Inactivation of the dnaK gene in Clostridium difficile 630 Δerm yields a temperature-sensitive phenotype and increases biofilm-forming ability (original) (raw)
Introduction
Clostridium difficile is recognised as the most common cause of infectious antibiotic-associated bacterial diarrhoea in healthcare settings worldwide1. During dysbiosis in the gut, C. difficile infects human colonic epithelial cells, whereupon its toxins disrupt epithelial cell ultrastructure and thus the integrity of the gut epithelial barrier2,3. Symptoms include mild, self-limiting diarrhoea, cramping and low-grade fever (up to 40.6 °C); however, untreated C. difficile infection (CDI) can be life threatening4. Treatment generally comprises oral administration of antibiotics such as metronidazole, vancomycin or the recently introduced fidaxomycin5,6.
Cases of CDI have been exacerbated by the recent emergence of new, hypervirulent strains of the organism, and are associated with more severe infections, higher recurrence rates and higher mortality7. Antibiotic resistance plays an important role in driving these epidemiological changes, but despite extensive characterisation of the organism’s pathogenesis8,9,10,11, and its epidemiology and global spread12,13,14,15,16, the survival strategy of C. difficile in the challenging gut environment still remains incompletely understood17,18.
Genomic investigations have shown that, worldwide, a variety of lineages of C. difficile exist with differences in genome content16,19,20. Post-genomic comparative approaches have subsequently provided insights into genes, pathways and metabolic processes modulated under clinically relevant in vitro culture conditions (e.g. heat, antibiotics, oxygen, pH) or in in vivo models of CDI21,22,23,24,25,26,27,28. However, the precise function of clostridial genes has been difficult to determine considering the lack of genetic manipulation tools. Since the 1990s, techniques including physical and chemical mutagenesis29, homologous recombination, antisense RNA, mobile group II introns (ClosTron) and more recently, CRISPR-Cas9 genome editing tools have been deployed for ever more precise genome editing in clostridia30,31. The ClosTron, developed by Heap et al.32,33, utilises a retargeted mobile group II intron to allow targeted, permanent gene disruptions and the introduction of an erythromycin resistance gene, ermB, that enables positive selection of mutants. ClosTron disruption mutants generated for a variety of genes involved in infection, virulence and primary metabolism have allowed insights into their individual roles and their influence on the global physiology of the cell. The reader is referred to Kuehne and Minton34 for a comprehensive summary of the ClosTron technology, intron design procedures and mutant nomenclature.We previously demonstrated up-regulation of class I heat shock genes in C. difficile strain 630 in response to mild, clinically relevant heat-stress ranging from 37 °C to 41 °C26,27,28. Class I heat shock genes are members of the heat-inducible HrcA regulon and chaperone proteins encoded by the groESL and dnaJK–GrpE operons play pivotal roles in refolding denatured cellular proteins under stressful conditions such as pH (acid/alkali), O2 or antibiotic stresses21,26,27,28. To dissect the C. difficile heat-stress response in detail, we utilised ClosTron to attempt to create knockout mutants of the class I molecular chaperones dnaK and groEL, in addition to their negative transcriptional regulator hrcA.
Results
We previously reported on the effects of clinically relevant heat-stress on the proteome and transcriptome of C. difficile strain 630, showing that a 4 °C temperature upshift (37–41 °C) resulted in a classical heat-stress response characterised by the up-regulation of various class I and III chaperones and cell-surface adhesins in addition to increased expression of Fe-only hydrogenases. A decrease in expression was noted for peptidyl prolyl cis-trans isomerases, tcdA, various cellular transport systems and certain motility-associated genes, including the flagellar gene fliC 26,27,28. In the current work, we hypothesised that disruption of key cellular chaperones would lead to pleiotropic changes in the physiology of C. difficile.
ClosTron mutant construction
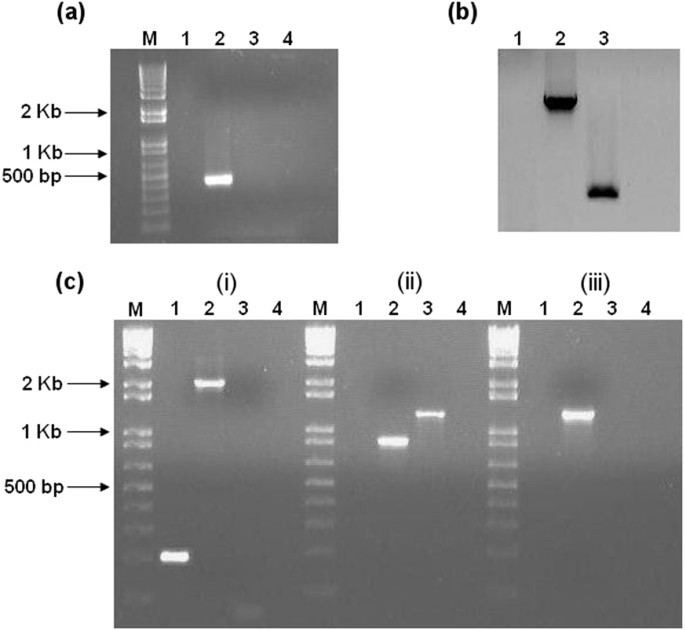
For the dnaK gene (target site 722|723a; score 6.925), PCR screening of erythromycin-resistant colonies confirmed the generation of a ClosTron knockout mutant (Fig. 1a). Southern blot analysis (Fig. 1b) using an intron-specific probe for ErmRAM further verified the existence of a single copy of the insertion element. As expected, the probe did not hybridise to genomic DNA from the Δ_erm_ strain, but hybridised as a single band to genomic DNA from the dnaK mutant strain, thus confirming the insertion of the group II intron into the desired target gene. The insertion site was verified by sequencing across intron–exon junctions (Supplementary Data S1), and confirmatory PCR of the ErmRAM region was also performed (Fig. 1c). In the case of groEL (target site 600|601 s; score 8.766) and hrcA (target site 285|286 s; score 7.971), genomic DNA from >100 erythromycin-resistant clones was PCR screened. Despite this, no gene-specific disruptions could be identified. Another intron insertion site was then chosen for groEL (target site 688|689a; score 6.18) and hrcA (target site 199|200a; score 4.19), and plasmids were ordered from DNA2.0, as per Heap et al.33. Despite multiple attempts and intensive PCR screening, it was not possible to isolate verifiable disruption mutants of either groEL or hrcA in C. difficile using the ClosTron system.
Figure 1
Validation of C. difficile 630 Δ_erm::dnaK_ 723a mutant by PCR screening and Southern blotting. Lanes for each gel/experiment were loaded with PCR products as follows: M, 1 kb Plus DNA ladder (Invitrogen); Lane 1, C. difficile 630 Δ_erm_; Lane 2, dnaK mutant; Lane 3, pMTL007C-E2 plasmid DNA; Lane 4; negative control (water). (a) PCR across the intron-exon junction using EBS universal and Cdi-_dnaK_-R primers generated a 428 bp product from dnaK mutant (lane 2) showing presence of the intron; (b) Southern blot analysis to confirm single genomic insertion of the intron: An intron-specific probe for the ErmRAM was hybridised to Hin_dIII-digested: genomic DNA extracted from C. difficile 630 Δ_erm (Lane 1), pMTL007C-E2 plasmid DNA (Lane 2, positive control), and genomic DNA from the dnaK mutant (Lane 3). (c) Additional confirmatory PCR: (i) PCR using Cdi-_dnaK_-F and Cdi-dnaK_-R primers generated a 210 bp product from C. difficile 630 Δ_erm (lane 1), whereas the dnaK mutant produced a 2059 bp product, indicating the insertion of the group II intron (lane 2); (ii) PCR using ErmRAM-F and ErmRAM-R primers generated a 900 bp product from the dnaK mutant (lane 2) indicative of splicing out of the td group I intron, whereas unmodified pMTL007C-E2 template generated a 1300 bp product (lane 3), (iii) PCR across the other intron-exon junction using ErmRAM-R and Cdi-dnaK_-F primers generated a 1300 bp product from the dnaK mutant only (lane 2). These experiments confirm insertion of the group II intron into the C. difficile 630 Δ_erm chromosome at the desired site and in the correct orientation, resulting in dnaK inactivation.
Other researchers have attributed the inability to recover ClosTron mutants to functional inefficiency of group II introns35; however, it is known that the integration frequency of retargeted introns varies over several orders of magnitude, and sometimes the integration frequency is too low to detect32. Whether HrcA and GroEL are essential in _C. difficile—_as reported for certain other bacteria36—remains unclear, but further attempts to isolate groEL or hrcA mutants were not pursued.
Growth characteristics of the dnaK mutant
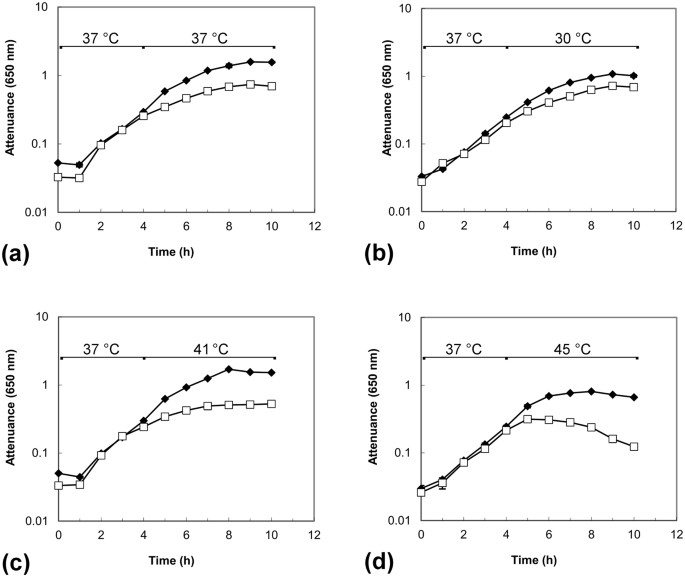
Having isolated and verified the construction of C. difficile strain 630 dnaK::Ll.ltrB-erm (hereafter, the dnaK mutant), we investigated phenotypic changes compared to the Δ_erm_ strain, initially considering growth rates and temperature sensitivity in BHIS broth. When grown at 37 °C (Fig. 2a), the dnaK mutant exhibited a temperature-sensitive phenotype, growing more slowly, and to a lower final attenuance, than C. difficile 630 Δ_erm_. In further experiments, cells were grown to early exponential phase (D650nm~0.3) at 37 °C, followed by transfer to 30 °C, 41 °C, or 45 °C. Upon transfer to 30 °C, both C. difficile 630 Δ_erm_ and the dnaK mutant grew in a comparable manner (Fig. 2b). Raw attenuance data, with associated standard error of the mean values for these experiments can be found online in Supplementary Data S2. We previously determined using C. difficile strain 630 that there was no substantial difference in either growth rate or biomass production when the growth temperature was shifted from 37 °C to 41 °C26, indicating a certain robustness of this strain to temperature upshift. In the current work, however, we observed a considerable difference in the growth rate of the dnaK mutant as compared to the parental Δ_erm_ strain following the induction of heat stress (Fig. 2c,d). This altered growth behaviour and thermosensitivity of the dnaK mutant could be interpreted as a direct consequence of dnaK inactivation.
Figure 2
Growth of C. difficile 630Δ_erm_ (◆) and C. difficile 630 Δ_erm::dnaK_ 723a mutant (□) in BHIS broth at different temperatures. Temperature shifts were induced at early exponential phase, 4 h. (a) When grown at 37 °C, the dnaK mutant exhibited a temperature-sensitive phenotype, growing more slowly than C. difficile 630 Δ_erm_. (b) Cells grown to early exponential phase at 37 °C and then transferred to 30 °C grew in a comparable manner. Cells grown to early exponential phase at 37 °C were challenged by transfer to temperatures of (c) 41 °C and (d) 45 °C, respectively, where temperature sensitivity of the dnaK mutant was more pronounced. D650nm values are plotted on a logarithmic scale and are averages of D650nm measurements from biological triplicate cultures; error bars represent the standard error of mean.
Disruption of dnaK results in impaired motility due to a _FliC_-deficient phenotype
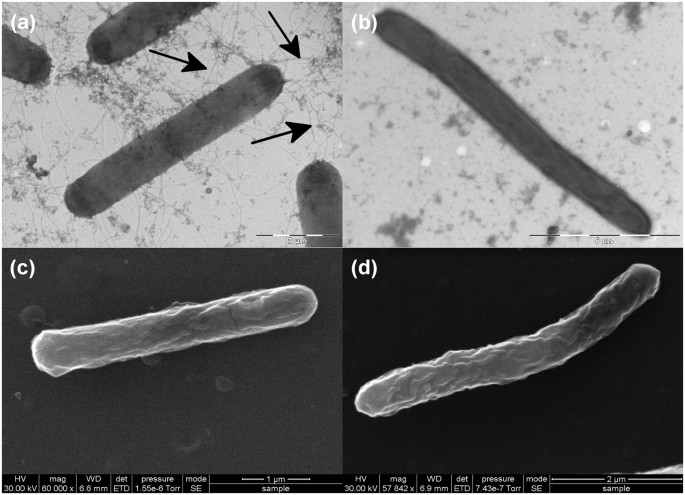
The sensitivity of the dnaK mutant to elevated temperatures led to the hypothesis that a defect in DnaK function places the cells in a ‘heat-stress’ mode. This, we posited, would lead to a similar physiological response—including down regulation of fliC_—to that observed in our earlier heat-stress experiments, where the expression of the gene encoding fliC was down-regulated. Thus, we assessed cellular motility by the method of Tasteyre et al.37 by stab inoculating C. difficile strains into motility agar tubes (in three replicates) and assessing growth following anaerobic incubation at 37 °C for 48 h. The parental C. difficile 630 Δ_erm strain displayed a diffuse spreading pattern, with clear evidence of growth away from the inoculum stab, indicative of a motile phenotype (Fig. 3a). In contrast, the dnaK mutant (Fig. 3b) failed to produce the spreading pattern typical of motile organisms37,38,39. We hypothesised that this lowered motility could be due to the reduced expression of fliC or the lack of flagella on the dnaK mutant cell surface. This hypothesis was tested using both transmission and scanning electron microscopy (TEM and SEM, respectively) on cells grown at 37 °C. TEM with negative staining using phosphotungstic acid revealed that the parental C. difficile 630 Δ_erm_ cells were peritrichously flagellated (Fig. 4a), while dnaK mutant cells did not have any visible flagella (Fig. 4b). This observation validated our hypothesis that the reduced motility of the dnaK mutant is attributable to the loss of the structural flagella machinery. TEM images indicated that dnaK disruption also resulted in a filamentous phenotype in the mutant (Fig. 4b), an observation further investigated using SEM, which clearly showed that cells of the dnaK mutant (Fig. 4d) were longer than those of the Δ_erm_ strain [mutant cells, 9.04 ± 1.42 µm in length; wild-type cells, 6.72 ± 1.28 µm in length; 12 cells of each strain were measured]. In general, both wild-type and mutant cells also appeared slightly wrinkled, a phenomenon that can be attributed to the acetone dehydration step during the critical-point drying (CPD) process prior to SEM.
Figure 3
Motility of C. difficile strains in BHIS agar (0.175%). (a) C. difficile 630 Δ_erm_, (b) dnaK mutant. Motility was visualised as a diffuse spreading pattern from the point of stab inoculation.
Figure 4
Electron microscopic analysis of C. difficile 630 Δ_erm_ and C. difficile 630 Δ_erm_::dnaK 723a mutant. (a) Transmission electron microscopy image of C. difficile 630 Δ_erm_. (b) Transmission electron microscopy image of dnaK mutant. Arrows indicate flagellar filaments. (c) Scanning electron microscopy image of C. difficile 630 Δ_erm_. (d) Scanning electron microscopy image of dnaK mutant. The images depict the filamentous phenotype of the dnaK mutant in comparison to the wild-type.
Chaperone genes and fliC are differentially expressed in the dnaK mutant
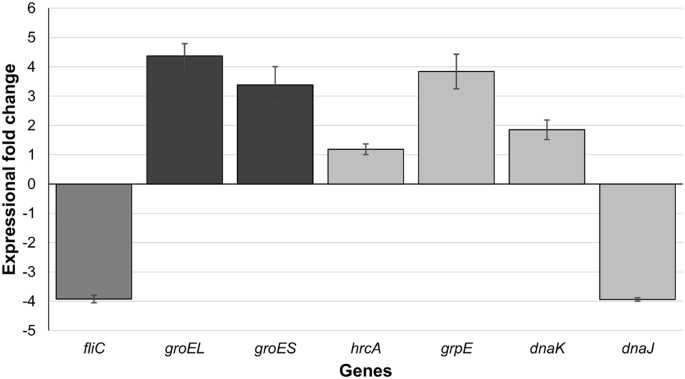
The lack of both motility and observable flagella, combined with the temperature sensitivity exhibited by the dnaK mutant, led us to the hypothesis that genes associated with these processes would be altered in the mutant. If, as we hypothesised, the dnaK mutant was in the ‘heat-stress mode’, then it was to be expected that expression of other chaperones would be increased as well. We also hypothesised that the lack of observable flagella could be underpinned by a decrease in fliC expression. Independent biological duplicate cultures of C. difficile 630 Δ_erm_ and the dnaK mutant were grown at 37 °C and total RNA was isolated from cells harvested at the late-log phase, reverse transcribed to cDNA and the relative expression of chaperone genes and fliC was analysed with tpi as reference (see Supplementary Data S3 for ratios). Expression of groEL, groES, and grpE was increased in the dnaK mutant, whereas dnaJ expression decreased by more than 4-fold (Fig. 5). This is in broad accordance with what has been reported by other researchers, where increases in transcription of heat shock genes, compared to wild-type strains, were observed in dnaK null mutants grown at optimal growth temp eratures40,41,42. In addition, expression of fliC was 4-fold lower in the dnaK mutant, confirming that lower level of fliC transcript, as opposed to some defect in either translation or in the export of FliC monomers, was the primary reason for lack of flagellar motility.
Figure 5
Expressional changes in class 1 chaperone genes and the flagellar filament gene, fliC, in the C. difficile 630 Δ_erm_::dnaK 723a mutant. RNA was extracted and reverse transcribed from biological duplicate cultures and cDNA was quantified in technical triplicate qPCR reactions. The ‘calibrator normalised relative quantification including efficiency correction’ experimental mode assessed gene expression using the tpi gene, whose expression did not change, as a reference. Bars represent average fold-changes in gene expression in the dnaK mutant compared with the Δ_erm_ parental strain. Error bars represent standard deviation of the mean.
The dnaK mutant exhibits an increased biofilm-forming phenotype
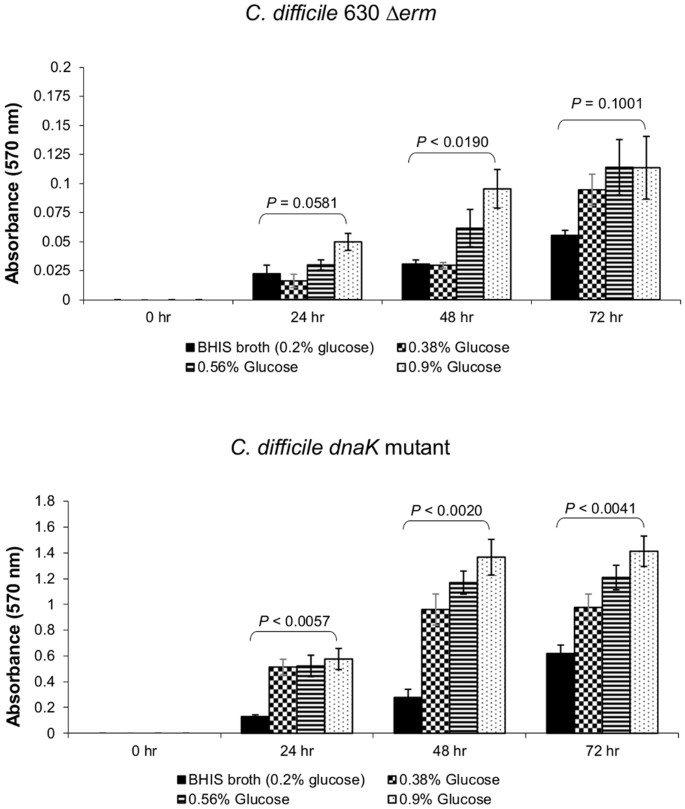
In many bacteria, including Pseudomonas aeruginosa, both flagella and type IV pili contribute to motility and biofilm-forming ability43. We therefore assessed the ability of C. difficile strains to form biofilms using a modified version of the method of O’Toole and Kolter44, with the expectation that the flagella-deficient dnaK mutant would exhibit reduced biofilm-forming ability. To assess biofilm development, assays were performed in 96-well polystyrene microtiter plates (Orange Scientific, Alpha Technologies, UK) with measurements at 24, 48 and 72 h (see Supplementary Data S4). We observed that the C. difficile 630 Δ_erm_ strain formed weak biofilms (A 570 < 0.5, per the classification of Varga et al.45) (Fig. 6). In contrast, the dnaK mutant formed moderate to strong biofilms, and this ability was significantly enhanced at 24 h (p = 0.0057), 48 h (p = 0.0020) and 72 h (p = 0.0041) by the addition of 0.9% glucose to the BHIS broth (Fig. 6). By contrast, the effect of 0.9% glucose addition on biofilm production by C. difficile 630 Δ_erm_ was significant only at 48 h (p = 0.0190), although biofilm biomass was increased at 24 h and 72 h compared to the BHIS control.
Figure 6
Biofilm-forming ability of C. difficile 630 Δ_erm_ and C. difficile 630 Δ_erm_::dnaK 723a mutant. Biofilm assays were performed in biological triplicates, each with 6 independent technical replicates. Strains were classified as strong- (A 570 > 1), moderate- (A 570 = 0.5−1), or weak- (A 570 < 0.5) biofilm producers45. P values represent statistical comparison (Student’s _t_-test, 2 tailed) between BHIS broth and BHIS broth with 0.9% (w/v) additional glucose.
Discussion
Here we report for the first time the successful construction, validation and phenotypic characterisation of a C. difficile dnaK disruption mutant using ClosTron. Our observations of impaired growth rates and lowered temperature stress tolerance with the dnaK mutant correspond with those of Selby et al.46 who made dnaK and hrcA mutants in C. botulinum. The C. difficile dnaK mutant had a lower growth rate and produced less biomass at temperatures between 30 °C and 45 °C (Fig. 2) and in addition was also less able to tolerate heat stress (Fig. 2), emphasising the importance of the DnaK chaperone system in protein folding, especially in relation to core cellular housekeeping functions. The results obtained using these two clostridial strains correspond with observations in other bacteria. In Escherichia coli, dnaK mutants reportedly grow more slowly and exhibit lower viability than the wild-type, exhibiting severe defects in DNA and RNA synthesis that account for the inhibited growth and reduced viability47,48,49. Accordingly, we noted that following lethal stress (2 min at 64 °C) and plating on BHIS agar, no C. difficile dnaK mutant cells were recovered, suggesting that dnaK mutation is deleterious to sporulation. We subsequently verified the presence of spores in cultures of the C. difficile dnaK mutant, but nonetheless, this markedly altered thermotolerance further emphasises the requirement for a functional DnaK chaperone in C. difficile physiology. Heat sensitivity has also been reported in Lactococcus lactis 41 and Staphylococcus aureus 50 dnaK mutants, yet in Bacillus subtilis, dnaK mutants were thermotolerant and could grow up to 52 °C, in addition to exhibiting a filamentous morphology51. Our motility experiments (Fig. 3) showed that the C. difficile dnaK mutant was less motile than the parental Δ_erm_ strain. Electron microscopy revealed for the first time in C. difficile that the C. difficile dnaK mutant had no flagella (Fig. 4b) and that the mutant cells were approximately 50% longer than the parental Δ_erm_ cells (Fig. 4d). This filamentous phenotype, reported in several other bacterial dnaK mutants31,35,52, could be due to either the accumulation of mutant DnaK proteins52 or some as yet unidentified defect in the expression/function of FtsZ, a highly conserved prokaryotic cytoskeleton protein which is the first protein to localise to the site of bacterial cell division and in turn defines the plane of cell division53. Sugimoto et al.54 reported that an E. coli dnaK deletion mutant displayed a filamentous morphology that was attributable to increased GrpE abundance which in turn interfered with DnaK chaperone system functionality: the imbalance resulted in defective cell division mediated via abnormal FtsZ localisation. The C. difficile dnaK mutant exhibited 3- to 4-fold increases in the expression of all class I heat shock genes (Fig. 5), with the exception of dnaJ, the expressions of which was 4-fold lower. We therefore indicate that intracellular concentrations of the molecular chaperones encoded by the dnaK operon may directly influence the activity and localisation of FtsZ (encoded by CD2646) in C. difficile. The reduction in dnaJ expression in the dnaK mutant is similar to that observed in E. coli 49 and could be due to either differential transcription or transcriptional attenuation. Our previous work reported differential expression of dnaK operon transcripts and proteins in C. difficile cells26,27,28. In B. subtilis, both transcriptional and post-transcriptional controls adjust cellular quantities of proteins derived from the dnaK operon, and a strategy of differential segmental mRNA stability is in place to fine-tune the expression of individual dnaK operon genes. The B. subtilis dnaK operon transcripts contain stemloop structures that act as rho-independent transcription terminators with one of these predicted upstream of dnaJ, and there is also a vegetative promoter just upstream of dnaJ in both Bacillus and Clostridium that is not regulated by heat stress55,56. Consequently, dnaK disruption may have wider effects on C. difficile transcription factors or mRNA processing. The possibility of dnaK disruption also giving rise to a polar effect on dnaJ cannot be discounted. While the dnaK ORF is disrupted and thus must be non-functional, a polar effect on the expression of neighbouring genes–including _dnaJ_–could be an alternative explanation for the observed decrease in dnaJ expression. In Salmonella enterica Serovar Typhimurium an insertion in the dnaK gene led to depletion of both DnaK and DnaJ57 and thus it could be argued that there may be a dnaJ polar effect component to the observed phenotype in C. difficile. The C. difficile dnaK mutant exhibited 3- to 4-fold increased expression of the groESL operon at 37 °C. A number of possibilities could explain this. In E. coli, the DnaK chaperone system is involved in the negative regulation of heat shock response by controlling the synthesis and stability of σ32, the positive regulator42. The absence of a functional DnaK protein leads to σ32 overproduction and thus E. coli dnaK mutants exhibit increased expression of molecular chaperones even at optimal growth temperatures40. This is what we observed in case of the C. difficile dnaK mutant. However, the regulation of heat shock response in Gram-positive microorganisms such as B. subtilis is much more complex, as multiple classes of heat shock genes have been identified58. Transcription of class I heat-inducible genes encoded by the groE and dnaK operon genes is negatively regulated by the HrcA repressor protein in conjunction with the CIRCE element, a palindromic sequence present in the promoter region of these operons59,60. In B. subtilis, GroEL is required for the stabilisation of HrcA, which in turn binds to the CIRCE element, blocking the transcription of class I heat-inducible genes at normal growth temperatures61. During stress, accumulation of unfolded proteins sequesters the activity of GroEL, causing inactivation of HrcA and allowing active transcription of the groE and dnaK operons. Thus, dnaK inactivation in B. subtilis does not result in an abnormal expression of class I heat shock proteins51,62. If the same mode of regulation holds for C. difficile, then dnaK disruption would not be expected to result in the overexpression of the groESL/dnaK operons. The C. botulinum hrcA mutant46 was reported to overexpress all six class I heat shock genes, as would be expected. Our observation that expression of the groESL operon was 4-fold higher in the C. difficile dnaK mutant suggests that in this organism, DnaK, rather than GroES/GroEL, might have a role to play in the stabilisation of HrcA and thus in the correct regulation of class I heat shock operons. There is clearly considerable diversity in the regulation networks and physiological roles of dnaK in different organisms, and regardless of the reasons for altered gene expression, the temperature-sensitive phenotype of the C. difficile dnaK mutant suggests that the protein folding defect resulting from dnaK disruption is only partially restored by subsequent increases in GroEL/GroES.
In C. difficile, the flagellum is an accessory virulence factor that promotes adherence to colonic epithelial cells at a level 10-fold higher than that of non-flagellated strains37,38,63. In the current work, the C. difficile dnaK mutant strain was non-motile, lacked surface flagella and fliC mRNA expression was 4-fold lower than that in the parental Δ_erm_ strain. These observations are consistent with those reported for B. subtilis 51 and E. coli 64 where dnaK inactivation also resulted in a non-motile phenotype. It could be hypothesised that non-flagellated C. difficile cells would adhere weakly and thus be less virulent. We used a biofilm assay model to test adherence in vitro and showed that the dnaK mutant formed much more biofilm than the parental Δ_erm_ strain (Fig. 6). This observation initially appears to be at odds with the literature consensus, that cell surface structure-driven motility is a vital factor in biofilm formation43,44,45,65,66. However, Hennequin et al.67 demonstrated that upon exposure of C. difficile to heat-shock conditions, GroEL was released into the extracytoplasmic space and became cell-surface adsorbed. They also provided evidence that in C. difficile, GroEL plays a role in adhesion, and further proposed that GroEL was, by default, associated with the membrane because of its chaperone activities67. The increased GroEL expression in the C. difficile dnaK mutant and this protein’s known role as an adhesin in C. difficile and also in other organisms such as Salmonella typhimurium 68, Helicobacter pylori69 and Lactobacillus johnsonii 70 suggest that increased GroEL expression is at least one of the factors responsible for the increased adherence and biofilm formation by the dnaK mutant.
To summarise, this paper reports for the first time the construction and characterisation of a ClosTron dnaK mutant in C. difficile. Our phenotypic characterisation clearly demonstrates that while DnaK is not essential for the viability of the organism, defects in DnaK functionality lead to altered expression of class I heat shock and motility genes, perturbations to the cell surface and adhesion and considerable disruption of global cellular physiology and homeostasis.
Materials and Methods
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this work are listed in Table 1. C. difficile strains were anaerobically grown on BHIS agar or broth, as previously described71. For heat-stress experiments, liquid cultures growing at 37 °C were transferred to a recirculating 41 °C water-bath set at the appropriate temperature, as per Jain et al.26. C. difficile 630 Δ_erm_ 72 was employed to allow selection of ClosTron mutants, E. coli TOP10 was used as the cloning host and E. coli CA43473 strain was the donor for conjugative transfer of plasmids to C. difficile 630 Δ_erm_.
Table 1 Strains/Plasmids used in this work.
ClosTron mutagenesis procedure
C. difficile 630 Δ_erm_ 72 was used and potential intron target sites in genes of interest were identified using the intron design tool available at http://clostron.com. Intron fragments for target genes were amplified by splicing overlap of extension (SOE) PCR, Hin_dIII and Bsr_GI digested and then ligated into the ClosTron plasmid pMTL007C-E233. The construct was electroporated into E. coli TOP10 and transformants selected on LB agar with 25 µg/ml chloramphenicol and 8 µg/ml Xgal. The PCR product-derived portion of the retargeted plasmid was verified by sequencing with cspfdx-seq-F1 and pMTL007-R1 primers (Table 2). Retargeted, sequence-verified ClosTron plasmids for dnaK, groEL and hrcA were retransformed into electrocompetent E. coli CA434 cells, and the transformation mixtures were then used to inoculate 5 ml of sterile LB broth supplemented with 12.5 µg/ml chloramphenicol. Following overnight incubation (37 °C, 200 rpm), cells from 1 ml of culture were collected by centrifugation at 4 °C, washed in 0.5 ml sterile PBS and resuspended in 200 µl of an overnight culture of C. difficile 630 Δ_erm. The entire conjugation mixture was pipetted onto fresh BHIS agar as a discrete drop and plates were anaerobically incubated for 8–10 h at 37 °C to allow conjugal transfer of the retargeted pMTL007C-E2 plasmid from E. coli CA434 to C. difficile 630 Δ_erm.
Table 2 Oligonucleotides used in this work.
Following incubation, the mating mixture was recovered from the conjugation plates using a sterile loop and resuspended in 1 ml sterile PBS. To counter-select against E. coli, 200 µl of this conjugation slurry was spread onto fresh BHIS agar supplemented with C. difficile selective supplement (250 µg/ml of D-cycloserine and 8 µg/ml of cefoxitin; Oxoid), in addition to 15 µg/ml thiamphenicol to select for the retargeted pMTL007C-E2 plasmid. Following anaerobic incubation at 37 °C for 24–72 h, single, isolated, thiamphenicol-resistant colonies were re-streaked and grown on the same medium for a further 24 h. Integrants were then isolated by resuspending thiamphenicol-resistant colonies in 300 µl sterile PBS and plating (100 µl neat and 100 µl of 10-fold diluted) onto BHIS agar supplemented with 10 µg/ml of erythromycin to select for the presence of the spliced erythromycin retrotransposition-activated selectable marker (ErmRAM). Following anaerobic incubation at 37 °C for 24–72 h, erythromycin-resistant integrant colonies were re-streaked on the same medium for 24 h to ensure purity. A few integrant colonies were also replica plated onto BHIS-thiamphenicol (15 µg/ml) agar to screen for plasmid loss by the thiamphenicol-sensitive phenotype. Several erythromycin-resistant, thiamphenicol-sensitive clones were subsequently used to inoculate 1 ml of sterile BHIS broth, and cultures were incubated anaerobically at 37 °C overnight.
Screening PCR
To confirm the generation of ClosTron mutants, the correct position of the intron in C. difficile mutant genomes was verified by PCR to determine whether the retargeted plasmid was present, whether the ClosTron had integrated and, more importantly, whether integration had taken place into the desired target gene in the C. difficile 630 Δ_erm_ genome. Initial screening of mutants involved forward and reverse primers for the respective genes (Table 2) designed to yield ~200 bp amplicons from wild-type dnaK, groEL and hrcA genes and a larger ~2 kbp amplicon from ClosTron mutants due to intron insertion in these genes. Additional PCRs across the intron–exon junctions and to demonstrate a spliced—and therefore integrated—RAM were also performed. Screening PCRs used genomic DNA of wild-type C. difficile 630 Δ_erm_ as a positive control, retargeted plasmid DNA to demonstrate a full-length RAM, or genomic DNA from mutant clones; PCR products were purified from agarose gels and sequenced to verify that the intron had indeed integrated into the correct target site (Supplementary Data S1).
Southern blotting
Southern hybridisations used an intron-specific probe for the ermB marker to confirm integration of the group II intron into the desired target gene of mutants. The Digoxigenin (DIG) High Prime DNA Labelling and Detection Starter Kit II (Roche Diagnostics, Hertfordshire, UK) was used as per manufacturer instructions. A 900 bp probe for the ermB marker was created using ErmRAM-F and -R primers, and genomic DNA template from the dnaK mutant strain; 1 µg of this was labelled with DIG High Prime and a 20 µl aliquot hybridised to _Hin_dIII-digested DNA (500 ng) of C. difficile strains on a nylon membrane at 42 °C overnight. Anti-DIG antibody conjugated to alkaline phosphatase in combination with chemiluminescent substrate for alkaline phosphatase (CSPD) was used to develop the blot with exposure on a sheet of an X-ray film (Amersham Hyperfilm™ ECL, GE Healthcare, UK) for 5–10 min at room temperature, and subsequent development in a Kodak X-OMAT 1000 automated processor.
Motility assays
Motility assays were performed as previously described by Tasteyre et al.37 Briefly, fresh BHIS broth with 0.175% technical agar No III (Oxoid) was placed in sterile glass test-tubes which were then stab inoculated with a clean toothpick using an actively growing colony of the respective strain. The tubes were incubated under strict anaerobic conditions at 37 °C for 48 h, following which growth of the mutant strain was visually compared to that of the wild-type. Motility assays were performed in three independent biological replicates.
Electron microscopy
All microscopy-related work was performed at Ulster’s FEI Centre for Advanced Bioimaging. For SEM, glass cover slips (10 mm diameter) were cleaned using 10% Decon 90 detergent, rinsed and then soaked in a solution of 2% (v/v) 3-aminopropyltriethoxysilane (APES, Sigma Aldrich) and 100% methanol for 5 s. Following rinsing with 100% methanol and then deionised water, the cover slips were dried overnight at 37 °C. C. difficile cells from overnight cultures were collected by centrifugation (8000 × g) and washed with ice-cold PBS, and a drop of cell suspension was applied to the APES-coated coverslip and incubated at room temperature for 2 h. The liquid was removed and a drop of paraformaldehyde fixative (4% v/v in PBS) was added for 2 h. The fixative was removed and the cover slips were gently rinsed with PBS, followed by critical point drying (Polaron E5000 critical point drier) using acetone as an intermediary fluid. The cover slips were then attached to adhesive carbon pads on aluminium SEM stubs and sputter coated with an Au/Pd target in a Polaron E5100 sputter coater. Cells were visualised in an FEI (FEI, Eindhoven, Netherlands) Quanta™ ESEM under high vacuum at 30 kV using spot size 2 in secondary electron mode using an Everhart–Thornley detector. Images were acquired using the integrated imaging software.
For TEM, a clean glass microscope slide was dipped into a 0.5% (w/v in chloroform) formvar solution for 30 s. Following drying, the film was cut at the edges of the glass and floated on deionised water. A 200-mesh thin-bar copper grid (Agar scientific Ltd., UK) was placed on the floating formvar using forceps, and the film/grid was removed from the water using a Whatman No. 1 paper disc that was then completely dried at room temperature prior to use. Overnight cultures of C. difficile were harvested by centrifugation (3000 × g) and washed with ice-cold PBS. Formvar-coated grids were floated on a 50 µl drop of PBS-washed C. difficile cell suspension for 2 min, following which the excess cell suspension was wicked off the grids. The cells were then stained by floating the grid on a drop of 0.25% (w/v) phosphotungstic acid for 1 min prior to visualisation in an FEI Tecnai 12 transmission electron microscope (FEI, Eindhoven, Netherlands) using a lanthanum hexaboride (LaB6) source. The instrument was operated at 120 kV using spot size 1 and images acquired with a Megaview III camera and analySIS® image capture software (Soft Imaging Systems GmbH, Műnster, Germany).
RNA isolation and qRT-PCR
RNA was extracted from late-log phase cultures, quality checked by agarose gel electrophoresis and ‘minus RT’-PCR with tpi primers, reverse transcribed using gene-specific primers (Table 3) and gene expression analysis was then performed as previously described26,27,28. The Relative Quantification (RelQuant) software (Roche Diagnostics) was used to assess gene expression levels, as per manufacturer instructions, and target gene expression was reported relative to the expression of the triose phosphate isomerase gene tpi (CD3172) in C. difficile 630 Δ_erm_.
Table 3 PCR primers.
Biofilm Assays
Biofilm formation assays were performed using a modified version of the method of O’Toole and Kolter44. Working anaerobically, overnight cultures of C. difficile 630Δ_erm_ and of the dnaK mutant strain were diluted 100–f old into sterile BHIS broth, as well as into BHIS broths supplemented with 1%, 2% and 5% of 1 M filter-sterilised glucose solution (final concentrations of 0.38%, 0.56%, and 0.9%, respectively). Subsequently, 100 µl aliquots of each diluted culture was added to wells of a 96-well flat-bottom polystyrene microtitre plate (Orange Scientific, Alpha Technologies Ltd., UK) in 6 replicates. Replicates of uninoculated BHIS broth were included as the negative controls and to prevent evaporation, microtitre plates were incubated anaerobically at 37 °C with lids in place in a humidified plastic lunchbox–type container. Biofilm biomass was quantified at 24 h intervals for up to 3 days. Outside the cabinet, planktonic cells were washed from the wells using sterile deionised water and the plates air-dried for 30 min at room temperature. A 125 µl aliquot of 0.1% crystal violet solution (filter sterilised, in deionised water) was added to each well and allowed to stain the attached bacterial biomass for 15 min. The excess crystal violet was removed and the wells washed twice with deionised water, following which the plates were allowed to air dry at room temperature overnight. The next day, 200 µl of 95% ethanol was added to each well and the dye allowed to solubilise for 15 min. The crystal violet/ethanol solution in each well was mixed briefly using gentle pipetting and a 125 µl aliquot transferred to a separate 96-well microtitre plate to allow measurement of absorbance at 570 nm (A 570) using a FLUOstar Omega microplate reader (BMG LabTech, UK). Background due to nonspecific staining by crystal violet was accounted for by subtracting the average values obtained from the wells containing the uninoculated negative controls.
Equipment and settings
PCR gels were imaged under UV light in an AlphaImager™ 2200 (Alpha Innotech, CA, US) equipped with a 1.4 megapixel camera with 12 bit A/D, using default AlphaView Image Analysis Software settings, and exported in jpg format. The jpg images were imported into the GNU Image Manipulation Program (GiMP) 2.8 for generation of Fig. 1. Growth curve graphs shown in Fig. 2 were produced using MS Excel, individually exported in PDF and imported into GiMP 2.8 for construction and final labelling. Motility agar tubes were photographed using a Nikon D3 camera, 60 mm Nikkor macro lens, shutter speed 1/200 sec, aperture f/6.3, ISO 640, lit with studio flash and the resultant jpg images imported into GiMP 2.8 for construction and labelling of Fig. 3. Electron microscope images (tiff format) were imported into GiMP 2.8 for construction and labelling of Fig. 4. Gene expression data was used to construct a bar chart in MS Excel, prior to chart export in PDF; this was imported into GiMP 2.8 for final labelling of Fig. 5. Biofilm assay data was used to construct bar charts in MS Excel. The charts were individually exported in PDF and imported into GiMP for construction of Fig. 6. No alterations to brightness or contrast were made to any of the images during figure construction.
Data availability
All data generated or analysed during this study are included in this published article (and in Supplementary Information files).
References
- Rupnik, M., Wilcox, M. H. & Gerding, D. N. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7, 526–536 (2009).
Article CAS PubMed Google Scholar - Johanesen, P. A. et al. Disruption of the gut microbiome: Clostridium difficile infection and the threat of antibiotic resistance. Genes 6, 1347–1360 (2015).
Article CAS PubMed PubMed Central Google Scholar - Carter, G. P. et al. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio. 6, e00551–15 (2015).
Article CAS PubMed PubMed Central Google Scholar - Bartlett, J. G. Historical perspectives on studies of Clostridium difficile and C. difficile infection. Clin. Infect. Dis. 46, S4–11 (2008).
Article PubMed Google Scholar - Freeman, J. et al. Pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes’ study group. Pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes. Clin. Microbiol. Infect. 21, 248.e9–248.e16 (2015).
Article CAS Google Scholar - Kociolek, L. K. & Gerding, D. N. Breakthroughs in the treatment and prevention of Clostridium difficileinfection. Nat. Rev. Gastroenterol. Hepatol. 13, 150–160 (2016).
Article CAS PubMed Google Scholar - Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther. Adv. Infect. Dis. 3, 23–42 (2016).
Article CAS PubMed PubMed Central Google Scholar - Saujet, L., Monot, M., Dupuy, B., Soutourina, O. & Martin-Verstraete, I. The key sigma factor of transition phase, SigH, controls sporulation, metabolism, and virulence factor expression in Clostridium difficile. J. Bacteriol. 193, 3186–3196 (2011).
Article CAS PubMed PubMed Central Google Scholar - Hutton, M. L., Mackin, K. E., Chakravorty, A. & Lyras, D. Small animal models for the study of Clostridium difficile disease pathogenesis. FEMS Microbiol. Lett. 352, 140–149 (2014).
Article CAS PubMed Google Scholar - Bouillaut, L., Dubois, T., Sonenshein, A. L. & Dupuy, B. Integration of metabolism and virulence in Clostridium difficile. Res. Microbiol. 166, 375–383 (2015).
Article CAS PubMed Google Scholar - Sun, X. & Hirota, S. A. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol. Immunol. 63, 193–202 (2015).
Article CAS PubMed Google Scholar - Stabler, R. A. et al. Comparative phylogenomics of Clostridium difficile reveals clade specificity and microevolution of hypervirulent strains. J. Bacteriol. 188, 7297–7305 (2006).
Article CAS PubMed PubMed Central Google Scholar - Stabler, R. A. et al. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10, R102 (2009).
Article PubMed PubMed Central Google Scholar - Stabler, R. A. et al. Macro and micro diversity of Clostridium difficile isolates from diverse sources and geographical locations. PLOS ONE 7, e31559 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar - He, M. et al. Evolutionary dynamics of Clostridium difficile over short and long time scales. Proc. Natl. Acad. Sci. USA 107, 7527–7532 (2010).
Article ADS CAS PubMed PubMed Central Google Scholar - He, M. et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 45, 109–113 (2013).
Article CAS PubMed Google Scholar - Cartman, S. T., Heap, J. T., Kuehne, S. A., Cockayne, A. & Minton, N. P. The emergence of ‘hypervirulence’ In Clostridium difficile. Int. J. Med. Microbiol. 300, 387–395 (2010).
- Crowther, G. S. et al. Recurrence of dual-strain Clostridium difficile infection in an in vitro human gut model. J. Antimicrob. Chemother. 70, 2316–2321 (2015).
Article CAS PubMed Google Scholar - Sebaihia, M. et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38, 779–786 (2006).
Article PubMed Google Scholar - Forgetta, V. et al. Fourteen-genome comparison identifies DNA markers for severe-disease-associated strains of Clostridium difficile. J. Clin. Microbiol. 49, 2230–2238 (2011).
Article CAS PubMed PubMed Central Google Scholar - Emerson, J. E., Stabler, R. A., Wren, B. W. & Fairweather, N. F. Microarray analysis of the transcriptional responses of Clostridium difficile to environmental and antibiotic stress. J. Med. Microbiol. 57, 757–764 (2008).
Article CAS PubMed Google Scholar - Scaria, J. et al. Clostridium difficile transcriptome analysis using pig ligated loop model reveals modulation of pathways not modulated in vitro. J. Infect. Dis. 203, 1613–1620 (2011).
Article CAS PubMed PubMed Central Google Scholar - Scaria, J. et al. Differential stress transcriptome landscape of historic and recently emerged hypervirulent strains of Clostridium difficile strains determined using RNA-seq. PLOS ONE. 8, e78489 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar - Chen, J. W. et al. Proteomic comparison of historic and recently emerged hypervirulent Clostridium difficile strains. J. Proteome. Res. 12, 1151–1161 (2013).
Article PubMed Google Scholar - Janoir, C. et al. Adaptive strategies and pathogenesis of Clostridium difficile from in vivo transcriptomics. Infect. Immun. 81, 3757–3569 (2013).
Article CAS PubMed PubMed Central Google Scholar - Jain, S., Graham, C., Graham, R. L., McMullan, G. & Ternan, N. G. Quantitative proteomic analysis of the heat stress response in Clostridium difficile strain 630. J. Proteome. Res. 10, 3880–3890 (2011).
Article CAS PubMed Google Scholar - Ternan, N. G., Jain, S., Srivastava, M. & McMullan, G. Comparative transcriptional analysis of clinically relevant heat stress response in Clostridium difficile strain 630. PLOS ONE. 7, e42410 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar - Ternan, N. G., Jain, S., Graham, R. L. J. & McMullan, G. Semiquantitative analysis of clinical heat stress in Clostridium difficile strain 630 using a GeLC/MS workflow with emPAI quantitation. PLOS ONE. 9, e88960 (2014).
Article ADS PubMed PubMed Central Google Scholar - Xue, C., Zhao, X.-Q., Liu, C.-G., Chen, L.-J. & Bai, F.-W. Prospective and development of butanol as an advanced biofuel. Biotechnol.Adv. 31, 1575–1584 (2013).
Article CAS PubMed Google Scholar - Xue, C., Zhao, J., Chen, L., Yang, S.-T. & Bai, F. Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum. Biotechnol. Adv. 35, 310–322 (2017).
Article CAS PubMed Google Scholar - Li, Q. et al. CRISPR-based genome editing and expression control systems in Clostridium acetobutylicum and Clostridium beijerinckii. Biotechnol. J. 11, 961–972 (2016).
Article CAS PubMed Google Scholar - Heap, J. T., Pennington, O. J., Cartman, S. T., Carter, G. P. & Minton, N. P. The ClosTron: A universal gene knock-out system for the genus. Clostridium. J. Microbiol. Meth. 70, 452–464 (2007).
Article CAS Google Scholar - Heap, J. T. et al. The ClosTron: Mutagenesis in Clostridium refined and streamlined. J. Microbiol. Meth. 80, 49–55 (2010).
Article CAS Google Scholar - Kuehne, S. A. & Minton, N. P. ClosTron-mediated engineering of Clostridium. Bioengineered. 3, 247–254 (2012).
Article PubMed PubMed Central Google Scholar - Underwood, S. et al. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J. Bacteriol. 191, 7296–7305 (2009).
Article CAS PubMed PubMed Central Google Scholar - Susin, M. F., Baldini, R. L., Gueiros-Filho, F. & Gomes, S. L. GroES/GroEL and DnaK/DnaJ have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus. J. Bacteriol. 88, 8044–8053 (2006).
Article Google Scholar - Tasteyre, A., Barc, M. C., Collignon, A., Boureau, H. & Karjalainen, T. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 69, 7937–7940 (2001).
Article CAS PubMed PubMed Central Google Scholar - Tasteyre, A. et al. A Clostridium difficile gene encoding flagellin. Microbiology 146, 957–966 (2000).
Article CAS PubMed Google Scholar - Twine, S. M. et al. Motility and flagellar glycosylation in Clostridium difficile. J. Bacteriol. 191, 7050–7062 (2000).
Article Google Scholar - Straus, D., Walter, W. & Gross, C. A. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of sigma 32. Genes Dev. 4, 2202–2209 (1990).
Article CAS PubMed Google Scholar - Koch, B., Kilstrup., M., Vogensen, F. K. & Hammer, K. Induced levels of heat shock proteins in a dnaK mutant of Lactococcus lactis. J. Bacteriol. 180, 3873–3881 (1998).
CAS PubMed PubMed Central Google Scholar - Zhao, K., Liu, M. & Burgess, R. R. The global transcriptional response of Escherichia coli to induced sigma 32 protein involves sigma 32 regulon activation followed by inactivation and degradation of sigma 32 in vivo. J. Biol. Chem. 280, 17758–17768 (2005).
Article CAS PubMed Google Scholar - Deligianni, E. et al. Pseudomonas aeruginosa cystic fibrosis isolates of similar RAPD genotype exhibit diversity in biofilm forming ability in vitro. BMC Microbiol. 10, 38 (2010).
Article PubMed PubMed Central Google Scholar - O’Toole, G. A. & Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28, 449–461 (1998).
Article PubMed Google Scholar - Varga, J. J., Therit, B. & Melville, S. B. Type IV pili and the CcpA protein are needed for maximal biofilm formation by the Gram-positive anaerobic pathogen Clostridium perfringens. Infect. Immun. 76, 4944–4951 (2008).
Article CAS PubMed PubMed Central Google Scholar - Selby, K. et al. Important role of class I heat shock genes hrcA and dnaK in the heat shock response and the response to pH and NaCl stress of group I Clostridium botulinum strain ATCC 3502. Appl. Environ. Microbiol. 77, 2823–2830 (2011).
Article CAS PubMed PubMed Central Google Scholar - Itikawa, H. & Ryu, J. Isolation and characterization of a temperature-sensitive dnaK mutant of Escherichia coli B. J. Bacteriol. 138, 339–344 (1979).
CAS PubMed PubMed Central Google Scholar - Paek, K. H. & Walker, G. C. Escherichia coli dnaK null mutants are inviable at high temperature. J. Bacteriol. 169, 283–290 (1987).
Article CAS PubMed PubMed Central Google Scholar - Bukau, B. & Walker, G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J. Bacteriol. 171, 2337–2346 (1989).
Article CAS PubMed PubMed Central Google Scholar - Singh, V. K. et al. Role for dnaK locus in tolerance of multiple stresses in Staphylococcus aureus. Microbiology 153, 3162–3173 (2007).
Article CAS PubMed Google Scholar - Schulz, A., Tzschaschel, B. & Schumann, W. Isolation and analysis of mutants of the dnaK operon of Bacillus subtilis. Mol. Microbiol. 15, 421–429 (1995).
Article CAS PubMed Google Scholar - McCarty, J. S. & Walker, G. C. DnaK mutants defective in ATPase activity are defective in negative regulation of the heat shock response: expression of mutant DnaK proteins results in filamentation. J. Bacteriol. 176, 764–780 (1994).
Article CAS PubMed PubMed Central Google Scholar - Margolin, W. FtsZ and the division of prokaryotic cells and organelles. Nat. Rev. Mol. Cell Biol. 6, 862–871 (2005).
Article CAS PubMed PubMed Central Google Scholar - Sugimoto, S., Saruwatari, K., Higashi, C. & Sonomoto, K. The proper ratio of GrpE to DnaK is important for protein quality control by the DnaK-DnaJ-GrpE chaperone system and for cell division. Microbiology 154, 1876–1885 (1990).
Article Google Scholar - Homuth, G., Masuda, S., Mogk, A., Kobayashi, Y. & Schumann, W. The dnaK operon of Bacillus subtilis is heptacistronic. J. Bacteriol. 179, 1153–1164 (1997).
Article CAS PubMed PubMed Central Google Scholar - Homuth, G., Mogk, A. & Schumann, W. Post-transcriptional regulation of the Bacillus subtilis dnaK operon. Mol. Microbiol. 32, 1183–1197 (1999).
Article CAS PubMed Google Scholar - Takaya, A., Tomoyasu, T., Matsui, H. & Yamamoto, T. The DnaK/DnaJ chaperone machinery of Salmonella enterica serovar Typhimurium is essential for invasion of epithelial cells and survival within macrophages, leading to systemic infection. Inf. Immunity. 72, 1364–1373 (2004).
Article CAS Google Scholar - Hecker, M., Pané-Farré, J. & Völker, U. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61, 215–236 (2007).
Article CAS PubMed Google Scholar - Narberhaus, F. Negative regulation of bacterial heat shock genes. Mol. Microbiol. 31, 1–8 (1999).
Article CAS PubMed Google Scholar - Helmann, J. D. et al. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183, 7318–7328 (2001).
Article CAS PubMed PubMed Central Google Scholar - Mogk, A. et al. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 16, 4579–4590 (1997).
Article CAS PubMed PubMed Central Google Scholar - Schulz, A. & Schumann, W. hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat shock genes. J. Bacteriol. 178, 1088–1093 (1996).
Article CAS PubMed PubMed Central Google Scholar - Poutanen, S. M. & Simor., A. E. _Clostridium difficile_-associated diarrhea in adults. CMAJ 171, 51–58 (2004).
Article PubMed PubMed Central Google Scholar - Shi, W., Zhou, Y., Wild, J., Adler, J. & Gross, C. A. DnaK, DnaJ, and GrpE are required for flagellum synthesis in. Escherichia coli. J. Bacteriol. 174, 6256–6263 (1992).
CAS PubMed Google Scholar - Hossain, M. M. & Tsuyumu, S. Flagella-mediated motility is required for biofilm formation by Erwinia carotovora subsp. carotovora. J. Gen. Plant Pathol. 72, 34–39 (2006).
Article CAS Google Scholar - Houry, A., Briandet, R., Aymerich, S. & Gohar, M. Involvement of motility and flagella in Bacillus cereus biofilm formation. Microbiology 56, 1009–1018 (2010).
Article Google Scholar - Hennequin, C. F. et al. GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology 147, 87–96 (2001).
Article CAS PubMed Google Scholar - Ensgraber, M. & Loos, M. A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect. Immun. 60, 3072–3078 (1992).
CAS PubMed PubMed Central Google Scholar - Phadnis, S. H. et al. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64, 905–912 (1996).
CAS PubMed PubMed Central Google Scholar - Bergonzelli, G. et al. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect. Immun. 74, 425–434 (2006).
Article CAS PubMed PubMed Central Google Scholar - Jain, S., Graham, R. L., McMullan, G. & Ternan, N. G. Proteomic analysis of the insoluble subproteome of Clostridium difficile strain 630. FEMS Microbiol. Lett. 312, 151–159 (2010).
Article CAS PubMed Google Scholar - Hussain, H. A., Roberts, A. P. & Mullany, P. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Deltaerm) and demonstration that the conjugative transposon Tn916DeltaE enters the genome of this strain at multiple sites. J. Med. Microbiol. 54, 137–141 (2005).
Article CAS PubMed Google Scholar - Heap, J. T., Pennington, O. J., Cartman, S. T. & Minton, N. P. A modular system for Clostridium shuttle plasmids. J. Microbiol. Meth. 78, 79–85 (2009).
Article CAS Google Scholar - Lemée, L., Dhalluin, A., Pestel-Caron, M., Lemeland, J. & Pons, J. Multilocus sequence typing analysis of human and animal Clostridium difficile isolates of various toxigenic types. J. Clin. Microbiol. 42, 2609–2617 (2004).
Article PubMed PubMed Central Google Scholar - Lemée, L. et al. Multilocus sequence analysis and comparative evolution of virulence-associated genes and housekeeping genes of Clostridium difficile. Microbiol. 151, 3171–3180 (2005).
Article Google Scholar
Acknowledgements
S.J. was supported by a Vice Chancellor’s Research Scholarship award (2007–2010) for studies leading to the award of Ph.D from the University of Ulster and by a Society for General Microbiology President’s fund grant for a research visit to Nottingham (2009). D.S. was supported by a Department of Employment and Learning (Northern Ireland) Ph. D studentship (2011–2015).
Author information
Authors and Affiliations
- Nutrition Innovation Centre for Food and HEalth (NICHE), School of Biomedical Sciences,University of Ulster, Coleraine, Co. Londonderry, N. Ireland, BT52 1SA, UK
Shailesh Jain, Deborah Smyth, Barry M. G. O’Hagan & Nigel G. Ternan - Centre for Synthetic Biology and Innovation, Department of Life Sciences, Imperial College London, South Kensington Campus, London, SW7 2AZ, UK
John T. Heap - Institute for Global Food Security, School of Biological Sciences, Queen’s University Belfast, N. Ireland, United Kingdom, BT9 7BL, UK
Geoff McMullan - Clostridia Research Group, BBSRC/EPSRC Synthetic Biology Research Centre (SBRC), University of Nottingham, University Park, Nottingham, NG7 2RD, UK
Nigel P. Minton
Authors
- Shailesh Jain
You can also search for this author inPubMed Google Scholar - Deborah Smyth
You can also search for this author inPubMed Google Scholar - Barry M. G. O’Hagan
You can also search for this author inPubMed Google Scholar - John T. Heap
You can also search for this author inPubMed Google Scholar - Geoff McMullan
You can also search for this author inPubMed Google Scholar - Nigel P. Minton
You can also search for this author inPubMed Google Scholar - Nigel G. Ternan
You can also search for this author inPubMed Google Scholar
Contributions
N.G.T., G.M.C.M. and N.P.M. conceived and designed the experiments. S.J., D.S., B.M.G.O.H. and J.T.H. performed the experiments. N.G.T. prepared the figures. N.G.T., G.M., J.T.H. and N.P.M. wrote the paper. All authors reviewed the paper.
Corresponding author
Correspondence toNigel G. Ternan.
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jain, S., Smyth, D., O’Hagan, B.M.G. et al. Inactivation of the dnaK gene in Clostridium difficile 630 Δ_erm_ yields a temperature-sensitive phenotype and increases biofilm-forming ability.Sci Rep 7, 17522 (2017). https://doi.org/10.1038/s41598-017-17583-9
- Received: 01 September 2017
- Accepted: 27 November 2017
- Published: 13 December 2017
- DOI: https://doi.org/10.1038/s41598-017-17583-9