Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer (original) (raw)
Introduction
Angiogenesis, the formation of new blood vessels from preexisting vasculature, is essential for many physiological processes, such as wound healing, and also plays a critical role in many pathological conditions including diabetic retinopathy, age-related macular degeneration, and tumor growth. Angiogenesis is controlled by delicate balance between pro- and anti-angiogenic signals, thus elucidating the molecular mechanisms underlying normal and aberrant blood vessel growth may provide new therapeutic options for many human diseases1. Among the pro-angiogenic molecules, vascular endothelial growth factor (VEGF) has received a considerable amount of attention as a promising target for anti-angiogenic therapy. Anti-angiogenic drugs targeting the VEGF pathway, such as a humanized monoclonal antibody against VEGF, were developed rapidly and used in the clinical settings. However, anti-VEGF treatments often result in only modest improvement in progression-free survival, and tumors can eventually acquire resistance to anti-angiogenic therapies. To overcome these problems, a better understanding of the molecular mechanisms responsible for angiogenesis is necessary, which may afford the opportunity to develop new anti-angiogenic therapeutic strategies.
The most widely investigated angiogenesis inhibitors are the proteolytic cleavage products of extracellular matrix or serum components, such as endostatin, angiostatin, arresten, and tumstatin (reviewed in2,3). Multiple cytokines can also exert anti-angiogenic properties, including interferons and certain interleukins, which often act indirectly by limiting the expression of pro-angiogenic mediators or inducing anti-angiogenic molecules (reviewed in2,3). In contrast, there are very few known developmentally regulated, naturally occurring anti-angiogenic molecules, which include platelet factor 44, thrombospondin-15, and pigment epithelium-derived factor6, whose precise mechanism of action is not fully understood. In this regard, emerging evidence suggests that proteins involved in transmitting axonal guidance cues, including members of the netrin, slit, eph and semaphorin families, also play a critical role in blood vessel guidance during physiological and pathological blood vessel development7,8. In this review, we summarize the current knowledge on semaphorin family proteins in angiogenesis, with an emphasis on semaphorin-regulated anti-angiogenic signaling circuitries in endothelial cells. The possible role of semaphorin signaling in supressing lymphangiogenesis will also be discussed.
Semaphorins
Semaphorins are a family of cell surface and soluble proteins originally identified as axon guidance factors that control the development of central nervous system9. All semaphorins are characterized by an amino-terminal 500-amino acid Sema domain that is essential for signaling. Semaphorins are grouped into eight classes based on their structural domains, with classes 3-7 comprising the vertebrate semaphorins (Figure 1). Although initially identified as potent axon chemorepellents, several semaphorins can provide bifunctional guidance cues, functioning as repulsive or attractive molecules depending on the cell types and biological context10,11. The common targets of semaphorins are the actin cytoskeleton and focal adhesions; the latter are dynamic cell-to-extracellular matrix adhesive structures that are assembled upon integrin engagement. Semaphorin signaling affects focal adhesion assembly/disassembly and induces cytoskeletal remodeling, thus consequently affecting cell shape, attachment to the extracellular matrix, cell motility, and cell migration12,13.
Figure 1
Human Plexins, Nrps, and Semaphorins: Vertebrates express semaphorin classes 3 through 7, plexins A, B, C, D, and Nrps 1 and 2. Semaphorins and plexins are comprised of a Sema domain and variable repeats of the PSI domain, as indicated. Members of the semaphorin 3 (Sema3, A-G) class are secreted, while the semaphorins 4-7 (Sema4-7) are membrane bound. Plexins contain cytoplasmic tails that contain both GAP and GTPase-binding domains. Additionally, plexins B1, B2, B3, and D1 contain a PDZ binding domain. Nrp1 and Nrp2 are comprised of extracellular complement binding, FV/FVIII, and MAM domains, but have only a short cytoplasmic tail, requiring their association with plexin A1-4 to facilitate signaling.
Semaphorin receptors: plexins and neuropilins (Nrps)
Semaphorins signal through two major receptor families, plexins and Nrps. In vertebrates, two Nrps (Nrp1 and Nrp2) and nine plexins have been identified14 (Figure 1). Membrane-bound semaphorins bind directly to plexins, whereas secreted type of semaphorins (class 3 semaphorins; Sema3s) bind to a holoreceptor complex consisting of Nrps as ligand binding and plexins as signal transducing subunit. An exception to this rule is Sema3E, which binds and signals directly through plexin-D1, independently of Nrps15.
Plexins are single-pass transmembrane receptors and subdivided into four groups, type A, B, C, and D. Similar to semaphorins, plexins have extracellular Sema domains. In addition, plexins have PSI (plexins, Sema, integrins) and IPT (Ig-like, plexins, transcription factors) domains, and share homology in their extracellular segment with the Met family tyrosine kinase receptors14. The intracellular domains of plexins have weak sequence similarity to GTPase activating proteins (GAPs) and display GAP activity towards the small GTPase R-Ras16. Type A, B, and D plexins require the association of the Rnd family of Rho-related GTPases to function as R-Ras GAP, while plexin-C1 displays GAP activity without Rnd17. Plexin-B1 also possesses GAP activity for R-Ras3/M-Ras18.
Nrps are transmembrane proteins with a short cytoplasmic domain of about 40 amino acids, and their three C-terminal amino acids (S-E-A) constitute a PDZ-binding motif19. In addition to Sema3s, Nrps also bind to structurally unrelated molecules, such as VEGF family proteins, and serve as their co-receptors20,21. The extracellular domains of Nrps contain two complement-binding domains (a1/a2), two coagulation factor V/VIII homology domains (b1/b2), and a MAM domain (c). Sema3s principally bind to a1/a2 domains, and VEGFs bind to b1/b2 domains14. Genetic studies have shown that Nrp1 is required for vascular morphogenesis. Nrp1-knockout mice are embryonically lethal due to vascular remodeling and branching defects22,23, whereas Nrp2-knockout mice are viable and their vasculature is grossly normal. However, Nrp2 null mice show absence of or reduced lymphatic vessel sprouting during development24, suggesting that Nrp2 plays a key role in lymphangiogenesis (discussed below).
Anti-angiogenic semaphorins
Sema3s are the only secreted type of semaphorins in vertebrates. Seven Sema3s have been identified (designated by the letters A-G) and multiple Sema3s are reported to control physiological and pathological angiogenesis25,26. Nrps and the type A/D family plexins (plexin-A1, -A2, and -A3, and plexin-D1) act as receptors for Sema3s, and each Sema3 family member shows distinct binding preference for Nrps. For example, Sema3A binds to Nrp127,28, while Sema3F binds exclusively to Nrp229. Other Sema3s, such as Sema3B, can bind to both Nrps30,31. Each Sema3-Nrp complex associates with specific plexins to mediate downstream signaling. Some Sema3s, such as Sema3A and Sema3F, are expressed in endothelial cells, suggesting an endothelial-initiated autocrine regulation of angiogenesis32.
Sema3A
Sema3A regulates endothelial cell migration and survival in vitro33,34, and tumor-induced angiogenesis in vivo35. The information emerging from the analysis of Sema3A null mice and mutant mice lacking Sema3A-Nrp1 signaling suggests that Sema3A may not be required for the early stages of developmental angiogenesis, but rather that Sema3A contributes to the reshaping of the vasculature to form a mature vascular network, such as that in the heart and brain32,36,37. The molecular mechanisms underlying the anti-angiogenic effects of Sema3A are complex, as its receptor Nrp1 also controls VEGFR2 signaling by binding VEGF16538,39. Hence, it was initially suggested that Sema3s compete for the binding to Nrp1 with VEGF, thus inhibiting VEGF-induced angiogenesis. However, recent reports have shown that Sema3s and VEGF use different domains on Nrp1 for binding40. Consistent with a separate function of Sema3A on Nrp1, Sema3A increases vascular permeability, inhibits endothelial cell proliferation, and induces apoptosis even in the absence of VEGF34,41, suggesting Sema3A activates its own signaling routes. Interestingly, Sema3A impairs endothelial cell adhesion and migration by inhibiting integrin function32. The molecular mechanisms by which Sema3A regulates integrins are not fully understood. From the studies in neuronal cells, it is likely that activation of plexin-A1 by Sema3A induces the intrinsic R-Ras GAP activity of Plexin-A1, thus resulting in R-Ras inhibition16,42. As R-Ras is known to sustain integrin activation, the inactivation of R-Ras by plexin-A1 may lead to the inactivation of integrins, thereby inhibiting integrin-mediated cell adhesion.
Sema3B
Sema3B and Sema3F were identified as tumor suppressors that are deleted or inactivated in lung cancer43,44. Consistently, overexpression of Sema3B suppresses tumorigenesis in adenocarcinoma cell lines45, and Sema3B also decreases proliferation of lung and breast cancer cell lines30, suggesting Sema3B exerts direct effects on cancer cells. In addition to the inhibitory effect on cancer cells, Sema3B can repel endothelial cells mainly through Nrp1, and therefore functions as an angiogenesis inhibitor46. Interestingly, Sema3B activity is abrogated as a result of proteolytic cleavage by furin-like pro-protein convertases, which is associated with enhanced invasion and proliferation in many cancers46,47. Together, Sema3B may function as a tumor suppressor by working on both tumor cells and endothelial cells. Certainly, more work is necessary to define the precise signaling events that mediate this effect of Sema3B.
Sema3E
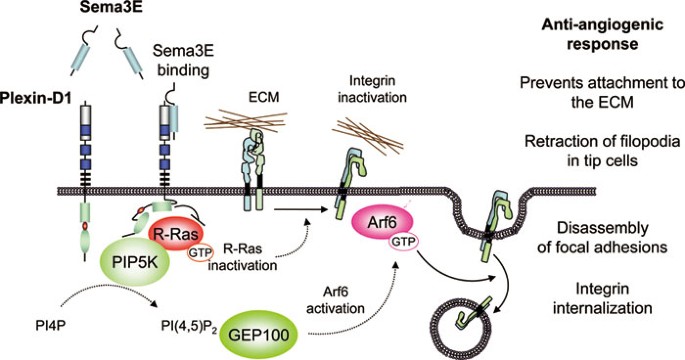
As described above, Sema3E binds directly to and activates plexin-D1 receptor. Plexin-D1 is highly expressed in endothelium during development48 and also in tumor-associated blood vessels49. Interestingly, the expression of plexin-D1 is dynamically regulated and increased in tip cells, which extend numerous filopodia and respond to attractive and repulsive guidance cues at the leading edge of the new branching blood vessels50. Sema3E controls vascular patterning during development by inhibiting the expansion of intersomitic vessels into the somites15,51, and causes endothelial-tip cell filopodial retraction in growing blood vessels52. These data suggest that Sema3E is a potent chemorepellent for plexin-D1-expressing endothelial cells. Indeed, Sema3E displays anti-angiogenic properties in several different in vivo angiogenesis models52,53. Furthermore, Sema3E is highly expressed in metastatic tumors, where a cleaved form of Sema3E may enhance tumor cell motility. Conversely, when wild-type Sema3E is overexpressed in cancer cell lines, Sema3E decreases tumor vessel density and tumor growth54. The interplay between cleaved products of Sema3E and potential receptors in cancer cells is an area of active investigation. More progress has been recently made on the study of how Sema3E prevents angiogenesis in endothelial cells, given the obligatory role of plexin-D1 but not of Nrps in this biological response. Sema3E may counteract the pro-angiogenic effects of VEGF by promoting the expression and release of a soluble VEGF receptor, thus inhibiting VEGF function in developing vessels55. In a more direct fashion in endothelial cells, upon stimulation with Sema3E, plexin-D1 initiates a two-pronged mechanism involving R-Ras inactivation and Arf6 activation, thereby affecting the activation status of β1 integrin and its intracellular trafficking, respectively52 (Figure 2). At the molecular level, Sema3E binding to plexin-D1 induces the activation of type I phosphatidylinositol-4-phosphate-5-kinase (PIP5K) β, which may stimulate the production of phosphatidylinositol 4,5-biphosphate (PI(4,5)P2) locally56. PI(4,5)P2 then binds to the PH domain of a guanine nucleotide exchange factor (GEF) for Arf6, guanine nucleotide exchange protein 100, thus increasing its GEF activity and activating Arf6. Sema3E-induced Arf6 activation induces rapid focal adhesion disassembly and endothelial cell collapse, thereby inhibiting angiogenesis56. In addition, recent reports revealed that PI(4,5)P2 acts as a second messenger and controls focal adhesion dynamics and actin cytoskeleton57, both of which are critical for cell migration. In neuronal cells, inhibition of another isoform of PIP5K, PIPKIγ661, is required for Sema3A-induced axonal repulsion and growth cone collapse42. These data suggest that semaphorins may commonly utilize phospholipid-regulated signaling pathways to regulate cell adhesion and migration, by regulating lipid kinases such as PIP5K.
Figure 2
Anti-angiogenic signaling by Sema3E-plexin D1 in endothelial cells. The activation of plexin D1 by Sema3E induces the association of the Ras GAP domain of plexin D1 with R-Ras. This inactivates integrins and enables their subsequent internalization by the plexin D1-mediated activation of Arf6, thus inhibiting endothelial cell adhesion to the extracellular matrix (ECM) by disrupting integrin-mediated adhesive structures, and causing filopodial retraction in endothelial tip cells. The pathway by which Sema3E stimulation of plexin D1 leads to Arf6 activation involves phosphatidylinositol-4-phosphate-5-kinase β activation, which generates PI(4,5)P2 locally. PI(4,5)P2 then binds to the PH domain of an Arf6 GEF, guanine nucleotide exchange protein 100, resulting in Arf6 activation. Sema3E-induced Arf6 activation induces rapid focal adhesion (FA) disassembly, integrin internalization, and endothelial cell collapse, thereby inhibiting angiogenesis.
Sema3F
Sema3F decreases tumor growth in a number of in vivo tumor models, and although Sema3F is capable of interacting with Nrp1, its higher-affinity interaction with Nrp2 appears to be required for its tumor-suppressive activity in many models58,59,60. Sema3F exerts a repulsive effect on breast cancer cells61 and reduces the growth and metastatic activity of colorectal carcinoma cells by modifying integrin αvβ362, suggesting that Sema3F affects tumor cells directly by controlling cell adhesion and migration. Similar to other Sema3s, the Sema3F-mediated suppression of tumor growth was associated with an anti-angiogenic phenotype, where Sema3F expression significantly decreased the vascularity of tumors25,58. Consistently, Sema3F can induce endothelial cell collapse63, repel endothelial cells and inhibit their survival59, and this anti-angiogenic effect is synergistically enhanced by the addition of Sema3A34. At the molecular level, it has been shown that Sema3F inhibits multiple signaling pathways in cancer cells63,64; however, whether these pathways are also affected in endothelial cells remains unclear. While it is clear that Sema3F can function as a potent tumor suppressor in vivo, further studies are required to understand the molecular mechanism behind this function and the role that Sema3F expression plays in cancer development and progression.
Sema3G
Far less is known about the role of Sema3G in cancer and signaling. Sema3G has been identified as a significant prognostic marker in glioma65. Sema3G appears to bind to Nrp2, but not Nrp166, and is able to suppress tumor growth and inhibit soft-agar colony formation only in cells expressing high levels of this receptor25. However, further work is necessary to determine whether Sema3G has anti-angiogenic effects in vivo and the mechanism by which it achieves its Nrp2-dependent tumor growth-suppressive activity.
Pro-angiogenic semaphorins
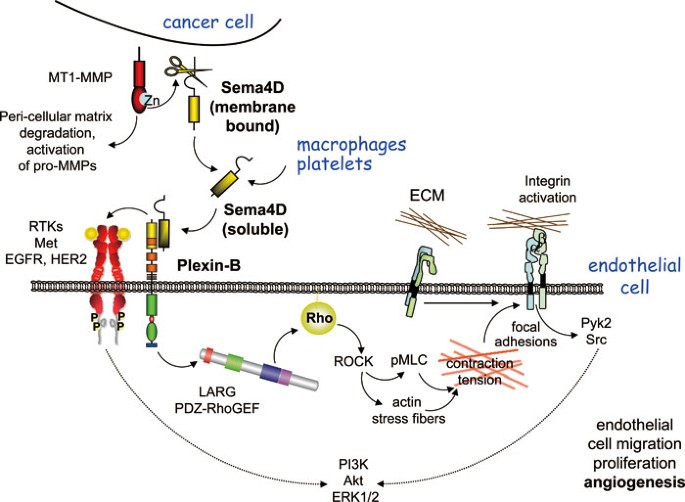
Some plexins are highly expressed in endothelial cells. Among them, plexin-B1 is a receptor for Sema4D, and it has been reported that Sema4D-Plexin-B1 signaling promotes endothelial cell migration and tube formation67,68. Interestingly, Sema4D expression is upregulated in head and neck squamous cell carcinoma as well as in some other solid tumors69,70. Sema4D is processed and released from the membrane by membrane type 1-matrix metalloproteinase, which is frequently overexpressed in malignant tumors, and activates plexin-B1 to elicit pro-angiogenic signaling in endothelial cells71. Similarly, Sema4D can be released from platelets by the action of metalloprotease ADAM17 (TACE), thereby acting on endothelial cells as well as platelets that also express Sema4D receptors72. The pro-angiogenic effect of Sema4D is mediated by the activation of a small GTPase RhoA through Rho-specific GEFs, leukemia-associated RhoGEF, and PDZ-RhoGEF, which bind to the C-terminal PDZ-binding motif of plexin-B167,73,74,75,76. Upon ligation of Sema4D, PDZ-RhoGEF and leukemia-associated RhoGEF are recruited to plexin-B1, thus stimulating RhoA and its downstream effector Rho kinase, and Rho kinase activation promotes angiogenic response through a chain of events involving myosin light chain phosphorylation, stress fiber contaction, non-receptor tyrosine kinase activation, and activation of the Akt and Erk pathways77 (Figure 3). RhoA/Rho kinase signaling also activates PIP5K α and leads to the generation of the lipid second messenger PI(4,5)P2, further supporting the idea that phosphoinositides may represent a common mediator for semaphorin signaling. In the case of Sema4D, PI(4,5)P2 serves as a substrate for PLCγ and increases intracellular calcium level, which is required for Sema4D-induced tube formation in vitro78. In addition to the direct effect of Sema4D on plexin-B1, this semaphorin can also promote endothelial cell migration through the activation of HGF receptor Met68, while in other cell types, plexin-B1 activates members of the EGF receptor family79 and inhibits RhoA through p190 RhoGAP80, resulting in changes in the actin cytoskeleton and cell migration.
Figure 3
Pro-angiogenic signaling by semaphorins in endothelial cells. Membrane-bound Sema4D expressed by cancer cells or by tumor-associated macrophages and platelets is cleaved by matrix metalloproteinases, and soluble Sema4D can then bind and activate plexin-B members expressed on endothelial cells. After stimulation by Sema4D, plexin-B can activate certain receptor tyrosine kinases, such as Met, EGFR, and Her2, in some cellular contexts, hence activating their regulated signaling pathways indirectly. Sema4D binding to plexin-B causes the recruitment and activation of PDZ-RhoGEF and leukemia-associated RhoGEF through association mediated by their PDZ domains. These GEFs then activate Rho and its downstream effector Rho kinase (ROCK). In turn, ROCK signaling promotes the phosphorylation of myosin light chain. This induces polymerization and contraction of actin/myosin stress fibers. The tension generated by contracting stress fibers promotes the assembly of mature focal adhesion complexes at the sites of cell contact with the extracellular matrix via integrins, thereby activating non-receptor tyrosine kinases such as Pyk2 and Src. Pyk2 activation results in the phosphorylation of phosphatidylinositol 3-kinase and the activation of the Akt and Erk1/2 pathways, leading to increased endothelial cell migration and proliferation, which contribute to promoting an angiogenic response.
Lymphangiogenesis and cancer
Like angiogenesis, lymphangiogenesis is both a physiological and pathological process required for the formation of new vasculature, and the regulation of this process is mediated by a balance of pro- and anti-lymphangiogenic factors. The lymphatic system is critical for the removal of waste products and transport of cells and proteins, and contributes to the immune response and cancer progression. During embryogenesis, expression of the transcription factor PROX1 leads to the differentiation of venous endothelial cells into lymphatic endothelial cells81,82,83,84. This differentiation is followed by sprouting and proliferation of the lymphatic vasculature, which is primarily attributed to signaling by VEGF-C through its receptor VEGFR385,86,87. VEGF-C binds to receptors VEGFR3 and Nrp2, and loss of these molecules results in a variety of lymphangiogenic defects. _Vegfc_−/− mice are able to commit to the lymphatic lineage, but cannot undergo lymphangiogenesis and die in utero88. VEGFR3-knockout mice display a more severe phenotype, where they lack lymphatic vasculature entirely and fail to undergo lymphangiogenesis89. In contrast, Nrp2 is not required for lymphangiogenesis but appears to be a key regulator of the process. Mice deficient in Nrp2 show a variety of lymphatic defects, including reduction of small lymphatic vessels and capillaries24. Further, Nrp2 appears to be selectively expressed in tumor-associated lymphatic vessels, and inhibition of Nrp2 function blocked VEGF-C/VEGFR3-mediated lymphangiogenesis and reduced metastasis in vivo90,91. By extension, this suggests that anti-angiogenic semaphorins like Sema3F can function as anti-lymphangiogenic signaling molecules in vivo through their interaction with Nrp2. Sema3F overexpression has been shown to have a direct chemorepulsive effect on lymphatic endothelial cells58. Sema3F appears to compete for Nrp2 binding with VEGF-C and decreases endothelial cell survival and migration92. Nevertheless, direct evidence for an anti-lymphangiogenic role of Sema3F is limited.
Indirectly, tumor-induced lymphangiogenesis is correlated with metastasis in a number of cancer models, including colorectal, breast, prostate, head and neck cancer, and melanoma93,94,95,96,97,98,99, and expression of Semaphorin molecules decreases the incidence of metastasis58,62,100. Inhibition of tumoral lymphangiogenesis through neutralization of VEGFR3 or knockdown of VEGF-C expression blocks metastasis in vivo101,102,103, indicating that this process is essential for metastasis in a variety of cancer models. Interestingly, this inhibition did not affect the normal lymphatic vasculature in adult mice, suggesting that alternative signaling mechanisms are employed after development, and targeting the VEGF-C/VEGFR3 signaling axis in adults may represent a potent means to target the tumor-associated lymphatics104. The precise molecular events that signal for lymphangiogenesis are poorly understood. Inhibition of the mTOR pathway decreases lymphangiogenesis and metastasis, and prolongs survival in a head and neck cancer animal model99. In support of this, a separate study found that inhibition of phosphatidylinositol 3-kinase/Akt similarly blocked lymphangiogenesis105. However, the specific signaling events required to initiate and sustain lymphangiogenesis in the context of tumor progression and metastasis are poorly defined. Given that tumoral lymphangiogenesis is likely a critical event in the progression of metastatic disease, the potential role of semaphorin-mediated regulation in this process warrants further investigation.
Concluding remarks
Recent studies have revealed the importance of semaphorins, plexins, and Nrps in tumor progression. These molecules directly control the behavior of tumor cells and also affect the other cell types which constitute the tumor microenviroment. In this review, we focused on vascular and lymphatic endothelial cells, both of which contribute to providing a suitable environment for tumor cells to grow and metastasize by inducing angiogenesis and lymphangiogenesis. A large fraction of the current information regarding semaphorin signaling was initially obtained from extensive studies in neuronal and cancer cells. It will be important to analyze whether the pathways deployed by the semaphorin-plexin signaling systems are shared by all cell types, with emphasis on those utilized in vascular and lymphatic endothelial cells to inhibit or promote angiogenesis and lymphangiogenesis. From the studies on Sema3B and Sema3F, loss of semaphorin function may represent an early event in cancer development, thus contributing to the acquisition of the angiogenic phenotype that characterizes most solid tumors. However, a number of questions remain: Which semaphorins are cleaved by furin-like proteases, and how does the proteolytic cleavage of semaphorins affect their function? Can the differential expression of these enzymatic factors account for the discrepancies in signaling and biological effects observed among different studies? Does differential expression of semaphorin receptors on tumor and endothelial cells affect their signaling potential? How does expression of competitive factors, such as VEGF, regulate the signaling capabilities of the semaphorins? Further, can their tumor-suppressive and anti-angiogenic roles be strengthened by multi-targeted approaches that take into account both the semaphorins and the regulators of their expression and function? What are the molecular differences in the signaling pathways activated by semaphorin binding, as opposed to the withdrawal of VEGF signaling? Semaphorin expression appears to affect a number of cellular phenotypes, including proliferation, survival, anchorage dependence, angiogenesis, and migration. Which among these represent the critical processes that are central to semaphorin-mediated tumor suppression? Answering these and other questions may be central to understanding the role of semaphorins in angiogenesis and cancer.
References
- Carmeliet P . Angiogenesis in life, disease and medicine. Nature 2005; 438:932–936.
Article CAS PubMed Google Scholar - Folkman J . Angiogenesis: an organizing principle for drug discovery?. Nat Rev Drug Discov 2007; 6:273–286.
Article CAS PubMed Google Scholar - Nyberg P, Xie L, Kalluri R . Endogenous inhibitors of angiogenesis. Cancer Res 2005; 65:3967–3979.
Article CAS PubMed Google Scholar - Maione TE, Gray GS, Petro J, et al. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science 1990; 247:77–79.
Article CAS PubMed Google Scholar - Good DJ, Polverini PJ, Rastinejad F, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA 1990; 87:6624–6628.
Article CAS PubMed PubMed Central Google Scholar - Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 1999; 285:245–248.
Article CAS PubMed Google Scholar - Carmeliet P, Tessier-Lavigne M . Common mechanisms of nerve and blood vessel wiring. Nature 2005; 436:193–200.
Article CAS PubMed Google Scholar - Adams RH, Eichmann A . Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2010; 2:a001875.
Article CAS PubMed PubMed Central Google Scholar - Kruger RP, Aurandt J, Guan KL . Semaphorins command cells to move. Nat Rev Mol Cell Biol 2005; 6:789–800.
Article CAS PubMed Google Scholar - Eichmann A, Makinen T, Alitalo K . Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev 2005; 19:1013–1021.
Article CAS PubMed Google Scholar - Derijck AA, Van Erp S, Pasterkamp RJ . Semaphorin signaling: molecular switches at the midline. Trends Cell Biol 2010; 20:568–576.
Article CAS PubMed Google Scholar - Gelfand MV, Hong S, Gu C . Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol 2009; 19:99–110.
Article CAS PubMed PubMed Central Google Scholar - Serini G, Napione L, Arese M, Bussolino F . Besides adhesion: new perspectives of integrin functions in angiogenesis. Cardiovasc Res 2008; 78:213–222.
Article CAS PubMed Google Scholar - Neufeld G, Kessler O . The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 2008; 8:632–645.
Article CAS PubMed Google Scholar - Gu C, Yoshida Y, Livet J, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 2005; 307:265–268.
Article CAS PubMed Google Scholar - Oinuma I, Ishikawa Y, Katoh H, Negishi M . The Semaphorin 4D receptor plexin-B1 is a GTPase activating protein for R-Ras. Science 2004; 305:862–865.
Article CAS PubMed Google Scholar - Uesugi K, Oinuma I, Katoh H, Negishi M . Different requirement for Rnd GTPases of R-Ras GAP activity of plexin-C1 and plexin-D1. J Biol Chem 2009; 284:6743–6751.
Article CAS PubMed PubMed Central Google Scholar - Saito Y, Oinuma I, Fujimoto S, Negishi M . Plexin-B1 is a GTPase activating protein for M-Ras, remodelling dendrite morphology. EMBO Rep 2009; 10:614–621.
Article CAS PubMed PubMed Central Google Scholar - Schwarz Q, Ruhrberg C . Neuropilin, you gotta let me know: should I stay or should I go? Cell Adh Migr 2010; 4:61–66.
Article PubMed PubMed Central Google Scholar - Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M . Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998; 92:735–745.
Article CAS PubMed Google Scholar - Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G . Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem 2000; 275:18040–18045.
Article CAS PubMed Google Scholar - Kawasaki T, Kitsukawa T, Bekku Y, et al. A requirement for neuropilin-1 in embryonic vessel formation. Development 1999; 126:4895–4902.
CAS PubMed Google Scholar - Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ . Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development 2005; 132:941–952.
Article CAS PubMed Google Scholar - Yuan L, Moyon D, Pardanaud L, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002; 129:4797–4806.
CAS PubMed Google Scholar - Kigel B, Varshavsky A, Kessler O, Neufeld G . Successful inhibition of tumor development by specific class-3 semaphorins is associated with expression of appropriate semaphorin receptors by tumor cells. PLoS ONE 2008; 3:e3287.
Article CAS PubMed PubMed Central Google Scholar - Serini G, Maione F, Bussolino F . Semaphorins and tumor angiogenesis. Angiogenesis 2009.
- He Z, Tessier-Lavigne M . Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997; 90:739–751.
Article CAS PubMed Google Scholar - Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD . Neuropilin is a semaphorin III receptor. Cell 1997; 90:753–762.
Article CAS PubMed Google Scholar - Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M . Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 1997; 19:547–559.
Article CAS PubMed Google Scholar - Castro-Rivera E, Ran S, Thorpe P, Minna JD . Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci USA 2004; 101:11432–11437.
Article CAS PubMed PubMed Central Google Scholar - Falk J, Bechara A, Fiore R, et al. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 2005; 48:63–75.
Article PubMed Google Scholar - Serini G, Valdembri D, Zanivan S, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 2003; 424:391–397.
Article CAS PubMed Google Scholar - Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M . Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol 1999; 146:233–242.
CAS PubMed PubMed Central Google Scholar - Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G . Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem 2007; 282:26294–26305.
Article CAS PubMed Google Scholar - Maione F, Molla F, Meda C, et al. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest 2009; 119:3356–3372.
CAS PubMed PubMed Central Google Scholar - Gu C, Rodriguez ER, Reimert DV, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell 2003; 5:45–57.
Article CAS PubMed PubMed Central Google Scholar - Arese M, Serini G, Bussolino F . Nervous vascular parallels: axon guidance and beyond. Int J Dev Biol 2011; 55:439–445.
Article CAS PubMed Google Scholar - Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M . VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem 2002; 85:357–368.
Article CAS PubMed Google Scholar - Whitaker GB, Limberg BJ, Rosenbaum JS . Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121). J Biol Chem 2001; 276:25520–25531.
Article CAS PubMed Google Scholar - Appleton BA, Wu P, Maloney J, et al. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J 2007; 26:4902–4912.
Article CAS PubMed PubMed Central Google Scholar - Acevedo LM, Barillas S, Weis SM, Gothert JR, Cheresh DA . Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 2008; 111:2674–2680.
Article CAS PubMed PubMed Central Google Scholar - Toyofuku T, Yoshida J, Sugimoto T, et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci 2005; 8:1712–1719.
Article CAS PubMed Google Scholar - Sekido Y, Bader S, Latif F, et al. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci USA 1996; 93:4120–4125.
Article CAS PubMed PubMed Central Google Scholar - Tomizawa Y, Sekido Y, Kondo M, et al. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci USA 2001; 98:13954–13959.
Article CAS PubMed PubMed Central Google Scholar - Tse C, Xiang RH, Bracht T, Naylor SL . Human Semaphorin 3B (SEMA3B) located at chromosome 3p21.3 suppresses tumor formation in an adenocarcinoma cell line. Cancer Res 2002; 62:542–546.
CAS PubMed Google Scholar - Varshavsky A, Kessler O, Abramovitch S, et al. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res 2008; 68:6922–6931.
Article CAS PubMed Google Scholar - Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ . Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog 2005; 44:151–161.
Article CAS PubMed Google Scholar - van der Zwaag B, Hellemons AJ, Leenders WP, et al. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Dev Dyn 2002; 225:336–343.
Article CAS PubMed Google Scholar - Roodink I, Raats J, van der Zwaag B, et al. Plexin D1 expression is induced on tumor vasculature and tumor cells: a novel target for diagnosis and therapy?. Cancer Res 2005; 65:8317–8323.
Article CAS PubMed Google Scholar - Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C . Semaphorin 3E-plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev 2011; 25:1399–1411.
Article CAS PubMed PubMed Central Google Scholar - Torres-Vazquez J, Gitler AD, Fraser SD, et al. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell 2004; 7:117–123.
Article CAS PubMed Google Scholar - Sakurai A, Gavard J, Annas-Linhares Y, et al. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol 2010; 30:3086–3098.
Article CAS PubMed PubMed Central Google Scholar - Fukushima Y, Okada M, Kataoka H, et al. Sema3E-plexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest 2011; 121:1974–1985.
Article CAS PubMed PubMed Central Google Scholar - Casazza A, Finisguerra V, Capparuccia L, et al. Sema3E-plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest 2010; 120:2684–2698.
Article CAS PubMed PubMed Central Google Scholar - Zygmunt T, Gay CM, Blondelle J, et al. Semaphorin-plexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev Cell 2011; 21:301–314.
Article CAS PubMed PubMed Central Google Scholar - Sakurai A, Jian X, Lee CJ, et al. Phosphatidylinositol-4-phosphate 5-Kinase and GEP100/Brag2 protein mediate antiangiogenic signaling by semaphorin 3E-plexin-D1 through Arf6 protein. J Biol Chem 2011; 286:34335–34345.
Article CAS PubMed PubMed Central Google Scholar - van den Bout I, Divecha N . PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J Cell Sci 2009; 122:3837–3850.
Article CAS PubMed Google Scholar - Bielenberg DR, Hida Y, Shimizu A, et al. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest 2004; 114:1260–1271.
Article CAS PubMed PubMed Central Google Scholar - Kessler O, Shraga-Heled N, Lange T, et al. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res 2004; 64:1008–1015.
Article CAS PubMed Google Scholar - Futamura M, Kamino H, Miyamoto Y, et al. Possible role of semaphorin 3F, a candidate tumor suppressor gene at 3p21.3, in p53-regulated tumor angiogenesis suppression. Cancer Res 2007; 67:1451–1460.
Article CAS PubMed Google Scholar - Nasarre P, Kusy S, Constantin B, et al. Semaphorin SEMA3F has a repulsing activity on breast cancer cells and inhibits E-cadherin-mediated cell adhesion. Neoplasia 2005; 7:180–189.
Article CAS PubMed PubMed Central Google Scholar - Wu F, Zhou Q, Yang J, et al. Endogenous axon guiding chemorepulsant semaphorin-3F inhibits the growth and metastasis of colorectal carcinoma. Clin Cancer Res 2011; 17:2702–2711.
Article CAS PubMed Google Scholar - Shimizu A, Mammoto A, Italiano JE Jr, et al. ABL2/ARG tyrosine kinase mediates SEMA3F-induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J Biol Chem 2008; 283:27230–27238.
Article CAS PubMed PubMed Central Google Scholar - Potiron VA, Sharma G, Nasarre P, et al. Semaphorin SEMA3F affects multiple signaling pathways in lung cancer cells. Cancer Res 2007; 67:8708–8715.
Article CAS PubMed Google Scholar - Karayan-Tapon L, Wager M, Guilhot J, et al. Semaphorin, neuropilin and VEGF expression in glial tumours: SEMA3G, a prognostic marker?. Br J Cancer 2008; 99:1153–1160.
Article CAS PubMed PubMed Central Google Scholar - Taniguchi M, Masuda T, Fukaya M, et al. Identification and characterization of a novel member of murine semaphorin family. Genes Cells 2005; 10:785–792.
Article CAS PubMed Google Scholar - Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS . Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res 2004; 64:5212–5224.
Article CAS PubMed Google Scholar - Conrotto P, Valdembri D, Corso S, et al. Sema4D induces angiogenesis through Met recruitment by plexin B1. Blood 2005; 105:4321–4329.
Article CAS PubMed Google Scholar - Basile JR, Castilho RM, Williams VP, Gutkind JS . Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA 2006; 103:9017–9022.
Article CAS PubMed PubMed Central Google Scholar - Ch'ng E, Tomita Y, Zhang B, et al. Prognostic significance of CD100 expression in soft tissue sarcoma. Cancer 2007; 110:164–172.
Article PubMed Google Scholar - Basile JR, Holmbeck K, Bugge TH, Gutkind JS . MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem 2007; 282:6899–6905.
Article CAS PubMed Google Scholar - Zhu L, Bergmeier W, Wu J, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci USA 2007; 104:1621–1626.
Article CAS PubMed PubMed Central Google Scholar - Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL . The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci USA 2002; 99:12085–12090.
Article CAS PubMed PubMed Central Google Scholar - Hirotani M, Ohoka Y, Yamamoto T, et al. Interaction of plexin-B1 with PDZ domain-containing Rho guanine nucleotide exchange factors. Biochem Biophys Res Commun 2002; 297:32–37.
Article CAS PubMed Google Scholar - Perrot V, Vazquez-Prado J, Gutkind JS . Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem 2002; 277:43115–43120.
Article CAS PubMed Google Scholar - Swiercz JM, Kuner R, Behrens J, Offermanns S . Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 2002; 35:51–63.
Article CAS PubMed Google Scholar - Basile JR, Gavard J, Gutkind JS . Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem 2007; 282:34888–34895.
Article CAS PubMed Google Scholar - Binmadi NO, Proia P, Zhou H, Yang YH, Basile JR . Rho-mediated activation of PI(4)P5K and lipid second messengers is necessary for promotion of angiogenesis by Semaphorin 4D. Angiogenesis 2011; 14:309–319.
Article CAS PubMed PubMed Central Google Scholar - Swiercz JM, Kuner R, Offermanns S . Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol 2004; 165:869–880.
Article CAS PubMed PubMed Central Google Scholar - Barberis D, Casazza A, Sordella R, et al. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci 2005; 118:4689–4700.
Article CAS PubMed Google Scholar - Wigle JT, Oliver G . Prox1 function is required for the development of the murine lymphatic system. Cell 1999; 98:769–778.
Article CAS PubMed Google Scholar - Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002; 21:1505–1513.
Article CAS PubMed PubMed Central Google Scholar - Petrova TV, Makinen T, Makela TP, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 2002; 21:4593–4599.
Article CAS PubMed PubMed Central Google Scholar - Hong YK, Harvey N, Noh YH, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 2002; 225:351–357.
Article CAS PubMed Google Scholar - Karpanen T, Egeblad M, Karkkainen MJ, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001; 61:1786–1790.
CAS PubMed Google Scholar - Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J 2001; 20:1223–1231.
Article CAS PubMed PubMed Central Google Scholar - He Y, Kozaki K, Karpanen T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst 2002; 94:819–825.
Article CAS PubMed Google Scholar - Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 2004; 5:74–80.
Article CAS PubMed Google Scholar - Haiko P, Makinen T, Keskitalo S, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol 2008; 28:4843–4850.
Article CAS PubMed PubMed Central Google Scholar - Caunt M, Mak J, Liang WC, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 2008; 13:331–342.
Article CAS PubMed Google Scholar - Xu Y, Yuan L, Mak J, et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol 2010; 188:115–130.
Article CAS PubMed PubMed Central Google Scholar - Favier B, Alam A, Barron P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood 2006; 108:1243–1250.
Article CAS PubMed Google Scholar - Nathanson SD . Insights into the mechanisms of lymph node metastasis. Cancer 2003; 98:413–423.
Article PubMed Google Scholar - Schietroma C, Cianfarani F, Lacal PM, et al. Vascular endothelial growth factor-C expression correlates with lymph node localization of human melanoma metastases. Cancer 2003; 98:789–797.
Article CAS PubMed Google Scholar - Dadras SS, Lange-Asschenfeldt B, Velasco P, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 2005; 18:1232–1242.
Article PubMed Google Scholar - Roma AA, Magi-Galluzzi C, Kral MA, Jin TT, Klein EA, Zhou M . Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod Pathol 2006; 19:392–398.
Article PubMed Google Scholar - He XW, Yu X, Liu T, Yu SY, Chen DJ . Vector-based RNA interference against vascular endothelial growth factor-C inhibits tumor lymphangiogenesis and growth of colorectal cancer in vivo in mice. Chin Med J 2008; 121:439–444.
Article CAS PubMed Google Scholar - Das S, Ladell DS, Podgrabinska S, et al. Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases. Cancer Res 2010; 70:1814–1824.
Article CAS PubMed PubMed Central Google Scholar - Patel V, Marsh CA, Dorsam RT, et al. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res 2011; 71:7103–7112.
Article CAS PubMed PubMed Central Google Scholar - Casazza A, Fu X, Johansson I, et al. Systemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor models. Arterioscler Thromb Vasc Biol 2011; 31:741–749.
Article CAS PubMed Google Scholar - Roberts N, Kloos B, Cassella M, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res 2006; 66:2650–2657.
Article CAS PubMed Google Scholar - Khromova N, Kopnin P, Rybko V, Kopnin BP . Downregulation of VEGF-C expression in lung and colon cancer cells decelerates tumor growth and inhibits metastasis via multiple mechanisms. Oncogene 2011 Aug 1. doi: 10.1038/onc.2011.330
- Kodera Y, Katanasaka Y, Kitamura Y, et al. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res 2011; 13:R66.
Article CAS PubMed PubMed Central Google Scholar - Karpanen T, Wirzenius M, Makinen T, et al. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol 2006; 169:708–718.
Article CAS PubMed PubMed Central Google Scholar - Yoo YA, Kang MH, Lee HJ, et al. Sonic hedgehog pathway promotes metastasis via activation of Akt, EMT, and MMP-9 in gastric cancer. Cancer Res 2011; 71:7061–7070.
Article CAS PubMed Google Scholar
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Dental and Craniofacial Research.
Author information
Authors and Affiliations
- Oral and Pharyngeal Cancer Branch, National Institute of Dental and Craniofacial Research, National Institutes of Health, 30 Convent Drive, Rm. 211, Bethesda, 20892, MD, USA
Atsuko Sakurai, Colleen Doci & J Silvio Gutkind
Authors
- Atsuko Sakurai
You can also search for this author inPubMed Google Scholar - Colleen Doci
You can also search for this author inPubMed Google Scholar - J Silvio Gutkind
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toJ Silvio Gutkind.
Rights and permissions
About this article
Cite this article
Sakurai, A., Doci, C. & Gutkind, J. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer.Cell Res 22, 23–32 (2012). https://doi.org/10.1038/cr.2011.198
- Published: 13 December 2011
- Issue Date: January 2012
- DOI: https://doi.org/10.1038/cr.2011.198