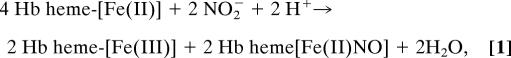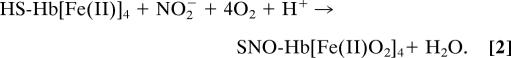An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate (original) (raw)
Abstract
Red blood cells (RBCs) act as O2-responsive transducers of vasodilator and vasoconstrictor activity in lungs and tissues by regulating the availability of nitric oxide (NO). Vasodilation by RBCs is impaired in diseases characterized by hypoxemia. We have proposed that the extent to which RBCs constrict vs. dilate vessels is, at least partly, controlled by a partitioning between NO bound to heme iron and to Cysβ93 thiol of hemoglobin (Hb). Hemes sequester NO, whereas thiols deploy NO bioactivity. In recent work, we have suggested that specific micropopulations of NO-liganded Hb could support the chemistry of _S_-nitrosohemoglobin (SNO-Hb) formation. Here, by using nitrite as the source of NO, we demonstrate that a (T state) micropopulation of a heme-NO species, with spectral and chemical properties of Fe(III)NO, acts as a precursor to SNO-Hb formation, accompanying the allosteric transition of Hb to the R state. We also show that at physiological concentrations of nitrite and deoxyHb, a _S_-nitrosothiol precursor is formed within seconds and produces SNO-Hb in high yield upon its prompt exposure to O2 or CO. Deoxygenation/reoxygenation cycling of oxyHb in the presence of physiological amounts of nitrite also efficiently produces SNO-Hb. In contrast, high amounts of nitrite or delays in reoxygenation inhibit the production of SNO-Hb. Collectively, our data provide evidence for a physiological _S_-nitrosothiol synthase activity of tetrameric Hb that depends on NO-Hb micropopulations and suggest that dysfunction of this activity may contribute to the pathophysiology of cardiopulmonary and blood disorders.
Keywords: hypoxic vasodilation, nitric oxide, _S_-nitrosohemoglobin, S-nitrosylation
Historically, red blood cells (RBCs) have been regarded as transporters of oxygen (O2) and carbon dioxide (CO2), with the uptake of one and release of the other being reciprocally related. With the advent of the field of nitric oxide (NO) biology, RBCs also were thought to be scavengers of NO that could effectively quench its bioactivity. Some years ago, we noted that this limited view was inconsistent with the role of hemoglobin (Hb) in O2 delivery, as sequestration of NO by Hb would lead to constriction of blood vessels, and this vasoconstriction, in turn, would limit the supply of O2 (1). We subsequently demonstrated that vasoconstriction by RBCs is seen only at relatively high partial pressures of O2 (pO2); at lower pO2s characteristic of tissues (5–20 mm Hg), RBCs dilate blood vessels (2, 3). Vasodilation (of aortic or pulmonary artery rings) by RBCs in bioassay is rapid, in keeping with the temporal requirements of arterial-venous transit (in seconds) (3, 4). Moreover, when infused into animals, RBCs increase blood flow and improve oxygenation, an indication that RBCs elicit vasodilation in both the systemic and pulmonary circulations (1, 4–6). Collectively, the observation of vasoconstriction by RBCs at higher pO2 but graded vasodilation with increasing hypoxia appears to provide a mechanism for matching blood flow to metabolic demand in the peripheral tissues and for matching ventilation to perfusion in the lungs (7).
Constriction of blood vessels by RBCs results from a lowering of the steady-state concentration of bioactive NO. The chemistry of vasoconstriction involves, among other reactions, NO binding to the ferrous hemes of Hb (1, 7–9). NO bound as a ferrous nitrosyl adduct [Fe(II)NO] does not exert direct vasodilatory activity (9–12), because ligand off rates are slow and any NO liberated is rapidly trapped by the surrounding hemes, which are present at relatively high concentrations (excess heme over NO: ≈10,000:1) (12). However, work from our laboratories has elucidated conditions under which β-chain heme Fe(II)NO complexes can be oxidatively converted to an _S_-nitrosothiol (CysβSNO), which maintains NO bioactivity (13–16).
S-nitrosylated Hb (SNO-Hb) is capable of transferring the NO group to cysteines of other peptides and proteins, such as glutathione and the RBC membrane protein AE1, which can convey the NO signal to the vessel wall (17). This exchange of NO groups occurs primarily in the T state (deoxyHb; high spin) of Hb (3, 6, 18), which exposes the β93cysNO to solvent and adjusts heme/SNO redox coupling (7, 18). As a result, NO group transfer is graded with the degree of hypoxia (6, 19). Studies in mice with a genetic deficiency in SNO turnover demonstrate the existence of an apparent equilibrium of NO between Hb and glutathione in vivo and provide strong evidence that _S_-nitrosothiols (SNOs) regulate vascular resistance (20).
Although the original problem of the coexistence of NO vasodilatory activity with Hb has been solved, the means by which SNOs are generated in Hb, particularly as it involves heme-to-thiol NO transfer, remains incompletely understood. Factors known to influence the efficiency of NO group transfer from hemes to thiols include the allosteric state of Hb, the disposition of NO within the tetramer (α- vs. β-chain), the concentration of NO, and its ratio to Hb (7). A further aspect of this chemistry is redox activation. Recent work by us (16) and others (21–23) has shown that oxidized hemes [Fe(III)], particularly β-chain hemes, are competent for this redox activity. Thus, βFe(III)NO meets both the oxidative and structural requirements for SNO-Hb production (7, 16, 23). In related studies, Pezacki et al. (18, 24) reported that βFe(III)NO accumulates during deoxygenation of SNO-Hb. Taken together (16, 24), these studies raise the idea of an equilibrium-like interrelationship between Hb[βFe(III)NO] and SNO-Hb. The intriguing suggestion of Rifkind et al. (25) that most HbNO in vivo is Hb[Fe(III)NO] {or Hb[Fe(II)NO]+} is of particular interest in this context.
To explore the role of Fe(III)NO in SNO-Hb production, we have extended our previous work on reactions of nitrite with deoxyHb (3, 7, 12, 16). Those studies showed that exposure of deoxyHb to limiting nitrite leads to production of Fe(III) and β Fe(II)NO, as described, in simplified terms, in Eq. 1:
with SNO-Hb produced upon prompt oxygenation (12, 15–17). However, our kinetic analysis (12), like other work on nitrite/Hb interactions (25–27), was performed at supraphysiological nitrite/Hb ratios (typically nitrite:Hb 1:10–10:1 vs. ≈1:1,000 in vivo), with a global spectral deconvolution approach that was not adapted to recognize “minority species.” We now examine the product distribution of reactions of nitrite and deoxyhemoglobin by using a modified approach in which spectral deconvolution is used to test for the presence of minority species formed at physiologically relevant concentrations, whereas studies of the chemical reactivity of these species are used to verify their identity. We show that product distributions vary substantially as a function of time after reagent mixing (seconds to hours), and nitrite to heme ratio (1:1,000–1:1). At physiological concentrations and time scales (approximate venous residence times in vivo), the reaction leads to high-yield production of a species with properties of Fe(III)NO that converts efficiently to SNO-Hb upon oxygenation (or CO exposure). In contrast, SNO-Hb production is suppressed at higher nitrite concentrations, apparently reflecting the formation of alternative NO-Hb micropopulations not competent for this chemistry. Moreover, delays in sample oxygenation drastically reduce SNO yields. This behavior presumably reflects the reactive nature of the SNO precursor that, absent prompt oxygenation, yields dead-end products. Overall, our data strengthen the case that Hb operates under physiological conditions as a SNO synthase, converting NO into SNOs through an allosterically modulated heme-NO redox reaction.
Experiments and Results
Reaction of NaNO2 with DeoxyHb Assessed by UV-Vis Spectroscopy.
Modified spectral analysis method.
We analyze product development in the reactions of nitrite (10–400 μM) with deoxyHb (250 μM) by spectral deconvolution of UV-Vis spectra in which experimental spectra are modeled as a linear combination of component spectra (12, 14, 16). In previous studies, we included in the basis of spectral components all Hb spectra that could be reasonably expected under the given experimental conditions. However, the calculated best-fit spectra still show small systematic deviations from experimental ones, both in our studies and related studies by others (28, 29). In estimating the precision of the fitted parameter values, we included effects of parameter correlation and systematic error of the model and, thus, reported conservative error estimates of ≈10 μM (a concentration exceeding the NO-Hb species of physiological relevance; refs. 3, 7, 15, and 17).
Here we adopt a use of the deconvolution method that is better adapted to recognize the minority species that form in physiological situations. First, we identify the minimal basis set that provides a reasonable simulation of the experimental spectrum. We then add additional suspected components to the spectral basis, repeat the analysis, and test for significance of such additions (30). In the present study, the minimal model includes deoxyHb, metHb, oxyHb, and Fe(II)NO Hb as basis spectra. With this procedure, we find that the addition of an Fe(III)NO component significantly improves the fit and provides a better improvement than addition of other possible components. Quantitatively, the improvement is significant with greater than 99.9% confidence in every experimental trial at the point of maximal Fe(III)NO concentration (F test). We report analyses made with the minimal basis augmented by the Fe(III)NO component. Additional experimental details are documented in Fig. 6, which is published as supporting information on the PNAS web site.
Results.
Under supraphysiological conditions of nitrite excess (400 μM), the ratio of products [Fe(III)]/[Fe(II)NO] was close to unity (1:1), in basic agreement with previous reports by several laboratories (refs. 12 and 26; Fig. 1A); a slight excess of Fe(III) over Fe(II)NO, however, was observed during the reaction. In addition, we detected, as described above, a trace concentration of a species that was analyzed as Fe(III)NO. The concentration of this species peaked after ≈40 min of reaction and subsequently decayed to zero (Fig. 1 A and B). With limiting nitrite, the ratio of [Fe(III)]/[Fe(II)NO] products showed substantial departures from unity, as exemplified in Fig. 1A (75 μM nitrite). Most interestingly, Fe(III)NO was observed to rise to a sustained level, without decay, under these reaction conditions (Fig. 1 A and B).
Fig. 1.
Reaction of deoxyHb with nitrite: Product distribution depends on nitrite concentration. (A) Reaction of deoxyHb (250 μM) with excess (400 μM; Left) or limiting (75 μM; Right) concentrations of nitrite. When excess nitrite is used, the ratio of Hb[Fe(II)NO] to Hb[Fe(III)] approaches 1:1 {and Hb[Fe(III)NO] is detected only at early time points and in trace amounts}. In contrast, with limiting nitrite, Hb[Fe(III)] is in significant excess over Hb[Fe(II)NO], and Hb[Fe(III)NO] accumulates over the time course of the reaction. (B) Hb[Fe(III)]NO production. With high nitrite relative to Hb (400 μM nitrite, 250 μM Hb; ◇), Hb[Fe(III)NO] concentrations rise (over time) until 50% of the hemes in Hb are either ligated by NO or oxidized (sum of all products divided by the heme concentration of 1 mM). Further reaction of nitrite with Hb (to achieve a concentration of products exceeding 50% of hemes) induces the allosteric transition, which is coupled to isomerization of Hb[Fe(III)NO] to SNO-Hb. With limiting nitrite (75 μM nitrite, 1 mM heme; black and white diamonds), Hb[Fe(III)NO] accumulates in T state. Results are presented as mean ± SE of three experiments.
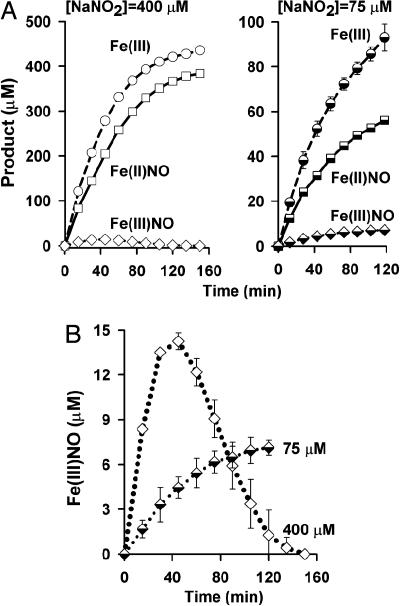
In Fig. 2 we illustrate the trends followed by the final levels of Fe(III)NO (Fig. 2A), Fe(III) and Fe(II)NO levels (Fig. 2B), and the [Fe(III)]/[Fe(II)NO] ratio (Fig. 2C) as a function of [NaNO2] over the range 10–400 μM (here and in subsequent descriptions of reaction conditions, [NaNO2] refers to the starting concentration of NaNO2). Although the ratio of [Fe(III)]/[Fe(II)NO] varies over this range, it appears to approach unity if [NaNO2] is in excess, or as [NaNO2]/[heme] approaches zero. The trend in Fe(III)NO levels also exhibits a biphasic response, increasing roughly linearly with increasing [NaNO2] up to 250 μM, but decreasing after this point. This result implies a connection to the occupancy of Hb heme by ligands {[Fe(II)NO]} or “holes” [Fe(III)] and, thus, to Hb allostery. The turning point in the trend occurs at a nitrite level at which the hemes are half-occupied, that is, at which further reaction would lead to a transformation of Hb from its T to R quaternary structure (15, 31). The same principle applies to time-dependent behavior of Fe(III)NO in reactions with excess nitrite, exemplified in Fig. 1 A and B (400 μM nitrite): After half-saturation of the protein, there is a decline in concentration of Fe(III)NO.
Fig. 2.
Reaction of deoxyHb and nitrite: distribution of products at completion of reaction as a function of initial [NaNO2]. (A) [NaNO2] vs. [Fe(III)NO]. Hb[Fe(III)NO] accumulates in reactions with limiting concentrations of nitrite (<250 μM). DeoxyHb, 250 μM. (B) [NaNO2] vs. [Fe(III)] and [Fe(II)NO]. Limiting (albeit supraphysiological) concentrations of nitrite yield an excess of Fe(III) over Fe(II)NO. DeoxyHb, 250 μM. (C) [NaNO2] vs. [Fe(III)]/[Fe(II)NO]. The ratio of Fe(III) to Fe(II)NO varies as a function of nitrite concentration. DeoxyHb, 250 μM. Results are presented as mean ± SE of three experiments.
SNO-Hb Formation.
Correlation with Fe(III)NO.
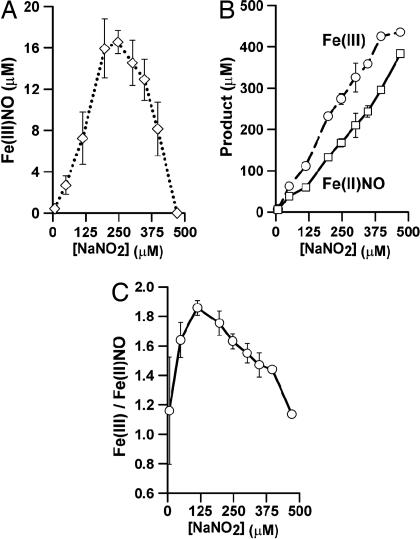
Inasmuch as heme-[Fe(III)NO] has been demonstrated previously to be an effective intermediary en route to SNO-Hb production (16), and the linkage of SNO-Hb formation to Hb allostery has been established (1, 7), we conjectured that the allosterically coupled loss of Fe(III)NO described above might reflect an allosterically linked conversion of the Fe(III)NO species to SNO-Hb. To investigate this possibility, we assessed SNO-Hb formation in reactions of deoxyHb with nitrite over the range 50–350 μM and compared the trends in SNO-Hb levels to trends in Fe(III)NO. The results are illustrated in Fig. 3A. Remarkably, the trend in SNO-Hb formation precisely complements the trend in Fe(III)NO loss: The amount of SNO formed is directly proportional to the deviation of Fe(III)NO levels from their linear dependence on [NaNO2] that is evident when [NaNO2] < [heme] (proportionality constant = 0.67 ± 0.05; R = 0.98). In Fig. 3A, we show the trends as a function of both [NaNO2] and heme occupancy to underscore the connection with Hb allostery.
Fig. 3.
Conversion of Hb[Fe(III)NO] to SNO-Hb with transition from T state to R state. The allosteric transition is induced by nitrite (A) or oxygen (B). (A) Fe(III)NO builds up and then isomerizes to SNO-Hb on transition from T state to R state under anaerobic conditions. Product concentrations (at reaction completion) are depicted as a function of percent ligation of all hemes ([DeoxyHb] = 250 μM; [heme] = 4[tetramer]). Nitrite in excess of 250 μM (the concentration producing 50% “ligation,” including both ligated and oxidized hemes) facilitates the allosteric transition from T to R state through an anaerobic buildup of product. (B) Anaerobic values of Hb[Fe(III)NO] concentration are predictive of [SNO-Hb] after oxygenation. Product concentrations are shown as a function of starting nitrite concentration. For [NaNO2] < 250 μM, Hb[Fe(III)NO] concentration preoxygenation (pre-O2) correlates directly with [SNO-Hb] after oxygenation (post-O2). For [NaNO2] > 250 μM, Hb[Fe(III)NO] concentration decreases to zero and [SNO-Hb] (after oxygenation) plateaus, indicating that an Fe(III)NO intermediate accumulates and then isomerizes to SNO-Hb after the allosteric transition to R state. [DeoxyHb] = 250 μM. Results are presented as mean ± SE of three to four experiments.
To explore this point further, we subjected mixtures of deoxyHb and limiting amounts of nitrite to oxygenation, after the nitrite reaction was completed (Fig. 3B). Again, remarkably, we found that SNO-Hb was produced after oxygenation in direct proportion to the concentration of Hb[Fe(III)NO] before oxygenation (proportionality constant = 0.89 ± 0.06; R = 0.97). In other words, declines in [Fe(III)NO] induced by ligation of vacant hemes are matched by an equivalent increase in [SNO-Hb]. Evidently, the heme-[(FeIII)NO] species is a precursor for SNO-Hb, with chemical interconversion coupled to the allosteric transition of Hb from T to R state.
Temporal characteristics and physiological conditions.
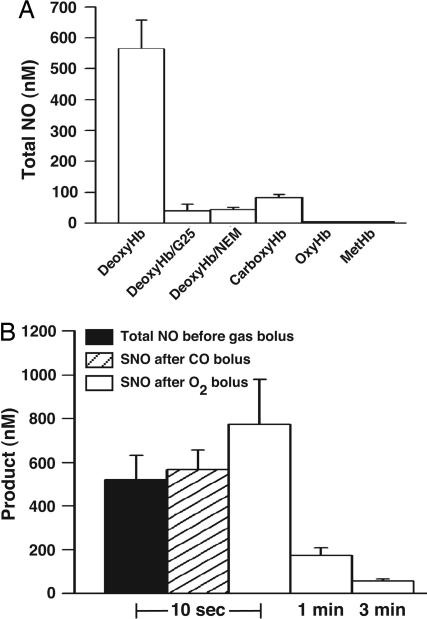
In the SNO-Hb paradigm (7, 13–17), SNO-Hb production would be optimized under physiological conditions, where nitrite or NO is ≈1 μM, Hb is in great excess, and deoxygenated Hb is available for no more than tens of seconds (the venous residence time is ≈30 seconds). To investigate the significance of this temporal constraint, a significance already suggested by the detrimental effect of aging of HbNO preparations on SNO formation (4), 250 μM deoxyHb and 1 μM NaNO2 were mixed and analyzed promptly by photolysis/chemiluminescence. Notably, at 10 seconds after mixing, a positive signal is obtained, amounting to roughly half of the nitrite concentration (565 nM ± 105; n = 5). Gel filtration (G25) eliminates the signal (Fig. 4A). Control experiments conducted with nitrite alone, or nitrite plus glutathione, gave essentially no photolysis/chemiluminescence signal (≤2% of nitrite concentration), establishing that the signal is derived from the protein. Similarly, no signals were obtained upon incubation of nitrite with metHb, carboxyHb, oxyHb, or from deoxyHb pretreated with _N_-ethylmaleimide. In further control experiments, we confirmed that signals produced by Hb[Fe(II)NO] and SNO-Hb are not altered by gel filtration. Collectively, the data indicate the rapid formation (within seconds) of a nitrite-derived species weakly associated with Hb, the generation of which is promoted by vacant reduced hemes and thiols and/or T state quaternary structure of Hb, and from which NO is liberated by photolysis.
Fig. 4.
DeoxyHb and nitrite react immediately to form a SNO-Hb precursor species (A) that produces SNO-Hb upon allosteric transition (B). (A) Samples produced by incubation of 1 μM NaNO2 with 250 μM deoxyHb for 10 sec yielded a photolysis-chemiluminescence signal representing 565 ± 105 nM NO, which was eliminated in samples desalted with G25. No R state Hb tetramer examined produced a comparable signal after reaction with NaNO2. Results are presented as mean ± SE; n = 5. (B) Samples produced by mixing 1 μM NaNO2 with 250 μM deoxyHb as in A were bolused for 10 sec with either CO or O2, beginning either 10 sec after mixing or after a delay of 1 or 3 min. When samples were bolused with either O2 or CO beginning 10 sec after mixing, the resultant SNO-Hb concentrations essentially matched SNO precursor concentrations before bolus. However, prolonging anaerobic incubation times before exposure to gas bolus markedly decreased SNO-Hb yields. Results are presented as mean ± SE of five experiments.
If this species were the SNO precursor characterized by UV-Vis spectroscopy, then occupation of the hemes should effect its conversion to SNO-Hb. To test this idea, mixtures of deoxyHb and nitrite, prepared as described above, were exposed (at 10 sec after mixing) to a bolus of O2 or CO, then purified via gel chromatography (G25) to isolate protein-bound NO products. Remarkably, a 10-sec exposure to CO or O2 produces SNO-Hb in concentrations equal to the SNO precursor concentrations measured before the gas bolus (Fig. 4B). Delaying oxygenation lowers SNO-Hb yields, presumably due to interfering chemical reaction(s) of the SNO precursor (Fig. 4B). In addition, raising the nitrite concentration led to diminished yields of SNO-Hb (approaching zero SNO at 50–100 μM nitrite), as previously described in experiments with NO (15, 17). For example, at 100 μM nitrite/250 μM deoxyHb (×10 sec), yields of SNO-Hb after oxygenation were 250 ± 212 nM (n = 3).
Oxygenation/deoxygenation cycling.
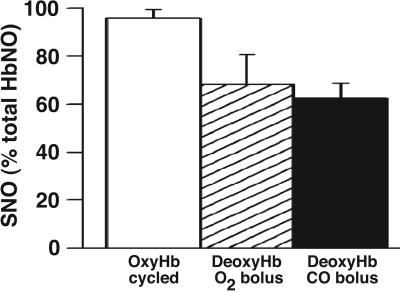
Because of chain heterogeneity in O2 dissociation rates, β-chains will preferentially deoxygenate under hypoxic conditions, effectively increasing β-chain interactions with nitrite relative to α-chains (16, 32). Thus, inasmuch as the efficiency of SNO-Hb production appears linked to NO localization to the β-chain (16), rapid deoxygenation of oxyHb in the presence of nitrite should increase the percent yield of SNO-Hb on reoxygenation. To test this prediction, 250 μM oxyHb and 1 μM NaNO2 were mixed, rapidly deoxygenated with argon (≈1 min), and reoxygenated and purified via G25 chromatography. OxyHb cycled (deoxygenation/reoxygenation) in the presence of nitrite produces SNO-Hb (206 nM ± 25; n = 4) that comprises 92% of all protein-bound NO (224 nM ± 10), in contrast to the lower SNO yields (65–70%) from deoxyHb samples incubated with nitrite for 10 sec and subsequently bolused with CO or O2 (Fig. 4).
Discussion
The data reported here provide considerable insight into the reaction of Hb with nitrite. The distribution of products, including nitrosylHb (FeNO) and metHb [Fe(III)], depends on the nitrite concentration. Further, not only is an intermediate with an Fe(III)NO spectrum detected, but its evolution has been monitored to show different fates depending on the allosteric state of Hb. In particular, when nitrosylHb and metHb accumulate in sufficient amounts to trigger a transition to the R state, the intermediate is lost with a corresponding gain in SNO product. The most significant feature of our results is the quantitative relationship between the SNO-Hb precursor formed in T state under physiological conditions and the SNO-Hb obtained from it after allosteric transition to the R state. Notably, Pezacki et al. (18, 24) have described a similar reaction in reverse: Upon deoxygenation, SNO-Hb furnishes a βFe(III)NO. The physiological significance of βFe(III)NO is suggested by the report of Nagababu et al. (25) that the in vivo HbFeNO pool is predominantly an EPR silent Fe(III)/NO hybrid.
Doyle (26) pioneered the quantitative exploration of interactions between deoxyhemoglobin and (excess) nitrite and described a heuristic reaction scheme in which one equivalent of nitrite yields one equivalent of Fe(III) and Fe(II)NO (Eq. 1). Subsequent work examining the kinetics and product distribution of this reaction under a range of conditions (12, 25, 33) has continued to employ nitrite concentrations and [NaNO2]/[deoxy-heme] ratios well above physiological values. A clue to the importance of reactant concentrations on this chemistry emerged from EPR studies, which revealed, in the aftermath of mixing very high concentrations of nitrite and deoxyHb, essentially equal subunit populations of nitrosyl heme [αFe(II)NO ≈ βFe(II)NO], whereas spectra obtained under conditions that simulate key aspects of the in vivo situation exhibit substantial βFe(II)NO preference (16). In addition, the importance of duration of reaction on product distribution was recognized: Aging of NO-deoxyHb samples that is incurred over the lengthy course of the nitrite reaction enables competing chemistry, including redistribution of NO from β- to α-chains, reductive loss of NO to HNO and quenching of radicals in Hb (4, 7, 13–15, 34). Because previous work has suggested collectively that the efficacy of transfer of NO groups from heme to βCys-93 (to form a bioactive SNO) might require not only physiological NO/Hb ratios and HbNO concentrations (< 1 μM) but also preferential processing of NO within the β-chain (14–17), the conditions used in prior work were not optimized for the study of SNO formation.
In the present study, the kinetics and product stoichiometry of nitrite reactions were reexamined, with a focus on physiological conditions. This focus includes the following: (i) variation of [NaNO2] from the supraphysiological range down to concentrations detected in vivo; (ii) examination of reactions over time intervals comparable with the physiological situation (venous residence time ≤30 sec); and (iii) oxygenation of reaction mixtures over seconds to simulate the lung (and use of Hb at concentrations compatible with full O2 saturation over such intervals) (4, 15, 17). Our results demonstrate that, under conditions that characterize the physiological realm, Hb operates on nitrite as a SNO synthase:
We have previously reported that Hb can synthesize SNO through a variety of processes involving βFe(III)NO and that nitrite-derived FeNO is converted to SNO after oxygenation (3, 16). These processes formally involve an Fe(III)NO intermediary. We and others (16, 25, 29), however, had not been able to identify the production of Fe(III)NO in reactions between Hb and nitrite, although Rifkind et al. (23, 25) have inferred its presence. Here we took an alternative approach to analysis of UV-Vis spectra that has enabled the detection of a minority species, identified as Fe(III)NO. This assignment is buttressed by the observed chemistry of this species, namely facile, quantitative, allosterically linked conversion to SNO-Hb.
Under physiological nitrite levels, we used photolysis-chemiluminescence and observed ≈50% conversion of nitrite to a photolyzable NO signal within seconds (Fig. 4), an observation in line with earlier ideas of Rifkind et al. (25), who proposed that nitrite is consumed rapidly by deoxyHb to form an EPR-silent Fe(II)NO+ equivalent in T state. We further determined that exposure of the weakly associated deoxyHb/nitrite-derived complex to O2 or CO produces an equivalent yield of SNO-Hb. Thus, the initial complex formed in deoxyHb may be viewed as a SNO precursor that is under allosteric control and whose reactivity is similar to Fe(III)NO or Fe(II)NO+. Notably, oxyHb deoxygenated in the presence of physiological amounts of nitrite, conditions that we have shown should favor NO binding to β- vs. α-chains (16), and then reoxygenated produces SNO-Hb concentrations nearing 100% of protein-bound NO (Fig. 5). Thus, in the realm of interactions of nitrite with hemoglobin of physiological relevance (physiological concentrations, ratios, and timescales), SNO-Hb is the major product. Raising the nitrite concentration to nonphysiological levels, or increasing incubation times with Hb, allows for alternative reactions yielding bio-inactive products [e.g., αFe(II)NO and N2O], thereby drastically reducing SNO-Hb yields upon oxygenation (Fig. 4B). Our results are reminiscent of previous studies that found a requirement for immediate oxygenation of submicromolar FeNO to efficiently produce SNO-Hb in vitro (7, 14, 15, 17) and to preserve endogenous levels of SNO-Hb in RBCs (4).
Fig. 5.
Cyclic deoxygenation/oxygenation of nitrite and hemoglobin produces SNO-Hb in yields approaching 100%. Rapid deoxygenation of oxyHb (250 μM) in the presence of nitrite (1 μM) followed by reoxygenation produces SNO-Hb effectively as the sole NO product (206 ± 25 nM SNO/224 ± 10 nM total protein-bound NO; open bar). Percent yields of SNO-Hb relative to total proteinbound NO are lower when nitrite is added directly to deoxyHb followed by bolus (as in Fig. 4B) with O2 (hatched bar) or CO (filled bar). Results are presented as mean ± SE of four to five experiments.
The distribution of met and nitrosyl species in the nitrite reaction can be rationalized in a model that includes the effects of quaternary structure and chain heterogeneity. In T state, _k_onα ≈ _k_onβ, _k_offβ > _k_offα, and _k_oxα > _k_oxβ (32, 35) (_k_on and _k_ox represent specific rate constants for ligand binding and for heme-iron oxidation, respectively, and the superscript refers to the Hb subunit). Under limiting conditions, nitrite will preferentially oxidize α-chains. If the NO lost from the αFe(III)NO intermediate in the oxidation tends to transfer within an α/β dimer (36), then this chemistry would furnish dimers of composition [αFe(III)][βFe(II)NO] (16), to the extent that the buildup of such dimers is faster than NO redistribution to αFe(II). As [NaNO2] increases into the intermediate range, however, oxidation on the β-chains will be increasingly competitive, which will result in a significant pool of dimers of composition [αFe(II)NO][βFe(III)]. Because heme irons within the α-chains are, in the T state, oxidized more rapidly that those of β-chains in T state (_k_oxα > _k_oxβ) (32), the αFe(II)NO that forms will be more likely to yield αFe(III) and HNO and, thus, add to the rate of metHb production while decreasing the total yield of Hb[Fe(II)NO]. Indeed, in previous analyses, both we and others (15, 26) have documented the coincident production of HNO (via detection of its byproducts, hydroxylamine and dinitrogen oxide) and metHb. Moreover, previous work by Gow and Stamler (15) emphasized not only the importance of chain heterogeneity in NO-mediated oxidation of hemes, but also the NO/Hb ratio (and the NO concentration), which determines the allosteric state of Hb. In the R state (high NO/Hb ratio), the ligand off rates are decreased, thus mitigating NO-mediated oxidation (15). Indeed, increasing [NaNO2] predictably leads to a predominately R state population with altered reactivity, as first noted by Doyle et al. (26) and recently detailed by others (27, 29). In this situation, a decrease in the rate of dissociation of NO/HNO decreases the NO-mediated oxidation; consequently, the [Fe(III)]/[Fe(II)NO] ratio shifts back toward unity. This model predicts a mass balance relationship between [NaNO2], the products Fe(III), Fe(II)NO, Fe(III)NO, and SNO reaction products that is in complete accord with all of our experimental results (see Fig. 7, which is published as supporting information on the PNAS web site).
In summary, the chemistry of nitrite/Hb interactions under physiological conditions (like that of NO/Hb interactions) is very different from that observed at the high nitrite to Hb ratios that have been used in most previous work (12, 26, 27, 29, 33). We have shown that conditions that closely replicate the physiological situation, entailing brief exposures of deoxyHb or rapidly saturated oxyHb to limiting (physiological) nitrite, and which include cycles of oxygenation or deoxygenation/reoxygenation, respectively, yield SNO-Hb as a major product. The amount of SNO-Hb produced is far greater than the amount of NO that would be predicted to form according to published rates of nitrite reduction (26), reflecting instead the production and reaction of an Fe(III)NO/SNO precursor. It is interesting to note that current methodologies used to assay NO species, with the exception of photolysis, cannot detect either Fe(III)NO or Fe(II)NO+ [and would not be anticipated to accurately assay SNOs that are in equilibrium with Fe(III)NO or Fe(II)NO+]. This inability may be of importance in understanding certain discrepancies between reported levels of NO species detected in biological systems (7). Finally, it should be noted that, although the results presented here coordinate the observation of Fe(III)NO with oxygenation-induced SNO-Hb formation, the possibility that a SNO precursor can involve a direct reaction of nitrites with thiols of Hb finds precedent in previous work (37, 38) and is not excluded by our experiments.
Materials and Methods
Preparation of Deoxyhemoglobin.
Highly purified adult human hemoglobin (HbA0; Apex Biosciences, Durham, NC) was reduced with sodium dithionite in an anaerobic glove box. Excess dithionite was removed with gel filtration chromatography (Sephadex G25 resin; Amersham Pharmacia Biosciences). Purified samples were prepared daily and stored anaerobically between experiments. In some experiments, deoxyHb samples were incubated with _N_-ethylmaleimide before starting the reaction. Excess _N_-ethylmaleimide was removed via gel chromatography.
Preparation of Oxyhemoglobin.
Deoxyhemoglobin was prepared as described above and subsequently oxygenated with room air.
Preparation of Carboxyhemoglobin.
Deoxyhemoglobin was prepared as described above and placed in a 3-ml septum-top glass cuvette. Samples were treated with a gentle stream of 1,000 ppm anaerobic CO gas for 30 min before measurement.
Preparation of Methemoglobin.
HbA0 was incubated with an excess of potassium ferricyanide for 5 min. Excess potassium ferricyanide subsequently was removed via gel chromatography.
Reaction of Deoxyhemoglobin and Nitrite.
Five hundred micromolar deoxyhemoglobin and an equal volume of NaNO2 buffer solution (NaNO2 plus 100 mM phosphate, pH 7.4/0.1 mM EDTA/0.1 mM diethylenetriaminepentaacetate) were mixed by vortexing inside an anaerobic glove box. Samples were placed in a 0.5 mm, 0.1 mm, or 0.2 mm screw-top cuvette (Spectrocell, Oreland, PA). Initial nitrite concentrations used in spectroscopic measurements varied from 1–500 μM, with deoxyHb concentration constant at 250 μM, giving [NaNO2]i/[DeoxyHb]i concentrations from 1:250–2:1.
Reaction of Methemoglobin, Carboxyhemoglobin, and Oxyhemoglobin.
Five hundred micromolar of the relevant form of Hb was combined with an equal volume of NaNO2 buffer containing 2 μM NaNO2, and the mixture was immediately subjected to photolysis-chemiluminescence. Samples containing oxyhemoglobin were measured by using compressed air as the carrier flow, whereas all others used a helium carrier flow.
Oxygenation/Deoxygenation Cycling of OxyHb.
Five hundred micromolar oxyhemoglobin was combined with an equal volume (25 μl) of NaNO2 buffer containing 2 μM NaNO2 in a 3-ml septum-top glass cuvette, giving 250 μM oxyhemoglobin and 1 μM NaNO2. Fifty-microliter aliquots were rapidly deoxygenated for 1 min with argon and subsequently reoxygenated rapidly with a gentle flow of room air for 15 sec.
CO/O2 Bolus of Deoxyhemoglobin/Nitrite Samples.
Fifty-microliter samples were treated with a gentle flow of 1,000 ppm anaerobic CO gas for 1 min or room air for 15 sec.
UV-Vis Wavelength Spectroscopy.
UV-Vis spectroscopy was conducted as described in detail in Supporting Text, which is published as supporting information on the PNAS web site. Deconvolution analysis of full spectrum scans was performed by using methods similar to those published in ref. 16, as elaborated in Supporting Text.
Measurement of SNO-Hb.
SNO-Hb production was measured via photolysis-chemiluminescence as described in ref. 1.
Supplementary Material
Supporting Information
Acknowledgments
We thank Irwin Fridovich for critically reviewing the manuscript and for discussions. This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL42444 (to J.S.S.) and National Science Foundation Grant MCB 00981228 (to D.J.S.).
Abbreviations
SNO
_S_-nitrosothiol
SNO-Hb
S-nitrosylated Hb.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Jia L., Bonaventura C., Bonaventura J., Stamler J. S. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J. S., Jia L., Eu J. P., McMahon T. J., Demchenko I. T., Bonaventura J., Gernert K., Piantadosi C. A. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 3.McMahon T. J., Moon R. E., Luschinger B. P., Carraway M. S., Stone A. E., Stolp B. W., Gow A. J., Pawloski J. R., Watke P., Singel D. J., et al. Nat. Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 4.McMahon T. J., Ahearn G. S., Moya M. P., Gow A. J., Huang Y.-C., Luchsinger B. P., Nudelman R., Yan Y., Krichman A. D., Bashore T. M., et al. Proc. Natl. Acad. Sci. USA. 2005;102:14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich H. H., Ellsworth M. L., Sprague R. S., Dacey R. G., Jr Am. J. Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 6.Doctor A., Platt R., Sheram M. L., Eischeid A., McMahon T., Maxey T., Doherty J., Axelrod M., Kline J., Gurka M., et al. Proc. Natl. Acad. Sci. USA. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singel D. J., Stamler J. S. Annu. Rev. Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 8.Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Proc. Natl. Acad. Sci. USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty D. H., Doyle M. P., Curry S. R., Vali R. J., Fattor T. J., Olson J. S., Lemon D. D. Nat. Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 10.Pawloski J. R., Hess D. T., Stamler J. S. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 11.James P. E., Tufnell-Barret T., Milsom A. B., Frenneaux M. P., Lang D. Circ. Res. 2004;95:e8–e9. [PubMed] [Google Scholar]
- 12.Luchsinger B. P., Rich E. N., Yan Y., Williams E. M., Stamler J. S., Singel D. J. J. Inorg. Biochem. 2005;99:912–921. doi: 10.1016/j.jinorgbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Gow A. J., Buerk D. G., Ischiropoulos H. J. Biol. Chem. 1997;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 14.Gow A. J., Luchsinger B. P., Pawloski J. R., Singel D. J., Stamler J. S. Proc. Natl. Acad. Sci. USA. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gow A. J., Stamler J. S. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 16.Luchsinger B. P., Rich E. N., Gow A. J., Williams E. M., Stamler J. S., Singel D. J. Proc. Natl. Acad. Sci. USA. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawloski J. R., Hess D. T., Stamler J. S. Proc. Natl. Acad. Sci. USA. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pezacki J. P., Ship N. J., Kluger R. J. Am. Chem. Soc. 2001;123:4615–4616. doi: 10.1021/ja015716o. [DOI] [PubMed] [Google Scholar]
- 19.McMahon T. J., Exton Stone A., Bonaventura J., Singel D. J., Solomon Stamler J. J. Biol. Chem. 2000;275:16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 20.Liu L., Yan Y., Zeng M., Zhang J., Hanes M. A., Ahearn G., McMahon T. J., Dickfeld T., Marshall H. E., Que L. G., Stamler J. S. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 21.Herold S., Rock G. Arch. Biochem. Biophys. 2005;436:386–396. doi: 10.1016/j.abb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Herold S., Rock G. J. Biol. Chem. 2003;278:6623–6634. doi: 10.1074/jbc.M210275200. [DOI] [PubMed] [Google Scholar]
- 23.Nagababu E., Ramasamy S., Rifkind J. M. Nitric Oxide. 2006 doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Ship N. J., Pezacki J. P., Kluger R. Bioorg. Chem. 2003;31:3–10. doi: 10.1016/s0045-2068(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 25.Nagababu E., Ramasamy S., Abernethy D. R., Rifkind J. M. J. Biol. Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 26.Doyle M. P., Pickering R. A., DeWeert T. M., Hoekstra J. W., Pater D. J. Biol. Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 27.Kim-Shapiro D. B., Gladwin M. T., Patel R. P., Hogg N. J. Inorg. Biochem. 2005;99:237–246. doi: 10.1016/j.jinorgbio.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Fago A., Crumbliss A. L., Peterson J., Pearce L. L., Bonaventura C. Proc. Natl. Acad. Sci. USA. 2003;100:12087–12092. doi: 10.1073/pnas.2032603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang K. T., Keszler A., Patel N., Patel R. P., Gladwin M. T., Kim-Shapiro D. B., Hogg N. J. Biol. Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 30.Kirkwood B., Sterne J. Essential Medical Statistics. Malden, MA: Blackwell; 2003. [Google Scholar]
- 31.Perutz M. In: Molecular Basis of Blood Diseases. Stammatayanopoulos G., editor. St. Louis: W.B. Saunders; 1987. pp. 127–177. [Google Scholar]
- 32.Edelstein S. J., Gibson W. H. J. Biol. Chem. 1975;250:961–965. [PubMed] [Google Scholar]
- 33.Huang Z., Shiva S., Kim-Shapiro D. B., Patel R. P., Ringwood L. A., Irby C. E., Huang K. T., Ho C., Hogg N., Schechter A. N., Gladwin M. T. J. Clin. Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y., Houk K. N. J. Am. Chem. Soc. 2006;128:1422–1423. doi: 10.1021/ja057097f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassoly R., Gibson Q. J. Mol. Biol. 1975;91:301–313. doi: 10.1016/0022-2836(75)90382-4. [DOI] [PubMed] [Google Scholar]
- 36.Holt J. M., Ackers G. K. Biochemistry. 2005;44:11939–11949. doi: 10.1021/bi050710n. [DOI] [PubMed] [Google Scholar]
- 37.Doyle M. P., Pickering R. A., da Conceicao J. J. Biol. Chem. 1984;259:80–87. [PubMed] [Google Scholar]
- 38.Doyle M. P., Herman J. G., Dykstra R. L. J. Free Radical Biol. Med. 1985;1:145–153. doi: 10.1016/0748-5514(85)90019-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information