Cellular Bioenergetics as a Target for Obesity Therapy (original) (raw)
. Author manuscript; available in PMC: 2011 Jun 1.
Published in final edited form as: Nat Rev Drug Discov. 2010 Jun;9(6):465–482. doi: 10.1038/nrd3138
Summary
Obesity develops when energy intake exceeds energy expenditure. While most current obesity therapies are focused on reducing caloric intake, recent data suggest that increasing cellular energy expenditure (bioenergetics) may be an attractive alternative approach. This is especially true for adaptive thermogenesis - the physiological process whereby energy is dissipated in the form of heat in response to external stimuli. There have been significant recent advances in identifying factors that control the development and function of these tissues and in techniques to measure brown fat in human adults. In this review, we integrate these developments in relation to the classic understandings of cellular bioenergetics to explore the potential for developing novel anti-obesity therapies that target cellular energy expenditure.
Introduction
Obesity is occurring at epidemic rates in the United States and worldwide. According to the World Health Organization, more than 1 billion adults (~15% of the world population) are overweight (body mass index (BMI) >25) and over 300 million rank as truly obese (BMI>30); and these numbers are expected to increase by more than half again by the year 2025. Obesity represents a major risk factor for the development of many of our most common medical conditions, including type 2 diabetes mellitus, dyslipidemias, non-alcoholic fatty liver and gallstones, cardiovascular disease, Alzheimer’s disease and even some cancers 1.
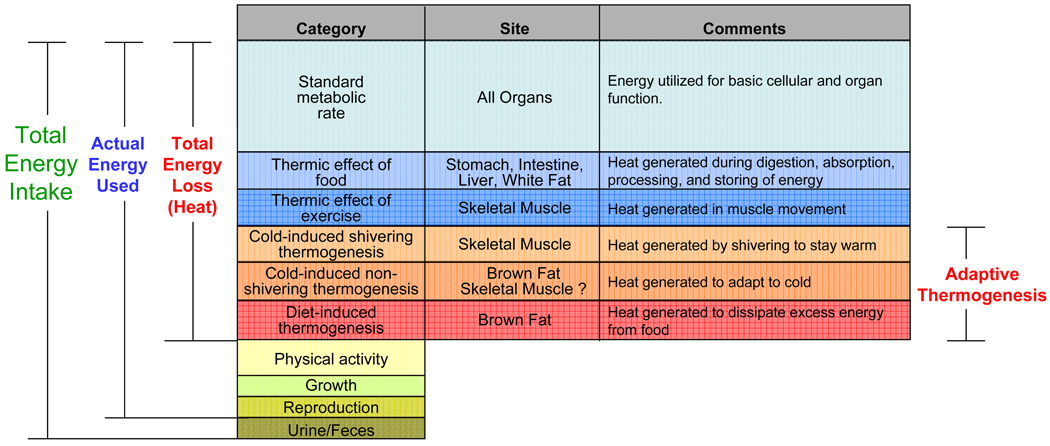
Obesity develops when energy intake exceeds energy expenditure. Of the nutrient energy intake, a small portion is lost in feces and urine; a portion is used for physiological needs (growth, pregnancy, or lactation); a variable, and unfortunately decreasing, portion is used in physical activity; while the majority is used for metabolic processes or is lost in the production of heat (Figure 1). The standard or basal metabolic rate (SMR or BMR) is the rate of energy utilized by an organism in the awake but resting state, not actively digesting food and at thermoneutrality 2. Every organ of the body contributes to SMR because nearly every enzymatic reaction is thermogenic. Of the remaining energy loss, there is the heat produced during digestion and absorption of food (thermic effect of food), the thermic effect of exercise, and the energy dissipated in response to the environmental changes, such as cold temperature and diet. These latter forms of regulated heat production are referred to as adaptive thermogenesis 3, and occur primarily in the mitochondria of skeletal muscle and brown fat, which are distinct from other body tissues in that their thermogenesis is finely regulated and therefore has the potential to be manipulated therapeutically to serve as a target for obesity treatment.
Figure 1. Cellular energy utilization.
Of the nutrient energy intake of an individual organism, a small portion is lost in the feces and urine; a portion is used for physiological needs, such as growth, pregnancy, or lactation; a variable portion is used in physical activity; while the majority of ingested calories is used for metabolic processes or is lost in the production of heat. Based on the function and tissues of heat production, thermogenesis can be further classified into six categories. Adaptive thermogenesis is defined as regulated heat production in response to environmental temperature or diet. There are three subcategories of adaptive thermogenesis. Cold exposure induces shivering thermogenesis in skeletal muscle, and non-shivering thermogenesis in brown fat. While current evidence does not indicate a role of muscle in non-shivering thermogenesis, indirect evidence suggests that such mechanisms may exist. Overfeeding triggers diet-induced thermogenesis; this is also a function of brown fat.
Current treatments of obesity
Since the laws of thermodynamics must be obeyed, any treatment for obesity must reduce energy intake, increase energy expenditure, or have an effect on both. Despite this simple reality, treatment of obesity remains one of the most important challenges facing the health care system. Current approved clinical approaches for the treatment of obesity include diet and exercise, medical therapies aimed at reducing caloric intake or absorption, which are of limited effectiveness, and bariatric surgery for extremely obese individuals. Unfortunately, only a small portion of individuals on dietary and/or exercise programs maintain a long-term weight loss 4. Although bariatric surgery has gained popularity for very obese patients, it is not without significant risk.
The only three drugs currently approved by the FDA specifically for weight loss decrease energy intake, either by acting at satiety centers in the brain (sibutramine and phentermine) or by reducing the efficiency of intestinal absorption (orlistat) 5, thereby reducing the elevated energy intake that is critical in maintaining the obese state 6. However, because of the unacceptable side effects or inadequate long-term clinical efficacy, these medications have thus far met limited success 7. There are over two-dozen treatments in at least Phase 1 clinical trials, and more in the pipeline (Table 1A, Table 1B, and Table 2), almost all of which are aimed at reducing energy intake.
Table 1.
| Table 1A. Anti-obesity Treatments that Decrease Energy Intake via Appetite Suppression | ||
|---|---|---|
| Class | Drug | Principal Mechanism |
| Anticonvulsant | Topiramate | Enhances GABA-activated chloride channels; inhibitsexcitatory neurotransmission through actions on kainateand AMPA receptors 173 |
| Zonisamide | Blocks voltage-gated Na and T-type Ca channels, blocksK-evoked glutamate release, modulates centraldopaminergic and serotonergic function 174 | |
| Enzyme activator | Metformin | AMPK activator 175,176 |
| Enzyme inhibitor | Trodusquemine | Inhibitor of protein tyrosine phosphatase 1B (PTP1B) 177 |
| Hormones | Adiponectin | Stimulates FA oxidation 178 |
| Exenatide, liraglutide | GLP-1 receptor agonist 179 | |
| Leptin, Metreleptin | Reflects size of fat depots 151,180 | |
| Oleoyl-estrone | Alters body weight set-point/ponderostat 181,182 | |
| Oxyntomodulin | Likely activates GLP-1 and other receptors 183 | |
| Pramlintide, AC2307 | Amylin receptor activator 180 | |
| NeurotransmitterReuptake inhibitor | Phentermine | Adrenergic reuptake blocker 184 |
| Rivastigmine | Cholinesterase inhibitor 185 | |
| Tesofensine | Blocker of presynaptic uptake of noradrenaline,dopamine, and serotonin186 | |
| Sibutramine | Monoamine reuptake blocker 10 | |
| Venlafaxine | Norepinephrine and 5HT reuptake blocker 187 | |
| NeurotransmitterReuptake inhibitor,Receptor antagonist | Bupropion | Norepinephrine and dopamine reuptake inhibitor, andnicotinic blocker 188 |
| Receptor activator | CE-326597 | CCK receptor activator 189, (NCT00542009) |
| Lorcaserin | 5-HT2C receptor activator 190 | |
| MK0493 | Melanocortin 4 (MCR4) receptor activator 191 | |
| TM30339 | NPY Y4 receptor activator 191 | |
| TTP435 | AgRP antagonist/MCR4 recepto activator 192,(NCT00779519) | |
| Receptor activatorand blocker | Betahistine | H1 receptor agonist,H3 receptor antagonist 193 |
| Receptor blocker | Naltrexone | Opioid receptor blocker 194 |
| Rimonabant, Taranabant,others | CB1 receptor blocker 10 | |
| Velneperit, MK0557 | NPY5 receptor blocker 191, (NCT00748605) |
| Table 1B. Anti-obesity Treatments that Decrease Energy Intake via Impaired Absorption | ||
|---|---|---|
| Class | Drug | Principal Mechanism |
| Enzyme inhibitor | Cetilistat, Orlistat, GT389–255 | Lumenal Intestinal and pancreatic lipases blocker 10 |
| Transporter inhibitor | R256918 | Gut-selective microsomal triglyceride transfer protein(MTP) 195, (NCT00622765) |
| GW869682,JNJ28431754 | Na-dependent glucose cotransporter (SGLT2) 196,(NCT00297180), (NCT00650806) | |
| Vaccine | Anti-ghrelin vaccine | Blocks ghrelin receptor binding 197 |
Table 2.
Anti-obesity Treatments that Increase Energy Expenditure
| Class | Drug | Principal Mechanism |
|---|---|---|
| Chemical Uncoupler | DNP | Uncouples of H+ gradient 18 |
| Enzyme inhibitor | INCB13739 | 11beta-HSD1 (11-beta hydroxysteroid dehydrogenasetype 1) inhibitor 198, (NCT00398619) |
| Hormone | GC-1, KB-141 | Thyroid Hormone Mimetics 145,146 |
| Somatotropin | Growth hormone receptor activator 199 | |
| Metabolic Target | AICAR, metformin | AMPK activator 200 |
| Desnutrin | Adipose triglyceride lipase activator 201 | |
| Resveratrol | SIRT1 activator 157,196 | |
| Receptor activator | Bromocriptine | Dopamine D2 receptor activator 202 |
| Bile acids, INT-777 | TGR5 receptor activator 148,149 | |
| Ephedrine | Mixed sympathomimetic 134 | |
| Ma Huang (herb) | Mixed sympathomimetic 134 | |
| BRL-26830, L-796568,N-5984 | Selective β3 adrenergic receptor activators 141 | |
| Transcription factoractivator | GW501516 | PPAR delta agonist 203 |
| Unknown | Reesterified long-chainn-3 polyunsaturated fattyacids (EPA, DHA) | Modifies Gene Expression 204 |
However, losing weight by only caloric restriction faces three conceptual challenges. First, mammals are designed to guard against starvation. While there is an active debate surrounding the mechanisms underlying this process 8,9, most agree that the human body is designed to defend against a lower bound of fat content. Therefore, redundant systems are in place to overcome any one pathway to appetite suppression, suggesting that a drug acting by one principal mechanism may be unlikely to have long-term efficacy. Second, as the experience with endocannabinoid receptor antagonists has shown, central satiety centers often interconnect with other core regulators in the brain, causing psychotropic side effects 10. Third, and probably most important, the body has homeostatic mechanisms such that weight loss produces an increase in caloric efficiency, i.e. a reduction in BMR, making further weight loss even more difficult 11,12.
Targeting energy expenditure, i.e., cellular bioenergetics, is therefore an attractive alternative strategy that could be used alone or in conjunction with other approaches for several reasons. First, from a practical perspective, few drugs in this class are currently available, so there is significant opportunity for novel treatments. Second, recent studies have shown that adult humans maintain potentially active brown adipose tissue, making this high energetic tissue a real therapeutic target 13–17. Third, increasing energy expenditure has already proved to be very effective in achieving weight loss. For example, dinitrophenol (DNP), a non-selective uncoupler of mitochondrial oxidation, effectively increases energy expenditure that could be sustained without tolerance 18. Unfortunately DNP produces unwanted side effects that preclude its use as therapy. Finally, increasing energy expenditure may be a way to combat the body’s own adaptive changes to lose weight. The integrated systems by which the brain and body communicate to regulate body weight may indicate single or multiple set-points for weight that may be difficult to adjust 12,19–21. Therapeutic interventions designed to increase energy expenditure may be able to reset an obese individual’s set-point for body weight back to a lower, healthier range. This raises the possibility that drug treatment might only need to be short-term or intermittent, reducing the risks and costs of lifelong exposure to medications.
Cellular bioenergetics and mitochondrial energy metabolism
Bioenergetics takes place largely within the mitochondria, where, through the tricarboxylic acid (TCA) cycle and the electron transport chain (ETC), energy from nutrients is released; oxygen is consumed; and water, carbon dioxide, and ATP are produced (Box 1, Figure 2a). Under normal circumstances, in most tissues, the release of the energy in chemical bonds is mediated enzymatically 22 as a series of interconnected reactions that permit the minimization of loss as heat and maximization of production of ATP. The ATP is distributed throughout the cell for maintenance of Na/K and calcium pumps (30%), protein synthesis (30%), and for gluconeogenesis, ureagenesis, and turnover of carbohydrate and lipid stores (20%) 23.
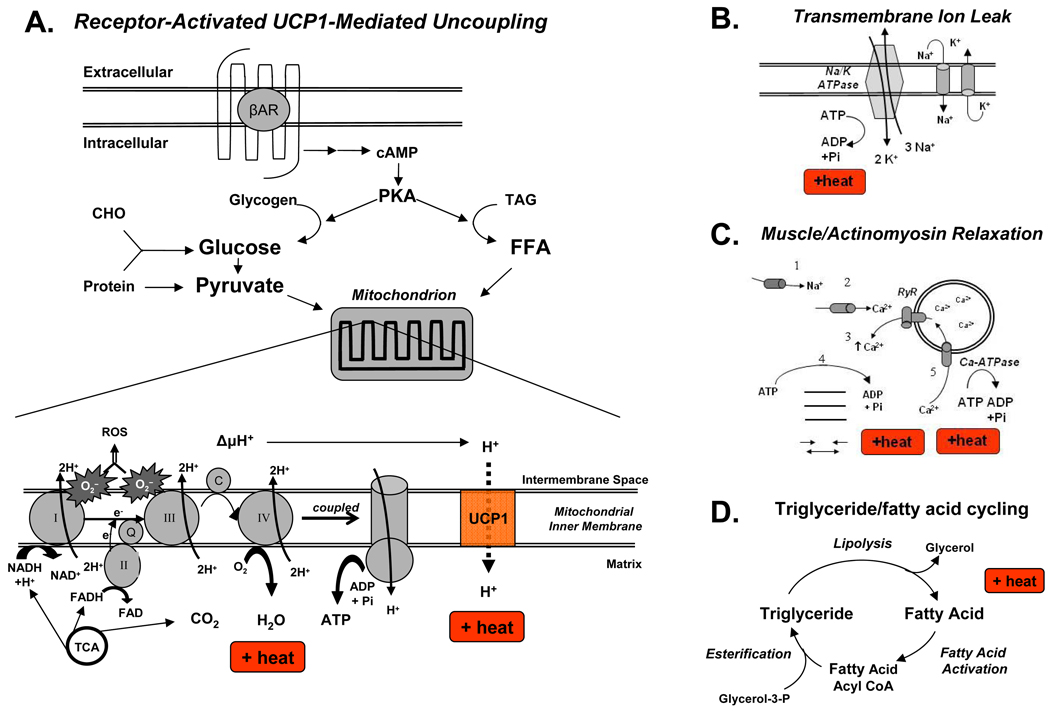
Figure 2. Molecular mechanisms of cellular thermogenesis.
(A) Regulated increases in thermogenesis occur in brown adipocytes with the stimulation of beta adrenergic receptors (βAR), starting a signal transduction cascade that produces cAMP and activates PKA, which then activates multiple enzymes responsible for converting the catabolic endproducts of macronutrients (carbohydrates (CHO), fats (TAG), and proteins) into mitochondrial fuel. The TCA cycle generates protons (H+) and electrons (e) that are carried by NADH and FADH to the ETC where the protons are transported to the mitochondrial intermembrane space, generating an electrochemical gradient (ΔµH+) that is used by the F0/F1-ATPase to convert that potential energy into the phosphoanhydride bonds in ATP. Meanwhile, the electrons are transported in successive steps through the ETC complexes until they are received by O2 to make H2O. The highly reactive electrons also lead to ROS, which can cause significant cellular damage. The TCA also produces CO2 as a byproduct. The respiratory quotient (RQ) is the ratio of CO2 produced / O2 consumed and typically ranges between 0.7 for fats and 1.0 for carbohydrates. Thus, RQ can help identify the mitochondrial fuel source.
(B) Multiple tissues, including muscle, generate heat via uncoupled processes such as leakage of ions (H+, Na+, K+, Ca2+) through channels back down their electrochemical gradients. Shown here is the ubiquitous Na/K ATPase releasing heat energy and Na+ and K+ leaking back to perpetuate this “futile” cycle.
(C) Myocytes can also increase thermogenesis through a series of uncoupled reactions. Neurotransmitter-mediated opening of (1) cell-surface Na+ channels leads to (2) release of Ca2+ into the cytoplasm from sources both outside the cell and (3) the sarcoplasmic reticulum via the ryanodine receptor (RyR). Dysfunction of this receptor leading to uncontrolled Ca2+ release underlies the thermogenesis in malignant hyperthermia 44. Ca2+ leads to heat generation from (4) ATP hydrolysis during both muscle relaxation and actinomyosin cross-bridge cycling during sustained contraction. Additional heat energy is released (5) when Ca2+ ions are pumped back into the sarcoplasmic reticulum by the sarcoplasmic reticulum calcium ATPases (Ca-ATPase).
(D) Triglyceride/fatty acid cycling is an example of a “futile” cycle involving muscle and adipose tissue in which esterification of triglycerides is followed by hydrolysis, leading to increased heat expenditure in processes as diverse as burn injuries, cancer cachexia, and after exercise.
Abbreviations: βAR, beta adrenergic receptors; Ca-ATPase, C, cytochrome C; CHO, carbohydrates; complex I, NADH–ubiquinone oxidoreductase; complex II, succinate–ubiquinone oxidoreductase; complex III, ubiquinone–cytochrome-c oxidoreductase; complex IV, cytochrome-c oxidase; ETC, electron transport chain; FFA, free fatty acids; PKA, protein kinase A; Q, ubiquinone; ROS, reactive oxygen species; RyR, Ryanodine receptor ; TAG, triacylglycerols; TCA, tricarboxylic acid cycle; UCP1, uncoupling protein 1
This interconversion of chemical energy is by nature an inefficient process. Even when reactions are tightly coupled, some energy is lost as heat. However, in the mammalian cell, there are certain processes that are entirely uncoupled, or “futile,” and therefore thermogenic or energy consuming. In all cells, H+, Na+, K+, and Ca2+ leak back across membrane channels down their electrochemical gradients. In mitochondria, the proton leak is typically as high as 20%, dissipating free energy substantially and reducing the amount of ATP actually generated for every molecule of oxygen split by the ETC. Muscle tissue has at least four uncoupled, or energy-burning, reactions, including (1) the energy dissipated as heat by the inwardly rectifying Ca2+ and Na+ channels; (2) cycling of actinomyosin during contraction and relaxation; (3) heat dissipated during physical work and (4) energy lost by triglyceride/fatty acid cycling (Figures 2b–2d) 2. The latter is an example of a “futile” cycle in which esterification of triglycerides is followed by hydrolysis, leading to heat expenditure, and was originally proposed over 30 years ago by Newslholme 24,25. This cycling is involved in the thermogenesis associated with burn injuries 26, cancer cachexia 27, and after exercise 28. Recent evidence has shown that this cycle is also present in human white adipocytes in vitro after treatment with the specific PPARα agonist, GW7647 29.
An obvious question is why are so many mammalian processes uncoupled? Teleological explanations include the flexibility that inefficiency may offer in levels of control of cell function, as well as the more rapid kinetics that energy-releasing reactions permit 2. However, there is one particular uncoupled reaction that occurs clearly by design: the regulated proton leak in brown adipose tissue (BAT) by uncoupling protein-1 (UCP1).
UCP1 is a 32 kDa inner mitochondrial transmembrane protein expressed only in brown adipocytes, which allows protons in the mitochondrial intermembrane space to re-enter the mitochondrial matrix without generating ATP, i.e. uncoupled, and heat is generated directly by protons rushing down their electrochemical gradient and also indirectly by the subsequent increase in flux through the ETC that follows. UCP1 is unique to BAT and is necessary to mediate BAT thermogenesis 30. UCP1-deficient mice are cold sensitive 31 and exhibit increased susceptibility to diet-induced obesity 32,33. Conversely, transgenic mice with UCP1 expression in white fat display lean phenotype 34,35. In addition to UCP1, two other uncoupling proteins have been identified. UCP2 is expressed at low levels in many tissues, while UCP3 is expressed preferentially in skeletal muscle. However, there is no convincing data to support their involvement in thermogenesis 18.
In rodents, the thermogenic capacity of BAT is enormous. In a cold-acclimatized rat weighting 350–400 g, oxygen consumption by 3 g of BAT is approximately twice the basal metabolic rate 36. Humans are quite different from rats, with much greater body mass (~200X), only moderately greater BAT mass (~10X), and much lower basal metabolic rate (~0.15X) 13,37,36. In this context, it has been estimated in humans that as little as 50g of BAT (less than 0.1% of body weight) could utilize up to 20% of basal caloric needs if maximally stimulated 38.
Regulated energy expenditure – Adaptive thermogenesis
Identical twin studies suggest that genetic factors account for 50–90% of the variance in weight gain 39,40. Part of this difference is due to differences in energy expenditure and adaptive thermogenesis. Indeed, >3-fold variations in energy expenditure and weight gain have been observed in response to overfeeding in normal lean individuals 39. Because of its cumulative nature, very small differences in energy expenditure can result in a large impact on body weight over time. For example, differences in energy balance as little as in 71 kJ/day (17 kcal), which is approximately the energy utilized in standing for one hour, and only about 0.6% of daily total energy expenditure, could theoretically lead to a weight gain or loss of 1 kg/year 23,41–43.
Categories of adaptive thermogenesis
In mammals, adaptive thermogenesis, defined as the heat production in response to environmental temperature or diet, occurs primarily in brown fat and skeletal muscle. Adaptive thermogenesis can be divided into three subtypes (Figure 1). Cold exposure induces shivering thermogenesis (ST), a function of skeletal muscle, and non-shivering thermogenesis (NST), a function of brown fat. Overfeeding triggers diet-induced thermogenesis (DIT); this is also a function of brown fat. While current evidence does not indicate a role of muscle in NST, this may be due to the lack of technologies allowing a direct measurement of muscle NST separated from other muscle thermogenic processes. Clearly, indirect evidence from malignant hyperthermia 44 and mild cold exposure in humans 45 suggest that such mechanisms may exist and that further investigation of this area is warranted.
Thermogenesis is essential for warm-blooded (endothermic/ homeothermic) animals, ensuring normal cellular and physiological function under conditions of environmental challenge. During prolonged cold exposure of rodents and humans, even as shivering disappears, energy expenditure remains elevated, due to NST 46. Newborn human infants cannot shiver, and thus maintenance of body temperature depends entirely on NST by brown fat 47.
Diet-induced thermogenesis was described over a century ago by Neumann as “luxuskonsumption”, i.e. a physiological mechanism exists that permits excessive caloric intake to be dissipated as heat, allowing individuals to eat without gaining weight 48. DIT was found to be tightly associated with the recruitment of brown adipose tissue by enhanced adrenergic activity 38. It has been proposed that differential responses to DIT may account for the large individual differences in weight gain in response to overfeeding and thus contribute to the development of obesity 49. Studies of pair-fed genetically obese mice have clearly shown a defect in BAT-mediated thermogenesis 50,51. In addition, nutritional components can influence DIT. For example, a diet rich in polyunsaturated fatty acids causes a greater induction of DIT than a diet rich in saturated fatty acids 52. The exact impact of other nutritional components on thermogenesis and systemic energy regulation remains to be elucidated.
DIT and NST share common features in that both occur in brown fat and both are regulated by the sympathetic nervous system. In healthy young adults, heat production in response to both overfeeding and mild cold exposure correlates closely with increased plasma norepinephrine concentrations 53. At the molecular level, animal experiments have indicated an indispensible role of UCP1 in mediating both NST and DIT. Thus, UCP1-ablated mice are more susceptible to cold and have to rely on shivering for thermoregulation 31. When kept at thermoneutrality (30 °C for mice), UCP1 knockout mice lack DIT and develop obesity 33, indicating that diet-induced thermogenesis is fully dependent on UCP1. Although these data suggest a convergence of NST and DIT around brown fat and UCP1, there is an important distinction between them. In non-shivering thermogenesis, heat produced is used to maintain body temperature. By contrast, heat produced by diet-induced thermogenesis is quickly dissipated to the environment to prevent body temperature from rising.
Neuronal and hormonal regulation of adaptive thermogenesis
Thermogenesis is under tight control by the nervous and endocrine systems. Hypothalamic nuclei in the central nervous system (CNS) integrate stimuli from two separate pathways to regulate thermogenesis. One is a feed-forward pathway involving cutaneous thermal receptors acting via thermosensory neurons. These cause GABAergic interneurons in the preoptic area (POA) of the hypothalamus to disinhibit thermogenesis-promoting neurons in the dorsomedial hypothalamus and thereby drive non-shivering thermogenesis in BAT 54,55 56. The second pathway is a negative feedback pathway involving temperature-sensitive neurons in the brain, which inhibits sympathetic nervous system (SNS) outflow to BAT. The pathways underlying shivering thermogenesis are less understood and involve signals which travel through the pontine parabrachial nucleus 57 and are then integrated in POA 58 to activate α-motoneurons that stimulate shivering 59,60.
A key question for drug development targeting adaptive thermogenesis is whether the central pathways stimulating ST, NST and DIT are necessarily connected or if they can be selectively activated. Clearly there are circumstances where BAT activation occurs independently of shivering. In humans, for example, when cold exposure is not extreme, NST precedes shivering thermogenesis 61 62. It is not yet known if this separation between NST and ST results from separated pathways or merely the magnitude of cold exposure.
Adaptive thermogenesis is regulated primarily by the sympathetic nervous system. In response to cold exposure or overfeeding, norepinephrine released from the SNS regulates brown adipocytes at multiple levels: it promotes proliferation and differentiation of brown preadipocytes, directly regulates the thermogenic program of BAT by activation of UCP1 gene, and protects brown adipocytes from TNFα-induced apoptosis 63.
Adaptive thermogenesis is also modulated by hormones. Type 2 iodothyronine deiodinase plays a critical role in regulating the amount of active thyroid hormone, triiodothyronine, in brown fat, thereby modulating adaptive thermogenesis 64,65. In addition, leptin, released by white adipocytes, regulates energy balance by effects on the hypothalamus that lead to inhibition of food intake and increased thermogenesis via activation of the SNS, though to date there is limited evidence that leptin mediates thermogenesis via the SNS in humans 66–70. Insulin can affect thermogenesis by increasing substrate uptake by BAT and increasing hypothalamic-mediated sympathetic activity 71, both of which may be connected to the thermic effect of food 72. Glucagon and epinephrine also increase oxygen consumption, but both may be permissive, rather than regulatory, in thermogenesis, in that they provide fuel for thermogenesis, but do not appear to have a primary role in temperature homeostasis. Glucocorticoids similarly do not directly increase thermogenesis, but they may have an important role in coordinating the thermogenic response to substrate and food availability 65.
Brown fat in human thermogenesis -- an old concept revisited
Rediscovery of brown fat in humans
Brown fat is important for a thermogenic response and energy balance in small mammals. Induction of BAT in mice promotes energy expenditure, reduces adiposity, and protects mice from diet-induced obesity 73,74. Conversely, ablation of BAT leads to reduced energy expenditure and increased obesity on high fat diet 75. In humans, the role of BAT has been more controversial,. Histological evidence has indicated that brown fat is present, albeit in small amounts, in adults, throughout life 76, but attempts to find functional BAT 77 or utilize its thermogenesis for weight loss 78,79 have been largely unsuccessful. This has led to the widely held belief that there is no functional BAT in normal adult humans 80,81
However, this dogma has recently been reversed by studies using PET/CT imaging. PET, or positron emission tomography, uses radiotracers such as 18F-fluorodeoxyglucose (18F-FDG) to measure the metabolic activity of different regions of the body. CT, or computed tomography, provides high-resolution anatomical detail. Fusion of the PET and CT images therefore simultaneously provides both functional and precise structural information, which has been mainly used for detection and staging of tumors 82. The possibility that this FDG-avid adipose tissue could represent functional BAT was first noted in the radiological literature 83,84 and a potential physiological role in humans was suggested by Nedergaard et al. 80,81. However, it was only during the past year PET/CT imaging was used to prove conclusively that adult humans possess physiologically active UCP1-positive BAT,13–17. This identification of functional brown adipose tissue in adult humans has led to a rethinking within the medical and scientific communities that BAT may play a role in normal physiology and could be a target for obesity treatment 81,85.
The location of BAT in adult humans was also unexpected. In rodents and human babies, adult human BAT is interscapular. In adult humans, on the other hand, the most common location for metabolically active BAT is the cervical–supraclavicular depot, in a distinct fascial plane in the front of the neck, sometimes extending into the thoracic and lumbar region. The percentage of adult humans with functional BAT under normal conditions is unresolved. Retrospective case series using 18F-FDG PET/CT report this to be less than 10% 13,83. However, histological analysis of cervical fat biopsies show rates three times higher 17, and prospective studies using cold stimulation to increase BAT activity and detection via PET/CT show that among younger people, 96% have functional BAT 14. This is in part due to fundamental limitations in PET/CT imaging technology for quantifying BAT mass and activity. CT alone cannot yet distinguish brown versus white fat. Complicating the PET image is the need to see a concentrated signal above background, and a variety of factors can alter 18F-FDG uptake, including dietary fatty acids and drugs such as beta-adrenergic blockers. In rodents, small collections of physiologically relevant BAT have been found in the hindlimb in intermuscular adipose tissue and mixed with white fat 86,87. Whether humans could have such scattered small collections of BAT remains unknown. Improvements in imaging technology will be essential to identify small or scattered depots and also essential for studies devoted to exploiting BAT energy expenditure for the treatment of obesity. Given the scanning data and the finding of brown adipocyte precursors in human tissues 17, it is likely that virtually every adult human has the capacity to develop some functional BAT.
Some parameters of human BAT function have already been defined. PET/CT positivity shows seasonal variation 13,16,88, indicating a role of BAT in normal adaptation to cold. More importantly, BAT activity correlates inversely with BMI13,14 and percentage body fat 13,14, suggesting a role in energy balance. This inverse correlation between BAT activity and obesity was seen despite the fact that the lean and overweight subjects had similar resting metabolic rates in both thermoneutral and cold exposure, suggesting that overweight people increase their energy expenditure using different physiological mechanisms 14. Functional BAT also decreases with age, and is rarely observed in non-stimulated [i.e. no investigator-controlled stimulation such as cold or pharmacological interventions], overweight adults over 64 years-old 13, suggesting a possible role of decreased BAT activity in the development of age-related obesity. Together, these findings suggest that drugs which can increase BAT activity may be useful in combating obesity, and in older adults, may help restore a component of normal human physiology.
Regulation of brown fat development
Cellular lineage specification
Adipose tissue is generally considered to arise from the multipotent mesenchymal stem cells of mesodermal origin 89. Emerging evidence suggests that brown adipocytes located in different anatomical locations may arise from different developmental origins. In vivo fate mapping has shown that progenitors derived from the central dermomyotome give rise to the interscapular brown fat cells 90, suggesting that the interscapular brown fat and skeletal muscle may share a common developmental ancestry. In support of this notion, cultured brown fat precursors appear to possess a myogenic signature, which includes myf5 91. Most recently, lineage-tracing studies have indicated that myf5-expressing progenitors can give rise to both skeletal muscle and the preformed BAT, in the interscapular and peri-renal depots 92 (Figure 3).
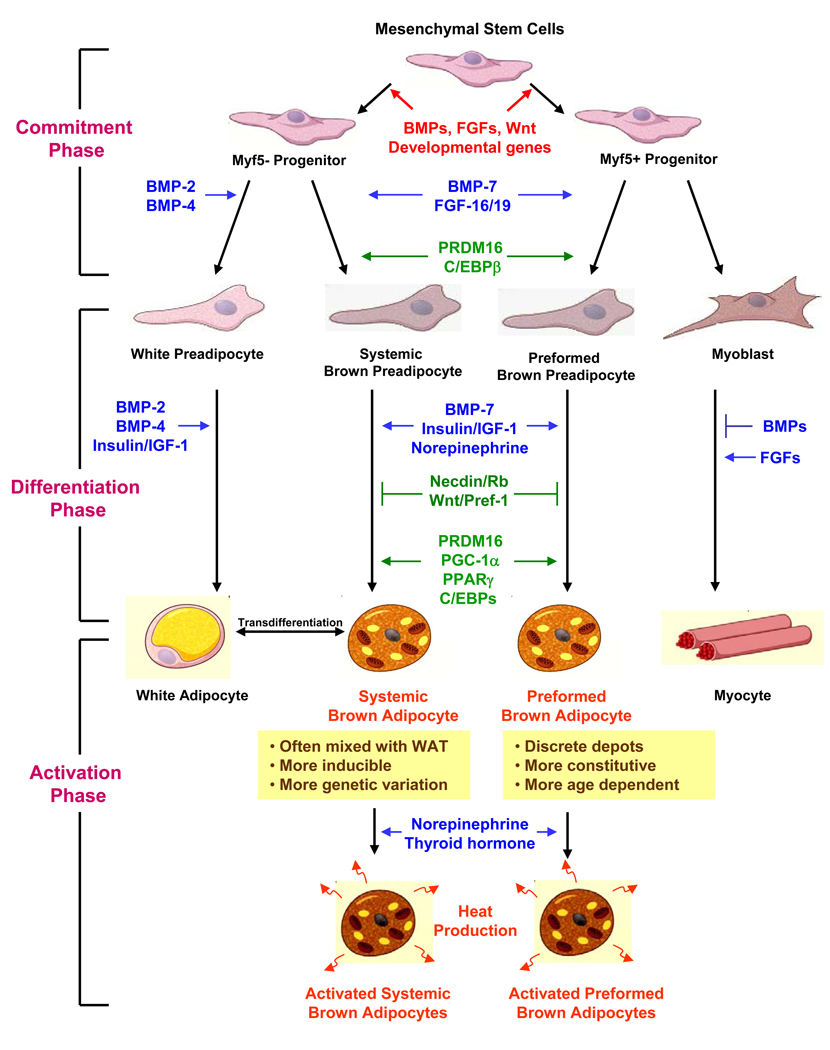
Figure 3. Lineage determination and control of brown adipocyte development.
In this model, we propose that there are distinct progenitors giving rise to the preformed versus systemic brown adipocytes. While the myf5-expressing progenitors give rise to skeletal muscle and interscapular brown fat 92, a distinct myf5-negative tissue resident progenitor serves as the common precursor for white adipocyte and the systemic brown adipocyte. The development of a fully functional brown adipocyte can be divided into three phases. The “commitment phase” is when multipotent mesenchymal stem cells become committed to brown adipocyte lineage in response to developmental cues, such as BMPs and FGFs. The “differentiation phase” is committed brown preadipocytes undergo a series of morphological and enzymatic changes to become rounded lipid-containing fat cells. This process is regulated by a number of growth factors and hormones and involved activation of transcriptional cascade. The “activation phase” refers as the stage when the maximal thermogenic capacity in matured brown adipocytes is turned on by hormonal or sympathetic stimulations.
However, not all brown fat cells are derived from myf5-expressing progenitors. For example, the brown fat cells emerging in white fat in response to β3-adrenergic stimulation are not marked by the myf5-driven fluorescent protein 92. When stimulated by PPARγ agonist, they express molecular characteristics distinct from the interscapular brown fat cells 93. Likewise, we have found that myf5-negative progenitors isolated from adult mouse skeletal muscle can differentiate into brown fat in vitro (Tseng et al., manuscript submitted). Thus, there exists a second class of progenitors that serve as a common precursor for white adipocytes and systemic brown adipocytes (Figure 3). It is also possible that some UCP1 positive brown fat cells found in WAT or skeletal muscle come from transdifferentiation of white adipocytes 94. Whatever their origin, these systemic brown fat cells are found in white fat and between muscle bundles, and possess distinct features compared with the interscapular brown adipocytes. These systemic brown adipocytes are often found admixed with white fat cells; are more sensitive to β3-adrenergic stimulation and cold exposure; and their thermogenic capacity appears to be regulated by genetic background 86,87,74. Interestingly, intermuscular brown adipocytes are more abundant in the obesity-resistant strain of mice 86, and high-fat feeding does not alter UCP1 expression in skeletal muscle 95, suggesting a critical role of these systemic brown adipocytes in protection against obesity. In humans, interscapular BAT is only a transient phenomenon in newborns 96 and is replaced in adults by BAT in the neck and other anatomical locations. Which, if either, population of progenitors gives rise to this adult human brown fat remains to be determined, however, the brown adipocytes present in the neck are often admixed with white adipocytes and appear to be very sensitive to activation by cold exposure.
Stages and signals inducing brown fat development
The development of fully functional brown adipocytes can be divided into three phases: a “commitment phase”, a “differentiation phase” and an “activation phase” (Figure 3). Several developmental signaling molecules implicated in the evolution of mesodermal tissue have been shown to impact early stages of brown fat development. These include nodal, wingless, members of the fibroblast growth factor (FGF), transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMPs) families and others 89. The exact effects of these factors depend on concentration, stage of differentiation, cell-cell interactions, and the nature of the extracellular matrix.
While TGF-β inhibits adipocyte differentiation in vitro 97 and TGF-β expression in fat is paradoxically increased with obesity in rodents and humans 98,99. BMPs are a family of developmental regulators belonging to the TGF-β superfamily, which promote adipogenesis at different stages 100. While BMP-2 and 4 promote white fat differentiation, BMP-7 serves as a potent inductive signal for brown adipogenesis 101. BMP-7 activates a full program of brown adipogenesis including induction of early regulators of brown fat fate - PRDM16 and PGC-1α, increased expression of adipogenic transcription factors - peroxisome proliferator-activated receptor (PPAR)γ and CCAAT/enhancer-binding proteins (C/EBPs), mitochondrial biogenesis, and increased expression of UCP1 (Figure 3). Moreover, BMP-7 triggers commitment of mesenchymal progenitor cells to a brown adipocyte lineage and also plays a critical role in embryonic brown fat development, as exemplified by the BMP-7 knockout embryo that shows a marked paucity of brown fat and near complete absence of UCP1 101. Adenoviral-mediated expression of BMP-7 in mice results in a significant increase in brown, but not white, fat mass and leads to an increase in energy expenditure and reduced weight gain 101. In addition, mice deficient in Growth Differentiation Factor (GDF) 3, another member of the TGF-β/BMP superfamily, are protected from diet-induce obesity due to an increased basal metabolism rate, which is presumably caused by the occurrence of UCP1-positive systemic brown adipocytes within white adipose tissue 102.
The other important developmental signaling system guiding brown fat development is the FGF family. bFGF stimulates the growth of brown fat precursors and may contribute to cold-induced enlargement of brown fat via an autocrine mechanism 103. FGF-16 mRNA is expressed predominantly in brown adipose tissue during embryonic days 17.5–19.5, and thereafter at lower levels into the neonatal period 104. Transgenic mice overexpressing FGF-19 have increased BAT mass and reduced susceptibility to diet-induced obesity 105. Interestingly, mice that overexpress FGF-21, a circulating FGF which is induced by a ketogenic diet 106, are lean and have increased brown fat 107, suggesting that the beneficial metabolic effects induced by a ketogenic diet are brought about in part by increases in BAT mass. Recent identification of the klotho gene family as cofactors essential for FGF function has added complexity to the role of FGF signaling in brown fat development. Disruption of the klotho gene in mice results in almost complete absence of WAT, while BAT is present but reduced in size and UCP1 protein expression. These mice also have lower core body temperature 108.
Norepinephrine plays an important role in regulating proliferation and differentiation of brown preadipocytes, as well as directly modulating the thermogenic function in mature brown adipocytes. In addition, the insulin/IGF-1 signaling system also exerts an essential role in brown fat differentiation and function (Figure 3). Brown preadipocytes lacking the insulin receptor substrate (IRS)-1 display a severe defect in differentiation 109; and IRS-2 null brown adipocytes have impaired glucose uptake function 110. Both insulin and IGF-1 protect these precursor cells from apoptosis, and this effect is mainly mediated by IRS-1 111.
Muscle as a thermogenic organ
After BAT, skeletal muscle is the other important organ for thermogenesis 46. Three types of thermogenesis occur in skeletal muscle: exercise-induced thermogenesis, non-exercise activity thermogenesis (NEAT), and cold-induced shivering thermogenesis (ST). There is no doubt that exercise has profound beneficial effects on virtually all biological systems, and is an effective way to burn excess energy. For an, 80 kg man, jogging 40 min at 6 miles (10 km) per hour will burn about 535 kcal (480 kcal above BMR). If he does this three times a week and does not increase food intake, he would be in negative caloric balance equivalent to 18.3 lbs (8.3 kg) of fat over the course of a year. NEAT is energy burned by movement other than exercise, such as fidgeting, maintenance of posture, and other activities of daily life, and has been found to play an important role in dissipating excess energy to preserve leanness in adult humans 43. NEAT is highly variable and ranges from ~ 15 % of total daily energy expenditure in very sedentary individuals to >50% in highly active persons. NEAT is also generally higher in lean than obese individuals. Indeed, lean and obese individuals are different in the energy dedicated to NEAT on average 350 kcal per day (equivalent to 30.3 pounds of fat over a year)112. ST is the physiological response to help protect body temperature from cold exposure. Shivering thermogenesis occurs in muscle and complements non-shivering thermogenesis, which occurs primarily in BAT in response to cold. Up to 250 kcal per hour are consumed by shivering 113, but this response is highly variable, usually of short duration, and not a likely method for therapeutic intervention.
Skeletal muscle contains different types of myofibers that differ in speed of contraction, mitochondrial content, and pattern of energy use. Type I (red) myofibers have a slow-twitch speed of contraction, a higher mitochondrial content, and thus a higher rate of oxidative metabolism. Type II (white) myofibers have a faster speed of contraction and both oxidative and glycolytic properties. Endurance exercise training triggers a remodeling of skeletal muscle by increasing expression of genes involved in mitochondrial respiration and fatty acid oxidation, which helps protect against obesity and related metabolic disorders 114. Chronic cold exposure may also trigger a switch from fast- to slow-twitch muscle with more oxidative myofibers by inducing the expression of nuclear coactivator PGC-1α, the same co-activator activated in brown fat in response to cold 115. Forced overexpression of PGC-1α in myotubes can produce the same change 116. On the other hand, thyroid hormone, which also increases thermogenesis, promotes formation of less oxidative fibers 46, indicating that different physiological stimuli regulate thermogenesis in muscle by different mechanisms.
Another important mechanism for heat production in skeletal muscle involves ATP turnover and maintenance of the calcium gradient mediated by the sarcoplasmic reticulum calcium ATPases (SERCA proteins) (Figure 2c). Neurotransmitter-mediated opening of cell-surface sodium channels leads to release of Ca2+ into the cytoplasm from sources both outside the cell and the sarcoplasmic reticulum via the ryanodine receptor (RyR). Dysfunction of this receptor leading to uncontrolled Ca2+ release and excessive thermogenesis can lead to malignant hyperthermia 44. Ca2+ leads to heat generation from ATP hydrolysis during both muscle relaxation and actinomyosin cross-bridge cycling during sustained contraction. Additional heat energy is released when Ca2+ ions are pumped back into the sarcoplasmic reticulum by SERCA. Cold exposure induces expression and increases activity of SERCA-1 in skeletal muscle to increase muscle oxidative metabolism and heat production 117.
Ephedrine is a mixed sympathomimetic capable of directly activating both α and β adrenergic receptors and enhancing release of norepinephrine from sympathetic neurons 118. Astrup et al. estimated that up to 50% of the increase in metabolism in lean men induced by ephedrine is attributable to skeletal muscle, and 24% is contributed by BAT 119. However, considering the relative mass of these tissues, BAT is 100–200 times more effective as a thermogenic organ per gram of tissue than muscle. Moreover, these calculations were performed focusing only on the minor perirenal BAT depot, suggesting that the contribution of total body BAT to thermogenesis is even greater. Mild cold exposure (16 °C) also induces muscle mitochondrial uncoupling and increases energy expenditure via NST 45. However, the recent identification of brown adipocytes interspersed between muscle bundles in mice 86 opens up the question whether some of the measured NST energy expenditure in skeletal muscle comes from these interspersed brown adipocytes. Since the extent of intermuscular brown fat is determined by genetic factors, this systemic brown adipocytes could play a role in the large variations in energy expenditure between individuals.
Therapeutically targeting bioenergetics
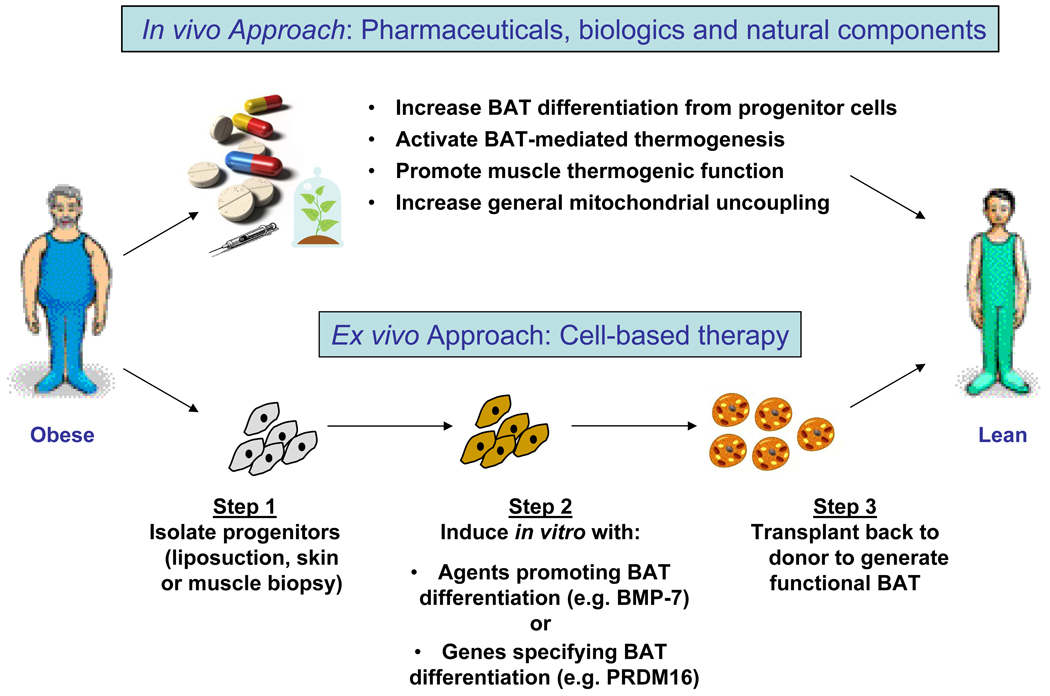
With the recognition that adult humans have brown adipose tissue, targeting cellular bioenergetics has become an increasingly attractive way to dissipate excess energy and provide a potential therapeutic approach for the treatment or prevention of obesity and it associated diseases. Approaches to increase adaptive thermogenesis may include small molecule pharmaceuticals, growth factors, and nutritional factors, as well as cell-based or ex vivo therapy (Figure 4). Based on the current knowledge of bioenergetics, four potential therapeutic approaches could be envisioned: (1) increasing brown fat differentiation from progenitor cells, (2) activating brown fat thermogenesis, (3) promoting skeletal muscle thermogenesis, or (4) increasing general mitochondrial uncoupling.
Figure 4. Approaches to increasing thermogenesis as an anti-obesity therapy.
Based on the current knowledge of bioenergetics, four potential therapeutic approaches could be envisioned: (1) increasing brown fat differentiation from progenitor cells, (2) activating brown fat thermogenesis, (3) promoting skeletal muscle thermogenesis: or (4) increasing general mitochondrial uncoupling. For skeletal muscle, there are three types of thermogenesis: exercise-induced thermogenesis, non-exercise activity thermogenesis, and cold-induced shivering thermogenesis. Thus, therapeutic interventions that mimic these mechanisms could potentially increase muscle’s thermogenic capacity and counteract obesity. This is especially beneficial to individuals with physical limitations in exercising or those who are genetically predisposed to obesity. All of these approaches can be applied in the conventional pharmaceutical approaches of developing drugs and/or using natural food components targeting key pathways of cellular bioenergetics. Alternatively, there is a cell-based therapy where progenitors are isolated from patients during liposuction or biopsy, manipulated ex vivo by treating them with factors that promote BAT differentiation or transfecting them with genes specifying BAT differentiation, then transplanted these cells back into the same individual to generate a functional brown fat to help dissipate excess energy.
Increasing brown fat differentiation from progenitor cells
Recently, brown fat progenitors have been identified in skeletal muscle and white fat of humans 120,121, suggesting that it may be possible to increase oxidative capacities in these tissues by targeting these endogenous precursors to differentiate in vivo, and produce energy-dissipating BAT. However, factors that regulate differentiation and function of these progenitors remain to be determined. Among the various newly identified factors that control the commitment and differentiation of brown fat progenitors discussed above, secreted proteins provide the most directly druggable agents. Indeed, both BMP-7 and FGFs are of direct therapeutic potential for obesity and its related metabolic disorders. Originally identified as a bone inducer, BMP-7 is now being recognized as a multifunctional cytokine and has been implicated as a potential therapeutic agent for cardiovascular, metabolic, and degenerative diseases as mostly validated in mouse models 122. Because of its important role in renal development and maintenance of normal kidney function in adult life, BMP-7 has been proposed as a therapeutic agent for chronic kidney disease, such as diabetic nephropathy 123,124. In addition, BMP-7 has been found to exert a neuroregenerative effect for treatment of ischemic stroke or Parkinson’s disease 125–127. BMP-7 has also been shown to reverse endothelial-to-mesenchymal transition associated with cardiac fibrosis 128 and facilitates liver regeneration 129. BMP-7 is pharmaceutically available and is already in use for orthopedic procedures in the US under Humanitarian Device Exemptions. When systemically expressed in mice, BMP-7 leads to reduced weight gain 101, suggesting that it may have anti-obesity potential, although more study is needed to determine an optimal therapeutic approach and if there will be any unwanted side effects. It is possible that tissue- or action-selective forms of BMP-7 could be developed, eliminating the bone-inducing effect, but retaining its brown adipogenic effects.
Recently, there is a growing interest in exploring the pharmacological potential of the FGF family in the treatment of metabolic diseases 130. As discussed above, FGF-16, FGF-19, and FGF-21, together with the klotho family of cofactors, have been implicated in the regulation of brown fat development. While the biology of this family of growth factors still needs to be fully exploited, development of recombinant FGFs and small molecule mimetics may hold therapeutic potential for the treatment of obesity. In addition to these secreted factors, the development of agents targeting key regulators of brown adipogenesis, such as PRDM16, PGC-1α, Rb or necdin 115,131–133, warrants further investigation.
Alternatively, an ex vivo approach could be used to create “thermogenic” cells that could be reimplanted in obese individuals (Figure 4). Recent advances in stem cell biology open up the possibility of isolating progenitor cells from an individual (by liposuction or muscle biopsy), stimulating them ex vivo by factors that promote BAT differentiation (such as BMP-7 or FGFs) or transfecting them with genes that specify BAT development (such as PRDM16 or PGC-1α). These cells could then potentially be transplanted back to the donor to allow in vivo expansion and differentiation into functional brown fat that could help burn energy. Because this approach involves minimal surgical procedures, it may represent an attractive alternative to those obese individuals who are unwilling to undergo liposuction or bariatric surgery for weight loss. Clearly, additional experiments in animal models will be needed to determine the optimal locations of engineered-cell implantation and assess the efficacy and potential side effects before this therapy becomes feasible.
Activating brown fat-mediated thermogenesis
Although it has only recently been proven that adult human BAT is functional and may be involved in protecting against weight loss 13–17, attempts to exploit BAT energy expenditure for treating obesity have been around for decades. Ephedra spp. is a family of plants of over 40 species, many indigenous to China, known as ma huang. Among its myriad of chemical compounds, ephedra contains the alkaloids ephedrine, pseudoephedrine, and other sympathomimetics that can induce central nervous system stimulation, bronchodilation, and vasoconstriction 118. A meta-analysis of several dozen trials showed that ephedrine promotes modest short-term weight loss, but there are no data on longer-term efficacy 134. PET/CT in rats shows that ephedrine’s effects on metabolism are mediated at least in part by activation of BAT 135. Unfortunately, ephedrine is associated with increased risk of psychiatric, autonomic, gastrointestinal, and cardiovascular symptoms 134, which likely will preclude the use of ephedrine itself for the treatment of obesity.
Caffeine is the most widely used psychoactive agent in the world 136. Caffeine is a trimethylxanthine that inhibits adenosine A2A receptors 137, thus stimulating the central nervous system and reducing the perception of fatigue. By itself, caffeine is not thought to stimulate the sympathetic nervous system enough to activate BAT 138. However, the combination of ephedrine and caffeine is a potent mediator of short-term weight loss 139 that likely involves activation of BAT by β3 adrenergic receptors 140.
Targeted approaches to activate BAT by selective sympathetic activation have had limited success thus far. In rodents, the β3-adrenergic receptor is found nearly exclusively on brown adipocytes, and treatment with the β3-specific agonist CL-316,243 substantially increases energy expenditure 18. Although human brown fat also expresses β3-adrenergic receptors 15, initial human trials using β3 receptor agonists were not successful, since human β3-adrenergic receptors have different binding characteristics than those in rodents 23. A second generation of β3 agonists with better binding properties had poor oral bioavailability or unfavorable pharmacokinetics 141. In addition, these studies were done before PET/CT scanning was known to be useful to specifically measure BAT function and mass. One β3 agonist, L-796568, increased lipolysis and energy expenditure in overweight men acutely 142, but its effect seemed to be lost after 28 days of treatment 79,141. Given the new ability to quantify human brown adipose tissue activity, attempts at therapeutically developing β3-adrenergic agonists and other drugs that activate BAT or stimulate BAT growth requires re-evaluation. For if one can measure BAT activity, then one can know whether or not a given intervention worked as designed. As an example, when testing beta3 adrenergic agonists designed to stimulate adipose tissue activity, quantifying BAT activity will validate whether any increases in energy expenditure were due to changes in WAT, BAT, both, or neither
The principal safety considerations regarding increasing BAT thermogenesis involve the thermodynamic implications of this therapy and raise a number of questions. How easily regulated is BAT thermogenesis? Would stimulated BAT induce necrosis? Could the body temperature rise to dangerous levels as seen with DNP 143? Will there be such an increased demand for cardiac output that treatment will be contraindicated in the elderly and those with heart disease? Going forward, these considerations must remain in the forefront of efforts designed to induce weight loss through BAT-mediated energy expenditure. Fortunately, BAT is not a simple combustion engine, but an exquisitely regulated biological tissue with internal negative feedback pathways 30,30.
Early attempts to use thyroid hormone (TH) to increase thermogenesis and induce weight loss were confounded by tachycardia, bone loss, and muscle catabolism 144. Current approaches focus on TH mimetics that selectively increase energy expenditure, in part by activating specific TH receptor isoforms. Indeed, two of the selective TH mimetics, GC-1 and KB141, can promote fat loss in rodents without causing unwanted effects on heart or muscle 145,146. Recently, a new role in TH-mediated thermogenesis was observed in response to bile acids 147. Bile acids are normally ligands for the nuclear hormone receptor farnesoid X receptor alpha, which regulates the enterohepatic lipid recycling and causes downregulation of hepatic fatty acid and triglyceride biosynthesis. Bile acids also increase energy expenditure in brown adipose tissue, preventing obesity and resistance to insulin. This effect is dependent on induction of the type 2 iodothyronine deiodinase and is mediated by the binding of bile acids to a novel G-protein-coupled receptor TGR5 148. In addition, TGR5 stimulates GLP-1 production in enteroendocrine cells that may improve glucose metabolism through its insulinotropic effects. TGR5 is therefore a new and attractive target for treating obesity, since it can theoretically protect against obesity and its complications via two distinct and potentially synergistic mechanisms: increased energy expenditure and improved insulin sensitivity 148. One promising TGR5 agonist, INT-777, has already shown efficacy in vivo, increasing energy expenditure and reducing adiposity in mice with diet-induced obesity 148,149.
The adipokine leptin both decreases appetite and increases energy expenditure and is able to completely reverse obesity in the leptin-deficient ob/ob mouse 150. However, leptin deficiency is rare in humans, and most obese humans suffer from leptin resistance,, so administration of leptin shows effects in only a small fraction of patients 151. Relative leptin deficiency, such as occurs in congenital or acquired lipodystrophies, thin, very athletic women with hypothalamic amenorrhea, and anorexia nervosa, does respond to leptin administration 152 153. Promise has been seen in an approach that combines metreleptin (recombinant human methionyl-leptin) treatment with the amylin analogue, pramlintide, which has been suggested to act as a leptin sensitizer 154 (Table 1A).
Promoting skeletal muscle’s thermogenic function
Given the numerous health benefits of exercise, the idea of a pill that mimics the effects of exercise is very attractive 46, especially in individuals with physical limitations in exercising or those who are genetically predisposed to obesity 114. Resveratrol (3,5,4'-trihydroxystilbene), a polyphenol found in grape skins, red wine, peanuts, and mulberries, can improve exercise endurance and protect mice against diet-induced obesity and insulin resistance 155. This effect is mediated by increasing mitochondrial biogenesis and oxidative phosphorylation via activation of the NAD+-dependent deacetylase SIRT1 and PGC-1α complex (Table 2). Resveratrol treatment leads to increased lifespan and improved metabolic profile and activity levels in mice with high fat diet-induced obesity 156. Preclinical observations have suggested that resveratrol and its analogues such as SRT1720 are safe and may have applications in the treatment of obesity and insulin resistance in humans. At this time, resveratrol has not yet been demonstrated to affect BAT directly. However, SRT1720 has been shown in BAT to modify lipid droplet size and gene expression 157,158.
Another enzyme central to cellular bioenergetics is AMP-activated protein kinase (AMPK), which detects the nutrient status of the cell and helps regulate glucose transport, fatty acid oxidation, and metabolic adaptations in skeletal muscle 159. Chronic activation of AMPK by 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR, Table 2) enhances mitochondrial function in skeletal muscle 159. Recent studies show that AMPK also enhances SIRT1 activity by increasing cellular NAD+ levels, resulting in the deacetylation and modulation of the activity of downstream SIRT1 targets 160. Pilot studies are currently underway using resveratrol (NCT00654667) or AICAR (NCT00168519) to treat metabolic diseases. Recently, Narkar et al., showed that treatment of mice with a combination of AICAR and GW1516, an agonist of muscle-specific transcriptional regulator PPARδ, synergistically increased oxidative myofibers and running endurance 161. While these data suggest a potential use of these compounds in improving skeletal muscle function and increasing energy expenditure, the safety issue of using AICAR or other drugs targeting AMPK needs to be considered 162. New promising candidates include A769662, a non-nucleoside compound thienopyridone that activates AMPK to stimulate glucose uptake in vitro 163.
Increasing general mitochondrial uncoupling
Increased mitochondrial uncoupling leads to energy inefficiency and increased energy expenditure. DNP, a non-selective uncoupler of mitochondrial oxidation, at 3–5 mg/kg, in humans led to a 20–30% increase in energy expenditure that could be sustained without tolerance 18. Unfortunately, DNP itself cannot be used as a therapy because of its narrow therapeutic window and serious adverse effects related to overdose 143. However, if safety can be proven, increasing mitochondrial uncoupling may represent a potential therapeutic approach. A new class of molecules that includes butylated hydroxytoluene utilizes the mitochondrial adenine nucleotide translocase to induce limited uncoupling at low concentrations and can have a dynamic range of more than a million fold in vitro. Though still years from clinical use, these compounds are attractive because of their small size and attractive pharmacokinetics 164.
Conclusions
With the growing worldwide epidemic of obesity, it is clear that new and effective anti-obesity therapies are desperately needed. Compelling data suggest that targeting cellular bioenergetics may provide an exciting new therapeutic approach for treatment or prevention of obesity. However, due to the high safety concerns for anti-obesity treatments, both the conventional pharmaceutical approach and the cell-based therapy require intensive benefit-risk assessments. Many questions remain to be answered before these therapies become possible. First, at the systemic level, it is not known whether increasing peripheral energy expenditure chronically will cause compensatory mechanisms, such as increased appetite, that might overcome its benefit. The exact contributions of brown fat and skeletal muscle in energy expenditure by adaptive thermogenesis in humans, especially in response to overfeeding, remain to be determined. At cellular and molecular levels, cellular lineage determination and factors determining the developmental fate of energy-dissipating brown fat need to be further elucidated. Together, answers to these questions would help in implementing the idea of targeting cellular bioenergetics to treat obesity and its many associated metabolic diseases.
Box 1 Mitochondrial ATP production
Carbohydrates and fats arrive into the cytoplasm for metabolism from two sources: uptake of extracellular substrates, such as glucose and free fatty acids, and intracellular release of substrates from the catabolism of cellular glycogen and lipid stores. Both processes are controlled by both nutrient availability and the action of hormones such as insulin, glucagon, and norepinephrine. In the cytoplasm, several preparatory steps take place before fuel substrates are transported into the mitochondrial matrix for energy production. Activated fatty acids and pyruvate are each metabolized to produce the same common intermediate, acetyl-CoA, which is fed into the tricarboxylic acid (TCA) cycle. Progressive steps of oxidization yield carbon dioxide and reduced forms of NADH and FADH, which deliver their electrons to the electron transport chain (ETC).
The ETC is the principal site for the regulated release and transfer of chemical bond energy in eukaryotes. Its goal is to preserve as much of the electrons’ potential energy for generation of ATP, the chemical currency of life, as possible. This is accomplished by passing electrons through macromolecular complexes until they are accepted by molecular oxygen, which is split to generate water. As the ETC shuttles electrons through its machinery, hydrogen ions are drawn from the mitochondrial matrix and deposited in the intermembrane space, creating an electrochemical gradient. The potential energy of this gradient is harnessed by the F1F0-ATPase, which straddles the inner mitochondrial membrane and converts the proton-motive force into the chemical bond energy of ATP. In the resting state, about 90% of cellular oxygen consumption takes place in the mitochondria, and 80% of this is coupled to ATP synthesis 2.
Box 2 Molecular controls of brown fat development
At the molecular level, brown fat differentiation involves mechanisms common to both brown and white adipocyte differentiation, as well as some specific factors 89,165–167. Prior to adipogenic transcriptional cascade initiation, both brown and white preadipocytes need to be released from suppression and become committed to terminal differentiation. Among the known inhibitors of preadipocyte-adipocyte transition, proteins of the retinoblastoma (Rb) family, and necdin, a growth repressor functionally resembling Rb, selectively inhibit brown preadipocyte differentiation 132,133,168
After release from suppression, the committed brown preadipocytes initiate a transcriptional cascade involving the transcription factors C/EBPs and PPARγ to turn on lipid synthesis and other adipocyte specific programs. A number of transcription factors and co-regulators appear to play particularly important roles in the final stages of differentiation of BAT and modulation of the expression of thermogenic genes, especially UCP1. Nuclear co-repressor RIP140 directs histone and DNA methylation to silence UCP1 expression and suppress mitochondrial biogenesis in white adipocytes 169,170. The zinc-finger containing protein, PRDM16, which is expressed at higher levels in brown compared with white adipocytes 131, has been shown to drive differentiation of white preadipocytes or myoblasts into functional brown adipocytes. This effect depends on the interaction of PRDM16 with PGC-1α/β, PPARγ and C/EBPβ, while binding of PRDM16 to CtBP-1 and CtBP-2 suppresses expression of white fat-selective genes 171,172. Current data suggest that in rodents, PRDM16 plays an important role in specifying brown fat cell fate for the interscapular, but not systemic brown adipose depots 92.
Adaptive thermogenesis: Heat production in response to environmental temperature or diet. It serves the purpose of protecting the organism from cold exposure or regulating energy balance after changes in diet. Brown fat and skeletal muscle are the two principal sites of adaptive thermogenesis.
Bioenergetics studies the flow of chemical bond energy within organisms. In a living cell, the principal reactions of fuel metabolism take place in the mitochondria, where food energy is released; oxygen is consumed; and water and carbon dioxide are produced.
Basal metabolic rate (BMR): The energy expended by an individual when physically and mentally at rest 12–18 hours after a meal in a thermoneutral environment. It is similar to SMR, although is now usually applied to human metabolism only.
Resting metabolic rate (RMR): is the amount of energy expended at rest. It is also similar to SMR, except the metabolic rate is measured while the organism is still digesting food.
Standard metabolic rate (SMR): The steady-state rate of energy utilized by a whole organism that is awake but resting, stress free, not actively digesting food, and is at thermoneutrality.
Diet-induced thermogenesis: is the heat produced in response to diet that permit excessive caloric intake. It primarily occurs in brown fat.
Facultative energy expenditure: The energy spent in excess of the obligatory requirements; it is the energy expended above that required to maintain the BMR. It is controlled by the nervous system.
Obligatory energy expenditure: The minimal heat produced by all those processes that maintain the body in a basal state, fasting, at thermoneutral temperature.
Thermoneutrality: The environmental temperature where heat production is not stimulated, e.g., ~28C for adult humans. In general, humans, usually make the microclimate thermoneutral through clothing choices.
Acknowledgements
We thank L. J. Goodyear for a critical reading of the manuscript. This work was supported in part by NIH grants DK077097 (Y.-H.T.), DK082659 (C.R.K), DK046200, DK081604, RR025757 (A.M.C.) and Joslin Diabetes Center’s Diabetes and Endocrinology Research Center (P30 DK036836 from the NIDDK), and by funding from Harvard Stem Cell Institute (to Y.-H.T.), the Harvard Catalyst/Harvard Clinical and Translational Science Center, RR025758 (to Y.-H.T. and A.M.C.),. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National NIDDK or the NIH.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 3.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 4.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82 doi: 10.1093/ajcn/82.1.222S. 222S–225S. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan LM. Pharmacological therapies for obesity. Gastroenterol. Clin North Am. 2005;34:91–104. doi: 10.1016/j.gtc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Welle S, Forbes GB, Statt M, Barnard RR, Amatruda JM. Energy expenditure under free-living conditions in normal-weight and overweight women. Am J Clin Nutr. 1992;55:14–21. doi: 10.1093/ajcn/55.1.14. [DOI] [PubMed] [Google Scholar]
- 7.Melnikova I, Wages D. Anti-obesity therapies. Nat. Rev. Drug Discov. 2006;5:369–370. doi: 10.1038/nrd2037. [DOI] [PubMed] [Google Scholar]
- 8.Wells JC. Thrift: a guide to thrifty genes, thrifty phenotypes and thrifty norms. Int. J Obes. (Lond.) 2009 doi: 10.1038/ijo.2009.175. [DOI] [PubMed] [Google Scholar]
- 9.Speakman JR. A nonadaptive scenario explaining the genetic predisposition to obesity: the "predation release" hypothesis. Cell Metab. 2007;6:5–12. doi: 10.1016/j.cmet.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 11.Redman LM, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS. ONE. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 13.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 15.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 16.Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zingaretti MC, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009 doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 18.Harper ME, Green K, Brand MD. The efficiency of cellular energy transduction and its implications for obesity. Annu Rev. Nutr. 2008;28:13–33. doi: 10.1146/annurev.nutr.28.061807.155357. [DOI] [PubMed] [Google Scholar]
- 19.Gosselin C, Cote G. Weight loss maintenance in women two to eleven years after participating in a commercial program: a survey. BMC. Womens Health. 2001;1:2. doi: 10.1186/1472-6874-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam J, Fukumura D, Jain RK. A mathematical model of murine metabolic regulation by leptin: energy balance and defense of a stable body weight. Cell Metab. 2009;9:52–63. doi: 10.1016/j.cmet.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow CC, Hall KD. The dynamics of human body weight change. PLoS. Comput. Biol. 2008;4:e1000045. doi: 10.1371/journal.pcbi.1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green DE, Zande HD. Universal energy principle of biological systems and the unity of bioenergetics. Proc Natl. Acad Sci U S. A. 1981;78:5344–5347. doi: 10.1073/pnas.78.9.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clapham JC, Arch JR. Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes. Metab. 2007;9:259–275. doi: 10.1111/j.1463-1326.2006.00608.x. [DOI] [PubMed] [Google Scholar]
- 24.Randle PJ, Garland PB, Hales CN, Newsholme FA. The glucose fatty-acid cycle: Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 25.Newsholme EA, Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem. Soc Symp. 1976:61–109. [PubMed] [Google Scholar]
- 26.Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–408. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 27.Klein S, Wolfe RR. Whole-body lipolysis and triglyceride-fatty acid cycling in cachectic patients with esophageal cancer. J Clin Invest. 1990;86:1403–1408. doi: 10.1172/JCI114854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990;258:E382–E389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- 29.Mazzucotelli A, et al. The transcriptional coactivator peroxisome proliferator activated receptor (PPAR)gamma coactivator-1 alpha and the nuclear receptor PPAR alpha control the expression of glycerol kinase and metabolism genes independently of PPAR gamma activation in human white adipocytes. Diabetes. 2007;56:2467–2475. doi: 10.2337/db06-1465. [DOI] [PubMed] [Google Scholar]
- 30.Golozoubova V, et al. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 31.Enerback S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 32.Kontani Y, et al. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging. Cell. 2005;4:147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 33.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonardsson G, et al. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl. Acad Sci U S. A. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can. J Physiol. Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 37.Mifflin MD, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 38.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond.) 1983;64:19–23. doi: 10.1042/cs0640019. [DOI] [PubMed] [Google Scholar]
- 39.Bouchard C, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 40.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav. Genet. 1997;27:325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 41.Christiansen E, Garby L, Sorensen TI. Quantitative analysis of the energy requirements for development of obesity. J Theor. Biol. 2005;234:99–106. doi: 10.1016/j.jtbi.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Human energy requirements: report of a joint FAO/ WHO/UNU Expert Consultation. Food. Nutr Bull. 2005;26:166. [PubMed] [Google Scholar]
- 43.Levine JA. Nonexercise activity thermogenesis--liberating the life-force. J Intern Med. 2007;262:273–287. doi: 10.1111/j.1365-2796.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- 44.Stowell KM. Malignant hyperthermia: a pharmacogenetic disorder. Pharmacogenomics. 2008;9:1657–1672. doi: 10.2217/14622416.9.11.1657. [DOI] [PubMed] [Google Scholar]
- 45.Wijers SL, Schrauwen P, Saris WH, Marken Lichtenbelt WD. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS. ONE. 2008;3:e1777. doi: 10.1371/journal.pone.0001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Himms-Hagen J. Exercise in a pill: feasibility of energy expenditure targets. Curr Drug Targets. CNS. Neurol Disord. 2004;3:389–409. doi: 10.2174/1568007043337076. [DOI] [PubMed] [Google Scholar]
- 47.Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond.) 1986;71:291–297. doi: 10.1042/cs0710291. [DOI] [PubMed] [Google Scholar]
- 48.Neumann RO. Experimentelle Beitrage Zur Lehre von den taglichen Nahrungsbedarf des Menschen unter besonderer Bernuksichtigung der notwendigen Eiweissmenge. Archiv fur Hygeine. 1902;45:1–87. [Google Scholar]
- 49.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 50.Trayhurn P, Thurlby PL, James WP. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- 51.Trayhurn P, Goodbody AE, James WP. A role for brown adipose tissue in the genesis of obesity? Studies on experimental animals. Proc Nutr Soc. 1982;41:127–131. doi: 10.1079/pns19820022. [DOI] [PubMed] [Google Scholar]
- 52.Mercer SW, Trayhurn P. Effect of high fat diets on energy balance and thermogenesis in brown adipose tissue of lean and genetically obese ob/ob mice. J Nutr. 1987;117:2147–2153. doi: 10.1093/jn/117.12.2147. [DOI] [PubMed] [Google Scholar]
- 53.Wijers SL, Saris WH, Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab. 2007;92:4299–4305. doi: 10.1210/jc.2007-1065. [DOI] [PubMed] [Google Scholar]
- 54.Hosaka T, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl. Acad Sci U S. A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol. Regul. Integr. Comp. Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat. Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? J Physiol. 1995;485((Pt 1)):195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka M, Owens NC, Nagashima K, Kanosue K, McAllen RM. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphe. J Physiol. 2006;572:569–583. doi: 10.1113/jphysiol.2005.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphe disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol. Regul. Integr. Comp. Physiol. 2008;294:R884–R894. doi: 10.1152/ajpregu.00655.2007. [DOI] [PubMed] [Google Scholar]
- 61.Vybiral S, Lesna I, Jansky L, Zeman V. Thermoregulation in winter swimmers and physiological significance of human catecholamine thermogenesis. Exp Physiol. 2000;85:321–326. [PubMed] [Google Scholar]
- 62.van Ooijen AM, Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR. Cold-induced heat production preceding shivering. Br. J Nutr. 2005;93:387–391. doi: 10.1079/bjn20041362. [DOI] [PubMed] [Google Scholar]
- 63.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 64.Golozoubova V, et al. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol Endocrinol. 2004;18:384–401. doi: 10.1210/me.2003-0267. [DOI] [PubMed] [Google Scholar]
- 65.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 66.Mistry AM, Swick AG, Romsos DR. Leptin rapidly lowers food intake and elevates metabolic rates in lean and ob/ob mice. J Nutr. 1997;127:2065–2072. doi: 10.1093/jn/127.10.2065. [DOI] [PubMed] [Google Scholar]
- 67.Commins SP, et al. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology. 1999;140:4772–4778. doi: 10.1210/endo.140.10.7043. [DOI] [PubMed] [Google Scholar]
- 68.Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nature Neuroscience. 1998;1:445–449. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 69.Harris RB. Leptin--much more than a satiety signal. Annu Rev. Nutr. 2000;20:45–75. doi: 10.1146/annurev.nutr.20.1.45. [DOI] [PubMed] [Google Scholar]
- 70.Rosenbaum M, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrannini E, et al. Insulin: new roles for an ancient hormone. Eur J Clin Invest. 1999;29:842–852. doi: 10.1046/j.1365-2362.1999.00536.x. [DOI] [PubMed] [Google Scholar]
- 72.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem. Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 74.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lowell BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 76.Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- 77.Astrup A. Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinol Suppl (Copenh.) 1986;278:1–32. [PubMed] [Google Scholar]
- 78.Weyer C, Tataranni PA, Snitker S, Danforth E, Jr, Ravussin E. Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective beta3-adrenoceptor agonist in humans. Diabetes. 1998;47:1555–1561. doi: 10.2337/diabetes.47.10.1555. [DOI] [PubMed] [Google Scholar]
- 79.Larsen TM, et al. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76:780–788. doi: 10.1093/ajcn/76.4.780. [DOI] [PubMed] [Google Scholar]
- 80.Cunningham S, et al. The characterization and energetic potential of brown adipose tissue in man. Clin Sci (Lond.) 1985;69:343–348. doi: 10.1042/cs0690343. [DOI] [PubMed] [Google Scholar]
- 81.Nedergaard J, Bengtsson T, Cannon B. Unexpected Evidence for Active Brown Adipose Tissue in Adult Humans. Am J Physiol. Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 82.Schoder H, Larson SM, Yeung HW. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl. Med. 2004;45 Suppl 1 72S–81S. [PubMed] [Google Scholar]
- 83.Hany TF, et al. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur. J Nucl. Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 84.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat ("USA-Fat"): description on 18F-FDG PET/CT. J Nucl. Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 85.Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes. Rev. 2009;10:265–268. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl. Acad Sci U S. A. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xue B, et al. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid. Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Cohade C, Mourtzikos KA, Wahl RL. "USA-Fat": prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl. Med. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 89.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 90.Atit R, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 91.Timmons JA, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl. Acad Sci U S. A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petrovic N, et al. Chronic PPAR{gamma} Activation of Epididymally Derived White Adipocyte Cultures Reveals a Population of Thermogenically Competent, UCP1-containing Adipocytes Molecularly Distinct From Classical Brown Adipocytes. J Biol Chem. 2009 doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cinti S. Transdifferentiation properties of adipocytes in the Adipose Organ. Am J Physiol. Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 95.Fink BD, et al. Mitochondrial proton leak in obesity-resistant and obesity-prone mice. Am J Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1773–R1780. doi: 10.1152/ajpregu.00478.2007. [DOI] [PubMed] [Google Scholar]
- 96.Lean MEJ, James WPT. Brown Adipose Tissue. In: Trayhurn P, Nicholls DG, editors. London: Edward Arnold; 1986. pp. 339–365. [Google Scholar]
- 97.Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem. 2003;278:9609–9619. doi: 10.1074/jbc.M212259200. [DOI] [PubMed] [Google Scholar]
- 98.Alessi MC, et al. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes. 2000;49:1374–1380. doi: 10.2337/diabetes.49.8.1374. [DOI] [PubMed] [Google Scholar]
- 99.Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3:37–48. [PMC free article] [PubMed] [Google Scholar]
- 100.Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine. Growth Factor. Rev. 2009;20:523–531. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tseng YH, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen JJ, et al. Deficiency of growth differentiation factor 3 protects against diet-induced obesity by selectively acting on white adipose. Mol Endocrinol. 2009;23:113–123. doi: 10.1210/me.2007-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamashita H, et al. Basic fibroblast growth factor (bFGF) contributes to the enlargement of brown adipose tissue during cold acclimation. Pflugers. Arch. 1994;428:352–356. doi: 10.1007/BF00724518. [DOI] [PubMed] [Google Scholar]
- 104.Konishi M, Mikami T, Yamasaki M, Miyake A, Itoh N. Fibroblast growth factor-16 is a growth factor for embryonic brown adipocytes. J Biol Chem. 2000;275:12119–12122. doi: 10.1074/jbc.275.16.12119. [DOI] [PubMed] [Google Scholar]
- 105.Tomlinson E, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 106.Badman MK, et al. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 107.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mori K, et al. Disruption of klotho gene causes an abnormal energy homeostasis in mice. Biochem. Biophys. Res. Commun. 2000;278:665–670. doi: 10.1006/bbrc.2000.3864. [DOI] [PubMed] [Google Scholar]
- 109.Tseng YH, Kriauciunas KM, Kokkotou E, Kahn CR. Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol Cell Biol. 2004;24:1918–1929. doi: 10.1128/MCB.24.5.1918-1929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fasshauer M, et al. Essential role of insulin receptor substrate-2 in insulin stimulation of glut4 translocation and glucose uptake in brown adipocytes. J Biol Chem. 2000;275:25494–25501. doi: 10.1074/jbc.M004046200. [DOI] [PubMed] [Google Scholar]
- 111.Tseng YH, Ueki K, Kriauciunas KM, Kahn CR. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J Biol. Chem. 2002;277:31601–31611. doi: 10.1074/jbc.M202932200. [DOI] [PubMed] [Google Scholar]
- 112.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 113.Badjatia N, et al. Predictors and clinical implications of shivering during therapeutic normothermia. Neurocrit. Care. 2007;6:186–191. doi: 10.1007/s12028-007-0011-2. [DOI] [PubMed] [Google Scholar]
- 114.Goodyear LJ. The exercise pill--too good to be true? N Engl J Med. 2008;359:1842–1844. doi: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]
- 115.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 116.Lin J, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 117.Arruda AP, et al. Cold tolerance in hypothyroid rabbits: role of skeletal muscle mitochondria and sarcoplasmic reticulum Ca2+ ATPase isoform 1 heat production. Endocrinology. 2008;149:6262–6271. doi: 10.1210/en.2008-0564. [DOI] [PubMed] [Google Scholar]
- 118.Nelson DL, Gehlert DR. Central nervous system biogenic amine targets for control of appetite and energy expenditure. Endocrine. 2006;29:49–60. doi: 10.1385/endo:29:1:49. [DOI] [PubMed] [Google Scholar]
- 119.Astrup A, Bulow J, Madsen J, Christensen NJ. Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. Am J Physiol. 1985;248:E507–E515. doi: 10.1152/ajpendo.1985.248.5.E507. [DOI] [PubMed] [Google Scholar]
- 120.Elabd C, et al. Human Multipotent Adipose-derived Stem Cells Differentiate into Functional Brown Adipocytes. Stem. Cells. 2009 doi: 10.1002/stem.200. [DOI] [PubMed] [Google Scholar]
- 121.Crisan M, et al. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem. Cells. 2008;26:2425–2433. doi: 10.1634/stemcells.2008-0325. [DOI] [PubMed] [Google Scholar]
- 122.Tobin JF, Celeste AJ. Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discov. Today. 2006;11:405–411. doi: 10.1016/j.drudis.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 123.Li T, Surendran K, Zawaideh MA, Mathew S, Hruska KA. Bone morphogenetic protein 7: a novel treatment for chronic renal and bone disease. Curr Opin Nephrol. Hypertens. 2004;13:417–422. doi: 10.1097/01.mnh.0000133974.24935.fe. [DOI] [PubMed] [Google Scholar]
- 124.Wang S, et al. Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney. Int. 2003;63:2037–2049. doi: 10.1046/j.1523-1755.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 125.Chou J, et al. Neuroregenerative effects of BMP7 after stroke in rats. J Neurol Sci. 2006;240:21–29. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 126.Harvey BK, et al. Neurotrophic effects of bone morphogenetic protein-7 in a rat model of Parkinson's disease. Brain Res. 2004;1022:88–95. doi: 10.1016/j.brainres.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 127.Zuch CL, et al. Beneficial effects of intraventricularly administered BMP-7 following a striatal 6-hydroxydopamine lesion. Brain Res. 2004;1010:10–16. doi: 10.1016/j.brainres.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 128.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 129.Sugimoto H, et al. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 2007;21:256–264. doi: 10.1096/fj.06-6837com. [DOI] [PubMed] [Google Scholar]
- 130.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seale P, et al. Transcriptional Control of Brown Fat Determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hansen JB, et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl. Acad Sci U S. A. 2004;101:4112–4117. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tseng YH, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat. Cell Biol. 2005;7:601–611. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- 134.Shekelle PG, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003;289:1537–1545. doi: 10.1001/jama.289.12.1537. [DOI] [PubMed] [Google Scholar]
- 135.Baba S, et al. Effect of nicotine and ephedrine on the accumulation of 18F-FDG in brown adipose tissue. J Nucl. Med. 2007;48:981–986. doi: 10.2967/jnumed.106.039065. [DOI] [PubMed] [Google Scholar]
- 136.Magkos F, Kavouras SA. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit. Rev. Food. Sci Nutr. 2005;45:535–562. doi: 10.1080/1040-830491379245. [DOI] [PubMed] [Google Scholar]
- 137.Huang ZL, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 138.Dhar R, et al. Cardiovascular toxicities of performance-enhancing substances in sports. Mayo. Clin Proc. 2005;80:1307–1315. doi: 10.4065/80.10.1307. [DOI] [PubMed] [Google Scholar]
- 139.Boozer CN, et al. Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int. J Obes. Relat Metab Disord. 2002;26:593–604. doi: 10.1038/sj.ijo.0802023. [DOI] [PubMed] [Google Scholar]
- 140.De Matteis R, et al. Immunohistochemical identification of the beta(3)-adrenoceptor in intact human adipocytes and ventricular myocardium: effect of obesity and treatment with ephedrine and caffeine. Int. J Obes. Relat Metab Disord. 2002;26:1442–1450. doi: 10.1038/sj.ijo.0802148. [DOI] [PubMed] [Google Scholar]
- 141.Arch JR. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from beta3-adrenoceptor agonists. Naunyn Schmiedebergs. Arch. Pharmacol. 2008;378:225–240. doi: 10.1007/s00210-008-0271-1. [DOI] [PubMed] [Google Scholar]
- 142.van Baak MA, et al. Acute effect of L-796568, a novel beta 3-adrenergic receptor agonist, on energy expenditure in obese men. Clin Pharmacol. Ther. 2002;71:272–279. doi: 10.1067/mcp.2002.122527. [DOI] [PubMed] [Google Scholar]
- 143.Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul. Toxicol. Pharmacol. 2007;48:115–117. doi: 10.1016/j.yrtph.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 144.Baxter JD, Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat. Rev. Drug Discov. 2009;8:308–320. doi: 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- 145.Villicev CM, et al. Thyroid hormone receptor beta-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J Endocrinol. 2007;193:21–29. doi: 10.1677/joe.1.07066. [DOI] [PubMed] [Google Scholar]
- 146.Bryzgalova G, et al. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. J Steroid Biochem. Mol Biol. 2008;111:262–267. doi: 10.1016/j.jsbmb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 147.Watanabe M, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 148.Thomas C, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tiwari A, Maiti P. TGR5: an emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov. Today. 2009;14:523–530. doi: 10.1016/j.drudis.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 151.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89 doi: 10.3945/ajcn.2008.26788B. p973S-979S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89 doi: 10.3945/ajcn.2008.26788E. p991S-997S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Welt CK, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 154.Roth JD, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl. Acad Sci U S. A. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 156.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21:1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- 158.Feige JN, et al. Specific SIRT1 Activation Mimics Low Energy Levels and Protects against Diet-Induced Metabolic Disorders by Enhancing Fat Oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 159.Koh HJ, Brandauer J, Goodyear LJ. LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr Opin Clin Nutr Metab Care. 2008;11:227–232. doi: 10.1097/MCO.0b013e3282fb7b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Narkar VA, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ruderman NB, Saha AK, Kraegen EW. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology. 2003;144:5166–5171. doi: 10.1210/en.2003-0849. [DOI] [PubMed] [Google Scholar]
- 163.Guigas B, et al. Beyond AICA riboside: in search of new specific AMP-activated protein kinase activators. IUBMB. Life. 2009;61:18–26. doi: 10.1002/iub.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lou PH, et al. Mitochondrial uncouplers with an extraordinary dynamic range. Biochem. J. 2007;407:129–140. doi: 10.1042/BJ20070606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat. Rev. Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 166.Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem. J. 2006;398:153–168. doi: 10.1042/BJ20060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Farmer SR. Molecular determinants of brown adipocyte formation and function. Genes. Dev. 2008;22:1269–1275. doi: 10.1101/gad.1681308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Scime A, et al. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 169.Kiskinis E, et al. RIP140 directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. EMBO J. 2007;26:4831–4840. doi: 10.1038/sj.emboj.7601908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Powelka AM, et al. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kajimura S, et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes. Dev. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kajimura S, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009 doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chiu YH, Lee TH, Shen WW. Use of low-dose topiramate in substance use disorder and bodyweight control. Psychiatry. Clin Neurosci. 2007;61:630–633. doi: 10.1111/j.1440-1819.2007.01718.x. [DOI] [PubMed] [Google Scholar]
- 174.Gadde KM, Franciscy DM, Wagner HR, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289:1820–1825. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]
- 175.Musi N, Goodyear LJ. Insulin resistance and improvements in signal transduction. Endocrine. 2006;29:73–80. doi: 10.1385/ENDO:29:1:73. [DOI] [PubMed] [Google Scholar]
- 176.English PJ, et al. Metformin prolongs the postprandial fall in plasma ghrelin concentrations in type 2 diabetes. Diabetes Metab Res. Rev. 2007;23:299–303. doi: 10.1002/dmrr.681. [DOI] [PubMed] [Google Scholar]
- 177.Ahima RS, et al. Appetite suppression and weight reduction by a centrally active aminosterol. Diabetes. 2002;51:2099–2104. doi: 10.2337/diabetes.51.7.2099. [DOI] [PubMed] [Google Scholar]
- 178.Sowers JR. Endocrine functions of adipose tissue: focus on adiponectin. Clin Cornerstone. 2008;9:32–38. doi: 10.1016/s1098-3597(08)60026-5. [DOI] [PubMed] [Google Scholar]
- 179.Bays HE. Current and investigational antiobesity agents and obesity therapeutic treatment targets. Obes. Res. 2004;12:1197–1211. doi: 10.1038/oby.2004.151. [DOI] [PubMed] [Google Scholar]
- 180.Ravussin E, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver. Spring.) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Remesar X, et al. Oral oleoyl-estrone induces the rapid loss of body fat in Zucker lean rats fed a hyperlipidic diet. Int. J Obes. Relat Metab Disord. 2000;24:1405–1412. doi: 10.1038/sj.ijo.0801393. [DOI] [PubMed] [Google Scholar]
- 182.McCarthy AA. When enough is too much: new strategies to treat obesity. Chem. Biol. 2004;11:1025–1026. doi: 10.1016/j.chembiol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 183.Chaudhri OB, Wynne K, Bloom SR. Can gut hormones control appetite and prevent obesity? Diabetes Care. 2008;31 Suppl 2:S284–S289. doi: 10.2337/dc08-s269. [DOI] [PubMed] [Google Scholar]
- 184.Glazer G. Long-term pharmacotherapy of obesity 2000: a review of efficacy and safety. Arch. Intern Med. 2001;161:1814–1824. doi: 10.1001/archinte.161.15.1814. [DOI] [PubMed] [Google Scholar]
- 185.Hansen RA, Gartlehner G, Lohr KN, Kaufer DI. Functional outcomes of drug treatment in Alzheimer's disease: A systematic review and meta-analysis. Drugs. Aging. 2007;24:155–167. doi: 10.2165/00002512-200724020-00007. [DOI] [PubMed] [Google Scholar]
- 186.Astrup A, et al. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1906–1913. doi: 10.1016/S0140-6736(08)61525-1. [DOI] [PubMed] [Google Scholar]
- 187.Appolinario JC, Bueno JR, Coutinho W. Psychotropic drugs in the treatment of obesity: what promise? CNS. Drugs. 2004;18:629–651. doi: 10.2165/00023210-200418100-00002. [DOI] [PubMed] [Google Scholar]
- 188.Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS. Drug Rev. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2007;14:63–67. doi: 10.1097/MED.0b013e3280122850. [DOI] [PubMed] [Google Scholar]
- 190.Smith SR, et al. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver. Spring.) 2009;17:494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- 191.Van der Ploeg LH, et al. Design and synthesis of (ant)-agonists that alter appetite and adiposity. Prog. Brain Res. 2006;153:107–118. doi: 10.1016/S0079-6123(06)53005-5. [DOI] [PubMed] [Google Scholar]
- 192.Adan RA, et al. The MC4 receptor and control of appetite. Br. J Pharmacol. 2006;149:815–827. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Barak N, Greenway FL, Fujioka K, Aronne LJ, Kushner RF. Effect of histaminergic manipulation on weight in obese adults: a randomized placebo controlled trial. Int. J Obes. (Lond.) 2008;32:1559–1565. doi: 10.1038/ijo.2008.135. [DOI] [PubMed] [Google Scholar]
- 194.Greenway FL, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver. Spring.) 2009;17:30–39. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 195.Li J, et al. In vitro and in vivo profile of 5-[(4'-trifluoromethyl-biphenyl-2-carbonyl)-amino]-1H-indole-2-carboxylic acid benzylmethyl carbamoylamide (dirlotapide), a novel potent MTP inhibitor for obesity. Bioorg. Med Chem. Lett. 2007;17:1996–1999. doi: 10.1016/j.bmcl.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 196.Idris I, Donnelly R. Sodium-glucose co-transporter-2 inhibitors: an emerging new class of oral antidiabetic drug. Diabetes Obes. Metab. 2009;11:79–88. doi: 10.1111/j.1463-1326.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 197.Carlson MJ, Cummings DE. Prospects for an anti-ghrelin vaccine to treat obesity. Mol Interv. 2006;6:249–252. doi: 10.1124/mi.6.5.5. [DOI] [PubMed] [Google Scholar]
- 198.Morton NM, Seckl JR. 11beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res. 2008;36:146–164. doi: 10.1159/000115363. [DOI] [PubMed] [Google Scholar]
- 199.Hartman ML, et al. Growth hormone replacement therapy in adults with growth hormone deficiency improves maximal oxygen consumption independently of dosing regimen or physical activity. J Clin Endocrinol Metab. 2008;93:125–130. doi: 10.1210/jc.2007-1430. [DOI] [PubMed] [Google Scholar]
- 200.Ruderman NB, et al. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol. Scand. 2003;178:435–442. doi: 10.1046/j.1365-201X.2003.01164.x. [DOI] [PubMed] [Google Scholar]
- 201.Ahmadian M, et al. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes. 2009;58:855–866. doi: 10.2337/db08-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kok P, et al. Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am J Physiol. Endocrinol Metab. 2006;291:E1038–E1043. doi: 10.1152/ajpendo.00567.2005. [DOI] [PubMed] [Google Scholar]
- 203.Wang YX, et al. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 204.Buckley JD, Howe PR. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes. Rev. 2009 doi: 10.1111/j.1467-789X.2009.00584.x. [DOI] [PubMed] [Google Scholar]