Brain Circuits Regulating Energy Homeostasis (original) (raw)
. Author manuscript; available in PMC: 2009 Aug 7.
Introduction
The past twenty years have witnessed tremendous advances in the understanding of the central mechanisms regulating food intake and energy balance, perhaps in response to the accelerated increase in the incidence of obesity in industrialized nations. Some of the most striking discoveries have included descriptions of hypothalamic neuropeptidergic circuits that respond to changes in peripheral metabolic signals, and that regulate metabolism through their multiple output pathways. In addition, the sophistication of research tools afforded by genetically engineered animals has provided a degree of certainty to the data that is unparalleled. Finally, much insight has been gained of the potential mechanisms that underlie the dynamic functioning of hypothalamic circuits. This chapter will attempt to provide a synopsis of these advances, leading to the idea that synaptic plasticity as an important factor in the regulation of food intake and energy homeostasis.
Hypothalamic Homeostatic Circuits
It is now well established that the hypothalamus plays a critical role in the regulation of energy balance. This was first suspected after descriptions of obesity in patients with hypothalamic tumors over a hundred years ago [1], but at the time, it was thought that the pituitary gland regulated most endocrine functions and that alterations of the pituitary lead to metabolic disorders [1]. Confirmation of the hypothalamus as important for regulation of food intake and energy balance was obtained from animal studies using brain lesions of hypothalamic structures [2–4]. In essence, evidence obtained from both the clinical descriptions in tumor patients, and from the lesion work, showed that gross damage to mediobasal hypothalamic areas, in particular the ventromedial hypothalamic nucleus (VMH), was clearly associated with increased food intake, morbid obesity and insulin resistance, while damage to more lateral hypothalamic structures was associated with anorexia and adipsia [5]. In turn, electrical stimulation of the VMH resulted in decreased feeding, whereas stimulation of the lateral hypothalamic region increased appetite [6–8]. As a whole, these data suggested that the mediobasal hypothalamus was a satiety center, and that the lateral hypothalamus was an orexigenic center [9, 10].
This dual center hypothesis dominated the field for several decades until a number of studies began to trickle data showing that neither the VMH and adjacent structures were solely satiety centers, nor was the lateral hypothalamus uniquely involved in appetite [11, 8]. For example, it was found that knife cuts that separated the ventral from the lateral hypothalamus without damage to the VMH were sufficient to cause hypothalamic obesity [12]. Similarly, vagotomy appeared to ameliorate obesity caused by VMH destruction [13, 14]. Finally, destruction of dopaminergic fibers of the medial forebrain bundle (mfb), which course through the lateral hypothalamus, resulted in animals that showed similar anorexic and adipsic symptoms as animals with lesions to the lateral hypothalamus [15]. Indeed, it seemed that disconnections of pathways coursing through these regions were as effective in inducing obesity or anorexia as the lesions themselves. For many years, the study of ingestive behavior and obesity focused on exploring the relative contribution of different neurotransmitter systems on the regulation of energy balance.
While a tremendous amount of data was obtained during this time, the discovery of neuropeptide Y (NPY) and leptin can be regarded as the most important discoveries in the past 25 years. First, NPY, a 36-amino acid peptide homologue of the pancreatic polypeptide family [16], was found to be produced within the brain primarily (although not uniquely) in the arcuate nucleus (ARC), a hypothalamic nucleus ventral to the VMH previously implicated in the regulation of body weight and energy balance [17]. When injected into the ventricles of rats or within other hypothalamic nuclei, NPY potently elicited food intake [17–21]. Moreover, NPY synthesis and content within the ARC was elevated in fasted and in genetically obese animals [22, 23]. NPY infusions also increased fat deposition and decreased brown fat thermogenesis and oxygen consumption, suggesting that NPY was not only an orexigenic peptide but also one important in the regulation of metabolism [24, 25].
A few years later, Dr. Jeff Friedman and his associates cloned the gene that produced leptin, a peptide hormone produced in adipocytes, and that was mutated in the ob/ob line of genetically obese mice [26]. Treatment with leptin reversed the phenotypic abnormalities seen in ob/ob mice and was also effective in reducing body weight and food intake while increasing energy expenditure in normal animals [27– 29]. A second line of genetically obese and diabetic mice known as the db/db, was soon after found to be the result of a deletion of the gene encoding the long form of the leptin receptor (ObRb) [30–32]. Finally, it was established that leptin targeted NPY neurons within the ARC to produce these dramatic changes in metabolism [33]. These groundbreaking discoveries laid the foundation of what could be termed as a renaissance in the study of neural control of obesity and energy balance. Reports of other peptides with either anorexic or orexigenic properties began to routinely appear in high impact journals, and continue to make headlines.
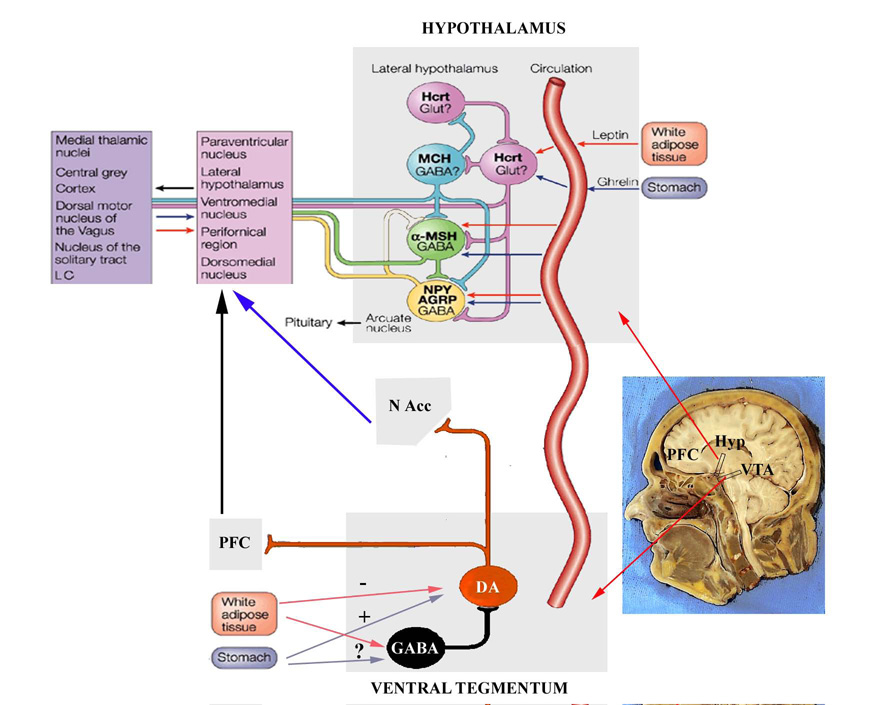
Because the ARC contains the largest concentration of cells that produce NPY and have the densest concentration of leptin sensitive neurons in the brain, it is generally accepted that this region is key to the regulation of energy balance (Fig. 1). This is supported by the fact that, in addition to NPY, the ARC also contains a second set of neurons that produce α?melanocyte stimulating hormone (α-MSH), an anorectic peptide formed from the cleavage of the proopiomelanocortin (POMC) protein [34]. This protein acts on melanocortin receptors types 3 and 4 (MC3/4, respectively) present in various hypothalamic nuclei to reduce food intake and energy expenditure in a manner similar to leptin [35]. Moreover the pharmacological blockade of MC3/4 receptors or the deletion of the gene encoding the MC4 receptor, result in obesity and leptin resistance in rodents and primates [36–38]. In addition, NPY neurons produce a second orexigenic peptide, the agouti related peptide (Agrp), an endogenous antagonist to the MC3/4 receptor [38]. This peptide, like NPY, increases food intake dramatically, but the increase in food intake produced by this peptide is long lasting, and effect that is still not well understood [39]. Similarly, POMC cells also synthesize a second anorexic peptide, the cocaine and amphetamine related transcript (CART) [40]. The relative contribution of CART versus α?MSH in the regulation of food intake and energy expenditure remains unexplained. What is known is that both NPY/Agrp and POMC/CART neurons within the ARC appear to primarily modulate food intake via their output targets (Fig. 1). Both POMC and NPY cells have a widespread projection field that has been implicated in a variety of physiological and behavioral events that include reproduction, water balance, body temperature and energy balance. The main output of both NPY/Agrp and POMC/CART cells appears to be the PVN where NPY, α-MSH and Agrp have strong effects on food intake and body temperature. These cells, however, target other hypothalamic nuclei like the VMH, dorsomedial hypothalamus (DMH), and LH among others to potentially modulate food intake end energy expenditure, and the relative contribution of these nuclei to produce the orexigenic or anorexic effects of these peptides continues to be investigated [41–43]. Finally, NPY/Agrp neurons the ARC appear to synapse onto neighboring POMC/CART cells to inhibit them using GABA as a neurotransmitter [44, 45].
Figure 1.
While the lateral hypothalamus had been previously described as a “hunger” center, it was not until recently that two orexigenic peptides, hypocretin/orexin and melanin concentrating hormone (MCH), were identified and localized within this area [46, 47]. Interestingly, both hypocretin/orexin and MCH increase food intake via different mechanisms. In the case of hypocretin/orexin neurons, their role in the regulation of food intake has been questioned given that their effects on food intake are short lived [48], and that ob/ob and db/db mice show lower levels of hypocretin/orexin mRNA and peptide content than their wild type littermates [49]. Nevertheless, mice with genetic deletion to the gene encoding the prepro-orexin peptide are hypophagic [50]. Hypocretin/orexin cells send projections to the ARC where they synapse onto NPY/Agrp cells, which in turn project back to hypocretin/orexin cells [51]. This particular circuit is thought to play an important role in hypocretin/orexininduced food intake [51–54]. Moreover, the presence of receptors for signals like leptin and ghrelin, as well as changes in electrophysiological activity of hypocretin/orexin neurons in response to these signals demonstrates that hypocretin/orexin cells can be directly modified by peripheral signals [55, 51]. Sakurai and his associates have determined that hypocretins/orexins play a crucial role in activating arousal circuits in response to energetic challenges resulting in food seeking behaviors and in food anticipatory behaviors [56, 57].
In contrast, the role of MCH hypothalamic neurons in the regulation of energy balance appears to be more straight forward. For example, ob/ob, db/db mice have high levels of MCH expression in the hypothalamus, and MCH transgenic mice are overweight and gain more weight under a high fat diet [58, 46]. In contrast, MCH or MCH receptor knockout mice are leaner, eat less and have increased metabolism than their wild type littermates [59, 60]. Interestingly, α-MSH/POMC cells inhibit the activity of MCH neurons, and thus prevent increases in food intake [61, 62]. Given the widespread distribution of both hypocretin/orexin and MCH projections [52], it has been suggested most aspects of food intake and energy regulation could be modulated by the interaction between these two cells groups at these target sites [63, 64], and given their close proximity and synaptic interconnections, perhaps by reciprocally modulating each other’s cellular activity [65–67].
The list of peripheral factors that, like leptin, target the ARC to modulate energy balance has also grown [68]. Metabolic signals such as glucose availability, insulin, cholecystokinin (CCK), pancreatic polypeptides (PP and PYY) and ghrelin have, among others, all been found to modulate NPY and POMC in the ARC to alter food intake and metabolism. Of these, ghrelin has received special attention given that, in contrast to the other peptides, ghrelin acts in NPY cells within the ARC to increase food intake, adiposity and the secretion of growth hormone [69–72]. Although ghrelin is produced primarily in the stomach [71, 73], a sub-set of ghrelin secreting neurons has been identified in the dorsal portion of the ARC and in the spaces that surround different hypothalamic nuclei implicated in the regulation of energy balance [55, 71]. The role of these neurons remains to be determined fully, but anatomically, it appears that these cells integrate metabolic and circadian outputs to regulate energy balance [55].
We are then left with a model where metabolic signals that monitor energetic state, signals like leptin, ghrelin, insulin and PYY, target the hypothalamus, and particularly the ARC to modulate the activity of NPY/Agrp and POMC neurons. The activation of these neurons by “satiety” signals leads to a reduction in NPY/Agrp and an increase in the release of α-MSH from POMC neurons. Consequently, α-MSH binds to MC3/4 receptors in MCH cells in the lateral hypothalamus to reduce food intake and with thyroid hormone and corticotropin releasing hormones (THS and CRH) in the PVN to increase energy expenditure. In contrast, hunger signals like a reduction in the glucose availability, or increased circulating ghrelin will lead to increases in ARC nucleus NPY release that inhibit POMC, THS and CRH and stimulate the secretion of hypocretin/orexin and MCH from the LH to ultimately increase food intake and reduce metabolic rate. The ARC appears to be, therefore, a brain nucleus orchestrating brain responses to changes in energy demands [34].
Tools for the Study of Feeding Circuits
In addition to improved lesion techniques and increased availability of agonists or antagonists that specifically target different neuropeptide receptors, the molecular biology and molecular genetics revolution have proven pivotal for the unveiling of feeding circuits. Molecular biological techniques have revealed that the ObRb leptin receptor belongs to the same family (gp130) of receptors associated with cytokines such as the interleukins [32]. Activation of this receptor by leptin can achieve gene transcription by at least three signaling cascades that include the activation of the JAK2/STAT3, the ERK/MAP kinase and the phosphoinositol 3 kinase (PI3K) pathways [74–76]. Much attention has been focused on the ability of leptin to activate STAT3 that, in turn, will act as a transcription factor for several genes that include the suppressor of cytokine signaling 3 (SOCS 3) gene, an intracellular protein that prevents further activation of the ObRb [77, 78]. The pivotal role of STAT3 as a transcription factor that mediates the effects of leptin on energy balance has been highlighted recently by the generation of mice with targeted deletions to different sites for STAT3 phosphorylation, rendering animals with deficient STAT 3 signaling. These mice are severely obese and insulin resistant, and show high expression of NPY and Agrp, and diminished expression of POMC in the ARC [79–81]. Several knock out mice lines have underlined the importance of the melanocortin system in the regulation of leptin’s effects and in energy balance in general. Thus, targeted deletions to the genes that encode α-MSH, MC4 receptor, and the specific deletion of the ObRb in POMC neurons also result in obese, hyperinsulinimic and leptin resistant mice [82, 83, 37]. Moreover, naturally occurring mutations of the Ob and α-MSH genes also produce the same symptoms in humans [84].
In contrast, deletions to the genes that encode NPY, ghrelin, or the active form of the ghrelin receptor (growth hormone secretagogue receptor 1a or GHS-R 1a) result in few phenotypic abnormalities [85–88]. Nevertheless, NPY /leptin double knockout animals show decreased food intake, body weight, and adiposity in comparison to the regular leptin (ob/ob) deficient mice [86], and ghrelin deficient animals appear to be slightly resistant to diet induced obesity [88]. Physiological responses of NPY, ghrelin and GHS-R deficient animals remain to be fully determined. In any event, there are a variety of mutations that lead to a lean phenotype (i.e. MCH KO mice), and some like in the dopamine deficient mice, become completely aphagic, needing dopamine replacement to continue eating [89, 90]. The relative contribution of these genes in the regulation of hypothalamic homeostatic circuits is a matter of continuous research efforts.
Finally, the development of reporter genes that can be used as tags has become a welcome addition to the study of hypothalamic circuits. For example, the gene that encodes the green fluorescent protein (GFP), a protein that is produced in a specific species of jellyfish, has been tagged onto the promoters of several of the peptides implicated in energy regulation. These gene “knock ins” have enabled the visualization of cells that synthesize neuropeptides such as NPY and POMC, or neurotransmitters like GABA that are difficult to visualize using immunocytochemical techniques. The use of mice with specific insertions of the GFP gene has proven invaluable to the study of anatomical and physiological properties of specific hypothalamic neuropeptides. For instance, Cowley and colleagues used mice with the GFP gene inserted in the POMC promoter to unveil the electrophysiological properties of POMC neurons in response to signals like leptin, NPY, ghrelin, and PYY [91, 92]. Friedman and colleagues have used mice with the GFP gene inserted in the NPY and POMC promoters to determine the mechanisms by which different metabolic signals and neurotransmitters act on NPY and POMC cells [93]. In collaboration with Friedman’s laboratory, we have used these mice lines crossbred with ob/ob mice to describe the dynamic synaptic remodeling that occurs in both POMC and NPY cells in response to leptin and ghrelin and that may be critical for the regulation of energy balance, a mechanisms that will be described in ensuing pages.
Synaptic Plasticity and Energy Balance
The concept of homeostasis implies that physiological events in all organisms necessitate a degree of plasticity or flexibility to allow for constant dynamic changes to achieve balance. Within the brain, this plasticity is afforded by systems that can change in response to given stimuli, and that rearrange in ways that allow for more efficient responses to future stimuli. In contrast to old dogma, it is now well accepted that connections between cells within the adult brain are capable to change in response to a variety of stimuli, and that these changes play an important role in critical brain functions as learning, memory, and motivated behavior. Such changes are referred to as synaptic plasticity.
Within the hypothalamus, synaptic changes have been implicated in a variety of processes that include osmoregulation, lactation, circadian rhythmicity, and reproductive function [94–100]. Interestingly, proteins that are commonly found in the developing brain and that are associated with the formation of new synapses are expressed selectively in the hypothalamus of adult organisms, and particularly in the ARC [101]. Interestingly, ultrastructural studies of the ARC revealed that synaptic remodeling occurs on cells within this region across the estrus cycle in female rats [99]. Garcia ?Segura and his associates then revealed that this effect was produced by estrogen, and that, in addition to rats, it was also observable across the reproductive cycle of non-human primates [101]. The ARC contains both estrogen receptor alpha and beta subtypes, yet the effects of estrogen on ARC nucleus cells can occur within minutes of the presence of estrogen in the media, and mimic those elicited in cells by growth factors [102]. While these studies were correlated with the onset and termination of the preovulatory luteinizing hormone surge, it has become clear that these changes may mediate the metabolic effects of estrogen.
Coinciding with these data, researchers soon discovered that leptin, like estrogen, targeted hypothalamic and extrahypothalamic structures that demonstrated a high degree of synaptic remodeling, including the ARC, VMH, and hippocampus [103–105]. Within the hippocampus, it has been demonstrated that leptin can lower the threshold for the induction of long term potentiation (LTP) after activation of the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors [106–108]. Because LTP is thought to result from synaptic changes, these data suggest that leptin can induce synaptic remodeling to increase sensitivity to excitatory stimulation.
Taken together, this information made it plausible that leptin, like estrogen, could target the ARC and other structures to modulate energy balance by actually remodeling inputs to the different cell groups in the ARC. In collaboration with Jeff Friedman, our laboratory engaged in a project examining the effects of leptin on the number and type of synapses contacting both POMC and NPY neurons [109]. To do this, mice in which the gene encoding the GFP protein was inserted in the promoter for either NPY or POMC were cross-bred with heterozygous leptin deficient (ob/ob) mice, to produce ob/ob GFP transgenic mice. Electron microscopic examination, determined that NPY cells in the ARC of ob/ob mice had more synapses than NPY cells of wild type mice. Surprisingly, POMC neurons of ob/ob mice had a lower number of synapses than those of wild type mice. Nevertheless, synapses onto POMC cells of ob/ob mice were predominantly putative inhibitory (symmetric), whereas NPY cells of ob/ob mice primarily exhibited putative excitatory (asymmetric). These data were consistent with electrophysiological recordings showing that the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) onto POMC cells of ob/ob mice was higher than that on POMC cells of wild type mice, with no significant differences in the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) on these cells. In contrast, the frequency of sEPSCs was increased and that of sIPSCs was decreased in NPY neurons of ob/ob mice compared to NPY neurons of wild type mice. Finally, leptin administration to ob/ob mice rapidly restored the balance of excitatory and inhibitory synapses to the levels observed in untreated wild type mice, whereas ghrelin treatment to wild type mice had just the opposite effect. The outcome of these experiments provided anatomical and electrophysiological evidence of a dynamic model of energy regulation in which hypothalamic neurons are in a constant “tug of war” between inhibitory and excitatory synapses, and where peripheral signals like leptin, ghrelin and estrogen shift the balance to ultimately increase or decrease food intake providing for a dynamic framework we have termed the ‘floating blueprint” [110].
Plasticity and mitochondrial UCP2
The plastic nature of ARC nucleus cells, and indeed that of any system that is capable of actual architectural remodeling, may involve high energy expenditure, which may be reflected in the activity as well as in the proliferation of the mitochondria. The mitochondria are involved in the generation of cellular metabolism, and optimal mitochondrial functioning determines the fate of individual cells [111]. Increased mitochondrial activity may, however, also result in the generation of free radicals that can lead to cellular stress and degeneration [111]. It has been suggested that uncoupling proteins (UCPs) are capable of preventing cell damage by dissociating the production of energy in the form of ATP and the resultant high levels of free radicals by regulating the proton leak from the inner membrane of the mitochondria [112, 113].
Of the different UCPs identified, UCP2 has been shown to play an important role in neuroprotection and may, as has been previously suggested, play a role in neurotransmission [114, 115]. This may indeed be the case in the mammalian hypothalamus, where UCP2 is constitutively expressed [115–117]. Within the ARC, UCP2 appears to be present in NPY/Agrp producing cells, as well as in estrogen and leptin sensitive cells, which could also be POMC secreting neurons [115]. The role of UCP2 in these systems remains to be determined although it has been suggested that locally produced active thyroid hormone (T3) activates UCP2 in NPY/Agrp cells, a response that may be critical to activate these cells during negative energy balance [110]. A role for UCP2 in obesity continues to be considered, although UCP2 knock out mice do not seem to be obese [113]. Nevertheless, spontaneously obese yellow agouti mice have a leaner phenotype when crossbred with mice that overexpress the human form of UCP2 (hUCP2) [118]. Interestingly, although these mice are heavier than their wild type littermates at the age of three months, they appear to have less body fat. As they age, hUCP2 transgenics do not continue to gain weight, and by the age of 10 months they are leaner than their wild type counterparts [118]. It is therefore tempting to suggest that UCP2 protects ARC cells from free radical damage that results from the high metabolic rate of these cells. As animals age, uncoupling mechanisms that include the induction of UCP2 and the production of new mitochondria may become deficient leading to alterations in cell function and ultimately obesity. Finally, it could be argued that UCP2 is a potential factor sustaining synaptic plasticity in the ARC. Recently, dendritic mitochondria have been directly implicated in the generation and maintenance of new synapses following hippocampal stimulation. In general it appears that increases the number of mitochondria present in dendrites is directly related to the number of synapses that are formed [119–121]. Because the induction of UCP2 also increases the number of mitochondria in hippocampal cells [122], one can speculate that UCP2 modulates synaptic remodeling through increases in the number of mitochondria.
Parallel Systems Regulating Food Intake and Body Weight
While it appears that the hypothalamus and in particular, the ARC, are key regions regulating energy balance, previous and emerging data demonstrate the existence of other circuits that, when activated, modulate food intake and body weight [63, 64, 123–125]. The importance of these circuits has often been overshadowed by the attention paid to hypothalamic homeostatic circuits, yet their study may prove to be more relevant to human obesity [63, 64]. In addition, these systems are often viewed as either secondary or connected in series with the hypothalamus, that is, they only function once the hypothalamus has been activated. Although these systems cannot be fully considered homeostatic, they may be activated in parallel with, and/or perhaps recruit homeostatic centers to modulate the ingestion of food. In addition, activation of these pathways may override regulatory signals from hypothalamic homeostatic centers to either increase or decrease appetite. For example, it is well established that rats whose brain stem is isolated continue to regulate the food they consume and even show affective responses to palatable foods [126]. Corticolimbic pathways are capable of integrating sensory inputs and produce cognitive as well as affective representations that are stored and used for making decisions, and lesions to various corticolimbic regions result in obesity [127–129]. Feeding is also associated with motivational mechanisms, the “liking” and “wanting”, which are required for the behavioral responses that are necessary to seek and obtain food [130, 131]. These mechanisms are commonly associated with mid brain and forebrain centers that regulate arousal, locomotor activity, mood, and reward. Reward pathways in particular have received special attention given the universality of food as a natural reinforcer. Dopamine produced in cells within the mid brain ventral tegmental area (VTA) is released into several forebrain structures like the hippocampus, ventral striatum, and prefrontal cortex, and this release is commonly associated with the experience or the expectation of reward [132–134]. Within the ventral striatum, dopamine release into the nucleus accumbens has been implicated in the rewarding aspects of food, sex, and drugs of abuse [135, 136]. Interestingly, genetic deletion of dopamine markedly suppresses food intake in a manner that is similar to that of lesions of the lateral hypothalamus [89, 90]. Numerous papers have appeared suggesting that hypothalamic peptides like NPY, α-MSH, Agrp, Orexin and MCH play an important role in modulating the activity of dopaminergic cells targeting the nucleus accumbens [137]. The idea in these papers is that the ARC funnels metabolic information from signals like leptin or ghrelin, to modulate the activity of the mesolimbic dopaminergic system via direct projections to the nucleus accumbens, or indirectly through the activation of hypocretin/orexin or MCH cells that also project to both the VTA and nucleus accumbens [63, 137]. Emerging evidence, however, supports the notion that at least the VTA is sensitive to leptin, insulin and ghrelin, and that the activity of dopaminergic cells within the VTA can be modulated by these signals [138, 139]. Further research may reveal that, in contrast to the funnel hypothesis, metabolic signals may act directly on reward systems to modulate motivational aspects of feeding in tandem with homeostatic systems to increase or reduce food intake.
Future Considerations
We believe that the ability of the ARC to dynamically rewire in response to ever changing signals is necessary for cells within this nucleus to efficiently modulate energy balance. Interestingly, synaptic plasticity also appears to be an important feature in extrahypothalamic circuits affecting food intake. For instance, synaptic rearrangement within the VTA and nucleus accumbens has been implicated in the mechanisms that lead to addiction to substances like opioids, cocaine and amphetamine [140, 141]. Within the VTA, the crosstalk between astrocytes and dopaminergic neurons appears to be important in the sensitization to amphetamine [142, 143]. Chronic cocaine stimulation leads to long lasting changes in gene expression within the nucleus accumbens that perhaps reflect permanent changes in the inputs to cells within this region [144]. We know that, in addition to targeting the ARC to modulate homeostatic pathways, leptin and ghrelin potentially reach cells in the VTA, where they may also alter their synaptic inputs to enhance or decrease their activity. Whether the modulation of synapses in the VTA and ucleus accumbens occurs in response to exposure to natural rewards, or in response to changes in metabolic signals like leptin or ghrelin, remain to be determined. In any event, the examination of this issue will lead to a better understanding of the mechanisms that cause food cravings, and those that increase or decrease the incentive value of palatable foods. They may also lead to insight in the study of eating disorders like obesity and anorexia nervosa, and ultimately lead to more efficient treatments for these disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brobeck JR. Mechanisms of the development of obesity in animals with hypothalamic lesions. Physiological Reviews. 1946;26:541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 2.Brobeck JR, Tepperman J, Long CNH. Experimental hypothalamic hyperphagia in the albino rat. Yale Journal of Biology and Medicine. 1943;15:831–853. [PMC free article] [PubMed] [Google Scholar]
- 3.Hetherington AW, Ranson SW. Hypothalamic lesions and adipocity in the rat. Anatomical Record. 1940;78:149. [Google Scholar]
- 4.Hetherington AW, Ranson SW. The relation of various hypothalamic lesions to adiposity in the rat. Journal of Comparative Neurology. 1942;76:475–499. [Google Scholar]
- 5.Anand BK, Brobeck JR. Localization of a feeding center in the hypothalamus of the rat. Proc. Soc. Exp. Biol. Med. 1951;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 6.Coons EE, Cruce JAF. Lateral hypothalamus:Food and current intensity in maintaining self stimulation of hunger. Science. 1968;159:1117–1119. doi: 10.1126/science.159.3819.1117. [DOI] [PubMed] [Google Scholar]
- 7.Valenstein ES, Cox VC, Kakolewski JW. Modification of motivated behavior elicited by electrical stimulation of the lateral hypothalamus. Science. 1968;159:1119–1121. doi: 10.1126/science.159.3819.1119. [DOI] [PubMed] [Google Scholar]
- 8.Valenstein ES, Mittleman G. Ingestive behavior evoked by hypothalamic stimulation and schedule-induced polydipsia are related. Science. 1984;224:415–417. doi: 10.1126/science.6710151. [DOI] [PubMed] [Google Scholar]
- 9.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 10.Grossman SP. The biology of motivation. Annu. Rev. Psychol. 1979;30:209–242. doi: 10.1146/annurev.ps.30.020179.001233. [DOI] [PubMed] [Google Scholar]
- 11.Stellar JR, Stellar E. The neurobiology of motivation and reward. New York, NY: Springer-Verlag; 1985. [Google Scholar]
- 12.Albert DJ, Storlien LH. Hyperphagia in rats with cuts between the ventromedial and lateral hypothalamus. Science. 1969;165:599–600. doi: 10.1126/science.165.3893.599. [DOI] [PubMed] [Google Scholar]
- 13.Bray GA, Inoue S, Nishizawa Y. Hypothalamic obesity. The autonomic hypothesis and the lateral hypothalamus. Diabetologia. 1981;20 Suppl:366–377. doi: 10.1007/BF00254505. [DOI] [PubMed] [Google Scholar]
- 14.Inoue S, Bray GA. The effects of subdiaphragmatic vagotomy in rats with ventromedial hypothalamic obesity. Endocrinology. 1977;100:108–114. doi: 10.1210/endo-100-1-108. [DOI] [PubMed] [Google Scholar]
- 15.Zigmond MJ, Stricker EM. Deficits in feeding behavior after intraventricular injection of 6-hydroxydopamine in rats. Science. 1972;177:1211–1214. doi: 10.1126/science.177.4055.1211. [DOI] [PubMed] [Google Scholar]
- 16.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y - a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 17.Nemeroff CB, Lipton MA, Kizer JS. Models of neuroendocrine regulation: use of monosodium glutamate as an investigational tool. Dev. Neurosci. 1978;1:102–109. doi: 10.1159/000112561. [DOI] [PubMed] [Google Scholar]
- 18.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 19.Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res. Bull. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 20.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 21.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci U S A. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahu A, Kalra PS, Kalra SP. Food deprivation and ingestion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides. 1988;9:83–86. doi: 10.1016/0196-9781(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 23.Sanacora G, Kershaw M, Finkelstein JA, White JD. Increased hypothalamic content of preproneuropeptide Y messenger ribonucleic acid in genetically obese Zucker rats and its regulation by food deprivation. Endocrinology. 1990;127:730–737. doi: 10.1210/endo-127-2-730. [DOI] [PubMed] [Google Scholar]
- 24.Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am. J. Physiol. 1991;260:R321–R327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- 25.Walker HC, Romsos DR. Similar effects of NPY on energy metabolism and on plasma insulin in adrenalectomized ob/ob and lean mice. Am. J. Physiol. 1993;264:E226–E230. doi: 10.1152/ajpendo.1993.264.2.E226. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 27.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 28.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 29.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 31.Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, Proenca R, Negrel R, Ailhaud G, Friedman JM. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 33.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, MacKellar W, Rosteck PR, Jr, Schoner B, Smith D, Tinsley FC, Zhang XY, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 34.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001;25 Suppl 5:S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 35.Boston BA, Blaydon KM, Varnerin J, Cone RD. Independent and additive effects of central POMC and leptin pathways on murine obesity. Science. 1997;278:1641–1644. doi: 10.1126/science.278.5343.1641. [DOI] [PubMed] [Google Scholar]
- 36.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 37.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 38.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 39.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 40.Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, Elmquist JK. Characterization of CART neurons in the rat and human hypothalamus. J. Comp. Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- 41.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol. Behav. 2001;74:703–708. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- 42.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 43.Leibowitz SF, Hoebel BG. Behavioral neuroscience and obesity. In: Bray GA, Bouchard C, editors. Handbook of Obesity. Etiology and Pathophysiology. New York, NY: Marcel Dekker; 2004. [Google Scholar]
- 44.Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology. 1992;131:2461–2467. doi: 10.1210/endo.131.5.1425443. [DOI] [PubMed] [Google Scholar]
- 45.Pu S, Jain MR, Horvath TL, Diano S, Kalra PS, Kalra SP. Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinology. 1999;140:933–940. doi: 10.1210/endo.140.2.6495. [DOI] [PubMed] [Google Scholar]
- 46.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 47.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 48.Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J. Endocrinol. 1999;160:R7–R12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- 49.Stricker-Krongrad A, Richy S, Beck B. Orexins/hypocretins in the ob/ob mouse: hypothalamic gene expression, peptide content and metabolic effects. Regul. Pept. 2002;104:11–20. doi: 10.1016/s0167-0115(01)00344-5. [DOI] [PubMed] [Google Scholar]
- 50.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 51.Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J. Neurosci. 1999;19:1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J. Comp. Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 53.Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, Sakurai T, Shioda S, Yada T. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur. J. Neurosci. 2004;19:1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- 54.Yamanaka A, Kunii K, Nambu T, Tsujino N, Sakai A, Matsuzaki I, Miwa Y, Goto K, Sakurai T. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 2000;859:404–409. doi: 10.1016/s0006-8993(00)02043-6. [DOI] [PubMed] [Google Scholar]
- 55.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 56.Sakurai T. Orexin: a link between energy homeostasis and adaptive behaviour. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6:353–360. doi: 10.1097/01.mco.0000078995.96795.91. [DOI] [PubMed] [Google Scholar]
- 57.Sakurai T. Reverse pharmacology of orexin: from an orphan GPCR to integrative physiology. Regul. Pept. 2005;126:3–10. doi: 10.1016/j.regpep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J. Clin. Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH, Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 61.Ludwig DS, Mountjoy KG, Tatro JB, Gillette JA, Frederich RC, Flier JS, Maratos-Flier E. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am. J. Physiol. 1998;274:E627–E633. doi: 10.1152/ajpendo.1998.274.4.E627. [DOI] [PubMed] [Google Scholar]
- 62.Tritos NA, Vicent D, Gillette J, Ludwig DS, Flier ES, Maratos-Flier E. Functional interactions between melanin-concentrating hormone, neuropeptide Y, and anorectic neuropeptides in the rat hypothalamus. Diabetes. 1998;47:1687–1692. doi: 10.2337/diabetes.47.11.1687. [DOI] [PubMed] [Google Scholar]
- 63.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 64.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol. Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 65.Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J. Physiol. 2001;533:237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan JL, Uehara K, Lu S, Wang QP, Funahashi H, Sakurai T, Yanagizawa M, Shioda S. Reciprocal synaptic relationships between orexin- and melanin- concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int. J. Obes. Relat. Metab. Disord. 2002;26:1523–1532. doi: 10.1038/sj.ijo.0802155. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 68.Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 69.Horvath TL, Diano S, Sotonyi P, Heiman M, Tschop M. Minireview: ghrelin and the regulation of energy balance--a hypothalamic perspective. Endocrinology. 2001;142:4163–4169. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 70.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 71.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 72.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 73.van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr. Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 74.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 75.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front. Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat. Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 77.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 78.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 79.Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- 80.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 81.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Butler AA, Cone RD. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann. N. Y. Acad. Sci. 2003;994:240–245. doi: 10.1111/j.1749-6632.2003.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 84.Barsh GS, Farooqi IS, O'Rahilly Genetics of body-weight regulation. Nature. 2000;404:644–651. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- 85.Erickson JC, Ahima RS, Hollopeter G, Flier JS, Palmiter RD. Endocrine function of neuropeptide Y knockout mice. Regul. Pept. 1997;70:199–202. doi: 10.1016/s0167-0115(97)01007-0. [DOI] [PubMed] [Google Scholar]
- 86.Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog. Horm. Res. 1998;53:163–199. [PubMed] [Google Scholar]
- 87.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci U S A. 2004;101:8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szczypka MS, Mandel RJ, Donahue BA, Snyder RO, Leff SE, Palmiter RD. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron. 1999;22:167–178. doi: 10.1016/s0896-6273(00)80688-1. [DOI] [PubMed] [Google Scholar]
- 90.Szczypka MS, Rainey MA, Kim DS, Alaynick WA, Marck BT, Matsumoto AM, Palmiter RD. Feeding behavior in dopamine-deficient mice. Proc Natl Acad Sci U S A. 1999;96:12138–12143. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann. N. Y. Acad. Sci. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 92.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 93.Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- 94.Flanagan-Cato LM. Estrogen-induced remodeling of hypothalamic neural circuitry. Front. Neuroendocrinol. 2000;21:309–329. doi: 10.1006/frne.2000.0204. [DOI] [PubMed] [Google Scholar]
- 95.Guldner FH, Ingham CA. Plasticity in synaptic appositions of optic nerve afferents under different lighting conditions. Neurosci. Lett. 1979;14:235–240. doi: 10.1016/0304-3940(79)96154-8. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S. Altered cortical synaptic morphology and impaired memory consolidation in forebrain- specific dominant-negative PAK transgenic mice. Neuron. 2004;42:773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Langle SL, Poulain DA, Theodosis DT. Neuronal-glial remodeling: a structural basis for neuronal-glial interactions in the adult hypothalamus. J. Physiol. Paris. 2002;96:169–175. doi: 10.1016/s0928-4257(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 98.Nishikawa Y, Shibata S, Watanabe S. Circadian changes in long-term potentiation of rat suprachiasmatic field potentials elicited by optic nerve stimulation in vitro. Brain Res. 1995;695:158–162. doi: 10.1016/0006-8993(95)00717-5. [DOI] [PubMed] [Google Scholar]
- 99.Olmos G, Naftolin F, Perez J, Tranque PA, Garcia-Segura LM. Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience. 1989;32:663–667. doi: 10.1016/0306-4522(89)90288-1. [DOI] [PubMed] [Google Scholar]
- 100.Theodosis DT, Poulain DA. Maternity leads to morphological synaptic plasticity in the oxytocin system. Prog. Brain. Res. 2001;133:49–58. doi: 10.1016/s0079-6123(01)33004-2. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Segura LM, Chowen JA, Parducz A, Naftolin F. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog. Neurobiol. 1994;44:279–307. doi: 10.1016/0301-0082(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 102.Garcia-Segura LM, Chowen JA, Duenas M, Parducz A, Naftolin F. Gonadal steroids and astroglial plasticity. Cell. Mol. Neurobiol. 1996;16:225–237. doi: 10.1007/BF02088178. [DOI] [PubMed] [Google Scholar]
- 103.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7:2635–2638. doi: 10.1097/00001756-199611040-00045. [DOI] [PubMed] [Google Scholar]
- 105.Shioda S, Funahashi H, Nakajo S, Yada T, Maruta O, Nakai Y. Immunohistochemical localization of leptin receptor in the rat brain. Neurosci. Lett. 1998;243:41–44. doi: 10.1016/s0304-3940(98)00082-2. [DOI] [PubMed] [Google Scholar]
- 106.Harvey J, Ashford ML. Leptin in the CNS: much more than a satiety signal. Neuropharmacology. 2003;44:845–854. doi: 10.1016/s0028-3908(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 107.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 108.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J. Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 110.Horvath TL, Diano S. The floating blueprint of hypothalamic feeding circuits. Nat. Rev. Neurosci. 2004;5:662–667. doi: 10.1038/nrn1479. [DOI] [PubMed] [Google Scholar]
- 111.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 112.BechmannI I, Diano S, Warden CH, Bartfai T, Nitsch R, Horvath TL. Brain mitochondrial uncoupling protein 2 (UCP2): a protective stress signal in neuronal injury. Biochem. Pharmacol. 2002;64:363–367. doi: 10.1016/s0006-2952(02)01166-8. [DOI] [PubMed] [Google Scholar]
- 113.Paradis E, Clavel S, Bouillaud F, Ricquier D, Richard D. Uncoupling protein 2: a novel player in neuroprotection. Trends. Mol. Med. 2003;9:522–525. doi: 10.1016/j.molmed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 114.Fuxe K, Rivera A, Jacobsen KX, Höistad M, Leo G, Horvath TL, Staines W, De la Calle A, Agnati LF. Dynamics of volume transmission in the brain. Focus on catecholamine and opioid peptide communication and the role of uncoupling protein 2. J. Neural. Transm. 2005;112:65–76. doi: 10.1007/s00702-004-0158-3. [DOI] [PubMed] [Google Scholar]
- 115.Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, Diano S. Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. J. Neurosci. 1999;19:10417–10427. doi: 10.1523/JNEUROSCI.19-23-10417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Diano S, Urbanski HF, Horvath B, Bechmann I, Kagiya A, Nemeth G, Naftolin F, Warden CH, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in the nonhuman primate brain and pituitary. Endocrinology. 2000;141:4226–4238. doi: 10.1210/endo.141.11.7740. [DOI] [PubMed] [Google Scholar]
- 117.Richard D, Rivest R, Huang Q, Bouillaud F, Sanchis D, Champigny O, Ricquier D. Distribution of the uncoupling protein 2 mRNA in the mouse brain. J. Comp. Neurol. 1998;397:549–560. [PubMed] [Google Scholar]
- 118.Horvath TL, Diano S, Miyamoto S, Barry S, Gatti S, Alberati D, Livak F, Lombardi A, Moreno M, Goglia F, Mor G, Hamilton J, Kachinskas D, Horwitz B, Warden CH. Uncoupling proteins-2 and 3 influence obesity and inflammation in transgenic mice. Int. J. Obes. Relat. Metab. Disord. 2003;27:433–442. doi: 10.1038/sj.ijo.0802257. [DOI] [PubMed] [Google Scholar]
- 119.Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int. Rev. Neurobiol. 2004;59:273–296. doi: 10.1016/S0074-7742(04)59011-6. [DOI] [PubMed] [Google Scholar]
- 120.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 121.Mattson MP, Liu D. Mitochondrial potassium channels and uncoupling proteins in synaptic plasticity and neuronal cell death. Biochem. Biophys. Res. Commun. 2003;304:539–549. doi: 10.1016/s0006-291x(03)00627-2. [DOI] [PubMed] [Google Scholar]
- 122.Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning. Endocrinology. 2003;144:5014–5021. doi: 10.1210/en.2003-0667. [DOI] [PubMed] [Google Scholar]
- 123.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front. Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 124.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 125.Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annu. Rev. Psychol. 2000;51:255–277. doi: 10.1146/annurev.psych.51.1.255. [DOI] [PubMed] [Google Scholar]
- 126.Grill HJ, Kaplan JM. Interoceptive and integrative contributions of forebrain and brainstem to energy balance control. Int. J. Obes. Relat. Metab. Disord. 2001;25 Suppl 5:S73–S77. doi: 10.1038/sj.ijo.0801917. [DOI] [PubMed] [Google Scholar]
- 127.Carr KD, Wolinsky TD. Regulation of feeding by multiple opioid receptors in cingulate cortex; follow-up to an in vivo autoradiographic study. Neuropeptides. 1994;26:207–213. doi: 10.1016/0143-4179(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 128.Stein EA, Carr KD, Simon EJ. Brain stimulation-induced feeding alters regional opioid receptor binding in the rat: an in vivo autoradiographic study. Brain Res. 1990;533:213–222. doi: 10.1016/0006-8993(90)91342-e. [DOI] [PubMed] [Google Scholar]
- 129.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 131.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol. Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 132.Wise RA. Dopamine and food reward: back to the elements. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R13. doi: 10.1152/ajpregu.00590.2003. [DOI] [PubMed] [Google Scholar]
- 133.Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 134.Wise RA, Rompre PP. Brain dopamine and reward. Annu. Rev. Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 135.Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann. N. Y. Acad. Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- 136.Mitchell JB, Gratton A. Involvement of mesolimbic dopamine neurons in sexual behaviors: implications for the neurobiology of motivation. Rev. Neurosci. 1994;5:317–329. doi: 10.1515/revneuro.1994.5.4.317. [DOI] [PubMed] [Google Scholar]
- 137.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 138.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain. Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 139.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain. Res. Mol. Brain. Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 140.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 141.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Suppl 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 142.Flores C, Stewart J. Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat. Psychopharmacology (Berl) 2000;151:152–165. doi: 10.1007/s002130000417. [DOI] [PubMed] [Google Scholar]
- 143.Flores C, Stewart J. Changes in astrocytic basic fibroblast growth factor expression during and after prolonged exposure to escalating doses of amphetamine. Neuroscience. 2000;98:287–293. doi: 10.1016/s0306-4522(00)00115-9. [DOI] [PubMed] [Google Scholar]
- 144.Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu. Rev. Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]