The Inhibitory Influence of the Lateral Habenula on Midbrain Dopamine Cells: Ultrastructural Evidence for Indirect Mediation via the Rostromedial Mesopontine Tegmental Nucleus (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 12.
Published in final edited form as: J Comp Neurol. 2011 Apr 15;519(6):1143–1164. doi: 10.1002/cne.22561
Abstract
The lateral habenula (LHb) provides an important source of negative reinforcement signals to midbrain dopamine (DA) cells in the substantia nigra and ventral tegmental area (VTA). This profound and consistent inhibitory influence involves a disynaptic connection from glutamate neurons in the LHb to some population of γ-aminobutyric acid (GABA) cells that, in turn, innervates DA neurons. Previous studies demonstrated that the GABA cells intrinsic to the VTA receive insufficient synaptic input from the LHb to serve as the primary source of this intermediate connection. In this investigation, we sought ultrastructural evidence supporting the hypothesis that a newly identified region of the brainstem, the rostromedial mesopontine tegmental nucleus (RMTg), is a more likely candidate for inhibiting midbrain DA cells in response to LHb activation. Electron microscopic examination of rat brain sections containing dual immunoreactivity for an anterograde tracing agent and a phenotypic marker revealed that: 1) more than 55% of the synapses formed by LHb axons in the RMTg were onto GABA-labeled dendrites; 2) more than 80% of the synapses formed by RMTg axons in the VTA contacted dendrites immunoreactive for the DA synthetic enzyme tyrosine hydroxylase; and 3) nearly all RMTg axons formed symmetric synapses and contained postembedding immunoreactivity for GABA. These findings indicate that the newly identified RMTg region is an intermediate structure in a disynaptic pathway that connects the LHb to VTA DA neurons. The results have important implications for understanding mental disorders characterized by a dysregulation of reward circuitry involving LHb and DA cell populations.
INDEXING TERMS: GABA, negative reinforcement, ventral tegmental area
Midbrain dopamine (DA) neurons provide forebrain targets with modulatory signals that facilitate learning in motor, affective, and cognitive domains (Wise, 2004; Redgrave and Gurney, 2006; Fields et al., 2007; Liu et al., 2008). DA cells fire in distinct activity modes that are associated with novel and reward-related stimuli (Ljungberg et al., 1992; Horvitz et al., 1997; Schultz, 1998; Hyland et al., 2002; Matsumoto and Hikosaka, 2009). Such behaviorally relevant firing patterns are shaped by excitatory and inhibitory synaptic inputs to DA neurons that interrupt tonic firing with bursts and pauses, respectfully (Grenhoff et al., 1988; Johnson and North, 1992; Overton and Clark, 1997; Pan and Hyland, 2005; Shepard et al., 2006; Tepper and Lee, 2007; Brazhnik et al., 2008; Sesack and Grace, 2010). The recent identification of multiple sources of afferents to the ventral tegmental area (VTA) DA system (Geisler and Zahm, 2005; Geisler et al., 2007) has opened the door to experiments designed to identify those inputs that are responsible for the complex activity patterns recorded in behaving animals (Dommett et al., 2005; Matsumoto and Hikosaka, 2008).
Significant attention has especially turned to consideration of the lateral habenula (LHb) as a major source of negative reinforcement signals to DA neurons in both the VTA and substantia nigra zona compacta (SNc). The LHb receives limbic and motor signals from diverse forebrain regions and projects mainly to brainstem monoamine cell groups including the VTA (Herkenham and Nauta, 1977, 1979; Araki et al., 1988; Geisler and Trimble, 2008; Hikosaka et al., 2008; Hong and Hikosaka, 2008; Kim, 2009). Although the innervation density is modest within the VTA and sparse within the SNc (Omelchenko et al., 2009; Brinschwitz et al., 2010), electrical stimulation of the LHb evokes short latency inhibition in most DA neurons throughout the ventral midbrain (Christoph et al., 1986; Ji and Shepard, 2007; Matsumoto and Hikosaka, 2007). The functional significance of this pervasive inhibitory influence is revealed by experiments in awake behaving primates indicating that the firing patterns of LHb and DA neurons are inversely correlated. More specifically, presentation of cues that signal no reward leads to activation of LHb cells and suppression of DA neuron firing with a time delay consistent with mediation by the LHb (Matsumoto and Hikosaka, 2007). Similarly, negative feedback enhances hemodynamic activity in the habenula in human imaging studies (Ullsperger and von Cramon, 2003; Shepard et al., 2006). The potential clinical importance of regulatory pathways originating from the LHb is underscored by studies in depressed patients reporting structural abnormalities in the habenula (Ranft et al., 2010) and increased blood flow to this region during the induction of depressive symptoms (Morris et al., 1999).
Reports that LHb activation consistently inhibits DA neurons at short latency are incongruent with the well-established glutamatergic phenotype of LHb cells (Fremeau et al., 2001; Herzog et al., 2004; Brinschwitz et al., 2005, 2010; Geisler et al., 2007; Omelchenko et al., 2009). Hence, one must suppose the existence of an intermediate neuron that converts LHb excitation into inhibition of DA cells. In a prior ultrastructural analysis (Omelchenko et al., 2009), we examined the hypothesis that the intermediate neurons were the γ-aminobutyric acid (GABA) cells that neighbor VTA DA neurons and innervate them through local collateral axons (Omelchenko and Sesack, 2009). However, the relatively sparse synaptic incidence of LHb axons in the ventral midbrain, and the fact that both DA and GABA cells were targeted with equal frequency, suggested that an intra- VTA circuit could not account for the consistency of LHb’s inhibitory effect. An independent laboratory has also reported a modest number of LHb synapses onto VTA GABA neurons and a few onto DA cells (Brinschwitz et al., 2010).
Quite recently, convergent work done in several laboratories (Jhou et al., 2009a,b; Kaufling et al., 2009, 2010) has led to the identification of a region in the brainstem reticular formation that 1) constitutes a terminal field of the LHb (Herkenham and Nauta, 1979; Araki et al., 1988); 2) contains primarily GABA neurons (Perrotti et al., 2005; Olson and Nestler, 2007); and 3) gives rise to extensive projections to the entire SNc-VTA complex (Ferreira et al., 2008). This region, termed either the rostromedial mesopontine tegmental nucleus (RMTg) (Jhou et al., 2009a,b) or the tail of the VTA (tVTA) (Kaufling et al., 2009, 2010), clearly constitutes a previously unappreciated major source of inhibitory signals to the midbrain. Moreover, light microscopic evidence suggests that this area is likely to serve as a critical intermediate between the LHb and VTA DA cells (Jhou et al., 2009b; Kaufling et al., 2009). We sought to obtain ultrastructural evidence consistent with this hypothesis by determining whether LHb inputs synapse onto GABA neurons in the RMTg and whether projections from the RMTg synapse onto VTA DA cells. A preliminary report of this study has appeared in abstract form (Balcita-Pedicino et al., 2009).
MATERIALS AND METHODS
Subjects and stereotaxic surgery
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Eighteen male Sprague-Dawley rats (Hilltop Lab Animals, Scottdale, PA), weighing 250–300 g, were single- or double-housed under a lighting schedule of 12-hour light/12-hour dark. The animals were given unrestricted access to food and water throughout the study. After surgery, the animals were housed singly.
Rats were injected i.m. with an anesthetic mixture of acetopromazine (1 mg/kg), ketamine (34 mg/kg), and xylazine (7 mg/kg). Rats were placed in a stereotaxic device adjusted to hold the skull flat. The scalp was retracted and a small burr hole was drilled in the skull for injection of the anterograde tracer, Phaseolus vulgaris leucoagglutinin (PHAL; Vector, Burlingame, CA; 2.5% in 0.01 M sodium phosphate buffer). The eight animals that received PHAL injections into the LHb were the same as those used in our previous analysis of LHb projections to the VTA (Omelchenko et al., 2009). Three of these rats were excluded from the study due to weak transport to the RMTg, leaving five rats for the present analysis. In addition, 10 rats received tracer injections into the RMTg; 4 received unilateral, and 6 received bilateral injections. Three of the bilateral cases were excluded from the study because the injections either were not optimally placed or were not sufficiently confined to the RMTg. This left 7 RMTg cases for electron microscopic examination and a total of 12 rats for the entire study.
The coordinates used to target the RMTg were 6.5 mm posterior to bregma, 0.5 mm lateral to bregma, and 8.0 mm ventral to the skull surface as measured at the exact drill site. PHAL was delivered by iontophoresis using a +5-µA current, alternating 10 seconds on/off for 10 minutes, passed through filament-containing borosilicate glass capillary tubes (World Precision Instruments, Sarasota, FL) with internal tip openings of 15–20 µm. Brain coordinates correspond to the atlas of Paxinos and Watson (1997).
Following the injection, the pipette was kept in place for 5 minutes before being lifted out of the brain. The exposed skull and wound were swabbed with saline and closed with surgical staples. A triple-antibiotic ointment was applied generously to the closed wound, and ketofen (2 mg/kg, i.m.) was administered immediately prior to removing the animal from the stereotaxic frame. Rats were allowed to recover over a heating pad until voluntarily mobile on all four limbs and then placed back into their home cages. Acetaminophen in drinking water (60–80 ml of 3.25 mg/ml) and approximately 30 g of food pellets soaked with the acetaminophen solution were placed in the cage, along with regular food pellets and water ad libitum.
Intracardial perfusion and tissue sectioning
After a survival period of 4–14 days, the rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.). The absence of pain reflexes was established prior to i.p. administration of the zinc chelator diethyldithiocarbamate (Sigma, St. Louis, MO; 1 g/kg) used to prevent the silver enhancement of endogenous zinc (Veznedaroglu and Milner, 1992). After 15 minutes, the rats were perfused transcardially with approximately 30 ml of heparin saline (1,000 U/ml) followed by fixative, either 1) 50 ml of 2% paraformaldehyde with 3.75% acrolein, followed by 250 ml of 2% paraformaldehyde (two animals with LHb tracer injections and one rat with RMTg injection) or 2) 500 ml of 4% paraformaldehyde with 1% glutaraldehyde (six rats each with LHb or RMTg injections of PHAL). The brains were removed, divided into coronal blocks using a brain mold, and postfixed for at least 30 minutes in 2–4% paraformaldehyde.
Tissue sections 50 µm thick were sliced through the rostral-caudal extent of the LHb, VTA, and RMTg by using a vibrating microtome (Vibratome). A series of six tissue sets was collected into chilled 0.1 M phosphate buffer (PB). All of the sections were treated with 1% sodium borohydride in PB for 30 minutes, followed by thorough rinsing with PB (4× 10 minutes). Certain tissue sets were placed in long-term cryoprotectant storage at −20°C. The remaining sections for immediate immunolabeling were placed in 0.1 M Tris-buffered saline (TBS; pH 7.6), rinsed 3× 5 minutes, and then incubated for 30 minutes in a blocking solution of TBS containing 3% normal goat serum, 1% bovine serum albumin (BSA), and Triton-X 100 (0.04% for electron microscopy or 0.3% for light microscopy).
Antibody characterization
The primary antibodies used in this study (Table 1) have been fully characterized and tested for specificity. The polyclonal antibody directed against PHAL (Vector, #AS-2300, used at 1:1,000) was raised in rabbits immunized with the purified lectin. It recognizes both PHAL and the related Phaseolous vulgaris erythroagglutinin, neither of which is found in mammalian brain. Hence, specificity is established by the absence of immunoreactivity for PHAL in brain sections from 1) naive animals, 2) cases in which uptake and transport of PHAL failed, and 3) regions that do not receive innervation from the area of tracer injection.
TABLE 1.
Primary Antibodies Used
| Antigen | Immunogen | Source | Dilution |
|---|---|---|---|
| GABA | GABA-BSA conjugate | Sigma (St. Louis, MO), #A0310, mouse monoclonal | 1:1,000 |
| GABA | GABA-BSA conjugate | Sigma, #A2052, rabbit polyclonal | 1:1,000 |
| PHAL | PHAL | Vector (Burlingame, CA), #AS2300, rabbit polyclonal | 1:1,000 |
| TH | TH purified from PC12 cells | Millipore/Chemicon (Billerica, MA), #MAB318, mouse monoclonal | 1:5,000 |
The monoclonal anti-tyrosine hydroxylase (TH) antibody raised in mouse (Millipore-Chemicon, Billerica, MA, #MAB318, used at 1:5,000) is directed against TH purified from PC12 cells. Western blot analysis indicates that this antibody specifically recognizes TH from isolated striatal dopaminergic synaptosomes and that the amount of this immunoreactivity is markedly reduced by lesioning the nigrostriatal pathway with the dopamine-selective neurotoxin 6-hydroxydopamine (Wolf and Kapatos, 1989). According to the supplier, the antibody does not recognize other monoamine enzymes, including dopamine-β-hydroxylase, phenethanolamine-_N_-methyl transferase, and trytophan hydroxylase. In our extensive experience with this antibody (Sesack et al., 1995; Carr and Sesack, 2000; Miner et al., 2006), we have observed regional patterns of immunoreactivity that are entirely consistent with recognition of catecholamine neurons and their terminal fields (Lindvall et al., 1978; Swanson, 1982; Pickel et al., 1996).
Two different antibodies directed against GABA were used in the present study, both from Sigma-Aldrich (St. Louis, MO). Pre-embedding immunolabeling employed a mouse monoclonal antibody (#A-0310; used at 1:1,000) that was raised against GABA conjugated to BSA. According to the supplier, the antibody recognizes GABA on a dot blot immunoassay and shows no cross-reaction to other structurally similar amino acid neurotransmitters, including glutamate, aspartate, and glycine. (Weak labeling of β-alanine was detected.) In sections through the amygdala, immunoreactivity produced by this antibody was abolished by preadsorption with BSA conjugated to GABA but not to glutamate (Lehmann et al., 1998). In the cerebral cortex, dual-immunofluorescence labeling experiments have confirmed the nearly complete overlap in immunoreactivity produced by this antibody and the polyclonal rabbit-anti GABA antibody from Sigma (Blurton-Jones and Tuszynski, 2006) (see below). Finally, in our experience, this antibody produces patterns of immunoreactivity within the forebrain and VTA that are indistinguishable from those observed with other GABA antibodies (Pickel et al., 1988; Sesack et al., 1995; Van Bockstaele and Pickel, 1995; Carr and Sesack, 2000).
For postembedding immunogold detection of GABA (see below), a polyclonal anti-GABA antibody raised in rabbit (Sigma, #A-2052, used at 1:1,000) was employed. This antibody was also generated against a GABA-BSA conjugate. Specificity has been demonstrated by dot blot assay, in which the antibody recognizes GABA and GABA-conjugates but not BSA. In neuronal tissue, immunoreactivity produced by this antibody is abolished by preadsorption with GABA or GABA conjugated to BSA but not by free or conjugated glycine (Ligorio et al., 2000). As described above, specificity has been further demonstrated by the overlap in cortical cellular labeling produced by this antibody and the monoclonal anti-GABA antibody (Blurton-Jones and Tuszynski, 2006). The results of the postembedding experiments themselves have further demonstrated specificity by the overlap of pre- and postembedding labeling for GABA in the same axons and by the relative absence of immunoreactivity from axons with evident non-GABAergic morphology (Omelchenko et al., 2009; Omelchenko and Sesack, 2009).
Immunolabeling and tissue processing for electron microscopy
The following section describes the double immunolabeling procedure by which tissue sections were processed for immunoperoxidase visualization of PHAL followed by silver enhancement of immunogold labeling for either GABA in RMTg cells or for TH in VTA DA neurons. Some sections run in parallel were processed only through the steps necessary to perform single labeling for PHAL, either by immunoperoxidase for light microscopic detection of injection sites and transport or by immunogold-silver for electron microscopic detection in combination with postembedding for GABA (see below).
After pretreatment in respective blocking solutions, the tissue was transferred to primary antibodies made in blocking solution and incubated overnight at room temperature. Sections were then rinsed in TBS (3× 10 minutes) and then incubated for 30 minutes in biotinylated secondary goat anti-rabbit antibody (Vector, 1:400). The tissue was again rinsed in TBS (3× 10 minutes), incubated for 30 minutes in avidin-biotin peroxidase complex (ABC Vectastain Elite at 1:100; Vector), and rinsed in TBS (3× 5 minutes). In order to visualize bound peroxidase, the tissue was treated with TBS containing 0.022% 3,3′-diaminobenzidine and 0.003% hydrogen peroxide for 3.5 minutes. The colorimetric reaction was stopped by TBS rinses (3× 5 minutes) followed by rinsing in 0.01 M phosphate-buffered saline (PBS, pH 7.4; 3× 5 minutes).
After this point, sections for light microscopy were mounted onto glass slides (SuperFrost Plus, Thermo Scientific, Waltham, MA) by using ethanol gelatin in PBS and allowed to dry before dehydration and coverslipping. Digital light micrographic images were captured by using an Olympus BX51 microscope with a Hamamatsu Orca ERGA digital cooled CCD camera. Images were adjusted in Adobe (San Jose, CA) Photoshop for white balance, evenness of illumination and contrast, and removal of obvious artifacts.
Sections for immunogold-silver staining and electron microscopic visualization were incubated for 30 minutes in a washing buffer made of PBS containing 0.8% BSA, 0.1% fish gelatin, and 3% normal goat serum. The tissue was then incubated at 4°C overnight in washing buffer containing 1 nm gold-conjugated secondary antibodies, either goat anti-rabbit or goat anti-mouse (1:50; Aurion, Electron Microscopy Sciences, Hatfield, PA).
The sections for electron microscopy were next rinsed in plain washing buffer (1× 1 minute, then 3× 5 minutes), followed by PBS (1× 1 minute, 3× 5 minutes), and then incubated in 2.5% glutaraldehyde in PBS for 10 minutes. The tissue was thoroughly rinsed of glutaraldehyde by using PBS (1× 1 minute, and then 6× 5 minutes). Proceeding onto the silver intensification of gold labeling, the sections were transferred to sterile, untreated culture well plates for a series of 1-minute rinses: once in PBS and three times in fresh 0.2 M sodium citrate buffer (pH 7.4). Rinses were followed by silver enhancement reaction at room temperature in IntenSE M silver kit reagents (GE Healthcare, Chalfont St. Giles, UK). The sections were handled with nonmetallic, wooden applicator sticks and gently swirled throughout the silver enhancement procedure. The incubation time of the silver reaction was chosen empirically based on test trials of varying times for each primary antibody, most typically 4–6 minutes. The silver reaction was stopped by rinsing twice in sodium citrate buffer and then twice in PB.
During the course of the study, the GE Healthcare silver enhancement product was discontinued. Therefore, an alternative enhancement procedure using RGENT-SEM kits (Aurion, Electron Microscopy Sciences) was tested alongside the GE Healthcare product for several cases. For the Aurion procedure, all kit reagents were taken out of cold storage and allowed to warm to room temperature. The enhancement conditioning solution (ECS) was diluted 10-fold with ultrapurified water, and the developer and silver enhancement solutions were prepared according to kit instructions. Silver enhancement proceeded by first rinsing sections in ECS (4× 5 minutes) in culture well plates. The tissue was then reacted in silver enhancement solution for 80 minutes and rinsed in ECS (4× 5 minutes) and then in PB (3× 5 minutes).
Single- and double-labeled tissue for electron microscopic analysis was transferred to fresh PB in shallow Coors plates. Sections were then incubated in 2% osmium tetroxide for 30 minutes (Aurion silver enhancement) or for 1 hour (silver kit from GE Healthcare). The tissue was rinsed in PB (2× 3 minutes), transferred to fresh Coors plates, dehydrated for 10 minutes each in a series of increasing ethanol concentrations (30, 50, 70, and 95%), and then placed into scintillation vials containing 100% ethanol (2× 10 minutes). Finally, the tissue sections were treated with propylene oxide (2× 10 minutes) and incubated overnight in a 1:1 mixture of propylene oxide and Durcupan resin (Electron Microscopy Sciences). This mixture was then replaced with straight Durcupan and allowed to infiltrate the sections for 2 hours. The tissue was embedded between sheets of commercial plastic, flattened underneath lead weights, and cured for at least 72 hours at 62°C.
Flat-embedded sections were glued onto resin blocks, trimmed to appropriate regions of interest, and sliced on an ultramicrotome at a thickness of 60–70 nm. The RMTg cannot be easily discerned from sections stained for Nissl or myelin. Hence, the trimming of this region was based on comparison with the dense projections of tracer from the LHb in immediately adjacent light microscopic sections that were carefully aligned to the plastic-embedded tissue. Most ultrathin sections were collected onto copper mesh grids in series of three to five sections per grid. After drying, approximately every fourth grid was counterstained with 5% uranyl acetate and Reynold’s lead citrate. Sections intended for postembedding immunolabeling (see below) were collected onto nickel grids.
Postembedding immunogold labeling for GABA
As described previously (Omelchenko and Sesack, 2009), the postembedding procedure provides superior sensitivity to pre-embedding methods for detection of GABA immunoreactivity within axon terminals, whereas the reverse is true for detection of GABA immunolabeling within dendrites (Shink and Smith, 1995; Van Bockstaele and Pickel, 1995; Jia et al., 2003; Omelchenko and Sesack, 2009). Consequently, VTA sections from three of the rats with optimal anterograde transport from the RMTg were single-labeled for PHAL by pre-embedding immunogold-silver and then prepared for postembedding immunolabeling by ultrathin sectioning, collection onto nickel mesh grids, and drying for several days. Postembedding labeling was performed on sheets of parafilm placed within humidified Petri dishes. For each incubation or rinse, fresh parafilm was used to stage the dropwise solutions. To begin, grids were placed tissue side down onto drops of 0.05 M TBS (pH 7.6) containing 0.1% Triton for 5 minutes. The grids were then incubated overnight on drops of primary antibody (rabbit anti-GABA, described above) made in TBS containing 0.01% Triton and 1% BSA.
Following exposure to the primary antibody, grids were rinsed on drops of TBS containing 0.01% Triton and 1% BSA for 2× 10 minutes and then 1× 30 minutes. Grids were then further rinsed on drops of 0.05 M TBS (pH 8.2) containing 0.1% Triton for 5 minutes, and then incubated on drops of 15-nm gold-conjugated goat anti-rabbit secondary (Ted Pella, Reading, CA; 1:25) for 90 minutes. The grids were rinsed on drops of plain TBS (pH 8.2; 2× 10 minutes), dipped one time each into five consecutive beakers of ultrapurified water, and then placed on distilled water drops for 5 minutes. The tissue was then counterstained by using the same procedure for grids containing pre-embedding immunolabeled tissue (see above).
Semiquantitative ultrastructural analysis
Tissue was analyzed by using an FEI Morgagni (Hillsboro, OR) 268 transmission electron microscope, and micrographs were captured by using an XP-60 digital camera from Advanced Microscopy Techniques (Danvers, MA). Adobe Photoshop was used to adjust image contrast and illumination.
A semiquantitative approach was taken in order to provide an approximation of the strength of synaptic connections from the LHb to the RMTg and from the latter structure to the VTA. A running tally was kept of the amount of tissue analyzed, and the number of anterogradely labeled axons encountered within this area was assessed as follows.
For each animal in the study, ultrathin sections taken from at least one to three Vibratome sections were examined. Ultrathin sections were scanned systematically for axons containing anterograde tracer from the LHb (in the case of RMTg sections) or from the RMTg (in sections through the VTA). The tissue was visible within square regions demarcated by the metal grid mesh (3,025 µm2 area), and grid squares were chosen for scanning based on whether the tissue they contained was close to the interface with the plastic embedding material. Specifically, grid squares were analyzed if they contained approximately 25–75% tissue (the remaining area being occupied by embedding resin) or if they contained 100% tissue but were immediately adjacent to the interface as just described. These steps ensured that sampling was conducted close to the tissue surface where antibodies penetrated most optimally. For the LHb to RMTg projection, a total of 1,202,059 µm2 was sampled, with an average area of 240,412 µm2 examined per rat. Approximately half of this sample was obtained from the rostroventral portions of the RMTg, and the remainder came from the caudodorsal regions. For the projection from the RMTg to the VTA, a total of 997,494 µm2 were examined, including an average of 142,499 µm2 per animal. For this pathway, roughly 75% of the electron microscopic sample came from the anterior and middle VTA, whereas-one quarter was taken from the posterior VTA and only from those rats in which the RMTg injection site (i.e., tracer-labeled cells) did not extend into this area.
Neuronal and glial profiles were identified as morphological compartments delineated by plasma membrane boundaries. Axonal profiles exhibited small clear vesicles and occasional mitochondria, had relatively uniform widths that changed only gradually, and sometimes contained visible microtubules. Notable swellings along the axon that were filled with vesicles constituted varicosities, whereas intervaricose segments contained few or no vesicles, had smaller diameters (arbitrarily defined as less than 0.2 µm), and often appeared in bundles of other axon fibers. All axons containing anterograde tracer were counted; however, only those classified as varicosities were analyzed for possible dendritic contacts (see below). Dendrites were identified as profiles having relatively large diameters, regular contours, and few or no small vesicles in the cytoplasm; dendrites were generally in receipt of synaptic input from axons. Neuronal soma had similar features but were distinguished by the presence of a nucleus. Glial processes were evident as thin profiles having a fairly clear cytoplasm and membranes that conformed to the irregular contours of adjacent structures and sometimes formed tight junctions.
Axon varicosities immunoreactive for PHAL were examined and photographed in adjacent serial sections whenever possible, being prevented mainly when obscured by the grid mesh. The collection of serial images aided in confirming the presence of labeling and the classification of dendritic contacts. Such contacts were identified as symmetric synapses when they exhibited widened, parallel membrane spacing, intercleft filaments, accumulated presynaptic vesicles, and little or no postsynaptic densities. Asymmetric synapses had similar features except for notably thickened postsynaptic densities. This latter morphological distinction has been correlated with an excitatory physiology versus a probable inhibitory action for symmetric synapses (Colonnier, 1968; Carlin et al., 1980). Nevertheless, and as noted previously (Omelchenko et al., 2009), many of the synapses formed by LHb axons in brainstem targets have either symmetric characteristics or exhibit an intermediate morphology that defies simple categorization. Dendritic contacts that lacked the aforementioned structural elements of synapses were classified as appositions.
Tracer-labeled varicosities were excluded from the final sample if dendrites containing specific immunogold-silver labeling were absent in the surrounding neuropil at a magnification of 18,000×. This was done to reduce false-negative outcomes by ensuring that the less sensitive immunogold-silver reagents (Chan et al., 1990) had penetrated to a comparable depth from the tissue surface as markers for immunoperoxidase. Specific pre-embedding gold labeling was defined as profiles containing at least three gold-silver particles in a single section. Whenever possible (see above), dendrites were examined in adjacent serial sections to confirm the presence of gold-silver labeling.
In the tissue labeled for GABA by postembedding immunogold, labeling of axons forming symmetric synapses was considered to be specific when it exceeded by a factor of at least 5 the density (number per unit area) of gold particles observed in axon terminals forming asymmetric synapses. This level of immunoreactivity typically exceeded that which was observed in adjacent dendrites by at least three times, consistent with the relative insensitivity of the postembedding method for detecting GABA in dendrites (Shink and Smith, 1995; Van Bockstaele and Pickel, 1995; Jia et al., 2003; Omelchenko and Sesack, 2009).
Image analysis
Axon profile diameter was measured as a preliminary characterization of the relative size of axon boutons originating from the RMTg. In addition, we measured the diameter of postsynaptic dendrites in order to estimate the relative distal (i.e., smaller diameter) to proximal (i.e., larger diameter) location of LHb synapses onto dendrites in the RMTg and RMTg synapses in the VTA. The size of immunoreactive dendrites that were synaptic targets was also compared with immunonegative dendrites receiving synaptic input and with immunoreactive dendrites in the adjacent neuropil that did not receive such contacts. These comparisons were made in order to determine whether LHb or RMTg axons demonstrated any specificity in the dendritic compartments targeted across different cell populations within each terminal region. The approximate size of axons and dendrites was estimated manually by using the maximum diameter along the short axis of each profile photographed at 11,000–18,000× magnification (Sesack et al., 1998).
We also endeavored to obtain an estimate of the relative TH content of the dendrites receiving RMTg synapses in the VTA, given that DA cell dendrites express varying levels of TH immunoreactivity (Bayer and Pickel, 1990; Sesack and Pickel, 1992) that correlate with the extent of synaptic input and, in particular, from GABAergic axons (Bayer and Pickel, 1991). To achieve this estimate, an image analysis system (Simple PCI, Hamamatsu, Bridge-water, NJ) was used to measure the density (number per unit area) of TH immunogold-silver particles in dendrites receiving synaptic input from RMTg axons versus other TH-labeled dendrites in the immediate vicinity. The dendrites of interest were outlined by using a binary overlay that avoided potential sources of false-positive counts, such as high contrast features like mitochondria and synaptic membranes. Gold particles within these regions were then counted automatically by thresholding the images with the gray minimum set to 0 and the maximum set to 100.
These various features were compared for statistical significance by using either Student’s t-test or the Wilcoxon rank-sum test with α equal to 0.05, two-tailed.
RESULTS
LHb projection to the RMTg: light microscopy
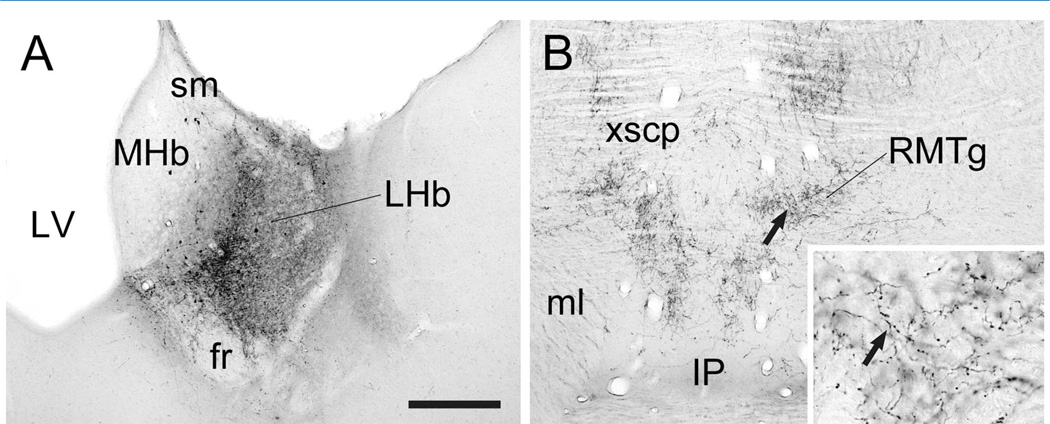
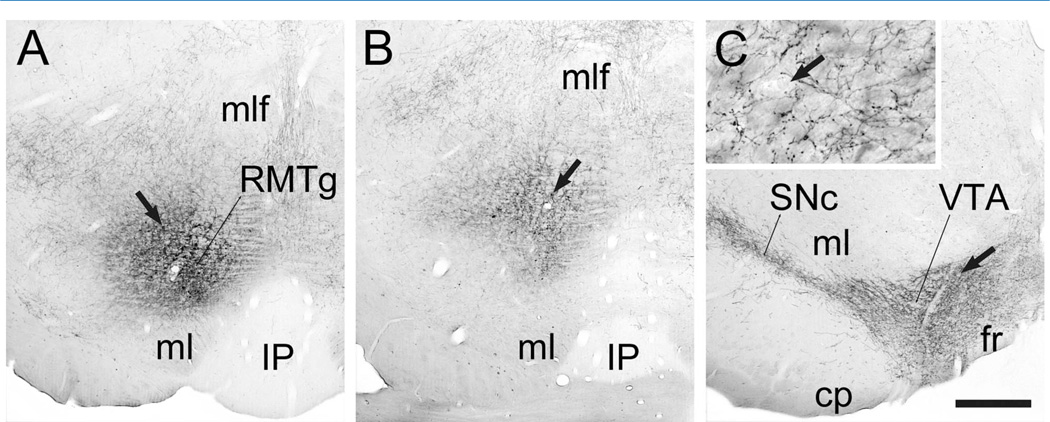
PHAL injections were targeted to the medial or lateral portions of the LHb (Fig. 1A). These injections resulted in anterograde transport to the brainstem RMTg that was bilaterally distributed with an ipsilateral predominance. The LHb terminal fields ranged from light (not shown) to heavy (Fig. 1B) depending on the size and placement of the injections, as previously described (Jhou et al., 2009b; Kaufling et al., 2009). As was noted earlier, these were the same animals as used for our prior analysis of the LHb projection to the VTA (Omelchenko et al., 2009), although a different case is illustrated here by light microscopy. Many of the LHb axons in the RMTg region exhibited extensive branching and beading suggestive of local terminations (Fig. 1B, inset).
Figure 1.
Light micrographic images of coronal sections through the rat lateral habenula (LHb; A) and rostromedial mesopontine tegmental nucleus (RMTg; B) illustrating immunoperoxidase labeling for PHAL. For both panels, medial is to the left. A: A unilateral injection of PHAL is centered in the medial portion of the caudal LHb (at approximately −3.8 mm posterior to Bregma) but also includes parts of the lateral LHb. It extends to the borders of the stria medullaris (sm) and fasciculus retroflexus (fr) but largely avoids the medial habenula (MHb) adjacent to the lateral ventricle (LV). B: Anterograde transport of PHAL to the RMTg (at approximately −6.8 mm caudal to Bregma) includes clusters of fibers near the crossing of the superior cerebellar peduncle (xscp). The fibers dorsal to the medial lemniscus (ml) and dorsolateral to the interpeduncular nucleus (IP) include branched axons with highly beaded morphology; arrows indicate the same axons at low and high magnification (inset). Scale bar in A = 300 µm in A, B; 60 µm in inset.
LHb projection to the RMTg: electron microscopy
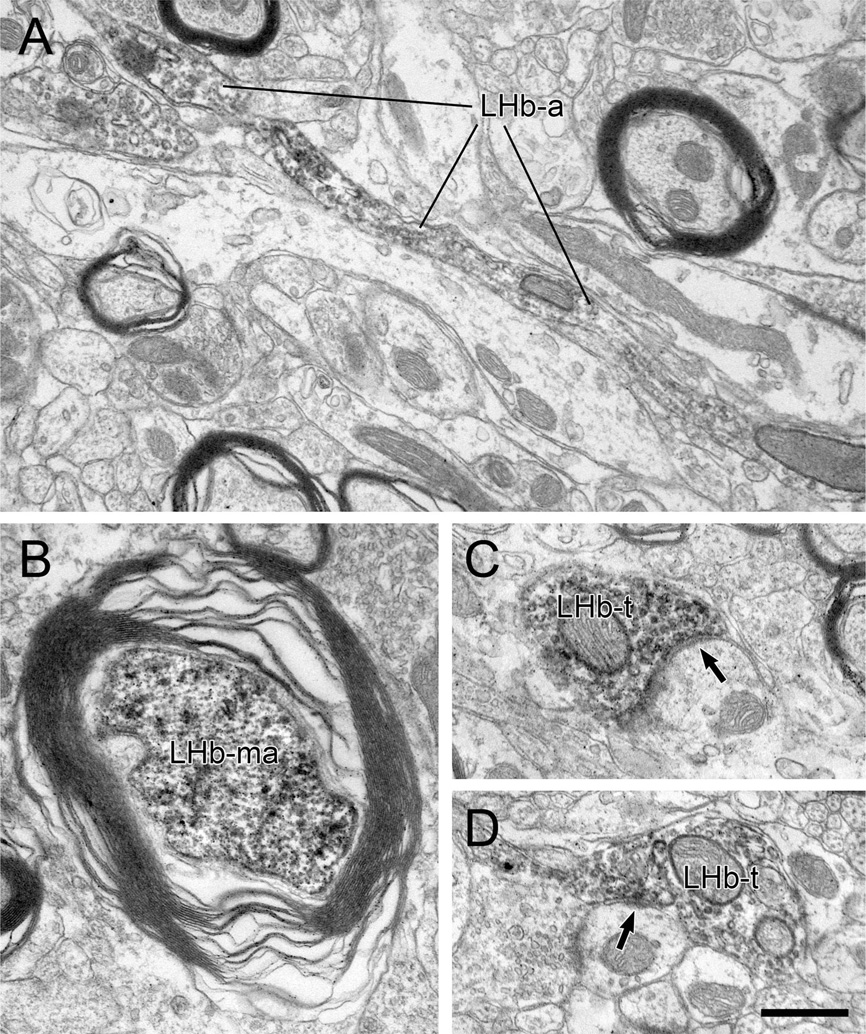
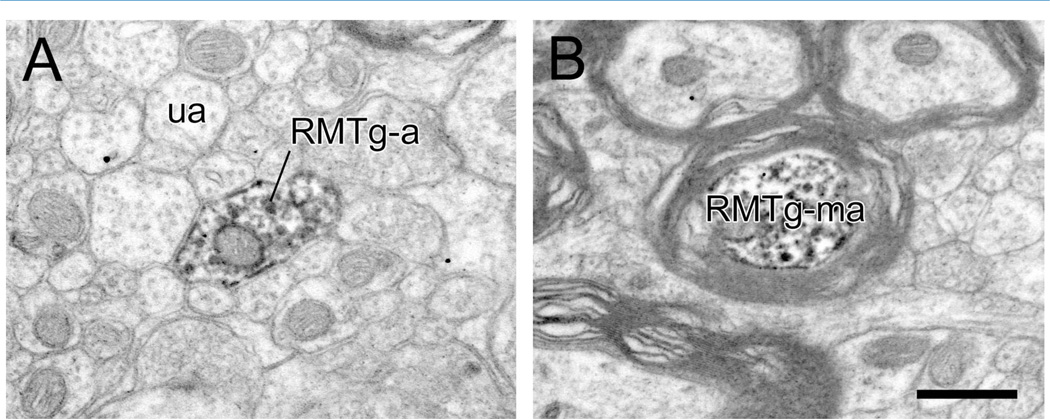
Both myelinated and unmyelinated axons containing PHAL transported from the LHb were observed within the RMTg (Fig. 2). Of 936 LHb axons, 35% (n = 325) were myelinated (Fig. 2B). The majority of these were recorded but not photographed. In a random sample of 29 myelinated axons illustrated in micrographs, the average outer diameter was 1.05 ± 0.63 µm (mean ± SD), with a range from 0.5 to 2.5 µm.
Figure 2.
A–D: Electron micrographs through the rat RMTg illustrating axons labeled by immunoperoxidase for PHAL transported anterogradely from the lateral habenula (LHb). Some LHb fibers are unmyelinated passing axons containing some vesicles but not forming synapses (LHb-a in A), whereas others exhibit distinct myelination (LHb-ma in B). Other LHb axons form synaptic contacts (black arrows) onto dendrites (LHb-t in C and D). Note the modest postsynaptic densities in both cases. Scale bar = 0.5 µm in D (applies to A–D).
The remaining 611 profiles containing anterograde tracer from the LHb were unmyelinated axons. Of these, 520 appeared either as small-diameter fibers or larger diameter varicosities that made no direct contacts onto dendrites. The morphological appearance of some of these unmyelinated axons was suggestive of fibers of passage (Fig. 2A).
The other tracer-labeled profiles (n= 91) were LHb axon varicosities that did directly contact dendrites (Table 2). Of these dendritic contacts, 41 exhibited synaptic junctions (Figs. 2C,D, 3), whereas the other 50 LHb profiles had membranes that were closely apposed to dendrites without obvious synaptic features (Fig. 3A, Table 2). Often, dense-cored vesicles were visible in addition to small, clear synaptic vesicles in LHb varicosities (Fig. 3C), although the former were sometimes masked by peroxidase reaction product. The morphology of the synaptic contacts formed by LHb axons varied from symmetric (i.e., with little or no postsynaptic thickening) to asymmetric (i.e., with dense thickenings), although the majority had intermediate characteristics that were not typical of either category, as exemplified in Figures 2 and 3. None of the observed LHb synapses had perforations, which are sometimes found on excitatory junctions (Jones and Harris, 1995). These morphological features, including the nonclassical synaptic junctions, are identical to those described for LHb axons in the VTA (Omelchenko et al., 2009).
TABLE 2.
Synaptic Contacts of LHb Axons in the Rat RMTg
| Dendrites immunolabeled | ||||||
|---|---|---|---|---|---|---|
| Unlabeled | GABA | |||||
| PHAL-containing LHb axons | No. | % | No. | % | No. | % |
| Total unmyelinated | 611 | |||||
| Total contacting dendrites | 91 | 15 | 43 | 47 | 48 | 53 |
| Total appositions | 50 | 55 | 25 | 50 | 25 | 50 |
| Total synapses | 41 | 45 | 18 | 44 | 23 | 56 |
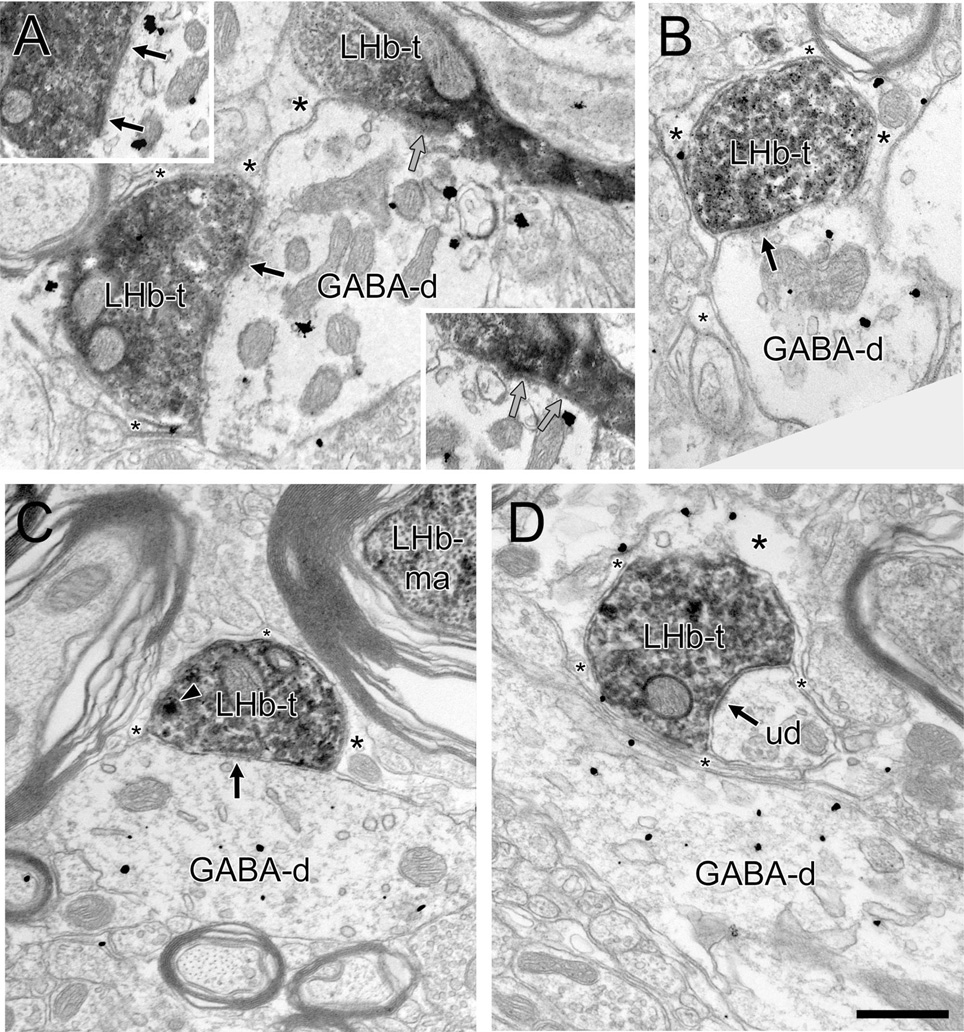
Figure 3.
A–D: Electron micrographs through the rat RMTg showing immunoperoxidase reaction product for PHAL in lateral habenula (LHb) axon terminals (LHb-t) in relation to dendrites containing immunogold-silver labeling for γ-aminobutyric acid (GABA) (GABA-d). LHb-ts often synapse (black arrows) onto GABA-ds (A–C). In some cases (A), multiple LHb-ts form convergent contacts onto common immunoreactive dendrites, although synaptic junctions are not always evident at each site. (Insets illustrate points of contact in adjacent serial sections; gray arrows illustrate an apposition that is not clearly synaptic.) In other instances (D), LHb-ts synapse onto unlabeled dendrites (ud) in close proximity to GABA-ds. Most LHb-ts and their postsynaptic targets are surrounded on multiple sides by glial processes (asterisks), some of which contain gold-silver immunolabeling for GABA (A,B,D). In C, a dense-cored vesicle (arrowhead) is evident within the LHb-t; a myelinated LHb axon (LHb-ma) can be seen nearby. Scale bar = 0.5 µm in D (applies to A–D).
The fact that the same animals were used for this investigation as for the prior study of the VTA (Omelchenko et al., 2009) allows for some rough comparison of the density of LHb synapses in the RMTg versus the VTA. For the study of the LHb projection to the VTA, 43 synapses were detected upon examination of 9,292,456 µm2 of tissue. For the LHb to RMTg analysis, we observed 41 synapses in 1,202,059 µm2 of tissue examined. Hence, the observed density of LHb synaptic input to the RMTg was approximately seven times greater than that detected in the VTA. Although crude, this estimate is consistent with light microscopic observations. A more precise density comparison will require unbiased stereological measures.
In tissue dually labeled for PHAL and GABA, 23 (56%) of the 41 dendrites postsynaptic to LHb varicosities were immunoreactive for GABA (Fig. 3A–C, Table 2). LHb axons also synapsed onto dendrites that were immunonegative for GABA (n = 18, 44%); this was observed even when GABA-labeled dendrites were present within the immediately adjacent neuropil (Fig. 3D). It was commonly seen that glial processes encompassed the LHb axons and their target dendrites, regardless of whether the postsynaptic structures were immunolabeled (Fig. 3). In many cases, immunoreactivity for GABA was detected within these astrocytic leaflets (Fig. 3A,B,D).
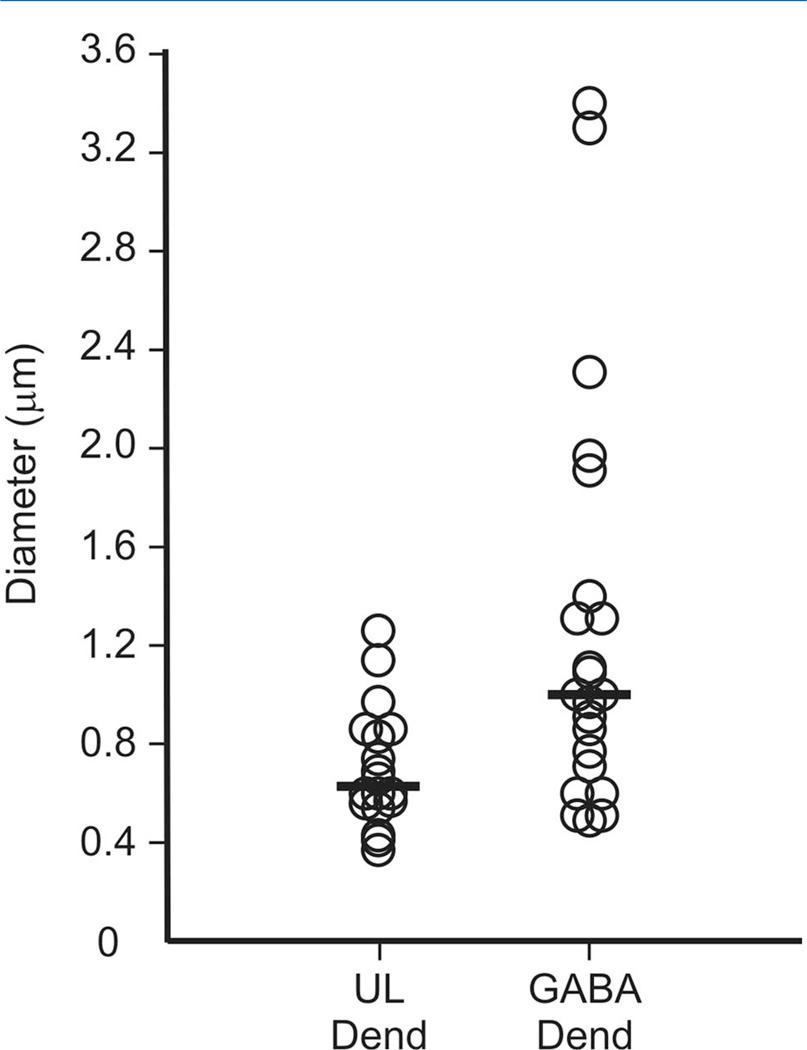
For those LHb axons forming synapses in the RMTg, we examined the diameter of the postsynaptic dendrites as an approximation of their distal to proximal location. For the 23 targeted dendrites that were GABA-immunoreactive, the average diameter was 1.52 ± 1.44 µm (range 0.49–7.00), whereas the average diameter of the 18 unlabeled dendritic targets of LHb axons was 0.70 ± 0.24 µm (range 0.37–1.26); this difference was significant by the Wilcoxon rank-sum test (P = 0.003). Figure 4 illustrates the distribution of diameters for GABA-immunoreactive and unlabeled dendrites that received synaptic input from the LHb and suggests that the larger dendrites were more likely to contain GABA immunoreactivity.
Figure 4.
Scatter plot showing the diameter of γ-aminobutyric acid (GABA)-immunoreactive or unlabeled (UL) dendrites (Dend) in the rat RMTg that received synaptic input from LHb axons. The group medians are indicated by horizontal lines. The largest diameter dendrite in the GABA-immunolabeled population (7.00 µm) was not included in the plot for ease of presentation, but was factored into the group median.
We also compared the approximate size of GABA-labeled dendrites in the RMTg that received LHb synapses versus other GABA-immunoreactive dendrites in the immediately adjacent neuropil that were not contacted by LHb axons. The mean diameter of the latter dendrites was 1.24 ± 0.43 (n = 43, range 0.43–2.10) and did not differ significantly from the former (P = 0.566) by the Wilcoxon rank-sum test. These findings suggest that LHb axons showed no distal to proximal preference in the location of their synapses onto RMTg neurons.
RMTg projection to the VTA: light microscopy
Most of the PHAL injections into the RMTg were targeted to the rostral portions that lie dorsal to the medial lemniscus and dorsolateral to the interpeduncular nucleus (Fig. 5A). Nevertheless, in some cases the zone of tracer uptake, as indicated by the presence of PHAL-positive soma, extended into more caudodorsal regions of the RMTg (Fig. 5B). Injections involving the RMTg produced anterograde transport throughout the VTA and SNc (Fig. 5C), and many of these axons were extensively branched and beaded (Fig. 5C, inset).
Figure 5.
Light micrographic images of coronal sections through the rat rostromedial mesopontine tegmental nucleus (RMTg; A,B) and ventral tegmental area (VTA; C) illustrating immunoperoxidase labeling for PHAL. In each panel, medial is to the right. A,B: A unilateral injection of PHAL is centered in the RMTg (at approximately −6.8 mm posterior to Bregma) just above the medial lemniscus (ml) and dorsolateral to the interpeduncular nucleus (IP). Arrows indicate cells that have taken up tracer. At the more caudal level shown in B (about −7.04 mm behind Bregma), a few such cells can be seen at more dorsal positions, consistent with the known caudodorsal shift of the RMTg. C: Anterograde transport of PHAL to the ventral midbrain includes the entire SNc-VTA complex (shown here at roughly −5.3 mm caudal to Bregma), outlining the region known to contain DA neurons. RMTg axons shown at higher magnification in the inset are extensively branched and beaded. (Arrows indicate the same capillary at low and high magnification.) Abbreviations: cp, cerebral peduncle; fr, fasciculus retroflexus; mlf, medial longitudinal fasciculus; SNc, substantia nigra zona compacta. Scale bar in C = 500 µm in A–C; 50 µm in inset.
For the three animals that received bilateral injections of PHAL into the RMTg, only the VTA in one hemisphere was analyzed for electron microscopy, based on which injection site was most optimally confined to the RMTg. Nevertheless, the midbrain projections from the RMTg are bilateral with an ipsilateral predominance (Ferreira et al., 2008; Jhou et al., 2009b; Kaufling et al., 2010), so that some of the tracer labeled axons in the VTA might have derived from the contralateral hemisphere in these three cases (see below).
RMTg projection to the VTA: electron microscopy
Ultrastructural examination of the VTA included primarily the paranigral and parabrachial pigmented subdivisions. Within these areas, the majority of RMTg axons detected were unmyelinated (n = 872/927, 94%; Fig. 6A), although occasional myelinated fibers were also observed (n = 55/927, 6%; Fig. 6B). A random photographic sample of 11 myelinated RMTg axons revealed their average outer diameter to be 1.44 µm (± 1.00 SD), with a range from 0.40 to 3.14 µm.
Figure 6.
A,B: Electron micrographic images of the rat VTA showing immunoperoxidase reaction product for PHAL transported anterogradely from the rostromedial mesopontine tegmental nucleus (RMTg). Tracer containing axons are either unmyelinated (RMTg-a), as shown in A in a field of unlabeled axons (ua) or occasionally myelinated as illustrated in B (RMTg-ma). Scale bar = 0.5 µm in B (applies to A,B).
Unmyelinated RMTg axons included a majority (n = 748) that were either small-diameter intervaricose segments or vesicle-containing boutons that did not contact surrounding dendrites in single or a small number of serial sections. RMTg axons contained mainly small, clear synaptic vesicles, with dense-cored vesicles rarely seen. The remaining 124 RMTg axons were varicosities in direct contact with dendrites in the vicinity (Figs. 7–9; Table 3), and these were observed in all seven of the animals with anterograde transport from the RMTg to the VTA. As this is the first ultrastructural description of projections arising from the RMTg, we estimated the relative size of these varicosities by measuring their short-axis diameter, which averaged 0.60 µm (± 0.16 SD) and ranged from 0.29 to 1.09 µm.
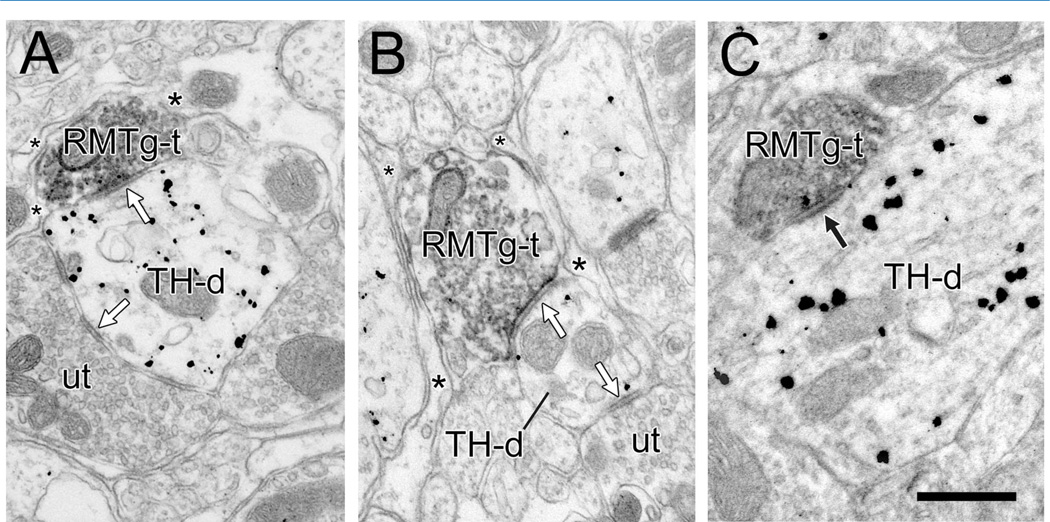
Figure 7.
Electron micrographs of the rat VTA showing rostromedial mesopontine tegmental nucleus (RMTg) axon varicosities labeled by immunoperoxidase for PHAL (RMTg-t) synapsing onto dendrites containing gold-silver labeling for tyrosine hydroxylase (TH-d). A,B: The synapses are symmetric (white arrows), the TH-ds receive additional symmetric synapses from unlabeled terminals (ut), and the RMTg-ts are mostly surrounded on nonsynaptic sides by glial processes (asterisks). In B, the small-diameter TH-d is relatively distal and contains fewer gold-silver particles for TH than adjacent dendrites. Nevertheless, the specific labeling of the target dendrite was confirmed in adjacent serial sections. C: The RMTg axon forms a synapse with a thicker postsynaptic density (black arrow) that is more typically asymmetric. Scale bar = 0.5 µm in C (applies to A–C).
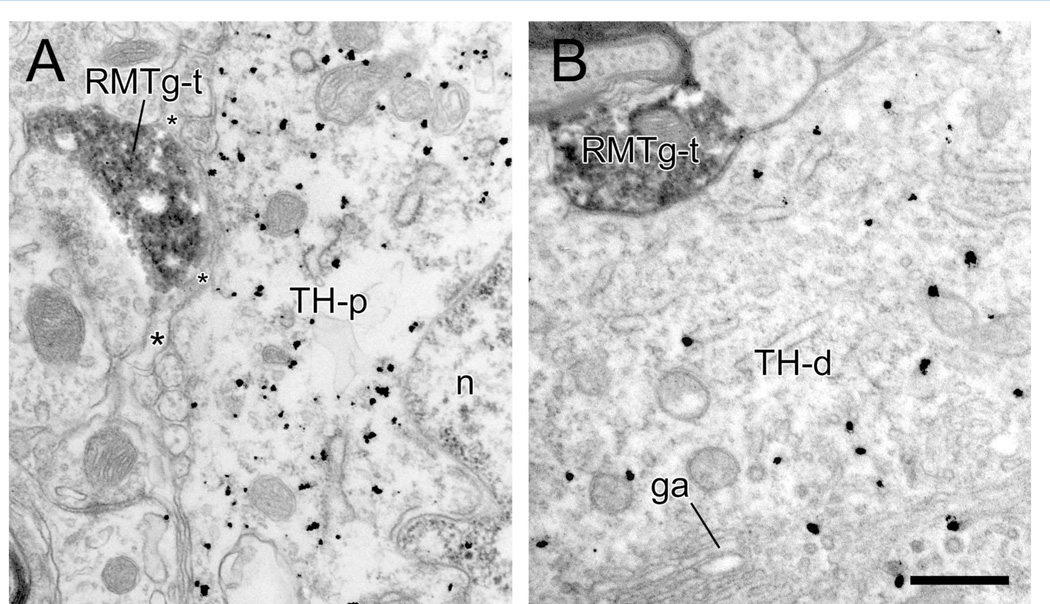
Figure 9.
Electron micrographs of the rat VTA showing rostromedial mesopontine tegmental nucleus (RMTg) axon varicosities (RMTg-t) in the vicinity of tyrosine hydroxylase (TH)-immunoreactive perikarya (TH-p; n represents the nucleus) or proximal dendrites (TH-d; ga represents a Golgi apparatus). A: Although the RMTg-t is closely adjacent to the TH-p, a thin glial leaflet (asterisks) separates the two structures. B: The RMTg-t is directly apposed to the proximal dendrite but without evidence of a junctional specialization in this or in adjacent serial sections. Scale bar = 0.5 µm in B (applies to A,B).
TABLE 3.
Synaptic Contacts of RMTg Axons in the Rat VTA
| Dendrites immunolabeled | ||||||
|---|---|---|---|---|---|---|
| Unlabeled | TH | |||||
| PHAL-containing RMTg axons | No. | % | No. | % | No. | % |
| Total unmyelinated | 872 | |||||
| Total contacting dendrites | 124 | 14 | 24 | 19 | 100 | 81 |
| Total appositions | 89 | 72 | 18 | 20 | 71 | 80 |
| Total synapses | 35 | 28 | 6 | 17 | 29 | 83 |
Of the 124 RMTg axons in contact with dendrites, 35 formed synaptic connections (Figs. 7, 8), whereas the other profiles exhibited membrane appositions without evidence of synaptic specializations (Table 3). The majority of synapses (n = 32, 91%) were of the symmetric type, with thin or absent postsynaptic densities (Figs. 7A,B, 8). A few RMTg axons formed synapses with thicker densities more typical of asymmetric synapses (Fig. 7C). Of the total number of synapses, 11 were from the three animals that received bilateral injections of PHAL. Although only the VTA ipsilateral to the best RMTg injection placement was examined in these cases, the bilateral nature of the projections from the brainstem suggests that some of these synapses might have originated from the contralateral tegmentum adjacent to the RMTg. Nevertheless, the remaining 24 synapses were from the four rats with unilateral, well-placed injections, indicating that the majority of synapses derived from the ipsilateral RMTg.
Figure 8.
Electron micrograph of the rat VTA illustrating an axon varicosity containing immunoreaction product for PHAL transported anterogradely from the rostromedial mesopontine tegmental nucleus (RMTg-t). Although the RMTg-t is in the field of a dendrite containing immunogold-silver labeling for tyrosine hydroxylase (TH-d), it synapses instead (white arrow) onto an unlabeled dendrite (ud). Note the small diameter of the ud. Scale bar = 0.5 µm.
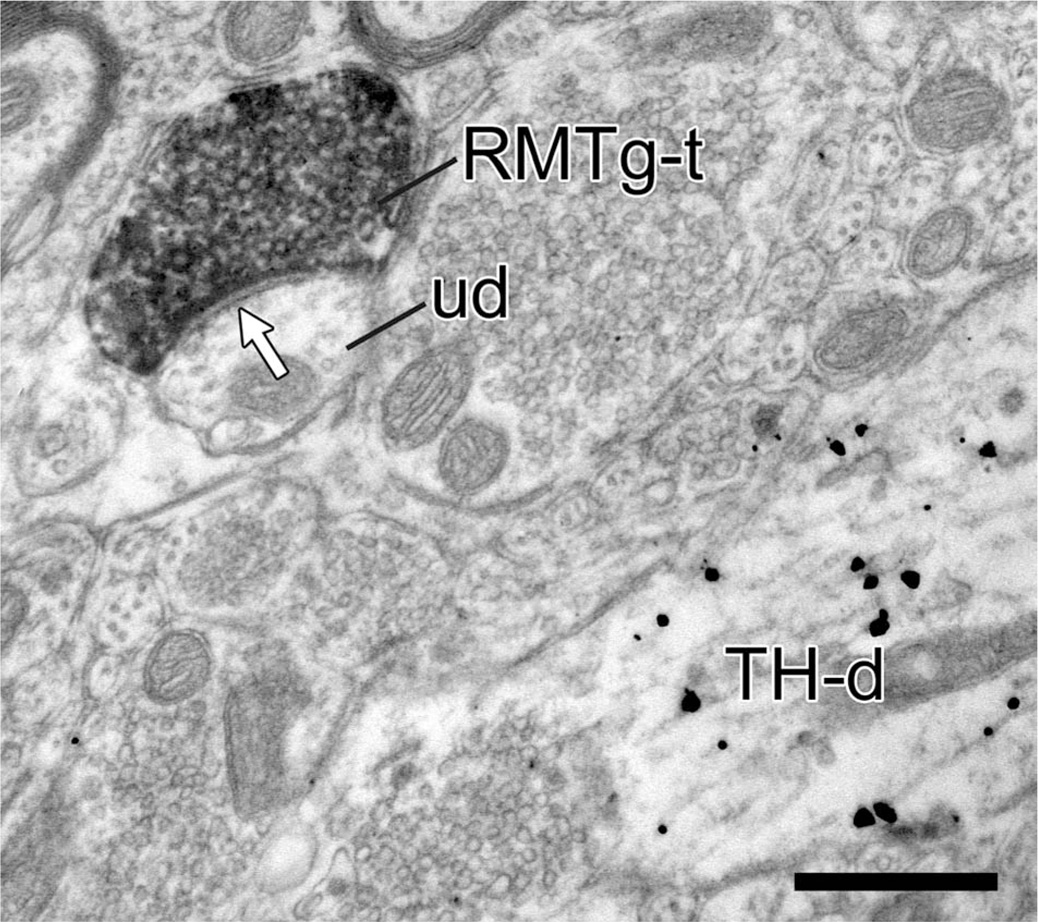
In tissue sections immunolabeled for TH, the large majority of synapses formed by RMTg axons were onto TH-positive dendrites (29/35, 83%; Fig. 7, Table 3). The target dendrites ranged in size and extent of immunolabeling for TH (see below). In a few cases, the dendrites postsynaptic to RMTg axons contained sparse immunogoldsilver particles, but these did not reach the criterion for specific labeling. Many of the dendrites postsynaptic to RMTg terminals received additional synaptic input from unlabeled axons (Fig. 7A,B). Other RMTg axon varicosities synapsed onto dendrites immunonegative for TH even when TH-labeled dendrites were located nearby (6/35, 17%; Fig. 8, Table 3).
In recent light microscopic studies describing the RMTg projection to the SNc-VTA complex (Jhou et al., 2009a; Kaufling et al., 2010), it has been suggested that RMTg axons contact DA cell bodies and proximal dendrites based on the extreme density of fibers in the immediate vicinity of TH-immunoreactive perikarya. Hence, in the present study, we paid particular attention to RMTg varicosities observed in the presence of these large-diameter structures. Nevertheless, we failed to detect clear evidence for synaptic contacts onto soma (identified by the presence of the nucleus) or proximal dendrites (identified by at least one Golgi apparatus). In some cases, vesicle-containing RMTg axons were found in close proximity to these potential targets, but they were either separated by thin glial leaflets (Fig. 9A) or were in appositional contact without evidence of synaptic junctions, even when examined in serial sections (Fig. 9B).
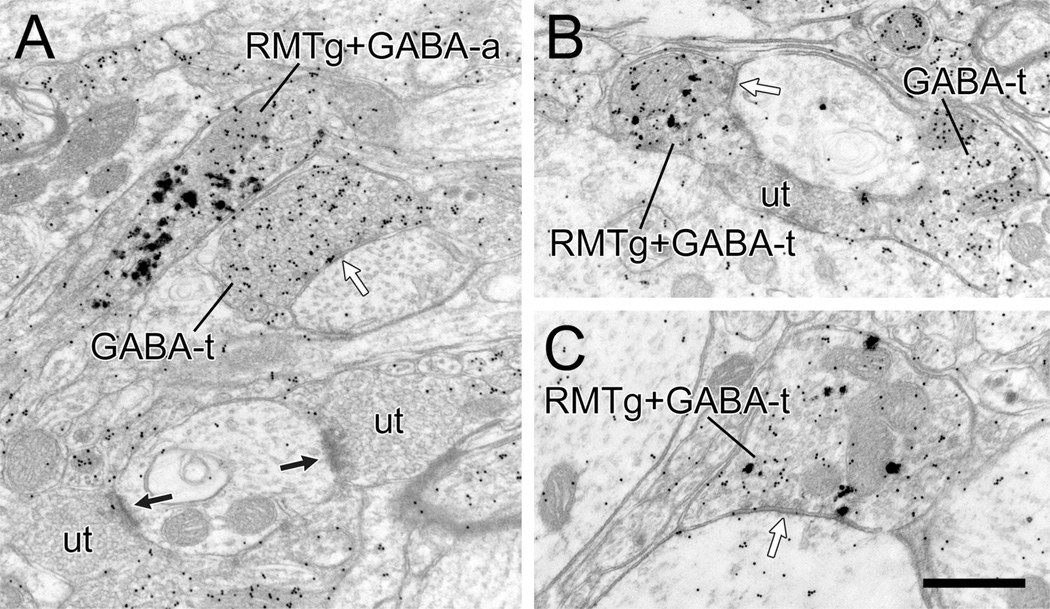
The symmetric synapses formed by most RMTg axons in the VTA suggest a possible GABAergic phenotype, consistent with prior studies using retrograde tract-tracing (Jhou et al., 2009a,b; Kaufling et al., 2010). In order to confirm this hypothesis in three animals with optimal anterograde transport from the RMTg, we performed postembedding immunogold labeling for GABA in combination with pre-embedding immunogold-silver labeling of PHAL (Omelchenko and Sesack, 2009). The analysis was confined to PHAL-containing axon varicosities that exhibited notable accumulations of vesicles; the restricted dimensions of small unmyelinated axons made them too difficult to assess for the presence of different sized silver particles. In this tissue, all 27 of the PHAL-labeled RMTg varicosities observed also contained postembedding immunoreactivity for GABA (Fig. 10). A few of these profiles did not contact dendrites (Fig. 10A), but the majority formed symmetric axodendritic synapses (Fig. 10B,C). A few myelinated axons containing PHAL transported from the RMTg also exhibited postembedding immunolabeling for GABA (not shown).
Figure 10.
Electron micrographic images of the rat VTA illustrating axons (RMTg+GABA-a) or terminal varicosities (RMTg+GABA-t) that contain both pre-embedding immunogold-silver labeling for PHAL (large, irregular particles) transported anterogradely from the RMTg and postembedding immunogold labeling for GABA (small, uniform particles). A: The RMTg+GABA-a is adjacent to an axon terminal that is singly labeled for GABA (GABA-t) and forms a symmetric synapse (white arrow) onto a dendrite. Two nearby unlabeled terminals (ut) forming asymmetric synapses are shown for comparison. B,C: The two RMTg+GABA-ts synapse (white arrows) onto dendrites. In B, a GABA-t and a ut are shown for comparison. Abbreviations: GABA, γ-aminobutyric acid; RMTg, rostromedial mesopontine tegmental nucleus. Scale bar = 0.5 µm in C (applies to A–C).
We considered whether RMTg axons exhibited any preference for synapsing onto specific populations of TH-immunoreactive dendrites as assessed by their distal to proximal position or relative content of TH (Bayer and Pickel, 1990). For the first estimate, we compared the average diameter of the TH-labeled dendrites receiving synapses from RMTg axons versus other immunoreactive dendrites in the immediate vicinity that were not contacted. TH-labeled dendrites that received RMTg synaptic input were on average larger (1.06 ± 0.64 µm, n = 29) than immediately adjacent TH-positive dendrites that showed no synaptic input in that plane of section (0.79 ± 0.34 µm, n = 94). This difference was significant by t-test (P = 0.004) and suggests that RMTg axons show some preference for synapsing onto larger, and therefore potentially more proximal dendrites. The unlabeled dendrites that received synaptic input from RMTg axons were not significantly smaller (0.73 ± 0.40 µm, n = 6; P = 0.24) than immunoreactive postsynaptic dendrites, perhaps due to the low number of immunonegative targets.
Regarding the relative TH content, this was estimated by using an image analysis system to calculate density, i.e., number of immunogold-silver particles per unit area of dendrite. For the 29 TH-positive dendrites that received synaptic input from RMTg axon varicosities, the mean gold particle density was 13.2 (± 8.5) and ranged from 1.1 to 41.9. For the 94 TH-positive dendrites that were in the neighboring neuropil but otherwise not contacted by RMTg axons, the mean gold particle density was 15.3 (± 12.0), and the minimum and maximum density range was 1.1–61.3 particles per unit area, respectively. Hence, the relative TH content did not differ significantly (t-test; P = 0.38) between TH-labeled dendrites that did or did not receive synaptic input from the RMTg.
DISCUSSION
Recent light microscopic studies have provided evidence suggestive of an indirect connection between the LHb and the midbrain DA system that involves a relay in a newly defined region of the brainstem tegmentum called the RMTg or tVTA (Jhou et al., 2009a,b; Kaufling et al., 2009, 2010). The present study provides the first ultrastructural data supporting the two key synaptic connections involved in this pathway, i.e., synapses from the LHb onto GABA neurons in the RMTg and synaptic connections from cells in the RMTg onto DA neurons in the VTA. Although it remains to be demonstrated specifically that the RMTg neurons receiving LHb synapses project in turn to the VTA, the striking density of these two pathways make this a likely outcome. Given the known phenotypes of the LHb and RMTg cells (Kaufling et al., 2009; Brinschwitz et al., 2010), this probable disynaptic pathway is a likely anatomical substrate for the nearly uniform inhibition of DA cells that is evoked by LHb stimulation (Christoph et al., 1986; Ji and Shepard, 2007) in the context of negative reinforcement (Matsumoto and Hikosaka, 2007).
Methodological considerations
The limitations of the methods utilized for this study are those that typically attend the application of tract-tracing and immunocytochemical techniques and have been discussed at length in our prior publications (Omelchenko and Sesack, 2005, 2007, 2009; Sesack et al., 2006). Specifically, the inability of tracers to label the entirety of a pathway, combined with the limited penetration of antibodies in tissue prepared for electron microscopy, increases the likelihood of false-negative outcomes. Nevertheless, all efforts were made to minimize false negatives by examining the regions of heaviest anterograde transport and by limiting the ultrastructural analysis to the surface of the tissue with optimal immunoreagent penetration. Consequently, the semiquantitative data provided here do allow some estimation of the relative extent of the demonstrated synaptic connections. These issues are further discussed below.
LHb projection to the RMTg
The relatively greater density of the LHb projection to the RMTg versus the more anterior VTA is evident from both light (Herkenham and Nauta, 1979; Jhou et al., 2009b; Kaufling et al., 2009; Omelchenko et al., 2009; Brinschwitz et al., 2010) and electron microscopy (present study). Despite this difference in density, the morphological features of LHb axons and synapses in the RMTg are identical to those described for the LHb projection to the VTA, including the frequent presence of dense-cored vesicles and modest postsynaptic densities that are atypical of symmetric and asymmetric categories (Omelchenko et al., 2009). Despite the infrequent presence of strongly thickened postsynaptic densities, pathways descending from the LHb are likely to be glutamatergic based on evidence that most LHb neurons contain the vesicular glutamate transporter 2 (VGlut2) or other markers of glutamate phenotype (Kalén et al., 1985; Behzadi et al., 1990; Fremeau et al., 2001; Kiss et al., 2002; Herzog et al., 2004; Geisler et al., 2007; Aizawa et al., 2008; Brinschwitz et al., 2010) and that VGlut2 is expressed in LHb axons within the adjacent VTA (Omelchenko et al., 2009; Brinschwitz et al., 2010). Moreover, the LHb contains only a small population of GABA neurons (Wang et al., 2006; Aizawa et al., 2008; Brinschwitz et al., 2010), which does not appear to contribute substantially to brainstem projections, based on the nearly complete absence of immunoreactivity for GABA in LHb axons within the VTA, as assessed by confocal (Brinschwitz et al., 2010) or electron microscopy (Omelchenko et al., 2009). Hence, it is most likely that the LHb exerts a purely excitatory glutamatergic influence on RMTg neurons, although this supposition requires explicit testing in electrophysiological studies.
It is tempting to speculate on the possible significance of the relatively weak postsynaptic densities exhibited by most LHb axons in the VTA and RMTg. Current understanding indicates that the size of the postsynaptic density reflects synaptic activity and in turn, contributes to synaptic strength (Okabe, 2007; Sheng and Hoogenraad, 2007). Hence, it is possible that the LHb synapses are relatively weak in electrophysiological terms, perhaps reflecting a history of limited usage in the naïve laboratory rat. This suggestion is consistent with the observations of Matsuda and Fujimura (1992) in the VTA that the excitatory postsynaptic potentials evoked by LHb stimulation were insufficient to bring neurons to threshold for generating action potentials. It would be interesting in future studies to examine whether LHb synapses become more pronounced in animals trained to perform behavioral tasks that are designed to recruit this pathway. Alternatively, one or more of the receptors, channels, scaffolding proteins, or signaling molecules that are typically associated with this electron-dense material (Yamauchi, 2002; Okabe, 2007; Sheng and Hoogenraad, 2007) may be different enough to change the structural appearance of LHb synapses. Heterogeneity of postsynaptic density protein composition has been reported previously for glutamate synapses (Cheng et al., 2006; Sheng and Hoogenraad, 2007).
As expected, given the dominant GABA phenotype of RMTg neurons (Perrotti et al., 2005; Olson and Nestler, 2007; Jhou et al., 2009b; Kaufling et al., 2009), the majority of LHb axons synapsing in this region contacted dendrites immunoreactive for GABA. Although the authors of a previous study claimed to have identified this synaptic connection, no quantitative or micrographic evidence for the input was provided in that paper (Brinschwitz et al., 2010). The fact that other targets of LHb axons were nonimmunoreactive for GABA suggests the possibility of synaptic input to a different cell type in this region. There are sparse, TH-labeled, presumed DA neurons in the RMTg (Kaufling et al., 2009); whether these receive synaptic input from the LHb requires further testing. Other neuronal phenotypes, however, have not yet been identified. This suggests an alternative interpretation consistent with the known limitations of electron microscopy, i.e., the possibility that false-negative outcomes lead to an underestimation of LHb synapses onto GABA neurons in the RMTg. Indeed, many of the GABA-labeled dendrites postsynaptic to LHb axons displayed relatively light immunoreactivity, consistent with the lower GABA levels reported in projection as opposed to local circuit interneurons (Van Bockstaele and Pickel, 1995; Van Bockstaele et al., 1996; Delle Donne et al., 1997; Steffensen et al., 1998).
Moreover, the fact that the postsynaptic GABA-labeled dendrites had a larger mean size compared with the unlabeled targets of LHb synapses suggests that GABA labeling in the distal dendrites of RMTg neurons may have fallen below detection limits. Hence, additional research using some means of amplifying the GABA signal in distal dendrites is required in order to determine the exact extent of LHb input to RMTg GABA cells.
RMTg projection to the VTA
The present study constitutes the first electron microscopic examination of RMTg neurons and their axonal projections to a principal target. The comparatively small size and limited degree of myelination indicates that the pathway from the RMTg to the VTA is likely to be relatively slowly conducting. This is especially the case compared with projections of the LHb, which exhibit more frequent myelination. The nearly exclusive formation of symmetric synapses, absence of discernible dense-cored vesicles, and demonstration of postembedding GABA immunoreactivity in all RMTg axons detected suggest that the projection from the RMTg to the VTA represents a relatively pure GABA pathway, consistent with light microscopic analyses to date (Jhou et al., 2009a,b; Kaufling et al., 2009, 2010). Future investigations will determine whether the ultrastructural features observed here agree with the electrophysiological characteristics of RMTg axons and their synaptically evoked responses. Further study is also needed to compare the anatomical characteristics of this dominant midbrain projection with other efferent pathways originating from the RMTg (Jhou et al., 2009b).
As predicted by the density of the RMTg projection to the midbrain and its remarkable overlap with the distribution of DA neurons (Ferreira et al., 2008; Jhou et al., 2009a; Kaufling et al., 2010), the considerable majority of RMTg axons in the VTA were observed to synapse onto the dendrites of DA cells. Our preliminary assessment of the SNc reveals that RMTg axons in this region also synapse mainly onto DA neurons (unpublished observations). These findings further suggest that the RMTg projection to the VTA synapses only infrequently onto non-DA cell populations. This supposition will be tested in future studies by examining whether GABA or glutamate is present in the dendrites postsynaptic to RMTg axons. DA cells constitute approximately 45–65% of the VTA population, depending on the subdivision (Swanson, 1982; McCormack et al., 2006; Nair-Roberts et al., 2008). Hence, the fact that RMTg synapses contacted TH-labeled dendrites 83% of the time suggests that there is selective targeting of DA neurons by this pathway. This finding is in marked distinction to all other afferents we have investigated to date, which either synapse somewhat equally onto DA and GABA cells (Carr and Sesack, 2000; Balcita-Pedicino and Sesack, 2007; Omelchenko and Sesack, 2007; Omelchenko et al., 2009) or synapse onto DA neurons in proportion to their representation in the VTA (Omelchenko and Sesack, 2005, 2006, 2010).
Our ultrastructural observations appear to support the light microscopic impression of many close contacts between RMTg axons and VTA DA neurons (Jhou et al., 2009a), including those that project to the nucleus accumbens (Kaufling et al., 2010). The images provided in those studies, however, suggest that the close contacts are onto DA cell soma and proximal dendrites, outcomes that we never observed by electron microscopy despite a concerted effort to examine these compartments. Still, the larger size of the immunoreactive dendrites receiving RMTg synapses versus those in the immediately adjacent neuropil do suggest that the RMTg input is relatively directed toward proximal as opposed to distal sites in the dendritic tree of VTA DA neurons.
Probable disynaptic pathway linking the LHb and VTA
As we did not perform retrograde tract-tracing from the VTA, it remains to be determined whether the RMTg neurons receiving LHb synaptic input are among those that project to the VTA. This aspect of a probable disynaptic connection will be investigated in future studies. Nevertheless, the RMTg is the most pronounced terminal field of the LHb (Herkenham and Nauta, 1979; Jhou et al., 2009b), and the ascending projection to the SNc/VTA complex is the single largest output of the RMTg (Ferreira et al., 2008; Jhou et al., 2009a,b; Kaufling et al., 2010). Hence, it is likely that a disynaptic pathway connecting the LHb to the VTA via the RMTg will ultimately be verified in future studies.
This circuit would explain the robust inhibition of most DA neurons evoked by electrical stimulation of the LHb or fasciculus retroflexus (Christoph et al., 1986; Ji and Shepard, 2007; Matsumoto and Hikosaka, 2007). Preliminary electrophysiological evidence in support of this pathway comes from recordings in awake monkeys (Hong and Hikosaka, 2009) in which electrical stimulation of the LHb produced a short-latency excitation of neurons in a region presumed to be the primate equivalent of the RMTg. Stimulation of this area subsequently induced short-latency inhibition in the majority of DA cells examined in the SNc (Hong and Hikosaka, 2009). Supportive data also come from electrophysiological recordings of the RMTg in behaving rats (Jhou et al., 2009a). A disynaptic projection system would also agree with neurochemical studies indicating that lesions of the LHb induce increased levels of DA in forebrain regions, suggesting removal of a tonic, pervasive inhibitory influence (Lisoprawski et al., 1980; Nishikawa et al., 1986; Lecourtier and Kelly, 2007; Lecourtier et al., 2008).
Alternative circuits linking the LHb and VTA DA neurons are also possible (Lecourtier and Kelly, 2007; Haber and Knutson, 2010), with the short latency of DA cell inhibition (Ji and Shepard, 2007) suggesting that only disynaptic connections be considered. We and others have previously demonstrated a projection from the LHb to VTA GABA neurons (Omelchenko et al., 2009; Brinschwitz et al., 2010). We have also established that the local collaterals of these GABA cells synapse onto neighboring DA neurons (Omelchenko and Sesack, 2009). Hence, both GABA cells within and caudal to the VTA may mediate the LHb-induced inhibition of DA neurons. However, it is worth noting that Matsuda and Fujimura (1992) found few short-latency inhibitory postsynaptic potentials in either DA or non-DA cells in an in vitro slice preparation that preserved the connection from the LHb to the VTA via the fasiculus retroflexus. This finding suggests that the dominant inhibitory effect of the LHb on DA neurons is not well preserved within the VTA itself. Other GABA neurons may yet be discovered that participate in the inhibitory actions of the LHb. For now, however, the RMTg remains the most viable intermediate structure for the conversion of LHb activation to inhibition of DA cells in situations of negative reinforcement (Ullsperger and von Cramon, 2003; Matsumoto and Hikosaka, 2007).
Functional implications
Given the large numbers of cells that project from the LHb to the RMTg (Jhou et al., 2009b; Kaufling et al., 2009), and from the RMTg to the VTA (Colussi-Mas et al., 2007; Geisler et al., 2008; Jhou et al., 2009a,b; Kaufling et al., 2010), a disynaptic circuit involving these regions is likely to contribute to many of the general functions reported for the LHb: avoidance learning, feeding and maternal behavior, stress, and sleep regulation (Sutherland, 1982; Klemm, 2004; Geisler and Trimble, 2008). Descending projections of the LHb to other brainstem structures probably also contribute to the same functions. Moreover, like the VTA, these probably involve both direct projections from the LHb and an indirect relay through the RMTg, which shares many of the same targets as the LHb (Jhou et al., 2009b).
The projections of the RMTg to the VTA may also provide DA neurons with ascending signals that are not initiated by LHb activation. In particular, the RMTg receives afferents from more caudal portions of the brainstem including the nucleus of the solitary tract, parabrachial nucleus, and medullary and pontine reticular formation. The RMTg appears to have a strong role in promoting passive avoidance behaviors in response to aversive and fear-provoking stimuli (Jhou et al., 2009a), making it likely that the RMTg conveys information regarding the aversive qualities of stimuli to DA neurons (Ungless et al., 2004; Jhou and Gallagher, 2007; Brischoux et al., 2009). The ensuing inhibition of DA cells may be instrumental in promoting avoidance learning.
The present findings reveal anatomical substrates for regulation of DA cell activity by the LHb and the RMTg and have important implications for mental health conditions. The identification of the RMTg as a key region of the brainstem tegmentum is too recent for this area to have been examined in pre- or postmortem studies of human brain or to have been linked with a particular mental or neurological disorder. Nevertheless, the consistent demonstration of c-fos activation by psychostimulants in this region (Scammell et al., 2000; Perrotti et al., 2005; Colussi-Mas et al., 2007; Geisler et al., 2008; Kaufling et al., 2010) suggests a potential role in substance abuse. Although speculative, it is possible that the RMTg has the potential to promote avoidance of addictive substances by inhibiting DA cell activity in response to the aversive properties of these drugs (Jhou et al., 2009a).
Malfunctions of the LHb have been suggested to contribute to the pathophysiology of both schizophrenia and depression (Shepard et al., 2006; Lecourtier and Kelly, 2007). Although early structural imaging studies reported calcification in the habenula of schizophrenic patients (Sandyk, 1992), more recent postmortem analyses have not found evidence for gross structural abnormalities in the habenular complex in this disorder (Ranft et al., 2010). Nevertheless, functional imaging experiments indicate an impairment of habenula recruitment during learning involving negative feedback in schizophrenic patients (Shepard et al., 2006). Such tasks also increase blood flow to the midbrain in healthy but not schizophrenic patients (Shepard et al., 2006). The latter observation is difficult to reconcile with the known inhibition of DA cells by LHb activation (Ji and Shepard, 2007). However, the resolution of these functional imaging studies may well be insufficient to differentiate signals from the closely adjacent RMTg and VTA.
Extensive evidence is now accumulating that the normal functions of the LHb are disrupted in major depressive disorder. There are structural abnormalities reported in this region (Ranft et al., 2010) in addition to altered blood flow in depressed patients that correlates with symptom severity (Morris et al., 1999). Moreover, several different animal models of depression highlight a substantial contribution by the LHb (Caldecott-Hazard et al., 1988; Shumake and Gonzalez-Lima, 2003), and lesions of the LHb prevent or improve the symptoms of depression in these models (Amat et al., 2001; Yang et al., 2008). Whether these effects are mediated through midbrain DA neurons and their powerful regulation by the LHb and RMTg is not yet known but merits further investigation. Certainly interest in the potential contribution of the DA system to the pathophysiology and treatment of major depressive disorder has re-emerged in the clinical literature (Kapur and Mann, 1992; Dunlop and Nemeroff, 2007; Montgomery, 2008; IsHak et al., 2009). In any case, the fact that the LHb is a target for deep brain stimulation treatment of major depression (Sartorius et al., 2010) indicates a clear need to understand more precisely the connectivity of this region and its ability to regulate the activity of RMTg GABA cells, midbrain DA neurons, and other direct and indirect downstream targets.
Acknowledgments
Grant sponsor: U.S. Public Health Service; Grant number: MH067937.
LITERATURE CITED
- Aizawa H, Isomura Y, Kobayashi M, Harukuni R, Tanaka S, Fukai T, Okamoto H. Heterogeneity of the lateral habenular neurons revealed by gene expression. Soc Neurosci Abstr. 2008:490.492. [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–330. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The rostromedial mesopontine tegmentum as a relay between the lateral habenula and dopamine neurons in the ventral tegmental area: ultrastructural evidence in the rat. Soc Neurosci Abstr. 2009:815.817. [Google Scholar]
- Bayer VE, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in the rat ventral tegmental area: relationship between immunolabeling density and neuronal associations. J Neurosci. 1990;10:2996–3013. doi: 10.1523/JNEUROSCI.10-09-02996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- Behzadi G, Kalen P, Parvopassu F, Wiklund L. Afferents to the median raphe nucleus of the rat: retrograde cholera toxin and wheat germ conjugated horseradish peroxidase tracing, and selective D-[3H]aspartate labelling of possible excitatory amino acid inputs. Neuroscience. 1990;37:77–100. doi: 10.1016/0306-4522(90)90194-9. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol. 2006;499:603–612. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- Brazhnik E, Shah F, Tepper JM. GABAergic afferents activate both GABAA and GABAB receptors in mouse sub-stantia nigra dopaminergic neurons in vivo. J Neurosci. 2008;28:10386–10398. doi: 10.1523/JNEUROSCI.2387-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinschwitz K, Lommel R, Penkalla A, Goertzen A, Geisler S, Veh RW. Glutamatergic fibers from the lateral habenula do not terminate on dopaminergic neurons in the ventral tegmental area. Soc Neurosci Abstr. 2005;31:891.823. [Google Scholar]
- Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–476. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J Neurosci. 1988;8:1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Geisler S, Zimmer L, Zahm DS, Berod A. Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur J Neurosci. 2007;26:1011–1025. doi: 10.1111/j.1460-9568.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Donne KT, Sesack SR, Pickel VM. Ultrastructural immunocytochemical localization of the dopamine D2 receptor within GABAergic neurons of the rat striatum. Brain Res. 1997;746:239–255. doi: 10.1016/s0006-8993(96)01226-7. [DOI] [PubMed] [Google Scholar]
- Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JE, Overton PG, Redgrave P. How visual stimuli activate dopaminergic neurons at short latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Ferreira JG, Del-Fava F, Hasue RH, Shammah-Lagnado SJ. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Geisler S, Trimble M. The lateral habenula: no longer neglected. CNS Spectr. 2008;13:484–489. doi: 10.1017/s1092852900016710. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat—anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33:2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Tung CS, Svensson TH. The excitatory amino acid antagonist kynurenate induces pacemaker-like firing of dopamine neurons in rat ventral tegmental area in vivo. Acta Physiol Scand. 1988;134:567–568. doi: 10.1111/j.1748-1716.1998.tb08535.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Negative reward signals from lateral habenula to dopamine neurons are mediated by a group of medial mesopontine neurons. Soc Neurosci Abstr. 2009:482.486. [Google Scholar]
- Horvitz JC, Stewart T, Jacobs BL. Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Res. 1997;759:251–258. doi: 10.1016/s0006-8993(97)00265-5. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- IsHak WW, Davis M, Jeffrey J, Balayan K, Pechnick RN, Bagot K, Rapaport MH. The role of dopaminergic agents in improving quality of life in major depressive disorder. Curr Psychiatry Rep. 2009;11:503–508. doi: 10.1007/s11920-009-0076-z. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Gallagher M. Paramedian raphe neurons that project to midbrain dopamine neurons are activated by aversive stimuli. Soc Neurosci Abstr. 2007;33:425.425. [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DG, Harris RJ. An analysis of contemporary morphological concepts of synaptic remodelling in the CNS: perforated synapses revisited. Rev Neurosci. 1995;6:177–219. doi: 10.1515/revneuro.1995.6.3.177. [DOI] [PubMed] [Google Scholar]
- Kalén P, Karlson M, Wiklund L. Possible excitatory amino acid afferents to nucleus raphe dorsalis of the rat investigated with retrograde wheat germ agglutinin and D-[3H]aspartate tracing. Brain Res. 1985;360:285–297. doi: 10.1016/0006-8993(85)91244-2. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol Psychiat. 1992;32:1–17. doi: 10.1016/0006-3223(92)90137-o. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier M-J, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Gamma-aminobutyric acid cells with cocaine-induced DeltaFosB in the ventral tegmental area innervate mesolimbic neurons. Biol Psychiat. 2010;67:88–92. doi: 10.1016/j.biopsych.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Kim U. Topographic commissural and descending projections of the habenula in the rat. J Comp Neurol. 2009;513:173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csaki A, Bokor H, Kocsis K, Kocsis B. Possible glutamatergic/aspartatergic projections to the supramammillary nucleus and their origins in the rat studied by selective [(3)H]D-aspartate labelling and immunocytochemistry. Neuroscience. 2002;111:671–691. doi: 10.1016/s0306-4522(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–RA273. [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Defrancesco A, Moghaddam B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur J Neurosci. 2008;27:1755–1762. doi: 10.1111/j.1460-9568.2008.06130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Ebert U, Loscher W. Amygdala-kindling induces a lasting reduction of GABA-immunoreactive neurons in a discrete area of the ipsilateral piriform cortex. Synapse. 1998;29:299–309. doi: 10.1002/(SICI)1098-2396(199808)29:4<299::AID-SYN2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ligorio MA, Akmentin W, Gallery F, Cabot JB. Ultra-structural localization of the binding fragment of tetanus toxin in putative gamma-aminobutyric acidergic terminals in the intermediolateral cell column: a potential basis for sympathetic dysfunction in generalized tetanus. J Comp Neurol. 2000;419:471–484. doi: 10.1002/(sici)1096-9861(20000417)419:4<471::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Björklund A, Divac I. Organization of catecholamine neurons projecting to the frontal cortex in the rat. Brain Res. 1978;142:1–24. doi: 10.1016/0006-8993(78)90173-7. [DOI] [PubMed] [Google Scholar]
- Lisoprawski A, Herve D, Blanc G, Glowinski J, Tassin JP. Selective activation of the mesocortico-frontal dopaminergic neurons induced by lesion of the habenula in the rat. Brain Res. 1980;183:229–234. doi: 10.1016/0006-8993(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Fujimura K. Action of habenular efferents on ventral tegmental area neurons studied in vitro. Brain Res Bull. 1992;28:743–749. doi: 10.1016/0361-9230(92)90254-u. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Negative motivational control of saccadic eye movement by the lateral habenula. Prog Brain Res. 2008;171:399–402. doi: 10.1016/S0079-6123(08)00658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Langston JW, Di Monte DA. Decreased susceptibility to oxidative stress underlies the resistance of specific dopaminergic cell populations to paraquat-induced degeneration. Neuroscience. 2006;141:929–937. doi: 10.1016/j.neuroscience.2006.03.069. [DOI] [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA. The under-recognized role of dopamine in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23:63–69. doi: 10.1097/YIC.0b013e3282f2b3cb. [DOI] [PubMed] [Google Scholar]
- Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. NeuroImage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for nature of the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral teg-mental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. J Neurosci Res. 2010;88:981–991. doi: 10.1002/jnr.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Towle A, Joh TH, Chan J. Gamma-aminobutyric acid in the medial rat nucleus accumbens: ultrastructural localization in neurons receiving monosynaptic input from catecholaminergic afferents. J Comp Neurol. 1988;272:1–14. doi: 10.1002/cne.902720102. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Nirenberg MJ, Milner TA. Ultrastructural view of central catecholaminergic transmission: immunocytochemical localization of synthesizing enzymes, transporters and receptors. J Neurocytol. 1996;25:843–856. doi: 10.1007/BF02284846. [DOI] [PubMed] [Google Scholar]
- Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein HG. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med. 2010;40:557–567. doi: 10.1017/S0033291709990821. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Sandyk R. Pineal and habenula calcification in schizophrenia. Int J Neurosci. 1992;67:19–30. doi: 10.3109/00207459208994773. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiat. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Dual ultrastructural localization of enkephalin and tyrosine hydroxylase immunoreactivity in the rat ventral tegmental area: multiple substrates for opiate-dopamine interactions. J Neurosci. 1992;12:1335–1350. doi: 10.1523/JNEUROSCI.12-04-01335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Guido MA, Levey AI. Dopamine axon varicosities in the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Miner LAH, Omelchenko N. Pre-embedding immunoelectron microscopy: applications for studies of the nervous system. In: Zaborszky L, Wouterlood FG, Lanciego JL, editors. Neuroanatomical tract-tracing 3: molecules, neurons, systems. New York: Springer; 2006. pp. 6–71. [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schiz Bull. 2006;32:417–421. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Smith Y. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J Comp Neurol. 1995;358:119–141. doi: 10.1002/cne.903580108. [DOI] [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Lee CR. GABAergic control of substantianigra dopaminergic neurons. Prog Brain Res. 2007;160:189–208. doi: 10.1016/S0079-6123(06)60011-3. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Chan J, Pickel VM. Pre- and postsynaptic sites for serotonin modulation of GABA-containing neurons in the shell region of the rat nucleus accumbens. J Comp Neurol. 1996;371:116–128. doi: 10.1002/(SICI)1096-9861(19960715)371:1<116::AID-CNE7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Veznedaroglu E, Milner TE. Elimination of artifactual labeling of hippocampal mossy fibers seen following pre-embedding immunogold-silver technique by pretreatment with zinc chelator. Microscopy Res Tech. 1992;23:100–101. doi: 10.1002/jemt.1070230110. [DOI] [PubMed] [Google Scholar]
- Wang DG, Gong N, Luo B, Xu TL. Absence of GABA type A signaling in adult medial habenular neurons. Neuroscience. 2006;141:133–141. doi: 10.1016/j.neuroscience.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Kapatos G. Flow cytometric analysis of rat striatal nerve terminals. J Neurosci. 1989;9:94–105. doi: 10.1523/JNEUROSCI.09-01-00094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T. Molecular constituents and phosphorylation-dependent regulation of the post-synaptic density. Mass Spectrom Rev. 2002;21:266–286. doi: 10.1002/mas.10033. [DOI] [PubMed] [Google Scholar]
- Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]