Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? (original) (raw)
This Article

Academic Content and Language Evaluation of This Article

CrossCheck and Google Search of This Article

Academic Rules and Norms of This Article

Citation of this article
Marisi G, Cucchetti A, Ulivi P, Canale M, Cabibbo G, Solaini L, Foschi FG, De Matteis S, Ercolani G, Valgiusti M, Frassineti GL, Scartozzi M, Casadei Gardini A. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J Gastroenterol 2018; 24(36): 4152-4163 [PMID: 30271080 DOI: 10.3748/wjg.v24.i36.4152]

Corresponding Author of This Article
Alessandro Cucchetti, MD, PhD, Adjunct Professor, Surgeon, Department of Medical and Surgical Sciences, Alma Mater Studiorum, University of Bologna, Via Albertoni 15, Granarolo Dell’Emilia, Bologna 40126, Italy. aleqko@libero.it

Research Domain of This Article
Gastroenterology & Hepatology

Article-Type of This Article
Minireviews

Open-Access Policy of This Article
This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/

Times Cited Counts in Google of This Article
Number of Hits and Downloads for This Article
- Total Article Views (12411)
All Articles published online
Publishing Process of This Article
Sep 28, 2018 (publication date) through Oct 10, 2024
Times Cited of This Article
Journal Information of This Article
Publication Name
World Journal of Gastroenterology
ISSN
1007-9327
Publisher of This Article
Baishideng Publishing Group Inc, 7041 Koll Center Parkway, Suite 160, Pleasanton, CA 94566, USA
Minireviews Open Access
Copyright ©The Author(s) 2018. Published by Baishideng Publishing Group Inc. All rights reserved.
World J Gastroenterol. Sep 28, 2018; 24(36): 4152-4163
Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4152
Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers?
, Alessandro Cucchetti, Paola Ulivi, Matteo Canale, Giuseppe Cabibbo, Leonardo Solaini, Francesco G Foschi, Serena De Matteis, Giorgio Ercolani, Martina Valgiusti, Giovanni L Frassineti, Mario Scartozzi, Andrea Casadei Gardini
Giorgia Marisi, Paola Ulivi, Matteo Canale, Serena De Matteis, Biosciences Laboratory, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola 47014, Italy
Alessandro Cucchetti, Leonardo Solaini, Giorgio Ercolani, Department of Medical and Surgical Sciences, Alma Mater Studiorum, University of Bologna, Bologna 40126, Italy
Alessandro Cucchetti, Leonardo Solaini, Giorgio Ercolani, General and Oncologic Surgery, Morgagni-Pierantoni Hospital, Forlì 47121, Italy
Giuseppe Cabibbo, Section of Gastroenterology, DI.BI.M.I.S., University of Palermo, Palermo 35628, Italy
Francesco G Foschi, Department of Internal Medicine, Degli Infermi Hospital, Faenza 48018, Italy
Martina Valgiusti, Giovanni L Frassineti, Andrea Casadei Gardini, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori IRCCS, Meldola 47014, Italy
Mario Scartozzi, Department of Medical Oncology, University of Cagliari, Cagliari 45698, Italy
Author contributions: All authors equally contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of final version.
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Correspondence to: Alessandro Cucchetti, MD, PhD, Adjunct Professor, Surgeon, Department of Medical and Surgical Sciences, Alma Mater Studiorum, University of Bologna, Via Albertoni 15, Granarolo Dell’Emilia, Bologna 40126, Italy. aleqko@libero.it
Telephone: +39-543-731111 Fax: +39-543-739123
Received: July 3, 2018
Peer-review started: July 3, 2018
First decision: July 17, 2018
Revised: August 6, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: September 28, 2018
Processing time: 84 Days and 18.1 Hours
Abstract
Sorafenib has been considered the standard of care for patients with advanced unresectable hepatocellular carcinoma (HCC) since 2007 and numerous studies have investigated the role of markers involved in the angiogenesis process at both the expression and genetic level and clinical aspect. What results have ten years of research produced? Several clinical and biological markers are associated with prognosis. The most interesting clinical parameters are adverse events, Barcelona Clinic Liver Cancer stage, and macroscopic vascular invasion, while several single nucleotide polymorphisms and plasma angiopoietin-2 levels represent the most promising biological biomarkers. A recent pooled analysis of two phase III randomized trials showed that the neutrophil-to-lymphocyte ratio, etiology and extra-hepatic spread are predictive factors of response to sorafenib, but did not identify any predictive biological markers. After 10 years of research into sorafenib there are still no validated prognostic or predictive factors of response to the drug in HCC. The aim of the present review was to summarize 10 years of research into sorafenib, looking in particular at the potential of associated clinical and biological markers to predict its efficacy in patients with advanced HCC.
Core tip: Sorafenib has been considered the standard of care for patients with advanced unresectable hepatocellular carcinoma, but after 10 years of research into sorafenib response or resistance, there are still no validated prognostic or predictive factors of response.
- Citation: Marisi G, Cucchetti A, Ulivi P, Canale M, Cabibbo G, Solaini L, Foschi FG, De Matteis S, Ercolani G, Valgiusti M, Frassineti GL, Scartozzi M, Casadei Gardini A. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J Gastroenterol 2018; 24(36): 4152-4163
- URL: https://www.wjgnet.com/1007-9327/full/v24/i36/4152.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i36.4152
INTRODUCTION
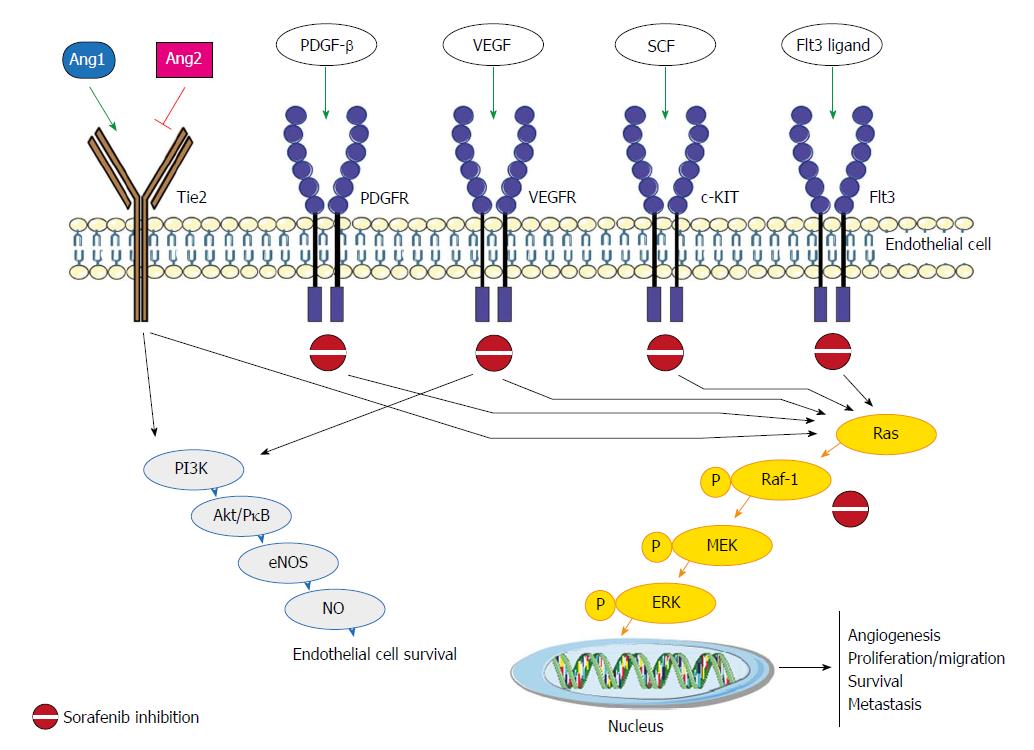
Sorafenib, an oral multikinase inhibitor, has been considered the standard of care for patients with advanced unresectable hepatocellular carcinoma (HCC) since 2007[1]. It works by inhibiting the activity of several tyrosine kinases involved in tumor angiogenesis and progression, including vascular endothelial growth factor receptor (VEGFR-2/3), platelet-derived growth factor receptor (PDGF-R), Flt3 and c-Kit, and also targets Raf kinases involved in the MAPK/ERK pathway[2] (Figure 1). The molecular mechanisms by which sorafenib exerts its activity have still not been fully elucidated, and both Raf/MEK/ERK-dependent and -independent mechanisms have been observed[3].
Figure 1 Sorafenib pathaway and the main molecular factors. Ang: Angiopoietin; Tie2: Tyrosine-protein kinase receptor; PDGFR: Platelet-derived growth factor receptors; VEGFR: Vascular endothelial growth factor receptor; SCF: Stem cell factor; PI3K: PhosphatidylInositol 3-Kinase; Akt/PKB: Protein-chinasi B; eNOS: Endothelial nitric oxide synthase; NO: Nitric oxide; P: Phospho-; MEK: Mitogen-activated protein kinase kinase; ERK: Extracellular signal–regulated kinase.
Sorafenib is expensive and associated with adverse events (AEs). Furthermore, a proportion of treated patients show no response to the drug. It would thus be useful to have predictive markers capable of identifying those who are more likely to benefit from therapy. The availability of more accurate predictive or prognostic factors would also help to spare potentially resistant patients from unnecessary toxicity.
Ten years have passed since sorafenib was first commercialized and about 2800 studies have been published on the kinase inhibitor. But how many associated prognostic and/or predictive markers have been identified? Numerous studies have focused on the role of markers involved in the angiogenesis process at both the expression and genetic levels. The largest biomarker study conducted to date is the SHARP trial[4], which included an adequate number of participants and a placebo-controlled group. Smaller single-arm studies exploring predictive or prognostic markers for sorafenib have also been conducted, but the results of these have yet to be validated.
The aim of the present review was to summarize 10 years of research into sorafenib, looking in particular at the potential of associated clinical and biological markers to predict its efficacy in patients with advanced HCC (Tables 1 and 2).
Table 1 Predictive and/or prognostic value of clinical markers in hepatocellular carcinoma patients.
| Clinical markers | Predictive value | Prognostic value | Ref. |
|---|---|---|---|
| Alpha-fetoprotein | No | Yes | [6] |
| Adverse events | |||
| Hand-foot skin reaction | No | Yes | [13] |
| Hypertension | No | Uncertain | [16,19,20] |
| Diarrhea | No | Yes | [21] |
| Child-Pugh A vs B | No | Yes | [27-29] |
| Macroscopic vascular invasion | No | Yes | [6] |
| BCLC B vs C | No | Yes | [6,29,32] |
| Starting dose and dose reduction | No | Yes | [29,32] |
| Etiology HCV vs HBV | Yes | Yes | [6] |
| Chronic treatment with metformin | No | Yes | [35,36] |
| Neutrophil-to-lymphocyte ratio | Yes | Yes | [6,41,44] |
| Extra hepatic spread | Yes | Yes | [6] |
Table 2 Predictive and/or prognostic value of biological markers in hepatocellular carcinoma patients.
| Biological markers | Predictive value | Prognostic value | Ref. |
|---|---|---|---|
| Serum and plasma proteins | |||
| VEGF-A | No | Uncertain | [4,57] |
| Ang-2 | No | Yes | [4] |
| IGF-1 | No | No | [55] |
| Single nucleotide polymorphisms | |||
| VEGF-A rs2010963 | No | Yes | [65] |
| VEGF-C rs4604006 | No | Yes | [65] |
| eNOS (_eNOS_-786/eNOS VNTR) | No | Yes | [66] |
| Ang-2 rs55633437 | No | Yes | [67] |
| HIF-1 alpha rs12434438 | No | Yes | [68] |
| Amplifications | |||
| VEGF | No | Uncertain | [70] |
| FGF3/FGF4 | No | Uncertain | [71] |
| miRNAs | |||
| miR-425-3p | No | Yes | [74] |
| miR-224 | No | Yes | [75] |
| miR-181a-5p | No | Yes | [77] |
| miR-339-5p | No | Yes | [77] |
| miR-423-5p | No | Yes | [78] |
| miR-10b-3p | No | Yes | [79] |
| miR-221 | No | Uncertain | [76] |
| Tissue biomarker expression | |||
| Phospho-ERK | Uncertain | Uncertain | [81,82] |
| PDGFR-b | No | Yes | [84] |
| c-Met | No | No | [84] |
| VEGFR | No | No | [84] |
| p-c-Jun | No | Yes | [85] |
CLINICAL PARAMETERS
Alpha-fetoprotein
Alpha-fetoprotein (AFP) is secreted by about 50% of all HCCs and is the main serological marker used for the diagnosis of the tumor[5]. The SHARP trial[4] showed that high baseline AFP plasma levels (> 200 ng/mL) had a negative impact on overall survival (OS), a finding recently confirmed in a pooled analysis of the SHARP trial and the Asia Pacific trial by Bruix _et al_[6]. High baseline serum AFP levels (≥ 400 ng/mL) also appear to be associated with shorter time-to-progression (TTP). Notably, in an analysis of six prospective phase II trials evaluating systemic therapies for patients with advanced HCC, no association was observed between baseline AFP levels and prognosis[7].
Several studies[8-10] have highlighted a consistent correlation between an early decrease of > 20% in AFP levels following sorafenib and objective response and better outcome in advanced HCC patients. Shao _et al_[8] evaluated for the first time this aspect and they observed that patients with early AFP response had an improved progression-free survival (PFS) (7.5 mo vs 1.9 mo) and OS (15.3 mo vs 4.1 mo). This data was confirmed by Personeni _et al_[10] a few years later. They reported that early responders had a significantly better median OS and TTP than non-responders (13.8 mo vs 8.2 mo, P = 0.022 and 7.9 mo vs 2.4 mo, P = 0.004; respectively). Conversely, Nakazawa _et al_[11] did not observe such an association.
Adverse events
The main AEs of Sorafenib are hand-foot skin reaction (HFSR), hypertension and diarrhea. Several papers have highlighted a consistent correlation between AEs and survival in patients treated with Sorafenib.
Vincenzi _et al_[12] evaluated for the first time the correlation between HSFR and outcome. They showed, in a small series of patients treated with sorafenib, that patients with HSFR had a significantly higher disease control rate with respect to patients without HSFR. This data was confirmed in a prospective study of 147 patients by Reig _et al_[13]. They reported different OS when patients were subdivided according to the presence or not of skin toxicity during the first 60 d of treatment (18.2 mo vs 10.1 mo, respectively)[13] . A recently meta-analysis confirmed that HSFR was a good indicator of outcome for OS and TTP in HCC patients receiving sorafenib[14]
Hypertension (HTN) is frequently associated with the use of angiogenesis inhibitors[15]. Casadei Gardini _et al_[16] showed that early HTN (15 d after the start of treatment) rather than later onset HTN vs patients without HTN was associated with better PFS (6.0 mo vs 2.5 mo; P < 0.001) and OS (14.6 mo vs 3.9 mo; P = 0.003). This finding has been confirmed in some studies[17,18] but not in others[19,20].
Bettinger _et al_[21] reported for the first time that diarrhea was an independent positive prognostic factor (HR = 0.41; P = 0.001) in 112 patients with advanced HCC, a finding also confirmed by Koschny _et al_[22].
Finally, other authors showed that the number of AEs was associated with predict survival in patients treated with sorafenib. In particular, Di Costanzo _et al_[23] evaluated the potential of pretreatment clinical variables to predict survival. Three groups of patients were taken into account: patients without AEs (group 0), patients with one AE (group 1) and patients with two to three AEs (group 2). The study reported a strong correlation between this classification and disease progression at 3 mo (41.9%, 25.9% and 12.7% of patients in groups 0, 1 and 2, respectively; P = 0.014). These data were subsequently confirmed in the validation cohort[24]. A recent meta-analysis by Abdel-Rahman _et al_[25] revealed an association between specific side-effects (hypertension, HFSR and diarrhea) and patient outcome (HR = 0.38; 95%CI: 0.30-0.48; P < 0.00001).
Stage, liver functionality and etiology
Child-Pugh A vs Child-Pugh B: In the SHARP trial[4] and the Asia Pacific trial[26], more than 95% of patients were classified as having Child-Pugh A cirrhosis, thus preventing the investigation of the potential benefits of sorafenib in Child-Pugh B patients.
Hollebecque _et al_[27] reported for the first time the results from a prospective study on sorafenib efficacy in 120 advanced HCC patients, 20 of whom Child-Pugh B cirrhosis. OS was 11.1 mo, with a significantly longer median survival in Child-Pugh A patients than Child-Pugh B patients (13 mo vs 4.5 mo, P = 0.0008). A few years later, Pressiani _et al_[28] studied clinical outcome in a population of 300 consecutive patients; PFS in the Child-Pugh A group was 4.3 mo vs 2.1 mo in the Child-Pugh B arm (HR = 3.23; 95%CI: 2.38-4.39; P < 0.001), TTP was 4.2 mo vs 3.8 mo and OS was 10.0 mo vs 3.8 mo, respectively (P < 0.001).
The most important work on the use of sorafenib in Child-Pugh subgroups was the GIDEON study published in 2016[29]. This study observed that median OS was significantly longer in patients with Child-Pugh A (13.6 mo) than in those with Child Pugh B (5.2 mo) or Child-Pugh C (2.6 mo).
Macroscopic vascular invasion: It is widely acknowledged that the presence of macroscopic vascular invasion leads to a poorer prognosis[26,30]. The meta-analysis by Peng _et al_[31] confirmed its prognostic value and the pooled analysis by Bruix _et al_[6] affirmed the importance of macroscopic vascular invasion as a predictor of survival but not of response to treatment.
BCLC stage: In the SHARP trial, patients with Barcelona Clinic Liver Cancer (BCLC) B had a median OS of 14.5 mo compared to 9.7 mo for those with BCLC C[4]. Later, SOFIA[32] and GIDEON study[29] confirmed this data. In the SOFIA trial[32] the OS was 8.4 mo in BCLC C vs 20.6 mo in BCLC B patients (P < 0.0001), but the time to radiologic progression did not differ significantly between the 2 groups. In the GIDEON study[29], median OS according to BCLC by Child-Pugh cross-classification followed a similar trend, i.e. patients with Child-Pugh A and BCLC stage B showed longer OS than those with Child-Pugh B and BCLC B (19.5 mo vs 10.0 mo); and patients with Child-Pugh A and BCLC stage C had longer OS than those with Child-Pugh B and BCLC stage C (11.2 mo vs 3.8 mo).
Recently, Bruix _et al_[6]’s pooled analysis confirmed that BCLC C patients had a poorer prognosis than those with BCLC B HCC (HR = 1.59; P = 0.02).
Sorafenib starting dose and dose escalation/reduction: The two most important studies that evaluated sorafenib starting dose and dose escalation/reduction are SOFIA[32] and GIDEON trial[29].
In the SOFIA trial[32] sorafenib was down-dosed in 161 (54%) patients because of AEs (133 patients, 83%) and a reduction in liver function (28 patients, 17%). Median OS of the 77 patients receiving a half-dose of sorafenib for 70% of the treatment period was 21.6 mo (95%CI: 13.6-29.6) compared with 9.6 mo (95%CI: 6.9-12.3) for the remaining 219 patients who had a dose reduction for < 70% of the treatment period or who maintained the full dosage.
A sub-analysis of the GIDEON study[29] evaluated the starting dose of sorafenib with respect to clinical outcome and toxicity. Patients starting on 400 mg/d were slightly older, had baseline characteristics indicative of greater disease progression and had a higher incidence of AEs than those with a starting dose of 800 mg/d (96% vs 88%). Treatment duration (18.0 wk vs 13.0 wk) and median OS (12.1 mo vs 9.4 mo) were longer in patients receiving 800 mg/d.
Etiology: In the subgroup analysis of the SHARP study[4], the HR for OS was 0.76 in HBV-positive patients (95%CI: 0.38-1.50, P = not significant) and 0.50 (95%CI: 0.32-0.77) in HCV-positive patients. Results were similar for TTP (HR = 1.03 and 0.43 for HBV-positive and HCV-positive patients, respectively). Similar data were obtained for HBV-positive HCC patients in the phase III randomized Asia Pacific trial, i.e. the HR for OS was 0.74 (95%CI: 0.51-1.06, not significant) with respect to patients with the other etiology, for which the HR was 0.57 (95%CI: 0.29-1.33)[26]. Bruix _et al_[6]’s pooled analysis of the SHARP/Asia Pacific trial results showed that the absence of HCV was a potential prognostic factor for poorer OS (HR = 0.7, P = 0.02). The same authors revealed that HBV-positive patients did not show a significant difference in treatment response with respect to their HBV-negative counterparts (HR = 0.78; 95%CI: 0.57-1.06) and OS (HR = 1.128, P = 0.4538). We believe that the 2 etiologic groups respond differently to sorafenib and that further investigation is warranted in specific studies[33].
Metformin treatment: Type 2 diabetes is a significant risk factor for the development of malignancies, including HCC[34]. Casadei Gardini _et al_[35] published findings of reduced sorafenib efficacy in HCC patients treated chronically with or without metformin for type II diabetes mellitus (PFS 2.6 mo vs 5.0 mo, respectively; and OS 10.4 mo vs 15.1 mo, respectively). The same authors validated these data in a series of more than 250 cases[36], also highlighting a possible role of sirtuin-3 in resistance to sorafenib[37]. Di Costanzo _et al_[38] recently reported an increase in TTP and OS in diabetic with respect to non-diabetic HCC patients. However, no distinction was made between the different hypoglycemic therapies administered.
Immune inflammation indicators
Systemic inflammatory responses have been shown to reflect the promotion of angiogenesis, DNA damage and tumor invasion through an upregulation of cytokines[39]. Previous research revealed that lymphocytes play a crucial role in tumor defense by inducing cytotoxic cell death and inhibiting tumor cell proliferation and migration[40]. Consequently, several inflammation and immune-based prognostic scores, such as lymphocyte count, neutrophil-lymphocyte ratio (NLR), and systemic immune-inflammation index (SII), have been developed to predict survival and recurrence in cancers, including HCC. Casadei Gardini _et al_[41] evaluated for the first time SII, NLR and platelet-lymphocyte ratio (PLR) in a small case series, observing that SII were independent prognostic factors for OS. Other studies showed that NLR was a significant independent risk factor for shorter survival[42,43]. NLR was also found to be an independent prognostic factor for both response and survival in Bruix _et al_[6]’s pooled analysis and Lue _et al_[44]’s retrospective study on Spanish patients.
IMAGING EXAMINATIONS
The response to sorafenib does not correlate with a change in lesion dimension, but it is more correlate with intralesional vascularization. For this reason, the RECIST criteria[45,46] usually used for tumor response evaluation is inappropriate to evaluate the response to sorafenib in patients with advanced HCC. The modified RECIST (mRECIST) appear more indicate for evalutation the response. They include vascularization and tumor arterial enhancement changes of the target lesion on computed tomographic (CT). Several studies have demonstrated the superiority of the mRECIST criteria with respect to the RECIST criteria in assessing the response to treatment with sorafenib[47]. Various functional imaging tools were proposed to evaluate the antiangiogenic effects, but none of these has entered normal clinical practice[48-52]. Finally, a recently study showed that texture features on pretreatment contrast material-enhanced CT images can help predict OS and TTP in these patients[53] .
BIOLOGICAL PARAMETERS
Serum and plasma proteins
Although plasma biomarkers are the best candidates for evaluating sorafenib efficacy, only the SHARP trial produced results with borderline significance[4]. Baseline angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A) plasma levels independently predicted survival in both the entire patient population and the placebo cohort. Conversely, none of the tested biomarkers significantly predicted response to sorafenib[4]. Insulin-like growth factor (IGF)-1 levels have been found to decrease in patients with cirrhosis of the liver or HCC[54], and high pretreatment levels of IGF-1 predict better PFS and OS in advanced HCC patients receiving first-line antiangiogenic therapy[55].
The role of serum cytokines as biomarkers for the prediction of sorafenib responses is interesting, in particular Kim _et al_[56] developed a new prediction model for sorafenib response that combines relevant serum markers, tumor related factors, and cirrhosis-related factors in a scoring system.
VEGF-A: Llovet _et al_[4] showed that, although baseline plasma VEGF-A concentrations did not exhibit a predictive value, low plasma VEGF-A was associated with improved prognosis (HR = 1.48, 95%CI: 1.08-2.03, P = 0.015). However, other authors did not find any association between VEGF-A and prognosis in patients treated with sorafenib[57]. Tsuchiya _et al_[58]’s analysis of plasma VEGF concentrations during sorafenib treatment revealed that a decrease in the protein 8 wk after the start of therapy predicted better overall survival in advanced HCC patients (30.9 mo vs 14.4 mo; P = 0.038).
Ang-2: In the presence of VEGF, Ang-2 destabilizes blood vessels, promotes vascular sprouting, and is associated with an invasive and metastatic cancer phenotype[59]. Llovet _et al_[4] demonstrated that high baseline Ang-2 levels were correlated with more aggressive disease (HR = 1.58, 95%CI: 1.20-2.07, P = 0.001). Moreover, levels of the protein increased during treatment in the placebo group, suggestive of poor outcome related to disease progression in this cohort, whereas they remained constant during treatment with sorafenib, reflecting the generally more favorable outcome of this group. Overall increased Ang-2 expression levels were associated with poorer outcome in both groups, suggesting that this marker could be useful in monitoring treatment response. In agreement with Llovet’s study, Miyahara _et al_[57] reported that high baseline Ang-2 serum levels were associated with poor outcome in advanced HCC patients receiving sorafenib (HR = 2.51, 95%CI: 1.01-6.57, P = 0.048). Although these results indicate the potential prognostic value of Ang-2 in HCC, its role in predicting response to sorafenib remains to be verified.
IGF-1: Shao _et al_[55] found that high-pretreatment serum levels of IGF-1 were associated with a better DCR and improved PFS and OS in patients undergoing antiangiogenic therapy. Although the study did not have a control arm, the substantial significant difference in DCR between patients with high and low levels of IGF-1 (71% vs 39%) denotes the potential usefulness of IGF-1 as a predictive biomarker of response to antiangiogenic therapy.
Multiple-factor analyses: By using baseline serum basic fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF) levels as covariates together etiology (B-viral), platelet count, BCLC stage and protein induced by vitamin K absence-II, Kim _et al_[56] reported that a total score of < 6 could be a relevant cutoff value for selecting patients who are most likely to benefit from sorafenib therapy. Moreover, Hayashi _et al_[60] found that serum interleukin (IL)-5, IL-8, CXCL9, PDGF-BB, TGF-α, and VEGF-A were elevated in the long survivors group among HCC patients who received sorafenib, potentially reflecting the activation of stromal signaling in the tumor microenvironment.
Genetic markers
Molecular and genomic analyses from tumor and non-tumor tissue have proven useful in evaluating prognosis and could open up new avenues for tailoring treatment[61]. Genetic alterations, such as single nucleotide polymorphisms (SNPs) in genes encoding for proteins involved in the angiogenic process, have been studied as potential biomarkers for anti-angiogenic therapy. SNP evaluation would seem to be more advantageous than protein or gene expression analyses as it can be performed at any time during the course of the disease, is not substantially influenced by laboratory biases, and is relatively inexpensive. Some authors have focused on molecular profiling in formalin-fixed paraffin-embedded (FFPE) samples, comparing the mutation profiles of HCC biopsy samples and the response to sorafenib treatment[62]. Gene amplification, gene mutations and expression profiling of tumors have now become a research priority and are expected to lead to personalized treatment for HCC patients[63,64].
SNPs: Specific SNPs in VEGF and VEGFR genes have been found to be correlated with PFS and OS in HCC patients treated with sorafenib. In multivariate analysis, VEGF-A rs2010963 and VEGF-C rs4604006 were found to be independent factors influencing outcome in terms of PFS (HR = 0.25, 95%CI: 0.19-1.02, P = 0.0376 and HR = 0.22, 95%CI: 0.14-0.81, P = 0.004, respectively) and OS (HR = 0.28, 95%CI: 0.23-0.96, P = 0.02 and HR = 0.25, 95%CI: 0.17-0.99, P = 0.04, respectively)[65]. In the Italian multicenter, retrospective ePHAS [endothelial nitric oxide synthase (eNOS) polymorphisms in HCC and sorafenib] study, eNOS polymorphisms were analyzed in relation to PFS and OS. In univariate analysis, training cohort patients homozygous for eNOS haplotype (HT1:T-4b at _eNOS_-786/eNOS VNTR) showed a lower median PFS (2.6 mo vs 5.8 mo, HR = 5.43, 95%CI: 2.46-11.98, P < 0.0001) and OS (3.2 mo vs 14.6 mo, HR = 2.35, 95%CI: 1.12-4.91, P = 0.024) than those with other haplotypes. These results were confirmed in a validation set and multivariate analysis further substantiated this haplotype as the only independent prognostic factor[66]. More recently, evidence emerged that patients homozygous for ANGPT2 (Ang2 gene) rs55633437 GG genotype showed significantly longer PFS (P < 0.001) and OS (P < 0.001) than those with the other genotypes (GT+TT)[67].
In the ALICE-2 study, Faloppi _et al_[68] investigated the role of hypoxia-inducible factor 1-alpha (_HIF-1_α) SNPs, confirming the results of the ALICE-1 study[65]. In multivariate analysis, rs12434438 of _HIF-1_α, rs2010963 of VEGF-A and rs4604006 of VEGF-C were confirmed as independent factors and may help to identify patients who are more likely to respond to sorafenib[68]. The prospective INNOVATE study is ongoing to validate the role of VEGF, eNOS, Ang-2 and _HIF-1_α SNPs in relation to clinical outcome in advanced HCC patients treated with sorafenib (NCT02786342)[69].
Gene amplification, gene mutations and RNA expression: A relation between VEGF-A gene amplification and response to sorafenib was observed in a study performed on a mouse model of HCC[70]. The authors found that HCC patients with tumor VEGF-A amplification showed markedly better survival than those with non-amplified tumors, highlighting that VEGFA amplification is a potential biomarker of response to _VEGF-A_-blocking drugs in HCC[70]. Arao _et al_[71] observed that FGF3/FGF4 amplification and multiple lung metastases were frequently observed in responders to sorafenib, although the sample size was relatively small.
Sakai _et al_[62] used targeted DNA and RNA sequencing in FFPE specimens from fine-needle biopsy to identify candidate biomarkers of response to sorafenib in 46 HCC patients. A significant difference was observed in the number of oncogene mutations between progressing and non-progressing patients (P = 0.045), suggesting that tumor mutational burden may be predictive of sorafenib effectiveness. Tumor gene expression of NRG1, TGFa, and PECAM1 would also seem to be a marker of treatment response and PFS.
ΜicroRNAs
MicroRNAs (miRNAs) affect drug response directly or indirectly by regulating the expression of genes involved in drug transportation, metabolism, and downstream signaling pathways. The deregulation of various miRNAs has been reported in in vitro, in vivo and population studies[72,73], confirming its correlation with response to sorafenib. Some authors have evaluated the predictive role of miRNA expression in HCC tissue[74,75], while others have studied circulating miRNA levels prior to sorafenib treatment[76]. To date, the most interesting tissue miRNAs are miR-425-3p[74] and miR-224[75]. High levels of miR-425-3p have been associated with longer TTP and PFS (HR = 0.4, 95%CI: 0.2-0.7, P = 0.0008 and HR = 0.5, 95%CI: 0.3-0.9, P = 0.007, respectively), and elevated miR-224 expression have been correlated with increased PFS and OS (HR = 0.28, 95%CI: 0.09-0.92, P = 0.029 and HR = 0.0.24, 95%CI: 0.07-0.79, P = 0.012, respectively). Circulating miRNAs have also been studied in the serum of HCC patients to predict early response to sorafenib treatment, with miR-181a-5p and miR-339-5p associated with partial response and disease progression[77], miR-423-5p with stable disease or partial response[78] and miR-10b-3p with shorter survival[79].
Another potentially interesting circulating miRNA is miR-221, which was studied by Fornari _et al_[76] in both animal models and in a patient population. Patients with radiologic disease progression after 2-mo treatment had higher pretreatment miR-221 levels than responders (P = 0.007).
Larger confirmatory studies are needed before miRNAs can be considered valid biomarkers for clinical practice.
Tissue biomarker expression
A number of studies have analyzed tissue biomarkers that may be very specific to the disease of interest[80]. In a phase II study of sorafenib in advanced HCC, Abou-Alfa _et al_[81] showed that patients whose tumors expressed higher baseline phospho-ERK levels had a longer TTP. However, other studies reported conflicting results[82,83]. With regard to the expression of angiogenic markers in tumor tissue, it has been observed that high platelet-derived growth factor receptor beta expression is correlated with poor OS but not with PFS in HCC patients receiving sorafenib. High expression of the proto-oncogene c-Met may predict the therapeutic effectiveness of sorafenib in HCC patients, but no differences in terms of outcome have been seen with respect to VEGFR-2 expression[84]. Hagiwara _et al_[85] studied another interesting tissue biomarker, phospho-c-Jun, reporting a significantly higher expression (P < 0.001) in non-responding compared to responding patients treated with sorafenib.
CONCLUSION
After 10 years of research into sorafenib, there are still no validated prognostic or predictive markers of response to sorafenib in hepatocellular carcinoma. Furthermore, the main results obtained to date come from 2 important randomized trials and from different subanalyses and pooled analyses rather than from normal clinical practice. The fact of there being only one drug for the treatment of these patients has certainly done nothing to stimulate research into identifying and validating predictors of response and prognosis. However, given the recent publication of a positive phase III trial[86] and the ongoing NCT01658878 immunotherapy study, the race is now on to see who will be the first to identify a prognostic and predictive factor for sorafenib and/or new drugs in this setting. In conclusion, the use of metabolomic profiling and whole genome analysis to examine the association between patient outcome and response to sorafenib could become alternative approaches to the search for new biomarkers in HCC.
ACKNOWLEDGMENTS
The authors thank Gráinne Tierney and Cristiano Verna for editorial assistance.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lin ZY, Sun XY, Yao DF S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
References
| 16. | Casadei Gardini A, Scarpi E, Marisi G, Foschi FG, Donati G, Giampalma E, Faloppi L, Scartozzi M, Silvestris N, Bisulli M. Early onset of hypertension and serum electrolyte changes as potential predictive factors of activity in advanced HCC patients treated with sorafenib: results from a retrospective analysis of the HCC-AVR group. Oncotarget. 2016;7:15243-15251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
|---|
| 18. | Howell J, Pinato DJ, Ramaswami R, Bettinger D, Arizumi T, Ferrari C, Yen C, Gibbin A, Burlone ME, Guaschino G. On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: a multi-centre, prospective study. Aliment Pharmacol Ther. 2017;45:1146-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
|---|
| 37. | De Matteis S, Granato AM, Napolitano R, Molinari C, Valgiusti M, Santini D, Foschi FG, Ercolani G, Vespasiani Gentilucci U, Faloppi L. Interplay Between SIRT-3, Metabolism and Its Tumor Suppressor Role in Hepatocellular Carcinoma. Dig Dis Sci. 2017;62:1872-1880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
|---|
| 45. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 47. | Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, Castera L, Vilgrain V, Belghiti J, Raymond E. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19:394-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 112] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
|---|
| 59. | Hu B, Cheng SY. Angiopoietin-2: development of inhibitors for cancer therapy. Curr Oncol Rep. 2009;11:111-116. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 84. | Chu JS, Ge FJ, Zhang B, Wang Y, Silvestris N, Liu LJ, Zhao CH, Lin L, Brunetti AE, Fu YL. Expression and prognostic value of VEGFR-2, PDGFR-β, and c-Met in advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
|---|