Reactive oxygen species mediate crosstalk between NF-κB and JNK (original) (raw)
Introduction
NF-_κ_B is a collective term used to describe members of the Rel family of dimeric transcription factors.1, 2 The Rel family regulates transcription of a large number of genes that control cell survival and differentiation including various proinflammatory cytokines, chemokines, and adhesion molecules. Many of these same proinflammatory molecules, including cytokines such as tumor necrosis factor (TNF)α and interleukin-1 (IL-1), are able to activate NF-_κ_B, initiating a signaling cascade of activation. NF-_κ_B can also be activated by Toll-like receptors that recognize pathogen-associated molecules or by cellular stress induced following UV or _γ_-irradiation. The recent identification of molecules, which regulate the activation of the NF-κ_B heterodimer, RelA(p65) and p50 has enhanced our understanding of the molecular mechanisms controlling inflammation (Figure 1). Signaling systems induced by a variety of stimuli activate two serine kinases, termed I_κ_B kinase (IKK)α and IKK_β (or IKK1; IKK2), which target the inhibitors of _κ_B (I_κ_B). The subsequent phosphorylation by these kinases leads to eventual ubiquitination and proteasome-dependent degradation of I_κ_B, releasing the latent dimeric transcription factor to the nucleus. A key mechanism by which NF-_κ_B controls cell survival3, 4, 5 is to enhance transcription of various antiapoptotic genes, including cellular FLICE-inhibitory protein (c-FLIP), Bcl-x L , A1 (also known as Bfl-1), and XIAP (X chromosome-liked inhibitor of apoptosis).6, 7
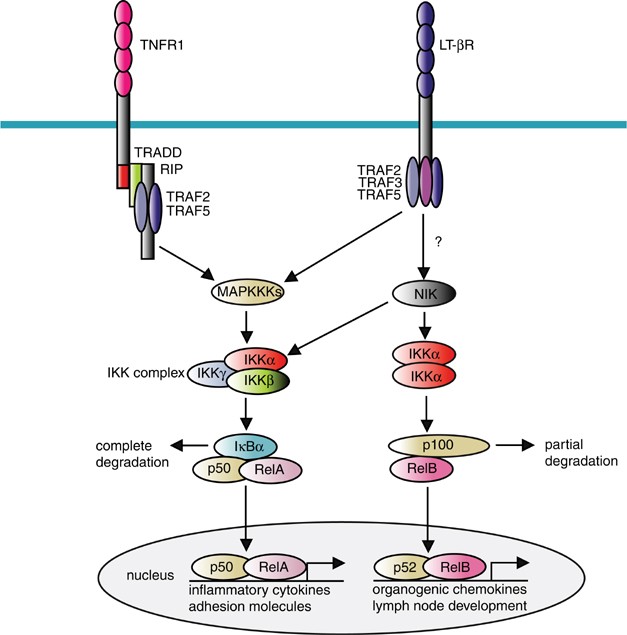
Figure 1
The classical and nonclassical NF-κ_B activation pathways. The classical NF-κ_B pathway is activated by inflammatory cytokines including TNF_α and IL-1. Activation of the classical pathway depends on TRAFs, MAPKKKs including TAK1 and MEKK3, and the IKK complex containing IKK_β and IKK_γ_ subunits. Activation of the IKK complex results in degradation of the inhibitor protein, I_κ_B_α_ and subsequent nuclear translocation of RelA/p50 dimers. The classical pathway mediates coordinate expression of inflammatory cytokines and adhesion molecules. The nonclassical pathway induces nuclear translocation of RelB/p52 dimers, is strictly dependent on IKK_α_ homodimers and is activated by members of the TNF receptor family, such as lymphotoxin-β receptor (LT-_β_R) and CD40 via NF-_κ_B-inducing kinase (NIK). NIK is also involved in activation of the classical pathway by CD27 and CD40, but not TNFR. The nonclassical pathway plays a central role in the expression of genes involved in development and maintenance of secondary lymphoid organs. The roles of TRAFs in activation of the nonclassical pathway remain unclear
Regulation of cell death and survival is also controlled in part by another signaling cascade activated by the mitogen-activated protein kinase (MAPK), which is induced following cellular stress or cytokine signaling.8, 9 In mammals, the MAPK cascades are composed of three distinct signaling modules, the c-Jun N-terminal kinase (JNK) cascade, the p38MAPK cascade, and the extracellular signal-regulated kinase (ERK) cascade. Each MAPK is activated by sequential protein phosphorylation through a MAPK module; for example MAPK kinase kinase (MAPKKK) phosphorylates MAPK kinase (MAPKK), which in turn phosphorylates MAPK (Figure 2). In the case of the JNK cascade, the MAPKKKs include apoptosis-signal regulating kinase (ASK)1, MAP/ERK kinase kinase (MEKK)s, MTK1 (also known as MEKK4), and TGF_β_-activated kinase (TAK)1. These MAPKKKs activate MKK4 and/or MKK7, which then in turn activate JNK, the targets of which include the AP1-related transcription factors, such as c-Jun.8, 9 Cytokines and growth factors including TNF_α_ and IL-1 induce rapid (within 10 min) yet transient activation of MAPK, whereas cellular stresses, such as UV or _γ_-irradiation, induce prolonged MAPK activation. Several lines of evidence suggest that transient MAPK activation is associated with gene expression, proliferation, and differentiation, whereas prolonged MAPK activation promotes cell death, by a mechanism that does not solely involve gene activation, and is cell type- and stimuli-dependent10, 11
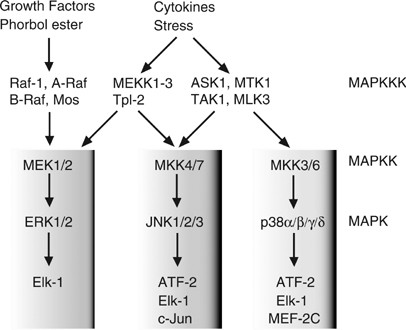
Figure 2
Three MAP kinase modules in mammals. Upon stimulation with growth factors, cytokines, or various inducers of cell stress, MAPKKKs are activated and subsequently phosphorylate MAPKKs, which in turn activate MAPKs. Activated MAPKs subsequently phosphorylate specific transcription factors and activate their transcriptional activity
Pro- and Antiapoptotic Roles of JNK
Although the activation mechanisms of JNK have been extensively investigated, the biological consequence of JNK activation in cell death is still controversial.7, 8, 12, 13 The most convincing evidence that JNK signaling promotes apoptosis comes from the experiments using mice deficient in the JNK activation cascade. In JNK1 and JNK2 double knockout mice, neuronal apoptosis is suppressed in the hindbrain, but increased in the forebrain, indicating that both JNK1 and JNK2 regulate region-specific apoptosis during early brain development.14 Moreover, murine embryonic fibroblasts (MEFs) from JNK1 and JNK2 double knockout mice are resistant to apoptosis induced by genotoxic stress including exposure to anisomycin, methylmethanesulfonate, and UV,15 although a recent study has challenged this conclusion.16 Consistent with these results, primary neurons from both neuron-specific JNK3 isoform knockout mice, and knockin mice expressing the nonphosphorylated form of c-Jun (c-JunAA), are resistant to excitotoxic glutamate-receptor agonist, kainate-induced apoptosis.17, 18 Moreover, MEFs from ASK1 knockout mice exhibit decreased sensitivity to TNF_α_- and H2O2-induced apoptosis.19
Several lines of evidence demonstrate that the proapoptotic JNK cascade ultimately induces apoptosis via the mitochondria-dependent pathway. JNK phosphorylates members of the Bcl-2 family of proteins, such as Bcl-2 and Bcl-xL, and inactivates their antiapoptotic function.20, 21, 22, 23, 24 Moreover, the ectopic expression of constitutively active JNK (using the MKK7-JNK1 fusion protein) efficiently induces apoptosis in wild-type cells, but not cells lacking the proapoptotic Bcl-2 family members, Bax and Bak, which are essential for the mitochondria-dependent apoptotic pathway.25 Furthermore, JNK activates proapoptotic members of the Bcl-2 family, Bim and Bmf, resulting in activation of Bax and Bak.26 Recently, Deng et al.27 revealed an unexpected role of JNK in the induction of the caspase 8-independent cleavage of Bid. Under conditions in which TNF_α_-induced NF-_κ_B activation is blocked, JNK induces caspase 8-independent cleavage of Bid at a different site, resulting in the production of jBid and not the previously described tBid. jBid translocates to the mitochondria leading to the preferential release of Smac (also known as DIABLO). Smac then disrupts a complex consisting of the TNF receptor-associated factor (TRAF)2 and the cellular inhibitors of apoptosis (c-IAPs) complex, resulting in caspase 8 activation and ultimately the induction of apoptosis. Finally, Tsuruta et al.28 have recently reported that JNK phosphorylates the 14-3-3 protein, a cytoplasmic anchor of Bax, and that phosphorylated 14-3-3 fails to sequestrate Bax into the cytoplasm, therefore inhibiting its translocation to the mitochondria.
In contrast to the proapoptotic function of JNK as described above, numerous studies demonstrate an antiapoptotic role for JNK. Nishina et al.29, 30 have illustrated that MKK4 knockout mice die due to massive hepatocyte apoptosis, and that MKK4-deficient T cells exhibit increased sensitivity to anti-Fas and anti-CD3-induced apoptosis, indicating that the JNK pathway mediates survival signals. Furthermore, differentiated embryonic stem (ES) cells lacking MEKK1 showed reduced oxidative stress-induced JNK activation and were more susceptible to apoptosis.31 Moreover, Lamb et al.32 have reported that JNK1 and JNK2 double knockout MEFs show increased sensitivity to TNF_α_-induced cell death, and that this increased sensitivity is due to defective JNK-mediated upregulation of c-IAP2. Finally, Yu et al.33 reported that JNK phosphorylates the Bcl-2 family protein, BAD and inactivates its proapoptotic function. Collectively, these data suggest that under certain experimental conditions JNK can protect cells from apoptosis.
To explain the apparently controversial findings described above, factor(s) other than JNK activation should be taken into account, such as the activation of other signaling cascades branching from the JNK pathway, including NF-_κ_B. Indeed, many stimuli such as TNF-related cytokines, simultaneously activate both JNK and NF-_κ_B pathways, but do not usually induce apoptosis in normal cells. In contrast, genotoxic stress preferentially activates the JNK pathway with marginal activation of NF-_κ_B, and thus apoptosis predominates. Although genotoxic stress induces translocation of NF-_κ_B, the NF-_κ_B complex containing RelA/p50 heterodimer turns out to be transcriptionally inactive.34 Thus, it is reasonable to speculate that molecules that are regulated by NF-_κ_B, could critically affect cell fate induced by the JNK cascade.
NF-_κ_B Downregulates JNK
In the past, the contributions of the NF-κ_B and JNK pathways to cell death have been discussed independently. However, two recent studies have revealed signaling crosstalk between the NF-κ_B and JNK pathways. Tang et al.35 and De Smaele et al.36 have independently demonstrated that TNF_α induces prolonged JNK activation in NF-κ_B activation-deficient cells, such as RelA and IKK_β knockouts, and cells stably expressing degradation-resistant I_κ_B_α. Consistent with previous studies,10, 11 this prolonged JNK activation was found to promotes apoptosis, suggesting that gene(s) are induced by TNF_α_ in an NF-κ_B-dependent fashion normally block JNK activation. Two target genes that they identified which block JNK activation were growth arrest and DNA damage-inducing protein (GADD45)β and XIAP. Given that GADD45_β was known to interact with and activate MTK1/MEKK4, (which triggers the p38 and JNK pathways),37 its inhibitory effect on JNK activation was unexpected. The other molecule identified, XIAP, had previously been shown to inhibit apoptosis by inhibiting activation of caspases by direct binding,38 thus, in this study Tang et al.35 reveal a novel antiapoptotic function of XIAP. Furthermore, Papa et al.39 showed that GADD45_β_ binds to and inhibits the JNK activator, MKK7 through the competitive inhibition of ATP. However, this inhibitory action of GADD45_β_ is cell-type specific, since TNF_α_-induced JNK activation is not prolonged in GADD45_β_ knockout MEFs or splenocytes.40 Collectively, these studies demonstrate a cell-type specific molecular link between NF-_κ_B and JNK.
In Drosophila, there are several counterparts of signaling components in the mammalian NF-_κ_B and MAPK pathways,41 such as DJNK, a homologue of JNK; IMD, a homologue of RIP; TAK1, a MAPKKK that activates JNK; and the NF-_κ_B homologue, Relish (Figure 3). The biological consequences of the JNK pathway (DJNK) in Drosophila are less complicated than in mammals, functioning to preferentially promote apoptosis. Park et al.42 demonstrated that JNK activation is prolonged in S2 cells lacking Relish. Moreover, they showed that Relish activation leads to degradation of TAK1, resulting in termination of JNK signaling. These results indicate that the regulatory crosstalk between the JNK and NF-_κ_B pathways is also conserved in Drosophila. Interestingly, two recent papers have demonstrated that the phosphorylated form of c-Jun is recognized by a specific ubiquitin ligase and is then subsequently degraded by the ubiquitin-proteasome pathway.43, 44 This indicates that apoptotic c-Jun-dependent transcription is negatively regulated by the ubiquitin-proteasome pathway. Although it is currently unknown whether this proteasome-dependent c-Jun degradation pathway is regulated by NF-_κ_B, this system is reminiscent of degradation of TAK1 by Relish in the Drosophila IMD pathway.
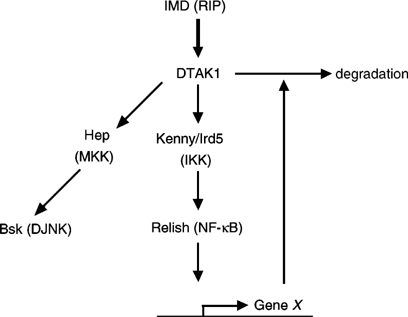
Figure 3
Crosstalk between NF-_κ_B and JNK in the Drosophila IMD pathway. IMD (a homologue of RIP) activates DTAK1, which in turn activates DJNK and Relish (a homologue of NF-_κ_B). Unidentified gene(s) (X) induced by Relish mediate the degradation of DTAK1, limiting the duration of JNK activation
Reactive Oxygen Species – Emerging Mediators of Prolonged JNK Activation
The two studies described above have convincingly demonstrated that NF-_κ_B downregulates JNK activation and have identified candidate molecules that inhibit JNK activation. Nevertheless, the molecular mechanisms underlying the NF-_κ_B-dependent inhibition have still been controversial. Reactive oxygen species (ROS) have emerged as bridging molecules mediating the crosstalk between NF-_κ_B and JNK.45, 46, 47 ROS, including superoxide anions, hydrogen peroxide and hydroxyl radicals, are accidentally generated in the mitochondria during the transport of electrons from the reducing equivalent (NADH-FADH2) to molecular oxygens through a mitochondrial respiratory chain of enzymatic complexes (I–IV) (Figure 4a).48, 49 Under normal physiological conditions, ROS are rapidly eliminated by antioxidant enzymes, including superoxide dismutases (SODs), catalase, glutathione peroxidase (GPx), and peroxiredoxin (PRx) (Figure 4b).48, 49 Dysregulation of electron transport through the mitochondrial respiratory chain or an impairment in the function of antioxidant enzymes results in the accumulation of ROS. In addition to the byproducts of ROS which accumulate in the mitochondria as described above, NADPH oxidase (NOX) enzymes, which are localized in the plasma membrane have also been identified as producers of ROS in various types of cells in response to growth factors, cytokines, or calcium signals.50 Moreover, several enzymes localized in the peroxisomes, cytoplasm, and endoplasmic reticulum may also generate ROS.48, 49, 51 Whether ROS play a central role in cytokine-induced MAPK activation under physiological conditions, however, remains controversial.
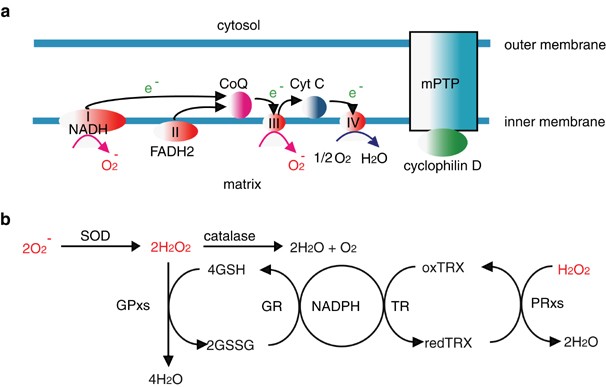
Figure 4
Generation of ROS in the mitochondria and their elimination by cellular antioxidants. (a) The mitochondrial respiratory chain consists of four multimeric complexes (complexes I–IV), coenzyme Q (CoQ), and cytochorome c (Cyt C). Electrons (e−) are transferred from the reducing equivalent (NADH-FADH2) to molecular oxygens through the mitochondrial respiratory chain, finally generating water at complex IV. During the electron transfer, reactive oxygen species (ROS) are accidentally generated at complexes I and III. The mitochondrial permeability transition pore (mPTP) is regulated by cyclophilin D, and opened by high calcium or oxidative stress. Opening of this pore results in massive loss of ions and metabolites from the matrix (see the text). (b) O2− is converted into H2O2 by superoxide dismutases (SODs). H2O2 is then eliminated by catalase, glutathione peroxidases (GPxs), and peroxiredoxins (PRxs). During elimination of H2O2, reduced glutathione (GSH) is converted to disulfide form (GSSG) by GPxs, and then GSSG is recycled to GSH by glutathione reductase (GR). However, PRxs also catalyze H2O2 into H2O by using reduced thioredoxin (TRx). Oxydized TRx is then recycled back to redTRx by thioredoxin reductase (TR). NADPH is essential for both recycling reactions. ROS are indicated using red characters
Previous studies have shown that ROS directly activate various kinases, including ASK1, MEKK1, c-src, EGFR, and PDGFR, which in turn activate the MAPK cascades.52 Consistent with a previous study,19 we and others45, 53, 54, 55 have demonstrated that NF-κ_B downregulates JNK activation by suppressing TNF_α_-induced ROS accumulation. Notably, TNF_α induces early and transient JNK activation in wild-type cells, whereas TNF_α_ induces ROS accumulation leading to prolonged JNK activation in NF-_κ_B activation-deficient cells. In accordance with these findings, prolonged JNK activation is inhibited by pretreatment of cells with antioxidants such as butylated hydroxylanisole (BHA) and _N_-acetyl cysteine (NAC), suggesting that the mechanisms of early/transient and prolonged JNK activation are qualitatively different. Moreover, extended JNK activation is still induced in TRAF2/TRAF5 double KO cells,45 in which TNF_α_-induced early/transient JNK activation is severely impaired.56 Collectively, these results demonstrate that early/transient JNK activation is dependent upon TRAF, whereas prolonged JNK activation is ROS-dependent.
Another important issue is how ROS induce long-lasting JNK activation. A previous study57 has shown that ASK1 responds to ROS and triggers the JNK and p38MAPK, but not ERK cascades. Given that TNF_α_ and arsenic induce both prolonged JNK and ERK activation in RelA KO and IKK_β_ KO MEFs,45, 58 a kinase that activates the ERK cascade, such as MEKK1 may also be involved in this prolonged MAPK activation. In addition, as ROS directly activate JNK, we need to consider the possibility that ROS may inactivate inhibitors, which normally suppress JNK activation, therefore resulting in prolonged JNK activation. In this respect, Kamata et al.55 reported that ROS inactivate MAP kinase phosphatases (MKPs)59 by oxidizing cysteine residues critical for their phosphatase activities. Moreover, oxidized MKPs are rapidly degraded by the ubiquitin-proteasome pathway. Collectively, these data suggest that ROS may utilize two different mechanisms in order to promote persistent JNK activation. ROS may either positively activate MAPKKKs, resulting in JNK activation, and/or inactivate MKPs that would otherwise dephosphorylate and inactivate JNK.
In addition to ROS, we do not formally exclude the possibility that activation of the caspase cascades also contributes to JNK activation. In this respect, several kinases, including MEKK1,60 MST,61 and PAK2,62 have been reported to be cleaved by caspases, resulting in their activation. Under the conditions in which NF-κ_B activation is impaired, TNF_α stimulation induces both caspase-dependent apoptosis and ROS-dependent necrosis. Therefore, activation of the caspase cascades may contribute to JNK activation in a stimuli-dependent fashion.
The Molecular Mechanisms of TNF_α_-Induced ROS Accumulation
Regarding the mechanism whereby TNF_α_ induces ROS accumulation in NF-_κ_B activation-deficient, but not wild-type cells, two possibilities need to be considered. Firstly, impaired induction of antioxidant enzymes or antioxidants that are induced by NF-κ_B under normal conditions might be responsible for ROS accumulation. Indeed, previous studies63, 64 have shown that various antioxidant enzyme genes including manganese-dependent SOD (MnSOD), metallothionein, glutathione S-transferase, and ferritin heavy chain (fhc), are induced by TNF_α in an NF-κ_B-dependent fashion. Pham et al.54 and Kamata et al.55 have also shown that the ectopic expression of fhc and MnSOD inhibits TNF_α_-induced ROS accumulation in RelA KO and IKK_β KO cells, respectively. However, given that complicated and multiple step reactions might be required for efficient elimination of ROS (Figure 5b), it is rather surprising that expression of a single gene, such as fhc or MnSOD is sufficient for ROS elimination. In fact, the inhibitory effect of MnSOD on TNF_α_-induced ROS accumulation is not complete,55 indicating that another molecule or mechanism might also be involved in this process.
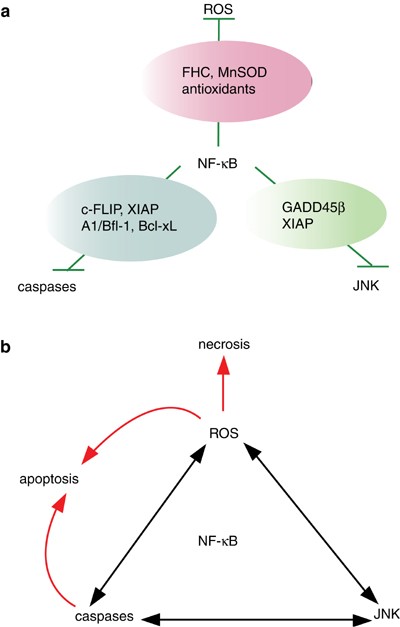
Figure 5
Signaling crosstalk between NF-κ_B and JNK. (a) Activation of NF-κ_B by TNF_α induces expression of GADD45_β and XIAP that downregulates JNK activation. NF-κ_B also induces expression of c-FLIP, Bcl-xL, and A1/Bfl-1 that inhibit caspase activation. Furthermore, NF-κ_B inhibits ROS accumulation by upregulating FHC and MnSOD. Under these conditions, TNF_α induces transient JNK activation and cell survival. (b) In cells unresponsive to NF-κ_B activation induced by TNF_α (TRAF2/TRAF5, RelA, and IKK_β KO cells), JNK activation is prolonged and caspase activation is induced due to the lack of induction of these molecules. Furthermore, ROS also promote JNK activation. Through the coordinate activation of these pathways, TNF_α_ induces apoptosis and necrosis
Another possibility is that latent signaling cascade(s) that are normally suppressed by NF-κ_B, may dominate over its inhibition and therefore induce ROS. Chen et al.58 have reported that TNF_α promotes expression of a member of the p450 family, cyp1b1 that generates ROS in IKK_β_ KO cells. Moreover, several studies have shown that ROS accumulation is induced during apoptotic processes. A recent study65 indicated that activated caspase 3 cleaves the p75 subunit of complex I of the mitochondrial electron transport chain, resulting in ROS accumulation. Similarly, Giorgio et al.66 have shown that proapoptotic signals induce release of p66Shc from a putative inhibitory complex, which in turn oxidizes reduced cytochrome c, thereby generating ROS. On the other hand, Ventura et al.53 have reported that TNF_α_-induced ROS accumulation is abolished in cells lacking JNK1 and JNK2, indicating that a central role for JNK in ROS accumulation. Given that ROS promote JNK activation, they hypothesized that activation of the JNK pathway induces ROS accumulation, which can in turn activate JNK in a positive feedback fashion. However, it remains unclear how JNK induces ROS accumulation. It is reasonable to surmise that the molecular mechanisms underlying TNF_α_-induced ROS accumulation are not due to a single mechanism, but are more likely to be cell-type specific. To determine the subcellular localization of ROS generation, such as the mitochondria, cytoplasm, or plasma membrane might provide valuable information needed to elucidate the mechanisms by which TNF_α_ induces ROS. Given that there is currently no reliable way to determine the subcellular location of ROS generation using oxidation-sensitive dyes, it is crucial to develop such detection systems to better understand the signaling specificity induced by various oxidative stresses as well as the mechanism whereby TNF_α_ induces ROS.
Do ROS Induce Apoptosis or Necrosis?
Under various pathological conditions such as ischemia, excessive amounts of accumulated ROS induce apoptosis or necrosis by activating the MAPK, caspase cascades, and /or by disrupting mitochondrial membrane potential.67 This contribution of ROS to apoptosis and necrosis is highly cell-type specific, and also depends on the amount of endogenously or exogenously generated ROS present. As ROS induce activation of the JNK cascade, apoptosis induced by ROS is likely to be dependent on the JNK-mediated mitochondria-dependent apoptotic pathway. Alternatively, ROS may act directly on the mitochondria, inducing the mitochondrial membrane permeability transition (mPT) and resulting in the release of apoptogenic factors, such as cytochrome c, apoptosis-inducing factor (AIF), and/or Smac/DIABLO.68, 69
The mechanisms underlying ROS-induced necrosis have been highly debated. It is well known that TNF_α_ induces ROS-dependent necrosis in murine fibrosarcoma, L929 cells.70 Geldanamycin treatment induces the degradation of heat shock protein (HSP)90 and its client protein, RIP, causing a shift from necrosis to apoptosis in L929 cells, indicating an essential role for RIP in TNF_α_-induced necrosis.71 Similarly, Holler et al.72 have also reported that a critical role for RIP in Fas-, TNF_α_-, and TRAIL-induced necrotic cell death. The contribution of ROS to receptor-mediated necrosis was not investigated in these studies; however, and the conclusion reached was that FADD and kinase activity of RIP, but not caspase 8, are essential for necrosis. Intriguingly, a recent report73 has highlighted that both cell death followed by ischemic brain injury and RIP-dependent necrotic cell death are tightly linked with autophagy. However, further studies will be required to address the role of autophagy in necrotic cell death.
Two recent studies74, 75 have shown that H2O2-induced necrosis, but not genotoxic stress-induced apoptosis is reduced in cells lacking cyclophilin D. As mPT induced by H2O2 is also severely impaired in cyclophilin D knockout cells, it appears that mPT is crucial for H2O2-induced necrosis. Therefore, one of the mechanisms of ROS-induced necrosis may be the opening of an mPT pore, resulting in the loss of membrane potential and causing extensive swelling of the mitochondria. However, it is currently unknown whether all RIP- and ROS-dependent pathways leading to necrotic cell death finally converge on mPT. To investigate this matter further, it would be interesting to test whether knockdown of cyclophilin D suppresses TNF_α_-induced ROS-dependent necrosis in L929 cells or NF-_κ_B activation-deficient cells.
Finally, we need to further our understanding of factor(s) that may affect the fate of cells exposed to ROS. Protein synthesis inhibitors, such as cycloheximide (CHX) and emetine, which are usually required for TNF_α_ to induce cell death in wild-type cells, might affect the fate of the TNF_α_-stimulated cells and determine whether they die from apoptosis or necrosis. Indeed, TNF_α_-induced necrotic cell death is preferentially observed in cells that are stimulated with TNF_α_ alone,53 and not in those stimulated with TNF_α_ plus CHX or emetine.53, 54 Therefore, TNF_α_-induced apoptosis might prevail over necrosis in the presence of protein synthesis inhibitors,76 although the detailed molecular mechanism remains unknown.
Concluding Remarks
Recent advances in gene targeting techniques convincingly demonstrate the proapoptotic and antiapoptotic function of the JNK signaling cascade. Although the caspase cascade is sufficient for the induction of apoptosis, the activation of the JNK pathway itself does not appear to be sufficient for determining cell fate. As Lin12 describes, the JNK cascade appears to regulate the path to cell death or survival. In this respect, the central checkpoint at which cell fate is determined involves NF-_κ_B. As described in Figure 5a, activation of NF-_κ_B is sufficient for inhibiting the cascades induced by proapoptosis-inducing molecules, caspases, JNK, and ROS in normal cells. Under the conditions, in which NF-_κ_B-mediated survival signals are blocked (such as cellular parasitism by viruses and other pathogens, or genotoxic stress), JNK and ROS promote cell death in a context-dependent manner (Figure 5b). More importantly, several studies indicate that treatment of cells with caspase inhibitors enhances ROS-dependent necrosis both in vitro and in vivo.70, 77 To understand the NF-_κ_B-mediated survival signals in more detail and to develop novel strategies to prevent excessive cell death under the pathological conditions, future studies will focus on identifying the molecules involved in JNK activation and ROS accumulation.
Abbreviations
JNK:
c-Jun N-terminal kinase
c-FLIP:
cellular FLICE-inhibitory protein
XIAP:
X chromosome-linked inhibitor of apoptosis
ROS:
reactive oxygen species
TNF:
tumor necrosis factor
IL-1:
interleukin-1
I_κ_B:
inhibitor of _κ_B
IKK:
I_κ_B kinase
MAPK:
mitogen-activated protein kinase
ERK:
extracellular signal-regulated kinase
ASK1:
apoptosis-signal regulating kinase 1
MEKK:
MAP/ERK kinase kinase
TAK1:
TGF_β_-activated kinase 1
MEFs:
murine embryonic fibroblasts
TRAF:
TNF rceptor-associated factor
c-IAP:
cellular inhibitor of apoptosis
ES:
embrynonic stem
GADD:
growth arrest and DNA damage-inducing protein
SOD:
superoxide dismutase
GPx:
glutathione peroxidase
PRx:
peroxiredoxin
NOX:
NADPH oxidase
BHA:
butylated hydroxylanisole
NAC:
_N_-acetyl cystein
MKP:
MAP kinase phosphatase
MnSOD:
manganese-dependent SOD
FHC:
ferritin heavy chain
mPT:
membrane permeability transition
AIF:
apoptosis-inducing factor
HSP:
heat shock protein
CHX:
cycloheximide
References
- Ghosh S, May MJ and Kopp EB (1998) NF-_κ_B and Rel proteins: evolutionarily conserved mediators of immune responses. Ann. Rev. Immunol. 16: 225–260
Article CAS Google Scholar - Ghosh S and Karin M (2002) Missing pieces in the NF-_κ_B puzzle. Cell 109 (Suppl): S81–S96
Article CAS PubMed Google Scholar - Beg AA and Baltimore D (1996) An essential role for NF-_κ_B in preventing TNF_α_-induced cell death. Science 274: 782–784
Article CAS PubMed Google Scholar - Van Antwerp DJ, Martin SJ, Kafri T, Green DR and Verma IM (1996) Suppression of TNF_α_-induced apoptosis by NF-_κ_B. Science 274: 787–789
Article CAS PubMed Google Scholar - Wang CY, Mayo MW and Baldwin Jr AS (1996) TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-_κ_B. Science 274: 784–787
Article CAS PubMed Google Scholar - Barkett M and Gilmore TD (1999) Control of apoptosis by Rel/NF-_κ_B transcription factors. Oncogene 18: 6910–6924
Article CAS PubMed Google Scholar - Karin M and Lin A (2002) NF-_κ_B at the crossroads of life and death. Nat. Immunol. 3: 221–227
Article CAS PubMed Google Scholar - Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252
Article CAS PubMed Google Scholar - Kyriakis JM and Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81: 807–869
Article CAS PubMed Google Scholar - Chen YR, Wang X, Templeton D, Davis RJ and Tan TH (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 271: 31929–31936
Article CAS PubMed Google Scholar - Guo YL, Baysal K, Kang B, Yang LJ and Williamson JR (1998) Correlation between sustained c-Jun N-terminal protein kinase activation and apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J. Biol. Chem. 273: 4027–4034
Article CAS PubMed Google Scholar - Lin A (2003) Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays 25: 17–24
Article PubMed Google Scholar - Varfolomeev EE and Ashkenazi A (2004) Tumor necrosis factor: an apoptosis JuNKie? Cell 116: 491–497
Article CAS PubMed Google Scholar - Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P and Flavell RA (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22: 667–676
Article CAS PubMed Google Scholar - Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA and Davis RJ (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288: 870–874
Article CAS PubMed Google Scholar - Wada T, Joza N, Cheng HY, Sasaki T, Kozieradzki I, Bachmaier K, Katada T, Schreiber M, Wagner EF, Nishina H and Penninger JM (2004) MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nat. Cell. Biol. 6: 215–226
Article CAS PubMed Google Scholar - Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P and Flavell RA (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389: 865–870
Article CAS PubMed Google Scholar - Behrens A, Sibilia M and Wagner EF (1999) Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 21: 326–329
Article CAS PubMed Google Scholar - Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T and Ichijo H (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2: 222–228
Article CAS PubMed PubMed Central Google Scholar - Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C, Gillieron C, Boschert U, Vial-Knecht E, Martinou JC and Arkinstall S (1997) Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. J. Biol. Chem. 272: 25238–25242
Article CAS PubMed Google Scholar - Pandey P, Avraham S, Place A, Kumar V, Majumder PK, Cheng K, Nakazawa A, Saxena S and Kharbanda S (1999) Bcl-xL blocks activation of related adhesion focal tyrosine kinase/proline-rich tyrosine kinase 2 and stress-activated protein kinase/c-Jun N-terminal protein kinase in the cellular response to methylmethane sulfonate. J. Biol. Chem. 274: 8618–8623
Article CAS PubMed Google Scholar - Yamamoto K, Ichijo H and Korsmeyer SJ (1999) BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol. Cell. Biol. 19: 8469–8478
Article CAS PubMed PubMed Central Google Scholar - Deng X, Xiao L, Lang W, Gao F, Ruvolo P and May Jr WS (2001) Novel role for JNK as a stress-activated Bcl2 kinase. J. Biol. Chem. 276: 23681–23688
Article CAS PubMed Google Scholar - Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA and Chambers TC (2000) Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J. Biol. Chem. 275: 29980–29985
Article CAS PubMed Google Scholar - Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D and Davis RJ (2002) The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol. Cell. Biol. 22: 4929–4942
Article CAS PubMed PubMed Central Google Scholar - Lei K and Davis RJ (2003) JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 100: 2432–2437
Article CAS PubMed PubMed Central Google Scholar - Deng Y, Ren X, Yang L, Lin Y and Wu X (2003) A JNK-dependent pathway is required for TNF_α_-induced apoptosis. Cell 115: 61–70
Article CAS PubMed Google Scholar - Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N and Gotoh Y (2004) JNK promotes Bax translocation to mitochondria through phosphorylation of 14–3–3 proteins. EMBO J 23: 1889–1899
Article CAS PubMed PubMed Central Google Scholar - Nishina H, Fischer KD, Radvanyi L, Shahinian A, Hakem R, Rubie EA, Bernstein A, Mak TW, Woodgett JR and Penninger JM (1997) Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature 385: 350–353
Article CAS PubMed Google Scholar - Nishina H, Vaz C, Billia P, Nghiem M, Sasaki T, De la Pompa JL, Furlonger K, Paige C, Hui C, Fischer KD, Kishimoto H, Iwatsubo T, Katada T, Woodgett JR and Penninger JM (1999) Defective liver formation and liver cell apoptosis in mice lacking the stress signaling kinase SEK1/MKK4. Development 126: 505–516
CAS PubMed Google Scholar - Minamino T, Yujiri T, Papst PJ, Chan ED, Johnson GL and Terada N (1999) MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc. Natl. Acad. Sci. USA 96: 15127–15132
Article CAS PubMed PubMed Central Google Scholar - Lamb JA, Ventura JJ, Hess P, Flavell RA and Davis RJ (2003) JunD mediates survival signaling by the JNK signal transduction pathway. Mol. Cell 11: 1479–1489
Article CAS PubMed Google Scholar - Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J and Lin A (2004) JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol. Cell 13: 329–340
Article CAS PubMed Google Scholar - Campbell KJ, Rocha S and Perkins ND (2004) Active repression of antiapoptotic gene expression by RelA(p65) NF-_κ_B. Mol. Cell 13: 853–865
Article CAS PubMed Google Scholar - Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M and Lin A (2001) Inhibition of JNK activation through NF-_κ_B target genes. Nature 414: 313–317
Article CAS PubMed Google Scholar - De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R and Franzoso G (2001) Induction of gadd45_β_ by NF-_κ_B downregulates pro-apoptotic JNK signalling. Nature 414: 308–313
Article CAS PubMed Google Scholar - Takekawa M and Saito H (1998) A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95: 521–530
Article CAS PubMed Google Scholar - Deveraux QL, Takahashi R, Salvesen GS and Reed JC (1997) X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388: 300–304
Article CAS PubMed Google Scholar - Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, Smaele ED, Tang WJ, D'Adamio L and Franzoso G (2004) Gadd45_β_ mediates the NF-_κ_B suppression of JNK signalling by targeting MKK7/JNKK2. Nat. Cell. Biol. 6: 146–153
Article CAS PubMed Google Scholar - Amanullah A, Azam N, Balliet A, Hollander C, Hoffman B, Fornace A and Liebermann D (2003) Cell signalling: cell survival and a Gadd45-factor deficiency. Nature 424: 741; discussion 742
Article CAS PubMed Google Scholar - Silverman N and Maniatis T (2001) NF-_κ_B signaling pathways in mammalian and insect innate immunity. Genes Dev. 15: 2321–2342
Article CAS PubMed Google Scholar - Park JM, Brady H, Ruocco MG, Sun H, Williams D, Lee SJ, Kato Jr T, Richards N, Chan K, Mercurio F, Karin M and Wasserman SA (2004) Targeting of TAK1 by the NF-_κ_B protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 18: 584–594
Article CAS PubMed PubMed Central Google Scholar - Nateri AS, Riera-Sans L, Da Costa C and Behrens A (2004) The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303: 1374–1378
Article CAS PubMed Google Scholar - Wertz IE, O'Rourke KM, Zhang Z, Dornan D, Arnott D, Deshaies RJ and Dixit VM (2004) Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science 303: 1371–1374
Article CAS PubMed Google Scholar - Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T and Nakano H (2003) NF-_κ_B inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 22: 3898–3909
Article CAS PubMed PubMed Central Google Scholar - Nakano H (2004) Signaling crosstalk between NF-_κ_B and JNK. Trends Immunol. 25: 402–405
Article CAS PubMed Google Scholar - Papa S, Zazzeroni F, Pham CG, Bubici C and Franzoso G (2004) Linking JNK signaling to NF-_κ_B: a key to survival. J. Cell. Sci. 117: 5197–5208
Article CAS PubMed Google Scholar - Balaban RS, Nemoto S and Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120: 483–495
Article CAS PubMed Google Scholar - Thannickal VJ and Fanburg BL (2000) Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279: L1005–L1028
Article CAS PubMed Google Scholar - Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4: 181–189
Article CAS PubMed Google Scholar - Haynes CM, Titus EA and Cooper AA (2004) Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 15: 767–776
Article CAS PubMed Google Scholar - Droge W (2002) Free radicals in the physiological control of cell function. Physiol. Rev. 82: 47–95
Article CAS PubMed Google Scholar - Ventura JJ, Cogswell P, Flavell RA, Baldwin Jr AS and Davis RJ (2004) JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 18: 2905–2915
Article CAS PubMed PubMed Central Google Scholar - Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV and Franzoso G (2004) Ferritin heavy chain upregulation by NF-_κ_B inhibits TNF_α_-induced apoptosis by suppressing reactive oxygen species. Cell 119: 529–542
Article CAS PubMed Google Scholar - Kamata H, Honda S, Maeda S, Chang L, Hirata H and Karin M (2005) Reactive oxygen species promote TNF_α_-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661
Article CAS PubMed Google Scholar - Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, Hashimoto H, Mak TW, Yagita H, Okumura K, Yeh WC and Nakano H (2001) Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-_κ_B activation and protection from cell death. J. Biol. Chem. 276: 36530–36534
Article CAS PubMed Google Scholar - Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K and Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17: 2596–2606
Article CAS PubMed PubMed Central Google Scholar - Chen F, Castranova V, Li Z, Karin M and Shi X (2003) Inhibitor of nuclear factor _κ_B kinase deficiency enhances oxidative stress and prolongs c-Jun NH2-terminal kinase activation induced by arsenic. Cancer Res. 63: 7689–7693
CAS PubMed Google Scholar - Farooq A and Zhou MM (2004) Structure and regulation of MAPK phosphatases. Cell Signal 16: 769–779
Article CAS PubMed Google Scholar - Widmann C, Gerwins P, Johnson NL, Jarpe MB and Johnson GL (1998) MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol. Cell. Biol. 18: 2416–2429
Article CAS PubMed PubMed Central Google Scholar - Lee KK, Murakawa M, Nishida E, Tsubuki S, Kawashima S, Sakamaki K and Yonehara S (1998) Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene 16: 3029–3037
Article CAS PubMed Google Scholar - Rudel T, Zenke FT, Chuang TH and Bokoch GM (1998) p21-activated kinase (PAK) is required for Fas-induced JNK activation in Jurkat cells. J. Immunol. 160: 7–11
CAS PubMed Google Scholar - Pahl HL (1999) Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18: 6853–6866
Article CAS PubMed Google Scholar - Sasazuki T, Okazaki T, Tada K, Sakon-Komazawa S, Katano M, Tanaka M, Yagita H, Okumura K, Tominaga N, Hayashizaki Y, Okazaki Y and Nakano H (2004) Genome wide analysis of TNF-inducible genes reveals that antioxidant enzymes are induced by TNF and responsible for elimination of ROS. Mol. Immunol. 41: 547–551
Article CAS PubMed Google Scholar - Ricci JE, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH and Green DR (2004) Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell 117: 773–786
Article CAS PubMed Google Scholar - Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F and Pelicci PG (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122: 221–233
Article CAS PubMed Google Scholar - Fiers W, Beyaert R, Declercq W and Vandenabeele P (1999) More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene 18: 7719–7730
Article CAS PubMed Google Scholar - Kroemer G and Reed JC (2000) Mitochondrial control of cell death. Nat. Med. 6: 513–519
Article CAS PubMed Google Scholar - Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev. 15: 2922–2933
CAS PubMed Google Scholar - Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W and Vandenabeele P (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187: 1477–1485
Article CAS PubMed PubMed Central Google Scholar - Vanden Berghe T, Kalai M, van Loo G, Declercq W and Vandenabeele P (2003) Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J. Biol. Chem. 278: 5622–5629
Article CAS PubMed Google Scholar - Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp J (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1: 489–495
Article CAS PubMed Google Scholar - Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchinson TJ, Moskowitz MA and Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chem. Biol. 1: 112–119
Article CAS Google Scholar - Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T and Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658
Article CAS PubMed Google Scholar - Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J and Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662
Article CAS PubMed Google Scholar - Nakano H (2005) A revival of old players. EMBO Rep. 6: 126–127
Article CAS PubMed PubMed Central Google Scholar - Cauwels A, Janssen B, Waeytens A, Cuvelier C and Brouckaert P (2003) Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat. Immunol. 4: 387–393
Article CAS PubMed Google Scholar
Acknowledgements
We thank CF Ware and S Shimizu for critically reading the manuscript. We also thank D Wallach for the comments on the manuscript. KO and HN are supported in part by a Grant-in-Aid for 21st Century COE Research and Scientific Research (B) from Japan Society for the Promotion of Science (JSPS), Japan. HN is supported by Takeda Science Foundation and by a Grant from Human Frontier Science Program (HFSP).
Author information
Authors and Affiliations
- Department of Immunology, Juntendo University School of Medicine, Tokyo, Japan
H Nakano, A Nakajima, S Sakon-Komazawa, J-H Piao, X Xue & K Okumura
Authors
- H Nakano
- A Nakajima
- S Sakon-Komazawa
- J-H Piao
- X Xue
- K Okumura
Corresponding author
Correspondence toH Nakano.
Additional information
Edited by G Kroemer
Rights and permissions
About this article
Cite this article
Nakano, H., Nakajima, A., Sakon-Komazawa, S. et al. Reactive oxygen species mediate crosstalk between NF-κB and JNK.Cell Death Differ 13, 730–737 (2006). https://doi.org/10.1038/sj.cdd.4401830
- Received: 23 September 2005
- Revised: 28 October 2005
- Accepted: 03 November 2005
- Published: 09 December 2005
- Issue Date: 01 May 2006
- DOI: https://doi.org/10.1038/sj.cdd.4401830