Colonoscopy: Background, Indications, Contraindications (original) (raw)
Overview
Background
Colonoscopy enables visual inspection of the entire large bowel (ie, the colon or large intestine) from the distal rectum to the cecum. It remains the gold standard for the detection of polyps and colorectal cancer (CRC). The procedure is a safe and effective means of evaluating the large bowel. The technology for colonoscopy has evolved to be capable of providing a very clear image of the mucosa through a video camera attached to the end of the scope. The camera connects to a computer, which can store and print color images selected during the procedure.
Screening for and surveillance of CRC are among the indications for colonoscopy. Although CRC is highly preventable, it remains the second most common cause of cancer death in the United States. Both men and women face a lifetime risk of nearly 6% for the development of invasive colorectal cancer. Proper screening can help reduce mortality at all ages, and colonoscopy plays an important role in this effort.
Additionally, advances in endoscopic resection methods, notably endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR), have positioned endoscopic resection as the primary approach for treating early-stage CRC. [1]
When assigning appropriate screening and surveillance intervals, endoscopists should follow current guidelines. Repeat colonoscopy should be performed in 3 years on all patients with advanced adenomas. If, in average-risk patients, screening colonoscopies are normal or only small distal hyperplastic polyps are present, repeat examinations should not be performed before 10 years. [2]
Compared with other imaging modalities, colonoscopy is especially useful for detecting small lesions (eg, adenomas) that can develop into cancer; however, its main advantage is that it also allows for intervention, because biopsies and polypectomies can be performed. Colonoscopy plays a crucial role in managing large-bowel obstruction (LBO), enabling not only the diagnosis of various LBO-related diseases but also the performance of therapeutic interventions (eg, balloon dilatation for benign strictures and insertion of endoluminal metal stents for malignant obstructions). [3]

Indications
Screening, evaluation, and follow-up of colorectal cancer
Screening in average-risk adults
Recommendations for CRC screening have varied among the leading organizations in this field—namely, the American Cancer Society (ACS), [4, 5] the World Health Organization (WHO), the US Preventive Services Task Force (USPSTF), the American College of Physicians (ACP), and the American Gastroenterological Association (AGA). [2] It is now generally recommended, however, that average-risk adults should begin CRC screening at age 45 years.
There are a few approved screening options, of which colonoscopy every 10 years is the most common in the United States. Screening for CRC is recommended to continue up to the age of 75 years for patients at average risk, provided they have a life expectancy of 10 years or more.
Other tests used to screen for CRC include annual fecal occult blood testing (FOBT), fecal immunohistochemical testing (FIT), and stool DNA testing (multitarget DNA testing). In current practice, barium enema is rarely performed; newer modalities, such as computed tomography (CT) colonography, have gained wider acceptance. It is crucial to counsel patients regarding these other CRC screening modalities. Randomized trials evaluating patient preferences for CRC screening tests found that participation rates were higher among those assigned to FIT than among those screened with stool guaiac tests, sigmoidoscopy, or colonoscopy. [6]
Evaluation and removal of polyps
The finding of a polyp larger than 1 cm in diameter during sigmoidoscopy is an indication for examination of the entire colon because 30-50% of these patients have additional polyps. Although controversy continues regarding whether colonoscopy is indicated for patients with a polyp or polyps smaller than 1 cm, the general belief is that most cancers arise in preexisting adenomatous polyps, which should lead to a full colonoscopic examination, regardless of size.
Polypoid lesions observed on barium enema may represent pseudopolyps, true polyps, or carcinomas. Colonoscopy can be used to differentiate among these and can similarly be used to distinguish between benign and malignant strictures, which cannot be accurately accomplished with radiologic studies alone.
When clinical signs and symptoms suggest colon cancer or when screening (by radiography or sigmoidoscopy) identifies a large-bowel tumor, a full colonoscopic examination should be performed to obtain biopsy samples and to search for synchronous lesions. Findings on colonoscopy may also have implications for the surgical treatment plan.
Histologic diagnosis should be based on examination of the completely excised polyp. In general, all polypoid lesions greater than 0.5 cm in diameter should be totally excised. After a large (>2 cm) sessile polyp has been removed or if there is concern that an adenoma was not completely excised, repeat colonoscopy should generally be performed in 3-4 months. If residual tissue remains, it should be resected and colonoscopy repeated again in another 3-4 months.
In patients with polyps identified on initial examination, the ACS has recommended that follow-up colonoscopy be performed on the basis of polyp number and type, as well as dysplasia grade, as follows [4] :
- Patients with small rectal hyperplastic polyps can be treated as patients with an average risk of cancer, with colonoscopy or other screening on a similar schedule
- Individuals with one or two sub-1-cm tubular adenomas with low-grade dysplasia should receive colonoscopy 5-10 years after polyp removal
- Patients who have three to 10 adenomas or one adenoma larger than 1 cm or who have any adenomas with high-grade or villous features should receive follow-up colonoscopy 3 years after removal
- Patients who have more than 10 adenomas on initial examination should undergo colonoscopy within 3 years
- Patients with sessile adenomas that are removed in pieces should undergo follow-up colonoscopy 2-6 months after removal
Endoscopic ultrasonography
Endoscopic ultrasonography (EUS) can be used to evaluate lesions that represent invasive cancer. The combination of endoscopy and ultrasonography (US) makes it possible to look closely at the different layers of the wall of the rectum (mucosa, submucosa, muscularis propria, etc). EUS is especially helpful in cases of rectal cancer, in that it can reliably identify the level of invasion of the carcinoma and accurately determine the stage. These findings have significant ramifications for subsequent steps in the treatment of rectal cancer.
Current or previous bowel resection for colon cancer
Because of the potential implications for the operative plan, preoperative colonoscopy should be performed in patients who are to undergo bowel resection for colon cancer. Patients who have already had a large-bowel cancer removed should have a colonoscopy performed 6 months to 1 year after surgery, followed by yearly colonoscopy on two occasions. Some authorities believe that colonoscopy should then be performed every 3 years if the results of all these studies are negative.
Family history of cancer
Individuals with a family history of familial adenomatous polyposis (FAP) or Gardner syndrome should undergo genetic testing and flexible sigmoidoscopy or colonoscopy every 12 months, beginning at age 10-12 years and continuing until age 35-40 years if negative. Total colectomy should be considered for these individuals because they have a nearly 100% risk of developing colon cancer by age 40 years. Colonoscopy is not as effective in preventing colon cancer under these circumstances as it is with polyps in general.
Individuals who have a first-degree relative diagnosed with colon cancer or adenomas when younger than 60 years, or who have multiple first-degree relatives diagnosed with colon cancer or adenomas, should undergo screening colonoscopy every 3-5 years, beginning either at age 40 years or at an age 10 years younger than that of the earliest familial diagnosis, whichever comes first. [7]
The diagnosis of hereditary nonpolyposis CRC (HNPCC) should be considered in people who have several relatives with CRC, particularly if one or more of the relatives developed cancer when younger than 50 years. HNPCC is an autosomal dominant disorder with an approximately 70% lifetime risk of developing CRC.
These patients should be evaluated colonoscopically every 1-2 years, beginning at age 20-25 years or at an age 10 years younger than that of onset in the index case (whichever comes first). Annual screening is indicated in patients older than 40 years.
Management of inflammatory bowel disease
Although in many cases, colonoscopy is not required for the diagnosis of inflammatory bowel disease (IBD), it is an important aid in the follow-up care and management of patients with ulcerative colitis (UC) or Crohn disease (CD). (See the images below.) Colonoscopy is more sensitive than barium enema in determining the anatomic extent of the inflammatory process and is useful when clinical, sigmoidoscopic, and radiologic studies are inadequate. Colonoscopy with multiple biopsies is indicated to differentiate UC from CD. Given that CD frequently affects the terminal ileum, it is crucial to intubate the terminal ileum during a colonoscopy.
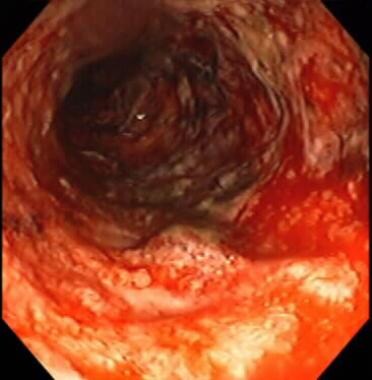
Inflammatory bowel disease. Severe colitis noted during colonoscopy. Mucosa is grossly denuded, with active bleeding noted. Patient had her colon resected very shortly after this view was obtained.
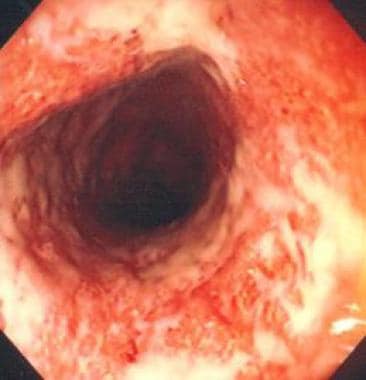
Colonoscopy. Ulcerative colitis as visualized with colonoscope.
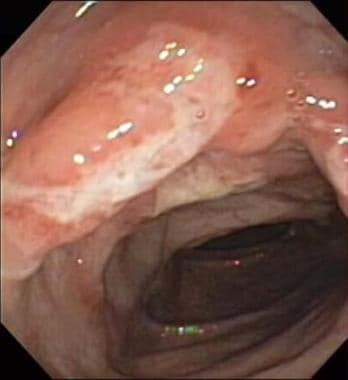
Colonoscopy. Colonoscopic image of large ulcer and inflammation of descending colon in 12-year-old boy with Crohn disease.
The cancer surveillance schedule varies in patients with IBD. Patients with pancolitis for more than 7-10 years and patients with left-side UC for more than 15 years are at increased risk for the development of colon cancer. The current recommendation for screening colonoscopy for these groups is every 1-2 years. For patients with CD of the colon, the same schedule of colonoscopic surveillance is warranted. The current recommendation for screening colonoscopy for patients with IBD is to start 8 years after the onset of symptoms.
Ideally, because differentiating inflammatory changes from premalignant ones can be difficult, colonoscopy for surveillance purposes should not be performed during periods of active colitis, and biopsies from areas of less inflammation should be preferred. It has been suggested that as many as 64 biopsies are needed to achieve 95% sensitivity in surveying for dysplasia in patients with IBD.
Newer technologies, including chromoendoscopy, magnification endoscopy, and narrow-band imaging, may improve detection of dysplasia during surveillance colonoscopy and allow endoscopists to take fewer but higher-yield biopsies.
Endoscopic dilation for inflammatory bowel disease
IBD, particularly CD, often leads to complications such as stricture, which can affect as many as 33% of CD patients within 10 years after the diagnosis is made. Strictures necessitate surgical or endoscopic intervention, but repeated surgical procedures raise the risk of short-bowel syndrome. Endoscopic balloon dilatation, which offers a technical success rate of 89% and a clinical success rate of 81%, is preferred for its conservation of bowel length, though it is contraindicated for patients who have fistulas, deep ulcers, or strictures longer than 5 cm. [8, 9]
Identification and treatment of acute bleeding sites
In the case of lower gastrointestinal (GI) bleeding, colonoscopy can be useful not only for localizing the site of bleeding but also, potentially, for enabling therapeutic intervention. Endoscopic therapy using injection of epinephrine, electrocauterization, argon plasma coagulation (APC), band therapy, or combinations thereof can be applied to the treatment of various causes of lower GI bleeding, including postpolypectomy coagulation syndrome, diverticula, arteriovenous malformations (AVMs), hemorrhoids, and radiation-induced mucosal injury.
In the acute setting, the endoscopist may be limited by poor visualization in an unprepared colon and by the risks of sedation in an acutely bleeding patient. A purge preparation may be considered, using 4 L of polyethylene glycol (eg, GoLYTELY, CoLyte) either orally over 2 hours or via a nasogastric tube, as tolerated by the patient.
If the bleeding source cannot be determined by means of colonoscopy, angiography or a nuclear medicine scan may be required. Radiographic studies should be performed before colonoscopy when perforation or obstruction is suspected.
In managing lower GI bleeding, endoscopic treatments adhere to general hemostasis protocols, employing a variety of techniques (eg, epinephrine injections, thermal tools, and mechanical interventions such as endoclipping and band ligation). The optimal approach is determined by the practitioner's expertise and the specific characteristics of the bleeding lesion. [10] Whereas epinephrine injection is often the initial choice, by virtue of its effectiveness in stopping bleeding promptly, it carries an increased risk of subsequent bleeding (6-36%) and therefore should be used in conjunction with other hemostasis methods. [11]
Decompression of colon
Patients with a volvulus typically present with abdominal pain, nausea or vomiting, obstipation, and abdominal distention. For a cecal volvulus, surgical intervention is generally recommended. In the case of a sigmoid volvulus, decompression of the colon can generally be achieved with colonoscopy or sigmoidoscopy by advancing the endoscope through the torsed segment of bowel. A large expulsion of air indicates a successful reduction.
Endoscopic decompression is not recommended in cases of peritonitis due to perforation, suspected necrosis of the intestine indicated by symptoms of sepsis, or continuous severe bleeding with hemodynamic instability from the rectum. In such situations, prompt stabilization and surgical intervention are necessary. Given its high relapse rates (30-90%), endoscopic decompression usually does not provide a lasting solution. Accordingly, to prevent further recurrence, laparoscopic surgery (sigmoid resection) is generally advised within 2-5 days after endoscopic relief is attempted. [12]
Acute colonic pseudo-obstruction (Ogilvie syndrome) is a clinical condition characterized by signs and symptoms of an acute LBO in the absence of a mechanical cause. When supportive treatment fails, endoscopic decompression may be considered to prevent bowel ischemia and perforation. This is a technically difficult procedure and should be performed by using minimal air insufflation and without preceding oral laxative preparation.
Whereas colonoscopy appears to be beneficial in the management of patients with Ogilvie syndrome, it is associated with a greater risk of complications, and randomized trials have not been done to establish its efficacy.
Endocytoscopy
Endocytoscopy represents a newer advance in the diagnosis of colorectal lesions (particularly CRC). It offers higher-resolution imaging than can be obtained with traditional magnifying endoscopy and electronic chromoendoscopy. This high-magnification system enables optical biopsies, allowing for the visualization of cellular-level images. It provides accurate real-time assessments of mucosa, effectively distinguishing between normal and abnormal cells. [13]

Contraindications
Pregnancy is considered to increase the risk associated with colonoscopy. Guidelines for colonoscopy during pregnancy are not available, because of insufficient data. The largest reported series included eight colonoscopies performed during pregnancy. In this study, six patients delivered healthy infants after colonoscopy. One patient experienced a miscarriage unrelated to colonoscopy, and another had an elective abortion.
In general, colonoscopy may be considered for severe life-threatening conditions during pregnancy when the only alternative is colonic surgery or when colon cancer is suspected. The procedure is best performed in a hospital setting rather than in a doctor’s office. Surveillance colonoscopy for prior history of cancer or polyps, abdominal pain, or change in bowel habits should be deferred until the postpartum period.
Other relative contraindications for colonoscopy include known or suspected colonic perforation, toxic megacolon, and fulminant colitis or severe IBD with ulceration; these conditions increase the risk that the procedure will result in perforation. [14]

Technical Considerations
Best practices
For a colonoscopy to be effective, the bowel preparation (cleansing) must be adequate, [15] or else visualization will suffer. Preprocedural patient instructions are important to ensure good colon preparation. Of the numerous regimens that exist, polyethylene glycol (PEG) is still the most cost-effective preparation. PEG is an osmotic laxative and works by causing watery diarrhea so that the stool can be emptied from the colon. The medication also contains electrolytes to prevent dehydration and other serious side effects that may be caused by fluid loss as the colon is emptied.
The quality of bowel preparation should be measured by endoscopy units, on a unit level, at least annually. Screening and surveillance colonoscopies should be associated with adequate bowel preparation (ie, a Boston Bowel Preparation Scale [BBPS] score ≥6, with each segment score ≥2) in at least 90% of procedures, with the aspirational target being 95% or above. [2]
In patients undergoing colonoscopy, split-dose bowel preparation should serve as the endoscopy unit's standard preparation strategy. [2]
High-definition colonoscopes should be used by endoscopy units for screening and surveillance colonoscopy. [2]
Many patients choose to have anesthesia for their colonoscopy. This can take the form either of conscious sedation with midazolam and fentanyl or moderate sedation handled by means of anesthesia.
Inflating the colon with air to facilitate visualization of the mucosa simulates abdominal cramps and can be uncomfortable.
The patient is most commonly positioned in the left lateral decubitus position. A digital rectal examination (DRE) is mandatory. The scope is inserted gently and then advanced to the cecum. The cecum can be identified by three landmarks: the cecal strap, the appendiceal orifice, and the ileocecal valve. If it is difficult to identify one of the landmarks, transillumination (visualizing the light of the scope in the right lower quadrant) can be used as an aid for determining the location. The endoscopist should photographically document the cecum with a landmark.
The 2021 AGA colonoscopy clinical practice guidelines included the following recommendations [2] :
- To improve polyp detection, endoscopists should give the right colon a second look, either in retroflexed or forward view
- Endoscopy units should, at the endoscopist and unit level, routinely measure the adenoma detection rate and provide feedback on it, doing so at least annually or when 250 screening colonoscopies have been accrued by the endoscopists
- Individual endoscopists should have a goal adenoma detection rate of 30% or above, with the aspirational target being at least 35%; when these thresholds are not met, endoscopists "may consider extending withdrawal times, self-learning regarding mucosal inspection and polyp identification, peer feedback, and other educational interventions"
- Serrated lesion detection rates should be measured by endoscopy units on an endoscopist and unit level, with the unit providing feedback on these values; for serrated lesion detection, an individual endoscopist should have a goal rate of 7% or higher, with the aspirational target being at least 10%, and low rates should be addressed with improvement efforts oriented toward colonoscopists and pathologists
- For nonpedunculated polyps 3-9 mm in size, cold snare polypectomy should be employed, with aim taken at "a small rim of normal tissue around the polyp"; polyps larger than 2 mm generally should not be addressed with forceps
- If overt malignant endoscopic features are not present and patient pathology is not consistent with invasive adenocarcinoma, individuals with complex polyps should be evaluated by an expert in polypectomy with regard to the use of endoscopic resection
The withdrawal time should exceed 6 minutes so as to maximize the rate of polyp detection. Using minimal air during advancement keeps the colon length short and makes the procedure more comfortable for the patient.
Water immersion colonoscopy and water exchange colonoscopy are two newer techniques that use water instead of air. These techniques result in less colon distention and patient discomfort and can enhance visualization.
Complication prevention
Several organizations and authors have made recommendations for safe colonoscopy during the COVID-19 pandemic. [16] The European Centre for Disease Prevention and Control (ECDC) has recommended using a filtering face piece of respiratory class 2 or 3, goggles or a face shield to protect the eyes, and long-sleeved water-resistant gowns and gloves. Use of a class 2 or 3 filtering face piece is recommended during interrogation and when the colonoscopy report is being written.

Outcomes
Colonoscopy is safe and effective and rarely leads to complications. There is a real risk of colon injury during the procedure, [17] but with only about one in 1750 cases resulting in perforation, colonoscopy is, on the whole, extremely safe. Currently, more aggressive techniques of polyp removal, such as EMR and ESD, are becoming more widely performed; these are associated with a higher rate of colonic perforation than routine screening is.
A randomized controlled trial (N = 84,585) by Bretthauer et al evaluated colonoscopy screening in presumptively healthy men and women between the ages of 55 and 64 years. [18] Patients were divided into an invited group (n = 28,220, of whom 11,843 underwent screening), who received an invitation to undergo a single screening colonoscopy, and a usual-care group (n = 56,3650), who received no invitation or screening. The primary end points were the risks of CRC and related death, and the secondary end point was death from any cause.
The authors found that the risk of CRC at 10 years was 0.98% in the invited group and 1.2% in the usual-care group. [18] In the analysis adjusted to estimate the effect of screening on CRC risk, with the assumption that all participants assigned to the screening group actually underwent screening, the risk of CRC decreased by 31%, and the risk of CRC-related death decreased by 50%.
Such findings are crucial for public health strategies and guidelines, in that they provide evidence-based support for the implementation and encouragement of regular screening programs to reduce the burden of CRC in the population. The reduction in risk cited in this study highlights the importance of adherence to screening recommendations, which can significantly impact cancer prevention and early detection efforts. [18]

- Ebigbo A, Probst A, Messmann H. Endoscopic treatment of early colorectal cancer - just a competition with surgery?. Innov Surg Sci. 2018 Mar. 3 (1):39-46. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Keswani RN, Crockett SD, Calderwood AH. AGA Clinical practice update on strategies to improve quality of screening and surveillance colonoscopy: expert review. Gastroenterology. 2021 Aug. 161(2):701-11. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] van Hooft JE, Veld JV, Arnold D, Beets-Tan RGH, Everett S, Götz M, et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020 May. 52 (5):389-407. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] American Cancer Society recommendations for colorectal cancer screening. American Cancer Society. Available at https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html. January 29, 2024; Accessed: June 27, 2024.
- [Guideline] Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018 Jul. 68 (4):250-281. [QxMD MEDLINE Link]. [Full Text].
- Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012 Feb 23. 366 (8):697-706. [QxMD MEDLINE Link].
- [Guideline] Colorectal screening and surveillance: clinical guideline and rationale. American Society of Colon and Rectal Surgeons. Available at https://fascrs.org/healthcare-providers/education/clinical-practice-guidelines/colorectal-cancer-screening-and-surveillance-clini. 2024; Accessed: June 27, 2024.
- Paine E, Shen B. Endoscopic therapy in inflammatory bowel diseases (with videos). Gastrointest Endosc. 2013 Dec. 78 (6):819-835. [QxMD MEDLINE Link].
- Bettenworth D, Gustavsson A, Atreja A, Lopez R, Tysk C, van Assche G, et al. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn's Disease. Inflamm Bowel Dis. 2017 Jan. 23 (1):133-142. [QxMD MEDLINE Link].
- Gralnek IM, Neeman Z, Strate LL. Acute Lower Gastrointestinal Bleeding. N Engl J Med. 2017 Mar 16. 376 (11):1054-1063. [QxMD MEDLINE Link].
- Liou TC, Lin SC, Wang HY, Chang WH. Optimal injection volume of epinephrine for endoscopic treatment of peptic ulcer bleeding. World J Gastroenterol. 2006 May 21. 12 (19):3108-13. [QxMD MEDLINE Link].
- Perrot L, Fohlen A, Alves A, Lubrano J. Management of the colonic volvulus in 2016. J Visc Surg. 2016 Jun. 153 (3):183-92. [QxMD MEDLINE Link].
- Misawa M, Kudo SE, Takashina Y, Akimoto Y, Maeda Y, Mori Y, et al. Clinical Efficacy of Endocytoscopy for Gastrointestinal Endoscopy. Clin Endosc. 2021 Jul. 54 (4):455-463. [QxMD MEDLINE Link].
- Bhagatwala J. Colonoscopy - indications and contraindications. Ettarh R, ed. Screening for Colorectal Cancer with Colonoscopy. 3rd ed. Rijeka, Croatia: InTech; 2015.
- Harrison NM, Hjelkrem MC. Bowel cleansing before colonoscopy: Balancing efficacy, safety, cost and patient tolerance. World J Gastrointest Endosc. 2016 Jan 10. 8 (1):4-12. [QxMD MEDLINE Link]. [Full Text].
- Iacucci M, Cannatelli R, Labarile N, Mao R, Panaccione R, Danese S, et al. Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period. Lancet Gastroenterol Hepatol. 2020 Jun. 5 (6):598-606. [QxMD MEDLINE Link]. [Full Text].
- Lohsiriwat V. Colonoscopic perforation: incidence, risk factors, management and outcome. World J Gastroenterol. 2010 Jan 28. 16 (4):425-30. [QxMD MEDLINE Link]. [Full Text].
- Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022 Oct 27. 387 (17):1547-1556. [QxMD MEDLINE Link].
- DiPalma JA, Wolff BG, Meagher A, Cleveland Mv. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol. 2003 Oct. 98 (10):2187-91. [QxMD MEDLINE Link].
- Prepopik (sodium picosulfate/magnesium oxide/anhydrous citric acid) [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc. July 2012. Available at [Full Text].
- Flemming JA, Vanner SJ, Hookey LC. Split-dose picosulfate, magnesium oxide, and citric acid solution markedly enhances colon cleansing before colonoscopy: a randomized, controlled trial. Gastrointest Endosc. 2012 Mar. 75 (3):537-44. [QxMD MEDLINE Link].
- Samarasena JB, Muthusamy VR, Jamal MM. Split-dosed MiraLAX/Gatorade is an effective, safe, and tolerable option for bowel preparation in low-risk patients: a randomized controlled study. Am J Gastroenterol. 2012 Jul. 107 (7):1036-42. [QxMD MEDLINE Link].
- Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000 Sep. 52 (3):346-52. [QxMD MEDLINE Link].
- Lee SE, Oh DJ, Nam JH, Cho H, Kim JH, Lee JK, et al. Taking Oral Sulfate Tablets with Simethicone for Bowel Preparation Leads to Higher Adenoma Detection Rate than Polyethylene Glycol: A Propensity Score Analysis. Dig Dis Sci. 2023 Mar. 68 (3):867-876. [QxMD MEDLINE Link].
- Markowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005 Nov. 16 (11):3389-96. [QxMD MEDLINE Link].
- Alvarez-Gonzalez MA, Pantaleon MA, Flores-Le Roux JA, Zaffalon D, Amorós J, Bessa X, et al. Randomized Clinical Trial: A Normocaloric Low-Fiber Diet the Day Before Colonoscopy Is the Most Effective Approach to Bowel Preparation in Colorectal Cancer Screening Colonoscopy. Dis Colon Rectum. 2019 Apr. 62 (4):491-497. [QxMD MEDLINE Link].
- Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. Circulation. 1997 Jul 1. 96 (1):358-66. [QxMD MEDLINE Link].
- Anderson AB, Slaven SE, Watson NL, Cody JP, McGill RJ, Potter BK, et al. Periprosthetic Joint Infection in Patients With Arthroplasty Undergoing Perioperative Colonoscopy. JAMA Netw Open. 2024 May 1. 7 (5):e2410123. [QxMD MEDLINE Link].
- [Guideline] Wilson W, Taubert KA, Gewitz M, et al, American Heart Association. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007 Oct 9. 116 (15):1736-54. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Li PK, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022 Mar. 42 (2):110-153. [QxMD MEDLINE Link].
- Ul-Haque I, Shaikh TG, Ahmed SH, Waseem S, Qadir NA, Bin Arif T, et al. Efficacy of Remimazolam for Procedural Sedation in American Society of Anesthesiologists (ASA) I to IV Patients Undergoing Colonoscopy: A Systematic Review and Meta-Analysis. Cureus. 2022 Mar. 14 (3):e22881. [QxMD MEDLINE Link].
- Li J, Wang X, Liu J, Wang X, Li X, Wang Y, et al. Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: A multi-centre, non-inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. 2022 Aug. 131 (2):138-148. [QxMD MEDLINE Link].
- Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000 Jan. 51 (1):33-6. [QxMD MEDLINE Link].
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006 Dec 14. 355 (24):2533-41. [QxMD MEDLINE Link].
- Low DJ, Hong Z, Jugnundan S, Mukherjee A, Grover SC. Automated Detection of Bowel Preparation Scoring and Adequacy With Deep Convolutional Neural Networks. J Can Assoc Gastroenterol. 2022 Dec. 5 (6):256-260. [QxMD MEDLINE Link].
- Liu P, Wu J, He C, Wang W. ENDOANGEL versus water exchange for the detection of colorectal adenomas. Therap Adv Gastroenterol. 2023. 16:17562848231218570. [QxMD MEDLINE Link].
- Fernandez-Urien I, Carretero C, Borda A, Muñoz-Navas M. Colon capsule endoscopy. World J Gastroenterol. 2008 Sep 14. 14 (34):5265-8. [QxMD MEDLINE Link]. [Full Text].
- Song HJ, Shim KN. Current status and future perspectives of capsule endoscopy. Intest Res. 2016 Jan. 14 (1):21-9. [QxMD MEDLINE Link].
- Buchner AM, Shahid MW, Heckman MG, McNeil RB, Cleveland P, Gill KR, et al. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010 Apr. 8 (4):364-70. [QxMD MEDLINE Link].
- Tribonias G, Theodoropoulou A, Konstantinidis K, Vardas E, Karmiris K, Chroniaris N, et al. Comparison of standard vs high-definition, wide-angle colonoscopy for polyp detection: a randomized controlled trial. Colorectal Dis. 2010 Oct. 12 (10 Online):e260-6. [QxMD MEDLINE Link].
- Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001 Feb. 53 (2):216-20. [QxMD MEDLINE Link].
- Taghiakbari M, Mori Y, von Renteln D. Artificial intelligence-assisted colonoscopy: A review of current state of practice and research. World J Gastroenterol. 2021 Dec 21. 27 (47):8103-8122. [QxMD MEDLINE Link].
- Li YK, Guo CG, Cheung KS, Liu KSH, Leung WK. Risk of Postcolonoscopy Thromboembolic Events: A Real-World Cohort Study. Clin Gastroenterol Hepatol. 2023 Nov. 21 (12):3051-3059.e4. [QxMD MEDLINE Link].
- Inflammatory bowel disease. Severe colitis noted during colonoscopy. Mucosa is grossly denuded, with active bleeding noted. Patient had her colon resected very shortly after this view was obtained.
- Colonoscopy. Stricture in terminal ileum noted during colonoscopy. Narrowed segment visible upon intubation of terminal ileum with colonoscope. Relatively little active inflammation is present, indicating that this is cicatrix stricture.
- Colonoscopy. Inflammation in terminal ileum noted during colonoscopy. Areas of inflammation, friability, and ulceration in terminal ileum are consistent with mild-to-moderate Crohn disease.
- Colonoscopy. Ulcerative colitis as visualized with colonoscope.
- Colonoscopy. Colonoscopic image of large ulcer and inflammation of descending colon in 12-year-old boy with Crohn disease.
- Colonoscopy. Colonoscopy video depicts arteriovenous malformation (AVM) in colon. Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.
- Colonoscopy. Colonoscopy video shows removal of small polyp from cecum with snare polypectomy technique. Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.
- Colonoscopy. Colonoscopy video shows mass in colon suspicious for colon cancer. Mass is too large to remove endoscopically and will have to be tattooed so that surgeons can find it easily. Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.
- Colonoscopy. Colonoscopy video shows pseudopolyps in colon. This is usually seen in inflammatory bowel disease (eg, Crohn disease or ulcerative colitis). Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.
- Colonoscopy. Colonoscopy video shows narrowed area in colon in setting of diverticulitis. Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.
- Colonoscopy. Colonoscopy video shows narrowed area in colon in setting of diverticulitis. Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.
- Colonoscopy. Colonoscopy video shows removal of large polyp by hot snare polypectomy technique. Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.
- Colonoscopy. Colonoscopy video shows placement of tattoo adjacent to colon mass in order to make it visible and easy to locate for surgeons. Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.
- Colonoscopy. Colonoscopy video shows diverticulosis (pockets within colon that can bleed or become infected). Video courtesy of Dawn Sears, MD, and Dan C Cohen, MD, Division of Gastroenterology, Scott & White Healthcare.


Author
Coauthor(s)
Jonah A Schindelheim, DO Resident Physician, General Surgery Department, MedStar Union Memorial Hospital
Jonah A Schindelheim, DO is a member of the following medical societies: American College of Surgeons
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Chief Editor
John Geibel, MD, MSc, DSc, AGAF Vice Chair and Professor, Department of Surgery, Section of Gastrointestinal Medicine, Professor, Department of Cellular and Molecular Physiology, Yale University School of Medicine; Director of Surgical Research, Department of Surgery, Yale-New Haven Hospital; American Gastroenterological Association Fellow; Fellow of the Royal Society of Medicine
John Geibel, MD, MSc, DSc, AGAF is a member of the following medical societies: American Gastroenterological Association, American Physiological Society, American Society of Nephrology, Association for Academic Surgery, International Society of Nephrology, New York Academy of Sciences, Society for Surgery of the Alimentary Tract
Disclosure: Nothing to disclose.
Additional Contributors
David Greenwald, MD Professor of Clinical Medicine, Fellowship Program Director, Department of Medicine, Division of Gastroenterology, Montefiore Medical Center, Albert Einstein College of Medicine
David Greenwald, MD is a member of the following medical societies: Alpha Omega Alpha, New York Society for Gastrointestinal Endoscopy, American College of Gastroenterology, American College of Physicians, American Gastroenterological Association, American Society for Gastrointestinal Endoscopy
Disclosure: Nothing to disclose.
Alberto J Parra Vitela, MD, MSc Resident Physician, Department of Surgery, Medstar Union Memorial Hospital
Alberto J Parra Vitela, MD, MSc is a member of the following medical societies: American College of Surgeons
Disclosure: Nothing to disclose.
Acknowledgements
Michael A Grosso, MD Consulting Staff, Department of Cardiothoracic Surgery, St Francis Hospital
Michael A Grosso, MD is a member of the following medical societies: American College of Surgeons, Society of Thoracic Surgeons, and Society of University Surgeons
Disclosure: Nothing to disclose.
Alex Jacocks, MD Program Director, Professor, Department of Surgery, University of Oklahoma School of Medicine
Disclosure: Nothing to disclose.
Burton I Korelitz, MD Director of GI Research, Chief, Department of Medicine, Section of Gastroenterology, Lenox Hill Hospital, Clinical Professor, Department of Medicine, State University of New York at Brooklyn
Disclosure: Nothing to disclose.
Acknowledgments
Medscape Reference also thanks Dawn Sears, MD, Associate Professor of Internal Medicine, Division of Gastroenterology and Hepatology, Scott and White Memorial Hospital; and Dan C Cohen, MD, Fellow in Gastroenterology, Scott and White Hospital, Texas A&M Health Science Center College of Medicine, for assistance with the video contribution to this article.