Phylotranscriptomics unveil a Paleoproterozoic-Mesoproterozoic origin and deep relationships of the Viridiplantae (original) (raw)
- Article
- Open access
- Published: 11 September 2023
Nature Communications volume 14, Article number: 5542 (2023)Cite this article
- 6602 Accesses
- 10 Citations
- 28 Altmetric
- Metrics details
Subjects
Abstract
The Viridiplantae comprise two main clades, the Chlorophyta (including a diverse array of marine and freshwater green algae) and the Streptophyta (consisting of the freshwater charophytes and the land plants). Lineages sister to core Chlorophyta, informally refer to as prasinophytes, form a grade of mainly planktonic green algae. Recently, one of these lineages, Prasinodermophyta, which is previously grouped with prasinophytes, has been identified as the sister lineage to both Chlorophyta and Streptophyta. Resolving the deep relationships among green plants is crucial for understanding the historical impact of green algal diversity on marine ecology and geochemistry, but has been proven difficult given the ancient timing of the diversification events. Through extensive taxon and gene sampling, we conduct large-scale phylogenomic analyses to resolve deep relationships and reveal the Prasinodermophyta as the lineage sister to Chlorophyta, raising questions about the necessity of classifying the Prasinodermophyta as a distinct phylum. We unveil that incomplete lineage sorting is the main cause of discordance regarding the placement of Prasinodermophyta. Molecular dating analyses suggest that crown-group green plants and crown-group Prasinodermophyta date back to the Paleoproterozoic-Mesoproterozoic. Our study establishes a plausible link between oxygen levels in the Paleoproterozoic-Mesoproterozoic and the origin of Viridiplantae.
Similar content being viewed by others
Introduction
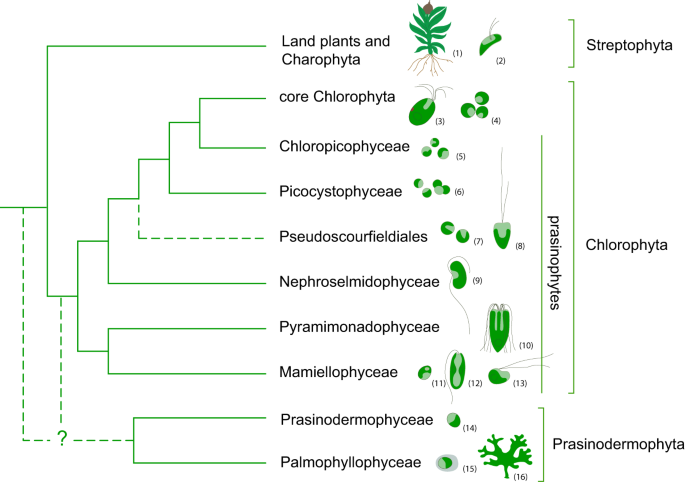
The Viridiplantae, commonly known as green plants, including green algae and land plants, are a major group of photosynthetic eukaryotes that have played a prominent and uninterrupted role in the ecosystems globally at least since the Paleozoic1,2. Traditionally two main lineages have been recognized in the Viridiplantae, the Chlorophyta and the Streptophyta. Chlorophyta, initially diversified as unicellular planktonic algae mostly in marine environments3, and gave rise to the modern prasinophytes and the morphologically and ecologically diverse core Chlorophyta4,5. Previous phylogenetic studies reported prasinophytes as a paraphyletic assemblage composed of several clades, which only recently were classified as separate classes, Mamiellophyceae, Pyramimonadophyceae, Nephroselmidophyceae, Palmophyllophyceae, Chloropicophyceae, and Picocystophyceae, as well as several environmental sequences lacking formal description6,7,8,9,10,11,12,13 (Fig. 1). The traditional dichotomy of freshwater streptophytes and an early marine diversification of prasinophytes was recently shattered by phylogenomic analyses, which resolved Palmophyllophyceae and Prasinodermophyceae as a distinct phylum (Prasinodermophyta), diverging before the classic Chlorophyta and Streptophyta split14. Prasinodermophyta includes planktonic non-flagellate coccoids and benthic macroscopic species. Prasinophytes are also composed of planktonic non-flagellate coccoids and unicellular flagellates15,16,17,18,19,20. Prasinodermophytes and most prasinophytes live in marine environments and are globally distributed21. They are important contributors to primary productivity in marine systems and play a vital role in global elemental cycles22,23,24,25. For example, Mamiellophyceae and Chloropicophyceae (both in Chlorophyta) are major components of picoplanktonic communities in coastal and oligotrophic oceanic waters, significantly contributing to global carbon cycling23,25,26,27. In addition to their ecological significance, they represent excellent model systems for plant biology owing to their small-sized genomes with low gene redundancy21,28. Despite their non-negligible ecological significance and great value as model systems, the phylogenetic relationships and the origins of these green algae remain controversial.
Fig. 1: Current knowledge of phylogenetic relationships among the main lineages of Viridiplantae based on previous studies2,5,11,12,14,18,29.
Uncertain phylogenetic relationships are indicated by dashed lines. Drawings illustrate representative genera of each clade (not drawn to scale): (1) Physcomitrella, (2) Mesostigma, (3) Tetraselmis, (4) Chlorella, (5) Chloropicon, (6) Picocystis, (7) Pycnococcus, (8) Pseudoscourfieldia, (9) Nephroselmis, (10) Pyramimonas, (11) Ostreococcus, (12) Monomastix, (13) Dolichomastix, (14) Prasinoderma, (15) Prasinococcus, (16) Verdigellas.
The phylogenetic relationships within Prasinodermophytes and prasinophytes are difficult to resolve, as has been exemplified by the fact that different data types and analytical approaches have produced considerable nuclear-nuclear and plastid-nuclear discordance29. Plastid phylogenies consistently recovered the Prasinodermophyta as sister to Chlorophyta11,29,30, while nuclear analyses yielded inconsistent positions concerning the Prasinodermophyta14,29. Nuclear rDNA analyses and multigene concatenated analyses generally supported the Prasinodermophyta as sister to all other green plants11,14,29. Conversely, in the coalescent analyses from 1KP initiative29, Prasinodermophyta was allied with Streptophyta, whereas Li et al.14 recovered Prasinodermophyta as sister to all other green plants. This incongruence may result from low taxon sampling, long-branch attraction (LBA), weak phylogenetic signal and/or various biological processes such as incomplete lineage sorting (ILS) and gene flow (both hybridization and introgression)31,32,33,34,35.
Estimating the timeframe of Viridiplantae early divergences is important because the origin and early diversification of green plants set the stage for the transition from a cyanobacterial to a eukaryotic algal-dominated world36. Establishing the evolutionary timescale of Viridiplantae has been a difficult task, due to the poor fossil record of green algae. The sparse Proterozoic fossil record of green algae is further compromised by their simple morphologies and contentious interpretations. For example, acritarchs such as Pterospermella and Tamanites have been allied with Pterosperma and Pachysphaera, respectively, of the Pyramimonadophyceae37,38,39; however, these interpretations are uncertain as they rely on comparisons of simple morphological characters such as the presence of an annular membranous “wing” around the cell or pores through the cell wall40,41,42. Given the contentious taxonomic affinities of these Precambrian fossils, Nie et al.30 found that the use of Precambrian fossils could generate older divergence-time estimates than generally accepted for crown-group green plants30. A well-preserved multicellular Precambrian fossil of green algae, Proterocladus antiquus, was found in the ca. 1.0 Ga Nanfen Formation from North China43. P. antiquus shares several morphological features with species of extant Cladophorales (Ulvophyceae), and has been interpreted as either a member of or as an extinct relative to the cladophoraleans, the ulvophyceans, the chlorophytes or the Viridiplantae33,43. Evaluation of different phylogenetic interpretations of this fossil are useful to quantify its effect on the time estimates of the origin and early diversification of the Viridiplantae33,43. Recently, a unicellular green alga fossil from the latest Ediacaran of South China, Protocodium sinense, has received attention for its taxonomic interpretation. This fossil can be conservatively used as a minimal calibration point for the origin of Bryopsidales44, providing a new opportunity for reliable time estimates of the early diversification of the Viridiplantae. In general, fossils only provide a set of minimum age constraints which are insufficient for the dating analysis45. Therefore, a maximum age constraint on the root is particularly important in divergence-time estimation. The root maximum constraint can effectively restrict the age range of the entire tree. Different maximum constraints on the root may have large impacts on the same descendant clade age45,46, which needs to be taken into account in dating analysis.
Biomarkers, or fossilized organic compounds derived from biomolecules, can also be used to constrain the age of green plant clades. Caution must be exercised, however, because biomarkers may not be clade-specific, can be contaminated by later sources or be affected by preservational biases, and may reflect ecological dominance, rather than phylogenetic divergence47. Thus, biomarkers should be scrutinized for clade specificity, contamination, and preservational biases. Authentic biomarkers, like body fossils, should be regarded as minimum age constraints on clade divergence. With these caveats in mind, we note that early reports of eukaryotic biomarkers from Archean sediments48 were later determined to be contaminants49. Instead, the abundant occurrence of eukaryotic biomarkers began in the Tonian Period around 820 Ma50, although emerging data indicate that fossil sterols, which are mostly if not exclusively derived from eukaryotes, are abundant in Mesoproterozoic sediments 1.6–1.0 Ga51. Relevant to this study, the occurrence of abundant green algal biomarkers in Cryogenic rocks around 660 Ma1,36,50 sets a minimum age for the divergence of Viridiplantae.
In this study we apply concatenation- and coalescent-based approaches to reconstruct deep relationships in the green plant phylogeny, and explore the causes for deep discordance concerning the phylogenetic position of Prasinodermophyta. Additionally, we estimate the timescale for Viridiplantae evolution by implementing four fossil calibration strategies based on different interpretations of P. antiquus and different maximum-age root constraints.
Results and discussion
Phylogenomic analyses of green plants
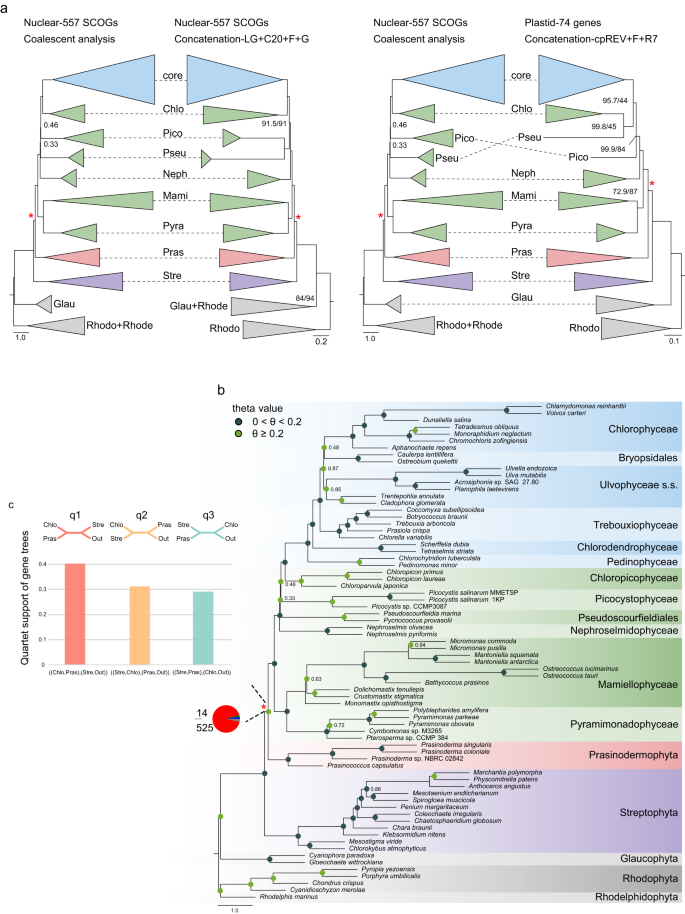
Our nuclear dataset consisted of 557 single-copy orthologous genes (SCOGs) derived from 68 genomes and transcriptomes. Phylogenetic relationships inferred from 557 SCOGs using concatenation-based (site-heterogeneous LG + C20 + F + G model) and coalescent-based approaches were highly congruent at the class and order level of green plants. However, certain discrepancies were observed for the positions of Picocystophyceae and Pseudoscourfieldiales relative to the other prasinophytes, as well as the position of Bryopsidales with respect to Ulvophyceae and Chlorophyceae, and Pedinophyceae and Chlorodendrophyceae relative to the other core Chlorophyta (Fig. 2a, b, Supplementary Fig. 1). Notably, the Prasinodermophyta consistently emerged as the sister clade to Chlorophyta with strong support (local posterior probability, LPP = 0.97) in the coalescent species tree (Fig. 2b). Likewise, the concatenation analyses using site-heterogeneous and site-homogeneous models all resolved Prasinodermophyta as the sister group to Chlorophyta, with Streptophyta as their common sister group (SH-aLRT = 100, BS = 100) (Fig. 2a, Supplementary Figs. 1 and 2). Our results deviate from the nuclear phylogeny reported by Li et al.14, which showed that the Prasinodermophyta diverged prior to the split between the Chlorophyta and Streptophyta, suggesting the need to establish a separate phylum. In contrast, our phylogenomic analyses indicate that a three-phylum classification for the Viridiplantae may not be necessary, and that instead the traditional two-phylum model could be retained. The determination of whether to recognize one or two classes (Palmophyllophyceae and Prasinodermophyceae) is primarily a matter of taxonomic preference. However, considering the unresolved position of Prasinococcus within the clade (Supplementary Figs. 3 and 4) and the comparable genetic divergence observed within the Prasinodermophyta and the Mamiellophyceae, it may be preferable to adopt a single class, Palmophyllophyceae (including Prasinoderma, Prasinococcus, Palmophyllum, and Verdigellas), as initially defined by Leliaert et al.11.
Fig. 2: Tree reconstruction of the Viridiplantae.
a Topological comparison between coalescent and concatenation analyses based on nuclear and plastid datasets. Support values are shown only for nodes receiving less than 95% LPP (in coalescent-based analysis) and SH-aLRT/BS (in concatenation-based analyses). Chlo Chlorodendrophyceae, core core Chlorophyta, Pico Picocystophyceae, Neph Nephroselmidophyceae, Pseu Pseudoscourfieldiales, Mami Mamiellophyceae, Pyra Pyramimonadophyceae, Stre Streptophyta, Pras Prasinodermophyta, Glau Glaucophyta, Rhodo Rhodophyta, Rhode Rhodelphidophyta. b The phylogenetic tree constructed using ASTRAL based on 557 individual gene trees with 10% BS value cutoff. Support values are shown only for nodes receiving less than 95% support from LPP analysis. The pie chart at the ancestral branch of Prasinodermophyta and Chlorophyta indicates the proportion of gene trees concordance and conflict against the coalescent species tree: blue, the proportion of gene trees supporting the species tree; green, the proportion of gene trees supporting the most common conflicting topology; red, the proportion of gene trees supporting all other conflicting topologies; dark gray, the proportion of the uninformative gene trees; light gray, the proportion of missing data. The color of circle point on each node represents two different intervals of theta values. The red asterisks in a and b indicate the ancestral branch consisting of Prasinodermophyta and Chlorophyta. c The quartet frequency (q1–q3) around the focal branch of ASTRAL tree based on the 72-taxon dataset. Each internal branch with four neighboring branches leads to three possible quartets.
Apart from the positions of Picocystophyceae and Pseudoscourfieldiales, the branching order of the prasinophyte grade was identical in the concatenation and coalescent trees (Fig. 2a). In the concatenation tree, navigating the topology from the root towards the tips, we can successively identify a Pyramimonadophyceae + Mamiellophyceae clade, the Nephroselmidophyceae, a Pseudoscourfieldiales + Picocystophyceae clade, and Chloropicophyceae sister to core Chlorophyta, consistent with current understanding of prasinophyte phylogeny2. The Pseudoscourfieldiales + Picocystophyceae clade here recovered differs from the nuclear and plastid rDNA concatenated phylogeny of Li et al.14. This discrepancy may be attributed to the expanded sampling of Picocystophyceae and prasinophytes in our study. Yet, our coalescent analysis recovered Picocystophyceae as sister to a Chloropicophyceae + core Chlorophyta clade (Fig. 2b), consistent with coalescent tree inference of 1KP initiative29. In addition, our coalescent analysis weakly supported Pseudoscourfieldiales as sister to a Picocystophyceae + Chloropicophyceae + core Chlorophyta clade (LPP = 0.33) (Fig. 2b), while the coalescent analysis of 1KP initiative29 weakly supported Pseudoscourfieldiales as sister to a Pyramimonadophyceae + Mamiellophyceae clade.
In addition, we compiled a plastid dataset comprising 74 plastid genes from 63 genomes. Phylogenetic relationships within prasinophytes were similar across different models (the site-homogeneous and site-heterogeneous models) except for the position of Pseudoscourfieldiales (Supplementary Figs. 3 and 4). Plastid topologies consistently recovered Prasinodermophyta as sister to Chlorophyta with strong support (SH-aLRT ≥ 95, BS ≥ 95) (Fig. 2a, Supplementary Figs. 3 and 4), consistent with our nuclear topologies and previously reported plastid topologies11,29.
Conflicting signal
The statistics of gene tree-coalescent tree and quartet tree discordance for each internal branch in the coalescent tree based on the 557 SCOGs dataset indicated rampant gene tree conflict at the deep internal branches (backbone) of our phylogeny (Supplementary Figs. 5 and 6), including the ancestral branch consisting of Prasinodermophyta and Chlorophyta (indicated with red asterisks in Fig. 2a, b). This focal branch associated with the position of Prasinodermophyta exhibited a high proportion of conflicting bipartitions (94.25%), while the concordant proportion was remarkably low (2.51%) (Fig. 2b). This conflicting phylogenetic signal regarding the position of the Prasinodermophyta was observed despite consistent recovery of Prasinodermophyta as sister to Chlorophyta in both concatenation and coalescent trees. To gain further insight into the distribution of phylogenetic signal in 557 SCOGs dataset, we quantified phylogenetic signal for each nuclear gene with respect to three alternative hypotheses for the position of the Prasinodermophyta. The fraction of loci strongly supporting Prasinodermophyta as sister to Chlorophyta (T1) was larger (19.89%) than the fraction of loci strongly supporting the other two alternative topologies (T2, 19.52% and T3, 9.76%) (Supplementary Fig. 7). Additionally, we conducted statistical analyses on the subsets of genes that strongly or weakly supported T1, T2, or T3, focusing on the distribution of parsimony informative sites, evolutionary rates, and saturation. Median values for parsimony informative sites showed that genes with strong signal (median value = 229–235) possessed a higher number of informative sites compared to those with weak signal (median value = 176.5–219) (Supplementary Fig. 7). In addition, both genes with strong signal (median value = 1.46–1.61) and genes with weak signal (median value = 1.44–0.47) exhibited rapid evolutionary rates. Similarly, there were non-negligible levels of substitution saturation in genes with both strong signal (median value = 0.14–0.15) and weak signal (median value = 0.15–0.16) (Supplementary Fig. 7). Thus, a large proportion of genes with weak signal (50.83%) in our dataset, as previously mentioned, can be attributed to a smaller number of informative sites, rather than higher levels of saturation or faster evolutionary rates.
Potential causes of phylogenetic discordance regarding the position of Prasinodermophyta
Quartet frequencies (q1, q2 and q3) surrounding the focal branch (the ancestral branch consisting of Prasinodermophyta and Chlorophyta) showed the high extent of symmetry in two minor quartet frequencies (q2 and q3). This finding suggests that ILS may have occurred in the early evolutionary history in green plants (Fig. 2c). Given that ILS can be the underlying cause of gene tree conflict regarding the position of Prasinodermophyta, we calculated the theta value of each internal branch based on a 72-taxon dataset of 557 SCOGs. The theta value of this focal branch (0.29) exceeded the average theta value of all internal branches (0.21), indicating that ILS cannot be disregarded (Fig. 2b).
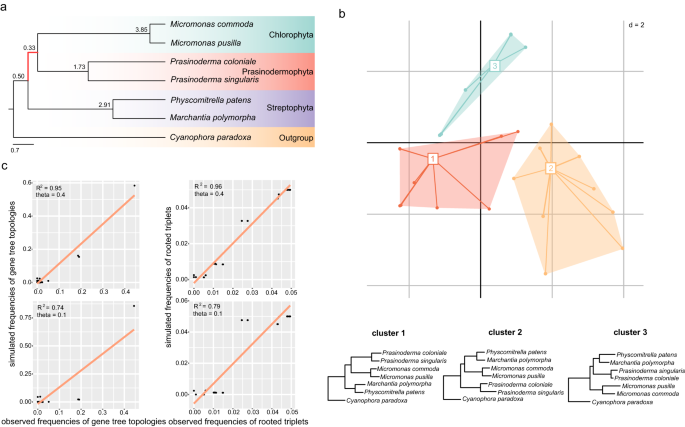
To further explore the topological variability of phylogenetic trees, we explored landscapes (topological variability) of phylogenetic trees and performed coalescent simulations based on a 7-taxon dataset (Fig. 3a) due to computational burden. In the landscape analysis of phylogenetic trees, when the number of clusters was set to 3, the maximum clade credibility (MCC) tree (or the consensus tree) topology of clusters 1, 2, and 3 represented three alternative hypotheses regarding the position of Prasinodermophyta, respectively (Fig. 3b). This analysis showed that three alternative hypotheses for the position of the Prasinodermophyta were evident in terms of topological variability.
Fig. 3: The coalescent simulations and exploration of landscapes of phylogenetic trees.
a Phylogenetic relationships of a 7-taxon dataset based on the multispecies coalescent model. The short ancestral branch of Prasinodermophyta and Chlorophyta is highlighted in red. b Two-dimensional metric multidimensional scaling (MDS) plot of 434 gene trees based on the 7-taxon dataset. A consensus tree of each cluster in MDS plot is displayed below. c Comparison of simulated frequencies of gene tree topologies and rooted triplets with the corresponding observed frequencies. The two figures above and below show the correlations obtained by setting theta value to 0.4 and 0.1 on the ancestral branch consisting of Prasinodermophyta and Chlorophyta, respectively.
Furthermore, we performed coalescent simulations by generating 20,000 gene trees under high ILS conditions in Phybase and the multispecies coalescent model. The topological frequencies and triplet frequencies of simulated gene trees were in agreement with the empirical gene trees (R2 = 0.95/0.96) (Fig. 3c). To assess the explanatory power of the coalescent model for the observed gene tree heterogeneity in the 7-taxon dataset, we calculated unweighted RF distances for empirical gene trees and simulated gene trees with high ILS conditions (theta = 0.4). The results indicated that the coalescent model accounted for 66% of gene tree variation observed for 434 gene trees under high ILS conditions (Supplementary Fig. 8). We also simulated 20,000 gene trees with a low level of ILS (theta = 0.1) for the same dataset, but these simulated gene trees exhibited a lower correlation (R2 = 0.74/0.79) with the empirical gene trees (Fig. 3c). These results indicate the ILS incorporated in the coalescent model could considerably account for gene tree discordance, especially the presence of two alternative topologies (T2 and T3). We therefore conclude that ILS is a major factor driving the gene tree conflict concerning the position of the Prasinodermophyta.
In addition to ILS, gene flow can also introduce gene tree discordance and lead to inaccurate species tree estimation52,53. We applied two approaches to investigate the level of gene flow causing discordance regarding the position of the Prasinodermophyta in the presence of ILS. Firstly, we measured the difference between the frequencies of two discordant topologies (three possible unrooted topologies of four taxa include one concordant and two discordant topologies) by calculating the Quartet Differential (QD) value in Quartet Sampling54 (e.g., QD < 1 can be indicative of gene flow). Secondly, we performed the chi-square test of the two minor quartet frequencies between empirical gene trees and simulated gene trees with ILS (e.g., _P_-value > 0.1 can be indicative of ILS). For each empirical gene tree, we collapsed branches with BS support below 10% to reduce gene tree estimation error. The ancestral branch (number 52 in Supplementary Fig. 9) of Prasinodermophyta and Chlorophyta (_P_-value = 0.71, QD value = 0.97) showed no significant difference between the observed and simulated dataset with respect to the symmetry in the two minor quartet frequencies (_P_-value > 0.1), and no significant difference between the frequencies of two discordant topologies in the observed dataset (QD value > 0.8) (Supplementary Fig. 9).
The coalescent simulations, quartet sampling, and chi-square tests indicate an important contribution of ILS to phylogenetic discordance surrounding this focal branch. The occurrence of ILS across the short ancestral branch of Prasinodermophyta and Chlorophyta suggests that the ancestor of green plants underwent an ancient radiation event, leading to rapid divergence into the major clades. The phylogenomic analyses support the sister relationship between Prasinodermophyta and Chlorophyta, indicating that the classical two-phylum model of the Viridiplantae can be retained. Our analyses highlight the importance of exploring multiple potential factors contributing to phylogenetic discordance in phylogenomic analyses, particularly when dealing with deep nodes exhibiting substantial variation in gene trees.
Key evolutionary events in the history of the Viridiplantae
Our inferred phylogenetic relationships provided a solid framework for tracing key evolutionary events in green plant lineages, including freshwater-marine (or freshwater-terrestrial) transitions, the evolution of complex cyto-morphologies, and the loss of flagella (Supplementary Figs. 10 and 11, Supplementary Table 1). Ancestral state estimation suggested that the ancestor of green plants was most likely a unicellular freshwater alga with flagella, or at least with a flagellate stage in its life cycle, consistent with earlier conclusions drawn from chloroplast phylogenomic analyses of Archaeplastida55.
Transitions from freshwater to marine or terrestrial habitats occurred independently multiple times within the Chlorophyta and Streptophyta (Supplementary Fig. 10). Multicellularity emerged from multiple independent origins, and complex cyto-morphologies evolved repeatedly in the core Chlorophyta (Supplementary Fig. 10), likely from unicellular ancestors, in agreement with previous analyses56,57. While the majority of green algae either possess flagella or exhibit flagellate stages in their life cycle, certain groups such as the Prasinodermophyta (Palmophyllophyceae and Prasinodermophyceae), some prasinophytes (e.g., Mamiellophyceae: Ostreococcus and Bathycoccus, Chloropicophyceae, Picocystophyceae, and Pycnococcus) and some lineages of Trebouxiophyceae and Chlorophyceae, have never been reported to have flagellate stages12,16,58,59,60,61. Our results indicate that the loss of flagella occurred independently in these lineages, as well as certain lineages of the Streptophyta (Supplementary Fig. 11). However, these results obtained from the ancestral state estimation should be interpreted with caution.
Although flagella were most likely present in a life cycle stage of the green plant ancestor, it is plausible that this ancestor was a non-motile unicellular organism with transient flagellate stages18. This hypothesis finds support in the identification of numerous genes encoding proteins involved in flagellar structure within the genomes of Prasinoderma (Prasinodermophyta) and Chloropicon (Chloropicophyceae), implying the possible existence of cryptic flagellate stages in these species14,62,63.
The evolutionary timescale of green plants
It is worth mentioning that a number of organic-walled microfossils or acritarchs found in sedimentary rocks dating back approximately 1.8–2.0 Ga have been interpreted by some researchers as green algae41,64, although diagnostic green algal features are lacking65. Several acritarch genera (e.g., Tasmanites, Valeria, and Pterospermopsimorpha) have been interpreted as green microalgae or even as prasinophytes39,66. If these interpretations are correct, then the occurrence of these acritarchs in 1.6–1.8 Ga rocks indicates that the divergence of green plants and prasinophytes may extend as the Paleoproterozoic66,67. It is important to note that the green algal interpretation of these acritarchs remains uncertain due to their relatively simple morphologies and the possibility of convergent evolution. Consequently, it is critical to establish a timescale of green algal evolution using molecular data, as we have attempted in this study.
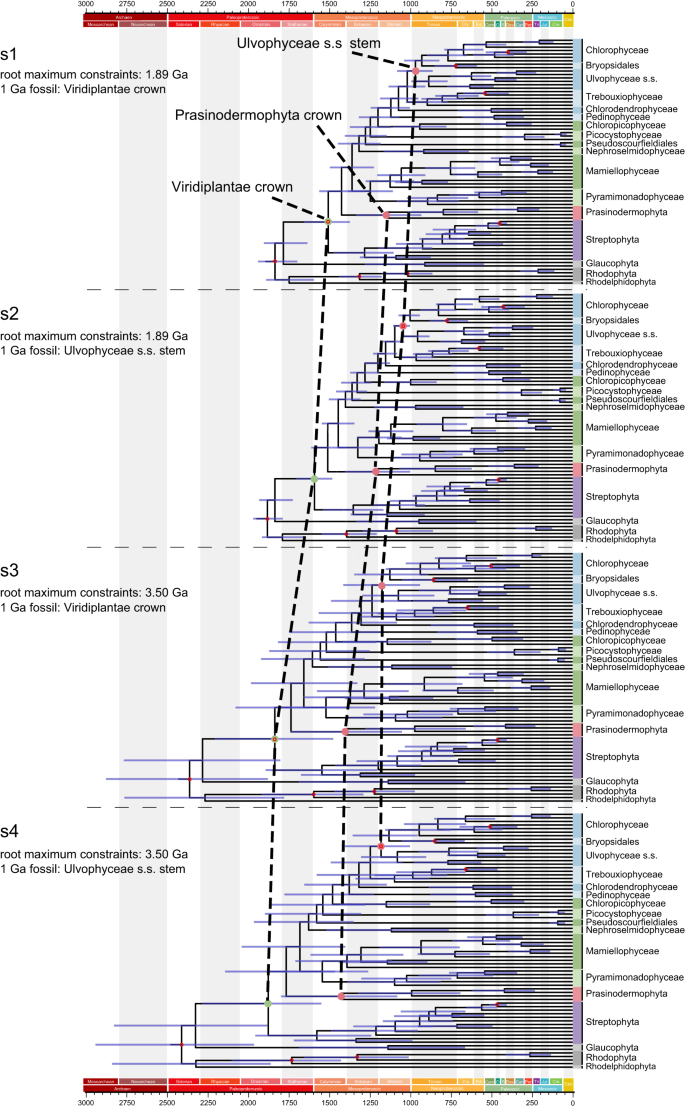
To estimate a timeframe for the evolution of green plant lineages, we conducted molecular dating analyses employing four calibration strategies. Strategies 1 and 2 set the maximum constraint for the root age at 1.89 Ga68 and placed the P. antiquus fossil at either node 4–1 or node 4–2 (Table 1, Fig. 4, Supplementary Fig. 12). In contrast, strategies 3 and 4 differed from the first two by setting the root maximum constraints to 3.50 Ga, representing the oldest known fossil evidence of life on Earth69 (Table 1, Fig. 4, Supplementary Fig. 13). For each strategy, we compared the posterior distribution and the effective prior distribution of different calibrated nodes. Most of the fossil-calibrated nodes showed a significant shift of the posterior relative to the effective prior, indicating that the molecular data could provide information (Supplementary Fig. 14). By comparing divergence time estimates between strategies 1 and 2 (or between strategies 3 and 4), we found that the placement of the P. antiquus fossil significantly influenced the age estimates of most nodes. For strategies 1 and 2, the maximum value of the estimated 95% highest posterior density (HPD) interval of the root age all exceeded 1.89 Ga (Fig. 4, Supplementary Table 2), suggesting that older root maximum constraints may better account for the uncertainties. Therefore, our time estimation exploration suggests that green plants originated somewhere between the early Paleoproterozoic and early Mesoproterozoic (2.28–1.48 Ga) based on strategies 3 and 4. Similarly, strategies 3 and 4 provided divergence-time estimates for crown-group Prasinodermophyta ranging from the late Paleoproterozoic to late Mesoproterozoic (1.79–1.05 Ga). The origin of prasinophytes was also dated back to between the middle Paleoproterozoic and middle Mesoproterozoic (1.87–1.22 Ga), followed by a Mesoproterozoic origin of core Chlorophyta (1.65–1.08 Ga) (Supplementary Table 2). In addition to macrofossils like P. antiquus and P. sinense with specific taxonomic explanations, there are well-preserved fossils from the Dolores Creek Formation (0.95–0.90 Ga) that exhibit characteristics of green macroalgae70,71,72. Moreover, the macrofossil Horodyskia from the Tonian successions (0.95–0.72 Ga) in North China was tentatively interpreted as a multinucleate (coenocytic) green macroalga73. These fossils collectively indicate the early diversification of the Ulvophyceae, which can be traced back to at least the early Neoproterozoic, thus supporting our estimated origin of the Ulvophyceae s.s. (1.30–0.85 Ga).
Table 1 Information of the calibration nodes
Fig. 4: Comparison of divergence time estimates from four fossil assignment strategies.
Node ages are plotted at the posterior means, with horizontal bars representing 95% credibility intervals (n = 10,000 samples for the MCMC process). The calibration nodes are represented by red hollow circles. The 1 Ga fossil refers to Proterocladus antiquus.
Geochemical evidence for the origin of green plants
Our molecular timeline presents older age estimates for crown-group green plants compared to previous large-scale analyses of eukaryotes74, as well as studies focusing on land plants30 or green algae57. The divergence of eukaryotes has traditionally been associated with the increase of atmospheric oxygen levels75, although the causality of this relationship remains a topic of debate76. Geochemical data reveal two episodes of stepwise increase in atmospheric oxygen levels, known as the Great Oxidation Event at 2.3 Ga and the Neoproterozoic Oxygenation Event, c. 850–542 Ma[77](/articles/s41467-023-41137-5#ref-CR77 "Large, R. R. Evolution of Earth’s atmosphere. In Encyclopedia of Geology (Second Edition) (eds. Alderton, D. & Elias, S. A.) 571–584 (Academic Press, 2021). https://doi.org/10.1016/B978-0-12-409548-9.11853-6
."). During the intervening interval, atmospheric oxygen levels are believed to have been low, ranging between 0.1 to 1% of the present atmospheric level (PAL)[78](/articles/s41467-023-41137-5#ref-CR78 "Planavsky, N. J. et al. Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635–638 (2014)."),[79](/articles/s41467-023-41137-5#ref-CR79 "Cole, D. B. et al. A shale-hosted Cr isotope record of low atmospheric oxygen during the Proterozoic. Geology 44, 555–558 (2016)."), although some studies suggest higher atmospheric oxygen levels of 1–10% PAL during the Mesoproterozoic[80](#ref-CR80 "Canfield, D. E. The early history of atmospheric oxygen: Homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 33, 1–36 (2005)."),[81](#ref-CR81 "Holland, H. D. The oxygenation of the atmosphere and oceans. Philos. Trans. R. Soc. B Biol. Sci. 361, 903–915 (2006)."),[82](#ref-CR82 "Kump, L. R. The rise of atmospheric oxygen. Nature 451, 277–278 (2008)."),[83](#ref-CR83 "Large, R. R. et al. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean–atmosphere evolution. Earth Planet. Sci. Lett. 389, 209–220 (2014)."),[84](/articles/s41467-023-41137-5#ref-CR84 "Large, R. R. et al. Atmosphere oxygen cycling through the Proterozoic and Phanerozoic. Miner. Deposita 54, 485–506 (2019)."). Oxygen levels between 1% and 10% PAL would certainly fulfill the respiration requirements of eukaryotic algae[85](/articles/s41467-023-41137-5#ref-CR85 "Müller, M. et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 76, 444–495 (2012)."). Even at the lower end of atmospheric oxygen levels (0.1–1% PAL), localized oxygen-rich oases may have existed in the surface ocean to support eukaryotic life[86](/articles/s41467-023-41137-5#ref-CR86 "Reinhard, C. T., Planavsky, N. J., Olson, S. L., Lyons, T. W. & Erwin, D. H. Earth’s oxygen cycle and the evolution of animal life. Proc. Natl. Acad. Sci. 113, 8933–8938 (2016)."). Considering the oxygen availability in the Paleoproterozoic-Mesoproterozoic oceans, it is plausible that these environments were hospitable to green algae. Consequently, our estimated divergence times for crown-group green plants (2.28–1.48 Ga) and crown-group Prasinodermophyta (1.79–1.05 Ga) align well with geochemical evidence, which suggest that the oceans of the Paleo- and Mesoproterozoic provided a suitable habitat for early Viridiplantae, considering the surface redox conditions of the Earth.Methods
Taxon sampling and orthology inference
Taxon sampling of the nuclear dataset was sourced from 35 publicly available genomes and 37 transcriptomes, covering all classes of Prasinodermophyta, Chlorophyta, Charophyta, and Bryophyta, as well as two glaucophytes, four red algae and Rhodelphis marinus (Rhodelphidophyta) as the outgroups (Supplementary Data 1). We inferred single-copy orthologous genes (SCOGs) using OrthoFinder v2.4.187 from eight published genomes of prasinophytes (Micromonas commoda and Chloropicon primus), prasinodermophytes (Prasinoderma coloniale), core chlorophytes (Chlamydomonas reinhardtii, Chlorella variabilis, and Ulva mutabilis) and streptophytes (Physcomitrella patens and Marchantia polymorpha). Then, HMMER v3.3.188 (with parameter -T 50) was used to identify 677 putative SCOGs from 64 additional genomes and transcriptomes. Redundancy in transcriptomes was reduced using CD-HIT v4.8.189 (with parameters -c 0.99 and -l 99). Each SCOG was aligned with the L-INS-I algorithm in MAFFT v7.47190 and poor alignments with pairwise identity below 20% were removed. Then, each remaining alignment was pruned using trimAl v1.491 with the heuristic automated method. In order to reduce the influence of long branch attraction on the nuclear dataset, TreeShrink v1.3.292 was used with a threshold of 0.05 to identify abnormal sequences that resulted in unrealistically long branch lengths within each gene. Each SCOG with abnormal sequences removed was re-aligned and re-trimmed using MAFFT and trimAl. Besides, to reduce the impact of missing data on phylogenomic analyses, sequences in the trimmed alignments with taxon occupancy below 50% and lengths less than 100 amino acids were filtered out, resulting in a dataset of 557 SCOGs selected from 72 genomes and transcriptomes for the downstream phylogenomic analyses.
Taxon sampling of the plastid dataset included 63 plastid genomes downloaded from NCBI-GenBank, covering all classes of Prasinodermophyta, Chlorophyta, Charophyta, and Bryophyta, as well as two glaucophytes and three red algae as the outgroups (Supplementary Data 1). The amino acid sequence of each plastid gene was aligned with the L-INS-I algorithm in MAFFT. Then each alignment was pruned using trimAl91 with the heuristic automated method. The influence of long branch attraction on the plastid dataset was reduced by TreeShrink with a threshold of 0.05. A plastid dataset of 74 genes with taxon occupancy above 50% was ultimately generated to reconstruct the plastid phylogeny.
Tree reconstruction
Multispecies coalescent model and concatenation approaches were used to infer phylogenetic trees based on the dataset of 557 SCOGs. To estimate a coalescent-based tree, we firstly inferred 557 individual gene trees using RAxML v8.2.1193 with the best-fit substitution model, and 100 rapid bootstrap (BS) replicates for clade support. In order to reduce the influence of gene tree estimation error, branches in gene trees with bootstrap support below 10% were collapsed by Newick Utilities v1.694 prior to the coalescent-based tree inferred by ASTRAL v5.7.495. Local posterior probabilities96 (LPP) were used to assess clade support for the ASTRAL tree. For the concatenation approach, the 557 SCOG alignments were concatenated to reconstruct the maximum likelihood (ML) trees with three types of evolutionary models and two partitioning schemes fed to IQ-TREE v2.0.797: (1) a single partition with the best-fit substitution model searched by IQ-TREE (parameter -MFP): the site-homogeneous LG + F + R10 model; (2) a single partition with the site-heterogeneous LG + C20 + F + G model; (3) a gene-wise partitioned analysis using the best-fit model estimated to each partition. Supports of each analysis were estimated with 1,000 ultrafast bootstrap and SH-aLRT branch test replicates98.
For the plastid dataset, 74 plastid gene alignments were concatenated into a supermatrix and then applied to reconstruct concatenation trees with three types of evolutionary models by IQ-TREE: (1) a single partition with the best-fit substitution model searched by IQ-TREE: the site-homogeneous cpREV + F + R7 model; (2) a single partition with the site-heterogeneous cpREV + F + G + C20 model; (3) a gene-wise partitioned analysis using the best-fit model estimated to each partition. Supports of each analysis were estimated with 1000 ultrafast bootstrap and SH-aLRT branch test replicates.
Detection of gene tree conflict and quartet tree discordance
We used PhyParts99 to map individual gene trees on the coalescent tree with a bootstrap support (BS) threshold of 10% to examine patterns of gene tree-coalescent tree concordance and conflict within the nuclear dataset of 557 SCOGs. The pie chart of each internal branch (node) was summarized and generated by PhyPartsPieCharts and ETE3100 (https://github.com/mossmatters/MJPythonNotebooks). For the same purpose of an interpretable measure of gene-tree heterogeneity specific to each internal branch, the quartet frequency around each internal branch of the coalescent tree reconstructed by 557 SCOGs was summarized and generated by ASTRAL and AstralPlane v0.1.1 (https://github.com/chutter/AstralPlane) with a bootstrap support threshold of 10%.
Dissection of phylogenetic signal
Gene-wise log-likelihood scores among the three constraint topologies (T1, T2, and T3) for each gene in the dataset of 557 SCOGs were calculated to quantify the distribution of the phylogenetic signal over three alternative hypotheses. If a gene satisfied lnL(T1)-lnL(T2) ≥ 2 and lnL(T1)-lnL(T3) ≥ 2, this gene is supposed to strongly support T1. If a gene satisfied 0 <lnL(T1)-lnL(T2) < 2 and lnL(T1)-lnL(T3) ≥ 2, 0 <lnL(T1)-lnL(T2) < 2 and 0 <lnL(T1)-lnL(T3) < 2, or lnL(T1)-lnL(T2) ≥ 2 and 0 <lnL(T1)-lnL(T3) < 2, this gene is supposed to weakly support T1. By analogy, we then identified other genes that strongly or weakly support T2 or T3. The number of genes that strongly or weakly support one of three topologies was calculated. We also explored the correlation of the strength of the gene signal with parsimony informative sites, substitution saturation, and evolutionary rates. Calculations of substitution saturation and evolutionary rates are based on TreSpEx v1.1101. The level of saturation is measured by the slope of the patristic distances (PD) calculated from the tree against the uncorrected genetic distance (p) calculated from the alignment. The evolutionary rate of each gene is derived from the average PD between any pair of taxa in the corresponding gene tree.
Detection of ILS and gene flow
In order to explore if gene tree discordance, concerning the position of the Prasinodermophyta was related to incomplete lineage sorting, we implemented the following analyses: (1) We calculated the population size parameter θ of each internal branch based on the nuclear dataset of 557 SCOGs. A ML tree with branch lengths in mutational units (μT) was inferred with RAxML-NG v.1.2.0102 by constraining a tree search to the ASTRAL tree topology and using a partition-by-gene scheme with best fit model for each partition (with parameters -evaluate and -brlen scaled). The population size parameter θ (θ = μ T/τ103) reflecting the ILS level for each internal branch was calculated from dividing the RAxML-NG mutational branch length by the ASTRAL coalescent branch length35,104. (2) For giving our main focus on identifying potential ILS among major clades of green plants, we explored phylogenetic tree landscapes and performed the coalescent simulation on the 7-taxon dataset. We reduced our taxon sampling to one outgroup (one species of the Glaucophyta) and six ingroup taxa (two species of the Prasinodermophyta, two species of the Chlorophyta, two species of the Streptophyta). After aligning, trimming and outlier removal with TreeShrink, 434 genes with a minimum of 100 aligned amino acid pairs and 100% taxa occupancy were retained for reconstructing individual RAxML gene trees. For the investigation of phylogenetic tree landscapes, the metric multidimensional scaling (MDS) plot and median tree plot were generated based on these rooted RAxML gene trees using the function findGroves (with the parameter method = “treeVec”, nf = 4, nclust = 3) and the function med.trees in TREESPACE v1.1.4.2105. For the analysis of coalescent simulation, the population size parameter θ of each internal branch was calculated by the RAxML-NG tree and ASTRAL tree, and the theta value for terminal branches was set to a constant value of 1. Then, combining the constraint tree and the estimated theta values, we simulated 20,000 gene trees with the sim.coaltree.sp function in Phybase v.1.5106. We also simulated 20,000 gene trees with a low ILS level (set the theta value on the ancestral branch composed of Prasinodermophyta and Chlorophyta to 0.1). The topological frequencies of simulated gene trees and empirical gene trees were calculated. In addition to topological frequencies, we summarized the triplet frequencies from gene trees. Rooted triplets were generated by pruning all possible combinations of three ingroup taxa plus an outgroup from each gene tree with ETE3100, and triplet frequencies of simulated gene trees and empirical gene trees were calculated. Lastly, the correlation tests were performed for topological frequencies and triplet frequencies using the cor.test function in R. To estimate how much ILS contributes to gene tree heterogeneity observed, we calculated unweighted RF distances for empirical gene trees and simulated gene trees in the 7-taxon dataset.
Furthermore, we explored whether ILS or gene flow was a main cause of discordance concerning the position of Prasinodermophyta. Based on the theta value of each internal branch and the RAxML-NG tree (the constraint tree) inferred from the nuclear dataset of 557 SCOGs, 20,000 gene trees were simulated using the sim.coaltree.sp function in Phybase v.1.5. The theta value for terminal branches was set to a constant value of 1. The two minor quartet frequencies (quartet frequencies of two minor topologies) of 557 empirical gene trees and 20,000 simulated gene trees with ILS were calculated by the parameter “-t 32” in ASTRAL. Then, a two-sided chi-squared test was performed on those quartet frequencies. We also used Quartet Sampling v1.3.1 (https://github.com/fephyfofum/quartetsampling) to calculate Quartet Differential (QD) score which measures the difference between the two minor quartet frequencies of 557 empirical gene trees. Finally, it is determined whether a significant level of ILS has been observed in the focal ancestral branch related to the position of Prasinodermophyta based on the criteria of whether _P_-value greater than 0.1, QD value greater than 0.8, similar to Ma et al.107 and Suvorov et al.108.
Ancestral state reconstruction
We coded habitat, flagellate, and morphological characters of 72 species for ancestral state reconstruction (Supplementary Table 1). Habitat characters include freshwater, terrestrial, and marine. Morphological characters include unicellular, colonial, multicellular, siphonous, and siphonocladous. Flagellate characters refer to whether flagellate stages can be observed in the life cycle. The ultrametric trees were used to guide the ancestral state reconstruction of three traits (habitat, flagellate stages and cell morphology) in phytools v1.5.1 R package109. We estimated the discrete character evolution with ARD model which allows every possible type of transition has a different rate. The posterior probabilities of each character for each ancestral node were calculated from summaries of 1000 simulations of stochastic character mapping under ARD model (the function make.simmap).
Divergence time estimation
Dataset assembly for molecular dating
The most clock-like 100 genes were identified from the dataset of 557 SCOGs according to the priority order of the bipartition support, root-to-tip variance, and tree length by SortaDate110. Selecting the most clock-like genes could minimize the impact of model misspecification for the estimation of divergence times.
Rate priors
A relaxed molecular clock model that accounts for rate variation among lineages was set for divergence time estimation. We calculated the amino acid pairwise distance between Caulerpa lentillifera and Ulva mutabilis with LG + Γ4 + F model by the package CODEML in PAML package v4.9j111. According to P. antiquus fossil placement, the divergence time between the two species was about 1.0 Ga43, which indicated that the mean rate for branches (μ) was assigned to a gamma hyperprior G (2, 50.28) with 2/50.28 = 0.04 substitutions per site per time unit (100 My). The rate drift parameter was assigned gamma hyper-prior σ2 ~ G (1, 10) with the mean 0.1.
Time priors
The time prior depends on the information of the fossil calibration point and the birth-death process. Birth-death process includes three parameters: birth rate (λ), death rate (μ), and sample fraction (ρ). The ddBD tree prior was used to calculate the birth rate and death rate because it can produce accurate node ages and confidence intervals even with a few well-constrained calibrations112. The sampling fraction was the proportion of our ingroup sample size (65 taxa) to the number of extant species in the, Prasinodermophyta, Chlorophyta, Charophyta (https://www.algaebase.org) and Bryophyta113 (~33,413).
Fossil constraints
We estimated the evolutionary timeline of the Viridiplantae using four analyses: two different root soft maximum constraints (1.89 Ga and 3.50 Ga) × two different positions of P. antiquus fossil calibration (node 4–1 and 4–2). Not only are there few records of green algal fossils, but some Precambrian green algal fossils with simple morphology have low taxonomic reliability. Thus, eight fossil calibration points were carefully selected for divergence time estimation. According to phylogenetic justifications of these fossils, uniform priors with a hard minimum bound (pL = 1e−300) and a soft maximum bound (pU = 0.025) were used for node 1 and 5, while cauchy priors with a hard minimum bound (pL = 1e−300) were used for other nodes (Table 1).
Approximate likelihood calculation and convergence assessment
The approximate likelihood calculations in MCMCTree were implemented under the LG + Γ4 + F model using CODEML114. We performed two runs, each consisting of 1 × 107 iterations after a burn-in of 1 × 106 iterations and sampling every 1000. Both independent runs using Tracer v. 1.7.2115 showed evidence of convergence (effective sample sizes, ESS values > 200). Calibration densities of the effective prior and posterior distributions were plotted by MCMCTreeR in R116.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data associated with the analyses are available from open-access repositories: https://figshare.com/s/a042722630e5ca0b2ba5. The data include sequence alignments, inferred phylogenies, simulated data, and time-calibrated trees. Taxon sampling of the nuclear and plastid datasets are provided in Supplementary Data 1.
References
- Brocks, J. J. et al. The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548, 578–581 (2017).
Article CAS PubMed ADS Google Scholar - Leliaert, F. Green algae: Chlorophyta and Streptophyta. In Encyclopedia of microbiology 457–468 (Academic Press, 2019).
- Zhang, Z. et al. Origin and evolution of green plants in the light of key evolutionary events. J. Integr. Plant Biol. 64, 516–535 (2022).
Article PubMed Google Scholar - Leliaert, F. et al. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 31, 1–46 (2012).
Article Google Scholar - Fang, L., Leliaert, F., Zhang, Z.-H., Penny, D. & Zhong, B.-J. Evolution of the Chlorophyta: Insights from chloroplast phylogenomic analyses. J. Syst. Evol. 55, 322–332 (2017).
Article Google Scholar - Nakayama, T. et al. The basal position of scaly green flagellates among the green algae (Chlorophyta) is revealed by analyses of nuclear-encoded SSU rRNA sequences. Protist 149, 367–380 (1998).
Article CAS PubMed Google Scholar - Fawley, M. W., Qin, M. & Yun, Y. The relationship between Pseudoscourfieldia marina and Pycnococcus provasolii (Prasinophyceae, Chlorophyta): evidence from 18S rDNA sequence data. J. Phycol. 35, 838–843 (1999).
Article CAS Google Scholar - Nakayama, T., Suda, S., Kawachi, M. & Inouye, I. Phylogeny and ultrastructure of Nephroselmis and Pseudoscourfieldia (Chlorophyta), including the description of Nephroselmis anterostigmatica sp. nov. and a proposal for the Nephroselmidales ord. nov. Phycologia 46, 680–697 (2007).
Article Google Scholar - Viprey, M., Guillou, L., Ferréol, M. & Vaulot, D. Wide genetic diversity of picoplanktonic green algae (Chloroplastida) in the Mediterranean Sea uncovered by a phylum-biased PCR approach. Environ. Microbiol. 10, 1804–1822 (2008).
Article CAS PubMed Google Scholar - Marin, B. & Melkonian, M. Molecular phylogeny and classification of the Mamiellophyceae class. nov. (Chlorophyta) based on sequence comparisons of the nuclear- and plastid-encoded rRNA operons. Protist 161, 304–336 (2010).
Article CAS PubMed Google Scholar - Leliaert, F. et al. Chloroplast phylogenomic analyses reveal the deepest-branching lineage of the Chlorophyta, Palmophyllophyceae class. nov. Sci. Rep. 6, 25367 (2016).
Article CAS PubMed PubMed Central ADS Google Scholar - Lopes dos Santos, A. et al. Chloropicophyceae, a new class of picophytoplanktonic prasinophytes. Sci. Rep. 7, 14019 (2017).
Article PubMed PubMed Central ADS Google Scholar - Daugbjerg, N., Fassel, N. M. D. & Moestrup, Ø. Microscopy and phylogeny of Pyramimonas tatianae sp. nov. (Pyramimonadales, Chlorophyta), a scaly quadriflagellate from Golden Horn Bay (eastern Russia) and formal description of Pyramimonadophyceae classis nova. Eur. J. Phycol. 55, 49–63 (2020).
Article Google Scholar - Li, L. et al. The genome of Prasinoderma coloniale unveils the existence of a third phylum within green plants. Nat. Ecol. Evol. 4, 1220–1231 (2020).
Article PubMed PubMed Central Google Scholar - Miyashita, H., Ikemoto, H., Kurano, N., Miyachi, S. & Chihara, M. Prasinococcus capsulatus gen. et sp. nov., a new marine coccoid prasinophyte. J. Gen. Appl. Microbiol. 39, 571–582 (1993).
Article CAS Google Scholar - Hasegawa, T. et al. Prasinoderma coloniale gen. et sp. nov., a new pelagic coccoid prasinophyte from the western Pacific Ocean. Phycologia 35, 170–176 (1996).
Article Google Scholar - Zechman, F. W. et al. An unrecognized ancient lineage of green plants persists in deep marine waters. J. Phycol. 46, 1288–1295 (2010).
Article Google Scholar - Leliaert, F., Verbruggen, H. & Zechman, F. W. Into the deep: new discoveries at the base of the green plant phylogeny. BioEssays 33, 683–692 (2011).
Article PubMed Google Scholar - Fawley, M. W., Yun, Y. & Qin, M. Phylogenetic analyses of 18s rDNA sequences reveal a new coccoid lineage of the Prasinophyceae (Chlorophyta). J. Phycol. 36, 387–393 (2001).
Article Google Scholar - Guillou, L. et al. Diversity of picoplanktonic prasinophytes assessed by direct nuclear SSU rDNA sequencing of environmental samples and novel isolates retrieved from oceanic and coastal marine ecosystems. Protist 155, 193–214 (2004).
Article CAS PubMed Google Scholar - Bachy, C. et al. The land–sea connection: insights Into the plant lineage from a green algal perspective. Annu. Rev. Plant Biol. 73, 585–616 (2022).
Article PubMed Google Scholar - Not, F., Simon, N., Biegala, I. & Vaulot, D. Application of fluorescent in situ hybridization coupled with tyramide signal amplification (FISH-TSA) to assess eukaryotic picoplankton composition. Aquat. Microb. Ecol. 28, 157–166 (2002).
Article Google Scholar - Not, F. et al. A single species, Micromonas pusilla (Prasinophyceae), dominates the eukaryotic picoplankton in the western English channel. Appl. Environ. Microbiol. 70, 4064–4072 (2004).
Article CAS PubMed PubMed Central ADS Google Scholar - Worden, A. Z., Nolan, J. K. & Palenik, B. Assessing the dynamics and ecology of marine picophytoplankton: the importance of the eukaryotic component. Limnol. Oceanogr. 49, 168–179 (2004).
Article CAS ADS Google Scholar - Rii, Y. M. et al. Diversity and productivity of photosynthetic picoeukaryotes in biogeochemically distinct regions of the South East Pacific Ocean. Limnol. Oceanogr. 61, 806–824 (2016).
Article ADS Google Scholar - Lopes dos Santos, A. et al. Diversity and oceanic distribution of prasinophytes clade VII, the dominant group of green algae in oceanic waters. ISME J. 11, 512–528 (2017).
Article PubMed Google Scholar - Not, F. et al. Diversity and ecology of eukaryotic marine phytoplankton. In Advances in Botanical Research vol. 64 1–53 (Elsevier, 2012).
- Faktorová, D. et al. Genetic tool development in marine protists: emerging model organisms for experimental cell biology. Nat. Methods 17, 481–494 (2020).
Article PubMed PubMed Central Google Scholar - One Thousand Plant Transcriptomes Initiative. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574, 679–685 (2019).
Article CAS Google Scholar - Nie, Y. et al. Accounting for uncertainty in the evolutionary timescale of green plants through clock-partitioning and fossil calibration strategies. Syst. Biol. 69, 1–16 (2020).
Article CAS PubMed Google Scholar - Fučíková, K. et al. New phylogenetic hypotheses for the core Chlorophyta based on chloroplast sequence data. Front. Ecol. Evol. 2, 2 (2014).
- Fang, L. et al. Improving phylogenetic inference of core Chlorophyta using chloroplast sequences with strong phylogenetic signals and heterogeneous models. Mol. Phylogenet. Evol. 127, 248–255 (2018).
Article CAS PubMed Google Scholar - Hou, Z. et al. Phylotranscriptomic insights into a Mesoproterozoic–Neoproterozoic origin and early radiation of green seaweeds (Ulvophyceae). Nat. Commun. 13, 1610 (2022).
Article CAS PubMed PubMed Central ADS Google Scholar - Zhou, B.-F. et al. Phylogenomic analyses highlight innovation and introgression in the continental radiations of Fagaceae across the Northern Hemisphere. Nat. Commun. 13, 1320 (2022).
Article CAS PubMed PubMed Central ADS Google Scholar - Morales-Briones, D. F. et al. Disentangling sources of gene tree discordance in phylogenomic data sets: Testing ancient hybridizations in Amaranthaceae s.l. Syst. Biol. 70, 219–235 (2021).
Article PubMed Google Scholar - Brocks, J. J. The transition from a cyanobacterial to algal world and the emergence of animals. Emerg. Top. Life Sci. 2, 181–190 (2018).
Article CAS PubMed Google Scholar - Tappan, H. N. The paleobiology of plant protists. (W. H. Freeman, San Francisco, 1980).
- Loron, C. C. & Moczydłowska, M. Tonian (Neoproterozoic) eukaryotic and prokaryotic organic-walled microfossils from the upper Visingsö Group, Sweden. Palynology 42, 220–254 (2018).
Article Google Scholar - Moczydłowska, M. Algal affinities of Ediacaran and Cambrian organic-walled microfossils with internal reproductive bodies: Tanarium and other morphotypes. Palynology 40, 83–121 (2016).
Article Google Scholar - Samuelsson, J., Dawes, P. R. & Vidal, G. Organic-walled microfossils from the Proterozoic Thule Supergroup, Northwest Greenland. Precambrian Res. 96, 1–23 (1999).
Article CAS ADS Google Scholar - Teyssèdre, B. Precambrian palaeontology in the light of molecular phylogeny – an example: the radiation of the green algae. Biogeosci. Discuss. 4, 3123–3142 (2007).
ADS Google Scholar - Javaux, E. J. & Knoll, A. H. Micropaleontology of the lower Mesoproterozoic Roper Group, Australia, and implications for early eukaryotic evolution. J. Paleontol. 91, 199–229 (2017).
Article ADS Google Scholar - Tang, Q., Pang, K., Yuan, X. & Xiao, S. A one-billion-year-old multicellular chlorophyte. Nat. Ecol. Evol. 4, 543–549 (2020).
Article PubMed PubMed Central Google Scholar - Chai, S., Aria, C. & Hua, H. A stem group Codium alga from the latest Ediacaran of South China provides taxonomic insight into the early diversification of the plant kingdom. BMC Biol. 20, 199 (2022).
Article CAS PubMed PubMed Central Google Scholar - Sauquet, H. A practical guide to molecular dating. Comptes Rendus Palevol 12, 355–367 (2013).
Article Google Scholar - Wang, S. & Luo, H. Dating Alphaproteobacteria evolution with eukaryotic fossils. Nat. Commun. 12, 3324 (2021).
Article CAS PubMed PubMed Central ADS Google Scholar - Cohen, P. A. & Kodner, R. B. The earliest history of eukaryotic life: uncovering an evolutionary story through the integration of biological and geological data. Trends Ecol. Evol. 37, 246–256 (2022).
Article PubMed Google Scholar - Brocks, J. J., Logan, G. A., Buick, R. & Summons, R. E. Archean molecular fossils and the early rise of eukaryotes. Science 285, 1033–1036 (1999).
Article CAS PubMed ADS Google Scholar - Rasmussen, B., Fletcher, I. R., Brocks, J. J. & Kilburn, M. R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455, 1101–1104 (2008).
- Zumberge, J. A., Rocher, D. & Love, G. D. Free and kerogen‐bound biomarkers from late Tonian sedimentary rocks record abundant eukaryotes in mid‐Neoproterozoic marine communities. Geobiology 18, 326–347 (2020).
Article CAS PubMed Google Scholar - Brocks, J. & Nettersheim, B. Lost world of complex life: molecular traces of our deepest eukaryotic ancestors. In Goldschmidt Abstracts 263–263 (Geochemical Society, 2020).
- Morales‐Briones, D. F., Liston, A. & Tank, D. C. Phylogenomic analyses reveal a deep history of hybridization and polyploidy in the Neotropical genus Lachemilla (Rosaceae). New Phytol. 218, 1668–1684 (2018).
Article PubMed Google Scholar - Rose, J. P., Toledo, C. A. P., Lemmon, E. M., Lemmon, A. R. & Sytsma, K. J. Out of sight, out of mind: Widespread nuclear and plastid-nuclear discordance in the flowering plant genus Polemonium (Polemoniaceae) suggests widespread historical gene flow despite limited nuclear signal. Syst. Biol. 70, 162–180 (2021).
Article CAS PubMed Google Scholar - Pease, J. B., Brown, J. W., Walker, J. F., Hinchliff, C. E. & Smith, S. A. Quartet Sampling distinguishes lack of support from conflicting support in the green plant tree of life. Am. J. Bot. 105, 385–403 (2018).
Article PubMed Google Scholar - Sánchez-Baracaldo, P., Raven, J. A., Pisani, D. & Knoll, A. H. Early photosynthetic eukaryotes inhabited low-salinity habitats. Proc. Natl. Acad. Sci. 114, E7737–E7745 (2017).
Article PubMed PubMed Central ADS Google Scholar - Cocquyt, E., Verbruggen, H., Leliaert, F. & De Clerck, O. Evolution and cytological diversification of the green seaweeds (Ulvophyceae). Mol. Biol. Evol. 27, 2052–2061 (2010).
Article CAS PubMed Google Scholar - Del Cortona, A. et al. Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds. Proc. Natl. Acad. Sci. 117, 2551–2559 (2020).
Article PubMed PubMed Central ADS Google Scholar - Chrétiennot-Dinet, M.-J. et al. A new marine picoeucaryote: Ostreococcus tauri gen. et sp. nov. (Chlorophyta, Prasinophyceae). Phycologia 34, 285–292 (1995).
Article Google Scholar - Lewin, R. A., Krienitz, L., Goericke, R., Takeda, H. & Hepperle, D. Picocystis salinarum gen. et sp. nov. (Chlorophyta) – a new picoplanktonic green alga. Phycologia 39, 560–565 (2000).
Article Google Scholar - Blanc, G. et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22, 2943–2955 (2010).
Article CAS PubMed PubMed Central Google Scholar - Roth, M. S. et al. Chromosome-level genome assembly and transcriptome of the green alga Chromochloris zofingiensis illuminates astaxanthin production. Proc. Natl. Acad. Sci. 114, E4296–E4305 (2017).
Article CAS PubMed PubMed Central Google Scholar - Eliáš, M., Klimeš, V., Derelle, R., Petrželková, R. & Tachezy, J. A paneukaryotic genomic analysis of the small GTPase RABL2 underscores the significance of recurrent gene loss in eukaryote evolution. Biol. Direct 11, 5 (2016).
Article PubMed PubMed Central Google Scholar - Lemieux, C., Turmel, M., Otis, C. & Pombert, J.-F. A streamlined and predominantly diploid genome in the tiny marine green alga Chloropicon primus. Nat. Commun. 10, 4061 (2019).
Article PubMed PubMed Central ADS Google Scholar - Teyssèdre, B. Are the green algae (phylum Viridiplantae) two billion years old? Carnets Géologie/Notebooks on Geology CG2006, 1–15 (2006).
- Knoll, A. H. The fossil record of microbial life. In Fundamentals of geobiology 297–314 (Wiley, 2012).
- Moczydłowska, M., Landing, E., Zang, W. & Palacios, T. Proterozoic phytoplankton and timing of Chlorophyte algae origins. Palaeontology 54, 721–733 (2011).
Article Google Scholar - Agić, H., Moczydłowska, M. & Yin, L. Diversity of organic-walled microfossils from the early Mesoproterozoic Ruyang Group, North China Craton – A window into the early eukaryote evolution. Precambrian Res. 297, 101–130 (2017).
Article ADS Google Scholar - Lamb, D. M., Awramik, S. M., Chapman, D. J. & Zhu, S. Evidence for eukaryotic diversification in the ∼1800 million-year-old Changzhougou Formation, North China. Precambrian Res. 173, 93–104 (2009).
Article CAS ADS Google Scholar - Westall, F. et al. Early Archean fossil bacteria and biofilms in hydrothermally-influenced sediments from the Barberton greenstone belt, South Africa. Precambrian Res. 106, 93–116 (2001).
Article CAS ADS Google Scholar - Maloney, K. M. et al. New multicellular marine macroalgae from the early Tonian of northwestern Canada. Geology 49, 743–747 (2021).
Article CAS ADS Google Scholar - Maloney, K. M., Schiffbauer, J. D., Halverson, G. P., Xiao, S. & Laflamme, M. Preservation of early Tonian macroalgal fossils from the Dolores Creek Formation, Yukon. Sci. Rep. 12, 6222 (2022).
Article CAS PubMed PubMed Central ADS Google Scholar - Maloney, K. M. et al. Systematic paleontology of macroalgal fossils from the Tonian Mackenzie Mountains Supergroup. J. Paleontol. 97, 499–515 (2023).
Article ADS Google Scholar - Li, G. et al. Tonian carbonaceous compressions indicate that Horodyskia is one of the oldest multicellular and coenocytic macro-organisms. Commun. Biol. 6, 399 (2023).
Article CAS PubMed PubMed Central Google Scholar - Strassert, J. F. H., Irisarri, I., Williams, T. A. & Burki, F. A molecular timescale for eukaryote evolution with implications for the origin of red algal-derived plastids. Nat. Commun. 12, 1879 (2021).
Article CAS PubMed PubMed Central ADS Google Scholar - Knoll, A. H. The early evolution of eukaryotes: a geological perspective. Science 256, 622–627 (1992).
Article CAS PubMed ADS Google Scholar - Lenton, T. M., Boyle, R. A., Poulton, S. W., Shields-Zhou, G. A. & Butterfield, N. J. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat. Geosci. 7, 257–265 (2014).
Article CAS ADS Google Scholar - Large, R. R. Evolution of Earth’s atmosphere. In Encyclopedia of Geology (Second Edition) (eds. Alderton, D. & Elias, S. A.) 571–584 (Academic Press, 2021). https://doi.org/10.1016/B978-0-12-409548-9.11853-6.
- Planavsky, N. J. et al. Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635–638 (2014).
Article CAS PubMed ADS Google Scholar - Cole, D. B. et al. A shale-hosted Cr isotope record of low atmospheric oxygen during the Proterozoic. Geology 44, 555–558 (2016).
Article CAS ADS Google Scholar - Canfield, D. E. The early history of atmospheric oxygen: Homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 33, 1–36 (2005).
Article CAS ADS Google Scholar - Holland, H. D. The oxygenation of the atmosphere and oceans. Philos. Trans. R. Soc. B Biol. Sci. 361, 903–915 (2006).
Article CAS Google Scholar - Kump, L. R. The rise of atmospheric oxygen. Nature 451, 277–278 (2008).
Article CAS PubMed ADS Google Scholar - Large, R. R. et al. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean–atmosphere evolution. Earth Planet. Sci. Lett. 389, 209–220 (2014).
Article CAS ADS Google Scholar - Large, R. R. et al. Atmosphere oxygen cycling through the Proterozoic and Phanerozoic. Miner. Deposita 54, 485–506 (2019).
Article CAS ADS Google Scholar - Müller, M. et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 76, 444–495 (2012).
Article PubMed PubMed Central Google Scholar - Reinhard, C. T., Planavsky, N. J., Olson, S. L., Lyons, T. W. & Erwin, D. H. Earth’s oxygen cycle and the evolution of animal life. Proc. Natl. Acad. Sci. 113, 8933–8938 (2016).
Article CAS PubMed PubMed Central ADS Google Scholar - Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Article PubMed PubMed Central Google Scholar - Eddy, S. R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011).
Article MathSciNet CAS PubMed PubMed Central ADS Google Scholar - Fu, L., Niu, B., Zhu, Z., Wu, S. & Li, W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012).
Article CAS PubMed PubMed Central Google Scholar - Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Article CAS PubMed PubMed Central Google Scholar - Capella-Gutierrez, S., Silla-Martinez, J. M. & Gabaldon, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Article CAS PubMed PubMed Central Google Scholar - Mai, U. & Mirarab, S. TreeShrink: fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 19, 272 (2018).
Article PubMed PubMed Central Google Scholar - Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Article CAS PubMed PubMed Central Google Scholar - Junier, T. & Zdobnov, E. M. The Newick Utilities: high-throughput phylogenetic tree processing in the UNIX shell. Bioinformatics 26, 1669–1670 (2010).
Article CAS PubMed PubMed Central Google Scholar - Zhang, C., Rabiee, M., Sayyari, E. & Mirarab, S. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19, 153 (2018).
Article PubMed PubMed Central Google Scholar - Sayyari, E. & Mirarab, S. Fast coalescent-based computation of local branch support from quartet frequencies. Mol. Biol. Evol. 33, 1654–1668 (2016).
Article CAS PubMed PubMed Central Google Scholar - Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Article CAS PubMed Google Scholar - Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Article CAS PubMed Google Scholar - Smith, S. A., Moore, M. J., Brown, J. W. & Yang, Y. Analysis of phylogenomic datasets reveals conflict, concordance, and gene duplications with examples from animals and plants. BMC Evol. Biol. 15, 150 (2015).
Article PubMed PubMed Central Google Scholar - Huerta-Cepas, J., Serra, F. & Bork, P. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 33, 1635–1638 (2016).
Article CAS PubMed PubMed Central Google Scholar - Struck, T. H. Trespex-detection of misleading signal in phylogenetic reconstructions based on tree information. Evol. Bioinforma. 10, EBO.S14239 (2014).
Article Google Scholar - Kozlov, A. M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453–4455 (2019).
Article CAS PubMed PubMed Central Google Scholar - Degnan, J. H. & Rosenberg, N. A. Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol. Evol. 24, 332–340 (2009).
Article PubMed Google Scholar - Cloutier, A. et al. Whole-genome analyses resolve the phylogeny of flightless birds (Palaeognathae) in the presence of an empirical anomaly zone. Syst. Biol. 68, 937–955 (2019).
Article PubMed PubMed Central Google Scholar - Jombart, T., Kendall, M., Almagro-Garcia, J. & Colijn, C. TREESPACE: statistical exploration of landscapes of phylogenetic trees. Mol. Ecol. Resour. 17, 1385–1392 (2017).
Article PubMed PubMed Central Google Scholar - Liu, L. & Yu, L. Phybase: an R package for species tree analysis. Bioinformatics 26, 962–963 (2010).
Article CAS PubMed Google Scholar - Ma, J. et al. The Chloranthus sessilifolius genome provides insight into early diversification of angiosperms. Nat. Commun. 12, 6929 (2021).
Article CAS PubMed PubMed Central ADS Google Scholar - Suvorov, A. et al. Widespread introgression across a phylogeny of 155 Drosophila genomes. Curr. Biol. 32, 111–123.e5 (2022).
Article CAS PubMed Google Scholar - Revell, L. J. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Article Google Scholar - Smith, S. A., Brown, J. W. & Walker, J. F. So many genes, so little time: a practical approach to divergence-time estimation in the genomic era. PLOS One 13, e0197433 (2018).
Article PubMed PubMed Central Google Scholar - Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Article CAS PubMed Google Scholar - Tao, Q., Barba-Montoya, J. & Kumar, S. Data-driven speciation tree prior for better species divergence times in calibration-poor molecular phylogenies. Bioinformatics 37, i102–i110 (2021).
Article CAS PubMed PubMed Central Google Scholar - Wang, Q.-H. et al. Diversity, phylogeny, and adaptation of bryophytes: insights from genomic and transcriptomic data. J. Exp. Bot. 73, 4306–4322 (2022).
Article CAS PubMed Google Scholar - Reis, M. D. & Yang, Z. Approximate likelihood calculation on a phylogeny for Bayesian estimation of divergence times. Mol. Biol. Evol. 28, 2161–2172 (2011).
Article PubMed Google Scholar - Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904 (2018).
Article CAS PubMed PubMed Central Google Scholar - Puttick, M. N. MCMCtreeR: functions to prepare MCMCtree analyses and visualize posterior ages on trees. Bioinformatics 35, 5321–5322 (2019).
Article CAS PubMed Google Scholar - Butterfield, N. J. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26, 386–404 (2000).
Article Google Scholar - Xiao, S., Knoll, A. H., Yuan, X. & Pueschel, C. M. Phosphatized multicellular algae in the Neoproterozoic Doushantuo Formation, China, and the early evolution of florideophyte red algae. Am. J. Bot. 91, 214–227 (2004).
Article PubMed Google Scholar - Guo, C.-Q. et al. Riccardiothallus devonicus gen. et sp. nov., the earliest simple thalloid liverwort from the Lower Devonian of Yunnan, China. Rev. Palaeobot. Palynol. 176–177, 35–40 (2012).
Article Google Scholar - Strother, P. K. Systematics and evolutionary significance of some new cryptospores from the Cambrian of eastern Tennessee, USA. Rev. Palaeobot. Palynol. 227, 28–41 (2016).
Article Google Scholar - Colbath, G. K. & Grenfell, H. R. Review of biological affinities of Paleozoic acid-resistant, organic-walled eukaryotic algal microfossils (including “acritarchs”). Rev. Palaeobot. Palynol. 86, 287–314 (1995).
Article Google Scholar - Nye, E., Feist-Burkhardt, S., Horne, D. J., Ross, A. J. & Whittaker, J. E. The palaeoenvironment associated with a partial Iguanodon skeleton from the Upper Weald Clay (Barremian, Early Cretaceous) at Smokejacks Brickworks (Ockley, Surrey, UK), based on palynomorphs and ostracods. Cretac. Res. 29, 417–444 (2008).
Article Google Scholar
Acknowledgements
This work is supported by the National Natural Science Foundation of China (32122010 and 31970229), Collaborative Innovation Center for Modern Crop Production co-sponsored by Province and Ministry (CIC-MCP), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We thank Lingxiao Yang, Zheng Hou, Alison Cloutier, Ya Yang and Diego Morales-Briones for helpful discussion.
Author information
Authors and Affiliations
- College of Life Sciences, Nanjing Normal University, Nanjing, China
Zhiping Yang, Xiaoya Ma, Qiuping Wang, Xiaolin Tian, Jingyan Sun, Zhenhua Zhang & Bojian Zhong - Department of Geosciences and Global Change Center, Virginia Tech, Blacksburg, VA, USA
Shuhai Xiao - Phycology Research Group and Center for Molecular Phylogenetics and Evolution, Ghent University, Ghent, Belgium
Olivier De Clerck - Meise Botanic Garden, Meise, Belgium
Frederik Leliaert
Authors
- Zhiping Yang
You can also search for this author inPubMed Google Scholar - Xiaoya Ma
You can also search for this author inPubMed Google Scholar - Qiuping Wang
You can also search for this author inPubMed Google Scholar - Xiaolin Tian
You can also search for this author inPubMed Google Scholar - Jingyan Sun
You can also search for this author inPubMed Google Scholar - Zhenhua Zhang
You can also search for this author inPubMed Google Scholar - Shuhai Xiao
You can also search for this author inPubMed Google Scholar - Olivier De Clerck
You can also search for this author inPubMed Google Scholar - Frederik Leliaert
You can also search for this author inPubMed Google Scholar - Bojian Zhong
You can also search for this author inPubMed Google Scholar
Contributions
B.Z. conceived and designed the research; Z.Y., X.M., Q.W., X.T., J.S., and Z.Z. performed the experiments and analyzed the data; Z.Y. and B.Z. drafted the papers with input from all authors; Z.Y., S.X., O.D.C., F.L., and B.Z. revised and edited the paper. All of the authors read and approved the final paper.
Corresponding author
Correspondence toBojian Zhong.
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Z., Ma, X., Wang, Q. et al. Phylotranscriptomics unveil a Paleoproterozoic-Mesoproterozoic origin and deep relationships of the Viridiplantae.Nat Commun 14, 5542 (2023). https://doi.org/10.1038/s41467-023-41137-5
- Received: 15 December 2022
- Accepted: 23 August 2023
- Published: 11 September 2023
- DOI: https://doi.org/10.1038/s41467-023-41137-5