Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis (original) (raw)
Abstract
The atypical PKC-interacting protein, Par-4, inhibits cell survival and tumorigenesis in vitro, and its genetic inactivation in mice leads to reduced lifespan, enhanced benign tumour development and low-frequency carcinogenesis. Here, we demonstrate that Par-4 is highly expressed in normal lung but reduced in human lung cancer samples. We show, in a mouse model of lung tumours, that the lack of Par-4 dramatically enhances Ras-induced lung carcinoma formation in vivo, acting as a negative regulator of Akt activation. We also demonstrate in cell culture, in vivo, and in biochemical experiments that Akt regulation by Par-4 is mediated by PKCζ, establishing a new paradigm for Akt regulation and, likely, for Ras-induced lung carcinogenesis, wherein Par-4 is a novel tumour suppressor.
Keywords: Akt, lung cancer, NF-κB, Par-4, PKCζ, Ras
Introduction
Inactivating mutations and deletions of tumour suppressor genes along with gain-of-function mutations in proto-oncogenes give rise to cells that can grow independent of external proliferation and survival cues and result in tumour transformation and cancer (Hanahan and Weinberg, 2000). Among the proto-oncogenes most frequently altered in human tumours are the small GTPases of the Ras family, which have been found to be mutated in at least 25% of human lung adenocarcinomas (Bos, 1989). This is of particular interest because lung neoplasia is the leading cancer-related cause of death in the United States, with an estimated incidence of 213 380 new cases and 160 390 deaths in 2007 (http://www.cancer.gov/cancertopics/wyntk/lung). Mouse lung cancer models are available that reproduce the human disease relatively faithfully, thus allowing the study of the cellular and molecular basis of this neoplasia at an organismal level (Fisher et al, 2001; Meuwissen and Berns, 2005). The two main types of lung cancer are small-cell lung cancer and non-small-cell lung cancer (NSCLC). Among the latter, the most prevalent type is the adenocarcinoma, which accounts for more than 40% of all lung cancers (http://www.cancer.gov/cancertopics/wyntk/lung). Current treatments do not lead to a cure for most patients with lung cancer. Targeted anti-tumour therapies are likely to prove more effective, but their development will require a better understanding of the signalling cascades involved in the initiation and progression of this type of tumour. In this regard, Ras oncogenes trigger a myriad of signalling pathways, of which only a few have been characterized in detail, such as the Raf-MEK and PI3 Kinase-Akt signalling cascades (Downward, 2003; Malumbres and Barbacid, 2003). With regard to tumour suppressors, we have reported the reduction of Par-4 (also known as PAWR) levels in Ras-transformed cells (Barradas et al, 1999). In the case of cultured fibroblasts, this Par-4 reduction is a required event for Ras to manifest its full transforming potential (Barradas et al, 1999). However, its role in more physiological cancer models is still unclear. Par-4 was initially identified in an in vitro differential screen for pro-apoptotic genes in human carcinoma cell lines (Sells et al, 1994). The Par-4 gene maps to chromosome 12q21, a region frequently deleted in certain malignancies (Johnstone et al, 1998), and encodes a protein (38 kDa) containing a leucine zipper domain in the COOH-terminal region, which interacts with a variety of proteins (Moscat and Diaz-Meco, 2003), including the atypical protein kinases (aPKCs), PKCζ and PKCλ/ι (Diaz-Meco et al, 1996). Among the mechanisms by which Par-4 triggers apoptosis, the best established one, which is supported by studies in knockout (KO) mice, is through inhibition of the aPKCs and the ensuing downmodulation of NF-κB and its prosurvival transcriptional targets, such as X-linked inhibitor of apoptosis (XIAP) (Garcia-Cao et al, 2003, 2005; Lafuente et al, 2003). These previous studies all point to a link between Par-4 downregulation and cancer. Indeed, we have recently shown that _Par-4_-null mice develop spontaneous benign prostate neoplasias and endometrial carcinomas, thus implicating Par-4 deficiency in the development of tumours (Garcia-Cao et al, 2005). In addition, our recent data also demonstrate, using cDNA arrays, quantitative reverse-transcription PCR, and by immunohistochemistry (IHC), that Par-4 is downregulated in approximately 40% of human endometrial carcinomas (Moreno-Bueno et al, 2007).
Lungs, along with prostate (see below) and endometrium (Moreno-Bueno et al, 2007), are the tissues that exhibit the highest levels of Par-4. We reasoned that if Par-4 is a tumour suppressor, its loss in the lung would lead to increased tumorigenicity. In this study we provide compelling evidence that Par-4 deficiency in the lung leads not only to enhanced NF-κB but also to increased Akt activity, thus making Par-4-deficient lungs more sensitive to Ras-induced oncogenesis. We demonstrate that the negative actions of Par-4 on Akt are cell autonomous and mediated by the ability of PKCζ to phosphorylate Akt at Ser124, which impacts the phosphorylation status of Ser473 and Thr308, two critical residues for its enzymatic activity (Cantley, 2002). Akt's Thr308 is phosphorylated by PDK1 in response to activation of PI 3-kinase, which generates PIP3, which by binding the pleckstrin homology domain of Akt and PDK1, makes that residue accessible to the action of PDK1 (Manning and Cantley, 2007). Ser473 has been shown to be phosphorylated by an mTOR–Rictor complex, termed as TORC2, which in contrast to the TORC1 complex, composed of mTOR and raptor that phosphorylate the Ser396 of S6K1, is insensitive to rapamycin (Sarbassov et al, 2005; Bhaskar and Hay, 2007). Our observations reported here unveil a novel role for Par-4 and PKCζ and reveal a novel mechanism of action for the regulation of Akt involving PKCζ phosphorylation of Ser124, which is negatively regulated by Par-4 and which is likely important in lung cancer in vivo.
Results
Tissue distribution of Par-4 and loss in human tumours
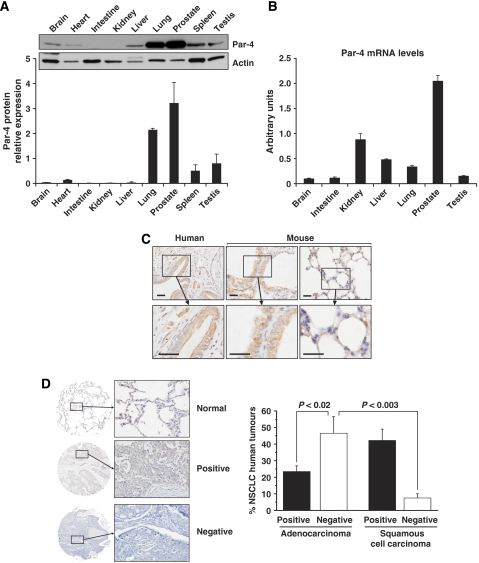
Our previous observations have demonstrated that Par-4 KO mice have decreased lifespan and display higher tumour incidence with ageing. These tumours are mainly prostate intraepithelial neoplasias (PIN) and endometrial carcinomas, and low-frequency lung adenocarcinomas (Garcia-Cao et al, 2005). This, together with the fact that Par-4 is absent in about 58% of human prostate cancers (our unpublished observations) and in about 40% of human endometrial carcinomas (Moreno-Bueno et al, 2007), indicates that Par-4 can be considered as a tumour suppressor. Thus, it is of interest to determine whether Par-4 also has tumour-suppressive activity in other organs. We reasoned that Par-4 will most likely have an important function as a tumour suppressor in those organs that exhibit the highest levels of Par-4 expression. Therefore, we analysed, by immunoblotting with a specific anti-Par-4 antibody, the levels of this protein in different normal mouse tissues. Interestingly, the Par-4 protein is highly expressed in the lung, with levels comparable with those of prostate (Figure 1A). Prostate displays the highest Par-4 mRNA levels, whereas lung, liver and kidney also expressed high levels as compared with other tissues such as brain and intestine (Figure 1B). On the basis of the high Par-4 protein levels in the lung in mouse samples, we determined the distribution of Par-4 in human and mouse lung sections by IHC. The data of Figure 1C show that Par-4 is expressed mostly in the epithelial cells of the airways both in human and mouse lung, as well as in the alveoli.
Figure 1.
Par-4 is highly expressed in the lung and is downregulated in human lung tumours. (A) Par-4 protein expression in normal mouse tissues. (B) Par-4 mRNA levels in normal mouse tissues. (C) Par-4 distribution in human and mouse tissues, determined by IHC. (D) Commercial tissue microarrays of NSCLC human samples (_n_=133) were stained with anti-Par-4 antibody. Par-4 expression was analysed in adenocarcinoma and squamous cell carcinoma tumours compared with normal lung tissue. A representative example of positive and negative tumour samples and normal control for Par-4 staining is shown at two magnifications ( × 2.5 and × 20). Scale bar=50 μm.
We hypothesized, then, that the loss of Par-4 in the lung would be instrumental for lung cancer initiation and progression. To begin addressing this possibility, we next determined Par-4 expression levels in tissue microarrays of human NSCLC. Interestingly, 47% (_n_=133) of NSCLC were negative for Par-4 as determined by IHC (Figure 1D). Importantly, there is a clear correlation between the loss of Par-4 and the type of tumour. That is, 41% of the adenocarcinomas were negative for Par-4 expression, whereas only 6% of squamous cell carcinomas show negative staining for Par-4. Also, when the adenocarcinomas are stratified by grade, it is clear that 74% of grade III tumours have lost Par-4 expression, whereas 59% of grade I-II are negative for Par-4. In summary, Par-4 levels are reduced in a significant number of human lung malignancies, strongly suggesting that this protein may have an important function in lung cancer prevention.
Par-4 deficiency enhances Ras-induced lung cancer in vivo
Par-4 deficiency leads to only benign tumorigenicity in prostates, and to low-frequency lung carcinomas (Garcia-Cao et al, 2005). As Par-4 is reduced most significantly in adenocarcinomas, which are the type of lung tumours that best correlate with the expression of mutant oncogenic Ras (Bos, 1989), we next wanted to test the hypothesis that loss of Par-4 favours the tumorigenic actions of this oncogene. To determine whether Ras-induced lung cancer may be influenced by Par-4, we asked whether in vivo genetic ablation of Par-4 would affect tumour development in lungs. To do this, we used a mouse model of pulmonary adenocarcinoma in which oncogenic Ras was introduced by a knock-in strategy and is inducibly expressed in an endogenous manner (Guerra et al, 2003). This model leads to lung adenomas and adenocarcinomas in which the likely target cell is the type II pneumocyte, evidenced by the observation that the resulting tumour cells express surfactant protein C, a marker of type II pneumocytes (Guerra et al, 2003; Tuveson et al, 2004). This is a physiologically relevant model for human cancer, as it has been reported that, in addition to Clara cells, type II pneumocytes are the most likely precursors of human lung adenocarcinomas (Fisher et al, 2001; Tuveson et al, 2004; Meuwissen and Berns, 2005).
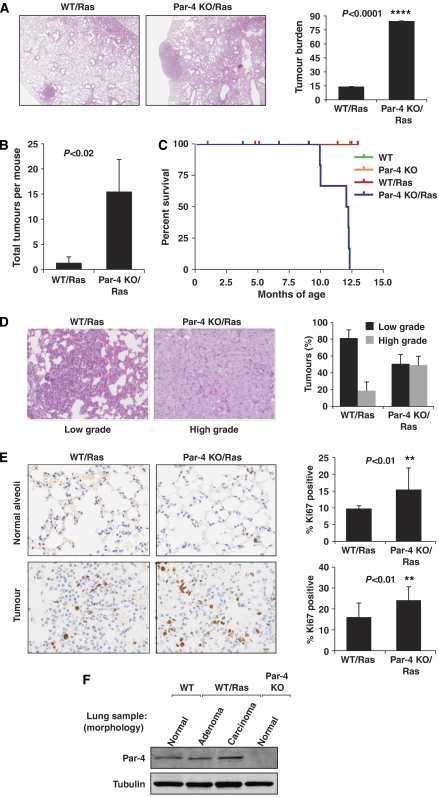
To address whether the role of Par-4 is to physiologically restrain Ras-induced lung tumorigenesis, we tested the hypothesis that expression of oncogenic Ras in a _Par-4_−/− background would increase tumour burden as compared with that in Ras-expressing WT mice. Therefore, Par-4 KO mice were bred to the mice expressing oncogenic Ras in the lung. In this model, a limited percentage of oncogenic Ras-expressing lung broncho-alveolar cells undergo malignant transformation leading to adenomas and adenocarcinomas (Guerra et al, 2003). Therefore, this is an ideal in vivo model to determine whether the loss of Par-4 under these conditions would lead to increased tumorigenicity in the lung. Results in Figure 2A demonstrate that the tumour burden in oncogenic-Ras-expressing WT lungs is approximately 18% of the total lung tissue, whereas that parameter is significantly higher in Par-4 KO mice (about 75%). Tumour incidence in the Ras-expressing Par-4 KO lungs was significantly higher as compared with the Ras-expressing WT controls (Figure 2B). The lifespan was dramatically reduced in Ras-expressing Par-4 KO mice as compared with the other genotypes (Figure 2C). The loss of Par-4 also accelerated the progression of Ras-initiated lung adenocarcinomas, as the percentage of high-grade tumours was significantly higher in the Par-4 KO mice as compared with their corresponding WT controls (Figure 2D). Consistent with increased tumorigenesis in the Par-4 KO lungs, proliferation was also enhanced, as determined by increased Ki67 staining compared with WT controls, both in the normal alveolar tissue (Figure 2E, upper panels) and in the tumours (Figure 2E, lower panels) of Ras-expressing lungs. We have reported previously in vitro downregulation of Par-4 in cultured, oncogenic Ras-expressing fibroblasts (Barradas et al, 1999). Surprisingly, the levels of Par-4 were similar in normal lung of WT mice and in cancer tissues of the oncogenic Ras-expressing mice (Figure 2F). Therefore, our observation that the loss of Par-4 dramatically enhances Ras tumorigenic potential in lung (Figure 2A and B) is consistent with: (1) oncogenic Ras expression not being sufficient to downregulate Par-4 in vivo; and (2) the ability of Par-4 inactivation in tumours to facilitate the development of Ras full-transforming potential. Globally, these data strongly suggest that Par-4 is a physiologically relevant negative regulator of lung tumorigenesis.
Figure 2.
Par-4 cooperates with Ras-induced tumorigenesis in the lung. (A) H&E staining of lungs from WT and Par-4 KO mice crossed with K-ras+/V12, RERT2T/T mice and analysed 5 months after activation of the K-rasV12 allele by injection of 4-hydroxytamoxifen (_n_=5 per genotype). Overall tumour burden was determined by quantification of the tumour area as a percentage of total area of H&E-stained tissue (right panel). (B) Number of total tumours per mouse in WT and Par-4 KO mice. (C) Survival of mice of different genotypes represented as percentage of total; the cause of death was asphyxiation. (D) Loss of Par-4 leads to increased Ras-induced high-grade lung adenocarcinomas. (E) Representative sections and quantification of proliferation index measured as percentage of positive nuclear staining for Ki67 in lung sections from normal alveolar or tumour tissue from WT and Par-4 KO Ras-expressing mice. (F) Par-4 expression levels in the lung from different mouse genotypes. **, P<0.01; ****, P<0.0001.
Signalling alterations in Par-4-deficient lungs
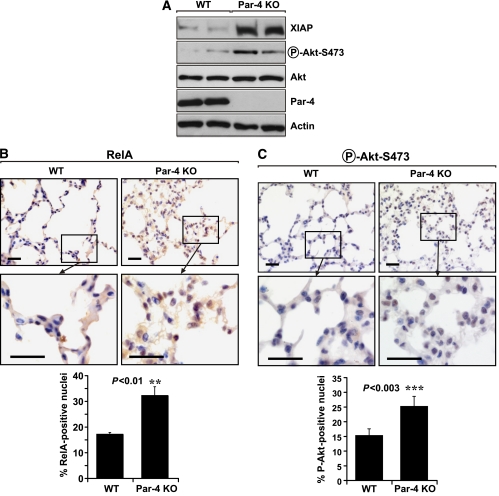
To gain understanding of the molecular and cellular mechanism(s) whereby Par-4 restrains lung tumorigenesis, we next analysed the state of different signalling pathways in lungs from WT and Par-4-deficient mice under basal conditions. We have recently demonstrated in cell cultures of Par-4-deficient EFs, and in Par-4 KO prostates and uteri, that the loss of Par-4 produces enhanced NF-κB activation, which leads to the increased expression of XIAP, a well-established anti-apoptotic downstream target of NF-κB (Garcia-Cao et al, 2003, 2005). Consistent with this, our analysis of lung extracts from Par-4 KO mice revealed increased levels of XIAP as compared with lung extracts from WT controls (Figure 3A). Par-4 constitutively inhibits aPKCs, as demonstrated in vivo by increased phospho-PKCζ staining in lung sections from Par-4 KO mice as compared with WT controls, both in the alveoli and in the airways (Supplementary Figure 1A). Consistently, PKCζ activity in lung extracts is also increased in the Par-4 KO mice, as determined in an in vitro enzymatic assay in PKCζ immunoprecipitates (Supplementary Figure 1B).
Figure 3.
Increased levels of XIAP and activation of RelA and Akt in lungs of Par-4 KO mice. (A) The levels of XIAP, phospho-Akt-S473, Akt, Par-4 and actin were determined in lung extracts from WT and Par-4 KO mice. These are representative experiments where there were at least two others with similar results. (B, C) Sections of alveolar lung tissue from WT and Par-4 KO mice were stained by IHC with anti-RelA (B) or anti-phospho-Akt-S473 (C) antibody and scored for the number of cells with nuclear staining. Results are the mean±s.d. of 10 different fields per mouse, with a total of five mice for each condition. Scale bar=50 μm. **, P<0.01; ****, P<0.003.
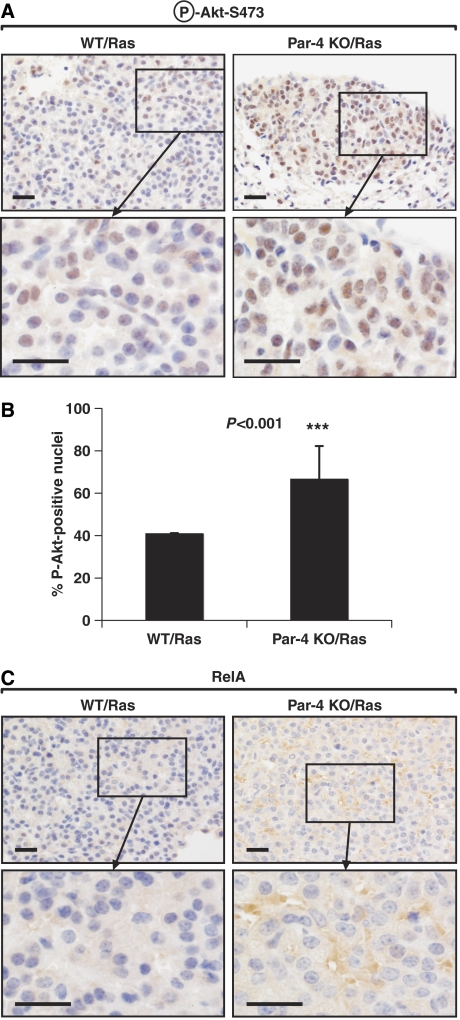
As PKCζ is required for the nuclear translocation of NF-κB in lung (Leitges et al, 2001), we next determined by IHC analysis whether the loss of Par-4 in the lung leads to enhanced nuclear levels of RelA in this tissue. The percentage of cells staining positive for nuclear RelA (also known as p65) was significantly increased in Par-4 KO lungs as compared with WT controls (Figure 3B). Therefore, the loss of Par-4 enhances basal NF-κB activity in lungs, which most likely accounts for the increased XIAP levels (Figure 3A). These results are consistent with the increased proliferation observed in Par-4 KO lungs, as enhanced NF-κB would result in higher cell survival during the oncogenic transformation process (Karin, 2006a, 2006b). This observation provides in vivo confirmation of our previous observations on the effect of Par-4 deficiency in EFs, prostate and uterus (Garcia-Cao et al, 2003, 2005), and extends their relevance to the lung. When the activation of other signalling molecules related to cell survival and proliferation was determined in lung extracts, we observed that the lack of Par-4 correlated with increased Akt phosphorylation of Ser473 in lung extracts (Figure 3A). Ser473 phosphorylation is a bona fide marker of Akt activation (Cantley, 2002), indicating that the Par-4 KO lungs have increased levels of Akt activity under basal conditions. These results were confirmed by IHC analysis of lung sections, which demonstrated increased phospho-Akt-Ser473 staining in vivo in _Par-4_−/− lung alveolar (Figure 3C) and bronchiolar (not shown) cells, as compared with WT controls. Importantly, levels of phospho-Akt-Ser473 were also significantly augmented in Ras-expressing Par-4 KO tumours as compared with WT tumours (Figure 4A and B). Surprisingly, nuclear RelA levels were not detectable in Ras-expressing WT or Par-4 KO tumours (Figure 4C), although the Par-4 KO cells in those tumours showed enhanced cytosolic staining for RelA (Figure 4C). However, increased RelA nuclear translocation was observed in the Par-4 KO, oncogenic Ras-expressing alveolar normal and hyperplastic tissues as compared with WT controls (Supplementary Figure 2A). Phospho-Akt-Ser473 levels in the Par-4 KO mice were increased as compared with WT controls in all oncogenic Ras-expressing lung tissues analysed (Supplementary Figure 2B). These results suggest that increased levels of Akt activity, but probably not of NF-κB, correlate with Par-4 KO-enhanced Ras tumorigenesis. These are important observations because they establish Par-4 as a novel critical negative regulator, in the lung, of two essential prosurvival cascades, NF-κB and Akt. The mechanism whereby Par-4 regulates NF-κB is well established and most likely involves PKCζ (Leitges et al, 2001; Garcia-Cao et al, 2003). However, the results of this article unveil an unexpected regulatory role of Par-4 as a negative modulator of Akt that deserves further analysis.
Figure 4.
Increased activation of Akt but not RelA in Ras-expressing Par-4 KO lung tumours. Sections of lung tumours from WT and Par-4 KO mice expressing Ras were stained by IHC with anti-phospho-Akt-S473 (A) or anti-RelA (C) antibody. (B) Quantitation of cells showing positive nuclear staining for phospho-Akt-S473. Results are the mean±s.d. of 10 different fields per mouse, with a total of five mice for each condition. Scale bar=50 μm. ***, P<0.001.
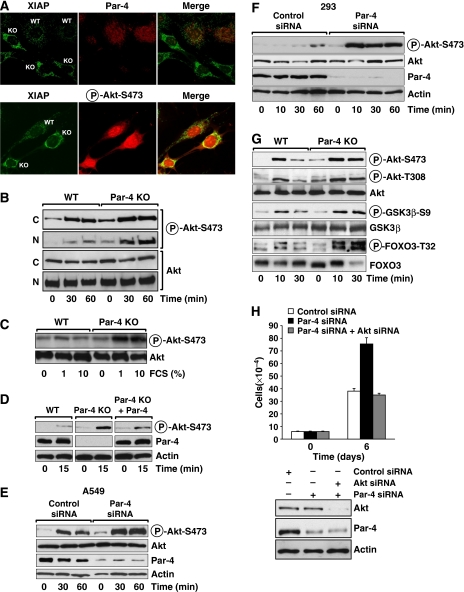
Cell-autonomous inhibition of Akt by Par-4
To investigate how Par-4 influences Akt Ser473 phosphorylation, we first determined whether the upregulation of Akt activity by Par-4 deficiency is cell autonomous, and if it can be reverted in KO cells by ectopically reintroducing Par-4. To address these two important issues, we used anti-Par-4 and anti-XIAP antibodies to perform immunofluorescence analysis of EFs from WT and Par-4-deficient mice. Both types of fibroblasts were co-cultured on the same coverslips so that the staining conditions for the different types of cells (WT and KO) would be identical for the different antibodies. Results in Figure 5A (upper panel) show that KO cells (which are easily identified because they stain negatively for Par-4) give a much stronger signal for XIAP, consistent with Par-4 being a negative regulator of XIAP expression (Garcia-Cao et al, 2003, 2005). As the antibodies to detect phospho-Akt-Ser473 are not compatible with those that detect Par-4, but are compatible with those for XIAP, we assessed phospho-Akt levels in cells with elevated XIAP levels (thus identified as _Par-4_−/−). The lower panel of Figure 5A shows a representative experiment. Interestingly, cells that were _Par-4_−/− displayed higher phospho-Akt-Ser473 levels, demonstrating a clear inverse correlation between Par-4 and Akt activity. Furthermore, when EFs were made quiescent by serum starvation and subsequently activated by serum, the levels of phospho-Akt were more potently stimulated in the Par-4 KO cells than in the WT controls, in both time- and dose-dependent manners, and in both the cytoplasm and the nucleus (Figure 5B and C). Importantly, when Par-4 was re-expressed in KO EFs, the activation of Akt was severely compromised (Figure 5D). These results support the notion that Par-4 is a cell-autonomous negative regulator of Akt in the lung and EFs. To test whether this is also true in human cells, we used a Par-4 siRNA to deplete endogenous Par-4 levels in human 293 cells and in the A549 human lung adenocarcinoma cell line. Cells were treated with control or Par-4-specific siRNAs, after which they were kept for 24 h in serum-free medium conditions and then stimulated with serum. Data in Figure 5E and F clearly demonstrate that the knockdown of Par-4 provokes enhanced serum-activated phospho-Akt-Ser473 levels in A549 and 293 human cells, respectively. These data strongly indicate that the extent of phosho-Akt-Ser473 activation is linked to Par-4 levels in several cell systems, including human lung cancer cells.
Figure 5.
Par-4 deficiency induces increased nuclear phospho-Akt in vivo. (A) Confocal immunofluorescence on WT and Par-4 KO EFs seeded on the same coverslip, double-stained for Par-4 and XIAP (upper panels), or XIAP and phospho-Akt-S473 (lower panels). WT and Par-4 KO EFs stimulated with serum (FCS) for different durations or a dose–response for 15 min (C) in total extracts (C, D) or in nuclear and cytosol extracts (B). Reconstitution of Par-4 KO EFs with Par-4 restored phospho-Akt-S473 levels to basal levels (D). (E, F) A549 or 293 cells treated with a control siRNA or with Par-4-specific siRNA were stimulated with serum for different times and the levels of phospho-Akt-S473 were determined. Knockdown of Par-4 was confirmed by immunoblot. (G) WT and Par-4 KO EFs stimulated with serum were analysed by immunoblot for phospho-Akt-S473 and phospho-Akt-T308 levels as well as the Akt substrates, Gsk3β-S9 and Foxo3-T32. (H) Knockdown of Akt blocks the increased cell proliferation induced by knockdown of Par-4 in A549 cells. Cell number was determined by trypan blue exclusion. Knockdown of Par-4 and Akt was analysed by immunoblot. These are representative experiments where there were at least two others with similar results.
Thr308 is also critical for Akt activity and function and is phosphorylated by PDK1 (Manning et al, 2002). To test whether the loss of Par-4 impacts not only Akt's Ser473 but also Thr308, we determined by immunoblotting with a specific anti-phospho-Akt-Thr308 antibody the levels of this phosphorylation in EFs from Par-4 KO and WT controls that have been either serum-starved or restimulated for 10 and 30 min with serum. We also tested in these extracts the phosphorylation levels of GSK3β and FOXO3, two direct substrates of Akt. From the data of Figure 5G, it is apparent that the lack of Par-4 leads to increased levels of phospho-Akt-Thr308, phospho-GSK3β and phospho-FOXO3. The enhanced phosphorylation of the two Akt substrates is consistent with the enhanced phosphorylation of Akt at its two critical residues and reflects increased Akt activity in the Par-4 KO cells.
The fact that Akt's Thr308 phosphorylation is also enhanced in the mutant cells is puzzling and suggests that Par-4 might be controlling directly or indirectly the phosphorylation of both sites or that their phosphorylation might be interconnected. Akt activation lies downstream of PI 3-kinase whose stimulation can be detected by increased production of PIP3 (Manning et al, 2002). A potential explanation for the fact that Akt phosphorylation at Ser473 and Thr308 is enhanced in Par-4 KO cells would be a similarly activated PI 3-kinase activity in the mutant cells. However, when PIP3 levels were measured in Par-4 KO and WT cells, we did not detect an increase in Par-4 KO cells but, on the contrary, serum-induced PIP3 levels were reduced in Par-4 KO cells. These results suggest that an increase in PI-3 kinase activity could not account for the hyperactivation of Akt in Par-4-deficient cells, and that feedback loop mechanisms may take place in an attempt to control a deregulated and constitutive Akt activation in these cells (Supplementary Figure 3).
Collectively, these results demonstrate that Par-4 deficiency leads to the activation of the Akt pathway in vivo and in several cell systems, including human lung cancer cells, which may offer a biochemical explanation for the increased tumorigenicity observed in Par-4 KO lungs. This previously unrecognized link between Par-4 and Akt is functionally relevant as the knockdown of Akt significantly reduces the increased cell proliferation produced by the knockdown of Par-4 (Figure 5H).
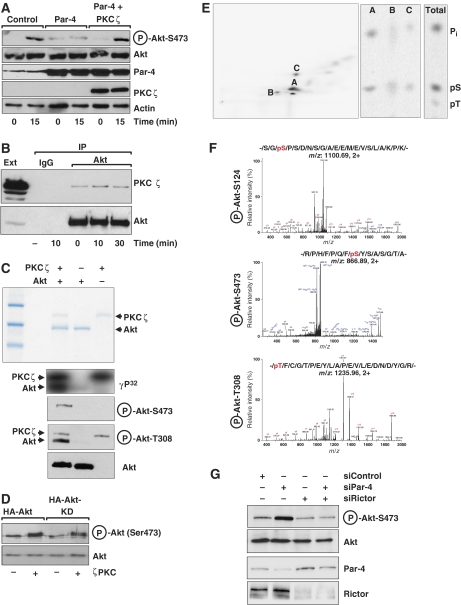
Role of PKCζ in the negative regulation of Akt by Par-4
As others and we have reproducibly detected a specific interaction between PKCζ and Par-4 (Diaz-Meco et al, 1996; Goswami et al, 2005), which leads to the inhibition of PKCζ activity in vitro and in vivo (Diaz-Meco et al, 1996), we reasoned that Par-4 regulation of Akt might be mediated by PKCζ. Results of Supplementary Figure 1 demonstrate increased PKCζ activation in Par-4 KO lung extracts, confirming and extending to an in vivo lung system our previous observations in EFs (Garcia-Cao et al, 2003). We thought it conceivable that PKCζ could be an Akt kinase and that its regulation by Par-4 could account for the role of this tumour suppressor as a negative modulator of Akt activity in vitro and in vivo. To address this possibility, we first transfected 293 cells with Par-4 along with a plasmid control or a PKCζ expression vector. We reasoned that if Par-4 reduces Akt's Ser473 phosphorylation through its ability to inhibit PKCζ, the overexpression of PKCζ should impair Par-4 inhibitory actions on Akt's Ser473 phosphorylation. The data in Figure 6A show that this is, indeed, the case. That is, the expression of Par-4 impairs phospho-Akt-Ser473 phosphorylation in serum-activated 293 cells, whereas the cotransfection of PKCζ with Par-4 completely reverses that effect (Figure 6A), strongly suggesting the existence of a Par-4/PKCζ/Akt cascade. The results of Figure 6B demonstrate that there is a specific and mostly constitutive interaction between Akt and PKCζ. We next tested the hypothesis that Akt could be a direct substrate of PKCζ. For this, we incubated recombinant pure PKCζ with recombinant purified His-Akt in vitro in a kinase assay using γP32-ATP. As shown in Figure 6C, PKCζ in addition to undergoing autophosphorylation was able to directly phosphorylate Akt, with a stoichiometry of 0.4±0.1 moles of phosphate per mole of Akt. Part of the kinase reaction was analysed by immunoblotting and revealed that PKCζ was capable to induce the phosphorylation of Akt's Ser473 and Thr308. Note that the anti-phospho-Thr308 antibody cross-reacts in this in vitro assay with activated PKCζ, which is phosphorylated at Thr410, the equivalent to Akt's Thr308 in their activation loops (Figure 6C). These results demonstrate that PKCζ is capable of directly phosphorylating Akt at both critical residues. To determine whether or not this is due to a hypothetical increase in autophosphorylation of Akt, we performed an in vitro kinase assay with recombinant pure PKCζ and either WT Akt or a kinase-inactive (KD) version of that enzyme. Interestingly, PKCζ phosphorylated both Akt forms with the same efficiency (Figure 6D). The same results were obtained when the levels of phospho-Akt-Thr308 were determined in a similar experiment (not shown). Phosphopeptide analysis of Akt phosphorylated with PKCζ as shown in Figure 6C reveals three major peptides that, according to a theoretical map, correspond to phosphorylation of Ser124 (Figure 6E). This is consistent with the phospho-amino-acid analysis of these peptides that gives Ser as the predominantly phosphorylated amino acid. Mass-spectrometry analysis of PKCζ-phosphorylated Akt demonstrates that Ser124, Ser473 and Thr308 were phosphorylated (Figure 6F), Ser124 phosphopeptide being the most abundantly detected. Analysis of these sites in untreated non-phosphorylated Akt by mass spectrometry revealed that Ser473 and Thr308 were both absent in the untreated control; however, we detected Ser124 phosphopeptide. To quantify the level of phosphorylation in Ser124 upon PKCζ incubation, we used the spectral total ion count (TIC) method, which is a label-free quantitation method that relies on spectral counting and TIC to calculate the relative intensities of phosphorylation (Asara et al, 2008). We observed a spectral TIC ratio of threefold for S124 phosphopeptide in PKCζ-phosphorylated Akt compared with untreated Akt. Phosphorylation of Ser124 has previously been shown to be constitutive and to regulate the extent of Akt activation (Manning et al, 2002). These results could be interpreted as that the loss of Par-4 leads to increased PKCζ activity, which by phosphorylation of Ser124 modulates the extent of Akt activation. However, the fact that PKCζ directly phosphorylates Akt at Thr308 and Ser473 could suggest that it could have an important function on Akt activation. However, a more plausible explanation to these data is that, although PKCζ phosphorylates Ser473 in vitro, it does it inefficiently in vivo in the presence of the TORC2 complex, its role being limited to phosphorylate Ser124, which nonetheless will impact the total Ser473 phosphorylation by the TORC2 complex. If this is the case, we predicted that the knockdown of Rictor should abolish Akt phosphorylation and Ser473 even in the absence of Par-4. Results of Figure 6G demonstrate that this is actually the case. Also, this model is consistent with the fact that Ser124 phosphopeptide was most abundantly detected in the mass-spectrometry analysis and in the phosphopeptide map of Figure 6E.
Figure 6.
PKCζ directly interacts and phosphorylates Akt. (A) Overexpression of Par-4 in 293 cells inhibited serum-induced phospho-Akt-S473 phosphorylation and was reversed by PKCζ co-expression. (B) Serum-stimulated 293 cells extracts were immunoprecipitated with anti-Akt or IgG control antibody and the immunoprecipitates were analysed by immunoblot with anti-PKCζ or anti-Akt, as a loading control. (C) In vitro phosphorylation of recombinant Akt by recombinant PKCζ with γP32-ATP. Part of the assay reaction was also immunoblotted with anti-phospho-Akt-Ser473, anti-phospho-Akt-T308 and anti-Akt antibodies. (D) Immunoprecipitates of WT or KD Akt were phosphorylated in vitro by PKCζ and phospho-Akt-Ser473 levels were determined. (E) Phosphopeptide map of in vitro PKCζ phosphorylated-Akt (left panel). Phospho-amino-acid analysis of peptides A, B, C and the total reaction. (F) MS/MS spectra of the identified phosphopeptides corresponding to sites S124, S473 and T308 phosphorylated in Akt by PKCζ. (G) A549 cells treated with a control siRNA, Par-4–siRNA, Rictor-siRNA or both siRNAs were stimulated with serum and the levels of phospho-Akt-S473 were determined. Knockdown of Par-4 and Rictor was confirmed by immunoblot. These are representative experiments where there were at least two others with similar results.
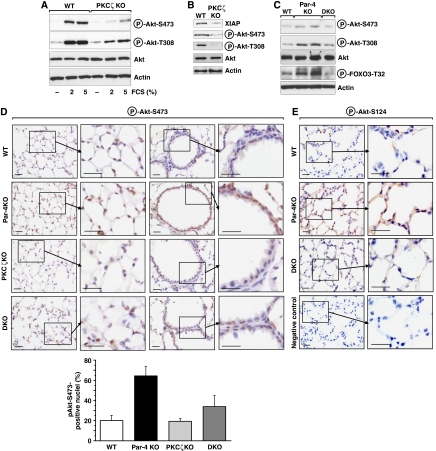
Collectively, these results indicate that PKCζ is an upstream regulator of Akt activation by phosphorylating Ser124. To demonstrate that PKCζ is actually required in vivo for the phosphorylation of Akt's Ser473 and Thr308, we analysed these parameters in EFs WT and KO for PKCζ either untreated or stimulated with different doses of serum. Of relevance, the lack of PKCζ severely impairs Akt phosphorylation at both critical residues (Figure 7A). The analysis of lung extracts from PKCζ-deficient mice supports a critical role for this kinase in Akt activation in vivo (Figure 7B). To demonstrate that PKCζ is actually required for Par-4 regulation of Akt phosphorylation at its two critical residues, we generated a Par-4/PKCζ double KO (DKO) mice cell line. We then analysed the levels of phospho-Akt-Ser473 and phospho-Akt-Thr308 in lung extracts from WT, Par-4 KO and DKO mice. Data of Figure 7C show that the increased phosphorylation in these two residues observed in the Par-4 KO lung extracts is abolished in lung extracts of DKO mice. Also, FOXO3 phosphorylation that is increased in the Par-4 KO lung extracts is similarly inhibited in the DKO mice (Figure 7C). Similar results were obtained when phospho-Akt-Ser473 and phospho-Akt-Ser124 levels were analysed by IHC of lung sections of mice with different genotypes (Figure 7D and E).
Figure 7.
Activation of Akt by Par-4 deficiency is dependent on PKCζ in vivo. (A) WT and PKCζ KO EFs stimulated with serum were analysed by immunoblot for phospho-Akt-S473 and phospho-Akt-T308 levels. (B) Lung extracts from WT and PKCζ KO were immunoblotted with XIAP, phospho-Akt-S473 and phospho-Akt-T308 antibodies. (C–E) Phosphorylation of Akt in Par-4 KO is reverted in DKO (Par-4/PKCζ). Phosphorylation of Akt and its substrate FOXO3 were determined in lung extracts of the different KO mice (C). Lung sections were stained for phospho-Akt-S473. Quantitation of cells showing positive nuclear staining for phospho-Akt-S473 (D). Results are the mean±s.d. of 10 different fields per mouse with a total of five mice for each condition. (E) Lung sections were stained for phospho-Akt-S124. Negative control was performed on Par-4 KO sample with no primary antibody. These are representative experiments where there were at least two others with similar results. Scale=50 μm.
Collectively, all these results establish the Par-4/PKCζ cassette as an important regulator of Akt activation, most likely through the ability of PKCζ to directly phosphorylate Akt's Ser124 and its subsequent impact in the phosphorylation of residues Ser473 and Thr308, which are critical for the regulation of Akt activity and function.
Discussion
The balance between oncogenic and tumor-suppressive signals is central to the control of tumour initiation and progression (Hanahan and Weinberg, 2000). Our laboratory identified Par-4, a gene previously discovered in a screen for genes upregulated in cells undergoing apoptosis (Sells et al, 1994), as a critical negative regulator of aPKCs (Diaz-Meco et al, 1996). In overexpression experiments, Par-4 has also been reported to interact with other potential partners (Johnstone et al, 1996; Page et al, 1999; Kawai et al, 2003; Roussigne et al, 2003), but whether these interactions actually have an important function in vivo is still unclear. Subsequent studies demonstrated, in vivo and under physiological conditions, that PKCζ and Par-4 KO mice display opposite phenotypes (Moscat and Diaz-Meco, 2003; Moscat et al, 2006), lending support to the notion that Par-4 is a negative regulator of PKCζ. In addition, from those studies, it is clear that the Par-4/PKCζ cassette is relevant for NF-κB activation during cell survival (Garcia-Cao et al, 2003; Duran et al, 2004).
Par-4 is reduced in a significant number of endometrial (Moreno-Bueno et al, 2007), prostate (our unpublished observations) and lung (this study) human carcinomas. Furthermore, loss of Par-4 in mice leads to the spontaneous appearance of PIN and endometrial tumours (Garcia-Cao et al, 2005), and the expression of oncogenic Ras in fibroblast cultures leads to a significant reduction in Par-4 levels (Barradas et al, 1999). All these studies suggest that Par-4 exerts tumour-suppressive effects in human cancer and in cell culture systems, although its ability to restrain oncogenesis in physiologically relevant model systems has not been demonstrated yet. The data shown here demonstrate that, although Par-4 levels are reduced in fibroblast cell cultures expressing oncogenic Ras (Barradas et al, 1999), Par-4 levels remain unaltered in normal and tumour-derived lung tissues from mice expressing the oncogenic form of Ras. This suggests that, in vivo, inactivating mutations or deletions of Par-4 could be instrumental for Ras-induced tumour transformation. Here we addressed this important question by determining the ability of Ras to induce lung adenocarcinomas in Par-4 KO mice. The rationale for this experiment is that if Par-4's role is to suppress tumorigenicity, Ras-induced lung cancer would be enhanced by the lack of Par-4. The data shown here clearly support that hypothesis in that the tumour burden induced by Ras was significantly higher in Par-4 KO lungs than in the WT controls. Also, the loss of Par-4 resulted in a higher percentage of Ras-induced high-grade lung adenocarcinomas, indicating that the absence of Par-4 promotes lung tumour progression. These are important observations that, coupled with our data that Par-4 is absent in a significant proportion of human NSCLC, mostly in adenocarcinomas, strongly suggest that Par-4 is a relevant tumour suppressor in lung cancer.
From a mechanistic point of view, the data presented here unveil an unexpected and important role of Par-4 as a negative regulator of Akt that exerts an effect by controlling the phosphorylation of Akt's Ser473 and Thr308, two critical events in the activity of this kinase (Cantley, 2002). Our findings have to be put in the context of other observations demonstrating that the proto-oncogene Akt is activated in many human cancers, mostly due to loss of the PTEN tumour suppressor. Interestingly, in tumorigenesis, PTEN synergizes with a network of other tumour suppressors, all of them modulating Akt activation through different molecular mechanisms. For example, the tumour suppressor PML cooperates with PTEN inside the nucleus through the recruitment of Akt itself and the phosphatase PP2a into the PML nuclear bodies. This leads to the efficient inactivation of Akt by dephosphorylation (Trotman et al, 2006). On the other hand, the tumour suppressor NEP inhibits Akt by direct interaction with, and stabilization of, PTEN (Sumitomo et al, 2004). We show here that the effect of Par-4 on Akt activation is cell autonomous and is observed not only in mouse tissues and EFs, but also in human lung adenocarcinoma cells, thus supporting its physiological relevance. In this study, we confirm and extend our views through genetic evidence gathered in both in vivo and in vitro studies that Par-4 not only impinges on the PKCζ signalling pathway leading to NF-κB activation (Leitges et al, 2001; Garcia-Cao et al, 2003; Duran et al, 2004), but also on Akt activity. Akt Thr308 phosphorylation is mediated by PDK1 and activated as a consequence of the stimulation of PI 3-kinase (Manning et al, 2002). The kinase responsible for Akt phosphorylation at Ser473 has been the subject of intense research. The Sabatini laboratory has found that a Rictor-containing mTOR complex is responsible for this phosphorylation (Sarbassov et al, 2005). However, recent data from Tarakhowsky's laboratory show that a PKC isoform, PKCβ, accounts for Ser473 phosphorylation in vivo, in at least B cells (Patke et al, 2006). We show here that PKCζ overexpression suppresses Par-4's inhibitory effect on Akt phosphorylation at residues Ser473 and Thr308, as well as that both phosphorylation events are dramatically reduced in the lungs of PKCζ KO mice. We also demonstrate that the enhanced Akt activation produced by Par-4 ablation is almost completely reduced to WT levels in Par-4/PKCζ DKO lungs and cells. Interestingly, in vitro assays demonstrate that PKCζ directly interacts and phosphorylates Akt, supporting the existence of a Par-4/PKCζ cassette that directly targets Akt. Mass-spectrometric, peptide mapping, immunoblotting and phospho-amino-acid analyses of the PKCζ-phosphorylated Akt reveal that although recombinant pure PKCζ directly phosphorylates WT and kinase-dead Akt at Ser473, it also targets Thr308 and to a larger extent Ser124. The fact that PKCζ can directly phosphorylate Ser473 would be consistent with previous observations that PKCβ can also fulfil such a role (Patke et al, 2006). However, our data that inactivation of the TORC2 complex by the knockdown of Rictor in the Par-4 KO cells totally abolishes Akt's Ser473 phosphorylation clearly indicate that PKCζ cannot be the most relevant regulator of Ser473 in vivo in Par-4-inactivated cells and that TORC2 is essential also for that function. Interestingly, our experiments indicate that the Akt residue more abundantly phosphorylated by PKCζ is Ser124. This is very important because this residue along with Thr450 is constitutively phosphorylated and is necessary for an efficient phosphorylation of Ser473 and Thr308 and the subsequent full activation of Akt (Alessi et al, 1996; Bellacosa et al, 1998; Conus et al, 2002). These are relevant findings because the identity of the Ser124 and Thr450 kinases remained unknown. Previous data suggest that JNK is the Thr450 kinase (Shao et al, 2006). The results presented here establish that the Ser124 kinase is PKCζ.
Collectively considered, these results lead us to propose a model whereby the Par-4/PKCζ complex modulates the extent of Akt activation by directly targeting Ser124. This is relevant, as overactivation of Akt in Par-4-deficient cells could be an important contributor to the pro-neoplasic phenotype of Par-4-deficient mice. In this regard, and taking into account the essential role of Akt in Ras transformation (Malumbres and Barbacid, 2003), our data suggest that the mechanism whereby the loss of Par-4 enhances the Ras-transforming potential in lung cancer in vivo will likely be accounted for by increased Akt activation.
Materials and methods
Mice
Par-4 KO and PKCζ KO mice were described previously (Leitges et al, 2001; Garcia-Cao et al, 2003). Par-4 KO and PKCζ KO were crossed to generate DKO. K-ras+/V12, RERT2nT/T mice (Guerra et al, 2003) were crossed with Par-4 KO mice. The KRasV12 allele was activated when mice were 1 month old by a single intraperitoneal injection of 500 μg of 4-hydroxytamoxifen dissolved and sonicated in olive oil. All mice were born and kept under pathogen-free conditions. Animal-handling protocols conform to institutional guidelines. All genotyping was done by PCR.
Cell culture
Wild-type, Par-4 KO and PKCζ KO primary EFs were derived from E13.5 embryos (Garcia-Cao et al, 2003). Cells were maintained in DMEM (Gibco BRL) supplemented with 10% (v/v) fetal calf serum (FCS), 1% glutamine and 1% penicillin/streptomycin (Gibco-Invitrogen) in an atmosphere of 95% air and 5% CO2, and immortalized by retroviral infection with pBabeT-Ag followed by puromycin selection (1 μg/ml). The established cell lines represent pools of at least 100 independent clones. The HEK293-derived virus-packaging cell line 293T, and 293 and A549 cells were cultured in DMEM with 10% FCS. For knockdown of Par-4 and Rictor, two different human siRNAs were obtained from Qiagen with the following target sequences: Par-4: AAGTGGGTTCCCTAGATATAA and CACAGCCGTTTGAATATATTT; Rictor: AAACAAGGCTGTGATATTCTA and AAAGACTACAGCAACAAAGAA; Akt: AAGCACCTTCATTGGCTACAA and AAGGAGGGTTGGCTGCACAAA.
Similar results were obtained with both siRNA sequences. AllStars Negative Control siRNA (Qiagen) was used as the negative control. Transfection of sRNAi was performed by the calcium phosphate method. For Par-4 reconstitution experiments, the retroviral expression vector pWZL-Par-4 was used. Retroviruses were produced in 293T cells by transient transfection using Lipofectamine 2000 (Invitrogen). Culture supernatants were collected 24, 48 and 72 h post-transfection, filtered (0.45 μm) and then supplemented with 4 μg/ml polybrene. EFs were infected with three rounds of viral supernatants and selected with hygromycin (75 μg/ml). The following expression plasmids were used: CMV-6-hPar-4, pCDNA3-HA-PKCζ, pSG-HA-Akt and pSG-HA-Akt-KD. PIP3 levels were measured by ELISA, using PIP3 ELISA kit (Echelon).
Confocal analysis
Wild-type and Par-4 KO EFs seeded on coverslips were fixed with 4% formaldehyde and permeabilized with 0.1% Triton X-100. Free aldehyde groups were quenched with 50 mM NH4Cl. Endogenous peroxidase activity was quenched by treatment with 3% H2O2 in methanol for 15 min. Fixed cells were treated with blocking solution. Cells were incubated with monoclonal anti-XIAP and polyclonal anti-Par-4 or anti-phospho-Akt for 1 h at 37 °C. Anti-rabbit-Alexa-594 and anti-mouse-Alexa-488 were used as secondary antibodies. Glass cover slips were mounted on Mowiol and examined with a Zeiss LSM 510 Meta confocal system.
Western blotting
Cell extracts for western blot were prepared in RIPA buffer (1 × PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM phenyl methyl sulphonyl fluoride and protease inhibitors). Lysates were separated by SDS–PAGE and transferred to nitrocellulose-ECL membranes (GE Healthcare) and the immune complex was detected by chemiluminescence (GE Healthcare). The following primary antibodies were used for western blot: phospho-Akt-S473, phospho-Akt-T308, phospho-FOXO3-T32, phospho-GSK3β-S9, PKCζ and phospho-PKCζ-T410/403 from Cell Signalling; Akt, actin, HA and Par-4 antibodies from Santa Cruz, and XIAP from BD. All antibodies were used according to the manufacturers' instructions.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Information
Acknowledgments
We thank Maryellen Daston for editing this manuscript, Glenn Doerman for preparing the figures and Lyndsey Cheuvront and Michael Winstead for technical assistance. We thank Hongjun Shu for his help in mass-spectrometry experiments. This work was funded in part by the University of Cincinnati-Consejo Superior de Investigaciones Cientificas Research Collaborative Agreement and by the NIH grant R01-AI072581 (to JM). Research at the laboratory of MS is funded by the CNIO and by grants from the Spanish Ministry of Education, the European Union (INTACT and PROTEOMAGE) and the ‘Marcelino Botin' Foundation. We thank Mariano Barbacid for making the K-ras+/V12, RERT2nT/T mice available.
References
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- Asara JM, Christofk HR, Freimark LM, Cantley LC (2008) A label-free quantification method by MS/MS TIC compared to SILAC and spectral counting in a proteomics screen. Proteomics 8: 994–999 [DOI] [PubMed] [Google Scholar]
- Barradas M, Monjas A, Diaz-Meco MT, Serrano M, Moscat J (1999) The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J 18: 6362–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P (1998) Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 17: 313–325 [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N (2007) The Two TORCs and Akt. Dev Cell 12: 487–502 [DOI] [PubMed] [Google Scholar]
- Bos JL (1989) Ras oncogenes in human cancer: a review. Cancer Res 49: 4682–4689 [PubMed] [Google Scholar]
- Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655–1657 [DOI] [PubMed] [Google Scholar]
- Conus NM, Hannan KM, Cristiano BE, Hemmings BA, Pearson RB (2002) Direct identification of tyrosine 474 as a regulatory phosphorylation site for the Akt protein kinase. J Biol Chem 277: 38021–38028 [DOI] [PubMed] [Google Scholar]
- Diaz-Meco MT, Municio MM, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J (1996) The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell 86: 777–786 [DOI] [PubMed] [Google Scholar]
- Downward J (2003) Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3: 11–22 [DOI] [PubMed] [Google Scholar]
- Duran A, Rodriguez A, Martin P, Serrano M, Flores JM, Leitges M, Diaz-Meco MT, Moscat J (2004) Crosstalk between PKCzeta and the IL4/Stat6 pathway during T-cell-mediated hepatitis. EMBO J 23: 4595–4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE (2001) Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev 15: 3249–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cao I, Duran A, Collado M, Carrascosa MJ, Martin-Caballero J, Flores JM, Diaz-Meco MT, Moscat J, Serrano M (2005) Tumour-suppression activity of the proapoptotic regulator Par4. EMBO Rep 6: 577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cao I, Lafuente M, Criado L, Diaz-Meco M, Serrano M, Moscat J (2003) Genetic inactivation of Par4 results in hyperactivation of NF-κB and impairment of JNK and p38. EMBO Rep 4: 307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T, Rangnekar VM (2005) Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell 20: 33–44 [DOI] [PubMed] [Google Scholar]
- Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M (2003) Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell 4: 111–120 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Johnstone RW, See RH, Sells SF, Wang J, Muthukkumar S, Englert C, Haber DA, Licht JD, Sugrue SP, Roberts T, Rangnekar VM, Shi Y (1996) A novel repressor, par-4, modulates transcription and growth suppression functions of the Wilms' tumor suppressor WT1. Mol Cell Biol 16: 6945–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RW, Tommerup N, Hansen C, Vissing H, Shi Y (1998) Mapping of the human PAWR (par-4) gene to chromosome 12q21. Genomics 53: 241–243 [DOI] [PubMed] [Google Scholar]
- Karin M (2006a) NF-kappaB and cancer: mechanisms and targets. Mol Carcinog 45: 355–361 [DOI] [PubMed] [Google Scholar]
- Karin M (2006b) Nuclear factor-[kappa]B in cancer development and progression. Nature 441: 431–436 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S, Reed JC (2003) ZIP kinase triggers apoptosis from nuclear PML oncogenic domains. Mol Cell Biol 23: 6174–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente MJ, Martin P, Garcia-Cao I, Diaz-Meco MT, Serrano M, Moscat J (2003) Regulation of mature T lymphocyte proliferation and differentiation by Par-4. EMBO J 22: 4689–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J (2001) Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell 8: 771–780 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M (2003) RAS oncogenes: the first 30 years. Nat Rev Cancer 3: 459–465 [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC (2002) Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10: 151–162 [DOI] [PubMed] [Google Scholar]
- Meuwissen R, Berns A (2005) Mouse models for human lung cancer. Genes Dev 19: 643–664 [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, Tendero MJ, Rodriguez-Pinilla SM, Garcia-Cao I, Hardisson D, Diaz-Meco MT, Moscat J, Serrano M, Palacios J (2007) Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res 67: 1927–1934 [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT (2003) Par-4 keeps the atypical PKCs at bay. Cell Cycle 2: 71–72 [PubMed] [Google Scholar]
- Moscat J, Rennert P, Diaz-Meco MT (2006) PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ 13: 702–711 [DOI] [PubMed] [Google Scholar]
- Page G, Kogel D, Rangnekar V, Scheidtmann KH (1999) Interaction partners of Dlk/ZIP kinase: co-expression of Dlk/ZIP kinase and Par-4 results in cytoplasmic retention and apoptosis. Oncogene 18: 7265–7273 [DOI] [PubMed] [Google Scholar]
- Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A (2006) BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med 203: 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussigne M, Cayrol C, Clouaire T, Amalric F, Girard JP (2003) THAP1 is a nuclear proapoptotic factor that links prostate-apoptosis-response-4 (Par-4) to PML nuclear bodies. Oncogene 22: 2432–2442 [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101 [DOI] [PubMed] [Google Scholar]
- Sells SF, Wood DP Jr, Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA, Humphreys S, Rangnekar VM (1994) Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ 5: 457–466 [PubMed] [Google Scholar]
- Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, Namchuk M, Salituro F, Yao YM, Hou WM, Chen X, Aronovitz M, Tsichlis PN, Bhattacharya S, Force T, Kilter H (2006) c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res 98: 111–118 [DOI] [PubMed] [Google Scholar]
- Sumitomo M, Iwase A, Zheng R, Navarro D, Kaminetzky D, Shen R, Georgescu MM, Nanus DM (2004) Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. Cancer Cell 5: 67–78 [DOI] [PubMed] [Google Scholar]
- Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP (2006) Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441: 523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, Hingorani SR, Zaks T, King C, Jacobetz MA, Wang L, Bronson RT, Orkin SH, DePinho RA, Jacks T (2004) Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5: 375–387 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Information