DIFFERENTIAL AND AGE-DEPENDENT EXPRESSION OF HYPERPOLARIZATION-ACTIVATED, CYCLIC NUCLEOTIDE-GATED CATION CHANNEL ISOFORMS 1–4 SUGGESTS EVOLVING ROLES IN THE DEVELOPING RAT HIPPOCAMPUS (original) (raw)
. Author manuscript; available in PMC: 2011 Apr 28.
Abstract
Hyperpolarization-activated cation currents (_I_h) are found in several brain regions including thalamus and hippocampus. Important functions of these currents in promoting synchronized network activity and in determining neuronal membrane properties have been progressively recognized, but the molecular underpinnings of these currents are only emerging. _I_h currents are generated by hyperpolarization-activated, cyclic nucleotide-gated cation channels (HCNs). These channel proteins are encoded by at least four HCN genes, that govern the kinetic and functional properties of the resulting channels. Because of the potential impact of _I_h-mediated coordinated neuronal activity on the maturation of the functional hippocampal network, this study focused on determining the expression of the four members of the HCN gene family throughout postnatal hippocampal development at both the regional and single cell level.
The results of these experiments demonstrated that HCNs 1, 2 and 4 are differentially expressed in interneuronal and principal cell populations of the rat hippocampal formation. Expression profiles of each HCN isoform evolve during postnatal development, and patterns observed during early postnatal ages differ significantly from those in mature hippocampus. The onset of HCN expression in interneurons of the hippocampus proper precedes that in the dentate gyrus, suggesting that HCN-mediated pacing activity may be generated in hippocampal interneurons prior to those in the hilus.
Taken together, these findings indicate an age-dependent spatiotemporal evolution of specific HCN expression in distinct hippocampal cell populations, and suggest that these channels serve differing and evolving functions in the maturation of coordinated hippocampal activity.
Keywords: development, pacemaker, ion channel, synchronization, interneurons, plasticity
Hyperpolarization-activated cation currents, termed _I_h, are observed in a variety of both central and peripheral neurons (for review, see DiFrancesco, 1993; Pape, 1996). In spontaneously firing neurons, _I_h contributes to the pacemaker depolarization that generates rhythmic activity (Clapham, 1998; Lüthi and McCormick, 1998). In non-pacing cells, _I_h helps to determine the resting membrane properties and limits the extent of hyperpolarizing and depolarizing responses (Pape, 1996).
Four members of a gene family encoding mammalian hyperpolarization-activated, cyclic nucleotide-gated cation channels (HCN1–4) have recently been cloned (Santoro et al., 1997, 1998; Ludwig et al., 1998, 1999; Ishii et al., 1999; Seifert et al., 1999; Monteggia et al., 2000). Available information suggests that each of these genes encodes proteins that, in vivo, form homomeric channels consisting of four HCN ‘subunit’ molecules. The functional properties of the channels differ, depending on their subunit make-up: thus, channels composed of HCN1 subunits activate and deactivate much faster in response to hyperpolarization than channels consisting of subunits HCN2, 3 or 4 (Ludwig et al., 1998; Santoro et al., 1998; Jegla et al., 1999; Seifert et al., 1999). In addition, the activity of HCN channels comprised of specific subunits is differentially modulated by cAMP (for review, see Santoro and Tibbs, 1999). In situ hybridization studies have demonstrated that all four HCN iso-forms are expressed in the adult mouse and rat brain, although with different regional distributions and at different levels (Moosmang et al., 1999; Monteggia et al., 2000; Santoro et al., 2000).
A growing body of evidence suggests that the currents produced by the HCN channels (_I_h) are critical contributors to several aspects of nervous system development. Specifically, these currents govern spontaneous ‘pacemaker’ activity, and are involved in synchronization of neuronal firing, thus leading to coordinated synaptic circuitry. Thus, in the early postnatal hippocampal formation, synaptically or electrically coupled neurons generate spontaneous oscillatory activity that may be required for an activity-dependent maturation of the hippocampal network (Khazipov et al., 1997; Strata et al., 1997; Garaschuk et al., 1998). A hyperpolarization-activated current with properties of _I_h has been suggested as the pacemaker governing the frequency of these oscillations (Strata et al., 1997).
Understanding the contribution of HCN channels to the development of coordinated hippocampal activity requires information about their expression and distribution within the diverse neuronal populations which make up this complex network. Therefore, the present study investigated the developmental profile and selective distributions of the four known HCN channel isoforms in rat hippocampus. The choice of the hippocampal formation as the focus of this study was determined not only by the established importance of these channels for hippocampal maturation, but also by recent findings that intense neuronal activity (some seizure types) can alter the properties of HCN channels in the developing hippocampus persistently, leading to an overall hyperexcitable state of the hippocampal network (Chen et al., 2001). Studying HCN expression in the developing hippocampus may therefore not only provide insight into processes that underlie hippocampal maturation, but may also reveal important information about molecular components subject to disruption by pathological alteration of normal hippocampal activity.
EXPERIMENTAL PROCEDURES
Animals and tissue processing
Immature Sprague–Dawley rats (Zivic-Miller, Zelienople, PA, USA) were born in our federally approved facility (day of birth = day 0). All experiments aimed to minimize pain or discomfort, were approved by the UCI Animal Care Committee and conformed to NIH guidelines. Animals were deeply anesthetized using sodium pentobarbital and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer on postnatal days (P) 2, 5, 11, 18, 24 or 60 (n = 4 per time point). Brains were removed, postfixed in perfusion buffer (4 h), cryoprotected, and frozen in −50°C isopentane. Coronal sections (50 μm) were cut using a cryostat and collected in diethylpyrocarbonate-treated 2×saline sodium citrate (SSC; 0.3 M sodium chloride, 0.03 M sodium citrate). Sections were then divided into four groups and each group was used for in situ hybridization for one of the four HCN isoform mRNAs. These groups were always processed in parallel.
Non-radioactive in situ hybridization probes and procedures
Antisense and sense riboprobes were generated from transcription vectors containing cDNA of mouse HCN1 (corresponding to amino acids 636–722), HCN2 (322–481), HCN3 (235–386) or HCN4 (400–690). They were labeled with digoxigenin (3.5:6.5 digoxigenin-UTP/UTP) according to the manufacturer’s instructions (Roche, Indianapolis, IN, USA). In situ hybridization was performed as described previously (Bender et al., 2000), with minor modifications. In brief, free-floating sections were washed in 2×SSC for 30 min, then subjected to an additional 30 min incubation in a solution composed of 2×SSC/prehybridization solution (1:1). Prehybridization took place for 1 h at 65°C in a humid chamber. The prehybridization solution consisted of 50% formamide, 4×SSC, 1×Denhardt’s solution, 5% dextran sulfate, 250 μg/ml yeast tRNA and 100 μg/ml salmon sperm DNA. For hybridization, digoxigenin-labeled RNA probes were added (final concentration: 10 ng per ml prehybridization buffer) and sections were incubated at 65°C for at least 12 h. For all steps, RNase-free solutions and sterile six-well plates were used. Following hybridization, sections were subjected to washes of increasing stringency including 2×SSC at room temperature (2×15 min), 50% formamide/2×SSC at 70°C for 60 min, 50% formamide/0.1×SSC at 70°C for 60 min and 0.1×SSC at 70°C for 30 min. Hybrid molecules were detected using an anti-digoxigenin antibody tagged with alkaline phosphatase (Roche). Staining was carried out using 4-nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (Roche) as chromogens. Color reaction was stopped when distinct, blue cytoplasmic in situ hybridization signals were clearly recognizable in circumscribed thalamic or hypothalamic nuclei that characteristically expressed the HCN isoform under study (see Results). Reaction was stopped for all isoforms after 2–4 h (P2–P11), and after 4–6 h for sections from older rats. Subsequently, sections were mounted on glass slides, dehydrated and embedded with Permount (Fisher, Pittsburgh, PA, USA). Specificity of the hybridization reaction was verified by substituting labeled sense probes for the antisense probes and by omitting either the antisense probe or alkaline phosphatase-conjugated antibody. No labeling was observed under these conditions.
Non-radioactive in situ hybridization combined with immunocytochemistry
For double-labeling, free-floating sections were first processed for in situ hybridization as described above. Sections were then processed for parvalbumin or somatostatin immunocytochemistry. For immunocytochemistry, sections were rinsed 2×15 min in 0.1 M phosphate buffer, before being incubated with primary antibodies (parvalbumin: mouse monoclonal IgG, 1:7000; somatostatin: rat monoclonal IgG, 1:500; Chemicon, Temecula, CA, USA) for 48 h at 4°C. Primary antibodies were then detected using biotinylated anti-mouse (parvalbumin) or anti-rat IgGs (somatostatin; 1:400 for both; Vector Laboratories, Burlingame, CA, USA). The immunoreaction product was visualized using an avidin–biotin–peroxidase detection system (Vectastain, ABC-Kit-Elite; Vector) and 3,3′-diaminobenzidine (0.02%) and H2O2 (0.005%) as chromogens. The peroxidase-mediated reaction resulted in a light brown product that was evident throughout somata and processes of labeled cells yet allowed detection of the blue, cytoplasmic HCN in situ hybridization signal.
Limited quantitative analysis
The confounding factors in quantitative analysis of immunocytochemistry and non-radioactive in situ hybridization have been well established. Therefore, in this study, we did not aim to provide absolute quantitative measures of HCN isoform expression, or to determine the relative levels of expression of one isoform compared to the others. However, in some instances, to highlight relative age- or isoform-related differences, numbers of HCN-expressing cells were determined in given hippocampal regions or layers. Thus, numbers of cells expressing the isoforms HCN2 or 4 were counted and compared in CA1 stratum oriens and in hilus at two different ages, P11 and P24. These cell counts were carried out in five anatomically matched sections per animal (n = 3 for each age group) and isoform.
RESULTS
Specificity of HCN in situ hybridization
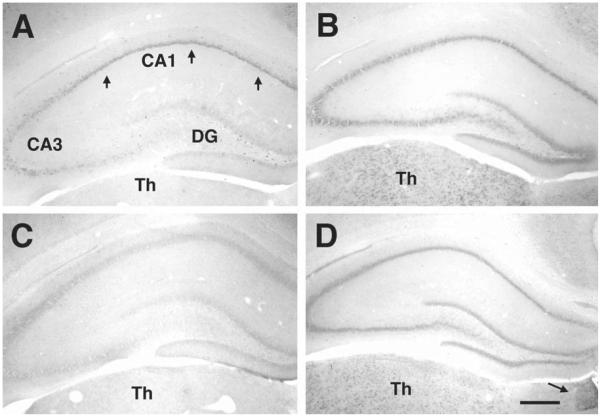
Members of the HCN gene family share a significant degree of homology in their base composition (Santoro and Tibbs, 1999; Monteggia et al., 2000), necessitating high stringency in the in situ hybridization conditions. The distinctive expression patterns of each HCN isoform in thalamus proved to be a useful indicator for the specificity of the in situ hybridization procedure: in the adult brain, HCN1 mRNA was not expressed in thalamus (Fig. 1A) (note that it was strongly expressed in the anterodorsal thalamic nucleus in the immature brain, Fig. 3A). HCN2 and HCN4 mRNAs were both expressed in several thalamic nuclei (Figs. 1B, D and 3B, D). Importantly, only HCN4 mRNA was expressed in the medial habenula (arrow, Fig. 1D). HCN3 was expressed weakly in hippocampus (Fig. 1C). However, its distinctive expression in the hypothalamic paraventricular nucleus was noted (data not shown).
Fig. 1.
Expression patterns of the four HCN mRNA isoforms in mature hippocampus. By the end of the third postnatal week, mRNA expression patterns of each HCN isoform resemble the adult patterns. Coronal sections of dorsal hippocampus from 24 day old rats, shown here, were subjected to in situ hybridization histochemistry for each of the HCN mRNAs: (A) HCN1 mRNA: robust expression is visible in the CA1 pyramidal cell layer (arrows), involving both pyramidal cells and in interneurons (see also Fig. 2B). In contrast, only interneurons express HCN1 in the CA3 pyramidal cell layer (see also Fig. 2A) and no HCN1 mRNA expression is observed in dentate gyrus (DG) granule cells. (B) HCN2 mRNA is homogenously expressed throughout the principal layers. (C) HCN3 mRNA hybridization signal is negligible. (D) HCN4 mRNA expression pattern in hippocampus generally resembles that of HCN2 mRNA. Differential thalamic (Th) expression patterns confirm probe specificity: virtually no HCN1 and HCN3 hybridization signals are detected in adult thalamus. In addition, HCN2 (B) and HCN4 (D), both expressed in thalamic nuclei, are distinguished by the selective expression of HCN4 mRNA in the medial habenula (arrow in D). Note: the findings for P24 were confirmed in P60 rats (not shown). Scale bar = 250 μm.
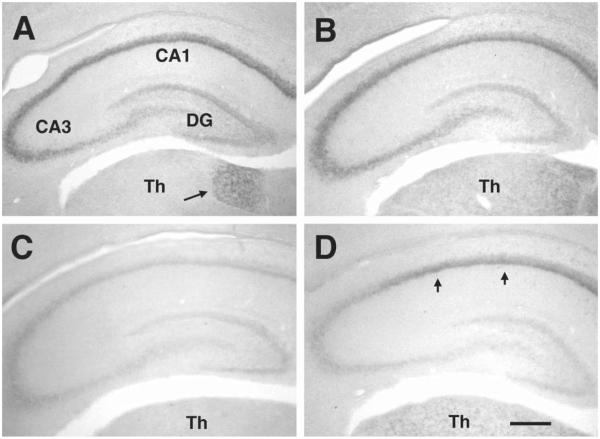
Fig. 3.
Expression of HCN1–4 mRNAs in the hippocampus of the 5 day old rat. In the early postnatal hippocampus, HCN expression patterns differ significantly from those seen later: (A) HCN1 mRNA is strongly expressed in the pyramidal cell layer, involving pyramidal cells in CA1 and – unlike in the adult – also in CA3. (B) The homogenous expression of HCN2 mRNA throughout the pyramidal cell layer, observed in mature hippocampus (Fig. 1B), is already established by P5. (C) As found in the adult, HCN3 mRNA is poorly detectable in the early postnatal hippocampus. (D) HCN4 mRNA expression in the P5 rat is confined to the CA1 region (arrows), whereas expression of this isoform is robust also in the CA3 pyramidal cell layer of the mature hippocampus (see Fig. 1D). No HCN isoform is expressed in the dentate gyrus (DG, neither in granule cells nor in interneurons) during this age. Differential expression in thalamic nuclei (Th) is evident. Interestingly, HCN1 is prominently expressed in the anterodorsal thalamic nucleus (arrow in A) during the first and second postnatal weeks, while this isoform is not found in adult thalamus. HCN2 and HCN4 expression in thalamus generally approximates the mature pattern (Fig. 1B, D). Scale bar = 250 μm.
Distribution of HCN1–4 mRNA expression in mature rat hippocampus
HCN distribution in rodent hippocampus has been described in detail for the mouse only. Therefore, as a preamble to analyzing the developmental expression patterns of the HCN isoforms, we determined their expression in mature rat hippocampus.
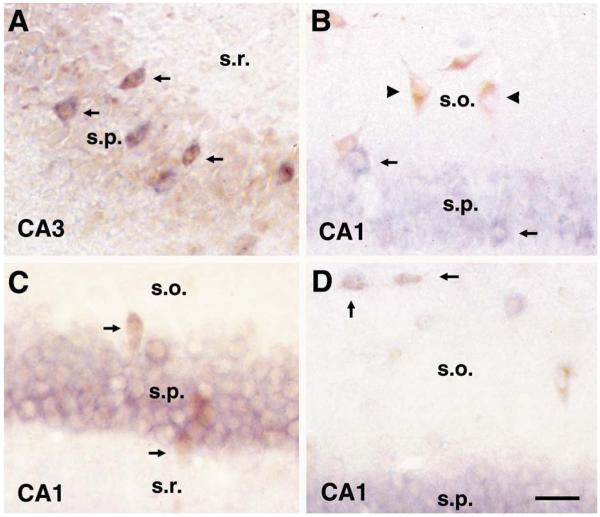
HCN1 mRNA was strongly expressed in CA1 pyramidal neurons, but was undetectable in dentate gyrus granule cells (Fig. 1A). Selectivity of this isoform’s expression was notable even within the pyramidal layer of Ammon’s horn: in contrast to the robust expression in CA1 pyramidal cells, CA3 pyramidal neurons were devoid of HCN1 mRNA (Fig. 1A, see also higher magnifications in Figs. 2A and 4C). Interneuronal expression of HCN1 was similarly selective: HCN1 mRNA was expressed in the vast majority of interneurons located within or adjacent to the principal cell layers, i.e. basket and axo-axonic (chandelier) cells (Fig. 2A). In contrast, somatostatin-immunoreactive interneurons in CA1 stratum oriens rarely expressed HCN1 mRNA (Fig. 2B).
Fig. 2.
HCN isoform expression in hippocampal interneurons is specific to neurochemically and anatomically defined populations. (A, B) HCN1 mRNA: (A) combined HCN1 mRNA (blue) and parvalbumin immunocytochemistry (brown) demonstrates co-expression of this HCN isoform in the vast majority of CA3 basket cells (arrows) in the 24 day old rat, while no expression is evident in CA3 pyramidal cells. In contrast to co-localization with PV-containing interneurons in principal cell layers, (B) shows little overlap of HCN1 mRNA (blue) and somatostatin expression (brown) in interneurons of CA1 stratum oriens (arrowheads). Thus, these two panels indicate that HCN1 mRNA is expressed in CA1 (B) but not CA3 (A) pyramidal cells, as well as in typically large-sized neurons in or closely adjacent to the pyramidal layer (arrows in A and B), which are likely basket and chandelier cells. (C, D) HCN2 mRNA: (C) combined HCN2 mRNA (blue) and parvalbumin immunocyto-chemistry (brown, arrows) reveals that HCN2 is rarely expressed in basket and chandelier cells, while (D) it is frequently expressed in somatostatin-labeled neurons at the O/A border in CA1 (O/A neurons; arrows). Abbreviations: s.o., stratum oriens; s.p., stratum pyramidale; s.r., stratum radiatum. Scale bar = 25 μm.
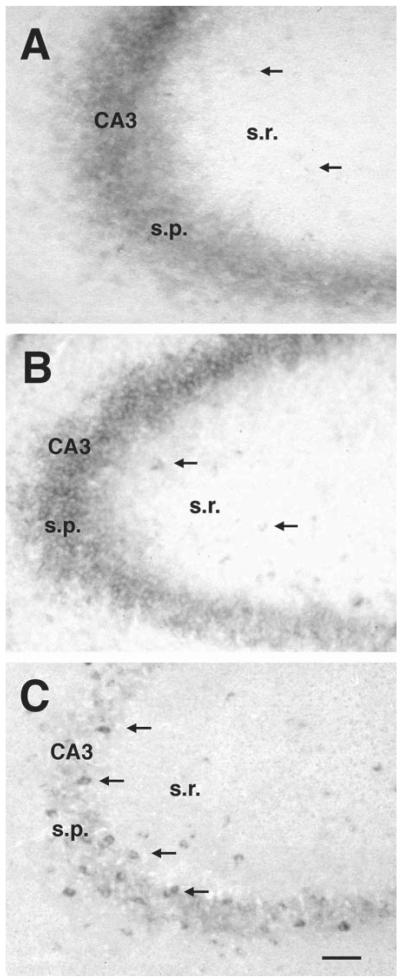
Fig. 4.
Evolution of the HCN1 mRNA expression pattern in CA3 of the developing hippocampus. (A) Prominent expression of HCN1 mRNA is observed as early as P2 in pyramidal neurons (s.p.). However, only faint staining in stratum radiatum (arrows) suggests the onset of this isoform’s expression in a few interneurons at that age. (B) By P5, distinct interneuronal expression was evident in stratum radiatum (arrows). Note that during this age, pyramidal cells still express high levels of the HCN1 isoform. (C) By P18, a large number of interneurons, mostly associated with the pyramidal layer, express HCN1 mRNA. However, pyramidal cells no longer express this isoform. Abbreviations: s.p., stratum pyramidale; s.r., stratum radiatum. Scale bar = 50 μm.
HCN2 mRNA was expressed homogenously throughout the pyramidal layer, and, unlike HCN1, was moderately expressed in the granule cell layer (Fig. 1B). Additionally, HCN2 mRNA was detected in diverse interneuronal populations, but its distribution pattern clearly differed from the pattern observed for HCN1 mRNA. Thus, unlike HCN1, HCN2 mRNA was frequently expressed in interneurons located at the border of strata oriens/alveus (O/A neurons) that co-express the neuropeptide somatostatin (Fig. 2D). In addition, unlike HCN1, it was rarely detected (~10%) in parvalbumin-immunoreactive basket or chandelier cells (Fig. 2C).
HCN3 mRNA was virtually undetectable in mature hippocampus under the hybridization conditions used (Fig. 1C). For HCN4, the mRNA expression pattern resembled that of HCN2 in hippocampal principal cell layers (Fig. 1D), but this isoform was less commonly observed in interneurons (72% and 74% in CA1 stratum oriens and hilus, respectively, compared to the numbers of interneurons expressing HCN2 mRNA).
Taken together, the data presented above indicate that in mature hippocampus, HCN1, HCN2 and HCN4 are all expressed in pyramidal cells, whereas HCN1 and HCN2 are the dominant forms in interneurons. The HCN3 expression pattern suggests that it contributes little to the make-up of HCN channels.
Developmental onset of HCN expression in hippocampal neurons
Expression of HCN1, 2 and 4 mRNAs was observed in the hippocampus already by P2, the earliest time point studied. Expression patterns evolved in the postnatal hippocampus until the end of the third postnatal week, when mature profiles were established. Little if any HCN3 mRNA was noted throughout postnatal development (Fig. 3C). For the other three isoforms, expression patterns observed throughout the first three postnatal weeks differed in several significant aspects from those in the adult.
Pyramidal neurons
HCN1 mRNA, detected in mature hippocampus in pyramidal neurons of CA1 only, was also expressed in CA3 pyramidal cells during development (see overview in Fig. 3A, and temporal evolution in Fig. 4A, B). In these cells, HCN1 mRNA was detected as early as P2 (Fig. 4A), and its expression was preserved throughout the first and second postnatal weeks (Figs. 3A and 4B). During the third postnatal week, HCN1 mRNA expression disappeared from CA3 pyramidal cells (Fig. 4C), while becoming increasingly robust in interneurons (see below).
HCN2 mRNA expression in pyramidal neurons did not differ significantly from the adult pattern (Fig. 3B): HCN2 mRNA was rather homogenously expressed throughout the pyramidal cell layer at all ages studied. For HCN4 mRNA, however, high levels of expression were observed in CA1 pyramidal neurons during the first and second postnatal weeks (Fig. 3D, and higher magnification in Fig. 5C), but no HCN4 mRNA expression was detected in CA3 at these ages. As for the HCN1 transcript, the mature expression pattern of HCN4 mRNA was achieved during the third postnatal week.
Fig. 5.
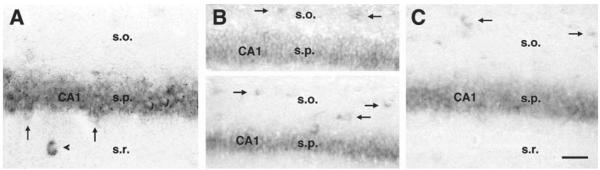
Differential expression of HCN mRNAs in developing CA1 interneurons and pyramidal cells. Cell specificity of HCN isoform expression was evident already by P11. (A) in situ hybridization for HCN1 demonstrates that this isoform is expressed in pyramidal cells, as well as in interneurons of stratum radiatum (arrowhead), but rarely in those of stratum oriens (s.o.). In addition, HCN1 mRNA is visible in typical large neurons at the base of the pyramidal cell layer (arrows), suggesting early onset of HCN1 mRNA expression in basket and chandelier cells. (B) Unlike HCN1, HCN2 mRNA is frequently expressed in interneurons of stratum oriens. This expression is evident already on P5 (arrows in top panel), and is robust by P11 (arrows in bottom panel). (C) HCN4 mRNA expression (arrows) resembles that of HCN2, but limited quantitative analysis suggests that fewer interneurons express this isoform compared with HCN2 mRNA (see text). Abbreviations: s.o., stratum oriens; s.p., stratum pyramidale; s.r., stratum radiatum. Scale bar (A, C) = 40 μm; (B) 50 μm.
Interneurons
Expression of HCN isoforms in hippocampal interneurons was generally observed later than in pyramidal neurons. Only a few, faintly stained cells in hippocampal polymorph layers suggested HCN mRNA expression already on P2 (arrows in Fig. 4A). Distinctly labeled interneurons were observed on P5, when, in addition to their abundance in CA3 stratum radiatum (arrows in Fig. 4B), labeled interneurons were particularly concentrated in CA1 stratum oriens (arrows in Fig. 5B, top). At this age, all three HCN isoforms were detected in these two interneuronal populations, and no differential expression of a given isoform in specific interneurons was evident. Differential expression, as discussed above for mature hippocampus, evolved by the second postnatal week: on P11, expression of HCN1 mRNA was confined primarily to interneurons located in or subjacent to the principal cell layers (Fig. 5A, arrows). In contrast, HCN2 and HCN4 mRNA expression was noted in O/A neurons (arrows in Fig. 5B, bottom, and in Fig. 5C). In addition, a more frequent expression of HCN2 compared with HCN4 in interneurons became evident during this period: in CA1 stratum oriens, for example, interneurons expressing HCN2 mRNA outnumbered those expressing HCN4 mRNA, in a pattern similar to that of mature hippocampus: numbers of HCN4-expressing interneurons were 72% of those expressing HCN2 mRNA. A similar relative abundance of HCN2 was noted in the hilus, where HCN2 mRNA-expressing interneurons outnumbered those expressing HCN4 mRNA by 10:6.9 on average.
Hilar interneurons were the last population to express HCN isoforms, suggesting that the onset of HCN expression in interneuronal populations follows the developmental gradient of the hippocampus (Bayer, 1980; Soriano et al., 1994). HCN expression was undetectable in the hilus during the first postnatal week (Fig. 6A, showing HCN2), and the characteristic expression pattern of each isoform developed during the second postnatal week. Similar to the findings in Ammon’s horn, HCN1 mRNA was preferentially expressed in interneurons spatially associated with the principal (granule cell) layer, although some interneurons in the deep hilus also expressed HCN1 (Fig. 6B). In contrast, HCN2 mRNA (Fig. 6C) and HCN4 mRNA-expressing neurons (not shown) were mainly localized to the deep hilus.
Fig. 6.
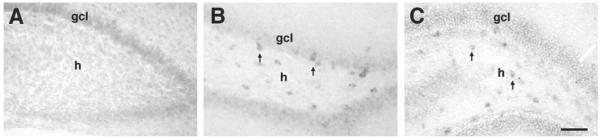
Evolution of HCN expression patterns in the dentate gyrus. In general, the onset of HCN mRNA expression in the dentate gyrus is delayed compared with the hippocampus proper. (A) HCN2 mRNA (as well as other isoforms, not shown) is not detectable in granule cells or in interneurons on P5. (B) By P11, HCN1 mRNA is expressed in interneurons, but not in granule cells. As demonstrated in other hippocampal regions, HCN1 mRNA is particularly prominent in interneurons associated with the principal (granule) cell layer (gcl, arrows). (C) In contrast to HCN1, HCN2 mRNA is more prevalent in interneurons residing in the deep hilus (h, arrows), and is also weakly detectable in granule cells. Scale bar = 60 μm.
Dentate gyrus granule cells
Expression of HCN isoforms in dentate gyrus granule cells was not detectable during the first postnatal week (Fig. 6A, as well as Fig. 3A–D), and developed during the second postnatal week. Only mRNAs encoding HCN2 and HCN4 were detected (Fig. 6C, as well as Fig. 1B, D). HCN1 mRNA was not expressed in the granule cell layer at any of the ages investigated.
DISCUSSION
This study examined in detail the mRNA expression patterns of the four known HCN isoforms in developing and mature rat hippocampus. The principal findings of this study are: (a) members of the HCN gene family are differentially expressed in interneuronal and principal cell populations of the rat hippocampal formation. (b) Expression profiles of each HCN isoform evolve during postnatal development, and patterns observed during early postnatal ages differ significantly from those in mature hippocampus. (c) Onset of HCN expression in interneurons of hippocampus proper precedes expression in the dentate gyrus. Taken together, these findings indicate an age-dependent spatiotemporal evolution of specific HCN expression in distinct hippocampal cell populations, suggesting that these channels serve differing and evolving functions in the maturation of coordinated hippocampal activity.
Potential roles of HCN isoforms in hippocampal interneurons
Detailed analyses of HCN hippocampal expression have to date been published only for the mouse (Moosmang et al., 1999; Santoro et al., 2000). Our observations in rat hippocampus are in general agreement with these reports, but also highlight significant differences. For example, partially overlapping expression of more than one HCN isoform was found in both species, as HCN2 and HCN4 were ubiquitously expressed throughout the pyramidal cell layer (Moosmang et al., 1999; Santoro et al., 2000; Fig. 1). However, in our data, HCN1 mRNA was confined to CA1 pyramidal cells, a result that differs from the reports for the mature mouse, where HCN1 mRNA was detected also in CA3 pyramidal cells (Moosmang et al., 1999; Santoro et al., 2000). Furthermore, this study confirmed and extended the reported differential expression of HCN isoforms in mouse interneuronal subtypes (Santoro et al., 1997, 2000) to the mature rat: HCN1 mRNA was detected in virtually all interneurons co-localizing the Ca2+-binding protein parvalbumin, a protein marker for basket and chandelier cells (Fig. 2A), but was less expressed in interneurons located in the polymorph layers. In contrast, expression in basket and chandelier cells was less prominent for HCN2 and HCN4 mRNAs, which were the most prevalent isoforms in interneurons residing in the polymorph layers, including the deep hilus. Thus, particularly for CA1 stratum oriens, HCN2 and HCN4 mRNA were frequently expressed in somatostatin-containing O/A neurons, whereas HCN1 expression was primarily confined to interneurons located in or close to the pyramidal cell layer.
It is acknowledged that mRNA expression does not always translate to functional proteins, and the distribution of the latter may differ from that of the cognate mRNA. However, the distinctive expression patterns of each HCN isoform, as shown here, may reflect different physiological roles: interneurons in CA1 stratum oriens have recently been classified using anatomical (Freund and Buzsáki, 1996; Katona et al., 1999) and physiological criteria (van Hooft et al., 2000). Most of the characteristically somatostatin-expressing neurons at the O/A border provide feedback inhibition to the distal dendrites of CA1 pyramidal cells (Blasco-Ibanez and Freund, 1995; Katona et al., 1999). These O/A neurons fire rhythmically, an activity which is at least partially mediated by slow-activating _I_h currents (Maccaferri and McBain, 1996), modulated by subcortical afferent input. Thus, the anatomical and physiological properties of these O/A neurons render them likely candidates to ‘pace’ and modulate synchronous activity of spatially related pyramidal cells, controlling the efficacy of entorhinal synaptic input (Katona et al., 1999). The slow-activating HCN2 and HCN4 channels, which are highly modulated by intracellular cAMP, are well suited to contribute to this role, a notion supported by the data presented here. Indeed, both of these isoforms contribute critically to the generation of rhythmic activity in thalamic and brainstem nuclei (Pape, 1996; Lüthi and McCormick, 1998; Franz et al., 2000; Monteggia et al., 2000). In contrast to O/A interneurons, basket and chandelier cells of stratum oriens demonstrate a characteristic high-frequency spiking pattern and only occasionally possess oscillatory currents (van Hooft et al., 2000). In these neurons, the fast-activating HCN1 isoform may predominate, as found in this study, and may subserve functions involving frequency regulation of tonic inhibitory input to principal cells, as shown for _I_h currents in cerebellar basket cells (Saitow and Konishi, 2000).
Potential roles of HCN isoforms in the developing hippocampus
The evolving expression profiles of hippocampal HCN isoforms during the first three postnatal weeks differed in several aspects from those in the adult, suggesting the possibility of evolving developmental functions of currents generated by HCNs during this period. The neonatal hippocampal network is organized in recurrent excitatory loops composed of pyramidal cells and interneurons, which are synchronously excited by the depolarizing effects of GABA and glutamate (Khazipov et al., 1997; Leinekugel et al., 1997; Strata et al., 1997; Garaschuk et al., 1998; Palva et al., 2000). These synchronized oscillations may be crucial for the formation and stabilization of this complex neuronal network (Goodman and Shatz, 1993; O’Donovan, 1999). Indeed, synchronous activity has been considered essential to the functional maturation of glutamatergic neurotransmission (Durand et al., 1996; Liao and Malinow, 1996). In addition, since the formation of stable synapses depends on a tight coincidence of pre- and postsynaptic activity, a sharply defined pattern of synchronous activity would allow neuronal ensembles to specifically select and stabilize connections with cells exhibiting matching activity patterns, and to suppress connections that fire ‘out of synch’ (Smith and Swann, 1999). The diverse combinations of HCN isoforms in hippocampal neurons, (coupled with other channel types (Pape, 1996; Talley et al., 1999)) provide the molecular machinery to shape these distinct activity patterns during development.
Indeed, slow network oscillations have been reported in CA1 pyramidal cells and interneurons during the first postnatal week (Garaschuk et al., 1998), decreasing in potency and disappearing by P14. Slow-activating channels composed of HCN4 subunits (Seifert et al., 1999), shown in the current study to demonstrate a strikingly parallel developmental expression profile in the same neurons, are thus excellent candidates to underlie these oscillations. In contrast, neonatal CA3 pyramidal cells fire mainly at γ frequencies, which are periodically synchronized into high-frequency population oscillations (100–400 Hz; Palva et al., 2000). The strong expression of the fast-activating HCN1 isoform in CA3 pyramidal neurons during the first postnatal days (shown here) might be required to enable these fast oscillations. Furthermore, the CA3 neuronal network undergoes fundamental remodeling during the third postnatal week, resulting in a less excitable state during adulthood (Smith et al., 1995), and the developmental expression profile of HCN1 may be related to these maturational changes in CA3 pyramidal cell connectivity.
Do HCN-expressing interneurons act as a pacemaker for hippocampal development?
It had been suggested that during the first postnatal days, hippocampal interneurons (particularly in the hilus) may provide _I_h current-mediated pacemaker activity which contributes to functional hippocampal maturation (Strata et al., 1997). Interestingly, the current study demonstrated little HCN expression in hilar interneurons prior to the second postnatal week. In contrast, interneurons residing in CA1 stratum oriens and CA3 stratum radiatum expressed substantial levels of HCN1, 2 and 4 isoform mRNAs already by P5. These data are consistent with a role for interneuronal populations of hippocampus proper in providing the stimulus for the giant GABA-mediated coordinated firing during this critical age for functional hippocampal maturation, or with the recent suggestion that multiple origins of this pacing activity exist (Menendez de la Prida and Sanchez-Andres, 2000).
In summary, this study demonstrates that HCN isoforms 1, 2 and 4 are expressed in complex, evolving and specific distribution patterns early in hippocampal development. Age- and region-specific expression of HCNs may determine and permit the generation of age- and region-specific coordinated activity patterns, which are necessary for orderly maturation of the hippocampal neuronal network. A disturbance of this process might result in long-lasting consequences, as has recently been shown in a rat model for developmental (complex febrile) seizures: a relatively short (~20 min) seizure, induced by hyperthermia on P10 (Dubé et al., 2000), caused persistent modification of HCN channel function (_I_h currents) in hippocampus (Chen et al., 2001), and led to hyperexcitability of the hippocampal network (Dubé et al., 2000). Therefore, further investigation of HCN expression and regulation in developing hippocampus may provide important insights into potentially pathological consequences of altered HCN channel function.
Acknowledgements
This study was supported by NIH NS 35439 (T.Z.B.) and by a postdoctoral fellowship from the Epilepsy Foundation of America (R.A.B.).
Abbreviations
HCN
hyperpolarization-activated, cyclic nucleotidegated cation channel
_I_h
hyperpolarization-activated cation current
O/A
oriens/alveus
P
postnatal day
SSC
saline sodium citrate
REFERENCES
- Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with [3H]thymidine autoradiography. J. Comp. Neurol. 1980;195:51–86. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Bender R, Hoffmann MC, Frotscher M, Nitsch C. Species-specific expression of parvalbumin in the entorhinal cortex of the Mongolian gerbil: dependence on local activity but not extrinsic afferents. Neuroscience. 2000;99:423–431. doi: 10.1016/s0306-4522(00)00208-6. [DOI] [PubMed] [Google Scholar]
- Blasco-Ibanez JM, Freund TF. Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur. J. Neurosci. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to excitability. Nat. Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Not so funny anymore: pacing channels are cloned. Neuron. 1998;21:5–7. doi: 10.1016/s0896-6273(00)80508-5. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- Dubé C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann. Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Franz O, Liss B, Neu A, Roeper J. Single-cell mRNA expression of HCN1 correlates with a fast gating phenotype of hyperpolarization-activated cyclic nucleotide-gated ion channels (Ih) in central neurons. Eur. J. Neurosci. 2000;12:2685–2693. doi: 10.1046/j.1460-9568.2000.00151.x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of the rat neonatal hippocampus. J. Physiol. 1998;507:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Takano M, Xie LH, Noma A, Ohmori H. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J. Biol. Chem. 1999;274:12835–12839. doi: 10.1074/jbc.274.18.12835. [DOI] [PubMed] [Google Scholar]
- Jegla T, Bachmann J, Silvia C, Stocker J, Wagoner PK. Cloning and expression of a novel hyperpolarization-activated cation channel, human HCN3. Soc. Neurosci. Abstr. 1999;25:893–6. [Google Scholar]
- Katona I, Acsády L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Leinekugel X, Khalilov I, Gaiarsa J-L, Ben-Ari Y. Synchronization of GABAergic interneuronal network in CA3 subfield of neonatal rat hippocampal slices. J. Physiol. 1997;498:763–772. doi: 10.1113/jphysiol.1997.sp021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+-oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Liao D, Malinow R. Deficiency in induction but not expression of LTP in hippocampal slices from young rats. Learn. Mem. 1996;3:138–149. doi: 10.1101/lm.3.2-3.138. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from heart with profoundly different activation kinetics. EMBO J. 1999;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens–alveus interneurons. J. Physiol. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez de la Prida L, Sanchez-Andres JV. Heterogeneous populations of cells mediate spontaneous synchronous bursting in the developing hippocampus through a frequency-dependent mechanism. Neuroscience. 2000;97:227–241. doi: 10.1016/s0306-4522(00)00029-4. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Mol. Brain Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Biel M, Hofmann F, Ludwig A. Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biol. Chem. 1999;380:975–980. doi: 10.1515/BC.1999.121. [DOI] [PubMed] [Google Scholar]
- O’Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr. Opin. Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Palva JM, Lamsa K, Lauri SE, Rauvala H, Kaila K, Taira T. Fast network oscillations in the newborn rat hippocampus in vitro. J. Neurosci. 2000;20:1170–1178. doi: 10.1523/JNEUROSCI.20-03-01170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu. Rev. Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Saitow F, Konishi S. Excitability increase induced by L-adrenergic receptor-mediated activation of hyperpolarization-activated cation channels in rat cerebellar basket cells. J. Neurophysiol. 2000;84:2026–2034. doi: 10.1152/jn.2000.84.4.2026. [DOI] [PubMed] [Google Scholar]
- Santoro B, Tibbs GR. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann. N.Y. Acad. Sci. 1999;868:741–764. doi: 10.1111/j.1749-6632.1999.tb11353.x. [DOI] [PubMed] [Google Scholar]
- Santoro B, Grant SG, Bartsch D, Kandel ER. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to eag and cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA. 1997;94:14815–14820. doi: 10.1073/pnas.94.26.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Lüthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J. Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Scholten A, Gauss R, Mincheva A, Lichter P, Kaupp UB. Molecular characterization of a slowly gating human hyperpolarization-activated channel predominantly expressed in thalamus, heart and testis. Proc. Natl. Acad. Sci. USA. 1999;96:9391–9396. doi: 10.1073/pnas.96.16.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KL, Swann JW. Long-term depression of perforant path excitatory postsynaptic potentials following synchronous network bursting in area CA3 of immature hippocampus. Neuroscience. 1999;89:625–630. doi: 10.1016/s0306-4522(98)00651-4. [DOI] [PubMed] [Google Scholar]
- Smith KL, Szarowski DH, Turner JN, Swann JW. Diverse neuronal populations mediate local circuit excitation in area CA3 of developing hippocampus. J. Neurophysiol. 1995;74:650–672. doi: 10.1152/jn.1995.74.2.650. [DOI] [PubMed] [Google Scholar]
- Soriano E, Del Rio JA, Martinez A, Supér H. Organization of the embryonicand early postnatal murine hippocampus. I. Immunocytochemical characterization of neuronal populations in the subplate and marginal zone. J. Comp. Neurol. 1994;342:571–595. doi: 10.1002/cne.903420406. [DOI] [PubMed] [Google Scholar]
- Strata F, Atzori M, Molnar M, Ugolini G, Tempia F, Cherubini E. A pacemaker current in dye-coupled hilar interneurons contributes to the generation of giant GABAergic potentials in developing hippocampus. J. Neurosci. 1997;17:1435–1446. doi: 10.1523/JNEUROSCI.17-04-01435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee J-H, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium-channels. J. Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooft JA, Giuffrida R, Blatow M, Monyer H. Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J. Neurosci. 2000;20:3544–3551. doi: 10.1523/JNEUROSCI.20-10-03544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]