Cytomegalovirus (CMV): Practice Essentials, Background, Pathophysiology (original) (raw)
Practice Essentials
Human Cytomegalovirus (CMV) is a member of the family Herpesviridae, also known as Human Herpesvirus 5 (HHV-5). It is the largest (220 nm in diameter) and most complex herpesvirus, with a 235,000 double-stranded DNA genome. CMV seroprevalence in immunocompetent adults varies from 40-100% globally. [1]
Signs and symptoms
CMV usually causes an asymptomatic infection or produces mild flulike symptoms; afterwards, it remains latent throughout life and may reactivate.
Most patients with CMV infection exhibit few clinical findings on physical examination.
- Primary CMV infection may be a cause of fever of unknown origin.
- Symptoms, when apparent, develop 9-60 days after primary infection.
- Pharyngitis may be present.
- Examination of the lungs may reveal fine crackles.
- The lymph nodes and spleen may be enlarged, so CMV should be included in the differential diagnoses of infections that produce lymphadenopathy.
In immunocompromised individuals, symptomatic disease usually manifests as a mononucleosis syndrome. Symptomatic CMV disease can affect almost every organ of the body, resulting in fever of unknown origin, pneumonia, hepatitis, encephalitis, myelitis, colitis, uveitis, retinitis, and neuropathy. Rarer manifestations of CMV infections in immunocompetent individuals include Guillain-Barré syndrome, meningoencephalitis, pericarditis, myocarditis, thrombocytopenia, and hemolytic anemia.
CMV is an opportunistic infection in patients with advanced HIV/AIDS and can effect multiorgan systems. The most common sites of CMV related gastrointestinal infection are the esophagus and the colon. These patients may have concurrent CMV reitinitis and should have formal opthalmologic screening.
See Clinical Presentation for more detail.
Diagnosis
Lab studies
CMV has been detected via culture, serologies, antigen assays, polymerase chain reaction (PCR), and cytopathology. In the transplant population, antigen assays or PCR is used (sometimes in conjunction with cytopathology) for diagnosis and treatment determinations.
Imaging studies
The diagnosis of CMV pneumonia can be suggested by chest radiography findings, but these findings cannot be used to differentiate between other common causes of pneumonia in immunocompromised hosts. A chest radiograph finding consistent with pneumonia and a bronchoalveolar lavage (BAL) result that is CMV positive is a common method for diagnosis.
See Workup for more details.
Management
Healthy people who are infected with CMV but who have no symptoms usually do not require medical treatment.
Antiviral treatment is used for immunocompromised individuals who have eye infections or life-threatening illnesses due to CMV. The drug of choice for prevention of CMV disease in solid-organ transplant patients is valganciclovir. [2] Other than CMV retinitis, however, ganciclovir remains the mainstay of treatment, at least initially.
Second-line treatments include foscarnet, cidofovir, or maribavir.
There is no vaccine to prevent CMV infection.
See Treatment and Medication for more detail.

Background
Cytomegalovirus (CMV) is a double-stranded DNA virus and is a member of the Herpesviridae family. The other family members include herpes simplex virus type 1 (HSV-1 or HHV-1) and herpes simplex virus type 2 (HSV-2 or HHV-2), varicella zoster virus (VZV), human herpes virus (HHV)–6, HHV-7, and HHV-8. CMV shares many attributes with other herpes viruses, including genome, virion structure, and the ability to cause latent and persistent infections. CMV has the largest genome of the herpes viruses. Replication may be categorized into immediate early, delayed early, and late gene expression based on time of synthesis after infection. The DNA is replicated by rolling circles. Human CMV grows only in human cells and replicates best in human fibroblasts.
At least 50-60% of the US population has been exposed to CMV, [3] with a prevalence of more than 90% in high-risk groups (eg, male homosexuals), and outside of the US prevalence can be more than 90%. [4, 5, 6] The prevailing age of infection varies worldwide. In developing countries, most infections are acquired during childhood, whereas in developed countries, up to 50% of young adults are CMV seronegative. The incidence of CMV seropositivity rises with age and in a US-based study was reported to increase from 36% in children aged 6-11 years to 91% in individuals older than 80 years. [7] Other factors associated with CMV seropositivity include ethnicity (77% in Mexican Americans and 71% in Blacks), [8] female sex, foreign-born status, and low socioeconomic status. [8]
CMV usually causes an asymptomatic infection; afterward, it remains latent throughout life and may reactivate. Infection is defined as isolation of CMV, its viral proteins, or its nucleic acid from any tissue sample or body fluid. [9] In immunocompetent individuals, symptomatic disease usually manifests as a mononucleosis syndrome, which was first described in adults in 1965. [10]
Clinically significant CMV disease (reactivation of previously latent infection or newly acquired infection) frequently develops in patients immunocompromised by HIV infection, solid-organ transplantation, or bone marrow transplantation, as well as in those receiving high-dose steroids, tumor necrosis antagonists, or other immunosuppressing medications for conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis, Crohn disease, or psoriasis, among others. In patients coinfected with HIV, CMV infection leads to progression to AIDS and eventually death, even in those receiving antiretroviral therapy (ART). [11]
Symptomatic CMV disease in immunocompromised individuals can affect almost every organ of the body, resulting in fever of unknown origin, pneumonia, hepatitis, encephalitis, myelitis, colitis, uveitis, retinitis, and neuropathy.
Individuals at an increased risk for CMV infection include individuals who attend or work at daycare centers, patients who undergo blood transfusions, persons who have multiple sex partners, and recipients of CMV mismatched organ or bone marrow transplants.
CMV is transmitted from person to person via close contact with an individual who is excreting the virus. It can be spread through the placenta, blood transfusions, organ transplantation, and breast milk. It also can be spread through sexual transmission.
In the United States, congenital CMV transmission from a mother with acute infection during pregnancy is a significant cause of neurologic abnormalities and deafness in approximately 8000 newborns annually. [12, 13]
Multiple genetically distinct strains of CMV exist. Differences in genotypes may be associated with differences in virulence. Infection with more than one strain of CMV is possible and has been observed in organ transplant recipients. Dual infection is a possible explanation for congenital CMV infection in children of CMV-seropositive mothers.

Pathophysiology
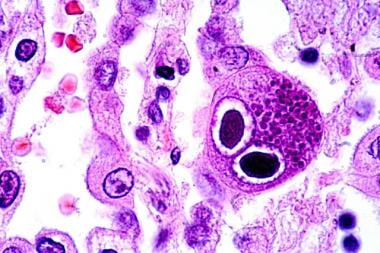
CMV is a lytic virus that causes a cytopathic effect in vitro and in vivo. The pathologic hallmark of CMV infection is an enlarged cell with viral inclusion bodies. Cells that exhibit cytomegaly also are seen in infections caused by other Betaherpesvirinae. The microscopic description given to these cells is most commonly an "owl's eye," depicted in the image below. Although considered diagnostic, such histologic findings may be minimal or absent in infected organs.
Hematoxylin-eosin–stained lung section showing typical owl-eye inclusions (480X). Courtesy of Danny L Wiedbrauk, PhD, Scientific Director, Virology & Molecular Biology, Warde Medical Laboratory, Ann Arbor, Michigan.
When the host is infected, CMV DNA can be detected with polymerase chain reaction (PCR) in all the different cell lineages and organ systems in the body. Upon initial infection, CMV infects the epithelial cells of the salivary gland, resulting in a persistent infection and viral shedding. Infection of the genitourinary system leads to clinically inconsequential viruria. Despite ongoing viral replication in the kidney, renal dysfunction is rare except in renal transplant recipients, in whom CMV is associated with rare cases of glomerulopathy and possible graft rejection.
Immunology
Primary CMV infection is defined as infection in an individual who was previously CMV seronegative. [9] In these patients, CMV immunoglobulin M (IgM) antibodies may be found as early as 4-7 weeks after initial infection and may persist as long as 16-20 weeks. Most neutralizing antibodies are directed against an envelope glycoprotein gB. Studies have shown that more than 50% of neutralizing activity in convalescent serum is attributable to glycoprotein gB. However, virion tegument proteins such as pp150, pp28, and pp65 evoke strong and durable antibody responses.
CMV is an immunomodulatory virus and may aggravate underlying immune disorders (eg, SLE).
The presence of CMV DNA in the blood and viruria are commonly found in healthy CMV seropositive women. Naturally acquired immunity to the virus does not seem to prevent reinfection or the duration of viral shedding. [14]
Cell-mediated immunity is considered the most important factor in controlling CMV infection. Patients deficient in cell-mediated immunity are at greatest risk for CMV disease. CMV-specific CD4+ and CD8+ lymphocytes play an important role in immune protection after primary infection or reactivation of latent disease. Studies of bone marrow transplant recipients have revealed that those who do not develop CMV-specific CD4+ or CD8+ cells are at higher risk for CMV pneumonitis. Additionally, no cases of CMV pneumonia have been reported in allogeneic marrow transplant recipients receiving infusions of CMV-specific CD8+ cells. [15]
Primary cytomegalovirus infection and viremia
Both replication of CMV DNA and morphogenesis of the virion capsid take place in the nucleus. Following maturation of the capsid, newly synthesized viral DNA is cleaved by an enzyme that results in packaging of linear genomic DNA. Subsequently, viral DNA-containing capsids acquire an inner layer of tegument proteins during their egress from the nucleus, including essential interactions between proteins and capsid protein, that stabilize the interaction between the capsid and the inner tegument layer of the virion. This then is transported along the cytoskeleton until the particle is enveloped. After this, the virus is released from the cell. [1]
Congenital cytomegalovirus disease
CMV is the leading cause of congenital infection worldwide (0.2-6.1% of live births), as well as the most common congenital viral infection in the United States (20000 to 30000 infants/year, mostly Black infants) and the leading cause of sensorineural hearing loss and neurodevelopmental delay in children. Congenital CMV-related sequelae affect over 5000 children per year and add significant cost in direct medical care in the United States. The transplacental transmission rate after maternal primary infection is around 32%. The risk for transmission is low following maternal infection occurring more than 11 weeks before conception. [16, 17, 18, 19]
Most infants are asymptomatic, however, symptomatic infants are seen in about 10% of the patients with a broad range of disease manifestations. These include thrombocytopenia, petechiae, hepatomegaly, splenomegaly, hepatitis, intrauterine growth restriction, CNS involvement (microcephaly, ventriculomegaly, intracerebral calcifications, white matter changes with seizures, and abnormal tone), ophthalmologic abnormalities (chorioretinitis, optic atrophy), and sensorineural hearing loss. Mortality due to congenital CMV infection is low (approximately 4% of infants). Symptomatic disease can be classified as moderate to severe (multiple manifestations with or without CNS involvement) or mild disease (1-2 manifestations with no CNS involvement). [16, 19]
In the pediatric population, congenital CMV infection is the most common cause of non-genetic sensorineural hearing loss (SNHL); this also is the most common permanent sequelae of congenital CMV infection. Around 40-60% of these neonates are at risk for permanent sequelae. [20] Other complications include cognitive impairment, chorioretinitis, and cerebral palsy. Other manifestations include motor deficits and seizures as well in 23% and 19%, respectively. CMV screening approaches could lead to the identification of many more infants with congenital CMV infection than are identified because of clinical signs. Trials are ongoing to formulate a vaccine for pregnant individuals. [16, 21, 22]
Increased rates of reactivation and cervical shedding are seen in advanced stages of gestation, and congenital infection is associated with sequelae previously described. Prevention includes hand hygiene to minimize occupationally acquired CMV (such as daycare centers), as well as limiting the number of sexual partners (during pregnancy). [23]
Cytomegalovirus pneumonia
This can be seen in an immunocompetent as well as an immunocompromised host, including hematopoietic stem cell transplantation (HSCT) recipients and solid organ transplant recipients. Pneumonitis is the most common manifestation of CMV infection in HSCT recipients and has a high mortality. Especially in neonates, it can lead to chronic lung disease and fibrosis. Symptoms include dry cough, shortness of breath, and fever. Imaging abnormalities include abnormal chest Xray with interstitial infiltrates as well as ground-glass opacities seen in computed tomography (CT) scans that could be nonspecific for CMV infections and should be correlated with serologic testing, viral load, respiratory samples, and histopathology. [24]
Cytomegalovirus hepatitis
CMV hepatitis is defined as elevated bilirubin and/or liver enzymes levels in combination with the detection of CMV in the absence of other causes for hepatitis. [9] CMV may be detected via culture, histopathology, immunohistochemistry, or in situ hybridization. CMV PCR alone is not satisfactory for diagnosis, as a positive result may reflect transient viral shedding. [9] The first described case of CMV hepatitis involved a child with chorioretinitis, hepatosplenomegaly, and cerebral calcifications.
Hepatitis has been commonly observed in patients with primary CMV infection and mononucleosis. Levels of hepatocellular enzymes may be mildly and transiently increased, and, in rare cases, jaundice may develop. The prognosis of CMV hepatitis in immunocompetent hosts typically is favorable, but death has been reported in immunosuppressed patients. Histology typically reveals mononuclear cell infiltration of the portal areas but also may reveal granulomatous inflammation. [25]
Cytomegalovirus gastritis and colitis
CMV GI disease is defined as the combination of symptoms of the upper and lower GI tract, mucosal lesions visible on endoscopy, and detection of CMV via culture, histopathology, immunohistochemistry, or in situ hybridization. [9] CMV colitis first was described in 1985 in 2 homosexual men who presented with abdominal pain, diarrhea, and hematochezia. [26] CMV PCR alone is insufficient for diagnosis, as a positive result may simply reflect transient viral shedding.
CMV may infect the GI tract from the oral cavity through the colon. The typical manifestation of disease is ulcerative lesions. In the oral cavity, these may be indistinguishable from ulcers caused by HSV or aphthous ulceration. Gastritis may present as abdominal pain and even hematemesis, whereas colitis more frequently presents as a diarrheal illness. CMV disease of the GI tract often is shorter-lived than that of other organ systems because of the frequent sloughing of infected cells of the GI mucosa.
Cytomegalovirus CNS disease
CMV CNS disease is defined as CNS symptoms in combination with CMV detection in CSF (culture, PCR) or brain biopsy tissue (culture, histopathology, immunohistochemistry, in situ hybridization). [9] The association between CMV and Guillain-Barré Syndrome involves 2 groups. Younger patients (typically < 35 y) present with sensory defects and facial palsy, antiganglioside (GM2) IgM response, and milder long-term sequelae. [27] A second group includes women older than 50 years. These observations were made in France and thus may not be applicable to other populations due to different ages of primary CMV exposure.
Cytomegalovirus retinitis
CMV retinitis is one of the most common opportunistic infections in persons with AIDS, typically those with CD4+ lymphocyte counts below 50 cells/µL. Although the number of cases has decreased with the use of antiretroviral therapy (ART), new cases continue to be reported. Individuals with CMV retinitis typically exhibit a progressive decrease in visual acuity, which may progress to blindness if untreated. Unilateral and bilateral disease may exist. Long-term CMV treatment is necessary to prevent retinitis relapse. All lesions suspected to be CMV retinitis must be confirmed by an ophthalmologist.
Immune reconstitution syndrome (IRIS) is reported in 16-63% of HIV-infected patients with CMV retinitis following the initiation of ART. [28, 29, 30] In one study, the median time to IRIS following ART initiation was 43 weeks but has been reported as early as 4 weeks or as late as 4 years. [31, 29] CMV IRIS may manifest as painless floaters, blurred vision, photopia, decreased visual acuity, or ocular pain. Some patients may develop macular edema leading to vision loss or proliferative vitreoretinopathy, spontaneous vitreal hemorrhage, and retinal detachment.
Cytomegalovirus nephritis
CMV nephritis is defined as CMV detection in combination with a renal biopsy showing CMV-associated changes in the setting of renal failure. [9] CMV PCR alone is inadequate for diagnosis. Of note, detection of CMV in the urine of a patient with renal failure does not meet diagnostic criteria for CMV nephritis. [9] CMV viremia has been associated with acute glomerular injury. [32]
Cytomegalovirus syndrome
In general, it is better to avoid this term in stem cell transplant recipients, as other viruses (eg, HHV-6) also can cause fever and bone marrow suppression. [9] However, in solid organ transplant recipients, CMV syndrome is better defined: fever (>38°C) for at least 2 days within a 4-day period, CMV detection in blood, and either neutropenia or thrombocytopenia. [9]
Graft versus host disease
CMV infection has been associated with acute graft verus host disease in bone marrow transplant recipients. Multiple genotypes (gB 1-4) of CMV exist, each with variations in the gene encoding envelope glycoprotein gB. The association of gB types with acute graft versus host disease and death related to myelosuppression has been examined. Taking into account disease type, donor-recipient HLA matching, donor CMV serostatus, and age, Torok-Storb et al (1997) found that gB3 and gB4 were linked to a higher degree of myelosuppression and death. [33] Interestingly, no specific CMV genotypes were linked to worse outcome in solid organ transplant recipients, although mixed gB genotype infections were associated with higher viral loads and delayed viral clearance. [34]

Frequency
United States
CMV infection is thought to be specific to humans. The age at presentation, clinical manifestations, and route of infection may vary from person to person, but very few people escape infection during their lifetime.
International
Serologic surveys conducted worldwide demonstrate CMV to be a ubiquitous infection of humans. Depending on the population surveyed, CMV may be found in more than 90% of people, depending on socioeconomic conditions.

Mortality/Morbidity
CMV seldom is associated with mortality in nonimmunocompromised hosts (< 1%). Substantial morbidity may occur in patients with a mononucleosis syndrome, as described in Adult Cytomegalovirus Infection in the Immunocompetent Host.
In both solid organ and marrow transplant recipients, CMV causes substantial morbidity and mortality. For example, even with antiviral therapy, the mortality rate in allogeneic marrow transplant recipients with interstitial pneumonia varies from 15-75%.
CMV RNA can be detected in 15% of fetal tissues or placentae, indicating that CMV infection during pregnancy contributes to stillbirths. [35]
Age
CMV prevalence increases with age. Age also has been found to be a risk factor for CMV disease in certain transplant populations.

Prognosis
The prognosis of CMV hepatitis generally is good. Most patients recover completely. Symptoms can persist, usually in the form of fatigue, for several months after primary infection.
CMV pneumonia in marrow transplant recipients once carried a mortality rate higher than 85%. The use of ganciclovir plus high-dose immune globulin for the treatment of CMV pneumonia in allogeneic marrow transplant recipients has lowered the mortality rate to 30-60%.
Because patients who develop CMV disease are immunocompromised, their prognosis may be determined by their underlying disease. The need for mechanical ventilation is a poor prognostic sign.

Patient Education
For excellent patient education resources, visit eMedicineHealth's patient education article Mononucleosis.

- Bennett J, Dolin R, Blaser M. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 9th edition. Elsevier/Saunders; 2015.
- Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005 Jun 18-24. 365(9477):2105-15. [QxMD MEDLINE Link].
- Zhang LJ, Hanff P, Rutherford C, Churchill WH, Crumpacker CS. Detection of human cytomegalovirus DNA, RNA, and antibody in normal donor blood. J Infect Dis. 1995 Apr. 171(4):1002-6. [QxMD MEDLINE Link].
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019 Sep. 33 (9):e13512. [QxMD MEDLINE Link].
- Collier AC, Meyers JD, Corey L, Murphy VL, Roberts PL, Handsfield HH. Cytomegalovirus infection in homosexual men. Relationship to sexual practices, antibody to human immunodeficiency virus, and cell-mediated immunity. Am J Med. 1987 Mar 23. 82(3 Spec No):593-601. [QxMD MEDLINE Link].
- Guinan ME, Thomas PA, Pinsky PF, Goodrich JT, Selik RM, Jaffe HW. Heterosexual and homosexual patients with the acquired immunodeficiency syndrome. A comparison of surveillance, interview, and laboratory data. Ann Intern Med. 1984 Feb. 100(2):213-8. [QxMD MEDLINE Link].
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006 Nov 1. 43(9):1143-51. [QxMD MEDLINE Link].
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010 Jun 1. 50(11):1439-47. [QxMD MEDLINE Link].
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002 Apr 15. 34(8):1094-7. [QxMD MEDLINE Link].
- Cunha BA. Cytomegalovirus pneumonia: community-acquired pneumonia in immunocompetent hosts. Infect Dis Clin North Am. 2010 Mar. 24(1):147-58. [QxMD MEDLINE Link].
- Deayton JR, Prof Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet. 2004 Jun 26. 363(9427):2116-21. [QxMD MEDLINE Link].
- Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986 Oct 10. 256(14):1904-8. [QxMD MEDLINE Link].
- Stagno S. Cytomegalovirus. Remington JS, Klein JO. Infectious Diseases of the Fetus and Newborn Infant. Philadelphia: WB Saunders; 2001. 389-424.
- Arora N, Novak Z, Fowler KB, Boppana SB, Ross SA. Cytomegalovirus viruria and DNAemia in healthy seropositive women. J Infect Dis. 2010 Dec 15. 202(12):1800-3. [QxMD MEDLINE Link]. [Full Text].
- Walter EA, Greenberg PD, Gilbert MJ. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995 Oct 19. 333(16):1038-44. [QxMD MEDLINE Link].
- Kabani N, Ross SA. Congenital Cytomegalovirus Infection. J Infect Dis. 2020. 221(Suppl 1):S9-S14. [Full Text].
- Institute of Medicine (US) Committee to Study Priorities for Vaccine Development, Stratton KR, Durch JS, Lawrence RS, eds. Vaccines for the 21st Century: A Tool for Decisionmaking. National Academies Press (US). 2000.
- Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med. 2006. 354(20):2151-2164. [Full Text].
- Leruez-Ville M, Foulon I, Pass R, Ville Y. Cytomegalovirus infection during pregnancy: state of the science. Am J Obstet Gynecol. 2020. 223(3):330-349. [Full Text].
- Sartori P, Baud D, de Tejada BM, Farin A, Rossier MC, Rieder W, et al. Cytomegalovirus infection during pregnancy: cross-sectional survey of knowledge and prevention practices of healthcare professionals in French-speaking Switzerland. Virol J. 2024 Feb 21. 21 (1):45. [QxMD MEDLINE Link].
- Dreher AM, Arora N, Fowler KB, et al. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr. 2014. 164(4):855-859. [Full Text].
- Chiopris G, Veronese P, Cusenza F, et al. Congenital Cytomegalovirus Infection: Update on Diagnosis and Treatment. Microorganisms. 2020. 8(10):1516. [Full Text].
- Davis NL, King CC, Kourtis AP. Cytomegalovirus infection in pregnancy. Birth Defects Res. 2017. 109(5):336-346. [Full Text].
- Fonseca Brito L, Brune W, Stahl FR. Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity. Int J Mol Sci. 2019. 20(16):3865. [Full Text].
- Bonkowsky HL, Lee RV, Klatskin G. Acute granulomatous hepatitis. Occurrence in cytomegalovirus mononucleosis. JAMA. 1975 Sep 22. 233(12):1284-8. [QxMD MEDLINE Link].
- Meiselman MS, Cello JP, Margaretten W. Cytomegalovirus colitis. Report of the clinical, endoscopic, and pathologic findings in two patients with the acquired immune deficiency syndrome. Gastroenterology. 1985 Jan. 88(1 Pt 1):171-5. [QxMD MEDLINE Link].
- Orlikowski D, Porcher R, Sivadon-Tardy V, et al. Guillain-Barre Syndrome following Primary Cytomegalovirus Infection: A Prospective Cohort Study. Clin Infect Dis. 2011 Apr. 52(7):837-44. [QxMD MEDLINE Link].
- Jabs DA, Van Natta ML, Kempen JH, Reed Pavan P, Lim JI, Murphy RL, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002 Jan. 133(1):48-61. [QxMD MEDLINE Link].
- Karavellas MP, Plummer DJ, Macdonald JC, Torriani FJ, Shufelt CL, Azen SP. Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis. 1999 Mar. 179(3):697-700. [QxMD MEDLINE Link].
- Wohl DA, Kendall MA, Owens S, Holland G, Nokta M, Spector SA. The safety of discontinuation of maintenance therapy for cytomegalovirus (CMV) retinitis and incidence of immune recovery uveitis following potent antiretroviral therapy. HIV Clin Trials. 2005 May-Jun. 6(3):136-46. [QxMD MEDLINE Link].
- Wright ME, Suzman DL, Csaky KG, Masur H, Polis MA, Robinson MR. Extensive retinal neovascularization as a late finding in human immunodeficiency virus-infected patients with immune recovery uveitis. Clin Infect Dis. 2003 Apr 15. 36(8):1063-6. [QxMD MEDLINE Link].
- Richardson WP, Colvin RB, Cheeseman SH. Glomerulopathy associated with cytomegalovirus viremia in renal allografts. N Engl J Med. 1981 Jul 9. 305(2):57-63. [QxMD MEDLINE Link].
- Torok-Storb B, Boeckh M, Hoy C. Association of specific cytomegalovirus genotypes with death from myelosuppression after marrow transplantation. Blood. 1997 Sep 1. 90(5):2097-102. [QxMD MEDLINE Link].
- Manuel O, Asberg A, Pang X, Rollag H, Emery VC, Preiksaitis JK. Impact of genetic polymorphisms in cytomegalovirus glycoprotein B on outcomes in solid-organ transplant recipients with cytomegalovirus disease. Clin Infect Dis. 2009 Oct 15. 49(8):1160-6. [QxMD MEDLINE Link].
- Iwasenko JM, Howard J, Arbuckle S, et al. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J Infect Dis. 2011 Jun. 203(11):1526-33. [QxMD MEDLINE Link].
- Klemola E, Von Essen R, Henle G, Henle W. Infectious-mononucleosis-like disease with negative heterophil agglutination test. Clinical features in relation to Epstein-Barr virus and cytomegalovirus antibodies. J Infect Dis. 1970 Jun. 121(6):608-14. [QxMD MEDLINE Link].
- Cohen JI, Corey GR. Cytomegalovirus infection in the normal host. Medicine (Baltimore). 1985 Mar. 64(2):100-14. [QxMD MEDLINE Link].
- Horwitz CA, Henle W, Henle G. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore). 1986 Mar. 65(2):124-34. [QxMD MEDLINE Link].
- Klemola E, Stenström R, von Essen R. Pneumonia as a clinical manifestation of cytomegalovirus infection in previously healthy adults. Scand J Infect Dis. 1972. 4(1):7-10. [QxMD MEDLINE Link].
- Jaber S, Chanques G, Borry J, Souche B, Verdier R, Perrigault PF. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005 Jan. 127(1):233-41. [QxMD MEDLINE Link].
- von Müller L, Klemm A, Weiss M, et al. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006 Oct. 12(10):1517-22. [QxMD MEDLINE Link]. [Full Text].
- Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med. 2009 Aug. 37(8):2350-8. [QxMD MEDLINE Link].
- Cook CH, Yenchar JK, Kraner TO, Davies EA, Ferguson RM. Occult herpes family viruses may increase mortality in critically ill surgical patients. Am J Surg. 1998 Oct. 176(4):357-60. [QxMD MEDLINE Link].
- De Vlieger G, Meersseman W, Lagrou K, et al. Cytomegalovirus serostatus and outcome in nonimmunocompromised critically ill patients. Crit Care Med. 2012 Jan. 40(1):36-42. [QxMD MEDLINE Link].
- Jerry Teng CL, Wang PN, Chen YC, Ko BS. Cytomegalovirus management after allogeneic hematopoietic stem cell transplantation: A mini-review. J Microbiol Immunol Infect. 2021. [Full Text].
- Reed EC, Bowden RA, Dandliker PS. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants. Ann Intern Med. 1988 Nov 15. 109(10):783-8. [QxMD MEDLINE Link].
- Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2010 Jan. 10(1):157-61. [QxMD MEDLINE Link].
- Dieterich DT, Rahmin M. Cytomegalovirus colitis in AIDS: presentation in 44 patients and a review of the literature. J Acquir Immune Defic Syndr. 1991. 4 Suppl 1:S29-35. [QxMD MEDLINE Link].
- McCutchan JA. Cytomegalovirus infections of the nervous system in patients with AIDS. Clin Infect Dis. 1995 Apr. 20(4):747-54. [QxMD MEDLINE Link].
- Shanahan A, Malani PN, Kaul DR. Relapsing cytomegalovirus infection in solid organ transplant recipients. Transpl Infect Dis. 2009 Dec. 11(6):513-8. [QxMD MEDLINE Link].
- Martín-Dávila P, Fortún J, Gutiérrez C, Martí-Belda P, Candelas A, Honrubia A, et al. Analysis of a quantitative PCR assay for CMV infection in liver transplant recipients: an intent to find the optimal cut-off value. J Clin Virol. 2005 Jun. 33(2):138-44. [QxMD MEDLINE Link].
- Aitken C, Barrett-Muir W, Millar C, Templeton K, Thomas J, Sheridan F. Use of molecular assays in diagnosis and monitoring of cytomegalovirus disease following renal transplantation. J Clin Microbiol. 1999 Sep. 37(9):2804-7. [QxMD MEDLINE Link]. [Full Text].
- Gerna G, Zipeto D, Parea M, Revello MG, Silini E, Percivalle E. Monitoring of human cytomegalovirus infections and ganciclovir treatment in heart transplant recipients by determination of viremia, antigenemia, and DNAemia. J Infect Dis. 1991 Sep. 164(3):488-98. [QxMD MEDLINE Link].
- Tanabe K, Tokumoto T, Ishikawa N, Koyama I, Takahashi K, Fuchinoue S. Comparative study of cytomegalovirus (CMV) antigenemia assay, polymerase chain reaction, serology, and shell vial assay in the early diagnosis and monitoring of CMV infection after renal transplantation. Transplantation. 1997 Dec 27. 64(12):1721-5. [QxMD MEDLINE Link].
- Anti-Cytomegalovirus (CMV) Immediate Early Antigen Monoclonal Antibody, Unconjugated, Clone 3G9.2 from CHEMICON. www.chemicon.com. Available at https://www.bio-medicine.org/biology-products/Anti-Cytomegalovirus--28CMV-29-Immediate-Early-Antigen-Monoclonal-Antibody--Unconjugated--Clone-3G9-2-from-CHEMICON-2132-1/. Accessed: March 17, 2010.
- Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. 2011 Jun 2. 364(22):2111-8. [QxMD MEDLINE Link]. [Full Text].
- Sanghavi SK, Abu-Elmagd K, Keightley MC, St George K, Lewandowski K, Boes SS. Relationship of cytomegalovirus load assessed by real-time PCR to pp65 antigenemia in organ transplant recipients. J Clin Virol. 2008 Aug. 42(4):335-42. [QxMD MEDLINE Link].
- Jabs DA, Martin BK, Forman MS, Ricks MO. Cytomegalovirus (CMV) blood DNA load, CMV retinitis progression, and occurrence of resistant CMV in patients with CMV retinitis. J Infect Dis. 2005 Aug 15. 192(4):640-9. [QxMD MEDLINE Link].
- Roche Molecular Diagnostics. COBAS AMPLICOR CMV MONITOR test. Available at https://molecular.roche.com/assays/Pages/COBASAMPLICORCMVMONITORTest.aspx. Accessed: July 10, 2012.
- Roche Molecular Diagnostics. COBAS AmpliPrep/COBAS TaqMan CMV test. Available at https://molecular.roche.com/assays/Pages/COBASAmpliPrepCOBASTaqManCMVTest.aspx. Accessed: July 10, 2012.
- Smith TF, Espy MJ, Mandrekar J, Jones MF, Cockerill FR, Patel R. Quantitative real-time polymerase chain reaction for evaluating DNAemia due to cytomegalovirus, Epstein-Barr virus, and BK virus in solid-organ transplant recipients. Clin Infect Dis. 2007 Oct 15. 45(8):1056-61. [QxMD MEDLINE Link].
- Razonable RR. Drug-resistant cytomegalovirus: clinical implications of specific mutations. Curr Opin Organ Transplant. 2018. 23(4):388-394. [Full Text].
- Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017 Dec 6. [QxMD MEDLINE Link]. [Full Text].
- Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011 Apr. 11(4):284-92. [QxMD MEDLINE Link].
- Li WW, Zhang YM, Shen MZ, Mo XD. Efficacy and safety of letermovir prophylaxis for cytomegalovirus infection after hematopoietic stem cell transplantation. Blood Sci. 2024 Jan. 6 (1):e00178. [QxMD MEDLINE Link].
- Acosta E, Bowlin T, Brooks J, et al. Advances in the Development of Therapeutics for Cytomegalovirus Infections. J Infect Dis. 2020. 221(Suppl 1):S32-S44. [Full Text].
- Limaye AP, Budde K, Humar A, Vincenti F, Kuypers DRJ, Carroll RP, et al. Letermovir vs Valganciclovir for Prophylaxis of Cytomegalovirus in High-Risk Kidney Transplant Recipients: A Randomized Clinical Trial. JAMA. 2023 Jun 6. [QxMD MEDLINE Link].
- No authors listed. Valganciclovir: new preparation. CMV retinitis: a simpler, oral treatment. Prescrire Int. 2003 Aug. 12(66):133-5. [QxMD MEDLINE Link].
- Caldés A, Gil-Vernet S, Armendariz Y, Colom H, Pou L, Niubó J, et al. Sequential treatment of cytomegalovirus infection or disease with a short course of intravenous ganciclovir followed by oral valganciclovir: efficacy, safety, and pharmacokinetics. Transpl Infect Dis. 2009 Dec 9. [QxMD MEDLINE Link].
- Dieterich DT, Chachoua A, Lafleur F. Ganciclovir treatment of gastrointestinal infections caused by cytomegalovirus in patients with AIDS. Rev Infect Dis. 1988 Jul-Aug. 10 Suppl 3:S532-7. [QxMD MEDLINE Link].
- Kalil AC, Mindru C, Florescu DF. Effectiveness of valganciclovir 900 mg versus 450 mg for cytomegalovirus prophylaxis in transplantation: direct and indirect treatment comparison meta-analysis. Clin Infect Dis. 2011 Feb. 52(3):313-21. [QxMD MEDLINE Link].
- Avery RK. Low-dose valganciclovir for cytomegalovirus prophylaxis in organ transplantation: is less really more?. Clin Infect Dis. 2011 Feb. 52(3):322-4. [QxMD MEDLINE Link].
- Legendre C, Pascual M. Improving outcomes for solid-organ transplant recipients at risk from cytomegalovirus infection: late-onset disease and indirect consequences. Clin Infect Dis. 2008 Mar 1. 46(5):732-40. [QxMD MEDLINE Link].
- Cytomegalovirus. Am J Transplant. 2004 Nov. 4 Suppl 10:51-8. [QxMD MEDLINE Link].
- Bodro M, Sabé N, Lladó L, et al. Prophylaxis versus preemptive therapy for cytomegalovirus disease in high-risk liver transplant recipients. Liver Transpl. 2012 Sep. 18(9):1093-9. [QxMD MEDLINE Link].
- Paudice N, Mehmetaj A, Zanazzi M, Moscarelli L, Piperno R, Di Maria L. Preemptive therapy for the prevention of cytomegalovirus disease in renal transplant recipients: our preliminary experience. Transplant Proc. 2009 May. 41(4):1204-6. [QxMD MEDLINE Link].
- Boeckh M, Gooley TA, Myerson D. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996 Nov 15. 88(10):4063-71. [QxMD MEDLINE Link].
- Ohmoto A, Fuji S. Letermovir for cytomegalovirus infection in allogeneic hematopoietic stem-cell transplantation: tips and notes for effective use in clinical practice. Expert Rev Anti Infect Ther. 2024 Mar 1. 1-10. [QxMD MEDLINE Link].
- Göhring K, Hamprecht K, Jahn G. Antiviral Drug- and Multidrug Resistance in Cytomegalovirus Infected SCT Patients. Comput Struct Biotechnol J. 2015. 13:153-9. [QxMD MEDLINE Link].
- Avery RK, Marty FM, Strasfeld L, Lee I, Arrieta A, Chou S. Oral maribavir for treatment of refractory or resistant cytomegalovirus infections in transplant recipients. Transpl Infect Dis. 2010 Dec. 12(6):489-96. [QxMD MEDLINE Link].
- Walti CS, Khanna N, Avery RK, Helanterä I. New Treatment Options for Refractory/Resistant CMV Infection. Transpl Int. 2023. 36:11785. [QxMD MEDLINE Link].
- Avery RK, Alain S, Alexander BD, Blumberg EA, Chemaly RF, Cordonnier C, et al. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial. Clin Infect Dis. 2022 Sep 10. 75 (4):690-701. [QxMD MEDLINE Link].
- Trofe J, Pote L, Wade E, Blumberg E, Bloom RD. Maribavir: a novel antiviral agent with activity against cytomegalovirus. Ann Pharmacother. 2008 Oct. 42(10):1447-57. [QxMD MEDLINE Link].
- John GT, Manivannan J, Chandy S, Peter S, Jacob CK. Leflunomide therapy for cytomegalovirus disease in renal allograft recepients. Transplantation. 2004 May 15. 77(9):1460-1. [QxMD MEDLINE Link].
- John GT, Manivannan J, Chandy S, Peter S, Fleming DH, Chandy SJ, et al. A prospective evaluation of leflunomide therapy for cytomegalovirus disease in renal transplant recipients. Transplant Proc. 2005 Dec. 37(10):4303-5. [QxMD MEDLINE Link].
- Levi ME, Mandava N, Chan LK, Weinberg A, Olson JL. Treatment of multidrug-resistant cytomegalovirus retinitis with systemically administered leflunomide. Transpl Infect Dis. 2006 Mar. 8(1):38-43. [QxMD MEDLINE Link].
- Battiwalla M, Paplham P, Almyroudis NG, McCarthy A, Abdelhalim A, Elefante A. Leflunomide failure to control recurrent cytomegalovirus infection in the setting of renal failure after allogeneic stem cell transplantation. Transpl Infect Dis. 2007 Mar. 9(1):28-32. [QxMD MEDLINE Link].
- Valantine HA, Luikart H, Doyle R, Theodore J, Hunt S, Oyer P. Impact of cytomegalovirus hyperimmune globulin on outcome after cardiothoracic transplantation: a comparative study of combined prophylaxis with CMV hyperimmune globulin plus ganciclovir versus ganciclovir alone. Transplantation. 2001 Nov 27. 72(10):1647-52. [QxMD MEDLINE Link].
- Go V, Pollard RB. A cytomegalovirus vaccine for transplantation: are we closer?. J Infect Dis. 2008 Jun 15. 197(12):1631-3. [QxMD MEDLINE Link].
- Schleiss MR. A cytomegalovirus vaccine tames the troll of transplantation. Lancet. 2011 Apr 9. 377(9773):1216-8. [QxMD MEDLINE Link].
- Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009 Mar 19. 360(12):1191-9. [QxMD MEDLINE Link]. [Full Text].
- Price NB, Prichard MN. Progress in the development of new therapies for herpesvirus infections. Curr Opin Virol. 2011 Dec. 1(6):548-54. [QxMD MEDLINE Link].
- Torres-Madriz G, Boucher HW. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis. 2008 Sep 1. 47(5):702-11. [QxMD MEDLINE Link].
- Wolf DG, Shimoni A, Resnick IB, Stamminger T, Neumann AU, Chou S. Human cytomegalovirus kinetics following institution of artesunate after hematopoietic stem cell transplantation. Antiviral Res. 2011 Jun. 90(3):183-6. [QxMD MEDLINE Link].
- Shapira MY, Resnick IB, Chou S, et al. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis. 2008 May 1. 46(9):1455-7. [QxMD MEDLINE Link].
- Kaul DR, Stoelben S, Cober E, Ojo T, Sandusky E, Lischka P. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant. 2011 May. 11(5):1079-84. [QxMD MEDLINE Link].
- Grossi P, Mohacsi P, Szabolcs Z, Potena L. Cytomegalovirus Immunoglobulin After Thoracic Transplantation: An Overview. Transplantation. 2016 Mar. 100 Suppl 3 (Suppl 3):S1-4. [QxMD MEDLINE Link].
- Barten MJ, Baldanti F, Staus A, Hüber CM, Glynou K, Zuckermann A. Effectiveness of Prophylactic Human Cytomegalovirus Hyperimmunoglobulin in Preventing Cytomegalovirus Infection following Transplantation: A Systematic Review and Meta-Analysis. Life (Basel). 2022 Mar 2. 12 (3):[QxMD MEDLINE Link].
Author
Coauthor(s)
Lisa Vanchhawng Pedroza, MD Assistant Professor of Medicine, Attending Physician, Division of Infectious Diseases, Cooper University Hospital
Lisa Vanchhawng Pedroza, MD is a member of the following medical societies: Infectious Diseases Society of America
Disclosure: Nothing to disclose.
Elvin Alfonso Colón Martínez, MD Resident Physician, Department of Internal Medicine, Sunrise Health GME Consortium, Mountain View Hospital
Elvin Alfonso Colón Martínez, MD is a member of the following medical societies: American College of Physicians
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
John W King, MD Professor of Medicine, Chief, Section of Infectious Diseases, Director, Viral Therapeutics Clinics for Hepatitis, Louisiana State University School of Medicine in Shreveport; Consultant in Infectious Diseases, Overton Brooks Veterans Affairs Medical Center
John W King, MD is a member of the following medical societies: American Association for the Advancement of Science, American College of Physicians, American Federation for Medical Research, American Society for Microbiology, Association of Subspecialty Professors, Infectious Diseases Society of America, Sigma Xi, The Scientific Research Honor Society
Disclosure: Nothing to disclose.
Chief Editor
Michael Stuart Bronze, MD David Ross Boyd Professor and Chairman, Department of Medicine, Stewart G Wolf Endowed Chair in Internal Medicine, Department of Medicine, University of Oklahoma Health Science Center; Master of the American College of Physicians; Fellow, Infectious Diseases Society of America; Fellow of the Royal College of Physicians, London
Michael Stuart Bronze, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Physicians, American Medical Association, Association of Professors of Medicine, Infectious Diseases Society of America, Oklahoma State Medical Association, Southern Society for Clinical Investigation
Disclosure: Nothing to disclose.
Additional Contributors
Todd S Wills, MD Associate Professor, Department of Medicine, Division of Infectious Disease and International Medicine, Program Director, Infectious Disease Fellowship Program, University of South Florida College of Medicine
Todd S Wills, MD is a member of the following medical societies: Infectious Diseases Society of America
Disclosure: Nothing to disclose.
Kauser Akhter, MD Assistant Professor, Department of Internal Medicine, Florida State University College of Medicine; Associate Program Director, Infectious Diseases Fellowship Program, Orlando Health
Disclosure: Nothing to disclose.
Acknowledgements
The authors and editors of Medscape Reference gratefully acknowledge the contributions of previous coauthor Todd S Wills, MD to the development and writing of this article.