Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection (original) (raw)
Abstract
Progressive loss of T cell functionality is a hallmark of chronic infection with human immunodeficiency virus 1 (HIV-1). We have identified a novel population of dysfunctional T cells marked by surface expression of the glycoprotein Tim-3. The frequency of this population was increased in HIV-1–infected individuals to a mean of 49.4 ± SD 12.9% of CD8+ T cells expressing Tim-3 in HIV-1–infected chronic progressors versus 28.5 ± 6.8% in HIV-1–uninfected individuals. Levels of Tim-3 expression on T cells from HIV-1–infected inviduals correlated positively with HIV-1 viral load and CD38 expression and inversely with CD4+ T cell count. In progressive HIV-1 infection, Tim-3 expression was up-regulated on HIV-1–specific CD8+ T cells. Tim-3–expressing T cells failed to produce cytokine or proliferate in response to antigen and exhibited impaired Stat5, Erk1/2, and p38 signaling. Blocking the Tim-3 signaling pathway restored proliferation and enhanced cytokine production in HIV-1–specific T cells. Thus, Tim-3 represents a novel target for the therapeutic reversal of HIV-1–associated T cell dysfunction.
It is clear from many studies that HIV-1– or SIV-specific CD8+ and CD4+ T cell responses have an important role in containing viral replication (1–5). However, in most cases cellular immunity to HIV-1 proves incapable of long-term control of viremia and, without antiretroviral therapy, progression to AIDS occurs. The failure of the host immune system to contain HIV-1 is related to the functional impairment of HIV-1–specific CD8+ and CD4+ T cells that accompanies progressive HIV-1 infection, a phenomenon which is referred to as T cell exhaustion (6–17). In HIV-1 infection, the deterioration of the T cell response follows a characteristic pattern: proliferative capacity, cytotoxic potential, and the ability to produce IL-2 are lost early, whereas the production of IFN-γ is more enduring. Ultimately, the majority of T cells chronically exposed to HIV-1 antigens enter into a state of dysfunction and, as disease advances, even the ability to produce IFN-γ is progressively impaired (7, 8, 18–22). The causal relationship between this progressive T cell exhaustion and high levels of HIV-1 replication in progressive infection remains unclear. Recently, signaling through PD-1 was shown to play an important role in T cell exhaustion in three models of chronic viral infection: LCMV in mice, SIV in rhesus macaques, and HIV-1 in humans (12, 13, 15, 23–25). Blockade of the PD-1–PD-L1 signaling pathway results in enhanced T cell responses and viral control in mouse LCMV infection, as well as in enhanced survival and proliferation of HIV-1–specific CD8+ T cells in vitro. Increased levels of total cytokine production and increased frequencies of cells producing cytokine in response to antigen are also induced in 6-d in vitro cultures treated with anti–PD-L1 (12, 15). However, it has been demonstrated that there is no direct relationship between the level of PD-1 expression of an HIV-1– or SIV-specific CD8+ T cell and the ability of that cell to produce cytokine upon ex vivo stimulation (13, 25). This has lead to the suggestion that the enhanced levels of total cytokine production observed in vitro with the addition of anti–PD-L1 is the result of greater survival and expansion of antigen-specific CD8+ T cells rather than improved functionality on a per-cell basis. These data suggest that PD-1 expression marks a population exhibiting features of relatively early T cell exhaustion, where cell survival and proliferation are impaired but cytokine production remains intact. Thus, the mechanisms leading to advanced stages of T cell exhaustion, where cytokine production becomes impaired, remain largely undefined.
Tim-3 (T cell immunoglobulin and mucin domain–containing molecule 3) is an Ig superfamily member that was identified as a specific cell surface marker of mouse Th1 CD4+ T cells (26). Interaction of mouse Tim-3 with its ligand, galectin-9, regulates Th1 responses by promoting the death of IFN-γ–producing Th1 cells (27). In mice, blocking the interaction of Tim-3 with its ligands prevents the acquisition of transplantation tolerance induced by costimulatory blockade (27, 28). Furthermore, Tim-3–deficient mice are refractory to the induction of high-dose tolerance in an experimental autoimmune encephalomyelitis model, and anti–Tim-3 monoclonal antibody treatment of SJL/J mice exacerbated experimental autoimmune encephalomyelitis (26, 29). These results indicate that Tim-3 plays a role in suppressing Th1-mediated immune responses, at least partially through the termination of effector Th1 cells. In humans, a defect in up-regulation of Tim-3 on IFN-γ–producing CD4+ T cells has been implicated as a contributing factor to the pathology associated with multiple sclerosis (30, 31). No study has yet examined the role of Tim-3 in chronic viral infection.
RESULTS
Tim-3 expression on T cells correlates with clinical parameters of progression in HIV-1–infected individuals
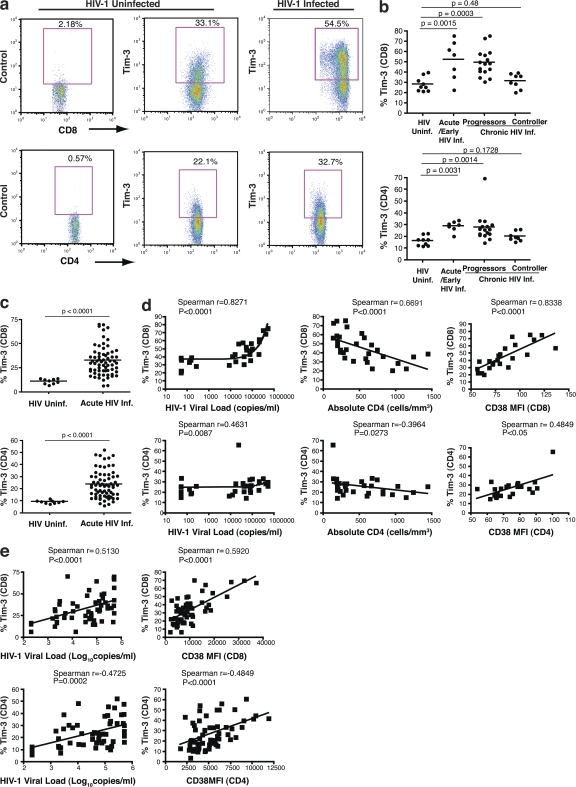
We profiled Tim-3 expression by flow cytometry on PBMC from 9 HIV-1–uninfected individuals and 31 treatment-naive, acute/early, and chronically HIV-1–infected subjects (Canadian Immunodeficiency Research Collaborative [CIRC] cohort) that included both viral controllers (nonprogressors) and progressors using a polyclonal anti–Tim-3 antibody. We observed elevated frequencies of Tim-3–expressing CD8+ T cells in acute/early and chronic progressive HIV-1–infected individuals, but not in viral controllers, relative to uninfected individuals (mean 28.5 ± SD 6.8% for HIV-1–uninfected versus 52.2 ± 19.0% for acutely/early infected individuals [P = 0.0015], 49.4 ± 12.9% for chronic progressors [P = 0.0003], and 31.6 ± 7.3% for viral controllers [P = 0.48]; Fig. 1, a and b). Tim-3 expression was also elevated on CD4+ T cells from acutely/early infected individuals and chronic progressors, as compared with both viral controllers and HIV-1–uninfected individuals (Fig. 1, a and b). The frequency of Tim-3+ CD8+ T cells correlated positively with HIV-1 viral load (P < 0.0001; Fig. 1 d) and inversely with absolute CD4+ T cell counts (P < 0.0001; Fig. 1 d). Similarly, the frequencies of Tim-3+ CD4+ T cells were significantly correlated with viral load (P = 0.0087) and absolute CD4+ T cell counts (P = 0.0273; Fig. 1 d). T cell activation, as reported by CD38 expression, is an additional predictor of disease progression (32). CD38 expression on CD8+ T cells correlated with the frequency of Tim-3+ CD8+ T cells (P < 0.0001; Fig. 1 d), and CD38 expression on CD4+ T cells correlated with the frequency of Tim-3+ CD4+ T cells (P < 0.05; Fig. 1 d). In acute/early and chronic progressive HIV-1 infection, increased expression of both Tim-3 and CD38 manifested as a frequent dual Tim-3+ CD38+ population of CD8+ T cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081398/DC1). In a separate cohort of 60 treatment-naive acutely/early HIV-1–infected individuals (OPTIONS cohort), we observed an analogous increase in the frequency of Tim-3+ CD8+ and CD4+ T cells as assessed with a monoclonal anti–Tim-3 antibody (Fig. 1 c and Fig. S2 a). Similar positive correlations between HIV-1 viremia, CD38, and Tim-3 expression on T cells were also observed in this acute/early infection cohort (Fig. 1 e).
Figure 1.
Tim-3 is up-regulated on T cells in HIV-1 infection, and its expression correlates with parameters of HIV-1 disease progression. (a) PBMCs from HIV-1–infected and uninfected subjects were stained with antibodies against CD4, CD8, CD3, and a biotinylated polyclonal goat anti–Tim-3 antibody, followed by a secondary streptavidin–APC conjugate. Plots show events gated on the CD3+ population, and subsequently on the CD8+ or CD4+ populations, from a representative HIV-1–uninfected subject and a chronically HIV-1–infected subject. Biotinylated normal goat serum was used as a negative control. (b) The percentages of Tim-3+ cells within CD8+ and CD4+ T cell populations are indicated for nine HIV-1–uninfected individuals and 31 individuals from the CIRC cohort separated into three groups: acute/early HIV-1 infected (7), HIV-1–infected chronic progressors (16), and HIV-1–infected viral controller (8), using polyclonal goat anti–Tim-3 antibody. (c) The percentages of Tim-3+ cells within CD8+ and CD4+ T cell populations are indicated for 60 treatment-naive HIV-1–infected individuals from the UCSF OPTIONS cohort of primary infection and 9 HIV-1–uninfected controls using PE-conjugated monoclonal anti–Tim-3 antibody. Subjects from the CIRC cohort were defined as follows: acute/early = infected with HIV-1 < 4 mo; chronic progressor = infected > 1 yr with CD4+ T cell count decline >50 cells/mm3/yr, viral controller infected >1 yr, no evidence of CD4+ T cell count decline, and viral load <5,000 copies/ml bDNA. Characteristics of the OPTIONS acute/early infection cohort are detailed in Materials and methods. Statistical analyses for both cohorts were performed using the Mann-Whitney test. (d and e) Correlations between Tim-3 expression on CD8+ and CD4+ T cells and viral load, CD4+ T cell counts, and levels of CD38 expression among individuals with available clinical data from the CIRC cohort (d) and OPTIONS cohort (e). Shown for the CIRC cohort are Tim-3 levels determined using a polyclonal anti–Tim-3 antibody. Confirmatory experiments were performed using a PE-conjugated monoclonal anti–Tim-3, and a tight correlation between the two datasets was observed, with slightly higher frequencies of Tim-3–expressing cells observed with polyclonal anti–Tim-3 antibody (Fig. S2 a). For the OPTIONS cohort, levels of Tim-3 expression were assessed using the monoclonal anti–Tim-3 antibody. Statistical analyses were performed using the Spearman's rank correlation test. Solid lines show the mean. Fig. S2 is available at http://www.jem.org/cgi/content/full/jem.20081398/DC1.
Tim-3 is up-regulated on HIV-1–specific CD8+ T cells in progressive HIV-1 infection
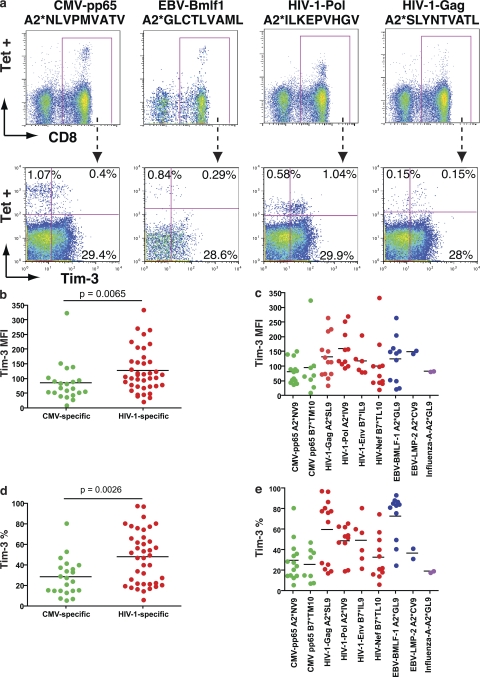
Tim-3 expression on antigen-specific CD8+ T cells was examined in HLA-A*0201+, HLA-B*0702+, and HLA-B*0801+ chronically HIV-1–infected individuals using matched MHC-I pentamers. We observed significantly higher levels of Tim-3 on HIV-1–specific versus CMV-specific CD8+ T cells (P = 0.0065 by mean fluorescence intensity [MFI], P = 0.0026 by percentage of Tim-3+; Fig. 2, a–e). CMV-specific CD8+ T cells exhibited low levels of Tim-3 expression, with the exception of one response to CMV-pp65-TPRVTGGGAM, which exhibited high levels of Tim-3 expression as measured by MFI, observed in cells from an individual with AIDS (absolute CD4 count, 132 cells/μl). Tim-3 expression was heterogenous among HIV-1–specific responses, with some exhibiting very high levels of Tim-3, whereas others exhibited only baseline levels (Fig. 2, c and e). The heterogeneity observed in Tim-3 expression levels on HIV-1–specific CD8+ T cells cannot be attributed solely to intersubject variability, as responses with high levels of Tim-3 expression were frequently observed contemporaneously with responses exhibiting low levels of Tim-3 expression within the same individual. This leads us to speculate that Tim-3 expression may mark HIV-1–specific T cells with differing functional capacities.
Figure 2.
Tim-3 is expressed at elevated levels on HIV-1–specific CD8+ T cells in progressive HIV-1 infection. PBMC from HLA-A*0201+, HLA-B*0702+, and HLA-B*0801+ chronically HIV-1–infected individuals from the CIRC cohort were stained with matched HLA pentamers presenting CMV, EBV, influenza, and HIV-1 epitopes, and with anti–Tim-3. (a) Shown are representative flow cytometry data from one HIV-1–infected chronic progressor using HLA-A*0201 pentamers presenting the CMV-pp65 epitope NLVPMVATV, the EBV-Bmlf1 epitope GLCTLVAML, the HIV-1–Pol epitope ILKEPVHGV, and the HIV-1–Gag epitope SLYNTVATL. (b–e) Compiled Tim-3 expression data from chronic progressors (n = 41) is shown for pooled Tim-3 expression on HIV-1 (b and d) and CMV-specific CD8+ T cell responses from chronic progressors' individual epitope responses (c and e). Statistical analyses comparing pooled responses were performed using the Mann-Whitney test. Solid lines show the mean.
Reduction of Tim-3 expression upon initiation of highly active antiretroviral therapy (HAART) is correlated with levels of ongoing T cell activation (CD38 expression)
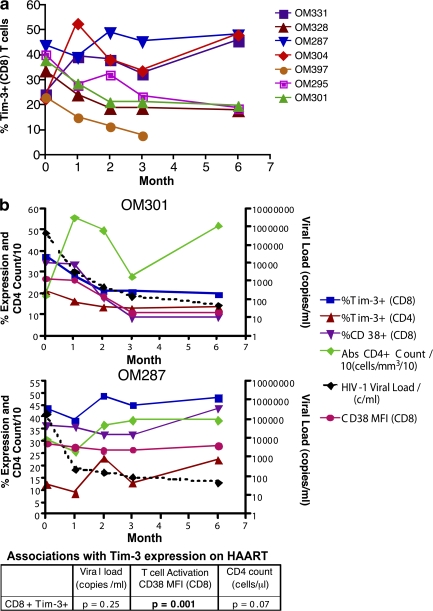
The effect of HAART on Tim-3 expression was studied in seven chronically HIV-1–infected individuals at baseline and at 1, 2, 3, and 6 mo after initiation of HAART (Fig. 3). Four subjects with chronic infection demonstrated a steady decline in Tim-3 levels on both CD4+ and CD8+ T cells with HAART, whereas three subjects (OM 304, 331, and 287) maintained high levels of Tim-3 expression despite achieving undetectable HIV-1 viral loads (<50 copies/ml branched-chain DNA [bDNA]; Fig. 3, a and b; and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081398/DC1). In a mixed-effects longitudinal analysis we observed that CD8+ T cell activation, as measured by CD38 expression, was significantly associated with Tim-3 expression over the period of HAART. Levels of CD38 expression on CD8+ T cells, as measured by either percentage or MFI, were associated with levels of Tim-3 expression on CD8+ T cells during therapy, with a 0.38% higher Tim-3 expression per 1% higher CD38 expression (SE = 0.11; P = 0.001; Fig. 3 b) and a 0.7% higher Tim-3 expression per unit higher CD38 MFI (SE = 0.19; P = 0.001). These effects remained unaltered when adjusted for CD4+ T cell count. In contrast, neither HIV-1 viral load (P = 0.25) nor absolute CD4+ T cell count (P = 0.07) were significantly associated with Tim-3 expression after HAART. Maintenance of high levels of Tim-3 expression in a subset of chronically HIV-1–infected individuals treated with HAART therapy is thus related to ongoing T cell activation (CD38 expression).
Figure 3.
Effect of HAART on levels of Tim-3 expression in chronic HIV-1 infection. Seven chronically HIV-1–infected individuals from the CIRC cohort were sampled at baseline and at 1, 2, 3, and 6 mo after initiation of HAART. (a and b) Shown are compiled Tim-3 expression on CD8+ T cells versus months after initiation of HAART (a) and Tim-3 and CD38 expression levels, as determined by flow cytometry, along with absolute CD4+ T cell count and HIV-1 viral load clinical data (b). The six individuals followed for 6 mo achieved undetectable viral loads (bDNA <50 copies/ml). The chart in b summarizes the p-values obtained from a mixed-effects longitudinal analysis studying associations between Tim-3 expression on CD8+ T cells with HIV-1 viral load, CD8+ T cell activation as measured by CD38 expression (MFI), and absolute CD4+ T cell count. The results of this analysis are further outlined in the text, and details are outlined in Materials and methods.
Tim-3 expression defines a population of dysfunctional Th1/Tc1 cells
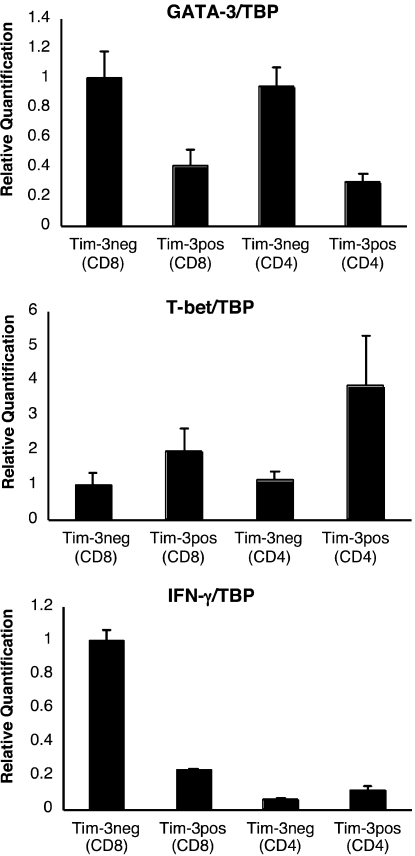
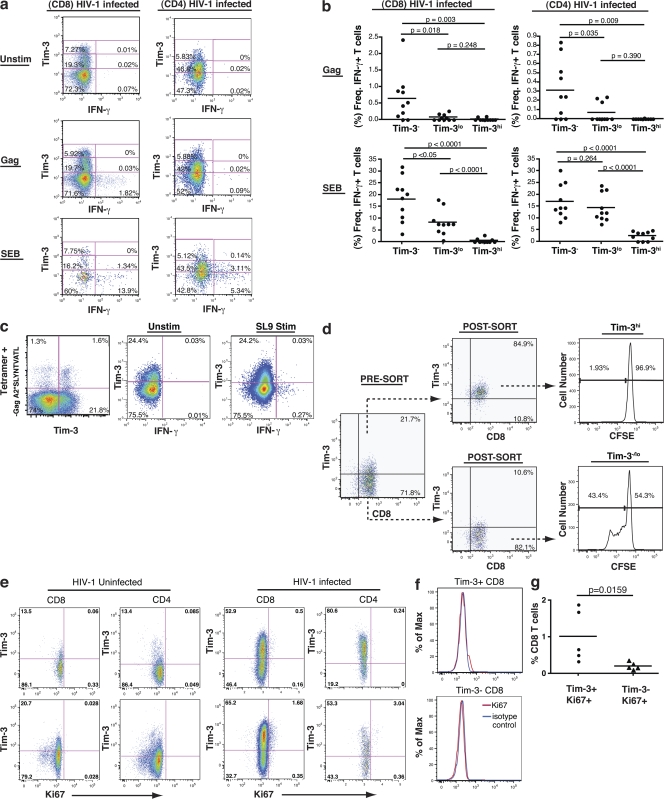
We sorted Tim-3+ from Tim-3− populations within both CD8+ and CD4+ T cell subsets using PBMC from both HIV-1–infected and –uninfected individuals and quantified T-bet (Th1), GATA-3 (Th2), and IFN-γ (Th1) messenger RNA (mRNA) by quantitative PCR. For both CD8+ and CD4+ T cell populations, GATA-3 was expressed at higher levels in the Tim-3− fraction than in the Tim-3+ fraction, whereas T-bet was more highly expressed in the Tim-3+ population (Fig. 4). Despite the Th1/Tc1 character of Tim-3+ cells, we detected the majority of IFN-γ mRNA in the Tim-3− CD8+ population. We then examined IFN-γ and TNF-α production in response to stimulation with pooled HIV-1–Gag peptides, CMV/EBV/Influenza (CEF) peptides, or staphylococcus enterotoxin B (SEB) in PBMC from 10 acutely/early HIV-1–infected individuals, 10 chronic progressors, 10 viral controllers, and 5 HIV-1–uninfected individuals. In both HIV-1–infected and –uninfected subjects, IFN-γ production from CD4+ and CD8+ T cells in response to stimulation was observed predominately from the Tim-3− population, with minimal cytokine production observed in either the Tim-3lo or Tim-3hi populations (Fig. 5, a and b). Analogous patterns of cytokine production were observed for acutely/early infected individuals, chronic progressors, viral controllers, and HIV-1–uninfected subjects (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20081398/DC1). TNF-α and CD107a expression in response to antigen were similarly restricted to Tim-3− cells (Figs. S4 and S5). As a corollary, we identified HIV-1–specific CD8+ T cells by staining with MHC-I tetramers and observed that, in response to cognate peptide, IFN-γ was produced only by the Tim-3−/lo fraction, with no IFN-γ production from tetramer+ Tim-3hi cells (Fig. 5 c). Thus, the lack of cytokine secretion from the Tim-3hi population cannot be attributed to an absence of antigen-specific cells. Tim-3hi CD8+ T cells were subsequently sorted from Tim-3−/lo CD8+ T cells using ex vivo PBMC from untreated chronic progressors. Both subsets were stimulated with anti-CD3/anti-CD28, and proliferation was assessed by CFSE dilution. Proliferation of the Tim-3−/lo cells was observed, whereas minimal proliferation was detected in the Tim-3hi population (Fig. 5 d).
Figure 4.
Quantitative PCR analysis of T-bet and GATA-3 mRNA in Tim-3+ versus Tim-3− T cell subsets. PBMCs were stained with monoclonal antibodies to CD3, CD4, CD8, and Tim-3 and sorted into Tim-3+CD8+, Tim-3−CD8+, Tim-3+CD4+, and Tim-3−CD4+ subsets by flow cytometry. RNA was isolated and reverse transcribed. GATA-3, T-bet, TBP, and IFN-γ transcripts were quantified in triplicate by SYBR real-time PCR. Levels of GATA-3, T-bet, and IFN-γ expression were normalized to TBP. Shown are normalized quantifications expressed relative to the mean of Tim-3−CD8+ from a representative HIV-1–infected chronic progressor (CIRC cohort). Error bars represent SE.
Figure 5.
Tim-3–expressing CD8+ and CD4+ T cell populations are dysfunctional. (a and b) PBMCs derived from HIV-1–infected and –uninfected individuals were stimulated with pooled peptides or SEB superantigen for 6 h, stained for IFN-γ, TNF-α, and Tim-3 using a polyclonal Tim-3 antibody, and analyzed by multiparametric flow cytometry. (a) Representative flow cytometry plots showing cytokine responses to pooled Gag peptides and SEB in CD8+ and CD4+ T cells from a chronically HIV-1–infected individual (CIRC cohort). We used a three-tiered gating system for analyzing cytokine secretion by Tim-3–expressing cells, considering Tim-3−, Tim-3lo, and Tim-3hi populations. The division between Tim-3− and Tim-3lo populations was determined based on a control normal goat serum staining (as in Fig. 1 a). The division between Tim-3lo and Tim-3hi was set arbitrarily and then consistently applied to all samples (all run in parallel). (b) Compiled flow cytometry data from 10 chronically HIV-1–infected progressors (CIRC cohort). IFN-γ response percentage for each subset is normalized to the total number of cells within that population. (c) PBMCs from three chronic progressors were stained with an HLA-A*0201-SLYNTVATL pentamer and stimulated with SLYNTVATL peptide or DMSO control. Shown are cytokine production and Tim-3 expression as determined by flow cytometry in a representative subject. (d) CD8+ T cells were sorted into purified Tim-3hiCD8+ T cells and Tim-3−/loCD8+ T cell populations and labeled with CFSE from one of three representative HIV-1–infected individuals. These two populations were then cultured in the presence of anti-CD3 and anti-CD28 monoclonal antibodies for 5 d. Cells where then assessed for the diminution of CFSE as a readout of cell division. (e) PBMCs from HIV-1–uninfected (n = 5) and HIV-1–infected (n = 5) subjects were assessed for levels of intracellular Ki67 antigen expression. Shown are flow cytometry plots displaying Ki67 expression (x axis) by Tim-3 expression as determined with monoclonal anti–Tim-3 (y axis). (f) Shown is a histogram presenting Ki67 staining in comparison to isotype controls in Tim-3− and Tim-3+ populations of CD8+ T cells. (g) Shown is compiled Ki67 staining data from five HIV-1–infected subjects broken down into Tim-3+ and Tim-3− populations of CD8+ T cells. Solid lines show the mean.
We costained ex vivo PBMC from five HIV-1–uninfected individuals and 5 HIV-1–infected chronic progressors with Tim-3 and Ki67 antigen. Ki67 antigen is a nuclear protein that is generally expressed only in cells in the late G1, S, G2, and M phases of cell cycle (33). Hence, it is generally used as a marker of proliferating cells. In chronic HIV-1 infection, however, it has been demonstrated that the large majority (92 ± 5%) of Ki67+ T cells in peripheral blood are activated cells that are arrested in the G0/G1 phases of cell cycle (34). Several studies have noted that Ki67 expression on T cells from HIV-1–infected individuals is associated with dysfunction or anergy (35–37). Consistently with previous studies, we observed elevated frequencies of Ki67+ cells in both the CD4+ and CD8+ T cell subsets of HIV-1–infected versus –uninfected PBMC (Fig. 5 e) (38). Although the large majority of Tim-3+ cells were Ki67−, Ki67+ T cells were greatly enriched for Tim-3–expressing cells (Fig. 5 g, P = 0.0159). Expression of Tim-3 on this population, which has been characterized as activated but arrested in cell cycle, is consistent with our in vitro data showing a lack of proliferation of Tim-3–expressing cells. Collectively, these studies indicate that Tim-3 expression defines a population of activated, but dysfunctional, T cells in HIV-1 infection.
Blocking the Tim-3–Tim-3L pathway enhances the functionality of HIV-1–specific T cells
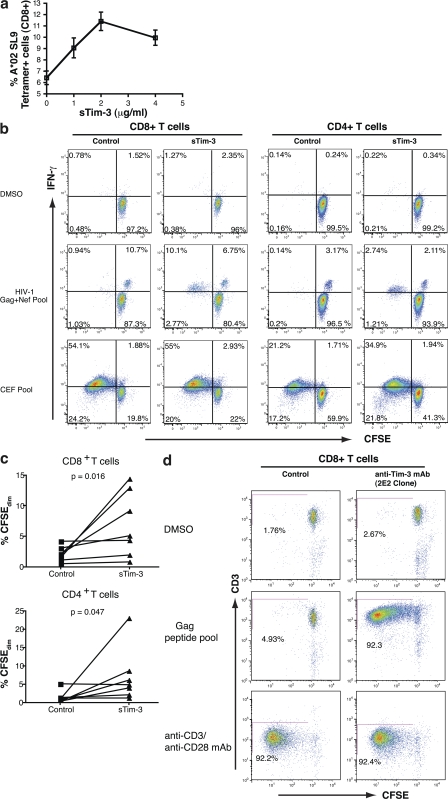
To delineate the causal relationship between Tim-3 expression and T cell dysfunction, we tested whether blocking the interaction of Tim-3 with its ligands would restore function in Tim-3–expressing cells. We used a recombinant soluble Tim-3 (sTim-3) glycoprotein to compete for Tim-3 ligands. Addition of sTim-3 enhanced the expansion of CD8+ T cells specific for the HLA-A*0201 restricted HIV-1–Gag epitope SLYNTVATL (SL9) in HIV-1–infected chronic progressors in a dose-dependent manner up to 2 μg/ml (Fig. 6 a). Enhanced proliferation of both CD8+ and CD4+ T cells was also observed when PBMCs from chronic progressors were stimulated with pooled Gag and Nef peptides (Fig. 6, b and c). We corroborated these data by using a blocking anti–Tim-3 mAb clone (2E2) to disrupt the Tim-3 pathway in an analogous proliferation assay experiment. Addition of 10 μg/ml of mAb 2E2 resulted in a profound rescue of HIV-1–Gag T cell proliferative responses (Fig. 6 d).
Figure 6.
Addition of sTim-3 enhances the proliferation of HIV-1–specific T cells. (a) PBMC from a chronically HLA-A0201+ HIV-1–infected individual were stimulated with the SLYNTVATL peptide in the presence of 4, 2, or 1 μg/ml sTim-3, or a control (see Tim-3 expression methods), for 6 d. Cells were stained with HLA-A*0201 SLYNTVATL tetramer and mAbs to CD3, CD8, and anti–Tim-3. Percentages of viable tetramer+ CD8+ T cells were determined by flow cytometry. Each condition was tested in independent triplicate. Shown are mean percentages of tetramer+ CD8+ T cells on day 6 of stimulation. Error bars represent SE. (b and c) PBMCs from HIV-1–infected patients were stained with CFSE, and the effect of 2 μg/ml sTim-3 on cytokine production and proliferation of PBMCs in response to antigen was determined in these individuals over a 5-d stimulation assay. (b) Shown are representative data from one chronically HIV-1–infected individual on day 5 of culture, showing CFSE (x axis) by IFN-γ production (y axis) in CD8+ and CD4+ T cell populations in response to DMSO (top), pooled HIV-1–derived Gag/Nef peptides (middle) or CEF pooled peptides (bottom) in the presence or absence of either 2 μg/ml sTim-3 or an equal volume of expression control (see Materials and methods). CFSE becomes diluted in cells undergoing proliferation. Thus, cells in the two left quadrants of each plot have proliferated. (c) Shown are summary data for the effect of 2 μg/ml sTim-3 on proliferation in response to Gag from seven chronically HIV-1–infected individuals. P-values were determined by the Wilcoxon matched pairs test. (d) PBMC from an individual with chronic progressive HIV-1 infection were stained with CFSE and stimulated for 7 d with DMSO and pooled HIV-1–Gag peptides or with anti-CD3 and anti-CD28. For each stimulation, the effect of 10 μg/ml of anti–Tim-3 mAb 2E2 was compared with 10 μg/ml of mouse IgG1 isotype control. Shown are flow cytometry plots showing CD3 by CFSE, where diminution of CFSE is indicative of proliferated cells.
An additional observation from these experiments is that cells that had undergone proliferation in vitro exhibited high levels of Tim-3 expression (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20081398/DC1). Tim-3 up-regulation in response to anti-CD3/anti-CD28 was observed as early as 20 h after stimulation and progressively increased out to at least 120 h (unpublished data). This is consistent with Tim-3 acting as a negative immune regulator, where antigen-stimulated cells perform effector functions and then up-regulate Tim-3 as a means of terminating responses. In reconciling our ex vivo data showing a lack of cytokine production from Tim-3+ cells with published in vitro data demonstrating an association between IFN-γ production and high levels of Tim-3 expression, there is an important distinction to make. Cells expressing Tim-3 ex vivo have been subjected to chronic stimulation in vivo and are dysfunctional to further in vitro stimulation. In contrast, when Tim-3− cultured cells are stimulated in vitro they perform effector functions, such as producing IFN-γ, and then up-regulate Tim-3 to dampen these responses. Thus, depending on when one observes these cultures, high levels of Tim-3 and IFN-γ could be observed in association. This model predicts that in addition to restoring functions of exhausted HIV-1–specific T cells, in vitro treatment with sTim-3 should prolong effector function in response to other antigens. This is supported by examining the level of IFN-γ production at day 5 of in vitro stimulation with anti-CD3/CD28. Under these conditions, all cells that have undergone division express high levels of Tim-3 (unpublished data). In the presence of sTim-3, these cells consistently express higher levels of IFN-γ than in the presence of a control (Fig. S7, available at http://www.jem.org/cgi/content/full/jem.20081398/DC1).
The Tim-3–expressing T cell population is distinct from the PD-1–expressing population.
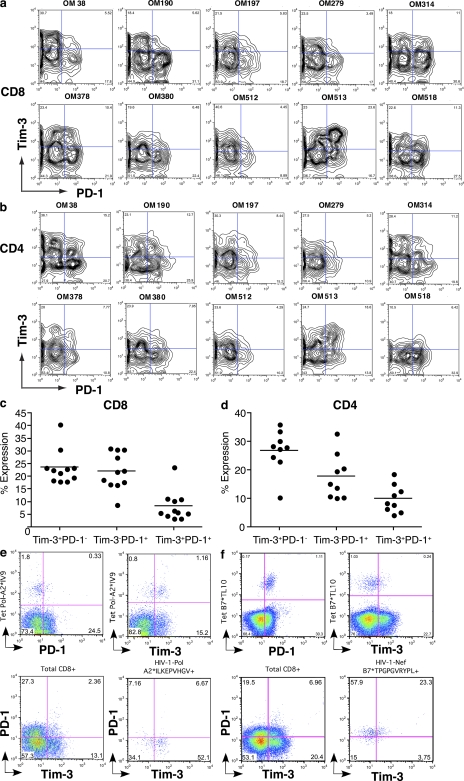
Because PD-1 has been identified as a marker of exhausted T cells in HIV-1 infection, we determined whether Tim-3 expression defines the same or a distinct population. PBMC from 10 individuals with chronic progressive HIV-1 infection were costained for Tim-3 and PD-1. Expression was analyzed by flow cytometry after gating on CD8+ or CD4+ T cells (Fig. 7). In 9/10 subjects, Tim-3 and PD-1 were primarily expressed by distinct populations of CD8+ T cells. One subject, OM513, displayed a frequent Tim-3+PD-1+ population (23.6%) but retained both Tim-3+PD-1− and Tim-3−PD-1+ populations (23 and 16.7%, respectively). Similarly, 9/10 subjects showed primarily divergent staining for PD-1 and Tim-3 on CD4+ T cells (Fig. 7, c and d). In HIV-1–specific CD8+ T cells, we observed two patterns of expression: tetramer+ populations were predominantly Tim-3+PD-1− (Fig. 7 e) or they were predominantly Tim-3− and PD-1+ (Fig. 7 f). In both patterns, a minority population coexpressed both Tim-3 and PD-1 (Fig. 7, e and f). Thus, Tim-3 and PD-1 expression define primarily distinct populations.
Figure 7.
Tim-3 and PD-1 are independent surface markers with divergent expression. (a–d) PBMCs were labeled with fluorochrome-conjugated mAbs to CD3, CD8, CD28, PD-1, and Tim-3. (a and b) Shown are flow cytometry plots from 10 chronically HIV-1–infected individuals (CIRC cohort), gated on either the CD3+CD8+ population (a) or the CD3+CD4+ population (b). (c and d) Shown is summary data of the flow cytometry plots displayed in a and b. (e and f) Shown are flow cytometry data analyzing coexpression of Tim-3 and PD-1 on HIV-1–specific CD8+ T cells in comparison with bulk CD8+ T cells in CD8+-enriched PBMC from individuals with chronic progressive HIV-1 infection. Solid lines show the mean. (e) HIV-1–Pol-ILKEPVHGV-specific CD8+ T cells were identified using HLA-A02 tetramers. (f) HIV-1–Nef-TGPGVRYPL-specific CD8+ T cells were identified using HLA-B07 tetramers.
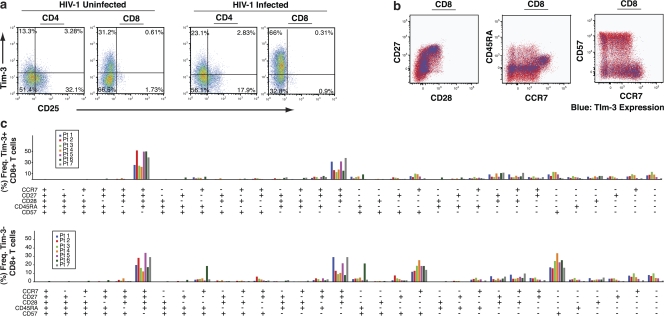
We performed dual staining for Tim-3 and CD25 on both CD4+ and CD8+ T cells (Fig. 8 a). We observed that Tim-3 and CD25 were primarily expressed by distinct populations of T cells. These data demonstrate that Tim-3 expression on CD4+ T cells does not mark a population of classical regulatory T cells. We then determined if the Tim-3hi population could be defined by other cell surface markers that have been used to define the maturation/differentiation status of T cells by costaining for CD57, CD45RA, CD27, CD28, and CCR7 (39–42). Tim-3–expressing CD8+ T cells from chronically HIV-1–infected individuals were distributed across a range of phenotypic profiles (Fig. 8, b and c).
Figure 8.
Tim-3–expressing CD8+ T cells are present in diverse phenotypic profiles. (a) PBMCs from HIV-1–uninfected (n = 3) and chronically HIV-1–infected (n = 3) individuals were labeled with fluorochrome-conjugated mAbs to CD3, CD8, CD4, CD25, and Tim-3. Shown are flow cytometry plots gated on the CD3+CD4+ population or the CD3+CD8+ population from two representative individuals. (b) PBMCs from a chronic progressor were labeled with fluorochrome-conjugated mAbs against Tim-3, CD3, CD8, CD28, CD27, CD45RA, CCR7, and CD57, as well as with a dead cell–discriminating marker. Gating was first performed to include only the viable CD3+CD8+ population in subsequent analyses. Shown are phenotypic representations of the Tim-3hi population (blue) versus Tim-3lo population (red). (c) Summary data showing phenotypic profiling for seven chronically HIV-1–infected individuals. Gating for maturation/differentiation markers was determined based on fluorescence minus one controls, and results were analyzed using SPICE software. Shown are the frequencies of populations with the corresponding combination of phenotypic markers, with each individual represented by a single bar.
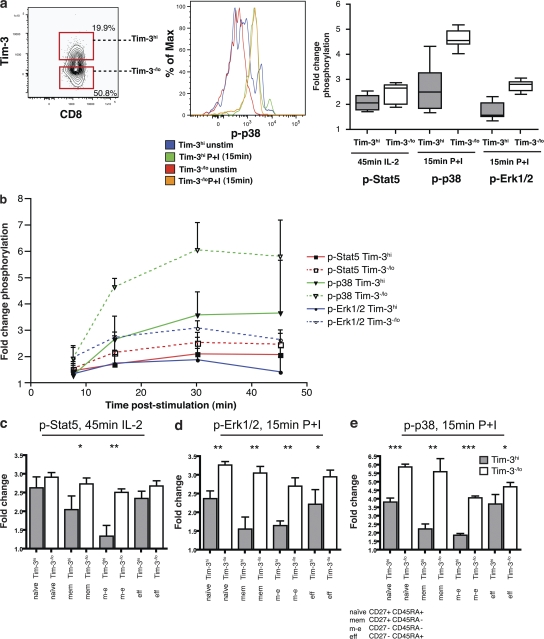
Tim-3+ T cells exhibit impaired Stat5, Erk1/2, and p38 signaling
We assessed the kinetics of STAT5, Erk1/2, and p38 phosphorylation (pSTAT5, pErk1/2, and p38, respectively) after stimulation in Tim-3hi versus Tim-3−/lo CD8+ T cells in three HIV-1–infected individuals (43). Tim-3hi CD8+ T cells had higher levels of basal phosphorylation of STAT5, p38, and ERK1/2 compared with Tim-3−/lo CD8+ T cells, and they exhibited lower fold changes in the phosphorylation of these molecules when stimulated in vitro with IL-2 for the STAT5 pathway and with PMA/Ionomycin (P+I) for p38 and ERK1/2 (MAP kinase pathway; Fig. 9, a and b). This impaired signaling response was seen in every stage of differentiation of Tim-3–expressing cells (Fig. 9, c–e). Thus, Tim-3–expressing CD8+ T cells exhibit a blunted change in phosphorylation of preactivated signaling proteins. This is consistent with the model recently proposed by Schweneker et al. (43), in which HIV-1 infection induces chronic activation of T cells, resulting in enhanced basal phosphorylation and perturbed signaling in response to restimulation. The intracellular domain of Tim-3 contains five conserved tyrosine residues but does not contain sequences corresponding to the ITIM consensus, and its downstream signaling targets remain unknown.
Figure 9.
Tim-3–expressing cells exhibit impaired Stat-5, p35, and Erk1/2 signaling in response to stimuli. Phosphorylation status of Stat5, p38, and Erk1/2 were analyzed by flow cytometry in Tim-3hi versus Tim-3−/lo CD8+ T cells from three HIV-1–infected subjects. Whole PBMCs were surface stained on ice, stimulated with either rIL-2 for 45 min or P+I for 15 min, and phosphorylation of Stat5 or Erk1/2 and p38, respectively, was analyzed with phosphospecific antibodies in CD3+CD8+ T cells and based on their Tim-3 expression. (a) Shown is a representative flow cytometry gating of Tim-3hi and Tim-3−/lo CD8+ PBMCs evaluating the fold change in p38 phosphorylation after 15 min of stimulation with P+I and summary of data from three chronically HIV-1–infected individuals, each analyzed in triplicates, with fold changes in phosphorylation of stimulated/unstimulated cells. (b) Shown is a representative time course depicting fold change in phosphorylation (stimulated/unstimulated cells) after 15, 30, and 45 min of stimulation. (c–e) Compiled data for Stat5 (c), Erk-1/2 (d), and p38 (e), showing differential levels of change in target phosphorylation (measured by change in mean fluorescence intensity) in Tim-3+ versus Tim-3− cells within each of the following CD3+CD8+ T cell subpopulations: naive (CD27+CD45RA+), memory (CD27+CD45RA−), effector memory (CD27−CD45RA−), or effector (CD27−CD45RA+). Statistical analyses were performed using a nonparametric two-tailed Mann Whitney U test. (*, P ≤ 0.05; **, P < 0.01; ***, P < 0.001) using Prism GraphPad. Error bars represent SE.
DISCUSSION
Together, these data support that Tim-3 acts to suppress effector functions of activated T cells in chronic uncontrolled viral infection with HIV-1. This complements and integrates previous studies that have identified an important role for Tim-3 in immunoregulation and have implicated defective Tim-3 signaling in the pathogenesis of multiple sclerosis and other autoimmune diseases (31, 44, 45). We show that in HIV-1 infection, the proportion of CD8+ and CD4+ T cells in peripheral blood that express Tim-3 can reach in excess of 70 and 30%, respectively (in contrast to means of 28.5 and 17.6% in HIV-1–uninfected individuals). As these frequencies exceed the proportion of HIV-1–specific cells in the periphery, suppression of T cell function by Tim-3 likely contributes not only to the loss-of-functional virus-specific responses but also to the impairment of responses to other antigens. This is supported by our observation that a subset of CMV and EBV-specific CD8+ T cells in chronic HIV-1–infected individuals expresses high levels of Tim-3 and is consistent with observations that HIV-1–infected individuals have reduced responses to recall antigens and vaccinations (46). The factors leading to this generalized expression of Tim-3 are unknown. Our data does, however, show a disproportionately high level of Tim-3 expression on HIV-1–specific CD8+ T cells, which is consistent with the preferential dysfunction of HIV-1–specific CD8+ T cells in chronic HIV-1 infection. We speculate that the heterogeneity that we observed in levels of Tim-3 expression on HIV-1–specific CD8+ T cell responses may reflect the relative functionality of that response, with greater frequencies of Tim-3–expressing antigen-specific cells associated with more advanced exhaustion and dysfunction. It will also be important to determine whether the fixation of escape mutations results in diminished Tim-3 expression on epitope-specific T cells and improvement in functionality, as has been described for PD-1 (47). These questions will be addressed by future studies.
We observed that the initiation of HAART in chronic progressive HIV-1 infection frequently resulted in a decline in Tim-3 expression (four out of seven individuals). However, we observed that a subset of chronically HIV-1–infected individuals on HAART therapy (three out of seven) retained high levels of Tim-3 expression despite suppression of HIV-1 viral load to undetectable levels. Maintenance of Tim-3 expression in the context of HAART was associated with sustained high levels of T cell activation (CD38 expression). Persistence of CD38 expression on T cells during HAART is predictive of disease (32, 48–50). In cases where HAART has failed to result in a reduction in CD38 expression, it has been demonstrated that intensification of HAART by eradicating persistent low-level replication can have a positive impact on immunological parameters, including diminishment of CD38 expression (51).
Our demonstration that blockade of the Tim-3 pathway can enhance HIV-1–specific T cell responses ex vivo clearly demonstrates that the Tim-3 pathway plays a critical role in suppressing the overall T cell response to HIV-1. It should be noted, however, that although T cell exhaustion is associated with increased viral replication, it is unclear whether this phenotype leads to loss of viral control in vivo or whether this loss results primarily from other factors, such as viral escape from CTL epitopes or the persistence of viral replication in sanctuaries inaccessible to CTL. The identification of Tim-3 as a novel mechanism of T cell exhaustion constitutes an important prerequisite for designing studies aimed at delineating the relative contributions of T cell exhaustion versus other factors in the overall inability of the cellular immune response to maintain control of HIV-1 replication.
An important implication of the present study is the possibility that pharmacological agents that block Tim-3 signaling may be of benefit in HIV-1 infection and potentially in other chronic viral diseases. However, it is unclear whether the high level of Tim-3 expressed in HIV-1 infection is the result of a pathological mechanism on the part of the virus to incapacitate the host immune system or if it is a physiological response to chronic immune activation necessary to hold immunopathology in check. With regard to the latter, recent data supporting that a dysregulation of the Tim-3 pathway may contribute to the pathology of multiple sclerosis highlights the importance of Tim-3 in regulating potentially harmful immune responses (30, 31). This situation is analogous to the considerations required in pursuing PD-1 as a therapeutic target. An important distinction of Tim-3 as a therapeutic target is its unique association with T cells that are impaired not only in their survival and proliferative potential but also in their ability to produce cytokine. Thus, blockade of the Tim-3 pathway carries the novel potential to enhance not only the numbers of T cells in HIV-1 infection but also to improve the functionality of both CD8+ and CD4+ T cells in HIV-1–infected individuals. Because a subset of subjects maintain high levels of Tim-3 expression despite seemingly effective HAART regimens, Tim-3 therapeutics may also play a role in reversing immune defects that persist with HAART.
The data presented in the present study clearly demonstrate that Tim-3 expression defines a distinct population of exhausted T cells from that of the recently identified PD-1–expressing population. This corroborates a recent study that reported that PD-1–expressing cells comprise only a subpopulation of dysfunctional HIV-1–specific CD8+ T cells in chronic progressors (52). The mechanisms leading to T cell exhaustion in the context of HIV-1 infection are clearly complex and cannot be attributed to a single pathway. It will be intriguing in future studies to explore the possibility of an additive or a synergistic effect of simultaneously blocking both the Tim-3 and PD-1 pathways. Such strategies may allow for a more comprehensive reversal of T cell exhaustion, potentially leading to potent combination therapies.
MATERIALS AND METHODS
Subjects.
Subjects were selected from participants in the CIRC Cohort, Toronto, Canada, and the OPTIONS Cohort, University of California San Francisco (UCSF). The CIRC cohort represented acutely/early HIV-1–infected subjects, HIV-1–infected chronic progressors, and HIV-1–infected viral controllers. Acute/early subjects were defined as individuals infected with HIV-1 within the last 4 mo. Chronic progressors were defined as individuals infected with HIV-1 for >1 yr with a CD4+ T cell count decline of >50 cells/mm3/year. Viral controllers were defined as individuals infected with HIV-1 for >1 yr, no evidence of CD4+ T cell count decline, and a viral load of <5,000 copies/ml bDNA. Clinical data for the cohort used in this study were the following: acute/early, absolute CD4+ T cell counts median = 542 and range = 180–1,240 cells/mm3, and viral loads median = 227,567 and range = 79,000 to >500,000 copies/ml; chronic progressors, absolute CD4+ T cell counts median = 250 and range = 132–660 cells/ml, and viral loads median = 50,000 and range = 290–500,000 copies/ml; and viral controllers, absolute CD4+ T cell counts median = 936 and range = 600–1,440 cells/mm3, and viral loads median = 100 and range = 50–250 copies/mm3. The subject with a viral load of 290 copies/ml, defined as a chronic progressor, was included in this patient group based on a CD4+ T cell count that had declined to 200 cells/mm3. The next lowest viral load in the chronic progressor group was 11,608 copies/ml. The chronic progressor with a relatively healthy absolute CD4+ T cell count of 660 cells/mm3 had a viral load of 51,250 copies/ml and exhibited CD4+ T cell count decline. The relatively high CD4+ T cell count in this individual was likely because of their relatively recent infection (13 mo). Controls were obtained from HIV-1–uninfected patients in the same demographic area with a similar age and sex profile and were processed in an identical manner. For the OPTIONS Cohort, baseline samples from all recruited subjects were evaluated to establish their HIV-1 infection status. Screened subjects must meet one of three criteria to be defined as having acute/early HIV-1 infection: (1) HIV-1 RNA >5,000 copies/ml with a negative or indeterminate HIV-1 antibody test; (2) a documented negative HIV-1 antibody test within 6 mo with current seroconversion; or (3) a history compatible with acute/early HIV-1 infection with laboratory confirmation based on a nonreactive less sensitive antibody test. All subjects discuss the advantages and disadvantages of early antiretroviral therapy with study staff and arrangements are made for therapy for those who elect to initiate treatment. Slightly over half of participants decline therapy. A total of 60 individuals with acute/early HIV-1 infection from the OPTIONS cohort were examined in this study. A median CD4+ T cell count of 544 (interquartile range 429.5, 721) cells/mm3 and median HIV-1 viral load of 4.7 (interquartile range 3.66, 5.2) log10 copies/ml. Controls were obtained from HIV-1–uninfected patients from both the Stanford Blood Bank and uninfected individuals from the cohort demographics. Additional subjects on HAART were recruited from these cohorts. This study was approved by the University of Toronto Institutional Review Board and by the UCSF Committee on Human Research, and subjects gave written informed consent. Studies were performed on cryopreserved PBMCs immediately after thawing. At the initiation of this study, a comparison between fresh and frozen PBMCs was performed, and it was found that Tim-3 levels remained proportional after freezing/thawing.
Peptides and stimulation reagents.
Overlapping HIV-1 Clade B Gag and Nef pooled peptides were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. CEF pooled peptides (AnaSpec), SEB (Sigma-Aldrich), and purified anti-CD3 and anti-CD28 monoclonal antibodies (BD) were used as additional reagents. Stimulations were performed with final concentrations of 10 μg/ml peptide.
Multicolor cytokine flow cytometry.
PBMCs from healthy HIV-1–uninfected and HIV-1–infected individuals were stained with fluorophore-conjugated monoclonal antibodies to CD4, CD8, CD57, CCR7, CD27, CD45RA, CD25, Ki67 (BD), CD28, PD-1 (BioLegend), CD3 (Beckman Coulter), and TIM-3 (R&D Systems) to determine phenotype assessment. An Aqua amine dye (Invitrogen) was used as a discriminating marker for live and dead cells. In some experiments, cells were stimulated after thawing with an HIV-1–Gag and –Nef peptide pool, a CEF peptide pool, or SEB followed by a fixation and permeabilization step. Intracellular staining for cytokines was performed using anti–TNF-α and IFN-γ (BD). Cells were fixed in PBS + 2% paraformaldehyde. Cells were acquired with a modified FACSAria, modified LSRII system, or FACSCalibur (BD). A total of >100,000 events were collected and analyzed with FlowJo software (Tree Star, Inc.). SPICE software (version 3.0; M. Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health) was used to assist in the organization and presentation of multicolor flow data.
Pentamer/tetramer analyses.
All pentamers were obtained from ProImmune Ltd and all tetramers were obtained from Beckman Coulter. Pentamers were used for the experiment displayed in Fig. 2, whereas tetramers were used for the experiment summarized in Fig. 7. Cryopreserved PBMC samples from chronically HIV-1–infected individuals were thawed and washed with 2 × 10 ml of 1% FBS PBS with 2 mM EDTA. Staining was performed immediately after thawing with fluorophore-conjugated antibodies against CD8 (BD), Tim-3 (R&D Systems), CD3 (BD), and the indicated pentamers (unlabeled), followed by a secondary staining step with APC-labeled pentamer fluorotags. Cells were washed two times with 1% FBS PBS and then fixed in 2% paraformaldehyde. Analysis was performed using a FACSCalibur instrument (BD).
Synthesis of recombinant Tim-3.
The expression vector pPA-TEV was previously derived from pIRESpuro3 (Clontech Laboratories, Inc.) and modified to incorporate the transin leader sequence and N-terminal protein A tag. The Tim-3 insert was obtained from PCR using the following primers : Tim-3 external forward, 5′-TTCGGCCGGCCCTCAGAAGTGGAATACAGAGCGG-3′; and Tim-3 external reverse, 5′-TGAGCGGCCGCTCATCATCTGATGGTTGCTCCAGAGTC-3′. For each primer, the underlined bases represent the template annealing sequence. Additional 5′ sequences comprise restriction sites and stop codons. The region amplified by these primers constitutes only the IgV and mucin domains of Tim-3. The resultant Tim-3 amplicon was cloned into the FseI–NotI cloning site of pPA-TEV. 10 μg of circular DNA plasmid was then transfected into HEK293T cells using the calcium phosphate method (Invitrogen). Expression of Tim-3 was confirmed by Western blot using a 1/5,000 dilution of a polyclonal anti–Tim-3 antibody (R&D Systems) and a 1/5,000 dilution of horseradish peroxidase–conjugated streptavidin (Thermo Fisher Scientific). Transfection was then repeated with linearized pPA–TEV–Tim-3 plasmid to generate stable cell lines. A parallel transfection was performed with empty linearized pPA-TEV. 3 d after transfection, puromycin drug selection was initiated by replacing the media with fresh media supplemented with 1–5 μg/ml puromycin. The media was exchanged with fresh puromycin-containing media every 2 d. 10 d later, six colonies from the pPA–TEV–Tim-3 transfection and six from the pPA-TEV transfection were isolated and expanded into 6-well tissue culture plates. Secreted proteins were detected by Western blot analysis using an anti–Tim-3 antibody for pPA–TEV–Tim-3 and an anti–protein-A antibody for pPA-TEV. A Tim-3–secreting clone (pPA–TEV–Tim-3 transfected) and a control protein A–secreting clone (pPA-TEV transfected) were selected and grown up in 2 liters each of CHO-SFM-II media supplemented with 2% FBS, penicillin, streptomycin, Hepes, l-glutamine, and 1 μg/liter apoprotinin (Sigma-Aldrich) in six T175 tissue culture flasks. Cells were plated at 50% confluency, and protein secretion was allowed to continue for 5 d. Supernatants were concentrated from 2 liters to 10 ml using Centricon Plus-70 centrifugal filter units (Millipore). Proteins were purified using IgG Sepharose 6 Fast Flow beads (GE Healthcare) as per the manufacturer's instructions. 200 μl of 0.33 mg/ml His-tagged TEV protease was then added to the beads, and cleavage was allowed to proceed overnight at 4°C. Supernatants were removed from beads, the beads were washed three times with 1 ml Tris-saline Tween, pH 7.2, and supernatants were pooled with wash eluates. This combined eluate was passed through a 1-ml nickel column (B-PER 6× His fusion protein purification kit; Thermo Fisher Scientific) to remove TEV protease and washed with 3 × 2 ml of wash buffer 2 from the same kit. The eluates were subsequently passed through Detoxi-Gel endotoxin removal columns (Thermo FIsher Scientific), according to the manufacturer's instructions, and then concentrated to 0.5 ml using Centricon plus-20 centrifugal filter units (Millipore). Volumes were then adjusted to 15 ml using sterile PBS and reconcentrated to 0.5 ml. The purity and identity of products were confirmed by SDS-PAGE and Western blot analysis. Protein concentration was determined by a Bradford assay. As expected, only small amounts of residual protein were detectable in the protein A control purification. This sample serves as a control for any effect of contaminant proteins or reagents from the purification process on proliferation or cytokine production.
Proliferation assay.
To track cell division, PBMCs from chronically HIV-1–infected individuals were labeled with 1 mM of the fluorescent intracellular dye CFSE (5-[and -6] carboxyfluorescein diacetate succinimidyl ester; Invitrogen) in PBS and mixed periodically for 10 min at room temperature. Labeling was quenched by addition of an equal volume of complete media (15% FBS in RPMI) for 2 min. The labeled cells were then washed twice, counted, and resuspended in cell culture media. CFSE-labeled cells were stimulated for 5–6 d with either DMSO alone, SLYNTVATL peptide, pooled HIV-1–derived Gag and Nef peptides, or CEF pooled peptides in the presence or absence of sTim-3, an equal volume of expression control, a monoclonal Tim-3–blocking antibody 2E2 (provided by V. Kuchroo, Center for Neurologic Diseases, Brigham and Women's Hospital, Boston, MA 02115), or an IgG1 isotype control. At the end of the culture period, cells were washed and incubated with a combination of the following conjugated anti–human monoclonal antibodies: CD4, CD8 (BD), and Tim-3 (R&D Systems). Intracellular staining for IFN-γ, IL-2 (BD), and CD3 (Beckman Coulter) was performed after cells were fixed and permeabilized. Cells were then washed in PBC with 2 mM EDTA and 1% BSA and fixed in 1% paraformaldehyde before being run on an LSRII flow cytometer (BD). Data were analyzed by using FlowJo Software (version 6.4; Tree Star, Inc.).
Signaling analyses.
Before analyses of cellular signaling, archived PBMCs that had been viably frozen were thawed in 15 ml RPMI cell culture medium (Mediatech, Inc.) containing 5% FBS (RPMI+; Thermo Fisher Scientific), washed in PBS containing 2% FBS (PBS+), and then rested at 5 × 106 cells/ml in RPMI+ at 37°C in 5% CO2 overnight. The next day, cells were washed with ice-cold PBS+, transferred to a 96-well V-bottom plate, and stained for cell surface markers with fluorophore-conjugated monoclonal antibodies against CD3, CD8, CD27, CD45RA, and Tim-3 on ice for 40 min. An amine-reactive dye (Invitrogen) was used to stain dead cells. After washing, cells were transferred to PBS containing IL-2 (final concentration, 100 ng/ml; Sigma-Aldrich) or P+I (final concentration, 100 ng/ml and 1 μg/ml, respectively; Sigma-Aldrich) at 37°C to induce signaling. Signaling was arrested after 15, 30, and 45 min by immediate fixation, adding 4% paraformaldehyde (final concentration, 2%). After 20-min fixation and subsequent washing, cells were permeabilized in 70% ice-cold methanol for 20 min on ice. Cells were washed and stained with an antibody cocktail containing the phosphospecific antibodies p-Erk1/2(pT202/pY204), p-p38(pT180/pY182), and p-Stat5(pY694) (BD) for 60 min on ice. Before analysis, cells were washed and resuspended in PBS+ with 0.05% formaldehyde. The unstimulated control cells underwent the same manipulations. Cells were analyzed on a customized LSR II Flow Cytometer (BD). Analysis of data was performed using FlowJo. Fold changes in phosphorylation were calculated as the ratio of MFI of stimulated cells over unstimulated cells.
Quantitative PCR.
Primer sequences were used as follows: TBP forward, GGGCATTATTTGTGCACTGAGA, and TBP reverse, TAGCAGCACGGTATGAGCAACT; GATA-3 forward, TGCATGACTCACTGGAGGAC, and GATA-3 reverse, TCAGGGAGGACATGTGTCTG; T-bet forward, GAGGCTGAGTTTCGAGCAGT, and T-bet reverse, CTGGCCTCGGTAGTAGGACA; and IFN-γ forward, TCCAAGTGATGGCTGAACTG, and IFN-γ reverse, CTTCGACCTCGAAACAGCAT. Manufacturer's protocols were followed where applicable, unless otherwise noted. RNA was isolated from samples with Trizol (Invitrogen), resuspended in 44 μl diethylpyrocarbonate water, and treated with DNase using DNA-Free (Applied Biosystems). RNA concentrations were determined by spectrophotometry and matched to the sample with the lowest concentration by dilution with diethylpyrocarbonate-treated water. 4 μl RNA were used for each Superscript III First-Strand Synthesis SuperMix (Invitrogen) RT reaction with 1 μl of 50 μM oligodT20. Parallel reactions lacking the RT enzyme were performed and consistently displayed no amplification in subsequent steps. Real-time PCRs were performed using the Prism 7900HT (Applied Biosystems) in 384-microwell plates. All samples, including the external standards, nontemplate control, and RT controls, were run in triplicate. Each 10-μl reaction contained PCR buffer (Invitrogen), 3 mM MgCl2, 0.2 mM dNTP (Applied Biosystems), 1 nM each of forward and reverse primers (Invitrogen), a 1/50 dilution of ROX reference dye (Sigma-Aldrich), a 3/100,000 dilution of SYBR Green I (Sigma-Aldrich), 0.05 U of Platinum Taq polymerase (Invitrogen), and template DNA. Template was a sevenfold serial dilution of genomic DNA for generation of standard curves, 5 μl of complementary DNA synthesis reactions, or 5 μl of matching RT control. Reaction conditions were 95°C for 3 min, followed by 36 cycles of 95°C for 15 s, 64°C for 15 s, and 72°C for 20 s. A final dissociation stage was run to generate a melting curve for verification of amplification product specificity. Real-time PCR was monitored and analyzed by the Sequence Detection System (version 2.0; Applied Biosystems).
Statistical analyses.
We used mixed effects longitudinal analyses to determine if CD8+ T cell activation levels independently associated with Tim-3 on CD8+ T cells during antiretroviral therapy. We specified a random effect for time and the individual. The models were run in the SAS System 9.2 under Proc Mixed. Other statistical tests used are identified in corresponding figure legends.
Online supplemental material.
Fig. S1 shows coexpression profiles of Tim-3 and CD38 in an HIV-1–uninfected individual, an HIV-1–infected chronic progressor with a moderate viral load, and an HIV-1–infected chronic progressor with advanced disease and a high viral load. Fig. S2 shows comparisons of alternative flow cytometry methodologies, including mAb versus polyclonal antibody Tim-3 staining, percentage of CD38 versus CD38 MFI, and percentage of Tim-3 versus Tim-3 MFI. Fig. S3 shows individual subject profiles for the effect of HAART on Tim-3 expression levels (as summarized in Fig. 3 a). Fig. S4 shows the relationship between Tim-3 expression and cytokine (TNF-α and IFN-γ) production in HIV-1–infected and uninfected subjects. Fig. S5 shows the relationship between Tim-3 expression and degranulation (CD107a staining) in HIV-1–infected and –uninfected subjects. Fig. S6 shows Tim-3 expression by CFSE diminution after 5 d of in vitro stimulation. Fig. S7 shows the effect of soluble on IFN-γ production after 6 d of in vitro stimulation with anti-CD28. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081398/DC1.
Acknowledgments
We thank Vijay Kuchroo for generously providing us with the monoclonal anti–Tim-3 antibody 2E2. We would like to thank Kelly S. Macdonald for the use of general laboratory equipment. GAG and NEF consensus peptides and CEF pooled peptides were obtained through the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health). We would like to thank Mario Roederer (Vaccine Research Center, National Institutes of Health) for use of the Spice software for data presentation. R.B. Jones, L.C. Ndhlovu, D.F. Nixon, M.A. Ostrowski, and J.M. Rini are named as inventors on a patent application based on this work, which was filed by their respective institutions.
This research was supported by funds from the Canadian Institutes for Health Research (CIHR), UCSF Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763), the UCSF AIDS Biology Program of the AIDS Research Institute (ARI), and the National Institutes of Health (AI60379, AI68498, AI64520, and AI066917). L.C. Ndhlovu was supported by the Irvington Institute/Dana Foundation Fellowship from the Cancer Research Institute. M.A. Ostrowski received salary support from the Ontario HIV Treatment Network (OHTN) and the CIHR. R.B. Jones receives a studentship from the OHTN. M.P. Sheth was supported by a grant from the University-wide AIDS Research Program (F05-GI-219). J.M. McCune was supported in part by National Institutes of Health grant U01 AI43641, by the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, and by the National Institutes of Health Director's Pioneer Award Program, which is part of the National Institutes of Health Roadmap for Medical Research (DPI OD00329). R. Kaul receives salary support from the Canada Research Chair Program and grant support from a CIHR Operating Grant (HOP-81735) and an OHTN Operating Grant. Biosafety level 3 laboratory space and equipment was provided by the Canadian Foundation for HIV Research (CANFAR) in partnership with the Canadian Foundation for Innovation and the Ontario Innovation Trust.
The authors state that they have no other conflicting financial interests.
Abbreviations used: bDNA, branched-chain DNA; CEF, CMV/EBV/Influenza; CIRC, Canadian Immunodeficiency Research Collaborative; HAART, highly active antiretroviral therapy; MFI, mean fluorescence intensity; mRNA, messenger RNA; P+I, PMA/Ionomycin; SEB, staphylococcus enterotoxin B; sTIM-3, soluble TIM-3.
R.B. Jones and L.C. Ndhlovu contributed equally to this paper.
References
- 1.Schmitz, J.E., M.J. Kuroda, S. Santra, V.G. Sasseville, M.A. Simon, M.A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B.J. Scallon, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 283:857–860. [DOI] [PubMed] [Google Scholar]
- 2.Metzner, K.J., X. Jin, F.V. Lee, A. Gettie, D.E. Bauer, M. Di Mascio, A.S. Perelson, P.A. Marx, D.D. Ho, L.G. Kostrikis, and R.I. Connor. 2000. Effects of in vivo CD8+ T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Exp. Med. 191:1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogg, G.S., X. Jin, S. Bonhoeffer, P.R. Dunbar, M.A. Nowak, S. Monard, J.P. Segal, Y. Cao, S.L. Rowland-Jones, V. Cerundolo, et al. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 279:2103–2106. [DOI] [PubMed] [Google Scholar]
- 4.Price, D.A., P.J. Goulder, P. Klenerman, A.K. Sewell, P.J. Easterbrook, M. Troop, C.R. Bangham, and R.E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA. 94:1890–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg, E.S., J.M. Billingsley, A.M. Caliendo, S.L. Boswell, P.E. Sax, S.A. Kalams, and B.D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 278:1447–1450. [DOI] [PubMed] [Google Scholar]
- 6.Shacklett, B.L., C.A. Cox, M.F. Quigley, C. Kreis, N.H. Stollman, M.A. Jacobson, J. Andersson, J.K. Sandberg, and D.F. Nixon. 2004. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J. Immunol. 173:641–648. [DOI] [PubMed] [Google Scholar]
- 7.Trimble, L.A., and J. Lieberman. 1998. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood. 91:585–594. [PubMed] [Google Scholar]
- 8.Appay, V., D.F. Nixon, S.M. Donahoe, G.M. Gillespie, T. Dong, A. King, G.S. Ogg, H.M. Spiegel, C. Conlon, C.A. Spina, et al. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson, J., H. Behbahani, J. Lieberman, E. Connick, A. Landay, B. Patterson, A. Sonnerborg, K. Lore, S. Uccini, and T.E. Fehniger. 1999. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS. 13:1295–1303. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley, J.M., N.J. Karandikar, M.R. Betts, D.R. Ambrozak, B.J. Hill, L.E. Crotty, J.P. Casazza, J. Kuruppu, S.A. Migueles, M. Connors, et al. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 101:2711–2720. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, D., P. Shankar, Z. Xu, B. Harnisch, G. Chen, C. Lange, S.J. Lee, H. Valdez, M.M. Lederman, and J. Lieberman. 2003. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 101:226–235. [DOI] [PubMed] [Google Scholar]
- 12.Day, C.L., D.E. Kaufmann, P. Kiepiela, J.A. Brown, E.S. Moodley, S. Reddy, E.W. Mackey, J.D. Miller, A.J. Leslie, C. DePierres, et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 443:350–354. [DOI] [PubMed] [Google Scholar]
- 13.Petrovas, C., J.P. Casazza, J.M. Brenchley, D.A. Price, E. Gostick, W.C. Adams, M.L. Precopio, T. Schacker, M. Roederer, D.C. Douek, and R.A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitcher, C.J., C. Quittner, D.M. Peterson, M. Connors, R.A. Koup, V.C. Maino, and L.J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518–525. [DOI] [PubMed] [Google Scholar]
- 15.Trautmann, L., L. Janbazian, N. Chomont, E.A. Said, S. Gimmig, B. Bessette, M.R. Boulassel, E. Delwart, H. Sepulveda, R.S. Balderas, et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198–1202. [DOI] [PubMed] [Google Scholar]
- 16.Hess, C., M. Altfeld, S.Y. Thomas, M.M. Addo, E.S. Rosenberg, T.M. Allen, R. Draenert, R.L. Eldrige, J. van Lunzen, H.J. Stellbrink, et al. 2004. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. Lancet. 363:863–866. [DOI] [PubMed] [Google Scholar]
- 17.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 96:3094–3101. [PubMed] [Google Scholar]
- 18.Kostense, S., K. Vandenberghe, J. Joling, D. Van Baarle, N. Nanlohy, E. Manting, and F. Miedema. 2002. Persistent numbers of tetramer+ CD8(+) T cells, but loss of interferon-gamma+ HIV-specific T cells during progression to AIDS. Blood. 99:2505–2511. [DOI] [PubMed] [Google Scholar]
- 19.Deeths, M.J., R.M. Kedl, and M.F. Mescher. 1999. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J. Immunol. 163:102–110. [PubMed] [Google Scholar]
- 20.Roos, M.T., R.A. van Lier, D. Hamann, G.J. Knol, I. Verhoofstad, D. van Baarle, F. Miedema, and P.T. Schellekens. 2000. Changes in the composition of circulating CD8+ T cell subsets during acute epstein-barr and human immunodeficiency virus infections in humans. J. Infect. Dis. 182:451–458. [DOI] [PubMed] [Google Scholar]
- 21.Gamadia, L.E., I.J. ten Berge, L.J. Picker, and R.A. van Lier. 2002. Skewed maturation of virus-specific CTLs? Nat. Immunol. 3:203. [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski, M.A., S.J. Justement, L. Ehler, S.B. Mizell, S. Lui, J. Mican, B.D. Walker, E.K. Thomas, R. Seder, and A.S. Fauci. 2000. The role of CD4+ T cell help and CD40 ligand in the in vitro expansion of HIV-1-specific memory cytotoxic CD8+ T cell responses. J. Immunol. 165:6133–6141. [DOI] [PubMed] [Google Scholar]
- 23.Barber, D.L., E.J. Wherry, D. Masopust, B. Zhu, J.P. Allison, A.H. Sharpe, G.J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. [DOI] [PubMed] [Google Scholar]
- 24.Freeman, G.J., E.J. Wherry, R. Ahmed, and A.H. Sharpe. 2006. Reinvigorating exhausted HIV-specific T cells via PD-1–PD-1 ligand blockade. J. Exp. Med. 203:2223–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrovas, C., D.A. Price, J. Mattapallil, D.R. Ambrozak, C. Geldmacher, V. Cecchinato, M. Vaccari, E. Tryniszewska, E. Gostick, M. Roederer, et al. 2007. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 110:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monney, L., C.A. Sabatos, J.L. Gaglia, A. Ryu, H. Waldner, T. Chernova, S. Manning, E.A. Greenfield, A.J. Coyle, R.A. Sobel, et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 415:536–541. [DOI] [PubMed] [Google Scholar]
- 27.Zhu, C., A.C. Anderson, A. Schubart, H. Xiong, J. Imitola, S.J. Khoury, X.X. Zheng, T.B. Strom, and V.K. Kuchroo. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6:1245–1252. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Fueyo, A., J. Tian, D. Picarella, C. Domenig, X.X. Zheng, C.A. Sabatos, N. Manlongat, O. Bender, T. Kamradt, V.K. Kuchroo, et al. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4:1093–1101. [DOI] [PubMed] [Google Scholar]
- 29.Sabatos, C.A., S. Chakravarti, E. Cha, A. Schubart, A. Sanchez-Fueyo, X.X. Zheng, A.J. Coyle, T.B. Strom, G.J. Freeman, and V.K. Kuchroo. 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4:1102–1110. [DOI] [PubMed] [Google Scholar]
- 30.Anderson, A.C., D.E. Anderson, L. Bregoli, W.D. Hastings, N. Kassam, C. Lei, R. Chandwaskar, J. Karman, E.W. Su, M. Hirashima, et al. 2007. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 318:1141–1143. [DOI] [PubMed] [Google Scholar]
- 31.Koguchi, K., D.E. Anderson, L. Yang, K.C. O'Connor, V.K. Kuchroo, and D.A. Hafler. 2006. Dysregulated T cell expression of TIM3 in multiple sclerosis. J. Exp. Med. 203:1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giorgi, J.V., Z. Liu, L.E. Hultin, W.G. Cumberland, K. Hennessey, and R. Detels. 1993. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J. Acquir. Immune Defic. Syndr. 6:904–912. [PubMed] [Google Scholar]
- 33.Gerdes, J., H. Lemke, H. Baisch, H.H. Wacker, U. Schwab, and H. Stein. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133:1710–1715. [PubMed] [Google Scholar]
- 34.Combadiere, B., C. Blanc, T. Li, G. Carcelain, C. Delaugerre, V. Calvez, R. Tubiana, P. Debre, C. Katlama, and B. Autran. 2000. CD4+Ki67+ lymphocytes in HIV-infected patients are effector T cells accumulated in the G1 phase of the cell cycle. Eur. J. Immunol. 30:3598–3603. [DOI] [PubMed] [Google Scholar]
- 35.van Oijen, M.G., R.H. Medema, P.J. Slootweg, and G. Rijksen. 1998. Positivity of the proliferation marker Ki-67 in noncycling cells. Am. J. Clin. Pathol. 110:24–31. [DOI] [PubMed] [Google Scholar]
- 36.Grossman, Z., and W.E. Paul. 2000. The impact of HIV on naive T-cell homeostasis. Nat. Med. 6:976–977. [DOI] [PubMed] [Google Scholar]
- 37.Grossman, Z., M.B. Feinberg, and W.E. Paul. 1998. Multiple modes of cellular activation and virus transmission in HIV infection: a role for chronically and latently infected cells in sustaining viral replication. Proc. Natl. Acad. Sci. USA. 95:6314–6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachsenberg, N., A.S. Perelson, S. Yerly, G.A. Schockmel, D. Leduc, B. Hirschel, and L. Perrin. 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J. Exp. Med. 187:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emu, B., E. Sinclair, D. Favre, W.J. Moretto, P. Hsue, R. Hoh, J.N. Martin, D.F. Nixon, J.M. McCune, and S.G. Deeks. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 79:14169–14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Appay, V., P.R. Dunbar, M. Callan, P. Klenerman, G.M. Gillespie, L. Papagno, G.S. Ogg, A. King, F. Lechner, C.A. Spina, et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379–385. [DOI] [PubMed] [Google Scholar]
- 41.Champagne, P., G.S. Ogg, A.S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G.P. Rizzardi, S. Fleury, M. Lipp, et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 410:106–111. [DOI] [PubMed] [Google Scholar]
- 42.Garrison, K.E., R.B. Jones, D.A. Meiklejohn, N. Anwar, L.C. Ndhlovu, J.M. Chapman, A.L. Erickson, A. Agrawal, G. Spotts, F.M. Hecht, et al. 2007. T cell responses to human endogenous retroviruses in HIV-1 infection. PLoS Pathog. 3:e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schweneker, M., D. Favre, J.N. Martin, S.G. Deeks, and J.M. McCune. 2008. HIV-induced changes in T cell signaling pathways. J. Immunol. 180:6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, L., D.E. Anderson, J. Kuchroo, and D.A. Hafler. 2008. Lack of TIM-3 immunoregulation in multiple sclerosis. J. Immunol. 180:4409–4414. [DOI] [PubMed] [Google Scholar]
- 45.Anderson, A.C., and D.E. Anderson. 2006. TIM-3 in autoimmunity. Curr. Opin. Immunol. 18:665–669. [DOI] [PubMed] [Google Scholar]
- 46.Lane, H.C., J.M. Depper, W.C. Greene, G. Whalen, T.A. Waldmann, and A.S. Fauci. 1985. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N. Engl. J. Med. 313:79–84. [DOI] [PubMed] [Google Scholar]
- 47.Streeck, H., Z.L. Brumme, M. Anastario, K.W. Cohen, J.S. Jolin, A. Meier, C.J. Brumme, E.S. Rosenberg, G. Alter, T.M. Allen, et al. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu, Z., W.G. Cumberland, L.E. Hultin, H.E. Prince, R. Detels, and J.V. Giorgi. 1997. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:83–92. [DOI] [PubMed] [Google Scholar]
- 49.Hunt, P.W., J.N. Martin, E. Sinclair, B. Bredt, E. Hagos, H. Lampiris, and S.G. Deeks. 2003. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187:1534–1543. [DOI] [PubMed] [Google Scholar]
- 50.Hunt, P.W., J. Brenchley, E. Sinclair, J.M. McCune, M. Roland, K. Page-Shafer, P. Hsue, B. Emu, M. Krone, H. Lampiris, et al. 2008. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolber, M.A., M.O. Saenz, T.J. Tanner, K.L. Arheart, S. Pahwa, and H. Liu. 2008. Intensification of a suppressive HAART regimen increases CD4 counts and decreases CD8+ T-cell activation. Clin. Immunol. 126:315–321. [DOI] [PubMed] [Google Scholar]
- 52.Wang, C., T. Wen, J.P. Routy, N.F. Bernard, R.P. Sekaly, and T.H. Watts. 2007. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J. Immunol. 179:8252–8263. [DOI] [PubMed] [Google Scholar]