for MAFLD has been approved and it is expected that other treatments will become available in the coming years. Holistic, patient-centered, and multidisciplinary management methods that focus on mitigating liver damage, treating metabolic dysfunction, and prioritizing patient-reported outcomes are necessary.
Abbreviations
APASL:
Asian Pacific Association for the Study of the Liver
ALT:
Alanine aminotransferase
AST:
Aspartate transaminase
BMI:
Body mass index
CAP:
Controlled attenuation parameter
CKD:
Chronic kidney disease
CVD:
Cardiovascular disease
ELF:
Enhanced liver fibrosis
FIB-4:
Fibrosis-4 Index
HCC:
Hepatocellular carcinoma
HSD17B13:
Hydroxysteroid 17-beta dehydrogenase-13
GCKR:
Glucokinase regulator
GLP-1RA:
Glucagon-like peptide-1 receptor agonists
LSM:
Liver stiffness measurement
MAFLD:
Metabolic dysfunction-associated fatty liver disease.
MASH:
Metabolic dysfunction-associated steatohepatitis
MBOAT7:
Membrane-Bound O-Acyltransferase Domain Containing 7
NITs:
Non-invasive tests
PNPLA3:
Patatin-like phospholipase domain-containing protein 3
SGLT2i:
Sodium-glucose cotransporter 2 inhibitors
T2DM:
Type 2 diabetes mellitus
TM6SF2:
Transmembrane 6 superfamily member 2
VCTE:
Vibration controlled transient elastography
References
- Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5(2):167–228
Article PubMed Google Scholar - Kawaguchi T, Tsutsumi T, Nakano D, Eslam M, George J, Torimura T. MAFLD enhances clinical practice for liver disease in the Asia-Pacific region. Clin Mol Hepatol. 2022;28(2):150–163
Article PubMed Google Scholar - Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323(7308):334–336
Article CAS PubMed PubMed Central Google Scholar - Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21(3):619–629
Article PubMed Google Scholar - Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10 739 607 individuals. J Clin Endocrinol Metab. 2022;107(9):2691–2700
Article PubMed Google Scholar - Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–1347
Article PubMed Google Scholar - Danpanichkul P, Kongarin S, Permpatdechakul S, Polpichai N, Duangsonk K, Manosroi W, et al. The Surreptitious Burden of Nonalcoholic Fatty Liver Disease in the Elderly in the Asia-Pacific Region: An Insight from the Global Burden of Disease Study 2019. J Clin Med. 2023;12(20).
- Chang M, Shao Z, Shen G. Association between triglyceride glucose-related markers and the risk of metabolic-associated fatty liver disease: a cross-sectional study in healthy Chinese participants. BMJ Open. 2023;13(5): e070189
Article PubMed PubMed Central Google Scholar - Chen YL, Li H, Li S, Xu Z, Tian S, Wu J, et al. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21(1):212
Article CAS PubMed PubMed Central Google Scholar - Prabhakar T, Prasad M, Kumar G, Kaushal K, Shenoy PS, Dubey S, Sarin SK. High prevalence of MAFLD in general population: A large cross‐sectional study calls for concerted public health action. Alimentary Pharmacology & Therapeutics. 2024.
- Alam S, Fahim SM, Chowdhury MAB, Hassan MZ, Azam G, Mustafa G, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in Bangladesh. JGH Open. 2018;2(2):39–46
Article PubMed PubMed Central Google Scholar - Kim M, Yoon EL, Cho S, Lee CM, Kang BK, Park H, et al. Prevalence of advanced hepatic fibrosis and comorbidity in metabolic dysfunction-associated fatty liver disease in Korea. Liver Int. 2022;42(7):1536–1544
Article PubMed Google Scholar - Fujii H, Fukumoto S, Enomoto M, Uchida-Kobayashi S, Kimura T, Tamori A, et al. The FibroScan-aspartate aminotransferase score can stratify the disease severity in a Japanese cohort with fatty liver diseases. Sci Rep. 2021;11(1):13844
Article CAS PubMed PubMed Central Google Scholar - Taheri E, Moslem A, Mousavi-Jarrahi A, Hatami B, Pourhoseingholi MA, Asadzadeh Aghdaei H, et al. Predictors of metabolic-associated fatty liver disease (MAFLD) in adults: a population-based study in Northeastern Iran. Gastroenterol Hepatol Bed Bench. 2021;14(Suppl1):S102–S111
PubMed PubMed Central Google Scholar - Yilmaz Y, Yilmaz N, Ates F, Karakaya F, Gokcan H, Kaya E, et al. The prevalence of metabolic-associated fatty liver disease in the Turkish population: A multicenter study. Hepatol Forum. 2021;2(2):37–42
PubMed PubMed Central Google Scholar - Kani HT, Demirtas CO, Keklikkiran C, Ergenc I, Mehdiyev S, Akdeniz E, et al. Evaluation of the Impact of Metabolic Syndrome on Fibrosis in Metabolic Dysfunction-Associated Fatty Liver Disease. Turk J Gastroenterol. 2021;32(8):661–666
Article PubMed Google Scholar - Vaz K, Kemp W, Majeed A, Lubel J, Magliano DJ, Glenister KM, et al. Non-alcoholic fatty liver disease prevalence in Australia has risen over 15 years in conjunction with increased prevalence of obesity and reduction in healthy lifestyle. J Gastroenterol Hepatol. 2023;38(10):1823–1831
Article CAS PubMed PubMed Central Google Scholar - Kemp W, Clayton-Chubb D, Majeed A, Glenister KM, Magliano DJ, Lubel J, et al. Impact of renaming NAFLD to MAFLD in an Australian regional cohort: Results from a prospective population-based study. J Gastroenterol Hepatol. 2022;37(2):395–403
Article PubMed Google Scholar - Eslam M, Sanyal AJ, George J. Toward More Accurate Nomenclature for Fatty Liver Diseases. Gastroenterology. 2019;157(3):590–593
Article PubMed Google Scholar - Eslam M, Newsome PN, Anstee QM, Targher G, Gomez MR, Zelber-Sagi S, et al. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020.
- Alharthi J, Gastaldelli A, Cua IH, Ghazinian H, Eslam M. Metabolic dysfunction-associated fatty liver disease: a year in review. Curr Opin Gastroenterol. 2022;38(3):251–260
Article CAS PubMed Google Scholar - Lee H, Lee HW, Kim SU, Chang KH. Metabolic Dysfunction-Associated Fatty Liver Disease Increases Colon Cancer Risk: A Nationwide Cohort Study. Clin Transl Gastroenterol. 2022;13(1): e00435
Article PubMed PubMed Central Google Scholar - Pan Z, Yilmaz Y, Al-Busafi SA, Eslam M. The MAFLD definition identifies three homogenous groups of patients. Liver Int. 2024;44(12):3287–3289
Article PubMed Google Scholar - Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14(6):889–919
Article PubMed Google Scholar - Yuan Q, Wang H, Gao P, Chen W, Lv M, Bai S, Wu J. Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease among 73,566 Individuals in Beijing, China. International journal of environmental research and public health. 2022;19(4).
- Fan J, Luo S, Ye Y, Ju J, Zhang Z, Liu L, et al. Prevalence and risk factors of metabolic associated fatty liver disease in the contemporary South China population. Nutr Metab (Lond). 2021;18(1):82
Article CAS PubMed Google Scholar - Kim D, Chung GE, Kwak MS, Seo HB, Kang JH, Kim W, et al. Body Fat Distribution and Risk of Incident and Regressed Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14(1):132-138.e134
Article PubMed Google Scholar - Eslam M, El-Serag HB, Francque S, Sarin SK, Wei L, Bugianesi E, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19(10):638–651
Article PubMed Google Scholar - En Li Cho E, Ang CZ, Quek J, Fu CE, Lim LKE, Heng ZEQ, et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut. 2023;72(11):2138–2148
Article PubMed Google Scholar - Ampuero J, Aller R, Gallego-Durán R, Banales JM, Crespo J, García-Monzón C, et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48(11–12):1260–1270
Article CAS PubMed Google Scholar - Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–752
Article PubMed Google Scholar - Kim D, Konyn P, Cholankeril G, Ahmed A. Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by FibroScan. Clin Gastroenterol Hepatol. 2022;20(6):e1438–e1455
Article CAS PubMed Google Scholar - Chun HS, Lee M, Lee HA, Oh SY, Baek HJ, Moon JW, et al. Association of physical activity with risk of liver fibrosis, sarcopenia, and cardiovascular disease in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2023;21(2):358-369.e312
Article CAS PubMed Google Scholar - Bali T, Chrysavgis L, Cholongitas E. Metabolic-associated fatty liver disease and sarcopenia. Endocrinol Metab Clin North Am. 2023;52(3):497–508
Article PubMed Google Scholar - Joo SK, Kim W. Interaction between sarcopenia and nonalcoholic fatty liver disease. Clin Mole Hepatol. 2023;29(Suppl):S68-s78
Article Google Scholar - Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology (Baltimore, MD). 2014;59(5):1772–1778
Article CAS PubMed Google Scholar - Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66(1):123–131
Article PubMed Google Scholar - Han E, Chun HS, Lee YH, Lee JS, Lee HW, Kim BK, et al. MAFLD might be better in identifying subjects with sarcopenia or cardiovascular risk than NAFLD: a nationwide study. J Gastroenterol Hepatol. 2023;38(9):1598–1609
Article CAS PubMed Google Scholar - Hsieh YC, Joo SK, Koo BK, Lin HC, Lee DH, Chang MS, et al. Myosteatosis, but not sarcopenia, predisposes nafld subjects to early steatohepatitis and fibrosis progression. Clin Gastroenterol Hepatol. 2023;21(2):388-397.e310
Article CAS PubMed Google Scholar - Lee YK, Koo BK, Joo SK, Lee DH, Jang H, Chai JW, et al. Low-quality muscle mass rather than normal-quality muscle mass determines fibrosis progression in biopsy-proven NAFLD. Aliment Pharmacol Ther. 2023;58(3):322–333
Article CAS PubMed Google Scholar - Kim HK, Bae SJ, Lee MJ, Kim EH, Park H, Kim HS, et al. Association of visceral fat obesity, sarcopenia, and myosteatosis with non-alcoholic fatty liver disease without obesity. Clin Moler Hepatol. 2023;29(4):987–1001
Article Google Scholar - Kwak JH, Jun DW, Lee SM, Cho YK, Lee KN, Lee HL, et al. Lifestyle predictors of obese and non-obese patients with nonalcoholic fatty liver disease: a cross-sectional study. Clin Nutr. 2018;37(5):1550–1557
Article PubMed Google Scholar - Ko E, Yoon EL, Jun DW. Risk factors in nonalcoholic fatty liver disease. Clin Mole Hepatol. 2023;29(Suppl):S79-s85
Article Google Scholar - Wang YY, Lin HL, Wang KL, Que GX, Cao T, Zhu LM, et al. Influence of gut microbiota and its metabolites on progression of metabolism-associated fatty liver disease. Chin Med Sci J. 2023;38:286
Article CAS PubMed Google Scholar - Alharthi J, Latchoumanin O, George J, Eslam M. Macrophages in metabolic associated fatty liver disease. World J Gastroenterol. 2020;26(16):1861
Article CAS PubMed PubMed Central Google Scholar - Huang J, Chen L, Li X, Chen M, Lin T, Chen G. Association between metabolic-associated fatty liver disease and obstructive sleep apnea: a cross-sectional study. Nat Sci Sleep. 2023;15:49–57
Article PubMed PubMed Central Google Scholar - Chen YL, Tian S, Wu J, Li H, Li S, Xu Z, et al. Impact of thyroid function on the prevalence and mortality of metabolic dysfunction-associated fatty liver disease. J Clin Endocrinol Metab. 2023;108(7):e434–e443
Article PubMed Google Scholar - Vidal-Cevallos P, Mijangos-Trejo A, Uribe M, Tapia NC. The interlink between metabolic-associated fatty liver disease and polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2023;52(3):533–545
Article PubMed Google Scholar - Mintziori G, Poulakos P, Tsametis C, Goulis DG. Hypogonadism and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42(2):145–150
Article PubMed Google Scholar - Koo BK, Joo SK, Kim D, Bae JM, Park JH, Kim JH, et al. Additive effects of PNPLA3 and TM6SF2 on the histological severity of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2018;33(6):1277–1285
Article CAS PubMed Google Scholar - Koo BK, Lee H, Kwak SH, Lee DH, Park JH, Kim W. Long-term effect of pnpla3 on the aggravation of nonalcoholic fatty liver disease in a biopsy-proven cohort. Clin Gastroenterol Hepatol. 2023;21(4):1105-1107.e1103
Article PubMed Google Scholar - Pan Z, El Sharkway R, Bayoumi A, Metwally M, Gloss BS, Brink R, et al. Inhibition of MERTK reduces organ fibrosis in mouse models of fibrotic disease. Sci Transl Med. 2024;16(741):eadj0133
Article CAS PubMed Google Scholar - Kang B, Kang B, Roh TY, Seong RH, Kim W. The chromatin accessibility landscape of nonalcoholic fatty liver disease progression. Mol Cells. 2022;45(5):343–352
Article CAS PubMed PubMed Central Google Scholar - Shin HK, Park JH, Yu JH, Jin YJ, Suh YJ, Lee JW, et al. Association between telomere length and hepatic fibrosis in non-alcoholic fatty liver disease. Sci Rep. 2021;11(1):18004
Article CAS PubMed PubMed Central Google Scholar - McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–1155
Article PubMed Google Scholar - Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59(3):550–556
Article CAS PubMed Google Scholar - Simon TG, Roelstraete B, Hagström H, Loomba R, Ludvigsson JF. Progression of non-alcoholic fatty liver disease and long-term outcomes: a nationwide paired liver biopsy cohort study. J Hepatol. 2023;79(6):1366–1373
Article PubMed PubMed Central Google Scholar - Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015. https://doi.org/10.1016/j.cgh.2014.04.014
Article PubMed PubMed Central Google Scholar - Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun. 2018;2(2):199–210
Article CAS PubMed Google Scholar - Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978
Article PubMed Google Scholar - Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol. 2022;20(2):283-292.e210
Article PubMed Google Scholar - Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128(10):2436–2443
Article CAS PubMed Google Scholar - Lee JS, Sinn DH, Park SY, Shin HJ, Lee HW, Kim BK, et al. Liver stiffness-based risk prediction model for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cancers (Basel). 2021;13(18):4567
Article CAS PubMed Google Scholar - Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61(3):409–415
Article PubMed Google Scholar - Mozes FE, Lee JA, Vali Y, Alzoubi O, Staufer K, Trauner M, et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(8):704–713
Article CAS PubMed Google Scholar - Chan WL, Chong SE, Chang F, Lai LL, Chuah KH, Nik Mustapha NR, et al. Long-term clinical outcomes of adults with metabolic dysfunction-associated fatty liver disease: a single-centre prospective cohort study with baseline liver biopsy. Hepatol Int. 2023;17(4):870–881
Article PubMed Google Scholar - Hussain A, Patel PJ, Rhodes F, Srivastava A, Patch D, Rosenberg W. Decompensated cirrhosis is the commonest presentation for NAFLD patients undergoing liver transplant assessment. Clin Med (Lond). 2020;20(3):313–318
Article PubMed Google Scholar - Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359–1368
Article CAS PubMed Google Scholar - Lai LL, Wan Yusoff WNI, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34(8):1396–1403
Article CAS PubMed Google Scholar - Huang DQ, Noureddin N, Ajmera V, Amangurbanova M, Bettencourt R, Truong E, et al. Type 2 diabetes, hepatic decompensation, and hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: an individual participant-level data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(9):829–836
Article CAS PubMed PubMed Central Google Scholar - Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int Off J Int Association Study Liver. 2013;33(9):1398–1405
CAS Google Scholar - Wong VW, Wong GL, Tsang SW, Hui AY, Chan AW, Choi PC, et al. Metabolic and histological features of non-alcoholic fatty liver disease patients with different serum alanine aminotransferase levels. Alimentary Pharmacol Therapeutics. 2009;29(4):387–396
Article CAS Google Scholar - Loo SY, Chan WK. Emerging new standard for non-invasive assessment of liver disease mortality in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2017;6(2):135–137
Article PubMed PubMed Central Google Scholar - Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56(1):234–240
Article PubMed Google Scholar - Mahady SE, Macaskill P, Craig JC, Wong GL, Chu WC, Chan HL, et al. Diagnostic accuracy of noninvasive fibrosis scores in a population of individuals with a low prevalence of fibrosis. Clin Gastroenterol Hepatol. 2017. https://doi.org/10.1016/j.cgh.2017.02.031
Article PubMed Google Scholar - Mozes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71(5):1006–1019
Article PubMed Google Scholar - Cholankeril G, Kramer JR, Chu J, Yu X, Balakrishnan M, Li L, et al. Longitudinal changes in fibrosis markers are associated with risk of cirrhosis and hepatocellular carcinoma in non-alcoholic fatty liver disease. J Hepatol. 2023;78(3):493–500
Article CAS PubMed Google Scholar - Kjaergaard M, Lindvig KP, Thorhauge KH, Andersen P, Hansen JK, Kastrup N, et al. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J Hepatol. 2023;79(2):277–286
Article CAS PubMed Google Scholar - Schreiner AD, Moran WP, Zhang J, Livingston S, Marsden J, Mauldin PD, et al. The association of fibrosis-4 index scores with severe liver outcomes in primary care. J Gen Intern Med. 2022;37(13):3266–3274
Article PubMed PubMed Central Google Scholar - Chan WK, Treeprasertsuk S, Goh GB, Fan JG, Song MJ, Charatcharoenwitthaya P, et al. Optimizing Use of Nonalcoholic Fatty Liver Disease Fibrosis Score, Fibrosis-4 Score, and Liver Stiffness Measurement to Identify Patients With Advanced Fibrosis. Clin Gastroenterol Hepatol. 2019;17(12):2570-2580 e2537
Article PubMed Google Scholar - Boursier J, Hagstrom H, Ekstedt M, Moreau C, Bonacci M, Cure S, et al. Non-invasive tests accurately stratify patients with NAFLD based on their risk of liver-related events. J Hepatol. 2022;76(5):1013–1020
Article CAS PubMed Google Scholar - Cassinotto C, Boursier J, Paisant A, Guiu B, Irles-Depe M, Canivet C, et al. Transient versus two-dimensional shear-wave elastography in a multistep strategy to detect advanced fibrosis in NAFLD. Hepatology. 2021;73(6):2196–2205
Article CAS PubMed Google Scholar - Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626-637.e627
Article PubMed Google Scholar - Lin H, Lee HW, Yip TC, Tsochatzis E, Petta S, Bugianesi E, et al. Vibration-controlled transient elastography scores to predict liver-related events in steatotic liver disease. JAMA. 2024;331(15):1287–1297
Article CAS PubMed PubMed Central Google Scholar - Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71(2):371–378
Article PubMed Google Scholar - Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65(3):570–578
Article PubMed Google Scholar - Boursier J, Guillaume M, Leroy V, Irles M, Roux M, Lannes A, et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol. 2019;71(2):389–396
Article PubMed Google Scholar - Eslam M, Wong GL-H, Hashem AM, Chan HL-Y, Nielsen MJ, Leeming DJ, et al. A sequential algorithm combining ADAPT and liver stiffness can stage metabolic-associated fatty liver disease in hospital-based and primary care patients. Off J Am College Gastroenterol| ACG. 2021;116(5):984–993
Article CAS Google Scholar - Tang L-J, Ma H-L, Eslam M, Wong GL-H, Zhu P-W, Chen S-D, et al. Among simple non-invasive scores, Pro-C3 and ADAPT best exclude advanced fibrosis in Asian patients with MAFLD. Metabolism. 2022;128:154958
Article CAS PubMed Google Scholar - Gracen L, Hayward KL, Aikebuse M, Williams S, Russell A, O’Beirne J, et al. An exploration of barriers and facilitators to implementing a nonalcoholic fatty liver disease pathway for people with type 2 diabetes in primary care. Diabet Med. 2022;39(6):e14799
Article PubMed PubMed Central Google Scholar - Zhang X, Yip TC, Wong GL, Leow WX, Liang LY, Lim LL, et al. Clinical care pathway to detect advanced liver disease in patients with type 2 diabetes through automated fibrosis score calculation and electronic reminder messages: a randomised controlled trial. Gut. 2023;72(12):2364–2371
Article CAS PubMed Google Scholar - Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51(6):1061–1067
Article PubMed PubMed Central Google Scholar - Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030
Article PubMed Google Scholar - Caussy C, Brissot J, Singh S, Bassirian S, Hernandez C, Bettencourt R, et al. Prospective, same-day, direct comparison of controlled attenuation parameter with the M vs the XL probe in patients with nonalcoholic fatty liver disease, using magnetic resonance imaging-proton density fat fraction as the standard. Clin Gastroenterol Hepatol. 2020;18(8):1842-1850.e1846
Article CAS PubMed Google Scholar - Wong VW, Petta S, Hiriart JB, Cammà C, Wong GL, Marra F, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017;67(3):577–584
Article PubMed Google Scholar - Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598-607.e592
Article PubMed Google Scholar - Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013;33(9):1398–1405
Article CAS PubMed Google Scholar - Ravaioli F, Dajti E, Mantovani A, Newsome PN, Targher G, Colecchia A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: a systematic review and meta-analysis. Gut. 2023;72(7):1399–1409
Article PubMed Google Scholar - Vali Y, Lee J, Boursier J, Petta S, Wonders K, Tiniakos D, et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol. 2023;8(8):714–725
Article CAS PubMed Google Scholar - Wong VW-S, Anstee QM, Nitze LM, Geerts A, George J, Nolasco V, et al. FibroScan-aspartate aminotransferase (FAST) score for monitoring histological improvement in non-alcoholic steatohepatitis activity during semaglutide treatment: post-hoc analysis of a randomised, double-blind, placebo-controlled, phase 2b trial. EClinicalMedicine. 2023;66:102310
Article Google Scholar - Huang DQ, Sharpton SR, Amangurbanova M, Tamaki N, Sirlin CB, Loomba R. Clinical utility of combined MRI-PDFF and ALT response in predicting histologic response in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2023;21(10):2682-2685.e2684
Article PubMed Google Scholar - Gawrieh S, Vilar-Gomez E, Wilson LA, Pike F, Kleiner DE, Neuschwander-Tetri BA, et al. Increases and decreases in liver stiffness measurement are independently associated with the risk of liver-related events in NAFLD. J Hepatol. 2024;81(4):600–608
Article PubMed Google Scholar - Wong VW, Chitturi S, Wong GL, Yu J, Chan HL, Farrell GC. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol. 2016;1(1):56–67
Article PubMed Google Scholar - Spinzi G, Terruzzi V, Minoli G. Liver biopsy. N Engl J Med. 2001;344(26):2030
Article CAS PubMed Google Scholar - Eslam M, Ampuero J, Jover M, Abd-Elhalim H, Rincon D, Shatat M, et al. Predicting portal hypertension and variceal bleeding using non-invasive measurements of metabolic variables. Ann Hepatol. 2013;12(4):588–598
Article PubMed Google Scholar - Berger D, Desai V, Janardhan S. Con: liver biopsy remains the gold standard to evaluate fibrosis in patients with nonalcoholic fatty liver disease. Clin Liver Disease. 2019;13(4):114–116
Article Google Scholar - Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474
Article CAS PubMed Google Scholar - Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321
Article PubMed Google Scholar - Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56(5):1751–1759
Article PubMed Google Scholar - Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open. 2019;2(10):e1912565–e1912565
Article PubMed PubMed Central Google Scholar - Pan Z, Fan J-G, Eslam M. An update on drug development for the treatment of metabolic (dysfunction) associated fatty liver disease: Progress and opportunities. Curr Opinion Pharmacol. 2021;60:170–176
Article CAS Google Scholar - Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47-64
Article PubMed Google Scholar - Pipitone RM, Ciccioli C, Infantino G, La Mantia C, Parisi S, Tulone A, et al. MAFLD: a multisystem disease. Therapeutic Adv Endocrinol Metab. 2023;14:20420188221145548
Article CAS Google Scholar - Kaya E, Yilmaz Y. Metabolic-associated fatty liver disease (MAFLD): a multi-systemic disease beyond the liver. J Clin Transl Hepatol. 2022;10(2):329–338
Article PubMed Google Scholar - Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, et al. MAFLD and risk of CKD. Metabolism. 2021;115: 154433
Article CAS PubMed Google Scholar - Chen S, Pang J, Huang R, Xue H, Chen X. Association of MAFLD with end-stage kidney disease: a prospective study of 337,783 UK Biobank participants. Hepatol Int. 2023;17(3):595–605
Article PubMed Google Scholar - Wang TY, Wang RF, Bu ZY, Targher G, Byrne CD, Sun DQ, et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. 2022;18(4):259–268
Article PubMed Google Scholar - Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: A 4.6-year cohort study in China. J Clin Endocrinol Metab. 2022;107(1):88–97
Article PubMed Google Scholar - Zhou XD, Cai J, Targher G, Byrne CD, Shapiro MD, Sung KC, et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc Diabetol. 2022;21(1):270
Article CAS PubMed PubMed Central Google Scholar - Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol Off Clin Prac J Am Gastroenterol Association. 2021;19(10):2138-2147.e2110
Google Scholar - Liao Y, Wang L, Liu F, Zhou Y, Lin X, Zhao Z, et al. Emerging trends and hotspots in metabolic dysfunction-associated fatty liver disease (MAFLD) research from 2012 to 2021: a bibliometric analysis. Front Endocrinol (Lausanne). 2023;14:1078149
Article PubMed Google Scholar - Yang K, Song M. New Insights into the pathogenesis of metabolic-associated fatty liver disease (MAFLD): gut-liver-heart crosstalk. Nutrients. 2023;15(18):3970
Article CAS PubMed PubMed Central Google Scholar - Sun D-Q, Targher G, Byrne CD, Wheeler DC, Wong VW-S, Fan J-G, et al. An international Delphi consensus statement on metabolic dysfunction-associated fatty liver disease and risk of chronic kidney disease. Hepatobiliary Surg Nutr. 2023;12(3):386
Article PubMed PubMed Central Google Scholar - Zhou X-D, Targher G, Byrne CD, Somers V, Kim SU, Chahal CAA, et al. An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol Int. 2023;17(4):773–791
Article PubMed Google Scholar - Chun HS, Kim MN, Lee JS, Lee HW, Kim BK, Park JY, et al. Risk stratification using sarcopenia status among subjects with metabolic dysfunction-associated fatty liver disease. J Cachexia, Sarcopenia Muscle. 2021;12(5):1168–1178
Article PubMed Google Scholar - Sharma P, Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metabolic Syndrome. 2020;14(5):825–827
Article PubMed PubMed Central Google Scholar - Tsutsumi T, Nakano D, Kawaguchi M, Hashida R, Yoshinaga S, Takahashi H, et al. MAFLD associated with COPD via systemic inflammation independent of aging and smoking in men. Diabetol Metabolic Syndrome. 2022;14(1):115
Article CAS Google Scholar - Yu Q, He R, Jiang H, Wu J, Xi Z, He K, et al. Association between Metabolic dysfunction-associated fatty liver disease and cognitive impairment. J Clin Transl Hepatol. 2022;10(6):1034–1041
PubMed PubMed Central Google Scholar - Hu J, Xu Y, He Z, Zhang H, Lian X, Zhu T, et al. Increased risk of cerebrovascular accident related to non-alcoholic fatty liver disease: a meta-analysis. Oncotarget. 2018;9(2):2752–2760
Article PubMed Google Scholar - Liu Z, Lin C, Suo C, Zhao R, Jin L, Zhang T, et al. Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metab Clin Exp. 2022;127:154955
Article CAS PubMed Google Scholar - Jung CY, Koh HB, Park KH, Joo YS, Kim HW, Ahn SH, et al. Metabolic dysfunction-associated fatty liver disease and risk of incident chronic kidney disease: a nationwide cohort study. Diabetes Metab. 2022;48(4): 101344
Article CAS PubMed Google Scholar - Jung CY, Ryu GW, Kim HW, Ahn SH, Kim SU, Kim BS. Advanced liver fibrosis measured by transient elastography predicts chronic kidney disease development in individuals with non-alcoholic fatty liver disease. Diabetologia. 2022;65(3):518–527
Article CAS PubMed Google Scholar - Jung CY, Chun HS, Lee M, Koh HB, Park KH, Joo YS, et al. Exercise reduces the risk of chronic kidney disease in individuals with nonalcoholic fatty liver disease: a nationwide cohort study. Diabetes Metab. 2022;48(5): 101362
Article PubMed Google Scholar - Mantovani A, Csermely A, Tilg H, Byrne CD, Targher G. Comparative effects of non-alcoholic fatty liver disease and metabolic dysfunction-associated fatty liver disease on risk of incident cardiovascular events: a meta-analysis of about 13 million individuals. Gut. 2023;72(7):1433–1436
Article PubMed Google Scholar - Chun HS, Lee M, Lee JS, Lee HW, Kim BK, Park JY, et al. Metabolic dysfunction associated fatty liver disease identifies subjects with cardiovascular risk better than non-alcoholic fatty liver disease. Liver Int. 2023;43(3):608–625
Article CAS PubMed Google Scholar - Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of insulin resistance in MAFLD. Int J Mole Sci. 2021;22(8):4156
Article CAS Google Scholar - Anwar SD, Foster C, Ashraf A. Lipid disorders and metabolic-associated fatty liver disease. Endocrinol Metab Clin North Am. 2023;52(3):445–457
Article PubMed Google Scholar - Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Experiment Mole Med. 2016;48(3): e218
Article CAS Google Scholar - Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77(4):1136–1160
Article CAS PubMed Google Scholar - Kuchay MS, Choudhary NS, Mishra SK. Pathophysiological mechanisms underlying MAFLD. Diabetes Metabolic Syndrome. 2020;14(6):1875–1887
Article PubMed Google Scholar - Eslam M, Ahmed A, Despres JP, Jha V, Halford JCG, Wei Chieh JT, et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol. 2021;6(9):743–753
Article PubMed Google Scholar - Zoncape M, Liguori A, Tsochatzis EA. Multi-disciplinary clinic models for the management of non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2022;11(4):586–591
Article PubMed PubMed Central Google Scholar - Mantovani A, Goyale A, Roccarina D, Prat LI, Guerrero M, Patel R, et al. A multidisciplinary approach to non-alcoholic fatty liver disease (NAFLD) improves cardiovascular risk factors. J Hepatol. 2020;73:S110
Article Google Scholar - Moolla A, Motohashi K, Marjot T, Shard A, Ainsworth M, Gray A, et al. A multidisciplinary approach to the management of NAFLD is associated with improvement in markers of liver and cardio-metabolic health. Frontline Gastroenterol. 2019;10(4):337–346
Article PubMed PubMed Central Google Scholar - Cobbold JF, Raveendran S, Peake CM, Anstee QM, Yee MS, Thursz MR. Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol. 2013;4(4):263–269
Article CAS PubMed PubMed Central Google Scholar - Kumar S, Wong R, Newberry C, Yeung M, Peña J, Sharaiha R. Multi-disciplinary clinic models: a paradigm of care for management of non-alcoholic fatty liver disease (NAFLD). Hepatology. 2021;74:3472
Article PubMed Google Scholar - Lazarus JV, Anstee QM, Hagström H, Cusi K, Cortez-Pinto H, Mark HE, et al. Defining comprehensive models of care for NAFLD. Nat Rev Gastroenterol Hepatol. 2021;18(10):717–729
Article PubMed Google Scholar - Eslam M, Ahmed A, Després J-P, Jha V, Halford JC, Chieh JTW, et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol. 2021;6(9):743–753
Article PubMed Google Scholar - Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378 e365
Article PubMed Google Scholar - Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–1344
Article PubMed Google Scholar - Tsompanaki E, Thanapirom K, Papatheodoridi M, Parikh P, Chotai de Lima Y, Tsochatzis EA. Systematic review and meta-analysis: the role of diet in the development of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2023;21(6):1462-1474 e1424
Article CAS PubMed Google Scholar - Zeng X-F, Varady KA, Wang X-D, Targher G, Byrne CD, Tayyem R, et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: an international multidisciplinary expert consensus. Metabolism. 2024;161: 156028
Article CAS PubMed Google Scholar - Vilar-Gomez E, Nephew LD, Vuppalanchi R, Gawrieh S, Mladenovic A, Pike F, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. 2022;75(6):1491–1506
Article PubMed Google Scholar - Laura Sol Grinshpan, Sigal Eilate-Adar, Dana Ivancovsky-Wajcman, Revital Kariv, Michal Gillon-Keren, Zelber-Ragi S. Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: a systematic review. JHEP Reports. 2023;In press.
- Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the american association for the study of liver diseases (AASLD). Endocr Pract. 2022;28(5):528–562
Article PubMed Google Scholar - Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD Practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–1835
Article PubMed Google Scholar - Francque SM, Marchesini G, Kautz A, Walmsley M, Dorner R, Lazarus JV, et al. Non-alcoholic fatty liver disease: a patient guideline. JHEP Rep. 2021;3(5): 100322
Article PubMed PubMed Central Google Scholar - Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schutz T, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38(2):485–521
Article PubMed PubMed Central Google Scholar - Properzi C, O’Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. 2018;68(5):1741–1754
Article CAS PubMed Google Scholar - Kawaguchi T, Charlton M, Kawaguchi A, Yamamura S, Nakano D, Tsutsumi T, et al. Effects of mediterranean diet in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression analysis of randomized controlled trials. Semin Liver Dis. 2021;41(3):225–234
Article PubMed Google Scholar - Haigh L, Kirk C, El Gendy K, Gallacher J, Errington L, Mathers JC, et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Clin Nutr. 2022;41(9):1913–1931
Article CAS PubMed Google Scholar - Del Bo C, Perna S, Allehdan S, Rafique A, Saad S, AlGhareeb F, et al. Does the Mediterranean diet have any effect on lipid profile, central obesity and liver enzymes in non-alcoholic fatty liver disease (NAFLD) subjects? A systematic review and meta-analysis of randomized control trials. Nutrients. 2023;15(10):2250
Article PubMed PubMed Central Google Scholar - Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, et al. Dietary patterns and risk of hepatocellular carcinoma among us men and women. Hepatology. 2019;70(2):577–586
Article CAS PubMed Google Scholar - Lange M, Nadkarni D, Martin L, Newberry C, Kumar S, Kushner T. Intermittent fasting improves hepatic end points in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Hepatol Commun. 2023;7(8):e0212
Article PubMed PubMed Central Google Scholar - Cunha GM, Guzman G, Correa De Mello LL, Trein B, Spina L, Bussade I, et al. Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Front Endocrinol (Lausanne). 2020;11:607
Article PubMed PubMed Central Google Scholar - Holmer M, Lindqvist C, Petersson S, Moshtaghi-Svensson J, Tillander V, Brismar TB, et al. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - a randomised controlled trial. JHEP Rep. 2021;3(3): 100256
Article PubMed PubMed Central Google Scholar - Niezen S, Mehta M, Jiang ZG, Tapper EB. Coffee consumption is associated with lower liver stiffness: a nationally representative study. Clin Gastroenterol Hepatol. 2022;20(9):2032-2040 e2036
Article CAS PubMed Google Scholar - Hayat U, Siddiqui AA, Okut H, Afroz S, Tasleem S, Haris A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: a meta-analysis of 11 epidemiological studies. Ann Hepatol. 2021;20: 100254
Article CAS PubMed Google Scholar - Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. British J Sports Med. 2011;45(6):473–474
Article Google Scholar - Keating SE, Sabag A, Hallsworth K, Hickman IJ, Macdonald GA, Stine JG, et al. Exercise in the management of metabolic-associated fatty liver disease (MAFLD) in adults: a position statement from exercise and sport science Australia. Sports Med. 2023;53(12):2347–2371
Article PubMed PubMed Central Google Scholar - Stine JG, Long MT, Corey KE, Sallis RE, Allen AM, Armstrong MJ, et al. Physical activity and nonalcoholic fatty liver disease: a roundtable statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2023;55(9):1717–1726
Article PubMed PubMed Central Google Scholar - Zelber-Sagi S, Godos J, Salomone F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: a review of observational studies and intervention trials. Therap Adv Gastroenterol. 2016;9(3):392–407
Article CAS PubMed PubMed Central Google Scholar - Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L, et al. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: systematic review. World J Gastroenterol. 2016;22(27):6318–6327
Article PubMed PubMed Central Google Scholar - Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–1375
Article CAS PubMed Google Scholar - Solomon TP, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2009;297(2):E552-559
Article CAS PubMed PubMed Central Google Scholar - Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000;72(2 Suppl):558S-563S
Article CAS PubMed Google Scholar - Langston PK, Sun Y, Ryback BA, Mueller AL, Spiegelman BM, Benoist C, et al. Regulatory T cells shield muscle mitochondria from interferon-gamma-mediated damage to promote the beneficial effects of exercise. Sci Immunol. 2023;8(89):eadi5377
Article CAS PubMed PubMed Central Google Scholar - Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15(1):96-102 e103
Article CAS PubMed PubMed Central Google Scholar - Stine JG, Munaganuru N, Barnard A, Wang JL, Kaulback K, Argo CK, et al. Change in MRI-PDFF and Histologic Response in Patients With Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2021;19(11):2274-2283 e2275
Article PubMed Google Scholar - Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174–182
Article PubMed Google Scholar - Sabag A, Barr L, Armour M, Armstrong A, Baker CJ, Twigg SM, et al. The effect of high-intensity interval training vs moderate-intensity continuous training on liver fat: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107(3):862–881
Article PubMed Google Scholar - Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 2016;176(8):1074–1082
Article PubMed Google Scholar - Keating SE, Croci I, Wallen MP, Cox ER, Thuzar M, Pham U, et al. High-intensity interval training is safe, feasible and efficacious in nonalcoholic steatohepatitis: a randomized controlled trial. Dig Dis Sci. 2023;68(5):2123–2139
Article PubMed Google Scholar - O’Gorman P, Naimimohasses S, Monaghan A, Kennedy M, Melo AM, Ni Fhloinn D, et al. Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment Pharmacol Ther. 2020;52(8):1387–1398
Article PubMed Google Scholar - Harris SJ, Smith N, Hummer B, Schreibman IR, Faust AJ, Geyer NR, et al. Exercise training improves serum biomarkers of liver fibroinflammation in patients with metabolic dysfunction-associated steatohepatitis. Liver Int. 2023;44:532
Article PubMed PubMed Central Google Scholar - Stine JG, DiJoseph K, Pattison Z, Harrington A, Chinchilli VM, Schmitz KH, et al. Exercise training is associated with treatment response in liver fat content by magnetic resonance imaging independent of clinically significant body weight loss in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Am J Gastroenterol. 2023;118(7):1204–1213
Article CAS PubMed Google Scholar - Zambon Azevedo V, Silaghi CA, Maurel T, Silaghi H, Ratziu V, Pais R. Impact of sarcopenia on the severity of the liver damage in patients with non-alcoholic fatty liver disease. Front Nutr. 2021;8: 774030
Article PubMed Google Scholar - El-Kotob R, Ponzano M, Chaput JP, Janssen I, Kho ME, Poitras VJ, et al. Resistance training and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020. https://doi.org/10.1139/apnm-2020-0245
Article PubMed Google Scholar - Abou Sawan S, Nunes EA, Lim C, McKendry J, Phillips SM. The health benefits of resistance exercise: beyond hypertrophy and big weights. Exercise Sports Movement. 2023;1(1):e00001
Google Scholar - Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66(1):142–152
Article PubMed Google Scholar - Keating SE, Croci I, Wallen MP, Cox ER, Coombes JS, Burton NW, et al. High-intensity Interval training for the management of nonalcoholic steatohepatitis: participant experiences and perspectives. J Clin Transl Hepatol. 2023;11(5):1050–1060
PubMed PubMed Central Google Scholar - Stine JG, Soriano C, Schreibman I, Rivas G, Hummer B, Yoo E, et al. Breaking down barriers to physical activity in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2021;66(10):3604–3611
Article PubMed Google Scholar - Glass O, Liu D, Bechard E, Guy CD, Pendergast J, Mae Diehl A, et al. Perceptions of exercise and its challenges in patients with nonalcoholic fatty liver disease: a survey-based study. Hepatol Commun. 2022;6(2):334–344
Article PubMed Google Scholar - Jirapinyo P, McCarty TR, Dolan RD, Shah R, Thompson CC. Effect of endoscopic bariatric and metabolic therapies on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(3):511-524 e511
Article CAS PubMed Google Scholar - Lee SY, Lai H, Chua YJ, Wang MX, Lee GH. Endoscopic bariatric and metabolic therapies and their effects on metabolic syndrome and non-alcoholic fatty liver disease - a systematic review and meta-analysis. Front Med (Lausanne). 2022;9: 880749
Article PubMed PubMed Central Google Scholar - Zhou H, Luo P, Li P, Wang G, Yi X, Fu Z, et al. Bariatric surgery improves nonalcoholic fatty liver disease: systematic review and meta-analysis. Obes Surg. 2022;32(6):1872–1883
Article PubMed Google Scholar - Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and meta-analysis. PLoS Med. 2020;17(7): e1003206
Article PubMed PubMed Central Google Scholar - Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, et al. 2022 American society for metabolic and bariatric surgery (ASMBS) and international federation for the surgery of obesity and metabolic disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis. 2022;18(12):1345–1356
Article PubMed Google Scholar - Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379–388
Article PubMed Google Scholar - Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(6):1040–10601011
Article PubMed Google Scholar - Verrastro O, Panunzi S, Castagneto-Gissey L, De Gaetano A, Lembo E, Capristo E, et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multicentre, open-label, randomised trial. Lancet. 2023;401(10390):1786–1797
Article PubMed Google Scholar - Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159(4):1290-1301 e1295
Article PubMed Google Scholar - Aminian A, Al-Kurd A, Wilson R, Bena J, Fayazzadeh H, Singh T, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy-proven nonalcoholic steatohepatitis. JAMA. 2021;326(20):2031–2042
Article PubMed PubMed Central Google Scholar - Bai J, Jia Z, Chen Y, Li Y, Zheng S, Duan Z. Bariatric surgery is effective and safe for obese patients with compensated cirrhosis: a systematic review and meta-analysis. World J Surg. 2022;46(5):1122–1133
Article PubMed Google Scholar - Agarwal L, Sahu AK, Baksi A, Agarwal A, Aggarwal S. Safety of metabolic and bariatric surgery in obese patients with liver cirrhosis: a systematic review and meta-analysis. Surg Obes Relat Dis. 2021;17(3):525–537
Article PubMed Google Scholar - Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–2307
Article CAS PubMed Google Scholar - Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–315
Article PubMed Google Scholar - Huang J-F, Dai C-Y, Huang C-F, Tsai P-C, Yeh M-L, Hsu P-Y, et al. First-in-Asian double-blind randomized trial to assess the efficacy and safety of insulin sensitizer in nonalcoholic steatohepatitis patients. Hepatol Int. 2021;15(5):1136–1147
Article PubMed Google Scholar - Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care. 2018;41(8):1801–1808
Article CAS PubMed Google Scholar - Cusi K, Bril F, Barb D, Polidori D, Sha S, Ghosh A, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21(4):812–821
Article CAS PubMed Google Scholar - Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21(2):285–292
Article CAS PubMed Google Scholar - Phrueksotsai S, Pinyopornpanish K, Euathrongchit J, Leerapun A, Phrommintikul A, Buranapin S, et al. The effects of dapagliflozin on hepatic and visceral fat in type 2 diabetes patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2021;36(10):2952–2959
Article CAS PubMed Google Scholar - Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4 placebo-controlled trial. Diabetes Care. 2020;43(2):298–305
Article CAS PubMed Google Scholar - Lai LL, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci. 2020;65(2):623–631
Article CAS PubMed Google Scholar - Harrison SA, Manghi FP, Smith WB, Alpenidze D, Aizenberg D, Klarenbeek N, et al. Licogliflozin for nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a study. Nat Med. 2022;28(7):1432–1438
Article CAS PubMed PubMed Central Google Scholar - Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42(8):1481–1488
Article CAS PubMed Google Scholar - Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Ghabril M, Saxena R, Cummings OW, et al. Vitamin E improves transplant-free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology. 2020;71(2):495–509
Article CAS PubMed Google Scholar - Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E cancer prevention trial (SELECT). JAMA. 2011;306(14):1549–1556
Article CAS PubMed PubMed Central Google Scholar - Haukeland JW, Konopski Z, Eggesbo HB, von Volkmann HL, Raschpichler G, Bjoro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44(7):853–860
Article CAS PubMed Google Scholar - Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79–104
Article CAS PubMed Google Scholar - Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659–1668
Article CAS PubMed PubMed Central Google Scholar - Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1(1):57–64
Article CAS PubMed Google Scholar - Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Eslam M, et al. Type 2 diabetes and metformin use associate with outcomes of patients with non-alcoholic steatohepatitis-related child-pugh a cirrhosis. Clin Gastroenterol Hepatol. 2020;19:136
Article PubMed Google Scholar - Zhou J, Ke Y, Lei X, Wu T, Li Y, Bao T, et al. Meta-analysis: The efficacy of metformin and other anti-hyperglycemic agents in prolonging the survival of hepatocellular carcinoma patients with type 2 diabetes. Ann Hepatol. 2020;19(3):320–328
Article CAS PubMed Google Scholar - Jang H, Kim Y, Lee DH, Joo SK, Koo BK, Lim S, et al. Outcomes of various classes of oral antidiabetic drugs on nonalcoholic fatty liver disease. JAMA Intern Med. 2024;184(4):375–383
Article CAS PubMed PubMed Central Google Scholar - Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98(11):2485–2490
Article CAS PubMed Google Scholar - Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685
Article CAS PubMed PubMed Central Google Scholar - Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38(2):134–143
Article CAS PubMed Google Scholar - Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31(7–8):923–930
Article CAS PubMed Google Scholar - Sarkhy AA, Al-Hussaini AA, Nobili V. Does vitamin E improve the outcomes of pediatric nonalcoholic fatty liver disease? A systematic review and meta-analysis. Saudi J Gastroenterol. 2014;20(3):143–153
Article PubMed PubMed Central Google Scholar - Amanullah I, Khan YH, Anwar I, Gulzar A, Mallhi TH, Raja AA. Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomised controlled trials. Postgrad Med J. 2019;95(1129):601–611
Article CAS PubMed Google Scholar - Zhang X, Wong GL, Yip TC, Tse YK, Liang LY, Hui VW, et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2022;76(2):469–482
Article CAS PubMed Google Scholar - Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Sargent R, Hawkins C, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int Off J Int Association Study Liver. 2015;35(3):979–985
CAS Google Scholar - Simon TG, Wilechansky RM, Stoyanova S, Grossman A, Dichtel LE, Lauer GM, et al. Aspirin for metabolic dysfunction-associated steatotic liver disease without cirrhosis: a randomized clinical trial. JAMA. 2024;331(11):920–929
Article CAS PubMed PubMed Central Google Scholar - Ayada I, van Kleef LA, Zhang H, Liu K, Li P, Abozaid YJ, et al. Dissecting the multifaceted impact of statin use on fatty liver disease: a multidimensional study. EBioMedicine. 2023;87: 104392
Article CAS PubMed Google Scholar - Boutari C, Pappas PD, Anastasilakis D, Mantzoros CS. Statins’ efficacy in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Clin Nutr (Edinburgh, Scotland). 2022;41(10):2195–2206
Article CAS Google Scholar - Cho Y, Rhee H, Kim YE, Lee M, Lee BW, Kang ES, et al. Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial (ESSENTIAL study). BMC Med. 2022;20(1):93
Article CAS PubMed PubMed Central Google Scholar - Harrison S, Bedossa P, Guy C, Schattenberg J, Loomba R, Taub R, et al. Primary results from MAESTRO-NASH a pivotal phase 3 52-week serial liver biopsy study in 966 patients with NASH and fibrosis. J Hepatol. 2023;78(Suppl 1):S1
Article Google Scholar - Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29(11):2919–2928
Article CAS PubMed PubMed Central Google Scholar - Newsome PN, Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol. 2023;79(6):1557–1565
Article CAS PubMed Google Scholar - Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124
Article CAS PubMed Google Scholar - Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjaer MS, Krarup N, et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(6):511–522
Article CAS PubMed PubMed Central Google Scholar - Loomba R, Hartman ML, Lawitz EJ, Vuppalanchi R, Boursier J, Bugianesi E, et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. 2024;391(4):299–310.
Article CAS PubMed Google Scholar - Sanyal AJ, Bedossa P, Fraessdorf M, Neff GW, Lawitz E, Bugianesi E, et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N Engl J Med. 2024;391:311
Article CAS PubMed Google Scholar - Sanyal AJ, Bedossa P, Fraessdorf M, Neff GW, Lawitz E, Bugianesi E, et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. New Engl J Med. 2024;391(4):311–319
Article CAS PubMed Google Scholar - Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, et al. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385(17):1547–1558
Article CAS PubMed Google Scholar - Harrison SA, Frias JP, Neff G, Abrams GA, Lucas KJ, Sanchez W, et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2023;8(12):1080–1093
Article CAS PubMed Google Scholar - Loomba R, Sanyal AJ, Kowdley KV, Bhatt DL, Alkhouri N, Frias JP, et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389(11):998–1008
Article CAS PubMed PubMed Central Google Scholar - Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroentero. 2018;24(30):3361
Article Google Scholar - Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1611-1625.e1612
Article CAS PubMed Google Scholar - Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161(5):1657–1669
Article CAS PubMed Google Scholar - Younossi ZM, Yilmaz Y, Yu ML, Wai-Sun Wong V, Fernandez MC, Isakov VA, et al. Clinical and Patient-Reported Outcomes From Patients With Nonalcoholic Fatty Liver Disease Across the World: Data From the Global Non-Alcoholic Steatohepatitis (NASH)/ Non-Alcoholic Fatty Liver Disease (NAFLD) Registry. Clin Gastroenterol Hepatol. 2022;20(10):2296-2306 e2296
Article PubMed Google Scholar - Gronkjaer LL, Lauridsen MM. Quality of life and unmet needs in patients with chronic liver disease: a mixed-method systematic review. JHEP Rep. 2021;3(6): 100370
Article PubMed PubMed Central Google Scholar - Younossi ZM, Stepanova M, Anstee QM, Lawitz EJ, Wai-Sun Wong V, Romero-Gomez M, et al. Reduced patient-reported outcome scores associate with level of fibrosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2019;17(12):2552-2560 e2510
Article PubMed Google Scholar - Beleigoli AM, Andrade AQ, Cancado AG, Paulo MN, Diniz MFH, Ribeiro AL. Web-based digital health interventions for weight loss and lifestyle habit changes in overweight and obese adults: systematic review and meta-analysis. J Med Internet Res. 2019;21(1): e298
Article PubMed PubMed Central Google Scholar - Kjaergaard M, Lindvig KP, Thorhauge KH, Johansen S, Hansen JK, Andersen P, et al. Screening for fibrosis promotes lifestyle changes: a prospective cohort study in 4796 individuals. Clin Gastroenterol Hepatol. 2023;22:1037
Article PubMed Google Scholar - Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20(12):2809–2817
Article PubMed Google Scholar - Loomba R, Wong R, Fraysse J, Shreay S, Li S, Harrison S, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of medicare data. Alimentary Pharmacol Therap. 2020;51(11):1149–1159
Article CAS Google Scholar - Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209
Article PubMed Google Scholar - Mendes FD, Suzuki A, Sanderson SO, Lindor KD, Angulo P. Prevalence and indicators of portal hypertension in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10(9):1028–1033
Article PubMed PubMed Central Google Scholar - Francque S, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, et al. Noncirrhotic human nonalcoholic fatty liver disease induces portal hypertension in relation to the histological degree of steatosis. Eur J Gastroenterol Hepatol. 2010;22(12):1449–1457
PubMed Google Scholar - Berzigotti A. Advances and challenges in cirrhosis and portal hypertension. BMC Med. 2017;15(1):200
Article PubMed PubMed Central Google Scholar - Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7(2):141–155
Article CAS PubMed Google Scholar - Nababan SHH, Lesmana CRA. Portal hypertension in nonalcoholic fatty liver disease: from pathogenesis to clinical practice. J Clin Transl Hepatol. 2022;10(5):979–985
Article PubMed Central Google Scholar - Lesmana CRA, Nababan SH, Kalista KF, Kurniawan J, Jasirwan COM, Sulaiman AS, et al. Impact of endoscopic ultrasound examination for deep esophageal collateral veins evaluation in liver cirrhosis patients prior to endoscopic treatment: a case series. Portal Hypertension Cirrhosis. 2022;1(1):76–81
Article Google Scholar - Lesmana CRA. Technique innovation of endoscopic ultrasound portal pressure gradient measurement using standard manometer set for portal hypertension assessment. Clin Case Rep. 2022;10(12): e6658
Article PubMed PubMed Central Google Scholar - Lesmana CRA, Kalista KF, Nababan SHH, Kurniawan J, Jasirwan COM, Sulaiman AS, et al. Innovations in endoscopic ultrasound for portal hypertension and its role in managing complications in clinical practice: lessons learned from a tertiary referral public hospital. Portal Hypertension Cirrhosis. 2024;3:31
Article Google Scholar - Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144(1):102–111
Article PubMed Google Scholar - Pons M, Rivera-Esteban J, Ma MM, Davyduke T, Delamarre A, Hermabessière P, et al. Point-of-care noninvasive prediction of liver-related events in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2024;22(8):1637–1645
Article CAS PubMed Google Scholar - Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SG, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Off J Am College Gastroenterol ACG. 2021;116(4):723–732
Article Google Scholar - Petta S, Sebastiani G, Vigano M, Ampuero J, Wai-Sun Wong V, Boursier J, et al. Monitoring occurrence of liver-related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced chronic liver disease. Clin Gastroenterol Hepatol. 2021;19(4):806–815
Article PubMed Google Scholar - Segna D, Mendoza YP, Lange NF, Rodrigues SG, Berzigotti A. Non-invasive tools for compensated advanced chronic liver disease and portal hypertension after Baveno VII - an update. Dig Liver Dis. 2023;55(3):326–335
Article PubMed Google Scholar - Lin H, Lai JC, Wong GL, Delamarre A, Ahn SH, Li G, et al. Risk and predictors of hepatic decompensation in grey zone patients by the Baveno VII criteria: a competing risk analysis. Aliment Pharmacol Ther. 2023;58(9):920–928
Article CAS PubMed Google Scholar - Dajti E, Ravaioli F, Marasco G, Alemanni LV, Colecchia L, Ferrarese A, et al. A combined baveno VII and spleen stiffness algorithm to improve the noninvasive diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2022;117(11):1825–1833
Article PubMed Google Scholar - Jachs M, Hartl L, Simbrunner B, Bauer D, Paternostro R, Balcar L, et al. Carvedilol achieves higher hemodynamic response and lower rebleeding rates than propranolol in secondary prophylaxis. Clin Gastroenterol Hepatol. 2023;21(9):2318–2326
Article CAS PubMed Google Scholar - Chen YG, Yang CW, Chung CH, Ho CL, Chen WL, Chien WC. The association between metabolic risk factors, nonalcoholic fatty liver disease, and the incidence of liver cancer: a nationwide population-based cohort study. Hepatol Int. 2022;16(4):807–816
Article PubMed Google Scholar - Johira Y, Nakahara T, Kinami T, Yamasaki S, Kosaka M, Shirane Y, et al. Impact and usefulness of the transition to the new MAFLD classification for non-B, non-C HCC: a retrospective cohort study. BMC Gastroenterol. 2023;23(1):222
Article PubMed PubMed Central Google Scholar - Lin YP, Wang PM, Chuang CH, Yong CC, Liu YW, Huang PY, et al. Metabolic risks are increasing in non-B non-C early-stage hepatocellular carcinoma: a 10-year follow-up study. Front Oncol. 2022;12: 816472
Article CAS PubMed PubMed Central Google Scholar - Myers S, Neyroud-Caspar I, Spahr L, Gkouvatsos K, Fournier E, Giostra E, et al. NAFLD and MAFLD as emerging causes of HCC: a populational study. JHEP Rep. 2021;3(2): 100231
Article PubMed PubMed Central Google Scholar - Shimose S, Tsutsumi T, Nakano D, Sano T, Amano K, Kawaguchi T. An ever-increasing metabolic dysfunction-associated fatty liver disease-related hepatocellular carcinoma: what are problems and countermeasures? Hepatobiliary Surg Nutr. 2023;12(6):941–944
Article PubMed PubMed Central Google Scholar - Vitale A, Svegliati-Baroni G, Ortolani A, Cucco M, Dalla Riva GV, Giannini EG, et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: the ITA.LI.CA database. Gut. 2023;72(1):141–152
Article PubMed Google Scholar - Pelusi S, Bianco C, Colombo M, Cologni G, Del Poggio P, Pugliese N, et al. Metabolic dysfunction outperforms ultrasonographic steatosis to stratify hepatocellular carcinoma risk in patients with advanced hepatitis C cured with direct-acting antivirals. Liver Int. 2023;43(7):1593–1603
Article CAS PubMed Google Scholar - Sano T, Amano K, Ide T, Isoda H, Honma Y, Morita Y, et al. Metabolic management after sustained virologic response in elderly patients with hepatitis C virus: A multicenter study. Hepatol Res. 2023;54(4):326–35.
Article PubMed Google Scholar - van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3(5): 100350
Article PubMed PubMed Central Google Scholar - Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Prognostic impact of MAFLD following surgical resection of hepatitis B virus-related hepatocellular carcinoma: a nationwide cohort Study. Cancers (Basel). 2022;14(20):5002
Article CAS PubMed Google Scholar - Huang SC, Su TH, Tseng TC, Chen CL, Hsu SJ, Liao SH, et al. Distinct effects of hepatic steatosis and metabolic dysfunction on the risk of hepatocellular carcinoma in chronic hepatitis B. Hepatol Int. 2023;17(5):1139–1149
Article PubMed Google Scholar - Xue J, Wang QX, Xiao HM, Shi MJ, Xie YB, Li S, et al. Impact of metabolic dysfunction associated fatty liver disease on the prognosis of patients with hepatitis b virus-related hepatocellular carcinoma based on propensity score matching analysis. Cancer Manag Res. 2022;14:2193–2202
Article PubMed PubMed Central Google Scholar - Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: a nationwide study. Hepatol Res. 2022;52(12):975–984
Article CAS PubMed Google Scholar - van der Spek DPC, Katwaroe WK, van Kleef LA, Brakenhoff S, de Man RA, de Knegt RJ, et al. Time-trends in disease characteristics and comorbidities in patients with chronic hepatitis B in the period 1980–2020. Eur J Intern Med. 2023;107:86–92
Article PubMed Google Scholar - Kim MN, Han K, Yoo J, Hwang SG, Zhang X, Ahn SH. Diabetic MAFLD is associated with increased risk of hepatocellular carcinoma and mortality in chronic viral hepatitis patients. Int J Cancer. 2023;153(8):1448–1458
Article CAS PubMed Google Scholar - Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681–693
Article PubMed Google Scholar - Prorok PC. Epidemiologic approach for cancer screening. Problems in design and analysis of trials. Am J Pediatr Hematol Oncol. 1992;14(2):117–128
Article CAS PubMed Google Scholar - Stefanini B, Tonnini M, Serio I, Renzulli M, Tovoli F. Surveillance for hepatocellular carcinoma: current status and future perspectives for improvement. Expert Rev Anticancer Ther. 2022;22(4):371–381
Article CAS PubMed Google Scholar - Giannini EG, Cucchetti A, Erroi V, Garuti F, Odaldi F, Trevisani F. Surveillance for early diagnosis of hepatocellular carcinoma: how best to do it? World J Gastroenterol. 2013;19(47):8808–8821
Article PubMed PubMed Central Google Scholar - Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922–1965
Article PubMed Google Scholar - European Association for the Study of the Liver. Electronic address eee, European association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236
Article Google Scholar - Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370
Article PubMed Google Scholar - Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75(6):1476–1484
Article PubMed Google Scholar - Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828–1837
Article PubMed Google Scholar - Castellana M, Donghia R, Lampignano L, Castellana F, Zupo R, Sardone R, et al. Prevalence of the absence of cirrhosis in subjects with nafld-associated hepatocellular carcinoma. J Clin Med. 2021;10(20):4638
Article PubMed PubMed Central Google Scholar - Best J, Bechmann LP, Sowa JP, Sydor S, Dechene A, Pflanz K, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2020;18(3):728–735
Article CAS PubMed Google Scholar - Singal AG, El-Serag HB. Rational HCC screening approaches for patients with NAFLD. J Hepatol. 2022;76(1):195–201
Article PubMed Google Scholar - Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S, et al. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B. 2022;12(2):558–580
Article CAS PubMed Google Scholar - Inamine S, Kage M, Akiba J, Kawaguchi T, Yoshio S, Kawaguchi M, et al. Metabolic dysfunction-associated fatty liver disease directly related to liver fibrosis independent of insulin resistance, hyperlipidemia, and alcohol intake in morbidly obese patients. Hepatol Res. 2022;52(10):841–858
Article CAS PubMed Google Scholar - Sano T, Amano K, Ide T, Isoda H, Honma Y, Morita Y, et al. Metabolic management after sustained virologic response in elderly patients with hepatitis C virus: a multicenter study. Hepatol Res. 2024;54(4):326–335
Article CAS PubMed Google Scholar - Fahira A, Hanifah RS, Wardoyo MP, Diba AD, Ramadhan R, Barliana JD, et al. Is higher BMI associated with worse overall mortality in hepatocellular carcinoma patients? An evidence based case report. Acta Med Indones. 2019;51(4):356–363
PubMed Google Scholar - Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131–140
Article CAS PubMed Google Scholar - Li Q, Xing H, Liu D, Li H. Negative impact of low body mass index on liver cirrhosis patients with hepatocellular carcinoma. World J Surg Oncol. 2015;13:294
Article PubMed PubMed Central Google Scholar - Hirota K, Kawaguchi T, Koya S, Nagamatsu A, Tomita M, Hashida R, et al. Clinical utility of the liver frailty index for predicting muscle atrophy in chronic liver disease patients with hepatocellular carcinoma. Hepatol Res. 2020;50(3):330–341
Article CAS PubMed Google Scholar - Nagamatsu A, Kawaguchi T, Hirota K, Koya S, Tomita M, Hashida R, et al. Slow walking speed overlapped with low handgrip strength in chronic liver disease patients with hepatocellular carcinoma. Hepatol Res. 2019;49(12):1427–1440
Article PubMed Google Scholar - Koya S, Kawaguchi T, Hashida R, Hirota K, Bekki M, Goto E, et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2019;34(3):580–588
Article PubMed Google Scholar - Koya S, Kawaguchi T, Hashida R, Goto E, Matsuse H, Saito H, et al. Effects of in-hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol Res. 2017;47(3):E22–E34
Article CAS PubMed Google Scholar - Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100(11):1523–1530
Article CAS PubMed Google Scholar - Harimoto N, Yoshizumi T, Shimokawa M, Sakata K, Kimura K, Itoh S, et al. Sarcopenia is a poor prognostic factor following hepatic resection in patients aged 70 years and older with hepatocellular carcinoma. Hepatol Res. 2016;46(12):1247–1255
Article PubMed Google Scholar - Nishikawa H, Nishijima N, Enomoto H, Sakamoto A, Nasu A, Komekado H, et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol Lett. 2017;14(2):1637–1647
Article PubMed PubMed Central Google Scholar - Ha Y, Kim D, Han S, Chon YE, Lee YB, Kim MN, et al. Sarcopenia predicts prognosis in patients with newly diagnosed hepatocellular carcinoma, independent of tumor stage and liver function. Cancer Res Treat. 2018;50(3):843–851
Article CAS PubMed Google Scholar - Imai K, Takai K, Watanabe S, Hanai T, Suetsugu A, Shiraki M, et al. Sarcopenia impairs prognosis of patients with hepatocellular carcinoma: the role of liver functional reserve and tumor-related factors in loss of skeletal muscle volume. Nutrients. 2017;9(10):1054
Article PubMed PubMed Central Google Scholar - Takada H, Kurosaki M, Nakanishi H, Takahashi Y, Itakura J, Tsuchiya K, et al. Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS ONE. 2018;13(6): e0198812
Article PubMed PubMed Central Google Scholar - Mardian Y, Yano Y, Ratnasari N, Choridah L, Wasityastuti W, Setyawan NH, et al. Sarcopenia and intramuscular fat deposition are associated with poor survival in Indonesian patients with hepatocellular carcinoma: a retrospective study. BMC Gastroenterol. 2019;19(1):229
Article CAS PubMed PubMed Central Google Scholar - Kong Q, Yi M, Teng F, Li H, Chen Z. Sarcopenia imperils postoperative long-term survival in HCC patients with metabolic dysfunction-associated fatty liver disease: a propensity score matching analysis. J Hepatocell Carcinoma. 2023;10:1367–1377
Article PubMed PubMed Central Google Scholar - Yoshio S, Shimagaki T, Hashida R, Kawaguchi T, Tsutsui Y, Sakamoto Y, et al. Myostatin as a fibroblast-activating factor impacts on postoperative outcome in patients with hepatocellular carcinoma. Hepatol Res. 2021;51(7):803–812
Article CAS PubMed Google Scholar - Hashida R, Kawaguchi T, Koya S, Hirota K, Goshima N, Yoshiyama T, et al. Impact of cancer rehabilitation on the prognosis of patients with hepatocellular carcinoma. Oncol Lett. 2020;19(3):2355–2367
CAS PubMed PubMed Central Google Scholar - Kawaguchi T, Kawaguchi A, Hashida R, Nakano D, Tsutsumi T, Kawaguchi M, et al. Resistance exercise in combination with aerobic exercise reduces the incidence of serious events in patients with liver cirrhosis: a meta-analysis of randomized controlled trials. J Gastroenterol. 2023;59(3):216–28.
Article PubMed Google Scholar - Shimose S, Koya S, Kawaguchi T, Hirota K, Yoshio S, Niizeki T, et al. Impact of branched-chain amino acids and frailty on the management of lenvatinib-related fatigue in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2021;27(4):616–619
Article PubMed PubMed Central Google Scholar - Pfister D, Nunez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumor surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456
Article CAS PubMed PubMed Central Google Scholar - Rimini M, Rimassa L, Ueshima K, Burgio V, Shigeo S, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open. 2022;7(6): 100591
Article CAS PubMed PubMed Central Google Scholar - Shimose S, Hiraoka A, Casadei-Gardini A, Tsutsumi T, Nakano D, Iwamoto H, et al. The beneficial impact of metabolic dysfunction-associated fatty liver disease on lenvatinib treatment in patients with non-viral hepatocellular carcinoma. Hepatol Res. 2023;53(2):104–115
Article CAS PubMed Google Scholar - Rimini M, Kudo M, Tada T, Shigeo S, Kang W, Suda G, et al. Nonalcoholic steatohepatitis in hepatocarcinoma: new insights about its prognostic role in patients treated with lenvatinib. ESMO Open. 2021;6(6): 100330
Article CAS PubMed PubMed Central Google Scholar - Younossi ZM, Stepanova M, Al Shabeeb R, Eberly KE, Shah D, Nguyen V, et al. The changing epidemiology of adult liver transplantation in the United States in 2013–2022: The dominance of metabolic dysfunction-associated steatotic liver disease and alcohol-associated liver disease. Hepatol Commun. 2024;8(1):e0352
Article PubMed Google Scholar - Verna EC, Phipps MM, Halazun KJ, Markovic D, Florman SS, Haydel BM, et al. Outcomes in liver transplant recipients with nonalcoholic fatty liver disease-related HCC: results from the US multicenter HCC transplant consortium. Liver Transpl. 2023;29(1):34–47
Article PubMed Google Scholar - Teo VXY, Heng RRY, Tay PWL, Ng CH, Tan DJH, Ong Y, et al. A meta-analysis on the prevalence of chronic kidney disease in liver transplant candidates and its associated risk factors and outcomes. Transpl Int. 2021;34(12):2515–2523
Article PubMed Google Scholar - Hegyi PJ, Soos A, Hegyi P, Szakacs Z, Hanak L, Vancsa S, et al. Pre-transplant sarcopenic obesity worsens the survival after liver transplantation: a meta-analysis and a systematic review. Front Med (Lausanne). 2020;7: 599434
Article PubMed PubMed Central Google Scholar - Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73:691
CAS PubMed Google Scholar - Jamil OK, Sandikci B, Faust N, Cotter TG, Paul S, di Sabato D, et al. Relatively poor long-term outcomes following liver transplantation for NASH in the United States. Transplantation. 2022;106(10):2006–2018
Article PubMed Google Scholar - Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European liver transplant registry study. J Hepatol. 2019;71(2):313–322
Article PubMed PubMed Central Google Scholar - Barman PM, VanWagner LB. Cardiac risk assessment in liver transplant candidates: current controversies and future directions. Hepatology. 2021;73(6):2564–2576
Article PubMed Google Scholar - Lim WH, Ng CH, Tan D, Tseng M, Xiao J, Yong JN, et al. Natural history of NASH cirrhosis in liver transplant waitlist registrants. J Hepatol. 2023;79(4):1015–1024
Article CAS PubMed Google Scholar - Linares I, Hamar M, Selzner N, Selzner M. Steatosis in liver transplantation: current limitations and future strategies. Transplantation. 2019;103(1):78–90
Article PubMed Google Scholar - Trakroo S, Bhardwaj N, Garg R, Modaresi EJ. Weight loss interventions in living donor liver transplantation as a tool in expanding the donor pool: a systematic review and meta-analysis. World J Gastroenterol. 2021;27(24):3682–3692
Article CAS PubMed PubMed Central Google Scholar - Lin JS, Muhammad H, Lin T, Kamel I, Baghdadi A, Rizkalla N, et al. Donor BMI and post-living donor liver transplantation outcomes: a preliminary report. Transpl Direct. 2023;9(2): e1431
Article Google Scholar - Goto R, Kawamura N, Watanabe M, Ganchiku Y, Nagatsu A, Okada K, et al. Long-term risk of a fatty liver in liver donors. Ann Gastroenterol Surg. 2023;7(4):645–653
Article PubMed PubMed Central Google Scholar - Akabane M, Imaoka Y, Esquivel CO, Melcher ML, Kwong A, Sasaki K. Overcoming the hurdles of steatotic grafts in liver transplantation: Insights into survival and prognostic factors. Liver Transpl. 2023;30(4):376–85.
Article PubMed Google Scholar - Widmer J, Eden J, Carvalho MF, Dutkowski P, Schlegel A. Machine perfusion for extended criteria donor livers: what challenges remain? J Clin Med. 2022;11(17):5218
Article PubMed PubMed Central Google Scholar - Lim WH, Ng CH, Tan DJH, Xiao J, Fu CE, Ong C, et al. Donor diabetes and steatosis affects recipient survival following liver transplantation based on etiology of liver cirrhosis. Transplantation. 2024;108(2):473–482
CAS PubMed Google Scholar - Taneja S, Roy A, Duseja A. NASH after liver transplantation: impact of immunosuppression. J Clin Exp Hepatol. 2023;13(5):835–840
Article CAS PubMed PubMed Central Google Scholar - Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: disease burden, current management and future challenges. JHEP Rep. 2020;2(6): 100192
Article PubMed PubMed Central Google Scholar - Tan HK, Teng MLP, Soh AYS, Cheo SHY, Fook-Chong S, Goh GBB, et al. Poor outcomes of cirrhosis due to nonalcoholic steatohepatitis compared with hepatitis b after decompensation with ascites. Am J Gastroenterol. 2021;116(7):1437–1446
Article PubMed Google Scholar - Mallet M, Silaghi CA, Sultanik P, Conti F, Rudler M, Ratziu V, et al. Current challenges and future perspectives in treating patients with NAFLD-related cirrhosis. Hepatology. 2023;80(5):1270–90.
Article PubMed Google Scholar - Younossi ZM, Zelber-Sagi S, Henry L, Gerber LH. Lifestyle interventions in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20(11):708–722
Article CAS PubMed Google Scholar - El Sherif O, Dhaliwal A, Newsome PN, Armstrong MJ. Sarcopenia in nonalcoholic fatty liver disease: new challenges for clinical practice. Expert Rev Gastroenterol Hepatol. 2020;14(3):197–205
Article PubMed Google Scholar - European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–193
Article Google Scholar - Stine JG, Long MT, Corey KE, Sallis RE, Allen AM, Armstrong MJ, et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol Commun. 2023;7(4):e0108
Article PubMed PubMed Central Google Scholar - Fernandez-Mincone T, Contreras-Briceno F, Espinosa-Ramirez M, Garcia-Valdes P, Lopez-Fuenzalida A, Riquelme A, et al. Nonalcoholic fatty liver disease and sarcopenia: pathophysiological connections and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2020;14(12):1141–1157
Article CAS PubMed Google Scholar - Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65(4):1293–1305
Article PubMed Google Scholar - Bassegoda O, Olivas P, Turco L, Mandorfer M, Serra-Burriel M, Tellez L, et al. Decompensation in advanced nonalcoholic fatty liver disease may occur at lower hepatic venous pressure gradient levels than in patients with viral disease. Clin Gastroenterol Hepatol. 2022;20(10):2276–2286
Article CAS PubMed Google Scholar - Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(10):897–901
Article PubMed Google Scholar - Goh GB, Schauer PR, McCullough AJ. Considerations for bariatric surgery in patients with cirrhosis. World J Gastroenterol. 2018;24(28):3112–3119
Article PubMed PubMed Central Google Scholar - Patton H, Heimbach J, McCullough A. AGA clinical practice update on bariatric surgery in cirrhosis: expert review. Clin Gastroenterol Hepatol. 2021;19(3):436–445
Article PubMed Google Scholar - Eslam M, Alkhouri N, Vajro P, Baumann U, Weiss R, Socha P, et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol. 2021;6(10):864–873
Article PubMed Google Scholar - Temple JL, Cordero P, Li J, Nguyen V, Oben JA. A guide to non-alcoholic fatty liver disease in childhood and adolescence. Int J Mol Sci. 2016;17(6):947
Article PubMed PubMed Central Google Scholar - Hegarty R, Kyrana E, Fitzpatrick E, Dhawan A. Fatty liver disease in children (MAFLD/PeFLD type 2): unique classification considerations and challenges. Ther Adv Endocrinol Metab. 2023;14:20420188231160388
Article CAS PubMed PubMed Central Google Scholar - Zhang L, El-Shabrawi M, Baur LA, Byrne CD, Targher G, Kehar M, et al. An international multidisciplinary consensus on pediatric metabolic dysfunction-associated fatty liver disease. Med. 2024;5(7):797-815.e2.
Article CAS PubMed Google Scholar - Carrieri L, Osella AR, Ciccacci F, Giannelli G, Scavo MP. Premenopausal syndrome and NAFLD: a new approach based on gender medicine. Biomedicines. 2022;10(5):1184
Article PubMed PubMed Central Google Scholar - Pan Z, Khatry MA, Yu M-L, Choudhury A, Sebastiani G, Alqahtani SA, et al. MAFLD: an ideal framework for understanding disease phenotype in individuals of normal weight. Therapeutic Adv Endocrinol Metab. 2024;15:20420188241252544
Article Google Scholar - Kim Y, Han E, Lee JS, Lee HW, Kim BK, Kim MK, et al. Cardiovascular risk is elevated in lean subjects with nonalcoholic fatty liver disease. Gut Liver. 2022;16(2):290–299
Article CAS PubMed Google Scholar - Eslam M, Fan J-G, Mendez-Sanchez N. Non-alcoholic fatty liver disease in non-obese individuals: the impact of metabolic health. Lancet Gastroenterol Hepatol. 2020;5(8):713–715
Article PubMed Google Scholar - Chung GE, Yu SJ, Yoo JJ, Cho Y, Lee KN, Shin DW, et al. Lean or diabetic subtypes predict increased all-cause and disease-specific mortality in metabolic-associated fatty liver disease. BMC Med. 2023;21(1):4
Article PubMed PubMed Central Google Scholar - Alarabi M, Pan Z, Romero-Gómez M, George J, Eslam M. Telomere length and mortality in lean MAFLD: the other face of metabolic adaptation. Hepatol Int. 2024;18(5):1448–58.
Article PubMed Google Scholar - Alharthi J, Pan Z, Gloss BS, McLeod D, Weltman M, George J, et al. Loss of metabolic adaptation in lean MAFLD is driven by endotoxemia leading to epigenetic reprogramming. Metabolism. 2023;144: 155583
Article CAS PubMed Google Scholar - Kaya E, Alphan E, Yilmaz Y. Role of intensive dietary and lifestyle interventions in the treatment of lean nonalcoholic fatty liver disease patients. Eur J Gastroen Hepat. 2019;32(10):1352–7.
Google Scholar - Shi JP, Fan JG, Wu R, Gao XQ, Zhang L, Wang H, et al. Prevalence and risk factors of hepatic steatosis and its impact on liver injury in Chinese patients with chronic hepatitis B infection. J Gastroenterol Hepatol. 2008;23(9):1419–1425
Article PubMed Google Scholar - Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67(4):862–873
Article PubMed Google Scholar - Hu D, Wang H, Wang H, Wang Y, Wan X, Yan W, et al. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model. Hepatol Int. 2018;12(5):438–446
Article PubMed Google Scholar - Zheng Q, Zou B, Wu Y, Yeo Y, Wu H, Stave CD, et al. Systematic review with meta-analysis: prevalence of hepatic steatosis, fibrosis and associated factors in chronic hepatitis B. Aliment Pharmacol Ther. 2021;54(9):1100–1109
Article CAS PubMed Google Scholar - Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, Bhanthumkomol P, Bandidniyamanon W, Pausawasdi N, et al. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int. 2017;37(4):542–551
Article CAS PubMed Google Scholar - Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–548
Article CAS PubMed Google Scholar - Huang CF, Liang PC, Tsai PC, Wei YJ, Huang CI, Wang CW, et al. The interplay of metabolic dysfunction-associated fatty liver disease and viral hepatitis on liver disease severity: a large community-based study in a viral endemic area. J Gastroenterol Hepatol. 2024;39(1):193–201
Article CAS PubMed Google Scholar - Wong YJ, Nguyen VH, Yang HI, Li J, Le MH, Wu WJ, et al. Impact of fatty liver on long-term outcomes in chronic hepatitis B: a systematic review and matched analysis of individual patient data meta-analysis. Clin Mol Hepatol. 2023;29(3):705–720
Article PubMed PubMed Central Google Scholar - Li J, Yang HI, Yeh ML, Le MH, Le AK, Yeo YH, et al. Association between fatty liver and cirrhosis, hepatocellular carcinoma, and hepatitis b surface antigen seroclearance in chronic hepatitis B. J Infect Dis. 2021;224(2):294–302
Article CAS PubMed Google Scholar - Oh JH, Lee HW, Sinn DH, Park JY, Kim BK, Kim SU, et al. Controlled attenuation parameter value and the risk of hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. Hepatol Int. 2021;15(4):892–900
Article PubMed Google Scholar - Mak LY, Hui RW, Fung J, Liu F, Wong DK, Li B, et al. Reduced hepatic steatosis is associated with higher risk of hepatocellular carcinoma in chronic hepatitis B infection. Hepatol Int. 2021;15(4):901–911
Article PubMed Google Scholar - Lee MH, Chen YT, Huang YH, Lu SN, Yang TH, Huang JF, et al. Chronic viral hepatitis B and C outweigh MASLD in the associated risk of cirrhosis and HCC. Clin Gastroenterol Hepatol Off Clin Practice J Am Gastroenterological Association. 2024;22:1275
Article CAS Google Scholar - Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56(3):533–540
Article PubMed Google Scholar - Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: a cohort study. Hepatology. 2017;65(3):828–835
Article CAS PubMed Google Scholar - Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26(9):1361–1367
Article PubMed Google Scholar - Diao Y, Tang J, Wang X, Deng W, Tang J, You C. Metabolic syndrome, nonalcoholic fatty liver disease, and chronic hepatitis B: a narrative review. Infect Dis Ther. 2023;12(1):53–66
Article PubMed Google Scholar - Kim DS, Jeon MY, Lee HW, Kim BK, Park JY, Kim DY, et al. Influence of hepatic steatosis on the outcomes of patients with chronic hepatitis B treated with entecavir and tenofovir. Clin Mol Hepatol. 2019;25(3):283–293
Article PubMed Google Scholar - Mak LY, Hui RW, Lee CH, Mao X, Cheung KS, Wong DK, et al. Glycemic burden and the risk of adverse hepatic outcomes in patients with chronic hepatitis B with type 2 diabetes. Hepatology. 2023;77(2):606–618
Article PubMed Google Scholar - Cheng PN, Feng IC, Chen JJ, Kuo HT, Lee PL, Yu ML, et al. Body weight increase and metabolic derangements after tenofovir disoproxil fumarate switch to tenofovir alafenamide in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2024;59(2):230–238
Article CAS PubMed Google Scholar - Yeh ML, Liang PC, Trinh S, Huang CI, Huang CF, Hsieh MY, et al. Body weight changes in treated hepatitis B patients switching to tenofovir alafenamide(☆). J Formos Med Assoc. 2022;121(7):1273–1282
Article CAS PubMed Google Scholar - Tai CM, Tu HP, Hwang JC, Yeh ML, Huang CF, Yu ML. HBV reactivation after bariatric surgery for HBV-infected obese patients. Obes Surg. 2022;32(10):3332–3339
Article PubMed Google Scholar - Attia D, Abdel Alem S, El-Akel W, Abdel-Razek W, Eslam M, Fouad Y, et al. Prevalence and clinical characteristics of patients with metabolic dysfunction-associated fatty liver disease with hepatitis C virus infection-a population-based study. Alimentary Pharmacol Therap. 2022;56(11–12):1581–1590
Article CAS Google Scholar - Cheng KL, Wang SW, Cheng YM, Hsieh TH, Wang CC, Kao JH. 2024 Prevalence and clinical outcomes in subtypes of metabolic associated fatty liver disease. J Formosan Med Assoc = Taiwan yi zhi. 2024;123(1):36–44
Article CAS PubMed Google Scholar - Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126(2):586–597
Article CAS PubMed Google Scholar - Huang CF, Dai CY, Yeh ML, Huang CI, Tai CM, Hsieh MH, et al. Association of diabetes and PNPLA3 genetic variants with disease severity of patients with chronic hepatitis C virus infection. J Hepatol. 2015;62(3):512–518
Article CAS PubMed Google Scholar - Younossi ZM, McCullough AJ, Ong JP, Barnes DS, Post A, Tavill A, et al. Obesity and non-alcoholic fatty liver disease in chronic hepatitis C. J Clin Gastroenterol. 2004;38(8):705–709
Article PubMed Google Scholar - Noureddin M, Wong MM, Todo T, Lu SC, Sanyal AJ, Mena EA. Fatty liver in hepatitis C patients post-sustained virological response with direct-acting antivirals. World J Gastroenterol. 2018;24(11):1269–1277
Article PubMed PubMed Central Google Scholar - Fouad Y, Lazarus JV, Negro F, Peck-Radosavljevic M, Sarin SK, Ferenci P, et al. MAFLD considerations as a part of the global hepatitis C elimination effort: an international perspective. Alimentary Pharmacol Therap. 2021;53(10):1080–1089
Article Google Scholar - Yen YH, Lin MT, Kuo FY, Chang KC, Tsai MC, Tseng PL, et al. The association between steatosis and diabetes with hepatocellular carcinoma in non-genotype 3 chronic hepatitis C patients. Liver Int. 2018;38(6):1064–1073
Article CAS PubMed Google Scholar - Cespiati A, Coelho Rodrigues I, Santos I, Policarpo S, Carvalhana S, Fracanzani AL, et al. Effect of HCV eradication by DAAs on liver steatosis, carotid atherosclerosis, and associated metabolic comorbidities: a systematic review. Liver Int Off J Int Association Study Liver. 2024;4:1075
Google Scholar - Huang CF, Dai CY, Yeh ML, Huang CI, Lee HC, Lai WT, et al. Cure or curd: Modification of lipid profiles and cardio-cerebrovascular events after hepatitis C virus eradication. Kaohsiung J Med Sci. 2020;36(11):920–928
Article CAS PubMed Google Scholar - Shih CI, Wu KT, Hsieh MH, Yang JF, Chen YY, Tsai WL, et al. Severity of fatty liver is highly correlated with the risk of hypertension and diabetes: a cross-sectional and longitudinal cohort study. Hepatol Int. 2024;18(1):138–154
Article PubMed Google Scholar - Tsai PC, Kuo HT, Hung CH, Tseng KC, Lai HC, Peng CY, et al. Metformin reduces hepatocellular carcinoma incidence after successful antiviral therapy in patients with diabetes and chronic hepatitis C in Taiwan. J Hepatol. 2023;78(2):281–292
Article CAS PubMed Google Scholar - Kalligeros M, Vassilopoulos A, Shehadeh F, Vassilopoulos S, Lazaridou I, Mylonakis E, et al. Prevalence and characteristics of nonalcoholic fatty liver disease and fibrosis in people living with HIV monoinfection: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21(7):1708–1722
Article CAS PubMed Google Scholar - Michel M, Labenz C, Armandi A, Kaps L, Kremer WM, Galle PR, et al. Metabolic dysfunction-associated fatty liver disease in people living with HIV. Sci Rep. 2023;13(1):9158
Article CAS PubMed PubMed Central Google Scholar - Eslam M, George J. Two years on, a perspective on MAFLD. eGastroenterology. 2023;1(2)
- Hirakawa M, Arase Y, Amakawa K, Ohmoto-Sekine Y, Ishihara M, Shiba M, et al. Relationship between alcohol intake and risk factors for metabolic syndrome in men. Int Med. 2015;54(17):2139–2145
Article CAS Google Scholar - Kim SK, Hong S-H, Chung J-H, Cho KB. Association between alcohol consumption and metabolic syndrome in a community-based cohort of Korean adults. Med Scie Monit Int Med J Exp Clin Res. 2017;23:2104
CAS Google Scholar - Åberg F, Puukka P, Salomaa V, Männistö S, Lundqvist A, Valsta L, et al. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology. 2020;71(3):835–848
Article PubMed Google Scholar - Bryazka D, Reitsma MB, Griswold MG, Abate KH, Abbafati C, Abbasi-Kangevari M, et al. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet. 2022;400(10347):185–235
Article Google Scholar - Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187–194
Article PubMed PubMed Central Google Scholar - Sharma M, Savage C, Nair M, Larsson I, Svedberg P, Nygren JM. Artificial intelligence applications in health care practice: scoping review. J Med Internet Res. 2022;24(10): e40238
Article PubMed PubMed Central Google Scholar - Rauschecker AM, Rudie JD, Xie L, Wang J, Duong MT, Botzolakis EJ, et al. Artificial intelligence system approaching neuroradiologist-level differential diagnosis accuracy at brain MRI. Radiology. 2020;295(3):626–637
Article PubMed Google Scholar - Lonardo A, Arab JP, Arrese M. Perspectives on precision medicine approaches to NAFLD diagnosis and management. Adv Ther. 2021;38(5):2130–2158
Article PubMed PubMed Central Google Scholar - Leow W-Q, Bedossa P, Liu F, Wei L, Lim K-H, Wan W-K, et al. An improved qFibrosis algorithm for precise screening and enrollment into non-alcoholic steatohepatitis (NASH) clinical trials. Diagnostics. 2020;10(9):643
Article CAS PubMed PubMed Central Google Scholar
Author information
Authors and Affiliations
- Storr Liver Centre, Westmead Institute for Medical Research, Westmead Hospital and University of Sydney, Westmead, NSW, 2145, Australia
Mohammed Eslam & Jacob George - Center for Fatty Liver, Department of Gastroenterology, Shanghai Key Lab of Pediatric Gastroenterology and Nutrition, Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Jian-Gao Fan - Hepatobiliary Division, Department of Internal MedicineCollege of Medicine and Center for Liquid Biopsy and Cohort ResearchFaculty of Internal Medicine and Hepatitis Research Center, School of Medicine, College of MedicineSchool of Medicine and Doctoral Program of Clinical and Experimental Medicine, College of Medicine and Center of Excellence for Metabolic Associated Fatty Liver Disease, Kaohsiung Medical University, National Sun Yat-Sen University, Kaohsiung, Taiwan
Ming-Lung Yu - Medical Data Analytics Centre, Department of Medicine and Therapeutics, State Key Laboratory of Digestive Disease, Institute of Digestive Disease, Chinese University of Hong Kong, Hong Kong, China
Vincent Wai-Sun Wong - Institute of Digestive and Liver Diseases, St. Luke’s Medical Center, Global City, Philippines
Ian Homer Cua - Division of Gastroenterology and Hepatology, Department of Internal MedicineHepatitis Research CenterGraduate Institute of Clinical Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
Chun-Jen Liu - Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Tawesak Tanwandee - Department of Internal Medicine, Hepatobiliary Division, Dr. Cipto Mangunkusumo National General Hospital, Universitas Indonesia, Pangeran Diponegoro Road No. 71St, Central Jakarta, 10430, Indonesia
Rino Gani - Department of Medicine, School of Clinical Medicine, State Key Laboratory of Liver Research, The University of Hong Kong, Hong Kong, China
Wai-Kay Seto - Department of Medicine, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
Wai-Kay Seto - Department of Hepatology, Bangabandhu Sheikh Mujib Medical University, Shahbag, Dhaka, Bangladesh
Shahinul Alam - Department of Medicine, Yong Loo Lin School of Medicine, National University Singapore, Singapore, Singapore
Dan Yock Young - Department of Medicine, Aga Khan University, Karachi, Pakistan
Saeed Hamid - MAFLD Research Center, Department of Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Key Laboratory of Diagnosis and Treatment for The Development of Chronic Liver Disease in Zhejiang Province, Wenzhou, China
Ming-Hua Zheng - Division of Gastroenterology, Department of Medicine, Kurume University School of Medicine, Kurume, Japan
Takumi Kawaguchi - Gastroenterology and Hepatology Unit, Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Wah-Kheong Chan - Department of Medicine, Cardinal Santos Medical Center, Mandaluyong, Philippines
Diana Payawal - Department of Hepatology, Selayang Hospital, Batu Caves, Malaysia
Soek-Siam Tan - Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore, Singapore
George Boon-bee Goh - Medicine Academic Clinical Program, Duke-NUS Medical School, Singapore, Singapore
George Boon-bee Goh - AW Morrow Gastroenterology and Liver Centre, Royal Prince Alfred Hospital, Sydney, NSW, Australia
Simone I. Strasser - Internal Medicine Faculty, Hanoi Medical University, Hanoi, Vietnam
Hang Dao Viet - Graduate Institute of Clinical MedicineDepartment of Internal MedicineHepatitis Research CenterDepartment of Medical Research, National Taiwan University College of Medicine, National Taiwan University, National Taiwan University Hospital, 1 Chang-Te Street, 10002, Taipei, Taiwan
Jia-Horng Kao - Division of Gastroenterology and Hepatology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul Metropolitan Government Boramae Medical Center, Seoul, Republic of Korea
Won Kim - Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, 50-1, Yonsei-Ro, Seodaemun-Gu, Seoul, 03722, Republic of Korea
Seung Up Kim - School of Human Movement and Nutrition Sciences, The University of Queensland, Brisbane, QLD, 4072, Australia
Shelley E. Keating - Department of Gastroenterology, School of Medicine, Recep Tayyip Erdoğan University, Rize, Turkey
Yusuf Yilmaz - National Medical Centre, Karachi, Pakistan
Lubna Kamani - Buddhist Tzu Chi Medical Foundation and School of Medicine, Taipei Tzu Chi Hospital, Tzu Chi University, Taipei, Taiwan
Chia-Chi Wang - Department of Gastroenterology, Hepatology and Endemic Medicine, Faculty of Medicine, Minia University, Cairo, Egypt
Yasser Fouad - Department of Hepatogastroenterology, Dr.Ziauddin University Hospital, Clifton, Karachi, Pakistan
Zaigham Abbas - Division of Gastroenterology, Chulalongkorn University, Bangkok, Thailand
Sombat Treeprasertsuk & Kessarin Thanapirom - Department of Hepatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
Mamun Al Mahtab - Department of Health Policy, School of Public Health, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
Undram Lkhagvaa - Department of Infectious Diseases, School of Medicine, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
Oidov Baatarkhuu - Department of Hepatology, Institute of Liver and Biliary Sciences, New Delhi, 110070, India
Ashok Kumar Choudhury - University of Otago, Christchurch Hospital, Christchurch, New Zealand
Catherine A. M. Stedman - Department of Hepatology, School of Digestive and Liver Diseases, Institute of Post Graduate Medical Education and Research, Kolkata, India
Abhijit Chowdhury - Department of Medicine, Ankara University School of Medicine, Ankara, Turkey
A Kadir Dokmeci - Senior Department of Infectious Diseases, The Fifth Medical Center of Chinese PLA General Hospital, National Clinical Research Center for Infectious Diseases, Chinese PLA Medical School, Chinese PLA General Hospital, Beijing, 100039, China
Fu-Sheng Wang - Division of Gastroenterology and Hepatology, Department of Medicine, Institute of Clinical Medicine, School of Medicine, Taipei Veterans General Hospital, National Yang-Ming Chiao Tung University, No. 201, Section 2, Shipai RdNo. 155, Section 2, Linong St, Beitou District, Taipei City, 112, Taiwan
Han-Chieh Lin - Hepatobiliary Division, Department of Internal MedicineCollege of Medicine and Center for Liquid Biopsy and Cohort ResearchFaculty of Internal Medicine and Hepatitis Research Center, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Jee-Fu Huang - Burnet Institute, Melbourne, VIC, 3004, Australia
Jess Howell - Department of Epidemiology and Preventive Medicine, Monash University, Clayton, VIC, 3008, Australia
Jess Howell - Department of Medicine, The University of Melbourne, Parkville, VIC, 3050, Australia
Jess Howell - Department of Gastroenterology, St Vincent’s Hospital Melbourne, Melbourne, VIC, 3165, Australia
Jess Howell - Liver Research Center, Beijing Key Laboratory of Translational Medicine On Liver Cirrhosis, Beijing Friendship Hospital, Capital Medical University, National Clinical Research Center of Digestive Diseases, Beijing, China
Jidong Jia - Department of Internal Medicine, Al-Azhar University, Cairo, 11884, Egypt
Mohamed Alboraie - Department of Gastroenterology and Hepatology, Central Clinical School, The Alfred, Monash University, Melbourne, Australia
Stuart K. Roberts - Department of Gastroenterology and Hepatology, Yokohama City University Graduate School of Medicine, Yokohama, 236-0004, Japan
Masato Yoneda - Gastroenterology and Hepatology Department, Yerevan Medical Scientific Center, Yerevan, Armenia
Hasmik Ghazinian & Aram Mirijanyan - Department of Traditional and Western Medical Hepatology, Third Hospital of Hebei Medical University, Shijiazhuang, China
Yuemin Nan - Department of Medicine, Medistra Hospital, Jakarta, Indonesia
Cosmas Rinaldi Adithya Lesmana - Medical School, Faculty of Medicine and Health Sciences, The University of Western Australia, Nedlands, WA, Australia
Leon A. Adams - Hepatology and Gastroenterology Unit, Internal Medicine Department, Faculty of Medicine, Mansoura University, Egyptian Liver Research Institute and Hospital (ELRIAH), Sherbin, El Mansoura, Egypt
Gamal Shiha - Department of Hepatology, Institute of Liver and Biliary Sciences, New Delhi, India
Manoj Kumar & Shiv K. Sarin - Department of Gastroenterohepatology, Istanbul Health and Technology University, Istanbul, Turkey
Necati Örmeci - Hepatopancreatobiliary Center, Beijing Tsinghua Changgung Hospital, Tsinghua University, Beijing, China
Lai Wei - Humanity and Health Medical Group, Humanity and Health Clinical Trial Center, Hong Kong SAR, China
George Lau - The Fifth Medical Center of Chinese, PLA General Hospital, Beijing, 100039, China
George Lau - Department of Gastroenterology, Yamanashi Central Hospital, Yamanashi, Japan
Masao Omata - University of Tokyo, Tokyo, Japan
Masao Omata
Authors
- Mohammed Eslam
- Jian-Gao Fan
- Ming-Lung Yu
- Vincent Wai-Sun Wong
- Ian Homer Cua
- Chun-Jen Liu
- Tawesak Tanwandee
- Rino Gani
- Wai-Kay Seto
- Shahinul Alam
- Dan Yock Young
- Saeed Hamid
- Ming-Hua Zheng
- Takumi Kawaguchi
- Wah-Kheong Chan
- Diana Payawal
- Soek-Siam Tan
- George Boon-bee Goh
- Simone I. Strasser
- Hang Dao Viet
- Jia-Horng Kao
- Won Kim
- Seung Up Kim
- Shelley E. Keating
- Yusuf Yilmaz
- Lubna Kamani
- Chia-Chi Wang
- Yasser Fouad
- Zaigham Abbas
- Sombat Treeprasertsuk
- Kessarin Thanapirom
- Mamun Al Mahtab
- Undram Lkhagvaa
- Oidov Baatarkhuu
- Ashok Kumar Choudhury
- Catherine A. M. Stedman
- Abhijit Chowdhury
- A Kadir Dokmeci
- Fu-Sheng Wang
- Han-Chieh Lin
- Jee-Fu Huang
- Jess Howell
- Jidong Jia
- Mohamed Alboraie
- Stuart K. Roberts
- Masato Yoneda
- Hasmik Ghazinian
- Aram Mirijanyan
- Yuemin Nan
- Cosmas Rinaldi Adithya Lesmana
- Leon A. Adams
- Gamal Shiha
- Manoj Kumar
- Necati Örmeci
- Lai Wei
- George Lau
- Masao Omata
- Shiv K. Sarin
- Jacob George
Corresponding authors
Correspondence toMohammed Eslam or Shiv K. Sarin.
Ethics declarations
Conflict of interest
LAA has served as an advisory board member or speaker for Novo Nordisk, CSL Behring and Gilead. ME has received personal fees from Pfizer and honoraria from Sanofi. JG is on advisory boards and receives honoraria for talks from Novo Nordisk, AstraZeneca, Roche, BMS, Pfizer, Cincera, Pharmaxis and Boehringer Mannheim. JH has received competitive grant funding from Gilead Sciences and speaker fee and ad board support from Roche and Astra-Zeneca, none relevant to this clinical guideline. JFH has received research grant from Gilead, Bristol-Myer-Squibb, serves as a consultant for Roche, Sysmex, Boehringer Ingelheim, Aligos, and has been a speaker for AbbVie, Gilead, Merck, Sysmex, and Novo Nordisk. WK has received honoraria and served in an advisory role for Gilead, Boehringer-Ingelheim, GSK, Novo Nordisk, Samil, Ildong, LG Chemistry, YUHAN, Hanmi, HK Inoen, Standigm, PharmaKing, KOBIOLABS, Olix Pharma, TSD Life Sciences, Daewoong, QUEST, Therasid Bioscience, Korea United Pharm, and Eisai; has received research funding from GSK, Gilead, Novartis, Pfizer, Roche, Springbank, Altimmune, Ildong, DaeWoong, Dicerna, Celgene, Hanmi, Novo Nordisk, Galmed, Enyo. TK received lecture fees from ASKA Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., Kowa Company, Ltd., AbbVie GK., Eisai Co., Ltd., Novo Nordisk Pharma Ltd., Janssen Pharmaceutical K.K., Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd. and KOBIOLABS; holds stock in KOBIOLABS and Lepidyne; and is the founder of Remedygen Incorporation. SKR is a member of the Advisory Board for Novo Nordisk. The other authors disclose no conflicts. SIS has received honoraria for advisory boards or speaking from Roche, Astra Zeneca, Ipsen, Eisai, Sirtex, BMS, MSD, AbbVie, Gilead Sciences, Norgine, Astellas, Novartis, Pfizer, CSL-Behring, Dr Falk Pharma, Chiesi, Novo Nordisk. WKS received speaker’s fees from Echosens, is an advisory board member and received speaker's fees from Abbott, received research funding from Astrazeneca, Alexion Pharmaceuticals, Boehringer Ingelheim, Pfizer and Ribo Life Science, and is an advisory board member, received speaker’s fees and research funding from Gilead Sciences. VW has provided consultancy services to AbbVie, AstraZeneca, Boehringer Ingelheim, Echosens, Eli Lilly, Gilead Sciences, Intercept, Inventiva, Merck, Novo Nordisk, Pfizer, Sagimet Biosciences, TARGET PharmaSolutions, and Visirna; has delivered lectures for Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk, and Unilab; has received research grants from Gilead Sciences; and he is a co-founder of Illuminatio Medical Technology, in which he holds stock. MLY has research support from Abbvie, Abbott Diagnostic, BMS, Gilead, Merck and Roche diagnostics; served as a consultant of Abbott, Abbvie, Abbott Diagnostic, BMS, Gilead, Roche and Roche diagnostics and a speaker of Abbvie, BMS, Eisai, Gilead, Roche and Roche diagnostics. YY has served as a consultant for Zydus, Cymabay, and Novo Nordisk, and as a speaker for Echosens. MHZ has received honoraria for lectures from AstraZeneca, Hisky Medical Technologies and Novo Nordisk, consulting fees from Boehringer Ingelheim. Outside their participation in this work. The other authors disclose no conflicts.
Ethical approval and Informed consent.
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Eslam, M., Fan, JG., Yu, ML. et al. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease.Hepatol Int 19, 261–301 (2025). https://doi.org/10.1007/s12072-024-10774-3
- Received: 06 September 2024
- Accepted: 28 December 2024
- Published: 27 February 2025
- Version of record: 27 February 2025
- Issue date: April 2025
- DOI: https://doi.org/10.1007/s12072-024-10774-3