Interferon-γ-Induced Oligodendrocyte Cell Death: Implications for the Pathogenesis of Multiple Sclerosis (original) (raw)
- Original Articles
- Published: 01 November 1995
Molecular Medicine volume 1, pages 732–743 (1995)Cite this article
- 1336 Accesses
- 284 Citations
- 9 Altmetric
- Metrics details
Abstract
Background
The histopathology of multiple sclerosis (MS) is characterized by a loss of myelin and oligodendrocytes, relative preservation of axons, and a modest inflammatory response. The reasons for this selective oligodendrocyte death and demyelination are unknown.
Materials and Methods
In light of the T lymphocyte and macrophage infiltrates in MS lesions and the numerous cytokines these cells secrete, the direct influence of cytokines on survival of cultured oligodendrocytes and sensory neurons was investigated. Expression of cytokines in vivo was determined by immunolabeling cryostat sections of snap-frozen tissue containing chronic active lesions from four different patients. The samples were also analyzed for the presence of apoptotic nuclei by in situ labeling of 3′-OH ends of degraded nuclear DNA.
Results
The results showed: (i) interferon-γ (IFN_γ_) to be a potent inducer of apoptosis among oligodendrocytes in vitro and that this effect can be reversed by leukemia inhibitory factor (LIF); (ii) IFN_γ_ has a minimal effect on the survival of cultured neurons; (iii) IFN_γ_ at the margins of active MS plaques but not in unaffected white matter; (iv) evidence for apoptosis of oligodendrocytes at the advancing margins of chronic active MS plaques.
Conclusions
Injury to a substantial number of oligodendrocytes in MS is the result of programmed cell death rather than necrotic cell death mechanisms. We postulate that IFN_γ_ plays a role in the pathogenesis of MS by activating apoptosis in oligodendrocytes.
Introduction
The pathologic hallmark of multiple sclerosis (MS) is a loss of oligodendrocytes, demyelination, and modest inflammation with relative preservation of neuronal elements (1–4). What allows for this remarkable specificity? A simple explanation for the specificity is that MS is a primary degenerative disease of oligodendrocytes. Death of oligodendrocytes would result in the release of encephalitogenic proteins which evoke an immune response as an epiphenomenon rather than the disease beginning with self-reactive T lymphocytes or antibodies (5). Adrenoleukodystrophy is an example of a disease in which death of oligodendrocytes yields a secondary inflammatory response (6). Another hypothesis is that focal damage to the integrity of the blood-brain barrier (BBB) may allow passage of molecules specifically toxic to oligodendrocytes (4,7,8). For example, oligodendrocytes have complement receptors, and complement, which is normally excluded by the intact BBB, could pass through a disrupted BBB to injure oligodendrocytes (8–10). The most studied current theory is that there is primary immune dysregulation in MS which unleashes an immune response that specifically targets oligodendrocyte/myelin antigens (11,12). It has been proposed that the pathogenic autoimmune response in MS is mainly cell mediated (11,12). However, the published evidence in support of this hypothesis is characterized by inconsistencies with regard to the specific T lymphocyte subsets present in MS lesions (13–15). Furthermore, macrophage/activated microglia, not T lymphocytes, may be the first immigrant cells to appear in active MS lesions (1–3,16).
Cell death is currently divided morphologically and mechanistically into the broad categories of necrotic cell death versus programmed or apoptotic cell death. Apoptosis is characterized by early condensation of chromatin, nuclear shrinkage, and fragmentation of DNA into nucleosome-sized components with preservation of the plasmalemma and mitochondria (17,18). Necrotic cell death is marked by early disruption of the plasma membrane, swelling of cytosol and mitochondria with nuclear changes occurring late (17,18). The molecular mechanisms of programmed cell death in mammalian cells are only currently being elucidated (18,19). Cell death in pathologic conditions can be either necrotic or apoptotic (20). During normal mammalian development the numbers of oligodendrocytes are in part controlled by programmed cell death, presumably as a result of competition for growth and survival factors (21). Oligodendrocyte survival is promoted by members of the leukemia inhibitory factor (LIF) family of growth factors and by insulin-like growth factor-1 (21,22).
In searching for a factor that might directly mediate oligodendrocyte injury in MS we set the following criteria: (i) it should injure isolated oligodendrocytes in vitro with cellular changes similar to those observed in MS lesions; (ii) it should be present in active recent and chronic active MS lesions; (iii) it should not affect or minimally affect cultured neurons. We have found that pure recombinant rat interferon-γ (IFN_γ_) kills rat oligodendrocytes and that this effect can be inhibited by anti-rat-IFN_γ_ antibodies. Oligodendrocytes exposed to IFN_γ_ have pyknotic nuclei and degradation of DNA into nucleosome-sized fragments consistent with apoptotic rather than necrotic cell death. IFN_γ_-treated oligodendrocytes can be partially rescued by treatment with LIF. However, IFN_γ_ does not injure cultured neurons. In fresh frozen autopsy tissue from MS patients, IFN_γ_ was localized to the advancing margins of chronic active lesions as previously described by Traugott and Lebon (23). Furthermore, apoptotic cells were found at the advancing margins of chronic active MS lesions, many of which were oligodendrocytes. On the basis of these results we propose that IFN_γ_ may be one of the factors that mediate the loss of oligodendrocytes in MS.
Materials and Methods
Antibodies
The galactosylcerebroside recognizing Ol antibody was generated by Dr. M. Schachner and colleagues (24), and the hybridoma was obtained from Dr. Stephen Pfeiffer (University of Connecticut, Farmington). Mouse anti-rat-IFN_γ_ (Gibco-BRL, Gaithersburg, MD, U.S.A.) was used at a concentration of 2 neutralizing units per unit of IFN_γ_ for in vitro blocking experiments to demonstrate specificity. Anti-human-IFN_γ_ (Genzyme, Cambridge, MA, U.S.A.) was used at a concentration of 5 _µ_g/ml for immunohistochemistry of MS lesions. Mouse anti-rat myelin-oligodendrocyte-glycoprotein (MOG) was used at a dilution of 1:200 (provided independently by Dr. Charissa Dyer and Dr. Minetta Gardinier). FITC-conjugated goat anti-rabbit antibodies, and FITC-conjugated goat anti-mouse antibodies were obtained from Sigma (St. Louis, MO, U.S.A.). Peroxidase-conjugated goat anti-mouse IgG (TAGO) was used at a dilution of 1:100.
Cytokines
Because of species specificity, all interferons used for in vitro experiments on rat glial cells were themselves from rat. Rat IFN_γ_ was recombinant (Gibco-BRL), and unless otherwise stated, was used at a concentration of 50 U/ml. Recombinant mouse tumor necrosis factor α (TNF_α_) (Gibco-BRL), which is active on rat cells, was used at a concentration of 100 ng/ml.
Histochemistry
Ten-micrometer cryostat sections of snap-frozen tissue from MS patients were mounted on gelatin-coated glass slides. Endogenous peroxidases were quenched by 30-min incubation with 3% H2O2 in PBS and 0.3% Triton X-100. Nonspecific sites were blocked with 5% milk in 0.3% Triton X-100 in PBS. Sections were incubated with primary antibodies overnight at 4°C and with peroxidase-conjugated secondary antibodies for 3 hr at room temperature. Peroxidase activity was detected by reaction with DAB and H2O2. Apoptotic nuclei were labeled in situ as previously described (25). Sections were permiabilized in ethanol:acetic acid (2:1), washed then incubated for 1 hr at 37°C with terminal deoxynucleotidyl transferase and Digoxigenin-dUTP. Labeled DNA was identified with peroxidase-conjugated goat anti-digoxegenin antibodies followed by reaction with H2O2 and DAB (Oncor, Gaithersberg, MD). Prior to incubation with anti-MOG antibodies for double labeling, remaining peroxidase activity was quenched with 3% H2O2.
Cell Culture
Oligodendrocyte progenitors were isolated from the forebrains of 1-day-old rats using a previously described method (26), with slight modifications (27). Cultures seeded with cells dissociated from P1 forebrains contain oligodendrocyte-type II astrocyte (O2A) progenitors, endothelial cells, microglia, neurons, and type II astrocytes, all of which grow on a monolayer of type I astrocytes. Following detachment of progenitors from the crude mixtures by a 15-hr 180 rpm shake, recovered cells, predominantly O2A progenitors, type II astrocytes, and microglia, were subjected to three successive incubations in 100 mm tissue culture dishes. This nonspecific panning enriched for O2A progenitors, because astrocytes, endothelial cells, and microglia adhere more rapidly to tissue culture plastic than do O2A progenitors. In addition, leucine methyl ester was added to the cells during panning, and similar to previous reports, this procedure reduced microglia to less than 0.5%, and astrocytes to less than 7% of the plating mixture (27). Viable cells remaining in suspension were then plated onto polyornithine-coated 25-mm glass coverslips in Dulbecco’s modified Eagle’s medium (DMEM) plus 5% fetal calf serum (Day 0). After 3 hr, plating medium was replaced with defined medium for oligodendrocytes (DMoli), which favors survival and growth of cells in the oligodendrocyte lineage or DMoli plus PDGF-AA and bFGF to maintain cells as O2A progenitors. DMoli contains DMEM (Gibco-BRL), 0.5% recrystallized BSA, 5 _µ_g/ml insulin, 100 _µ_M putrescine, 50 _µ_g/ml transferrin, 5 ng/ml selenium, 30 nM triiodothyronine, 20 nM progesterone, and 10 ng/ml D-biotin (Sigma). Prepared as described, cultures O2A cells or oligodendrocytes contained approximately 10% astrocytes as determined by GFAP staining, and approximately 0.5% ameboid microglia as determined by staining with diI-low-density lipoprotein (LDL).
Ameboid microglia were cultured as previously described (27). Mixed glial cultures were agitated on a rotary shaker for 15 hr at 37°C and 180 rpm. The cell suspension obtained was plated into new tissue culture flasks for 3 hr, after which flasks were gently rotated and nonadherent cells discarded. Adherent cells were removed by treatment with trypsin and vigorous shaking, and transferred to new tissue culture flasks containing fetal bovine serum (FBS) (15% final volume) for another three-hour interval. Non-adherent cells were removed, and adherent cells were composed of 95% dil-LDL positive microglia.
MTT AS A MEASURE OF CELL VIABILITY. Viability of cells was assessed using MTT, a tetrazolium salt cleaved with dehydrogenases of mitochondria to a water insoluble purple formazan (28,29). Three hours prior to the end of the incubation time the cultures were incubated with MTT to label living cells. Fifteen minutes prior to fixation the cultures were incubated with the O1 antibody to label surface galactocerebroside. At the end of the incubation time cultures were fixed with 4% paraformaldehyde followed with FITC-conjugated second antibody. Viable oligodendrocytes were defined by colabeling of MTT staining and Ol immunoreactivity.
DNA FRAGMENTATION. Oligodendrocytes were cultured as described above at a density of 5 × 104 cells/cm2 in 60-mm tissue culture dishes. Cultures were treated for 48 hr with either PBS (control), and both 24 and 48 hr with rat IFN_γ_ at 50 U/ml, then washed twice with ice-cold PBS (pH 7.4), and solubilized in lysis buffer (5 mM Tris, pH 7.4, 20 mM EDTA, 0.5% Triton X-100) as previously described (30). The supernatants from solubilized cells were extracted twice with Tris-buffered phenol (pH 8.0), then once with chloroform:isoamyl alcohol (24:1 v/v). Soluble nucleic acids were ethanol precipitated, resuspended in Tris-EDTA and digested with RNase A (50 _µ_g/ml) at 37°C for 30 min. DNA was resolved on 1.2% agarose gels, transferred to a Gene-screen Plus membrane (DuPont, Boston, MA), probed with random primed radiolabeled Rsal digested genomic rat DNA, and washed for 30 min at 50°C with 0.1X SSC and 0.1% SDS. Membranes were exposed to film for 12 hr with intensifying screens.
Results
Oligodendrocyte-type II astrocyte (O2A) progenitor cells are glial progenitors that can be isolated from 1-day-old rat forebrain (26) and derive their name from a bipotential nature in vitro (31). O2A cells are characterized by a distinct bipolar morphology and immunoreactivity with the A2B5 antibody (31). When cultured in a defined medium containing insulin or members of the LIF family of ligands (21,22), these cells pass through a pro-oligodendroblast stage (32) and differentiate into oligodendrocytes (31,32) characterized by extensive lace or sheet-like processes and expression of surface galactosylcerebroside recognized by the O1 antibody (33); when cultured in the presence of serum they differentiate into type II astrocytes (31) marked by stellate morphology and staining for glial fibrillary acidic protein (GFAP). The O2A cell can be perpetuated as a noncommitted bipotential progenitor when grown in the presence of PDGF-AA and bFGF (34). We studied the effects of recombinant rat IFN_γ_ on survival of rat O2A progenitors and oligodendrocytes derived from them. Because IFN_γ_ is a potent activator of blood borne monocytes (35,36), we sought to eliminate indirect effects by depleting the cultures of closely related ameboid microglia to less than 0.5% of the total number of cells (as determined by the presence of the high-affinity LDL receptor detected with dil-LDL [27]). Viability of O2A progenitors and oligodendrocytes was determined using the conversion of water soluble MTT into an insoluble purple precipitate by mitochondrial dehydrogenases (28,29). MTT-positive cells were also phase bright and excluded trypan blue.
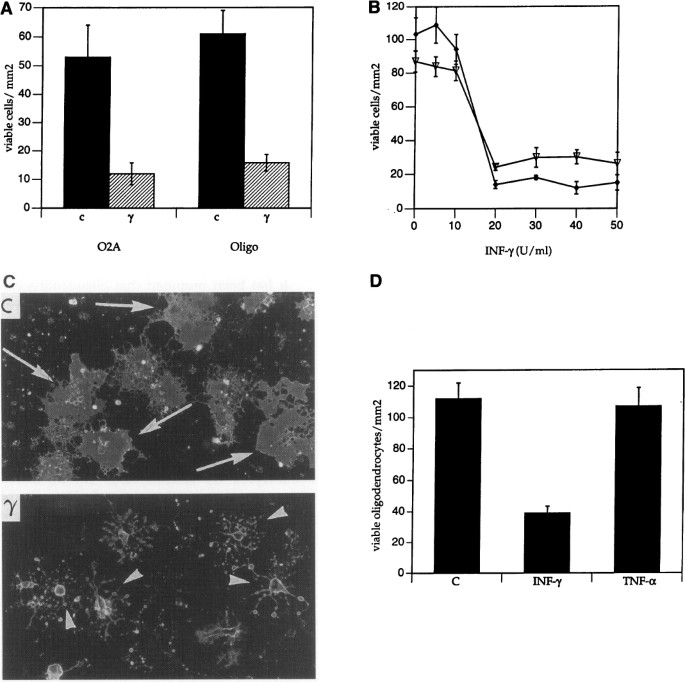
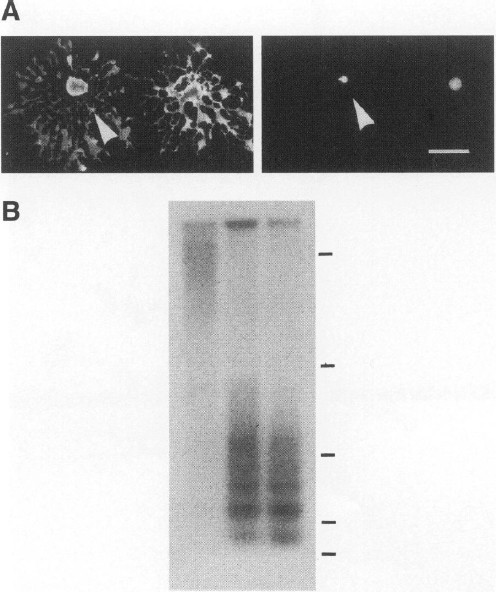
Exposure of cultures to rat IFN_γ_ (50 U/ml) for 2 days led to the death of 75% of O2A progenitors and oligodendrocytes (Fig. 1A). O2A progenitors are motile, and, prior to death, they migrated into clusters of 6–10 cells. The effect of IFN_γ_ on O2A progenitors and oligodendrocytes was dose dependent, with an EC50 of ∼15 U/ml (Fig. 1B). After 4 days of exposure to IFN_γ_, most of the cells had begun to demonstrate late morphologic changes of death, such as degeneration of processes into vacuoles (Fig. 1C).
Fig. 1
Effect of IFN γ on survival of O2A progenitors and oligodendrocytes.
(A) Number of viable O2A progenitor cells or oligodendrocytes as a function of a 48-hr exposure to control buffer or IFN_γ_ (γ). p < 0.01 for all comparisons. (B) O2A progenitor cell (♦) and oligodendrocyte (∇) survival as a function of IFN_γ_ concentration. (C) Micrographs of oligodendrocytes treated for four days with control buffer or IFN_γ_. Cells were stained for surface galactosylcerebroside with the O1 antibody. Viable oligodendrocytes with characteristic sheet-like processes (arrows) are shown in the control (c) panel. Late morphologic changes of cell death including degradation of processes into vacuoles are noted in the cultures treated with IFN_γ_ (γ) for 4 days (arrowheads). (D) Comparison of the effects of IFN_γ_ and TNF_α_ on oligodendrocyte survival.
It has been reported that TNF_α_, at concentrations of 100 ng/ml, may be toxic to oligodendrocytes (29,37). In cultures enriched for oligodendrocytes, with microglia accounting for less than 0.5% of the cells, we find no evidence for TNF_α_ (100 ng/ml)-induced oligodendrocyte cell death (Fig. 1C). Our preparation of TNF_α_ was active as it induced nitric oxide synthase activity in ameboid microglia.
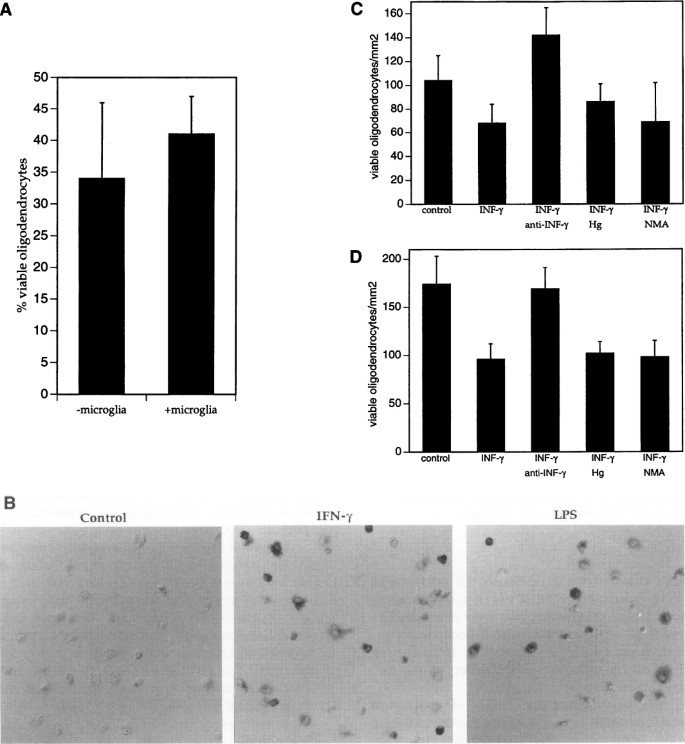
While we depleted oligodendrocyte cultures of microglia to approximately 0.5% of the cells, it was a remote possibility that IFN_γ_ was acting through a few contaminating microglia. To test this possibility, we performed the following experiments. First, we added equivalent numbers of ameboid microglia to cultures of oligodendrocytes and assessed the viability of oligodendrocytes with and without IFN_γ_ treatment. As noted in Fig. 2A, there was no significant difference in the percentage of oligodendrocyte killing by IFN_γ_ in the presence or absence of ameboid microglia.
Fig. 2
IFN γ -mediated oligodendrocyte death is a direct effect and not mediated through microglia or by nitric oxide
(A) Number of viable oligodendrocytes in cocultures of oligodendrocytes and ameboid microglia treated with IFN_γ_ or PBS for 48 hr. Oligodendrocytes and microglia were plated together each at a density of 10,000 cells/mm2 on glass coverslips. Cocultures were treated with IFN_γ_ or PBS. (B) NADPH diaphorase staining of rat ameboid microglia exposed to PBS (control), IFN_γ_, or LPS for 24 hr. NADPH diaphorase positive microglia stain intensly purple-black. (C) Number of viable oligodendrocytes 48 hr after coculture 1 mm beneath microglia containing inserts pretreated with IFN_γ_ or PBS. Ameboid microglia were cultured on membranes with 1.6 × 106 pores/cm2, and an average pore size of 0.45 µ_m. After treatment with IFN_γ or PBS for 24 hr, inserts were washed with five 30-min incubations in fresh culture medium. Washed inserts were then placed over coverslips of oligodendrocytes in the presence of PBS, anti-IFN_γ_ antibodies, hemoglobin (Hg) or N_ω_-methyl-L-arginine (NMA). (D) Number of viable oligodendrocytes 48 hr after coculture 1 mm beneath primary rat fibroblast containing inserts under conditions described above for Panel C.
It has been proposed that oligodendrocytes are susceptible to nitric oxide-mediated injury (38). Indeed, rat microglia express inducible nitric oxide synthase (NOS) in response to cytokines and lipopolysacharide (LPS). The presence of NOS can be detected by the NADPH diaphorase reaction which results in an insoluble purple-black precipitate (39,40). IFN_γ_ and LPS induce NOS activity in ameboid microglia, as determined by NADPH diaphorase staining (Fig. 2B). To assess the effects of nitric oxide on oligodendrocytes, we utilized tissue culture inserts with a highly permiable surface which can be placed in close apposition to cells grown in a tissue culture well. Microglia were cultured upon 0.45-µ_m high-density (1.6 × 106 pores/cm2) porous inserts, treated with IFN_γ or control solution for 24 hr, then washed five times with 30-min incubations in fresh culture medium. Inserts were then placed 1 mm above cultures of oligodendrocytes in the presence of nitric oxide scavengers (reduced hemoglobin, 500 µ_M), the NOS inhibitor N_ω_-methyl-L-arginine (500 µ_M) both during washes as well as coculture, anti-IFN_γ antibodies (200 neutralizing units/ml), or control buffer. After 48 hr of coculture we observed a 30%–40% decrease in the number of viable oligodendrocytes in all conditions except with anti-IFN_γ antibodies (Fig. 2C). The absence of reversal in the presence of nitric oxide and NOS inhibitors suggested to us that nitric oxide was not toxic to oligodendrocytes, but rather that IFN_γ_ was either inducing synthesis of IFN_γ_ in, or that it was adhering to microglia, being released during coculture and killing oligodendrocytes by a direct pathway. To assess these possibilities, we performed the same experiments as described for Fig. 2C except this time using fibroblasts in place of microglia. To our surprise, we obtained similar results (Fig. 2D), suggesting that IFN_γ_ was adhering to cells on the inserts since fibroblast do not synthesize IFN_γ_. Taken with the data in Fig. 1, our in vitro work supports direct killing of oligodendrocytes by IFN_γ_.
A major feature of the histopathology of MS is that there is little neuronal injury or axonal loss (1–3). We thus investigated the effects of rat IFN_γ_ on the viability of sensory neurons in vitro. Neurons isolated from E15 rat dorsal root ganglia were cultured for 2 weeks in the presence of nerve growth factor (NGF) (41). Contaminating fibroblasts and Schwann cells were eliminated by treatment of the cultures with 5-flourodeoxyuridine as previously described (41). IFN_γ_ did not significantly influence the viability of this cell population (Fig. 3).
Fig. 3
Effect of IFN γ on the survival of sensory neurons
DRG neurons were cultured in the presence of NGF on a polyornithine/laminin substratum. After 2 weeks, in vitro cultures were exposed to control solution, or IFN_γ_ for 7 days. Viability was assessed with MTT conversion to an insoluble purple precipitate shown above. There is a small decrease in the number of viable sensory neurons, which does not reach a statistical significance (controls, 126 ± 16 versus IFN_γ_, 103 ± 9.7) morphology and neuritic outgrowth from IFN_γ_ treated neurons is indistinguishable from controls. Bar = 10 _µ_m.
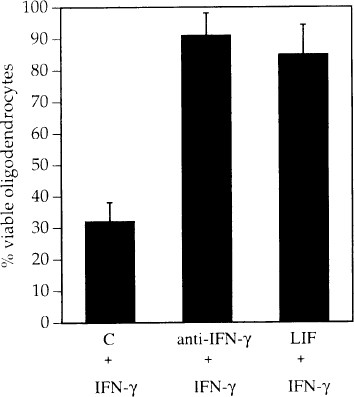
We investigated the ability of concurrent treatment with human LIF at a concentration of 1000 U/ml to protect oligodendrocytes from IFN_γ_ (50 U/ml)-mediated cell death. LIF increased the number of viable oligodendrocytes over cultures treated with IFN_γ_ alone (Fig. 4). The effect of IFN_γ_ was also blocked with pretreatment by a monoclonal anti-rat-IFN_γ_ antibody (Fig. 4).
Fig. 4
Reversal of IFN γ -induced oligodendrocyte death by human LIF (1000 U/ml) and anti-rat-IFN γ antibodies
Concentrations used were determined to be those which generated a maximal effect in dose-dependent assays (not shown). All cultures including control (c) received rat IFN_γ_ (1000 U/ml) plus the indicated reagents.
Oligodendrocytes treated for 18 hr with IFN_γ_ have pyknotic nuclei with preservation of the plasmalemma suggestive of apoptosis (Fig. 5A). Another feature frequently associated with apoptosis is degradation of DNA into nucleosome-sized fragments, which can be visualized as DNA ladders when resolved by agarose electrophoresis (42). We find that IFN_γ_-treated O2A cells and oligodendrocytes demonstrate degradation of DNA into nucleosome-sized fragments consistent with apoptosis (Fig. 5B).
Fig. 5
IFN γ -induced glial cell death is apoptotic
(A) IFN_γ_ treated oligodendrocytes have pyknotic nuclei. Oligodendrocytes were treated with IFN_γ_ for 18 hr and then stained with the O1 antibody detected with FITC-conjugated goat anti-mouse IgM (left panel). Nuclei were counterstained with Hoechst dye (right panel). The figure shows the same field of an IFN_γ_-treated culture. Arrow head indicates a cell with normal morphology with O1 staining but a pyknotic nucleus with Hoechst stain (bar = 10 µ_m). (B) DNA fragmentation in IFN_γ treated cultures. Oligodendrocytes were cultured as described in the methods section. Begining on Day 2 of culture, cells were treated with PBS for 48 hr (Lane 1) or IFN_γ_ for 24 and 48 hr (Lanes 2 and 3, respectively). Soluble DNA fragments were isolated and resolved on a 1.2% agarose gel, transfered to a Gene Screen Plus membrane, and probed with 32P-labeled rat genomic DNA. The membrane was exposed to film for 12 hr and the autoradiograph is shown. Bars at right represent the following sizes in base pairs from top to bottom: 9416, 2322, 872, 310, and 192.
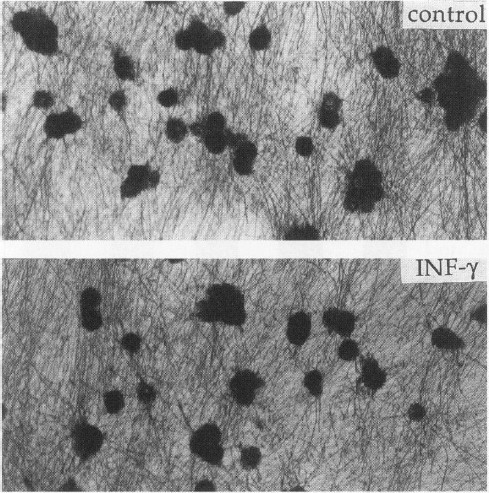
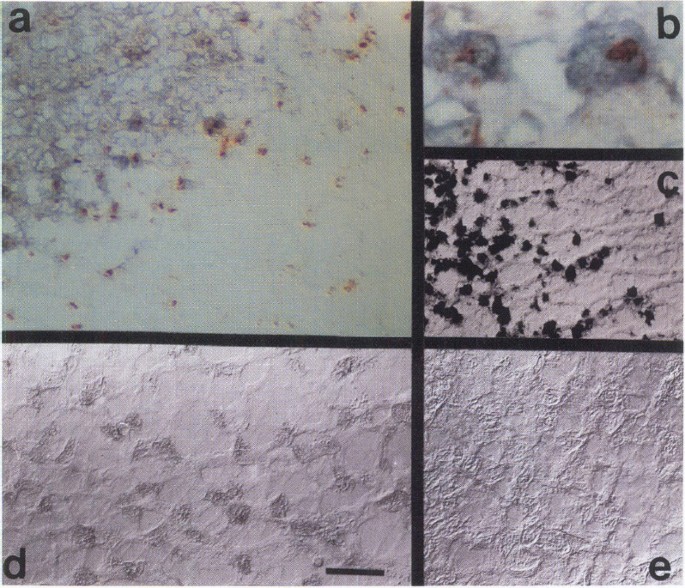
Degraded nuclear DNA of apoptotic cells can also be detected in situ by terminal labeling of the 3′-OH ends with digoxigenin-dUTP in the presence of terminal deoxynucleotide transferase (25). Tailed 3′-OH groups are identified with peroxidase-conjugated antibodies directed against digoxigenin and subsequent reaction with DAB and H2O2. This method was used to identify apoptotic cells, and staining with a monoclonal antibody against the MOG was used to label oligodendrocytes (43). Snap-frozen tissue containing four chronic active lesions from four different patients were obtained at autopsies that were performed between 4 and 12 hr after death (see Acknowledgments). These samples were studied, and all showed results similar to those in Figs. 6 and 7. The advancing margins of chronic active MS plaques, but not unaffected white matter, contain numerous pyknotic, apoptotic nuclei within MOG labeled cells (Fig. 6 a and b). The labeled nuclei are not clustered in a contiguous mass of dead cells as in necrosis but rather are scattered amongst normal cells throughout the advancing margin of the lesion, a finding consistent with apoptosis (17–20). Nuclei in adjacent unaffected white matter (Figs. 6 and 7) and normal controls (not shown) do not label with the terminal transferase technique.
Fig. 6
Identification of apoptotic oligodendrocytes and IFN γ -positive cells at the margins of chronic active MS lesions
Cryostat sections of fresh frozen tissue from MS patients (17) were processed for colabeling apoptotic nuclei (brown) and MOG (purple/black), (a and b), human IFN_γ_ (d and e), and oil-O-red to identify active phagocytic cells (c). Panels a–d show a chronic active MS lesion, whereas Panel e shows a region of unaffected white matter from the same patient. The tissue was removed at autopsy performed 4 hr after death and frozen. Some freezing artifacts are apparent on the sections.
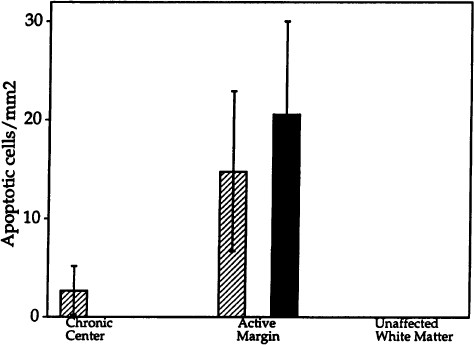
Fig. 7
Quantitation of apoptosis in the center and at the margin of the chronic active MS lesion shown in Fig. 6
Ten nonoverlapping 200X fields that comprised approximately half of the area occupied by the chronic center of the lesion and eight nonoverlapping contiguous 200X fields that comprised the majority of area occupied by the active margin were averaged. In addition, 10 random fields within unaffected white matter were counted. Apoptotic oligodendrocytes (filled bars) and apoptotic cells which did not colabel for the oligodendrocyte-specific protein MOG (hatched bars) were counted.
Our in vitro results suggest to us that IFN_γ_ could play a role in the pathogenesis of diseases that entail death of oligodendrocytes. Therefore, we searched for IFN_γ_ in snap-frozen tissue from autopsies of MS patients. Using a monoclonal antibody raised against recombinant human IFN_γ_ we find the cytokine at the advancing margins of active MS plaques but neither in adjacent unaffected white matter nor in the chronic portion of the plaques (Fig. 6 d and e). The IFN_γ_ is within cells with the morphology of macrophages or activated microglia (Fig. 6d).
Discussion
We have shown that IFN_γ_ induces programmed death of oligodendrocytes and their precursors in vitro. Furthermore, we and others (23) find IFN_γ_ in active MS lesions where we now find evidence for apoptosis of oligodendrocytes. Taken together, our data support the idea that IFN_γ_ may play a role in the pathogenesis of MS by inducing apoptosis of oligodendrocytes.
The antiviral effects of IFN_γ_ prompted its use in clinical trials in MS (44). Patients who received IFN_γ_ had an increased frequency and severity of attacks (44,45). Furthermore, a rise in cerebrospinal fluid (CSF) IFN_γ_ levels precedes exacerbations of MS (46–51). It has been suggested that the influence of IFN_γ_ on the clinical course of MS is mediated through its effects on cells of the immune system (52–55), or through induction of MHC class II antigens on oligodendrocytes (56). Our results suggest that the detrimental effects of IFN_γ_ on the course of MS may, at least in part, arise from direct toxicity to oligodendrocytes themselves. In fact, IFN_γ_ has also been shown to potentiate demyelination in Sprague-Dawley rats mediated by anti-MOG antibodies (55).
In addition to our results with IFN_γ_, glutamate has been shown to injure oligodendrocytes in vitro (57). Oligodendrocytes and O2A cells have well described glutamate receptors (58,59); however, glutamate-mediated toxicity occurs in many neuronal populations and is not likely to result in a specific oligodendrocyte death as seen in MS. It has been postulated that complement plays a role in the pathogenesis of multiple sclerosis (4,8,9), and complement alone is indeed toxic to oligodendrocytes in vitro (8,9). However, this theory would predict that any disruption of the blood brain barrier should result in injury to oligodendrocytes as an innocent bystander, which does not seem to be the case.
We do not observe a toxic effect of TNF_α_ on oligodendrocytes in vitro as previously reported (37,40). This difference in results might arise from the culture systems used. Investigators who have found an effect of TNF_α_ on oligodendrocytes have used either an O2A-like cell line, CG4, which may respond differently than primary O2A cells, or primary cultures from which microglia were not eliminated (37,39,40). Other laboratories have also failed to observe a direct toxic effect of TNF_α_ on oligodendrocytes (38). Cerebral malaria is as an example of a disease where TNF_α_ is present in high concentration and the blood-brain barrier is disrupted (60). However, there is no specific injury to the oligodendrocyte-myelin unit in cerebral malaria, and thus it seems that TNF_α_ is not always toxic to oligodendrocytes. This is not to say that in the context of other cytokines present in MS lesions TNF_α_ would not injure oligodendrocytes.
The effects of IFN_γ_ on oligodendrocytes were partially reversed with pretreatment or concurrent treatment with LIF. It is of interest that IFN_γ_, which adversely affected the outcome of MS, is also a potent inducer of oligodendrocyte death in vitro (45,61,62).
References
- Dawson JW. (1916) The histology of disseminated sclerosis. Trans. R. Soc. Edinburgh 50: 517–740.
Article Google Scholar - Lumsden CE. (1985) In: Vinken PJ, Bruyn GW (eds) Handbook of Clinical Neurology: The Neuropathology of Multiple Sclerosis. Elsevier Science, Amsterdam, Vol. 9, pp. 217–309.
- Raine CS, Scheinberg L, Waltz JM. (1981) Oligodendrocyte survival and proliferation in an active established lesion. Lab. Invest. 45: 534–546.
PubMed CAS Google Scholar - Matthews WB, Compston A, Allen IV, Martyn CN (eds). (1991) McAlpine’s Multiple Sclerosis. Churchill Livingstone, New York, pp. 379–390.
Google Scholar - Gulcher J, Vartanian T, Stefansson K. (in press) Is multiple sclerosis an autoimmune disease? Clin. Neurosci.
- Griffin DE, Moser HW, Mendoza Q, et al. (1985) Identification of the inflamatory cells in the central nervous system of patients with adrenoleukodystrphy. Ann. Neurol. 18: 660–669.
Article CAS PubMed Google Scholar - Hirano A, Dembitzer HM, Becker NH, et al. (1970) Fine structure alterations of the blood brain barrier in experimental allergic encephalomyelitis. J. Neuropathol. Exp. Neurol. 29: 432–440.
Article CAS PubMed Google Scholar - Compston A, Scolding N, Wren D, Noble M. (1991) The pathogenesis of demyelinating disease: Insights from cell biology. Trends Neurosci. 14: 175–182.
Article CAS PubMed Google Scholar - Wren DR, Noble M. (1989) Oligodendrocytes and O2A progenitor cells of adult rats are specifically susceptible to the lytic effects of complement in the absence of antibody. Proc. Natl. Acad. Sci. U.S.A. 86: 9025–9029.
Article CAS PubMed PubMed Central Google Scholar - Scolding NJ, Morgan BP, Houston WAJ, et al. (1989) Vesicular removal by oligodendrocytes of membrane attack complexes formed by complement. Nature 339: 620–622.
Article CAS PubMed Google Scholar - Reder AT, Arnason BGW. (1985) In: Vinken PJ, Bruyn GW (eds) Handbook of Clinical Neurology: Demyelinating Diseases. Elsevier Science, Amsterdam, Vol. 3, pp. 337–395.
- Hafler DA, Weiner HL. (1987) T cells in multiple sclerosis and inflammatory central nervous system diseases. Immunol. Rev. 100: 308–332.
Article Google Scholar - Hauser SL, Bhan AK, Gilles F, et al. (1986) Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann. Neurol. 19: 578–587.
Article CAS PubMed Google Scholar - Booss J, Esiri MM, Tourtellotte WW, et al. (1983) Immunohistochemical analysis of T-lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J. Neurol. Sci. 62: 19–32.
Article Google Scholar - Traugott U, Reinher EL, Raine CS. (1983) Multiple Sclerosis: Distribution of T-cell subsets within chronic active lesions. Science 219: 308–310.
Article CAS PubMed Google Scholar - Prineas JW, Kwon EE, Goldenberg PZ, et al. (1989) Multiple sclerosis: Oligodendrocyte proliferation and differentiation in fresh lesions. Lab. Invest. 5: 489–503.
Google Scholar - Kerr JF, Wyllie AH, Currie AR. (1972) Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue genetics. Br. J. Cancer 26: 239–257.
Article CAS PubMed PubMed Central Google Scholar - Raff MC. (1992) Social controls on cell survival and cell death. Nature 356: 397–400.
Article CAS PubMed Google Scholar - Williams GT, Smith CA. (1993) Molecular regulation of apoptosis: Genetic controls on cell death. Cell 74: 777–779.
Article CAS PubMed Google Scholar - Vaux DL. (1993) Toward an understanding of the molecular mechanisms of physiologic cell death. Proc. Natl. Acad. Sci. U.S.A. 90: 786–789.
Article CAS PubMed PubMed Central Google Scholar - Barres BA, Hart IK, Coles HSR, et al. (1992) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70: 31–46.
Article CAS PubMed Google Scholar - Barres BA, Schmid R, Sendtner M, Raff MC. (1993) Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 118: 283–295.
PubMed CAS Google Scholar - Traugott U, Lebon J. (1988) Interferon-γ and Ia antigen are present on astrocytes in active chronic multiple sclerosis lesions. Neurol. Sci. 84: 257–264.
Article CAS Google Scholar - Sommer I, Schachner M. (1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytochemical study in 742 the central nervous system. Dev. Biol. 83: 311–327.
Article CAS PubMed Google Scholar - Gavrieli Y, Sherman Y, Ben-Sasson A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell. Biol. 119: 493–501.
Article CAS PubMed Google Scholar - McCarthy K, de Vellis JJ. (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell. Biol. 85: 890–892.
Article CAS PubMed Google Scholar - Giulian D, Baker TJ. (1986) Characterization of ameboid mocroglia isolated from developing mammalian brain. J. Neurosci. 6: 2163–2178.
Article CAS PubMed Google Scholar - Carmichael J, DeGraff WG, Gazdar AF, et al. (1987) Evolution of tetrazolium-based semi-automated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 47: 936.
PubMed CAS Google Scholar - Louis J-C, Magal E, Takayama S, Varon S. (1993) CNTF protection of oligos against natural and tumor necrosis factor-induced death. Science 259: 689–692.
Article CAS PubMed Google Scholar - Rosenbaum DM, Michaelson M, Batter DK, et al. (1994) Evidence for hypoxia-induced programmed cell death of cultured neurons. Ann. Neurol. 36: 864–870.
Article CAS PubMed Google Scholar - Raff MC, Miller RH, Noble M. (1993) A glial progenitor cell that develops in vitro into and astrocyte or an oligodendrocyte depending on culture medium. Nature 303: 390–396.
Article Google Scholar - Gard AL, Pfeiffer SE. (1990) Two proliferative stages of the oligodendrocyte lineage (A2B5+O4− and O4+GalC−) under different mitogenic control. Neuron 5: 615–625.
Article CAS PubMed Google Scholar - Raff MC, Mirsky R, Fields KL, et al. (1978) Galacterocerebroside is a specific cell surface antigenic marker for oligodendrocytes in culture. Nature 274: 813–816.
Article CAS PubMed Google Scholar - Wren D, Wolswijk G, Noble M. (1992) In vitro analysis of the origin and maintenance of O2A (adult) progenitor cells. J. Cell. Biol. 116: 167–176.
Article CAS PubMed Google Scholar - Arenzana-Seisdedos F, Virelizier JL. (1983) Interferons as macrophage-activating factors II. Enhanced secretion of interleukin 1 by lipopolysaccharide-stimulated human monocytes. Eur. J. Immunol. 13: 437–440.
Article CAS PubMed Google Scholar - Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. (1993) Cytotoxicity of microglia. Glia 7: 111–118.
Article CAS PubMed Google Scholar - Selmaj KW, Raine CS. (1988) Tumor necrosis factor mediates myelin and olgiodendrocyte damage in vitro. Ann. Neurol. 23: 339–346.
Article CAS PubMed Google Scholar - Merrill JE, Ignarro LJ, Sherman MP, et al. (1993) Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J. Immunol. 151: 2132–2141.
PubMed CAS Google Scholar - Dawson TM, Bredt DS, Fotuhi M, et al. (1988) Nitric oxide synthase and neuronal NADPH diaphorase are identicle in brain and peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 88: 7797–7801.
Article Google Scholar - Hope BT, Michael GJ, Knigge KM, et al. (1988) Neuronal NADPH-diaphorase is a nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 88: 2811–2814.
Article Google Scholar - Kleitman N, Wood PM, Bunge RP. (1991) Tissue culture methods for the study of myelination. In: Banker G, Goslin K (Eds). Culturing Nerve Cells. MIT Press, Cambridge, MA pp. 335–377.
Google Scholar - Arends MJ, Morris RG, Wyllie AH. (1990) The role of the endonuclease. Am. J. Pathol. 136: 593–608.
PubMed PubMed Central CAS Google Scholar - Linington C, Webb M, Woodhams PL. (1984) A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J. Neuroimmunol. 6: 387–396.
Article Google Scholar - Panitch HS, Haley AS, Hirsch RL, Johnson KP. (1987) Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 1: 893–895.
Article CAS PubMed Google Scholar - Arnason BGW. (1993) Interferon beta in multiple sclerosis. Neurology 43: 641–643.
Article CAS PubMed Google Scholar - Salonen R. (1983) CSF and serum interferon in multiple sclerosis: A longitudinal study. Neurology 33: 1604–1606.
Article CAS PubMed Google Scholar - Beck J, Randot P, Catinot P, et al. (1988) Increased production of interferon-gamma and tumor necrosis factor preceding clinical manifestation in multiple sclerosis: Do cytokines trigger off exacerbations? Acta Neurol. Scand. 78: 318–323.
Article CAS PubMed Google Scholar - Maimone D, Gregory S, Arnason BGW, Reder AT. (1991) Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J. Neuroimmunol. 32: 67–74.
Article CAS PubMed Google Scholar - Weller M, Stevens A, Sommer N, et al. (1991) Comparative analysis of cytokine patterns in immunological, infectious, and oncological neurological disorders. Neurol. Sci. 104: 215–221.
Article CAS Google Scholar - Merrill JE. (1992) Proinflammatory and antiinflammatory cytokines in multiple sclerosis and central nervous system acquired immunodeficiency 743 syndrome. J. Immunotherapy 12: 167–170.
Article CAS Google Scholar - Imamura K, Suzamura A, Hayashi F, Marunouchi T. (1993) Cytokine production by peripheral blood monocytes/macrophages in multiple sclerosis patients. Acta Neurol. Scand. 87: 281–285.
Article CAS PubMed Google Scholar - Simmons RD, Willenborg DO. (1990) Direct injection of cytokines into the spinal cord causes autoimmune encephalomyelitis-like inflammation. J. Neurol. Sci. 100: 37–42.
Article CAS PubMed Google Scholar - Benveniste EN. (1992) Inflammatory cytokines within the central nervous system: Sources, function, and mechanism of action. Am. J. Physiol. 263: C1–C16.
Article CAS PubMed Google Scholar - Olson T. (1992) Cytokines in neuroinflammatory disease: Role of myelin autoreactive T cell production of interferon-gamma. J. Neuroimmunol. 40: 211–218.
Article Google Scholar - Vass K, Heininger K, Schafer B, et al. (1992) Interferon-γ potentiates antibody-mediated demyelination in vivo. Ann. Neurol. 32: 198–206.
Article CAS PubMed Google Scholar - Bergsteinsdottir K, Brennan A, Jessen KR, Mirsky R. (1992) In the presence of dexamethasone, gamma interferon induces rat oligodendrocytes to express major histocomplatibility complex class II molecules. Proc. Natl. Acad. Sci. U.S.A. 89: 9054–9058.
Article CAS Google Scholar - Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. (1993) Vulnerability of oligodendroglia to glutamate: Pharmacology, mechanisms, and prevention. J. Neurosci. 13: 1441–1453.
Article CAS PubMed Google Scholar - Eitan S, Zisling R, Cohen A, et al. (1992) Identification of an interleukin 2-like substance as a factor cytotoxic to oligodendrocytes and associated with central nervous system regeneration. Proc. Natl. Acad. Sci. U.S.A. 89: 5442–5446.
Article CAS PubMed PubMed Central Google Scholar - Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. (1994) Glial cells of the oligodendrocyte lineage express both kainate-and AMPA-preferring subtypes of the glutamate receptor. Neuron 12: 357–371.
Article CAS PubMed Google Scholar - Pende M, Holtzclaw LA, Curtis JL, Russell JT, Gallo V. (1994) Glutamate regulates intracellular calcium and gene expression in oligodendrocyte progenitors through the activation of AMPA receptors. Proc. Natl. Acad. Sci. U.S.A. 91: 3215–3219.
Article CAS PubMed PubMed Central Google Scholar - Grau GE, Fajardo LF, Piguet PF, et al. (1987) Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237: 1210–1212.
Article CAS PubMed Google Scholar - The IFN-β Multiple Sclerosis Study Group. (1993) Interferon beta-Ib is effective in relapsing-remitting multiple sclerosis. Neurology 43: 655–661.
Article Google Scholar - Paty DW, Li DKB. (1993) The IFN_β_ Multiple Sclerosis Study Group. Interferon beta-lB is effective in relapsing-remitting multiple sclerosis. Neurology 43: 662–667.
Article CAS PubMed Google Scholar
Acknowledgments
Tissue specimens obtained from the National Neurological Research Specimen Bank, VAMC, Los Angeles, CA 90073, which is sponsored by NINDS/NIMH, National Multiple Sclerosis Society, Hereditary Disease Foundation, Comprehensive Epilepsy Program, Tourette Syndrome Association, Dystonia Medical Research Foundation, and Veterans Health Services and Research Administration, Department of Veterans Affairs.
This work was supported in part by grants from the National Institutes of Health. T. Vartanian is a recipient of a Clinical Investigator Development Award from the National Institute of Neurologic Disorders and Stroke.
Author information
Authors and Affiliations
- Departments of Neurology and Pathology, Beth Israel Hospital, Boston, Massachusetts, USA
Timothy Vartanian, You Li, Meijuan Zhao & Kari Stefansson
Authors
- Timothy Vartanian
- You Li
- Meijuan Zhao
- Kari Stefansson
Additional information
Contributed by R. Cotran on July 8, 1995.
Rights and permissions
About this article
Cite this article
Vartanian, T., Li, Y., Zhao, M. et al. Interferon-_γ_-Induced Oligodendrocyte Cell Death: Implications for the Pathogenesis of Multiple Sclerosis.Mol Med 1, 732–743 (1995). https://doi.org/10.1007/BF03401888
- Published: 01 November 1995
- Issue Date: November 1995
- DOI: https://doi.org/10.1007/BF03401888