If phosphatidylserine is the death knell, a new phosphatidylserine-specific receptor is the bellringer (original) (raw)
- Review
- Published: 10 July 2001
Cell Death & Differentiation volume 8, pages 582–587 (2001)Cite this article
- 2201 Accesses
- 84 Citations
- Metrics details
Abstract
Recognition of phosphatidylserine (PtdSer) is essential for engulfment of apoptotic cells by mammalian phagocytes. Engagement of a new phosphatidylserine-specific receptor (PtdSerR) appears to be necessary for uptake of apoptotic cells. Many other mammalian receptors have been described to function in the clearance of apoptotic cells. The emerging picture is that many of these receptors may provide the strong adhesion needed to increase the likelihood of contact between the PtdSerR and its phospholipid ligand, which is required for uptake. Furthermore, stimulation of this receptor on different types of phagocytes by apoptotic cells, PtdSer-containing liposomes or an IgM monoclonal anti-PtdSer antibody initiates release of TGFβ, known to be involved in the anti-inflammatory effects of apoptotic cells. Although highly homologous genes exist in C. elegans and Drosophila melanogaster, their role in engulfment of apoptotic cells remains to be determined. Cell Death and Differentiation (2001) 8, 582–587
Similar content being viewed by others
Main
In order for phagocytes, including macrophages, to recognize and engulf them, apoptotic cells must lose phospholipid asymmetry and expose phosphatidylserine on the outer leaflet of the plasma membrane.1 Learning how macrophages and other phagocytes recognize this phospholipid has been a crusade for our laboratories and those of many other investigators. Many different types of receptors have been implicated in the uptake of apoptotic cells. An emerging pattern is that many of the same recognition receptors responsible for recognizing and engulfing pathogens also mediate recognition and engulfment of apoptotic cells, yet the outcome is very different. These include lectins, Class A and Class B scavenger receptors, CD68 and other receptors for oxidized LDL particles, CD14, selected integrins, C1q and collectin binding proteins, complement binding proteins, and β2GPI binding proteins.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 Uptake of apoptotic cells appears to be anti-inflammatory and possibly even immunosuppressive, at least with regard to self antigens. For example, it was recently shown that only necrotic tumor cells, but not apoptotic cells, could drive dendritic cell maturation and subsequent activation of T lymphocytes.23 In contrast, uptake of pathogens is proinflammatory and promotes an adaptive immune response. Understanding this conundrum is an important goal for investigators studying the clearance of apoptotic cells by phagocytes.
A brief history of the quest for a phosphatidylserine-specific receptor
We have recently cloned the gene for a novel protein that appears to mediate specific recognition of phosphatidylserine on apoptotic cells by phagocytes and is highly conserved throughout phylogeny.24 It was first reported as a gene of unknown function cloned from a human brain library; however, the authors made the observation that the gene was highly homologous to an undescribed gene from Caenorhabditis elegans contained on cosmid F2929.25 A highly homologous gene is also found in the genome of Drosophila melanogaster; its function in this organism is also unknown. For lack of a better name, since it does not fall into any of the known receptor families, we have called this protein the phosphatidylserine-specific receptor (PtdSerR).24
The PtdSerR is expressed on macrophages, fibroblasts, endothelial cells, epithelial cells, melanoma cells (Fadok, unpublished data), and human dendritic cells (Fadok, unpublished data); in short, on virtually all the cells described to mediate clearance of apoptotic bodies.24 It is not expressed on the surface of circulating cells such as lymphocytes, neutrophils, monocytes, or red blood cells. On macrophages, expression is variable. The expression on human monocyte-derived macrophages or mouse bone marrow-derived macrophages is low, unless they are stimulated with digestible particles such as β-glucan, zymosan, or even apoptotic cells themselves.24 In contrast, thioglycollate-elicited peritoneal macrophages express high surface levels of this receptor, which correlates with their ability to recognize apoptotic cells in a PtdSer-inhibitable manner. The human cDNA, when transfected into human Jurkat T cells and mouse M12.C3 B lymphocytes (two undisputedly nonphagocytic cell lines) enabled them to bind to and engulf apoptotic cells and PtdSer-expressing red blood cells.24
Several years ago we proposed the hypothesis that the ability to recognize PtdSer by macrophages was dependent on the macrophage subpopulation used.26,27 These observations were based on whether uptake of apoptotic cells could be inhibited by PtdSer-containing liposomes or not. Based on our current data and those of others, we believe it is time to dispel this notion. It has become clear that all macrophages, and in fact, all phagocytes, recognize phosphatidylserine on apoptotic cells. Our earlier misinterpretation arose from the fact that inhibition assays, particularly when using liposomes, are relatively insensitive in determining how a macrophage recognizes apoptotic cells. Insensitive though they are, inhibition assays were all we knew to use in the infancy of the study of clearance. Savill and coworkers had originally reported that human monocyte-derived macrophages (HMDM) could recognize apoptotic cells using a complex of receptors, the αvβ3 vitronectin receptor and CD36, which were bridged to the apoptotic cells by thrombospondin.7,8 Uptake by these cells, as well as by mouse bone marrow-derived macrophages (BMDM), was inhibited by the tetrapeptide RGDS and antibodies against αvβ3 or CD36, but not by PtdSer-containing liposomes.26,28 In contrast, HMDM or BMDM treated with TGFβ and β-glucan lost their ability to be inhibited by the αvβ3 antibodies, and acquired the ability to be inhibited by PtdSer-containing liposomes.26,28 Pradham and coworkers, however, later went on to demonstrate that while uptake of apoptotic cells by BMDM was not inhibited by PtdSer-containing liposomes, it could be inhibited by PtdSer-expressing symmetric red blood cells.29 They also observed that pretreatment with annexin V, PtdSer binding protein, could inhibit uptake of apoptotic cells by all macrophages studied.30,31
Recently, we reported that apoptotic cells which failed to express phosphatidylserine externally were not engulfed by either macrophages (stimulated or not to upregulate the PtdSerR) or fibroblasts.1 Restoration of PtdSer in the outer leaflet by liposome transfer or by differentiating the cells prior to induction of apoptosis restored recognition and uptake by either type of macrophage or by fibroblasts. Furthermore, only L stereoisomers were effective; 1-palmitoyl-2-oleoyl-sn-3 glycerophospho-L-serine (POP-L-PS) but not POP-D-PS was able to signal the phagocyte for uptake, and inhibition was inhibited by the monoclonal anti-PtdSerR antibody. All the data at this point, therefore, are most consistent with the hypothesis that phosphatidylserine on apoptotic cells must be engaged by the PtdSerR on phagocytes before engulfment can proceed.
Distribution, structure, and function of the mammalian PtdSer receptor
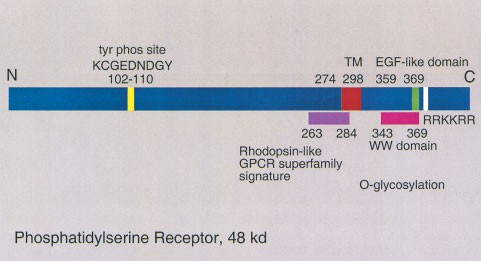
The gene for the PtdSer-specific receptor encodes a type II protein of approximately 48 kd. A simple bar structure of the protein is shown in Figure 1. There are multiple serines in the predicted extracellular domain which appear to be glycoslyated, as deglycosylation reduces the apparent molecular weight in Western blots from approximately 70 to 48 kd.24 There is at least one potential tyrosine phosphorylation site in the predicted intracellular domain. There are runs of basic residues in the extracellular domain, which could provide a binding site for the negatively charged phosphoserine head group of PtdSer. In addition, there is a weak WW domain as well; this domain has been implicated in the binding of phosphoserine and phosphotyrosine residues in proteins. It, therefore, could provide a potential binding site for phosphoserine in a phospholipid.32 This protein does not contain the proposed consensus binding sequence for phosphatidylserine (FxFxLKxxxKxR) found in protein kinase C isoforms, phospholipase C, and phosphatidylserine decarboxylase.33 Nor does it have any sequence similarities to the annexins, coagulation factors, vitronectin, or complement proteins known to bind to PtdSer.34,35,36,37,38
Figure 1
Bar diagram of the phosphatidylserine receptor, which is predicted to be a type II protein. The extracellular domain contains a weak motif for a WW domain which could represent a possible binding site for phosphoserine (see text). Alternatively, the run of basic residues (RRKKRR) could bind to the anionic head group of PtdSer. There is a potential tyrosine phosphorylation and multiple potential PKC phosphorylation site (not shown) in the predicted intracellular domain, which could provide signaling capabilities. TM=transmembrane domain. The significance of the rhodopsin GPCR-like motif and the EGF-like motif is not known at this time
As mentioned before, we have observed the PtdSerR on many different types of cells which engulf apoptotic bodies, including macrophages, fibroblasts, epithelial cells, and endothelial cells.24 Staining with the monoclonal anti-PtdSerR antibody on unfixed and nonpermeabilized cells has shown that the distribution of the PtdSer-specific receptor on the surface of fibroblasts, macrophages, and epithelial cells appears to be punctate rather than diffusely distributed around the membrane; other cell types remain to be tested. There are two possible explanations for this distribution which are currently being explored. First, the PtdSerR is associated with specific membrane lipid domains, such as caveolae or rafts; this remains to be explored but preliminary evidence obtained from mammary epithelial cells suggests that caveolin-1, a marker for caveolae, does not colocalize with the PtdSerR. Second, it is possible that PtdSer-containing vesicular debris from ongoing cell death in the cell cultures is continually cross-linking this receptor on the phagocyte surface. Figure 2 shows a fibroblast which has engulfed fluorescently-labeled apoptotic cells. In this case, the phagocytes were fixed prior to staining, to show that the PtdSerR clusters around the engulfed apoptotic bodies. With regard to tissue distribution, using RT–PCR, we have preliminary evidence that the gene is expressed in multiple tissues and organs, and as early as day 7 of embryogenesis in the mouse (unpublished data). This tissue expression pattern is consistent with our observation that the receptor is present on many different cell types.
Figure 2
Phosphatidylserine receptor clusters around apoptotic bodies during engulfment. NIH3T3 cells were exposed to apoptotic Jurkat T cells, then fixed and permeabilized briefly with 4% paraformaldehyde in sucrose. The cells were stained with Alexa 488-conjugated phalloidin (Molecular Probes, Eugene, OR, USA) to show actin, with DAPI to identify the nuclei of the phagocytes and the apoptotic bodies, and with mAb 217G8E9 followed by Cy3-labeled anti-mouse IgM (Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA). Apoptotic Jurkat T cells have very little cytoplasm, accounting for the apparent antibody staining around their nuclei
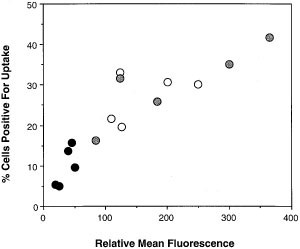
Using a novel biotinylated red cell system, we have created particles for uptake which will trigger single receptors using specific antibodies or natural ligands (Hoffman P, Fadok VA, Henson PM, unpublished data). The red blood cells are biotinylated; biotinylated antibodies are then attached via a streptavidin bridge. Antibodies against human CD36, αvβ3, CD68, CD14, or mouse SRA promoted binding but not uptake of these particles; anti-CD36, in particular, appeared to promote very strong binding. The red cells were only engulfed, however, if their outer plasma membranes had been reconstituted with phosphatidyl-L-serine; phosphatidyl-D-serine was not effective. Uptake was then inhibited by anti-PtdSerR antibody. As an alternative, NIH3T3 cells were transfected with vector containing the sense or antisense sequence of the PtdSer receptor. The antisense construct significantly reduced expression of the PtdSer receptor and significantly inhibited uptake of apoptotic cells (see Figure 3). Taken together, these data suggest that many of the receptors involved in apoptotic cell recognition promote strong adhesion, but that the PtdSer receptor must be engaged for engulfment to occur.
Figure 3
Expression levels of the PtdSerR is correlated with ability to take up apoptotic cells (_n_=5; _r_2=0.78; P<0.0001). Expression of the PtdSerR was reduced by transfecting NIH3T3 cells transiently with pcDNA3.1 containing the reverse sequence of the entire PtdSerR, and assessing uptake of apoptotic Jurkat T cells 18 h later. Expression of the PtdSerR was assessed by flow cytometry, and levels are shown as the relative mean fluorescence _(x_-axis). Uptake is expressed as per cent cells positive for uptake (_y_-axis). The black circles represent those cells transfected with the antisense construct; the grey circles represent those cells that were mock transfected; the open circles represent those cells transfected with empty vector. Note that loss of PtdSerR expression is associated with reduced ability to take up apoptotic cells
The anti-inflammatory properties of the phosphatidylserine receptor
Exposure to apoptotic cells is found to be not only non-inflammatory,49,50,51 but actively anti-inflammatory.52,53,54 By inducing the release of TGFβ and PGE2, apoptotic cells caused downregulation of chemokines, TNFα, IL1β, and IL10 from endotoxin-stimulated macrophages, as anti-TGFβ antibodies, indomethacin, and a platelet-activating factor receptor antagonist restored production of these cytokines.52 Exposure of mouse macrophages (J774 cells) to apoptotic cells also resulted in the release of TGFβ and reduction in proinflammatory cytokines.53 These effects could be mimicked by treatment with PtdSer-containing liposomes, as well as by mAb 217, suggesting that the interaction of phosphatidylserine on the apoptotic cells with this receptor on the phagocyte caused the release of anti-inflammatory mediators.24 This interpretation receives further support from two of our preliminary observations: first, that the fibroblasts transfected with anti-sense constructs for PtdSerR not only fail to engulf apoptotic cells but also fail to produce TGFβ, and second, that apoptotic cells which fail to express phosphatidylserine externally fail to induce TGFβ secretion.
Does the PtdSerR play a role in engulfment of apoptotic cells in invertebrates?
The role of this gene product in engulfment of apoptotic cells by phagocytes from other organisms remains to be determined. It is intriguing to note that there is high homology between the mammalian receptor and the genes of unknown function in Drosophila melanogaster and C. elegans. To date, the role of this gene is being actively studied in both these organisms. The notion that this gene would function in engulfment of apoptotic cells in nonmammalian organisms is appealing, as phosphatidylserine, at least with regard to its polar head group, is not polymorphic. This phospholipid is found in the plasma membranes of all eukaryotic cells. Exposure of phosphatidylserine in the membranes of Drosophila cells undergoing apoptosis has been convincingly demonstrated by van den Eijnde and colleagues,55 and it seems likely that worm cells would also undergo loss of phospholipid asymmetry, although this remains to be proven.
The study of cell corpse engulfment in C. elegans has revealed a wealth of information on the genes used by viable cells to recognize and engulf their dying neighbors, and it is encouraging to note that many similar genes are involved in mammalian recognition and engulfment.56,57,58,59,60 Pairing the genetic and molecular studies in C. elegans and Drosophila melanogaster with the mammalian functional studies has proved to be a powerful tool, as illustrated by the characterization of ced-7 and one of its human homologs the ABC1 transporter,58,61,62,63 ced-6 and its human homolog,56,57,64,65 and ced-2, ced-5, and ced-10 with CRKII, DOCK 180, and Rac-160,66,67,68,69 and fly croquemort and its human homolog CD36.8,70,71 Furthermore, the identity of ced-1 has recently been reported and the protein appears to be a surface receptor for recognition of apoptotic cells with homology to mammalian scavenger receptors.72
The future of the PtdSer-specific receptor
The PtdSer receptor we have identified will provide years of interesting work for us and hopefully others. There are many questions to be asked and answered. Our preliminary observations that this protein is expressed in intracellular membranes suggests a role in addition to recognition of apoptotic cells. It is possible that the PtdSerR could be important in intracellular phosphatidylserine transport, although it has no homologs in yeast as far as we have been able to determine and this is only speculation. How the protein recognizes phosphatidylserine and what accounts for the stereospecificity remains to be determined. It is unknown whether glycosylation is important for this function, or for some other function. The presence of charged residues within the predicted transmembrane domain suggests the potential for interaction with other proteins within the membrane, and the presence of cysteines in the extracellular domain suggest this protein may function as an oligomer, rather than a monomer.
What is clear at this point is that the PtdSer receptor binding to phosphatidylserine on the apoptotic cell is necessary for uptake of apoptotic cells and release of anti-inflammatory mediators. This PtdSer receptor is widespread, with regard to phylogeny, embryogenesis, tissue distribution, and cell type and is therefore likely to play a central role in clearance of apoptotic cells.
Abbreviations
β2GPI:
beta 2 glycoprotein 1
BMDM:
bone marrow-derived macrophages
HMDM:
human monocyte-derived macrophages
LDL:
low density lipoprotein
PAF:
platelet activating factor
PGE2:
prostaglandin E2
POP-L-PS:
1-palmitoyl-2-oleoyl-sn-3 glycerophospho-L-serine
POP-D-PS:
1-palmitoyl-2-oleoyl-sn-3 glycerophospho-D-serine
PtdSer:
phosphatidylserine
PtdSerR:
phosphatidylserine receptor
TGFβ:
transforming growth factor-beta
References
- Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL . 2001 Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts J. Biol. Chem. 276: 1071–1077
Article CAS PubMed Google Scholar - Dini L, Autuori F, Lentini A, Oliverio S, Piacentini M . 1992 The clearance of apoptotic cells in the liver is mediated by the asialoglycoprotein receptor FEBS Lett. 296: 174–178
Article CAS PubMed Google Scholar - Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, Vidal-Vanaclocha F . 1995 Phagocytosis of apoptotic bodies by liver endothelial cells J. Cell. Sci. 108: 967–973
CAS PubMed Google Scholar - Dini L . 1998 Endothelial liver cell recognition of apoptotic peripheral blood lymphocytes Biochem. Soc. Trans. 26: 635–639
Article CAS PubMed Google Scholar - Duvall E, Wyllie AH, Morris RG . 1985 Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology 56: 351–358
CAS PubMed PubMed Central Google Scholar - Falasca L, Bergamini A, Serafino A, Balabaud C, Dini L . 1996 Human Kupffer cell recognition and phagocytosis of apoptotic peripheral blood lymphocytes Exp. Cell. Res. 224: 152–162
Article CAS PubMed Google Scholar - Savill J, Dransfield I, Hogg N, Haslett C . 1990 Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis Nature 343: 170–173
Article CAS PubMed Google Scholar - Savill J, Hogg N, Haslett C . 1991 Macrophage vitronectin receptor, CD36, and thrombospondin cooperate in recognition of neutrophils undergoing programmed cell death Chest 99: 6S–7S
Article CAS PubMed Google Scholar - Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y . 1997 Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by Sertoli cells of the rat J. Biol. Chem. 272: 2354–2358
Article CAS PubMed Google Scholar - Shiratsuchi A, Kawasaki Y, Ikemoto M, Arai H, Nakanishi Y . 1999 Role of class B scavenger receptor type I in phagocytosis of apoptotic rat spermatogenic cells by Sertoli cells J. Biol. Chem. 274: 5901–5908
Article CAS PubMed Google Scholar - Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S . 1996 Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro Proc. Natl. Acad. Sci. USA 93: 12456–12460
Article CAS PubMed PubMed Central Google Scholar - Sambrano GR, Steinberg D . 1995 Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine Proc. Natl. Acad. Sci. USA 92: 1396–1400
Article CAS PubMed PubMed Central Google Scholar - Sambrano GR, Terpstra V, Steinberg D . 1997 Independent mechanisms for macrophage binding and macrophage phagocytosis of damaged erythrocytes. Evidence of receptor cooperativity Arterioscler. Thromb. Vasc. Biol. 17: 3442–3448
Article CAS PubMed Google Scholar - Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T . 1998 Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells Proc. Natl. Acad. Sci. USA 95: 9535–9540
Article CAS PubMed PubMed Central Google Scholar - Devitt A, Moffatt OD, Rakundalia C, Capra JD, Simmons DL, Gregory CD . 1998 Human CD14 mediates recognition and phagocytosis of apoptotic cells Nature 392: 505–509
Article CAS PubMed Google Scholar - Flora PK, Gregory CD . 1994 Recognition of apoptotic cells by human macrophages: inhibition by a monocyte/macrophage-specific monoclonal antibody Eur. J. Immunol. 24: 2625–2632
Article CAS PubMed Google Scholar - Mevorach D, Mascarenhas JO, Gershov D, Elkon KB . 1998 Complement-dependent clearance of apoptotic cells by human macrophages J. Exp. Med. 188: 2313–2320
Article CAS PubMed PubMed Central Google Scholar - Schwartz BR, Karsan A, Bombeli T, Harlan JM . 1999 A novel beta 1 integrin-dependent mechanism of leukocyte adherence to apoptotic cells J. Immunol. 162: 4842–4848
CAS PubMed Google Scholar - Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ . 2000 A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo J. Exp. Med. 192: 359–366
Article CAS PubMed PubMed Central Google Scholar - Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ . 1998 Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies [see comments] Nat. Genet. 19: 56–59
Article CAS PubMed Google Scholar - Balasubramanian K, Chandra J, Schroit AJ . 1997 Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of beta2-glycoprotein I in macrophage recognition J. Biol. Chem. 272: 31113–31117
Article CAS PubMed Google Scholar - Balasubramanian K, Schroit AJ . 1998 Characterization of phosphatidylserine-dependent beta2-glycoprotein I macrophage interactions. Implications for apoptotic cell clearance by phagocytes J. Biol. Chem. 273: 29272–29277
Article CAS PubMed Google Scholar - Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N . 2000 Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells [see comments] J. Exp. Med. 191: 423–434
Article CAS PubMed PubMed Central Google Scholar - Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekowitz RA, Henson PM . 2000 A receptor for phosphatidylserine-specific clearance of apoptotic cells [see comments] Nature 405: 85–90
Article CAS PubMed Google Scholar - Nagase T, Ishikawa K-I, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O . 1998 Prediction of the coding sequences of unidentified human genes. IX. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro DNA Res. 5: 31–39
Article CAS PubMed Google Scholar - Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM . 1992 Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells J. Immunol. 149: 4029–4035
CAS PubMed Google Scholar - Fadok VA, Laszlo DJ, Noble PW, Weinstein L, Riches DW, Henson PM . 1993 Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells J. Immunol. 151: 4274–4285
CAS PubMed Google Scholar - Fadok VA, Warner ML, Bratton DL, Henson PM . 1998 CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J. Immunol. 161: 6250–6257
CAS PubMed Google Scholar - Pradhan D, Krahling S, Williamson P, Schlegel RA . 1997 Multiple systems for recognition of apoptotic lymphocytes by macrophages Mol. Biol. Cell. 8: 767–778
Article CAS PubMed PubMed Central Google Scholar - Krahling S, Callahan MK, Williamson P, Schlegel RA . 1999 Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages Cell Death Differ. 6: 183–189
Article CAS PubMed Google Scholar - Callahan MK, Williamson P, Schlegel RA . 2000 Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes Cell Death Differ. 7: 645–653
Article CAS PubMed Google Scholar - Lu PJ, Zhou XZ, Shen M, Lu KP . 1999 Function of WW domains as phosphoserine- or phosphothreonine-binding modules Science 283: 1325–1328
Article CAS PubMed Google Scholar - Igarashi K, Kaneda M, Yarnaji A, Saido TC, Kikkawa U, Ono Y, Inoue K, Umeda M . 1995 A novel phosphatidylserine binding peptide motif defined by an anti-idiotypic monoclonal antibody. Localization of phosphatidylserine-specific binding sites on protein kinase C and phosphatidylserine decarboxylase J. Biol. Chem. 270: 29075–29078
Article CAS PubMed Google Scholar - Tait J, Sakata M, McMullen BA, Miao CH, Funakoshi T, Hendrickson LE, Fujikawa K . 1988 Placental anticoagulant proteins: isolation and comparative characterization four members of the lipocortin family Biochemistry 27: 6268–6276
Article CAS PubMed Google Scholar - Funakoshi T, Hendrickson LE, McMullen BA, Fujikawa K . 1987 Primary structure of human placental anticoagulant protein Biochemistry 26: 8087–8092
Article CAS PubMed Google Scholar - Funakoshi T, Heimark RL, Hendrickson LE, McMullen BA, Fujikawa K . 1987 Human placental anticoagulant protein: isolation and characterization Biochemistry 26: 5572–5578
Article CAS PubMed Google Scholar - Andree HA, Stuart MC, Hermens WT, Reutelingsperger CP, Hemker HC, Frederick PM, Willems GM . 1992 Clustering of lipid-bound annexin V may explain its anticoagulant effect J. Biol. Chem. 267: 17907–17912
CAS PubMed Google Scholar - Andree HA, Reutelingsperger CP, Hauptmann R, Hemker HC, Hermens WT, Willems GM . 1990 Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers J. Biol. Chem. 265: 4923–4928
CAS PubMed Google Scholar - Reutelingsperger CP, Hornstra G, Hemker HC . 1985 Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord Eur. J. Biochem. 151: 625–629
Article CAS PubMed Google Scholar - Tait JF, Gibson D . 1992 Phospholipid binding of annexin V: effects of calcium and membrane phosphatidylserine content Arch. Biochem. Biophys. 298: 187–191
Article CAS PubMed Google Scholar - Tait JF, Gibson D . 1990 Interaction of placental anticoagulant protein-I (lipocortin V) with model membranes Prog. Clin. Biol. Res. 349: 173–181
CAS PubMed Google Scholar - Tait JF, Gibson D, Fujikawa K . 1989 Phospholipid binding properties of human placental anticoagulant protein-I, a member of the lipocortin family J. Biol. Chem. 264: 7944–7949
CAS PubMed Google Scholar - Zwaal RFA . 1978 Membrane and lipid involvement in blood coagulation Biochim. Biophys. Acta 515: 163–205
Article CAS PubMed Google Scholar - Liu C, Marshall P, Schreibman I, Vu A, Gai W, Whitlow M . 1999 Interaction between terminal complement proteins C5b-7 and anionic phospholipids Blood 93: 2297–2301
CAS PubMed Google Scholar - Yoneda A, Ogawa H, Kojima K, Matsumoto I . 1998 Characterization of the ligand binding activities of vitronectin: interaction of vitronectin with lipids and identification of the binding domains for various ligands using recombinant domains Biochemistry 37: 6351–6360
Article CAS PubMed Google Scholar - Test ST, Mitsuyoshi J . 1997 Activation of the alternative pathway of complement by calcium-loaded erythrocytes resulting from loss of membrane phospholipid asymmetry J. Lab. Clin. Med. 130: 169–182
Article CAS PubMed Google Scholar - Liu D, Liu F, Song YK . 1995 Recognition and clearance of liposomes containing phosphatidylserine are mediated by serum opsonin Biochim. Biophys. Acta 1235: 140–146
Article PubMed Google Scholar - Wang RH, Phillips G, Medof ME, Mold C . 1993 Activation of the alternative complement pathway by exposure of phosphatidylethanolamine and phosphatidylserine on erythrocytes from sickle cell disease patients J. Clin. Invest. 92: 1326–1335
Article CAS PubMed PubMed Central Google Scholar - Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C . 1992 Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2 J. Leukoc. Biol. 52: 269–273
Article CAS PubMed Google Scholar - Stern M, Savill J, Haslett C . 1996 Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response Am. J. Pathol. 149: 911–921
CAS PubMed PubMed Central Google Scholar - Hughes J, Liu Y, Van Damme J, Savill J . 1997 Human glomerular mesangial cell phagocytosis of apoptotic neutrophils: mediation by a novel CD36-independent vitronectin receptor/thrombospondin recognition that is uncoupled from chemokine secretion J. Immunol. 158: 4389–4397
CAS PubMed Google Scholar - Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM . 1998 Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF J. Clin. Invest. 101: 890–898
Article CAS PubMed PubMed Central Google Scholar - McDonald PP, Fadok VA, Bratton DL, Henson PM . 1999 Transcriptional and translational regulation of inflammatory mediator production by endogenous TGFb in macrophages that have ingested apoptotic cells J. Immunol. in press
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I . 1997 Immunosuppressive effects of apoptotic cells [letter] Nature 390: 350–351
Article CAS PubMed Google Scholar - van den Eijnde SM, Boshart L, Baehrecke EH, De Zeeuw CI, Reutelingsperger CPM, Vermeij-Keers C . 1998 Phosphatidylserine exposure by apoptotic cells is phylogenetically conserved Apoptosis 3: 9–16
Article CAS PubMed Google Scholar - Liu QA, Hengartner MO . 1998 Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans Cell 93: 961–972
Article CAS PubMed Google Scholar - Liu QA, Hengartner MO . 1999 Human CED-6 encodes a functional homologue of the Caenorhabditis elegans engulfment protein CED-6 Curr. Biol. 9: 1347–1350
Article CAS PubMed Google Scholar - Wu YC, Horvitz HR . 1998 The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters Cell 93: 951–960
Article CAS PubMed Google Scholar - Wu YC, Horvitz HRC . 1998 C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK 180 [see comments] Nature 392: 501–504
Article CAS PubMed Google Scholar - Reddien PW, Horvitz HR . 2000 CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans Nat. Cell. Biol. 2: 131–136
Article CAS PubMed Google Scholar - Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G . 2000 ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine Nat. Cell. Biol. 2: 399–406
Article CAS PubMed Google Scholar - Marguet D, Luciani MF, Moynault A, Williamson P, Chimini G . 1999 Engulfment of apoptotic cells involves the redistribution of membrane phosphatidylserine on phagocyte and prey Nat. Cell. Biol. 1: 454–456
Article CAS PubMed Google Scholar - Luciani MF, Chimini G . 1996 The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death EMBO J. 15: 226–235
Article CAS PubMed PubMed Central Google Scholar - Su HP, Brugnera E, Van Criekinge W, Smits E, Hengartner M, Bogaert T, Ravichandran KS . 2000 Identification and characterization of a dimerization domain in CED-6, an adapter protein involved in engulfment of apoptotic cells J. Biol. Chem. 275: 9542–9549
Article CAS PubMed Google Scholar - Smits E, Van Criekinge W, Plaetinck G, Bogaert T . 1999 The human homologue of Caenorhabditis elegans CED-6 specifically promotes phagocytosis of apoptotic cells Curr. Biol. 9: 1351–1354
Article CAS PubMed Google Scholar - Albert ML, Kim JI, Birge RB . 2000 alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells Nat. Cell. Biol. 2: 899–905
Article CAS PubMed Google Scholar - Liu QA, Hengartner MO . 1999 The molecular mechanism of programmed cell death in C. elegans Ann. NY Acad. Sci. 887: 92–104
Article CAS PubMed Google Scholar - Driscoll M . 1996 Cell death in C. elegans: molecular insights into mechanisms conserved between nematodes and mammals Brain Pathol. 6: 411–425
Article CAS PubMed Google Scholar - Ellis RE, Jacobson DM, Horvitz HR . 1991 Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans Genetics 129: 79–94
CAS PubMed PubMed Central Google Scholar - Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA . 1996 Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells Immunity 4: 431–443
Article CAS PubMed Google Scholar - Franc NC, Heitzler P, Ezekowitz RA, White K . 1999 Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila Science 284: 1991–1994
Article CAS PubMed Google Scholar - Zhou Z, Hartwieg E, Hrovitz HR . 2001 CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans Cell 104: 43–56
Article CAS PubMed Google Scholar
Author information
Authors and Affiliations
- Department of Pediatrics, Program in Cell Biology, National Jewish Medical and Research Center, Denver, CO 80206, Colorado, USA
V A Fadok & P Henson - Molecular, Cellular, and Developmental Biology, University of Colorado, Boulder, Colorado, CO, USA
D Xue
Authors
- V A Fadok
- D Xue
- P Henson
Corresponding author
Correspondence toV A Fadok.
Additional information
Edited by M Piacentini
Rights and permissions
About this article
Cite this article
Fadok, V., Xue, D. & Henson, P. If phosphatidylserine is the death knell, a new phosphatidylserine-specific receptor is the bellringer.Cell Death Differ 8, 582–587 (2001). https://doi.org/10.1038/sj.cdd.4400856
- Received: 25 October 2000
- Revised: 08 February 2001
- Accepted: 19 February 2001
- Published: 10 July 2001
- Issue Date: 01 June 2001
- DOI: https://doi.org/10.1038/sj.cdd.4400856