Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy (original) (raw)
- Letter
- Published: 08 November 2009
- Hagit Katzov-Eckert1,2 na1,
- Marie-Pierre Dubé3,
- Beth Brooks4,
- S Rod Rassekh5,
- Amina Barhdadi3,
- Yassamin Feroz-Zada3,
- Henk Visscher1,2,
- Andrew M K Brown3,6,
- Michael J Rieder7,
- Paul C Rogers5,
- Michael S Phillips3,6,
- Bruce C Carleton2,8,9,
- Michael R Hayden1,2 &
- the CPNDS Consortium
Nature Genetics volume 41, pages 1345–1349 (2009)Cite this article
- 4565 Accesses
- 233 Citations
- 27 Altmetric
- Metrics details
A Corrigendum to this article was published on 26 April 2013
This article has been updated
Abstract
Cisplatin is a widely used and effective chemotherapeutic agent, although its use is restricted by the high incidence of irreversible ototoxicity associated with it1. In children, cisplatin ototoxicity is a serious and pervasive problem, affecting more than 60% of those receiving cisplatin2,3,4,5 and compromising language and cognitive development. Candidate gene studies have previously reported associations of cisplatin ototoxicity with genetic variants in the genes encoding glutathione S-transferases and megalin6,7,8. We report association analyses for 220 drug-metabolism genes in genetic susceptibility to cisplatin-induced hearing loss in children. We genotyped 1,949 SNPs in these candidate genes in an initial cohort of 54 children treated in pediatric oncology units, with replication in a second cohort of 112 children recruited through a national surveillance network for adverse drug reactions in Canada. We identified genetic variants in TPMT (rs12201199, P value = 0.00022, OR = 17.0, 95% CI 2.3–125.9) and COMT (rs9332377, P value = 0.00018, OR = 5.5, 95% CI 1.9–15.9) associated with cisplatin-induced hearing loss in children.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
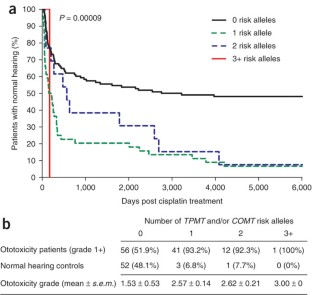
Figure 1: Cisplatin ototoxity and number of risk alleles.
Similar content being viewed by others
Change history
21 March 2013
In the version of this article initially published, the units for treatment duration in Table 1 were incorrectly given as weeks rather than months. This error has been corrected in the HTML and PDF versions of the article.
References
- Brock, P. & Bellman, S. Ototoxicity of cisplatinum. Br. J. Cancer 63, 159–160 (1991).
Article CAS Google Scholar - Li, Y., Womer, R.B. & Silber, J.H. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur. J. Cancer 40, 2445–2451 (2004).
Article CAS Google Scholar - Coradini, P.P., Cigana, L., Selistre, S.G., Rosito, L.S. & Brunetto, A.L. Ototoxicity from cisplatin therapy in childhood cancer. J. Pediatr. Hematol. Oncol. 29, 355–360 (2007).
Article CAS Google Scholar - Knight, K.R., Kraemer, D.F. & Neuwelt, E.A. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J. Clin. Oncol. 23, 8588–8596 (2005).
Article Google Scholar - Kushner, B.H., Budnick, A., Kramer, K., Modak, S. & Cheung, N.K. Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer 107, 417–422 (2006).
Article CAS Google Scholar - Oldenburg, J., Kraggerud, S.M., Cvancarova, M., Lothe, R.A. & Fossa, S.D. Cisplatin-induced long-term hearing impairment is associated with specific glutathione S-transferase genotypes in testicular cancer survivors. J. Clin. Oncol. 25, 708–714 (2007).
Article CAS Google Scholar - Peters, U. et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anti-Cancer Drugs. Anticancer Drugs 11, 639–643 (2000).
Article CAS Google Scholar - Riedemann, L. et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 8, 23–28 (2008).
Article CAS Google Scholar - Blakley, B.W., Gupta, A.K., Myers, S.F. & Schwan, S. Risk factors for ototoxicity due to cisplatin. Arch. Otolaryngol. Head Neck Surg. 120, 541–546 (1994).
Article CAS Google Scholar - Bess, F.H., Dodd-Murphy, J. & Parker, R.A. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 19, 339–354 (1998).
Article CAS Google Scholar - Ekborn, A. et al. Cisplatin-induced hearing loss: influence of the mode of drug administration in the guinea pig. Hear. Res. 140, 38–44 (2000).
Article CAS Google Scholar - Hirschhorn, J.N. & Daly, M.J. Genome-wide association studies for common diseases and complex traits. Nat. Rev. Genet. 6, 95–108 (2005).
Article CAS Google Scholar - Ujiie, S., Sasaki, T., Mizugaki, M., Ishikawa, M. & Hiratsuka, M. Functional characterization of 23 allelic variants of thiopurine S-methyltransferase gene (TPMT*2 - *24). Pharmacogenet. Genomics 18, 887–893 (2008).
Article CAS Google Scholar - Diatchenko, L. et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 14, 135–143 (2005).
Article CAS Google Scholar - Nackley, A.G. et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science 314, 1930–1933 (2006).
Article CAS Google Scholar - Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Article CAS Google Scholar - Visscher, H. et al. Application of principal component analysis to pharmacogenomic studies in Canada. Pharmacogenomics J. advance online publication, doi:10.1038/tpj.2009.36 (4 August 2009).
Article CAS Google Scholar - The International HapMap Consortium. A haplotype map of the human genome. Nature 437, 1299–1320 (2005).
- Shord, S.S., Thompson, D.M., Krempl, G.A. & Hanigan, M.H. Effect of concurrent medications on cisplatin-induced nephrotoxicity in patients with head and neck cancer. Anticancer Drugs 17, 207–215 (2006).
Article CAS Google Scholar - Krynetski, E.Y., Krynetskaia, N.F., Yanishevski, Y. & Evans, W.E. Methylation of mercaptopurine, thioguanine, and their nucleotide metabolites by heterologously expressed human thiopurine S-methyltransferase. Mol. Pharmacol. 47, 1141–1147 (1995).
CAS PubMed Google Scholar - Yates, C.R. et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann. Intern. Med. 126, 608–614 (1997).
Article CAS Google Scholar - Relling, M.V. et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J. Natl. Cancer Inst. 91, 2001–2008 (1999).
Article CAS Google Scholar - Weinshilboum, R.M. Pharmacogenomics: catechol O-methyltransferase to thiopurine S-methyltransferase. Cell. Mol. Neurobiol. 26, 539–561 (2006).
Article CAS Google Scholar - Weinshilboum, R.M. & Sladek, S.L. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am. J. Hum. Genet. 32, 651–662 (1980).
CAS PubMed PubMed Central Google Scholar - Ochoa, B., Bobadilla, N., Arrellin, G. & Herrera, L.A. S-Adenosyl-L-methionine increases serum BUN and creatinine in cisplatin-treated mice. Arch. Med. Res. 40, 54–58 (2009).
Article CAS Google Scholar - Ahmed, Z.M. et al. Mutations of LRTOMT, a fusion gene with alternative reading frames, cause nonsyndromic deafness in humans. Nat. Genet. 40, 1335–1340 (2008).
Article CAS Google Scholar - Du, X. et al. A catechol-O-methyltransferase that is essential for auditory function in mice and humans. Proc. Natl. Acad. Sci. USA 105, 14609–14614 (2008).
Article CAS Google Scholar - Nelson, M.R. et al. Genome-wide approaches to identify pharmacogenetic contributions to adverse drug reactions. Pharmacogenomics J. 9, 23–33 (2008).
Article Google Scholar - Link, E. et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N. Engl. J. Med. 359, 789–799 (2008).
Article CAS Google Scholar - Ross, C.J. et al. Genotypic approaches to therapy in children: a national active surveillance network (GATC) to study the pharmacogenomics of severe adverse drug reactions in children. Ann. NY Acad. Sci. 1110, 177–192 (2007).
Article CAS Google Scholar - Carlson, C.S. et al. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am. J. Hum. Genet. 74, 106–120 (2004).
Article CAS Google Scholar - Guo, S.W. & Thompson, E.A. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 48, 361–372 (1992).
Article CAS Google Scholar - Gao, X., Starmer, J. & Martin, E.R. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet. Epidemiol. 32, 361–369 (2008).
Article Google Scholar
Acknowledgements
We especially want to acknowledge the study participants and their families for their participation in the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) network; this work could not have been done without your help and support. We also want to acknowledge the support of the CPNDS active ADR surveillance consortium, as well as S. Langlois and B. Casey (Supplementary Note). The study was funded by Genome Canada, and additional funding was also provided by Genome British Columbia, Child and Family Research Institute, University of British Columbia Faculty of Pharmaceutical Sciences, Canadian Institutes of Health Research, Canada Foundation for Innovation, Canada Gene Cure Foundation, Canadian Society of Clinical Pharmacology, BC Clinical Genomics Network, C17 Research Network and Childhood Cancer Foundation–Candlelighters Canada, Michael Smith Foundation for Health Research, Health Canada, Pfizer, Eli Lilly, Merck Frosst and Janssen-Ortho. This work was funded as part of the peer-reviewed Genome Canada Applied Health Research Program; the pharmaceutical industry partners had no formal or informal role in this program of research.
Author information
Author notes
- Colin J D Ross and Hagit Katzov-Eckert: These authors contributed equally to this work.
Authors and Affiliations
- Department of Medical Genetics, University of British Columbia, Centre for Molecular Medicine and Therapeutics, Vancouver, British Columbia, Canada
Colin J D Ross, Hagit Katzov-Eckert, Henk Visscher & Michael R Hayden - Child and Family Research Institute, Children's and Women's Health Research Centre of British Columbia, Vancouver, British Columbia, Canada
Colin J D Ross, Hagit Katzov-Eckert, Henk Visscher, Bruce C Carleton & Michael R Hayden - Montreal Heart Institute and Université de Montreal, Montreal, Quebec, Canada
Marie-Pierre Dubé, Amina Barhdadi, Yassamin Feroz-Zada, Andrew M K Brown & Michael S Phillips - Audiology and Speech Pathology Department British Columbia Children's Hospital, Vancouver, British Columbia, Canada
Beth Brooks - Department of Pediatrics, Division of Pediatric Hematology/Oncology/Bone and Marrow Transplant, British Columbia Children's Hospital, Vancouver, British Columbia, Canada
S Rod Rassekh & Paul C Rogers - Montreal Heart Institute and Genome Québec Pharmacogenomics Centre, Montreal, Quebec, Canada
Andrew M K Brown & Michael S Phillips - Department of Paediatrics, Children's Hospital at the London Health Sciences Centre, London, UK
Michael J Rieder - Faculty of Pharmaceutical Sciences University of British Columbia, Vancouver, British Columbia, Canada
Bruce C Carleton - Department of Paediatrics, Pharmaceutical Outcomes Programme, University of British Columbia, Vancouver, British Columbia, Canada
Bruce C Carleton
Authors
- Colin J D Ross
You can also search for this author inPubMed Google Scholar - Hagit Katzov-Eckert
You can also search for this author inPubMed Google Scholar - Marie-Pierre Dubé
You can also search for this author inPubMed Google Scholar - Beth Brooks
You can also search for this author inPubMed Google Scholar - S Rod Rassekh
You can also search for this author inPubMed Google Scholar - Amina Barhdadi
You can also search for this author inPubMed Google Scholar - Yassamin Feroz-Zada
You can also search for this author inPubMed Google Scholar - Henk Visscher
You can also search for this author inPubMed Google Scholar - Andrew M K Brown
You can also search for this author inPubMed Google Scholar - Michael J Rieder
You can also search for this author inPubMed Google Scholar - Paul C Rogers
You can also search for this author inPubMed Google Scholar - Michael S Phillips
You can also search for this author inPubMed Google Scholar - Bruce C Carleton
You can also search for this author inPubMed Google Scholar - Michael R Hayden
You can also search for this author inPubMed Google Scholar
Consortia
the CPNDS Consortium
Contributions
B.C.C., S.R.R., M.J.R., P.C.R. and members of the CPNDS consortium recruited subject cohorts. B.B., B.C.C. and S.R.R. phenotyped subject cohorts. C.J.D.R., H.K.-E., H.V., A.M.K.B. and M.S.P. designed and performed genotyping studies. C.J.D.R., H.K.-E., H.V., A.B., Y.F.-Z. and M.-P.D. analyzed genotyping data. B.C.C. and M.R.H. planned and coordinated the study. All authors contributed to the final version of the manuscript.
Corresponding author
Correspondence toMichael R Hayden.
Additional information
A full list of members is provided in the Supplementary Note.
Supplementary information
Rights and permissions
About this article
Cite this article
Ross, C., Katzov-Eckert, H., Dubé, MP. et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy.Nat Genet 41, 1345–1349 (2009). https://doi.org/10.1038/ng.478
- Received: 15 May 2009
- Accepted: 01 October 2009
- Published: 08 November 2009
- Issue Date: December 2009
- DOI: https://doi.org/10.1038/ng.478