Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity (original) (raw)
. Author manuscript; available in PMC: 2015 Dec 1.
Published in final edited form as: Semin Immunol. 2014 Oct 26;26(6):454–470. doi: 10.1016/j.smim.2014.09.008
Abstract
Mendelian susceptibility to mycobacterial disease (MSMD) is a rare condition characterized by predisposition to clinical disease caused by weakly virulent mycobacteria, such as BCG vaccines and environmental mycobacteria, in otherwise healthy individuals with no overt abnormalities in routine hematological and immunological tests. MSMD designation does not recapitulate all the clinical features, as patients are also prone to salmonellosis, candidiasis and tuberculosis, and more rarely to infections with other intramacrophagic bacteria, fungi, or parasites, and even, perhaps, a few viruses. Since 1996, nine MSMD-causing genes, including seven autosomal (IFNGR1, IFNGR2, STAT1, IL12B, IL12RB1, ISG15, and IRF8) and two X-linked (NEMO, CYBB) genes have been discovered. The high level of allelic heterogeneity has already led to the definition of 18 different disorders. The nine gene products are physiologically related, as all are involved in IFN-γ-dependent immunity. These disorders impair the production of (IL12B, IL12RB1, IRF8, ISG15, NEMO) or the response to (IFNGR1, IFNGR2, STAT1, IRF8, CYBB) IFN-γ. These defects account for only about half the known MSMD cases. Patients with MSMD-causing genetic defects may display other infectious diseases, or even remain asymptomatic. Most of these inborn errors do not show complete clinical penetrance for the case-definition phenotype of MSMD. We review here the genetic, immunological, and clinical features of patients with inborn errors of IFN-γ-dependent immunity.
Keywords: BCG, mycobacteriosis, tuberculosis, IFN-γ, IL-12, ISG15, primary immunodeficiency
Mendelian susceptibility to mycobacterial disease (MSMD) is a rare inherited condition characterized by selective predisposition to clinical disease caused by weakly virulent mycobacteria, such as bacillus Calmette-Guerin (BCG) vaccines and non-tuberculous environmental mycobacteria (EM), in otherwise healthy patients with no overt abnormalities in routine hematological and immunological tests (Online Mendelian Inheritance in Man [OMIM 209950])[1–10]. Mycobacterial disease generally begins in childhood, more rarely during adolescence and adulthood, and has diverse manifestations, ranging from localized to disseminated infections with one or more mycobacterial species that may or may not recur [11–18]. The patients are also vulnerable to the more virulent Mycobacterium tuberculosis [19–28]. About half of them also suffer from clinical disease caused by non-typhoidal or, more rarely, typhoidal Salmonella [28–30]. Mild forms of chronic mucocutaneous candidiasis (CMC) have been described [31–36]. Other severe infections have been reported more rarely, typically in single patients, and include infections caused by various intramacrophagic bacteria (listeriosis, nocardiosis, klebsiellosis) [26, 37–39], fungi (candidiasis, histoplasmosis, paracoccidioidomycosis, coccidioidomycosis) [31–36, 40–43] and parasites (leishmaniasis, toxoplasmosis) [44, 45]. Viral infections have also been reported, including diseases caused by cytomegalovirus (CMV), human herpes virus 8 (HHV8), parainfluenza virus type 3 (PRV-3), respiratory syncitial virus (RSV) and varicella zoster virus (VZV) [46–49]. Six cases of malignancies, namely B-cell lymphoma, esophageal carcinoma, cutaneous squamous cell carcinoma, Kaposi sarcoma, liver cancer and pineal germinoma have also been reported [27, 50–54]. The pathogenesis of viral and tumoral diseases may not necessarily involve the underlying MSMD-causing inborn error, instead potentially involving an immunodeficiency acquired secondary to mycobacterial or other infections [55–61]. MSMD is strictly speaking a misnomer, as the clinical phenotype extends beyond mycobacterial diseases. However, this term remains useful, as mycobacterial diseases are by far the most common infections in these patients. It also serves as a useful reminder that isolated infectious diseases may be genetically driven [1, 12, 15]. Mycobacterial diseases are currently the most thoroughly analyzed human infectious diseases, and the results obtained provide support for a genetic theory of childhood infectious diseases [62–64].
The first genetic etiology of MSMD was discovered in 1996: bi-allelic null mutations of IFNGR1, which encodes the ligand-binding chain of the IFN-γ receptor (IFN-γR1) [65, 66]. MSMD-causing mutations have been identified in seven autosomal genes: IFNGR1 and IFNGR2, which encodes the accessory chain of IFN-γR; STAT1, encoding signal transducer and activator of transcription 1; IL12B, the p40 subunit common to IL-12 and IL-23; IL12RB1, encoding the β1 chain common to the receptors for IL-12 and IL-23; IRF8, a transcription factor inducible by IFN-γ, from the IRF family; and ISG15, an IFN-γ-inducing molecule that acts in synergy with IL-12; and in two X-linked genes: NEMO , encoding the Nuclear factor-kappa B (NF-κB) essential modulator, which mediates signaling in the NF-κB pathway; and CYBB (or gp91_phox_), which encodes the major component of the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (PHOX) complex [1, 12, 22, 28, 29, 67–69] (Table 1). MSMD is therefore allelic with other conditions caused by mutations at the IRF8, CYBB, STAT1, and NEMO loci [67, 70–75] (Figures 1–3) (Table 2). Allelic heterogeneity at these loci results in the definition of up to 18 different genetic etiologies, based on the impact of the mutation (null or hypomorphic), the mode of transmission (dominant or recessive), the expression of the mutant allele (e.g. expressed on the cell surface or not, for receptors), and the function affected (e.g. phosphorylation transcription factors, or both) (Table 1). Other primary immunodeficiencies (PID) underlie mycobacterial diseases, albeit typically in patients with many other infectious and immunological phenotypes [76, 77]. For example, known GATA2 mutations confer a predisposition to disseminated EM disease associated with warts and hematological disorders, including decreases in the numbers of circulating dendritic cells, monocytes, B cells and NK cells, and myelodysplasia or bone marrow hypoplasia, a phenotype referred to as MonoMAC syndrome, which is related to but different from MSMD [14, 78–82]. The products of the nine MSMD-causing genes are all involved in IFN-γ-mediated immunity, controlling the production of (IL12B, IL12RB1, IRF8, ISG15, NEMO) or response to (IFNGR1, IFNGR2, STAT1, IRF8, CYBB) IFN-γ. The human genetic of tuberculosis has been the subject of other review [83]. This review deals with the genetic, immunological, and clinical features of inborn errors of IFN-γ immunity, but not MSMD patients with no known genetic etiology.
Table 1.
Genetic etiologies of MSMD
| Gene | Inheritance | Defect | Protein |
|---|---|---|---|
| IFNGR1 | AR | C | E+ |
| AR | C | E− | |
| AD | P | E+++ | |
| AR | P | E+ | |
| IFNGR2 | AR | C | E+ |
| AR | C | E− | |
| AR | P | E+ | |
| AD | P | E+ | |
| STAT1 | AD | P | E+P− |
| AD | P | E+B− | |
| AD | P | E+P−B− | |
| IL12B | AR | C | E− |
| IL12RB1 | AR | C | E− |
| AR | C | E+ | |
| IRF8 | AD | P | E+ |
| ISG15 | AR | C | E− |
| CYBB | AR | C | E+* |
| NEMO | XR | P | E+ |
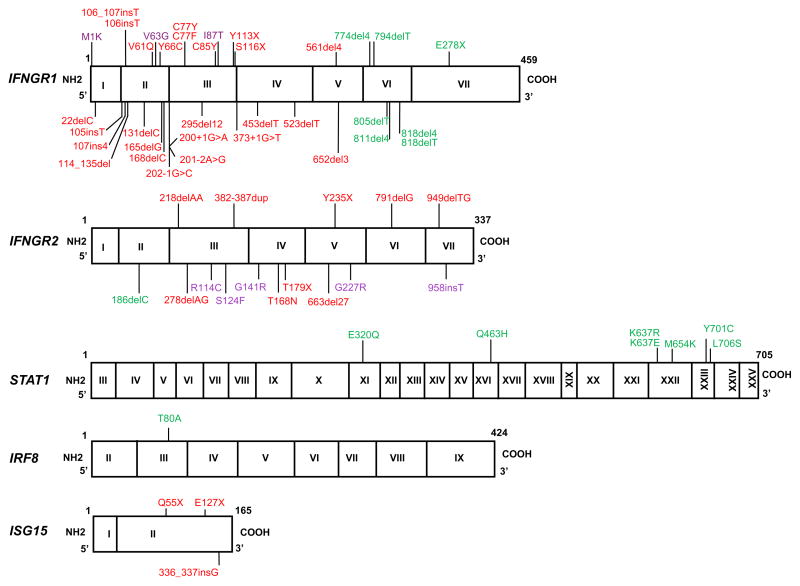
Figure 1. MSMD-causing mutations of IFNGR1, IFNGR2, STAT1, IL12B, IL12RB1, ISG15, IRF8, NEMO and CYBB.
The exons of each gene are shown, designated by roman numerals. Red: recessive loss-of-function mutations associated with complete defects. Purple: recessive mutations associated with partial deficiency. Green: dominant mutations causing partial deficiency.
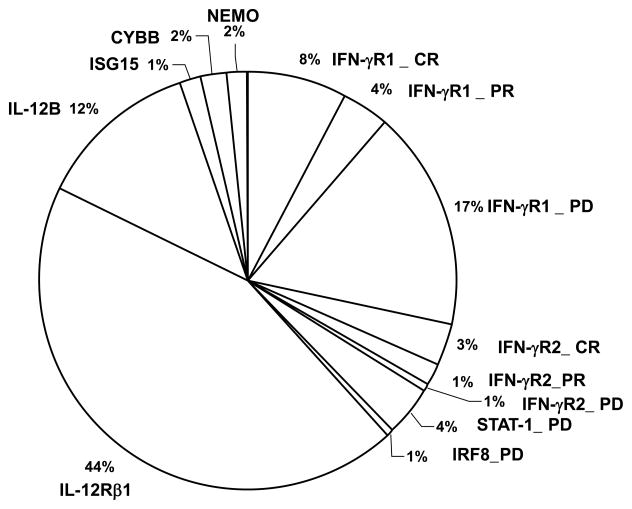
Figure 3. Distribution of genetic disorders in MSMD patients with known etiologies.
The genetic defects observed in 406 MSMD patients with known mutations are shown. The proportions of complete recessive (CR), partial recessive (PR), and partial dominant (PD) defects of autosomal genes (IFNGR1, IFNGR2, STAT1, IRF8, IL12B, IL12RB1 and ISG15), and X-linked recessive (XR) genes (NEMO and CYBB) are indicated.
Table 2.
Clinical diseases allelic with MSMD at the NEMO, CYBB, STAT1 and IRF8 loci
| Gene | Mutation | Inheritance | Infections | Disease | ||
|---|---|---|---|---|---|---|
| Mycobacteria | Pyogenic bacteria | Viruses | Fungi | |||
| STAT1 | Amorphic | AR | ++ | ++ | STAT1−/− (C) | |
| Hypomorphic | AR | ++ | +/− | + | STAT1−/− (P) | |
| Hypomorphic | AD | +++ | MSMD | |||
| Hypermorphic | AD | +/− | +++ | CMC | ||
| IRF8 | Amorphic | AR | ++ | ++ | IRF8−/− | |
| Hypomorphic | AD | ++ | MSMD | |||
| NEMO | Amorphic | XD | IP | |||
| Hypomorphic | XR | + | ++ | + | EDA-(OL)-ID | |
| Hypomorphic | XR | + | ++ | EDA-ID | ||
| Hypomorphic | XR | + | ++ | EDA like -ID | ||
| Hypomorphic | XR | +++ | MSMD | |||
| CYBB | Amorphic | XR | +/− | +++ | +++ | CGD |
| Hypomorphic | XR | +/− | +++ | +++ | Variant CGD | |
| Cell type specific | XR | +++ | MSMD | |||
IFN-γR1 deficiency
The first genetic etiology of MSMD was identified in 1996, with bi-allelic null mutations in the IFNGR1 gene, underlying autosomal recessive (AR) complete IFN-γR1 deficiency (Figure 1; table 1) [65, 66]. Thirty-one patients from 26 families and 25 different mutations (deletions _n_=10, insertions _n_=4, nonsense _n_=2, missense _n_=5 and splice site _n_=4) have been described to date (Figure 1). Two genetic forms of AR complete IFN-γR1 deficiency have been described, with [46, 53, 84, 85] or without surface expression of the receptor [37, 46, 48, 50, 65, 66, 86–101] (Table 1). A case of paternal uniparental disomy of chromosome 6, including IFNGR1, has been described in a patient with mycobacterial infectious disease and a complex phenotype including neonatal hyperglycemia, neuromuscular disease, and dysmorphic features [88]. The cellular phenotype of AR complete IFN-γR1 deficiency is characterized by a lack of response to IFN-γ in vitro, in terms of IL-12p70 production by leukocytes, gamma-activating factor (GAF: STAT1 homodimers) DNA-binding activity in Epstein-Barr virus-transformed lymphoblastic (EBV-B) cell lines, or HLA-II induction in fibroblasts [14, 46, 65, 84, 102, 103]. Plasma from patients contains high levels of IFN-γ [46, 104]. The clinical phenotype of the patients is characterized by early-onset, disseminated, life-threatening infections with BCG and/or EM (including species such as M. chelonae, M. fortuitum, M. mageritense, M. peregrinum, M. smegmatis, M. scrofulaceum) (Figure 4) [46, 90, 95, 96]. M. tuberculosis was identified in two patients, including one who died from disseminated disease despite antibiotic treatment [46, 87]. Infections typically begin in early childhood, before three years of age [46]. The clinical penetrance for MSMD complete in childhood. Granuloma lesions are poorly delineated and lepromatous-like; they contain multiple acid-fast bacilli and few, if any giant cells [105]. Other infections, caused by viruses (CMV, HHV8, RSV, PRV-3, VZV) [37, 46, 48, 53, 87, 93] and bacteria (Listeria monocytogenes) [37] have also been described. Salmonellosis has rarely been documented in these patients (_n_=3) [46, 65, 66]. One patient had a B-cell lymphoma and a second had a pineal germinoma [50, 54]. Treatment with IFN-γ is not indicated, owing to the lack of specific receptors. Treatment with IFN-α has been reported, but with variable clinical responses [106, 107], and recent evidence suggests that exogenous IFN-α treatment may aggravate mycobacterial disease [108–110]. Antibiotic treatment should not be stopped. Hematopoietic stem cell transplantation (HSCT) is the only known curative treatment [85, 111–113]. However, a high rate of graft rejection, even for transplants from an HLA-identical relative, has been observed [111], probably due to the high concentrations of IFN-γ in the plasma of the patients [46, 104, 114]. The overall prognosis is poor, with 17 deaths reported for the 31 known patients (58%) patients, including four deaths after HSCT. HSCT was considered successful for five patients at the time at which their cases were reported [85, 111–113]. The oldest surviving patient was 19 years old in 2007 and had suffered six episodes of mycobacterial infection, each treated with antibiotics for six to nine months [97].
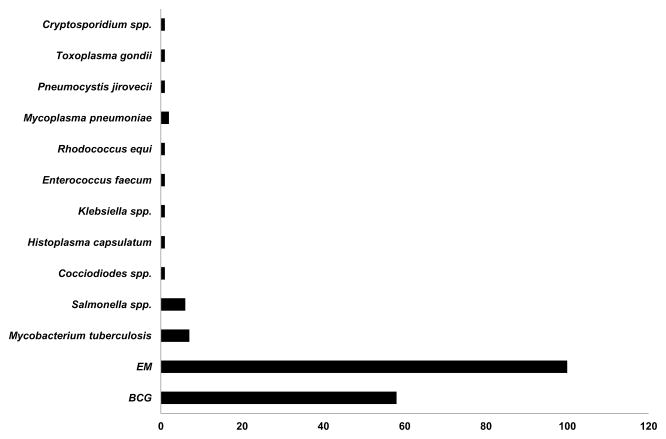
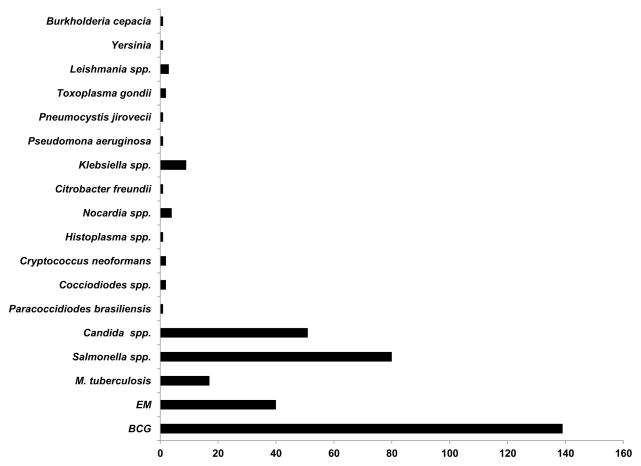
Figure 4. Infections in patients with deficiencies of IFN-γR and STAT1.
Infections in 115 patients with IFN-γR1 deficiencies (complete and partial), 21 patients with IFN-γR2 deficiencies (complete and partial) and 17 reported patients with partial dominant STAT1 deficiency. Some patients had multiple episodes of infectious diseases. BCG: bacillus Calmette-Guerin, EM: environmental mycobacteria.
Autosomal recessive partial (PR) IFN-γR1 deficiency results from any of three homozygous mutations: I87T, V63G, and M1K (Figure 1). The V63G mutation was found in five patients from four families from the Canary Islands and the I87T mutation was found in 13 patients from seven families from Portugal, Poland, Chile, and Colombia [23, 45, 115, 116]. The cells of these patients express the receptor on their surface, but display an impaired response to high concentrations of IFN-γ [45]. IFN-γ was detectable in plasma from these patients. A founder effect was documented for both the I87T and V63G mutations, probably dating back 1,600 (875–2,950) and 500 (200–1,275) years, respectively. The patients’ clinical phenotype is less severe than that of patients with AR complete IFN-γR1 deficiency. Patients suffer from mycobacterial infections caused by BCG and/or EM (M. avium, M. avium complex, M. abcessus, M. szulgai). Ten patients developed osteomyelitis [45, 116]. Infection with M. tuberculosis has been reported in a child who had not been vaccinated with BCG [23]. Other infectious agents have been described and include bacteria (_Haemophylus influenzae n_=1, _Klebsiella pneumoniae n_=1, Legionella spp. _n_=1, _Shigella sonnei n_=1, Salmonella spp. _n_=3, _Mycoplasma pneumoniae n_=2), viruses (VZV _n_=2, RSV _n_=1, _Molluscum contagiosum n_=1), and parasites (Cryptosporidium spp. _n_=1, _Toxoplasma gondii n_=1) (Figure 4). Treatment with antibiotics and IFN-γ for several years is necessary to contain and eventually control the infection [45]. HSCT is not indicated, given the relatively mild infectious phenotype. Only one of the 15 patients reported to date died (6.6%) and the oldest surviving patient was 31 years old in 2011 [45]. Prophylactic antibiotics are not required [14, 117]. A particular case of autosomal PR IFN-γR1 deficiency has been reported, caused by a germline mutation affecting the initiation codon, M1K [118]. The impact of the mutation depends on the cell type and tissue. IFN-γR1 expression is severely impaired in EBV-B cells, and abolished in fibroblasts [118]. The cellular phenotype is characterized by a severe impairment of STAT1 phosphorylation, very low levels of detectable interferon-Gamma Activated Sequence (GAS)-binding proteins in EBV-B cells, and a complete lack of detectable GAS-binding proteins in fibroblasts. The clinical phenotype of the patient is more severe than that of the previous patients described with PR IFN-γR1 deficiency, with severe mycobacterial infections caused by BCG and M. avium [118]. High levels of IFN-γ were detected in the plasma. The severe immunological and clinical status of this patient led to treatment by HSCT together with antibiotics [119].
An autosomal dominant (AD) form of partial IFN-γR1 deficiency was first identified in 1999 [120]. Mono-allelic mutations affect exon 6 and include a small deletion at a single mutation site, considered to be the first human small deletion hotspot [120]. Indistinguishable mutations, collectively described as “818del4”, account for 81% of the kindreds and 87% of the patients with AD IFN-γR1 deficiency [46, 120–124]. Other mutations in the immediate vicinity of 818del4 may also underlie AD IFN-γR1 deficiency (818delT, 794delT, E278X, 811del4, 774del4 and 805delT) [46, 120, 121, 125–130] (Figure 1). In total, 43 families containing 68 patients have been described, including four asymptomatic patients for the case-definition MSMD phenotype [41, 42, 46, 49, 86, 99, 120–123, 125–137]. Large amounts of IFN-γR1 protein are detected on the cell surface, due to the accumulation of truncated IFN-γR1 receptors lacking the recycling domain [120]. The accumulation of non-functional IFN-γR1 proteins lacking STAT1 and JAK1 docking sites impedes the normal function of IFN-γR1 dimers by negative dominance, despite the presence of receptors encoded by the wild-type IFNGR1 allele. All mutations confer a similar cellular phenotype, characterized by an impairment of the response in vitro to IFN-γ [46, 120]. The clinical features of the patients are less severe than those of patients with AR complete IFN-γR1 deficiency. Indeed, only one death has been reported among the 68 patients (1.5%). The oldest patient reported was 62 years old in 2004 [46]. Generally, patients are susceptible to BCG or EM (M. abcessus, M. avium complex, M. asiaticum, M. bohemicum, M. chelonei, M. gordonae, M. kansasii, M. scrofulaceum) (Figure 4). In 72% of patients, the infection affects the bone and some patients even develop osteomyelitis with no other organ involvement [41, 42, 46, 49, 86, 99, 120–123, 125–137]. Two patients with mycobacterial osteomyelitis were initially incorrectly diagnosed as having Langerhans cell histiocytosis and received chemotherapy [138]. Salmonella infection was reported in only 5% of cases [46]. The other associated pathogens detected are Cocciodiodes spp. [42], Histoplasma capsulatum [41] and VZV [49]. Two patients suffered from tuberculosis, one due to M. tuberculosis [126, 127] the other to M. bovis, corresponding to the only infection of this second patient [46] (Figure 4). In most cases, mycobacterial disease is well controlled by prolonged antibiotic treatment with or without recombinant IFN-γ treatment [117, 134, 139].
IFN-γR2 deficiency
AR IFN-γR2 deficiency is defined by bi-allelic mutations (Figure 1, table 1). Two forms of AR complete IFN-γR2 deficiency have been reported, depending on whether or not cell surface expression of the receptor is detectable [140, 141]. In seven patients from five kindreds, no protein is detected, as first documented in 1998 [47, 142–145]. The residual cell surface expression of non-functional IFN-γR2 has been described in six patients from five families [51, 140, 141]. Interestingly, three patients have a homozygous mutation, T168N, which creates a novel N-glycosylation site (N-X-S/T-X), abolishing the cellular response to IFN-γ although the protein continues to be expressed at the cell surface [141, 146]. This mutation is a gain-of-glycosylation mutation, and the novel glycan is both necessary and sufficient to cause disease. In another patient, the mutation (382–387dup) is not a gain-of–glycosylation mutation, instead resulting in a misfolded proteins; surprisingly, this mutation can also be rescued with inhibitors of glycosylation [140]. In all cases, the response to IFN-γ is abolished. An IFNGR2 null allele has also been reported to be dominant-negative in vitro in a healthy heterozygous relative of a patient with AR complete IFN-γR2 deficiency [143]. The clinical presentation of AR complete IFN-γR2 deficiency resembles that of complete IFN-γR1 deficiency. The disease manifests in early childhood, with poorly defined and multibacillary granulomas. The most commonly encountered microbial pathogens include BCG, M. abscessus, M. avium, M. fortuitum M. porcium, and M. simiae [51, 140, 141, 145, 147]. Severe infections have an early onset (all before the age of five years) and are often fatal. Six of the 13 patients identified have died. One of the other patients underwent HSCT in 2004 and was alive at the time of this report and the other six were alive when they were reported. The oldest of these patients was five years old in 2005. Only one genetically affected sibling of patients with symptomatic IFN-γR2 deficiency and without clinical disease was reported shortly after birth in 2013. BCG vaccination was contraindicated and this patient remained asymptomatic in 2013 [142]. Other infections are rare but include salmonellosis in one patient [145], and CMV disease in three patients [141, 147]. One patient presented multiple mycobacterial infections and cutaneous squamous cell carcinoma [51]. Antibiotic treatment should not be stopped, but IFN-γ treatment is not indicated, due to the lack of a functional receptor. As reported for IFN-γR1 deficiency, HSCT is the only curative treatment for these patients [14] whose prognosis remains poor.
A partial form of PR IFN-γR2 deficiency results from any of the following homozygous mutations: S124F, R114C, G141R, G227R and 958insT [145, 148–151]. Six patients have been reported to display partial AR IFN-γR2 deficiency (Figure 1). Mycobacterial infections were caused by BCG, M. abscessus, M. bovis, M. elephantis, M. fortuitum, and M. simiae. Two of the six patients described developed osteomyelitis [145, 149]. IFN-γR2 expression on the cell surface was weak but not abolished. The hypomorphic IFNGR2 missense alleles encode misfolded proteins that are abnormally N-glycosylated and largely retained in the endoplasmic reticulum [146, 149]. Impaired, but not abolished, responses to IFN-γ were observed in various cells from the patients: for GAS-binding activity of GAF and induction of GAF-dependent target genes in EBV-B cells, HLA-DR induction in fibroblasts and IL-12p70 production in whole-blood assays. Responses to IFN-γ in the patients’ cells were rescued with kifunensine, a modifier of N-glycosylation, as reported previously in some forms of complete IFN-γR2 deficiency [141, 149]. Two of the six reported patients (33%) have died, and the oldest surviving patient was 20 years old in 2000 [145, 150]. Antibiotics are indicated as an effective treatment for infection, with or without recombinant IFN-γ HSCT is not indicated [14]. A mono-allelic mutation of IFNGR2, 186delC, seems to contribute to an AD form of partial IFN-γR2 deficiency [142]. The mutation creates a premature codon stop upstream from the segment encoding the transmembrane domain. The 186delC was found in a Polish patient and her asymptomatic father. The patient presented a mild form of BCG disease. These and other individuals heterozygous for a loss-of-expression IFNGR2 allele were found to have low levels of IFN-γR2 expression on the cell surface. Their EBV-B cells displayed impaired STAT1 phosphorylation and GAF-DNA binding upon stimulation with IFN-γ and the induction of GAF-dependent target genes [142]. A more pronounced defect was observed in the presence of high doses of IFN-γ. Haploinsufficiency at the human IFNGR2 locus was restricted to EBV-B cells and T lymphocytes, but was not observed in monocytes and monocyte-derived macrophages (MDMs) [152]. The clinical penetrance of AD IFN-γR2 deficiency is very low, as only one of 18 heterozygous individuals was found to be affected, and the treatment of symptomatic individuals is based entirely on curative antibiotic treatments. This is the lowest penetrance reported for PIDs AD by haploinsufficiency [153]. As for most other PIDs AD by haploinsufficiency, the mechanism underlying the incomplete penetrance remains unknown [153].
AD STAT1 deficiency
STAT1 is a transcription factor involved in cellular responses mediated by cytokines including type I (IFN-α/β type II (IFN-γ), and type III (IFN-λ) IFNs [70]. Different forms of inherited STAT1 deficiency have been described in humans: bi-allelic mutations cause AR complete [154–156] or partial STAT1 deficiency [157–161]; mono-allelic mutations cause AD STAT1 deficiency [162] or AD STAT1 gain of activity [163, 164] (Figure 1, Table 2). AR complete STAT1 deficiency is characterized by the absence of WT protein expression and abolished cellular responses to antimycobacterial IFN-γ and antiviral IFN-α/β and IFN-λ [70, 154, 155]. The patients’ cells did not respond to IFN-γ and IFN-α in terms of GAF and interferon-stimulated gene factor 3 (ISGF3: STAT1/STAT2/p48) activity. The cells were unable to control the replication of the viruses tested in vitro, following treatment with IFN-α. Patients with AR complete STAT1 deficiency have a life-threatening susceptibility to both mycobacteria and viruses and are therefore clinically different from patients with MSMD [70, 154, 155]. PR STAT1 deficiency is conferred by bi-allelic hypomorphic mutations of STAT1 [157–161]. The response to IFN-γ and IFN-α is impaired but not abolished, and patients are susceptible to both intracellular bacteria (BCG, M. avium, M. szulgai, Salmonella) and viruses (EBV, CMV and VZV) [157–161]. Again, this phenotype is broader than that of MSMD. AD STAT1 gain of activity was first described in 2011, in patients with CMC [163, 165–167]. These mutations are gain-of-function (GOF), in terms of phosphorylation and GAS-binding activity; the cells of patients display a stronger response to IFN-γ, IFN-α and IL-27 [163, 165–183]. These three forms of inborn errors of STAT1 were actually described after AD STAT1 deficiency was discovered in children with MSMD [70, 162] (Table 1).
Indeed, AD STAT1 deficiency was first described in 2001 in two kindreds with MSMD [162]. In total, eight kindreds containing 17 genetically affected cases, including five asymptomatic individuals, have been reported [27, 162, 184–186]. The seven mutations are loss-of-function (either null, L706S, Q463H, M654K, Y701C, and K637E, or hypomorphic, E320Q and K673R) of STAT1 alleles. They are deleterious for both IFN-γ and IFN-α/β responses but, remarkably, have a negative dominant effect on IFN-γ but not IFN-α/β responses. The severity and underlying mechanism of the loss of function depend on the allele: the E320Q and Q463H mutations impair DNA-binding; the L706S, M654K, Y701C and K673R mutations affect the tyrosine phosphorylation of STAT1; the K637E mutation impairs both STAT1 phosphorylation and DNA-binding activity [27, 162, 184–186]. The principal reason for which these mutant alleles are intrinsically deleterious for IFN-γ and IFN-α/β responses but only dominant for IFN-γ responses is that there is no haploinsufficiency for STAT1, as shown by the normal GAF and ISGF3 DNA-binding activity in heterozygous cells [27]. Furthermore, we showed that some STAT1 mutants do not bind STAT2, whereas others bind STAT2 but do not impair the DNA-binding of the complex [27, 187], therefore being unable to alter ISGF3 activity. By contrast, all the mutations exert a dominant negative effect on GAF activation after IFN-γ stimulation, as only WT homodimers are functional, leading to only 25% the WT level of activation in the case of a functionally null mutant protein produced in normal amounts [162]. This dissociation of the two pathways accounts for the mycobacterial but not viral diseases in heterozygous individuals. The defect of the cellular IFN-γ response is partial, accounting for the relatively good prognosis of infections [1, 70, 92]. Patients with AD STAT1 deficiency have developed mycobacterial infections caused by BCG and EM (M. avium), but display no unusual susceptibility to severe viral infections. One patient suffered only from bona fide tuberculosis caused by M. tuberculosis [27]. As in patients with AD IFN-γR1 deficiency, multifocal osteomyelitis occurs frequently in these patients (in 6 of 12 patients) [162, 184]. It is intriguing, and perhaps not purely coincidental, that partial defects of two genes involved in the response to IFN-γ (IFNGR1 and STAT1) underlie the pathogenesis of osteomyelitis. Disease outcome is good, as no death related to MSMD has been reported in patients with STAT1 mutations. One patient died of liver cancer at the age of 49 years. The oldest surviving patient was 38 years old in 2005 [27]. Clinical penetrance is incomplete, with five of the 17 individuals identified remaining asymptomatic. Antibiotics and IFN-γ are effective treatments for infections.
Complete IL-12Rβ1 deficiency
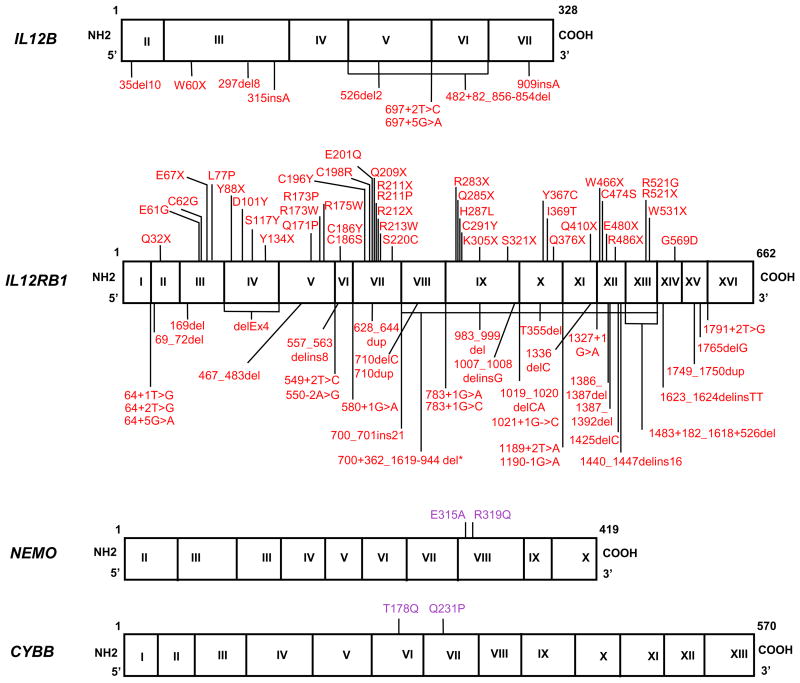
The most common genetic etiology of MSMD is AR complete IL-12Rβ1 deficiency, first reported in 1998 [188, 189]. The IL12RB1 gene encodes the IL-12Rβ1 chain, a gp130 protein, consisting of an extracellular N-terminal immunoglobulin (Ig)-like domain, a transmembrane domain and an intracellular domain. The combination of IL-12Rβ1 and IL-12Rβ2 is required for high-affinity IL-12 binding and signaling. IL-12Rβ1 acts in partnership with IL-23R, to recognize the IL-23 dimer formed from IL-12p40 and p19. Functional IL-12 receptors are expressed primarily on activated T and NK cells. In total, 180 patients from 136 kindreds have been described [2, 21, 25, 28, 30, 31, 34–36, 38–40, 43, 44, 86, 102, 188–233]. The list of known IL12RB1 mutations is increasing, with 78 identified to date, including nonsense (_n_=18), missense (_n_=24), and splice-site mutations (_n_=13), small deletions (_n_=16), large deletions (_n_=3) insertions (_n_=1), and duplications (_n_=3) (www.LOVD.nl/IL12RB1) [191] (Figure 1). A founder effect was demonstrated for the 1623_1624delinsTT mutation, which originated about 475 years ago and has been found in seven patients from Argentina and Belgium [197]. Most mutations result in complete lack of receptor expression, with the exception of one, large in–frame deletion of 12,165 nucleotides [195, 203]. All mutant alleles are loss-of-function and patients with bi-allelic mutations have AR complete IL-12Rβ1 deficiency [191, 234]. None of the patients tested respond to IL-12 and IL-23 and all produced low levels of IFN-γ [28, 102, 194].
The clinical phenotype of AR complete IL-12Rβ1 deficiency is very heterogeneous, ranging from early death in infancy to an asymptomatic course throughout adulthood. Indeed, 47 of the 179 patients died (26%), 8 are asymptomatic (the oldest being 22 years old in 2010) and 124 were alive at the time of their description, the oldest of these patients being 51 years old in 2010 [28, 198]. Mycobacterial infections are the most frequent infections observed in these patients (BCG, M. avium, M. avium intracellulare complex, M. chelonae, M. fortuitum, M. fortuitum_-chelonae complex, M. genevense, M. gordonae, M. tilburgii, M. triplex, M. simiae) [28, 34, 36, 86, 116, 190, 193, 194, 198, 199, 204, 206, 208–210, 214, 215, 235, 236]. Remarkably, BCG vaccination or disease protects against subsequent EM disease [28, 194] (Figure 5). Recurrent BCG disease is rare [28, 194]. These patients therefore display impaired immunity to primary infections caused by mycobacterial species but their immunity to latent or secondary mycobacterial infection seems to be intact. Severe TB has been diagnosed in rare patients with mutations of various MSMD-causing genes, including IFNGR1, STAT1, IL12B, CYBB, but the most commonly mutated gene underlying severe TB is IL12RB1. Six patients with AR complete IL-12Rβ1 deficiency presented with TB as their sole infectious phenotype, probably in the course of primary infection, providing proof-of-principle for the monogenic determinism of severe TB [20, 21, 24, 25, 83]. Interestingly, more than a third of all AR complete IL-12Rβ1-deficient patients (69 of 179 patients (38%)) have developed invasive salmonellosis [28, 30, 31, 39, 43, 188, 190, 196, 202, 206, 207, 233], associated with leukocytoclastic vasculitis in some cases [28, 196, 202]. Klebsiella pneumoniae is also pathogenic in patients with this deficiency [28, 31, 34, 38]. Pneumococcal disease and nocardiosis have each been reported once [39, 210]. A significant minority of patients (48 of 179, 27%) also suffered from mucocutaneous Candida infections, probably because of impaired IL-23-dependent IL-17 immunity [31–36]. Other fungal diseases have been observed in only one or two patients, and were caused by Paraccocidiodes brasiliensis, Coccidiodes spp., Histoplasma spp.,_ and Cryptococcus neoformans [35, 40, 43, 190]. Parasitic infections, such as toxoplasmosis and leishmaniasis, have been also reported in rare cases [19, 28, 44, 194] (and unpublished data) (Figure 5). The association of AR complete IL-12Rβ1 deficiency with other inherited diseases (due to mutations in other genes), including α1-antitrypsin deficiency [214], ataxia-telangiectasia [211], neurofibromatosis [39], and thrombophilia [36] has been reported; and this deficiency has also been reported to be associated with other diseases of no known genetic etiology, such as IgA deficiency [198]. One patient had a esophageal carcinoma [52]. AR complete IL-12Rβ1 deficiency displays incomplete penetrance for the case-definition phenotypes of disseminated BCG/EM [28]. Penetrance is 0.64 at five years of age, increasing to 0.79 by the age of 20 years. The prognosis of this immunodeficiency is variable, but good in most cases. Given the low penetrance of the disease, tests should be carried out to rule out this condition in healthy siblings of affected probands. Patients should be treated with prolonged and aggressive antibiotics against mycobacteria in addition to subcutaneous IFN-γ [237]. Abdominal surgery is indicated to remove the splenic and/or mesenteric lesions [11, 28, 32, 38, 199, 231](and unpublished data). Salmonellosis should also be treated with antibiotics and IFN-γ, such treatment often improving the vasculitis lesions. Prophylaxis with antibiotics should be considered if there are recurrent episodes of salmonellosis. HSCT is not indicated, although the overall mortality of 26% suggests that this option should perhaps be considered in selected cases, such as those in which there is an HLA-compatible donor available within the family and in which IFN-γ treatment is not readily available [14]. Despite the large number of patients with AR IL-12Rβ1 deficiency, no patient with AR complete IL-12Rβ2 deficiency has yet been identified among patients with MSMD. This may be because IL-12Rβ2 is required for IL-35 responses, impaired IL-23 responses contribute to the MSMD phenotype, the IL12RB1 locus is more prone to mutations than the IL12RB2 locus, or heterozygous lesions at the IL12RB2 locus are disease-causing (underlying MSMD or other phenotypes).
Figure 5. Infections in patients with IL-12Rβ1 and IL-12p40 deficiencies.
Infections in 180 patients with complete IL-12Rβ1 deficiency and in 50 reported patients with complete IL-12p40 deficiency. Some patients had multiple episodes of infectious diseases. BCG: bacillus Calmette-Guerin, EM: environmental mycobacteria.
Complete IL-12p40 deficiency
It was shown in 1998 that patients with MSMD may harbor mutations of the IL12B gene [238]. This condition was the first inherited cytokine defect to be identified (mutations of the genes encoding IL-17F and IL-21 have since been identified [239–241]). IL12 encodes IL-12p40, which is common to both IL-12 and IL-23. IL-12 binds to its receptors, IL-12Rβ1 and IL-12Rβ2, on T lymphocytes and NK cells and is a potent inducer of IFN-γ. IL-23 binds to its receptors, IL-12Rβ1 and IL-23, for IL-17 induction. Nine mutations of the IL12B gene have been identified in 50 patients from 31 kindreds with MSMD from five countries (India, Iran, Pakistan, Saudi Arabia and Tunisia) [26, 29, 216, 238, 242–244] (Figure 1). All patients with the same mutation also have the same ethnic origin, and the corresponding mutations are descended from a founder mutation that originated about 600 years ago in Iran, 1,100 years ago in Saudi Arabia, 700 years ago in India/Pakistan and 1,100 years ago in Tunisia [29, 243]. All the mutant alleles are null and patients with bi-allelic mutations display AR complete deficiency with an absence of the IL12p40, IL-12p70 and IL-23 proteins in leukocytes and EBV-B cells. AR complete IL-12p40 and IL-12Rβ1 deficiencies appear to be clinical phenocopies [28, 29]. BCG disease frequently occurs after vaccination (in 41 of the 42 patients vaccinated). Infections caused by M. tuberculosis and EM have been reported [29]. Multiple mycobacterial infections are rare [29]. Salmonellosis has been reported in 25% of the patients and was often recurrent (36%). Other infections caused by various pathogens, including fungi (Candida) and bacteria (Klebsiella and Nocardia) have been reported. IL-17 and IL-23 have been shown to be important for the immune response to Salmonella and Klebsiella in mice [245, 246] (Figure 5). Clinical penetrance reaches 50% before the age 12 months for IL-12p40 deficiency. Thirteen of the 50 patients died before the age of eight years, and one patient died at the age of 34 years. Five patients are asymptomatic, and the oldest of these patients was 26 years old in 2013. The other patients were still alive in 2013, the oldest of these patients being 24 years old. This disease, which closely mimics AR complete IL-12Rβ1 deficiency, generally has a good prognosis. The differences between these two disorders probably reflect the much lower allelic and ethnic diversity seen in patients with AR complete IL-12p40 deficiency. Patients are treated with prolonged courses of antibiotic treatment and recombinant IFN-γ. HSCT is not indicated in most cases [29]. Surprisingly, 50 patients carry mutations of the IL12B gene, whereas none carry mutations of the IL12A gene. This situation parallels the lack of reported AR IL-12Rβ2 deficiency, and the underlying reasons may be similar.
AD IRF8 deficiency
Interferon regulatory factor 8 (IRF8), also known as interferon consensus sequence-binding protein (ICSBP), is one of the nine members of the IRF family of transcription factors [247–249]. These proteins bind to IFN-stimulated response elements (ISRE) and regulate the expression of genes stimulated by IFN-α/β. IRF8 is expressed in macrophages and dendritic cells and plays an important role in several aspects of myeloid cells [250, 251]. Mutations of the human IRF8 gene underlie two different immunodeficiencies (Figure 1, Tables 1–2). AR complete IRF8 deficiency is caused by bi-allelic K108E mutation [67, 75]. The expression of the mutant IRF8 allele is comparable to WT but with a lower electrophoretic mobility. A recent functional characterization of this allele showed that the mutation resulted in a loss of nuclear localization and of transcriptional activity, together with lower stability of the protein, higher levels of ubiquitination and sumoylation, and enhanced proteosomal degradation [75]. A severe impairment of IL-12 and IFN-γ induction was observed in PBMCs stimulated with BCG, phytohemagglutinin (PHA), or lipopolysaccharide (LPS). This immunodeficiency is characterized by a complete absence of CD14+ and CD16+ circulating monocytes, CD11c+ conventional dendritic cells (DC) and CD11c+/CD123+ plasmacytoid DCs, whereas neutrophil counts are very high. The single patient reported also had normal number of T cells (CD4+ and CD8+), but they appeared to be anergic, probably due to the absence of myeloid antigen-presenting cells [75]. The patient had multiple infectious diseases, including disseminated BCG disease, oral candidiasis, and severe respiratory infections [67, 73]. AR complete IRF8 deficiency is not an etiology of MSMD. The patient received HSCT as a curative treatment [67], in addition to antibiotic and antifungal therapies.
An AD partial form of IRF8 deficiency was described in two unrelated patients from Brazil and Chile. Both were found to carry the same mono-allelic mutation (T80A) of IRF8 [67] (Figure 1, Tables 1–2). The mutations occurred de novo, as they were absent from the biological parents and siblings, who did not display MSMD. The T80A mutation maps to the conserved DNA-binding domain of IRF8, and the T80 residue is strictly conserved between orthologs, across all species. The expression of IRF8 in the patients’ EBV-B cells was normal. The T80A mutation has pleiotropic effects on IRF8 function, including a large decrease in DNA-binding, substantially reducing the potential of the protein to transactivate target genes, such as IL12B or NOS2. The mutant allele also has a dominant-negative effect on the transcriptional activity of the WT protein. Both patients have normal counts of circulating lymphocytes, granulocytes, and monocytes. Both the major (CD14+ CD16−) and minor (CD16+ and CD14dim) subsets of monocytes were present at the expected frequencies. However, the main subset of human blood myeloid DCs (MDCs) (DR+ CD11c+ CD1c+, or MDC1) was absent, in both patients [67]. These MDC1s are potent producers of IL-12. Interestingly, mice lacking Irf8 show a selective lack of CD8α+ lymphoid tissue-associated classical DC, which are also potent producers of IL-12 [247, 252]. This DC deficiency is different from that described in AR complete IRF8 and AD GATA2 deficiency, in terms of cellular and clinical phenotypes [253]. Clinically, both patients with AD IRF8 deficiency had recurrent episodes of disseminated BCG disease, without other infectious diseases (Table 2). These otherwise healthy individuals are now aged 18 and 44 years, and are well with no treatment. The management of infections is based on antimycobacterial antibiotics. IFN-γ does not appear to be required and HSCT is not indicated.
ISG15 deficiency
In 2012, whole-exome sequencing led to the identification of bi-allelic mutations of ISG15 [68, 254]. This gene encodes an interferon-induced ubiquitin-like protein that modifies substrates in a process similar to ubiquitination (referred to as ISGylation). ISG15 is present in the gelatinase and secretory granules, but not in the azurophilic or specific granules of steady-state neutrophils, which release this protein upon bacterial challenge [255]. ISG15 is also secreted by many other cell types, including myeloid cells, and it acts as a very potent IFN-γ-inducing cytokine in lymphocytes, acting in synergy with IL-12 in particular [256, 257]. Two bi-allelic mutations were found in two unrelated consanguineous families from Iran and Turkey, resulting in AR complete ISG15 deficiency (Figure 1). The three patients displayed BCG disease. More recently, three other patients from a Chinese kindred, without clinical mycobacterial infections, have also been shown to have AR complete ISG15 deficiency [258]. All three alleles resulted in an absence of ISG15 protein, as demonstrated by the transfection of HEK293T cells [68, 258]. The cellular phenotype is characterized by impaired, but not abolished IFN-γ production in response to the stimulation of whole blood with BCG plus IL-12, as in patients with deficiencies of IL-12p40 or IL-12Rβ1. The patients displayed impaired IFN-γ production by both NK cells and T lymphocytes, thereby accounting for mycobacterial disease [68]. The addition of recombinant extracellular ISG15 to the medium rescued the production of IFN-γ by T and NK cells from the patients. Surprisingly, another clinical phenotype was subsequently observed, resulting from the lack of intracellular, but not extracellular ISG15. All patients presented enhanced IFN-α/β immunity, as demonstrated by high levels of circulating IFN-α and/or leukocyte ISGs. The absence of intracellular ISG15 in the patients’ cells prevents the stabilization of USP18, a potent negative regulator of IFN-α/β signaling, leading to an amplification of IFN-α/β induced responses [258]. Clinically, the three Iranian and Turkish patients developed disseminated mycobacterial diseases after BCG vaccination, due to the lack of free extracellular ISG15, which is required to induce IFN-γ. The three Chinese patients subsequently identified have not been vaccinated with BCG and have not yet developed any mycobacterial infections. However the lack of intracellular free ISG15 led to intracranial calcifications in all six patients. The three Chinese children also suffered from epileptic seizures [68, 258]. Despite having been exposed to common childhood viruses, none of the patients displayed severe viral infectious diseases, contrasting with the reports for Isg15-deficient mice [259]. The evidence collected to date for the six ISG15-deficient individuals indicates that the lack of free secreted ISG15 underlies mycobacterial infection in these patients. This lack of intracellular free ISG15 prevents the accumulation of USP18, a known negative regulator of IFN-α/β, resulting in enhanced IFN-α/β immunity and autoinflammation, resembling Aicardi-Goutieres syndrome and spondyloenchondromatosis [258, 260, 261].
X-linked recessive NEMO deficiency
Germline mutations of NEMO and CYBB have been shown to cause X-linked recessive (XR) MSMD [22, 69, 262] (Figures 1–3, Tables 1–2). These two genes have long been implicated in other human diseases: incontinentia pigmenti (IP) and anhidrotic ectodermal dysplasia with immunodeficiency (EDA-ID) (NEMO) ) [263–265], and chronic granulomatous disease (CGD) (CYBB) [74, 266,267]. NEMO is a regulatory subunit of the inhibitor of NF-κB (IκB) kinase (IKK). It consists of a series of coiled-coil (CC) domains: CC1 in the N-terminal segment, HLX2 in the middle segment, a zinc finger domain (ZF) and the CC2-leucine zipper (LZ) regulatory domain in the C-terminal segment. Mutations of the NEMO gene confer different clinical and cellular phenotypes: null mutations abolish NEMO-dependent NF-κB activation and are associated with X-linked dominant incontinentia pigmenti (XD-IP) (OMIM 308300) in female subjects and in utero lethality in male subjects [265]; hypomorphic mutations impair, but do not abolish NF-κB signaling and are associated with the XR anhidrotic ectodermal dysplasia with immunodeficiency (XR-EDA-ID) syndrome in male individuals [71, 72]. This immunodeficiency results in an increase in susceptibility to a wide range of pathogens (pyogenic bacteria, mycobacteria and viruses), but most patients suffer from invasive pneumococcal disease. The extent and severity of the EDA define different clinical diseases: EDA-ID with osteopetrosis and/or lymphedema (XR-EDA-ID-OL), classic XR-EDA-ID, XR with mild-EDA-ID (e.g. conical incisors only), and ID without EDA (OMIM 300301, 300291, 300584, 300640) [263, 268–272].
The E315A and R319Q mutations of NEMO, affecting residues conserved in animal species [69], cause MSMD (Figure 1, Table 1). Six patients from three different kindreds from the USA, Germany and France have been described. These mutations disrupt the formation of the salt bridge normally formed between residues E315 and R319 within the LZ-helix of NEMO, interfering with the CD40-NEMO-NF-κB signaling pathway [69]. Studies based on pull-down assays have reported a milder defect of ubiquitin binding than for the mutations associated with EDA-ID [268, 273]. The mechanism underlying this susceptibility involves the impairment of CD40-dependent IL-12 production [69, 274–277]. The cellular phenotype includes low levels of IFN-γ and IL-12 production by the peripheral mononuclear cells of the patients in response to PHA or CD3-specific antibodies [69, 278–281]. The impaired production of IL-12 monocytes in response to T-cell activation was demonstrated in a co-culture system. Interestingly, the microbial stimulation-dependent production of IL-12 is conserved in the patients [69, 274–277]. These hypomorphic recessive mutations of NEMO selectively impair one of the two IL-12 production pathways. The T cell-dependent, CD40-dependent, c-Rel-mediated NF-κB pathway that controls IL-12 production in myeloid cells is impaired in these patients, and perhaps in patients with a NEMO mutation conferring a broader infection susceptibility [282, 283]. The patients developed disseminated mycobacterial diseases. M. avium complex infection is the most common mycobacterial infection (present in four of the six patients), one patient had a culture positive for M. avium and M. tuberculosis, and two patients had probable tuberculosis [12, 279, 284]. Only one patient from France was vaccinated with BCG. No other severe infection has been reported in these patients, with the exception of invasive Haemophilus influenzae type b infection in one patient [69, 279]. Only one of the patients has conical decidual incisors. Two of the six patients died, at the ages of 48 and 10 years [69]. Prognosis differs between patients, who may benefit from both antibiotics and IFN-γ treatment [139, 279].
X-linked recessive CYBB deficiency
CYBB (also known as gp91phox or NOX2) is an essential component of the NADPH oxidase complex. It encodes the β-chain of flavocytochrome b558. It is expressed in phagocytes, including granulocytes, monocytes and macrophages, but also, to a lesser extent, in other cells, such as dendritic cells and B lymphocytes. Germline mutations of CYBB are responsible for the most common form of CGD (OMIM 306400), a primary immunodeficiency disease in which phagocytic cells display little or no NADPH oxidase activity (Table 2). Three forms of XR-CGD have been described, based on X91 protein levels: X91° (no protein), X91− (low levels of protein) and X91+ (normal levels of protein). CGD patients suffer from recurrent life-threatening infections caused by multiple bacteria and fungi, including Staphylococcus and Aspergillus in particular [266, 267, 285–287]. Mycobacterial infections are not usually considered to be part of the typical clinical picture in CGD. However, the number of case reports from countries in which BCG vaccine is routinely administered has been increasing [288–295]. BCG disease had been reported in 38 CGD patients by 2007 [288]. Since 2007, 125 cases of BCG disease [288–292, 294, 296–298] and 42 cases of TB [288, 290–292, 299, 300] have been reported in CGD patients.
In 2011, a second form of XR-MSMD was described [22]. Seven male patients from two unrelated families who developed infections due to tuberculous mycobacteria were described [22] (Figure 1, Table 1). Six of these patients had BCG infections (BCG-osis in three patients and recurrent regional BCG-itis in three other patients) and the seventh developed a disseminated form of bona fide TB. Interestingly, this last patient was not vaccinated with BCG vaccine in infancy. None of the seven patients suffered from any other infectious diseases. These otherwise healthy individuals are now aged 61, 64, 59, 40 and 43 years, and all are well with no treatment. An obligate female carrier developed tuberculous salpingitis at the age of 29 years [22, 301]. A genome-wide linkage study led to the identification of two new hemizygous mutations of CYBB: Q231P and T178P [22]. These mutations were shown to affect respiratory burst function in MDMs and EBV-B cells. Indeed, when macrophages were activated with BCG, PPD (purified protein derivate from M. tuberculosis), or IFN-γ and triggered with phorbol ester, the respiratory burst was completely abolished. By contrast to what has been observed for CGD patients, neutrophils monocytes and monocytes-derived dendritic cells (MDCs) from patients with XR2-MSMD had a normal respiratory burst, as shown by measurements of superoxide and hydrogen peroxide production in response to phorbol ester induction and physiological stimuli [22, 302]. The specific impact of these mutations on MDMs and EBV-B is dependent on the levels of gp91_phox_ protein and flavocytochrome _b_558 and correlated with the defect in NADPH oxidase activity [22]. Functional studies on Chinese hamster ovary (CHO) epithelial cell lines and PLB-985 cell lines (a diploid myeloid leukemia cell line with granulocytic and monocytic differentiating capacity) also showed that these mutations had a selective, cell-specific impact. These results suggest that the respiratory burst in granulocytes and monocytes is critical for the control of fungi and pyogenic bacteria. By contrastt, the macrophage respiratory burst is essential for protective immunity to mycobacteria. The MSMD-causing CYBB mutations selectively impair the respiratory burst in one relevant cell type (macrophages, as we know from the various forms of agammaglobulinemia that B cells are not involved in protective immunity to BCG). Thus, these experiments of Nature are of general interest in the field of genetic diseases, especially in patients with narrow phenotypes, infectious or otherwise, in whom the possibility of subtle mutations, selectively affecting a single cell type, should not be ruled out [262].
Conclusions and future directions
Since the initial clinical description of MSMD, probably in 1951 [4], and the discovery of the first genetic etiology of this condition in 1996 [65, 66], 18 genetic etiologies of MSMD, including mutations in nine genes, have been described and characterized (Figures 1–3, Table 1). However, about half the MSMD patients known to us do not suffer from any of these 18 MSMD-causing defects, suggesting an even greater degree of genetic heterogeneity underlying MSMD. Investigations of MSMD patients have revealed that human IFN-γ mediated immunity is essential for the control of mycobacterial infections. IFN-γ-mediated immunity also seems to play a role in immunity to other intra-macrophagic pathogens, and perhaps to some viruses and tumors. At odds with the mouse Th1 paradigm, according to which IFN-γ is the signature cytokine of immunity to intracellular agents in general [303], human patients with inborn errors of IFN-γ immunity have a narrow infectious phenotype. They do not even display a massive Th2 bias, as allergy and IgE levels are not particularly high in these patients [304, 305]. The study of MSMD led to the discovery of autoantibodies against IFN-γ with late-onset mycobacterial diseases as phenocopies of MSMD, mimicking inborn errors of IFN-γ immunity [306–309]. The genetic dissection of MSMD has therefore had important immunological implications, derived from the dissection of human immunity in natura [1, 63, 310, 311]. The identification of these genetic diseases has also had important clinical implications. This series of studies has provided the most comprehensive genetic and immunological analysis of infectious diseases striking otherwise healthy individuals to date. The findings support the genetic theory of childhood infectious diseases, including, in particular, the notion that life-threatening primary infections in otherwise healthy children and young adults may be caused by single-gene inborn errors of immunity [62, 63]. Other examples include herpes simplex encephalitis, predisposition to Epstein-Barr virus or to oncogenic papillomaviruses in patients with epidermodysplasia verruciforme, CMC and invasive pneumococcal disease [72, 312–316]. These findings have facilitated genetic counseling for affected families and they guide the treatment of patients based on a rational understanding of the pathogenesis of mycobacterial disease. Patients with MSMD are currently treated with antibiotics, with or without recombinant IFN-γ In some cases, the prognosis remains poor. Finally, the genetic dissection of MSMD has paved the way for the genetic dissection of severe TB in otherwise healthy children [19, 24]. Proof-of-principle for a genetic basis of human TB was provided by IL-12Rb1-deficient patients [19, 24, 77, 83]. The advent of News on Genomic Studies (NGS), with Whole Exome sequencing (WES) and Whole Genome (WGS), will further boost the discovery of novel genetic disorders underlying MSMD [68, 145, 149, 258] and other infections, including TB [63, 254, 317–322].
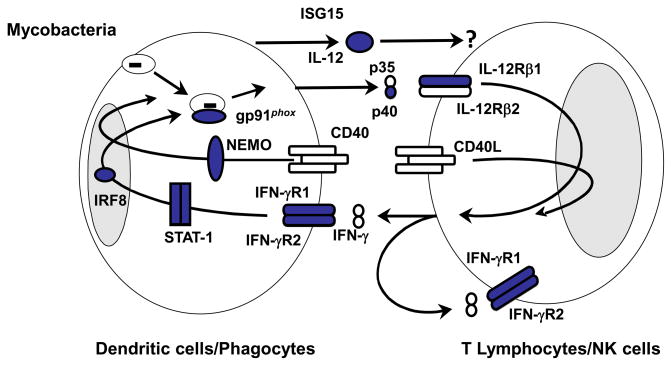
Figure 2. Cells producing and responding to IFN-γ.
Proteins for which mutations in the corresponding genes have been identified and associated with Mendelian susceptibility to mycobacterial diseases (MSMD) are indicated in blue. The allelic heterogeneity of nine genes results in the definition of 18 genetic disorders. MSMD-causing mutations of IFNGR1, IFNGR2, STAT1, IRF8 and CYBB impair the action of IFN-γ. MSMD-causing mutations of IL12B, IL12RB1, ISG15, IRF8 and NEMO impair the production of IFN-γ.
Highlights.
- The human IFN-γ-mediated immunity is essential for the control of mycobacterial infections.
- The high level of allelic heterogeneity has already led to the definition of 18 different disorders.
- The genetic dissection of MSMD has important immunological implications, derived from the dissection of human immunity in natura.
- The genetic dissection of MSMD has paved the way for the genetic dissection of severe tuberculosis in otherwise healthy children.
Acknowledgments
We thank all members of the laboratory for helpful discussions, and Yelena Nemirovskaya, Lahouari Amar, Martine Courat and Eric Anderson for administrative support; and all members of the laboratory involved in the study of patients with MSMD, including, in particular, Dusan Bogunovic, Caroline Deswarte, Jacqueline Feinberg Emmanuelle Jouanguy, Xiao-Fei Kong, Janet Markle, Rubén Martínez-Barricarte, Mélanie Migaud, Marcela Moncada-Vélez, Satoshi Okada, Capucine Picard and Guillaume Vogt. We thank the patients and their families, and referring physicians worldwide for their trust and collaboration over the years. This research was supported in part by grants from INSERM, Paris Descartes University, Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (ANR-10-LABX-62-IBEID), the French National Research Agency (ANR) under “the investments for the future” program (grant ANR-10-IAHU-01) and ANR grant IFNGPHOX number ANR 13-ISV-0001-01, the National Institute of Allergy and Infectious Diseases grant numbers 5R37AI095983 and 5R01AI089970, the National Center for Research Resources and the National Center for Advancing Sciences of the National Institutes of Health grant number 8UL1TR000043, and the St. Giles Foundation.
Abbreviations
AD
autosomal dominant
AR
autosomal recessive
BCG
bacillus Calmette-Guerin
CGD
chronic granulomatous disease
CMC
chronic mucocutaneous candidiasis
EM
environmental mycobacteria
GOF
gain-of-function
HSCT
Hematopoietic stem cell transplantation
IFN-γ
interferon gamma
IL
interleukin
MDCs
monocytes-derived dendritic cells
MDMs
monocytes and monocyte-derived macrophages
MSMD
Mendelian susceptibility to mycobacterial disease
NGS
News on Genomic Studies
PID
primary immunodeficiencies
PR
partial receissive
WES
Whole Exome sequencing
WGS
Whole Genome
XR
X-linked recessive
Footnotes
Conflict of interest
The authors have no financial or commercial conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 2.Levin M, Newport MJ, D’Souza S, Kalabalikis P, Brown IN, Lenicker HM, Agius PV, Davies EG, Thrasher A, Klein N, et al. Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet. 1995;345(8942):79–83. doi: 10.1016/s0140-6736(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 3.Casanova JL, Jouanguy E, Lamhamedi S, Blanche S, Fischer A. Immunological conditions of children with BCG disseminated infection. Lancet. 1995;346(8974):581. doi: 10.1016/s0140-6736(95)91421-8. [DOI] [PubMed] [Google Scholar]
- 4.Mimouni J. Our experiences in three years of BCG vaccination at the center of the O.P.H.S. at Constantine; study of observed cases (25 cases of complications from BCG vaccination) Alger Medicale. 1951;55(8):1138–1147. [PubMed] [Google Scholar]
- 5.Ulgenalp I, Yalcin M, Cetiner M, Ozgen M, Koseli I. Olumle sonuclanan jeneralize BCG enfeksiyonu. Tuberculoz ve Toraks. 1973;21:11–19. [Google Scholar]
- 6.Sicevic S. Generalized BCG tuberculosis with fatal course in two sisters. Acta Paediatr Scand. 1972;61(2):178–184. doi: 10.1111/j.1651-2227.1972.tb15922.x. [DOI] [PubMed] [Google Scholar]
- 7.Heyne K. Generalized familial semibenign BCG infection, salmonella osteomyelitis and intestinal pseudotuberculosis--due to a familial defect of the macrophage system? (author’s transl) Eur J Pediatr. 1976;121(3):179–189. doi: 10.1007/BF00445481. [DOI] [PubMed] [Google Scholar]
- 8.Van der Hoeven LH, Rutten FJ, Van der Sar A. An unusual acid-fast bacillus causing systemic disease and death in a child; with special reference to dissemintaed osteomyelitis and intracellular parasitism. Am J Clin Pathol. 1958;29:433–448. doi: 10.1093/ajcp/29.5.433. [DOI] [PubMed] [Google Scholar]
- 9.Buhler VB, Pollack A. Human infection with atypical acid-fast organism. Am J Clin Pathol. 1953;23:363–374. doi: 10.1093/ajcp/23.4.363. [DOI] [PubMed] [Google Scholar]
- 10.Engbaek HC. Three cases in the same family of fatal infection with M. avium. Acta Tuber Pneumol Scand. 1964;45:105–117. [PubMed] [Google Scholar]
- 11.Al-Muhsen S, Casanova JL. The genetic heterogeneity of mendelian susceptibility to mycobacterial diseases. J Allergy Clin Immunol. 2008;122(6):1043–1051. doi: 10.1016/j.jaci.2008.10.037. quiz 1052–1043. [DOI] [PubMed] [Google Scholar]
- 12.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, et al. Inborn errors of IL-12/23- and IFN-gamma- mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18(6):347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Holland SM. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol Res. 2007;38(1–3):342–346. doi: 10.1007/s12026-007-0045-8. [DOI] [PubMed] [Google Scholar]
- 14.Holland S, Casanova JL. Inherited disorders of the interleukin-12-interleukin-23/interferon-g-circuit. In: Ochs HD, Edvard Smith CI, Puck JM, editors. Primary Immunodeficiency disorders - A molecular approach. Chapter 35. 2014. pp. 450–466. [Google Scholar]
- 15.Rosenzweig SD, Holland SM. Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev. 2005;203:38–47. doi: 10.1111/j.0105-2896.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 16.Cottle LE. Mendelian susceptibility to mycobacterial disease. Clin Genet. 2011;79(1):17–22. doi: 10.1111/j.1399-0004.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- 17.Haverkamp MH, van de Vosse E, van Dissel JT. Nontuberculous mycobacterial infections in children with inborn errors of the immune system. J Infect. 2014;68 (Suppl 1):S134–150. doi: 10.1016/j.jinf.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez-Alejo N, Santos-Argumedo L. Innate defects of the IL-12/IFN-gamma axis in susceptibility to infections by mycobacteria and salmonella. J Interferon Cytokine Res. 2014;34(5):307–317. doi: 10.1089/jir.2013.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boisson-Dupuis S, El Baghdadi J, Parvaneh N, Bousfiha A, Bustamante J, Feinberg J, Samarina A, Grant AV, Janniere L, El Hafidi N, et al. IL-12Rbeta1 deficiency in two of fifty children with severe tuberculosis from Iran, Morocco, and Turkey. PLoS One. 2011;6(4):e18524. doi: 10.1371/journal.pone.0018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabarsi P, Marjani M, Mansouri N, Farnia P, Boisson-Dupuis S, Bustamante J, Abel L, Adimi P, Casanova JL, Mansouri D. Lethal tuberculosis in a previously healthy adult with IL-12 receptor deficiency. J Clin Immunol. 2011;31(4):537–539. doi: 10.1007/s10875-011-9523-9. [DOI] [PubMed] [Google Scholar]
- 21.Caragol I, Raspall M, Fieschi C, Feinberg J, Larrosa MN, Hernandez M, Figueras C, Bertran JM, Casanova JL, Espanol T. Clinical tuberculosis in 2 of 3 siblings with interleukin-12 receptor beta1 deficiency. Clin Infect Dis. 2003;37(2):302–306. doi: 10.1086/375587. [DOI] [PubMed] [Google Scholar]
- 22.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, Grant AV, Marchal CC, Hubeau M, Chapgier A, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12(3):213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M, et al. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J Clin Invest. 1997;100(11):2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcais A, Fieschi C, Abel L, Casanova JL. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med. 2005;202(12):1617–1621. doi: 10.1084/jem.20052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozbek N, Fieschi C, Yilmaz BT, de Beaucoudrey L, Demirhan B, Feinberg J, Bikmaz YE, Casanova JL. Interleukin-12 receptor beta 1 chain deficiency in a child with disseminated tuberculosis. Clin Infect Dis. 2005;40(6):e55–58. doi: 10.1086/427879. [DOI] [PubMed] [Google Scholar]
- 26.Picard C, Fieschi C, Altare F, Al-Jumaah S, Al-Hajjar S, Feinberg J, Dupuis S, Soudais C, Al-Mohsen IZ, Genin E, et al. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet. 2002;70(2):336–348. doi: 10.1086/338625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapgier A, Boisson-Dupuis S, Jouanguy E, Vogt G, Feinberg J, Prochnicka-Chalufour A, Casrouge A, Yang K, Soudais C, Fieschi C, et al. Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease. PLoS Genet. 2006;2(8):e131. doi: 10.1371/journal.pgen.0020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Janniere L, Rose Y, de Suremain M, et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89(6):381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prando C, Samarina A, Bustamante J, Boisson-Dupuis S, Cobat A, Picard C, AlSum Z, Al-Jumaah S, Al-Hajjar S, Frayha H, et al. Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine (Baltimore) 2013;92(2):109–122. doi: 10.1097/MD.0b013e31828a01f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozen M, Ceyhan M, Sanal O, Bayraktar M, Mesci L. Recurrent Salmonella bacteremia in interleukin-12 receptor beta1 deficiency. J Trop Pediatr. 2006;52(4):296–298. doi: 10.1093/tropej/fml001. [DOI] [PubMed] [Google Scholar]
- 31.Ouederni M, Sanal O, Ikinciogullari A, Tezcan I, Dogu F, Sologuren I, Pedraza-Sanchez S, Keser M, Tanir G, Nieuwhof C, et al. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor beta1 deficiency. Clin Infect Dis. 2014;58(2):204–213. doi: 10.1093/cid/cit722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aytekin C, Dogu F, Tuygun N, Tanir G, Guloglu D, Boisson-Dupuis S, Bustamante J, Feinberg J, Casanova JL, Ikinciogullari A. Bacille Calmette-Guerin lymphadenitis and recurrent oral candidiasis in an infant with a new mutation leading to interleukin-12 receptor beta-1 deficiency. J Investig Allergol Clin Immunol. 2011;21(5):401–404. [PMC free article] [PubMed] [Google Scholar]
- 33.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205(7):1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Alejo N, Blancas-Galicia L, Yamazaki-Nakashimada M, Garcia-Rodriguez SE, Rivas-Larrauri F, Paolo-Cienfuegos DP, Alcantara-Salinas A, Espinosa-Rosales F, Santos-Argumedo L. Molecular analysis for patients with IL-12 receptor beta1 deficiency. Clin Genet. 2013;86(2):161–166. doi: 10.1111/cge.12253. [DOI] [PubMed] [Google Scholar]
- 35.Rezai MS, Khotael G, Kheirkhah M, Hedayat T, Geramishoar M, Mahjoub F. Cryptococcosis and deficiency of interleukin12r. Pediatr Infect Dis J. 2008;27(7):673. doi: 10.1097/INF.0b013e318179263a. [DOI] [PubMed] [Google Scholar]
- 36.Akar HH, Kose M, Ceylan O, Patiroglu T, Bustamante J, Casanova JL, Akyildiz BN, Doganay S. Congenital IL-12R1beta receptor deficiency and thrombophilia in a girl homozygous for an IL12RB1 mutation and compound heterozygous for MTFHR mutations: A case report and literature review. Eur J Microbiol Immunol (Bp) 2014;4(1):83–87. doi: 10.1556/EuJMI.4.2014.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roesler J, Kofink B, Wendisch J, Heyden S, Paul D, Friedrich W, Casanova JL, Leupold W, Gahr M, Rosen-Wolff A. Listeria monocytogenes and recurrent mycobacterial infections in a child with complete interferon-gamma-receptor (IFNgammaR1) deficiency: mutational analysis and evaluation of therapeutic options. Exp Hematol. 1999;27(9):1368–1374. doi: 10.1016/s0301-472x(99)00077-6. [DOI] [PubMed] [Google Scholar]
- 38.Pedraza S, Lezana JL, Samarina A, Aldana R, Herrera MT, Boisson-Dupuis S, Bustamante J, Pages P, Casanova JL, Picard C. Clinical disease caused by Klebsiella in 2 unrelated patients with interleukin 12 receptor beta1 deficiency. Pediatrics. 2010;126(4):e971–976. doi: 10.1542/peds.2009-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luangwedchakarn V, Jirapongsaranuruk O, NiemeLa JE, Thepthai C, Chokephaibulkit K, Sukpanichnant S, Pacharn P, Visitsunthorn N, Vichyanond P, Piboonpocanun S, et al. A novel mutation of the IL12RB1 gene in a child with nocardiosis, recurrent salmonellosis and neurofibromatosis type I: first case report from Thailand. Asian Pac J Allergy Immunol. 2009;27(2–3):161–165. [PubMed] [Google Scholar]
- 40.Moraes-Vasconcelos D, Grumach AS, Yamaguti A, Andrade ME, Fieschi C, de Beaucoudrey L, Casanova JL, Duarte AJ. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the beta1 subunit of the interleukin (IL)-12/IL-23 receptor. Clin Infect Dis. 2005;41(4):e31–37. doi: 10.1086/432119. [DOI] [PubMed] [Google Scholar]
- 41.Zerbe CS, Holland SM. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2005;41(4):e38–41. doi: 10.1086/432120. [DOI] [PubMed] [Google Scholar]
- 42.Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2009;49(6):e62–65. doi: 10.1086/605532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinh DC, Schwartz B, Hsu AP, Miranda DJ, Valdez PA, Fink D, Lau KP, Long-Priel D, Kuhns DB, Uzel G, et al. Interleukin-12 receptor beta1 deficiency predisposing to disseminated Coccidioidomycosis. Clin Infect Dis. 2011;52(4):e99–e102. doi: 10.1093/cid/ciq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanal O, Turkkani G, Gumruk F, Yel L, Secmeer G, Tezcan I, Kara A, Ersoy F. A case of interleukin-12 receptor beta-1 deficiency with recurrent leishmaniasis. Pediatr Infect Dis J. 2007;26(4):366–368. doi: 10.1097/01.inf.0000258696.64507.0f. [DOI] [PubMed] [Google Scholar]
- 45.Sologuren I, Boisson-Dupuis S, Pestano J, Vincent QB, Fernandez-Perez L, Chapgier A, Cardenes M, Feinberg J, Garcia-Laorden MI, Picard C, et al. Partial recessive IFN-gammaR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum Mol Genet. 2011;20(8):1509–1523. doi: 10.1093/hmg/ddr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, Rosenzweig SD, Newport M, Levin M, Roesler J, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364(9451):2113–2121. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 47.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998;101(11):2364–2369. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorman SE, Uzel G, Roesler J, Bradley JS, Bastian J, Billman G, King S, Filie A, Schermerhorn J, Holland SM. Viral infections in interferon-gamma receptor deficiency. J Pediatr. 1999;135(5):640–643. doi: 10.1016/S0022-3476(99)70064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roesler J, Hedrich C, Laass MW, Heyne K, Rosen-Wolff A. Meningoencephalitis caused by varicella-zoster virus reactivation in a child with dominant partial interferon-gamma receptor-1 deficiency. Pediatr Infect Dis J. 2011;30(3):265–266. doi: 10.1097/INF.0b013e3181f6f78a. [DOI] [PubMed] [Google Scholar]
- 50.Bax HI, Freeman AF, Anderson VL, Vesterhus P, Laerum D, Pittaluga S, Wilson WH, Holland SM. B-cell lymphoma in a patient with complete interferon gamma receptor 1 deficiency. J Clin Immunol. 2013;33(6):1062–1066. doi: 10.1007/s10875-013-9907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toyoda H, Ido M, Nakanishi K, Nakano T, Kamiya H, Matsumine A, Uchida A, Mizutani H, de Beaucoudrey L, Vogt G, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon gamma receptor 2 (IFN gamma R2) deficiency. J Med Genet. 2010;47(9):631–634. doi: 10.1136/jmg.2009.072108. [DOI] [PubMed] [Google Scholar]
- 52.Cardenes M, Angel-Moreno A, Fieschi C, Sologuren I, Colino E, Molines A, Garcia-Laorden MI, Campos-Herrero MI, Andujar-Sanchez M, Casanova JL, et al. Oesophageal squamous cell carcinoma in a young adult with IL-12R beta 1 deficiency. J Med Genet. 2010;47(9):635–637. doi: 10.1136/jmg.2009.071910. [DOI] [PubMed] [Google Scholar]
- 53.Camcioglu Y, Picard C, Lacoste V, Dupuis S, Akcakaya N, Cokura H, Kaner G, Demirkesen C, Plancoulaine S, Emile JF, et al. HHV-8-associated Kaposi sarcoma in a child with IFNgammaR1 deficiency. J Pediatr. 2004;144(4):519–523. doi: 10.1016/j.jpeds.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Taramasso L, Boisson-Dupuis S, Garre ML, Bondi E, Cama A, Nozza P, Morana G, Casanova JL, Marazzi MG. Pineal Germinoma in a Child with Interferon-gamma Receptor 1 Deficiency. Case Report and Literature Review. J Clin Immunol. 2014 doi: 10.1007/s10875-014-0098-0. in press. [DOI] [PubMed] [Google Scholar]
- 55.de Bruin AM, Voermans C, Nolte MA. Impact of interferon-gamma on hematopoiesis. Blood. 2014 doi: 10.1182/blood-2014-04-568451. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Zhang Z, Zheng B, He Z, Winberg G, Ernberg I. An update on viral association of human cancers. Arch Virol. 2013;158(7):1433–1443. doi: 10.1007/s00705-013-1623-9. [DOI] [PubMed] [Google Scholar]
- 57.Sarid R, Gao SJ. Viruses and human cancer: from detection to causality. Cancer Lett. 2011;305(2):218–227. doi: 10.1016/j.canlet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novelli F, Casanova JL. The role of IL-12, IL-23 and IFN-gamma in immunity to viruses. Cytokine Growth Factor Rev. 2004;15(5):367–377. doi: 10.1016/j.cytogfr.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casanova JL, Fieschi C, Zhang SY, Abel L. Revisiting human primary immunodeficiencies. J Intern Med. 2008;264(2):115–127. doi: 10.1111/j.1365-2796.2008.01971.x. [DOI] [PubMed] [Google Scholar]
- 60.Sancho-Shimizu V, Perez de Diego R, Jouanguy E, Zhang SY, Casanova JL. Inborn errors of anti-viral interferon immunity in humans. Curr Opin Virol. 2011;1(6):487–496. doi: 10.1016/j.coviro.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, et al. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32(1–3):231–245. doi: 10.1385/ir:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 62.Alcais A, Abel L, Casanova JL. Human genetics of infectious diseases: between proof of principle and paradigm. J Clin Invest. 2009;119(9):2506–2514. doi: 10.1172/JCI38111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casanova JL, Abel L. The genetic theory of infectious diseases: a brief history and selected illustrations. Annu Rev Genomics Hum Genet. 2013;14:215–243. doi: 10.1146/annurev-genom-091212-153448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casanova JL, Abel L, Quintana-Murci L. Immunology Taught by Human Genetics. Cold Spring Harb Symp Quant Biol. 2013 doi: 10.1101/sqb.2013.78.019968. [DOI] [PubMed] [Google Scholar]
- 65.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335(26):1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 66.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335(26):1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 67.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365(2):127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bogunovic D, Byun M, Durfee LA, Abhyankar A, Sanal O, Mansouri D, Salem S, Radovanovic I, Grant AV, Adimi P, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337(6102):1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filipe-Santos O, Bustamante J, Haverkamp MH, Vinolo E, Ku CL, Puel A, Frucht DM, Christel K, von Bernuth H, Jouanguy E, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006;203(7):1745–1759. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L, Casanova JL. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol. 2012;24(4):364–378. doi: 10.1016/j.coi.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puel A, Picard C, Ku CL, Smahi A, Casanova JL. Inherited disorders of NF-kappaB-mediated immunity in man. Curr Opin Immunol. 2004;16(1):34–41. doi: 10.1016/j.coi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 72.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clin Microbiol Rev. 2013;24(3):490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salem S, Gros P. Genetic determinants of susceptibility to Mycobacterial infections: IRF8, a new kid on the block. Adv Exp Med Biol. 2013;783:45–80. doi: 10.1007/978-1-4614-6111-1_3. [DOI] [PubMed] [Google Scholar]
- 74.Roos D, de Boer M. Molecular diagnosis of chronic granulomatous disease. Clin Exp Immunol. 2014;175(2):139–149. doi: 10.1111/cei.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salem S, Langlais D, Lefebvre F, Bourque G, Bigley V, Haniffa M, Casanova JL, Burk D, Berghuis A, Butler KM, et al. Functional characterization of the human dendritic cell immunodeficiency associated with the IRF8K108E mutation. Blood. 2014 doi: 10.1182/blood-2014-04-570879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reichenbach J, Rosenzweig S, Doffinger R, Dupuis S, Holland SM, Casanova JL. Mycobacterial diseases in primary immunodeficiencies. Curr Opin Allergy Clin Immunol. 2001;1(6):503–511. doi: 10.1097/00130832-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Boisson-Dupuis S, Bustamante J, Abel L, Casanova JL. Inborn errors of immunity underling tuberculosis in childhood. Immunological Reviews. 2014 in press. [Google Scholar]
- 78.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, Spalding C, Hughes S, Pittaluga S, Raffeld M, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(8):1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, Frucht DM, Vinh DC, Auth RD, Freeman AF, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, Cookson S, Ferozepurwalla Z, Langridge A, Pagan S, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123(6):863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Camargo JF, Lobo SA, Hsu AP, Zerbe CS, Wormser GP, Holland SM. MonoMAC syndrome in a patient with a GATA2 mutation: case report and review of the literature. Clin Infect Dis. 2013;57(5):697–699. doi: 10.1093/cid/cit368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abel L, El-Baghdadi J, Bousfiha AA, Casanova JL, Schurr E. Human genetics of tuberculosis: a long and winding road. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130428. doi: 10.1098/rstb.2013.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jouanguy E, Dupuis S, Pallier A, Doffinger R, Fondaneche MC, Fieschi C, Lamhamedi-Cherradi S, Altare F, Emile JF, Lutz P, et al. In a novel form of IFN-gamma receptor 1 deficiency, cell surface receptors fail to bind IFN-gamma. J Clin Invest. 2000;105(10):1429–1436. doi: 10.1172/JCI9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chantrain CF, Bruwier A, Brichard B, Largent V, Chapgier A, Feinberg J, Casanova JL, Stalens JP, Vermylen C. Successful hematopoietic stem cell transplantation in a child with active disseminated Mycobacterium fortuitum infection and interferon-gamma receptor 1 deficiency. Bone Marrow Transplant. 2006;38(1):75–76. doi: 10.1038/sj.bmt.1705399. [DOI] [PubMed] [Google Scholar]
- 86.Lee WI, Huang JL, Lin TY, Hsueh C, Wong AM, Hsieh MY, Chiu CH, Jaing TH. Chinese patients with defective IL-12/23-interferon-gamma circuit in Taiwan: partial dominant interferon-gamma receptor 1 mutation presenting as cutaneous granuloma and IL-12 receptor beta1 mutation as pneumatocele. J Clin Immunol. 2009;29(2):238–245. doi: 10.1007/s10875-008-9253-9. [DOI] [PubMed] [Google Scholar]
- 87.Edeer Karaca N, Boisson-Dupuis S, Aksu G, Bustamante J, Kandiloglu G, Ozsan N, Hekimgil M, Casanova JL, Kutukculer N. Granulomatous skin lesions, severe scrotal and lower limb edema due to mycobacterial infections in a child with complete IFN-gamma receptor-1 deficiency. Immunotherapy. 2012;4(11):1121–1127. doi: 10.2217/imt.12.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prando C, Boisson-Dupuis S, Grant AV, Kong XF, Bustamante J, Feinberg J, Chapgier A, Rose Y, Janniere L, Rizzardi E, et al. Paternal uniparental isodisomy of chromosome 6 causing a complex syndrome including complete IFN-gamma receptor 1 deficiency. Am J Med Genet A. 2010;152A(3):622–629. doi: 10.1002/ajmg.a.33291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenzweig S, Dorman SE, Roesler J, Palacios J, Zelazko M, Holland SM. 561del4 defines a novel small deletion hotspot in the interferon-gamma receptor 1 chain. Clin Immunol. 2002;102(1):25–27. doi: 10.1006/clim.2001.5135. [DOI] [PubMed] [Google Scholar]
- 90.Marazzi MG, Chapgier A, Defilippi AC, Pistoia V, Mangini S, Savioli C, Dell’Acqua A, Feinberg J, Tortoli E, Casanova JL. Disseminated Mycobacterium scrofulaceum infection in a child with interferon-gamma receptor 1 deficiency. Int J Infect Dis. 2010;14(2):e167–170. doi: 10.1016/j.ijid.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 91.Cunningham JA, Kellner JD, Bridge PJ, Trevenen CL, McLeod DR, Davies HD. Disseminated bacille Calmette-Guerin infection in an infant with a novel deletion in the interferon-gamma receptor gene. Int J Tuberc Lung Dis. 2000;4(8):791–794. [PubMed] [Google Scholar]
- 92.Dupuis S, Doffinger R, Picard C, Fieschi C, Altare F, Jouanguy E, Abel L, Casanova JL. Human interferon-gamma-mediated immunity is a genetically controlled continuous trait that determines the outcome of mycobacterial invasion. Immunol Rev. 2000;178:129–137. doi: 10.1034/j.1600-065x.2000.17810.x. [DOI] [PubMed] [Google Scholar]
- 93.Holland SM, Dorman SE, Kwon A, Pitha-Rowe IF, Frucht DM, Gerstberger SM, Noel GJ, Vesterhus P, Brown MR, Fleisher TA. Abnormal regulation of interferon-gamma, interleukin-12, and tumor necrosis factor-alpha in human interferon-gamma receptor 1 deficiency. J Infect Dis. 1998;178(4):1095–1104. doi: 10.1086/515670. [DOI] [PubMed] [Google Scholar]
- 94.Vesterhus P, Holland SM, Abrahamsen TG, Bjerknes R. Familial disseminated infection due to atypical mycobacteria with childhood onset. Clin Infect Dis. 1998;27(4):822–825. doi: 10.1086/514939. [DOI] [PubMed] [Google Scholar]
- 95.Pierre-Audigier C, Jouanguy E, Lamhamedi S, Altare F, Rauzier J, Vincent V, Canioni D, Emile JF, Fischer A, Blanche S, et al. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin Infect Dis. 1997;24(5):982–984. doi: 10.1093/clinids/24.5.982. [DOI] [PubMed] [Google Scholar]
- 96.Koscielniak E, de Boer T, Dupuis S, Naumann L, Casanova JL, Ottenhoff TH. Disseminated Mycobacterium peregrinum infection in a child with complete interferon-gamma receptor-1 deficiency. Pediatr Infect Dis J. 2003;22(4):378–380. [PubMed] [Google Scholar]
- 97.Noordzij JG, Hartwig NG, Verreck FA, De Bruin-Versteeg S, De Boer T, Van Dissel JT, De Groot R, Ottenhoff TH, Van Dongen JJ. Two patients with complete defects in interferon gamma receptor-dependent signaling. J Clin Immunol. 2007;27(5):490–496. doi: 10.1007/s10875-007-9097-8. [DOI] [PubMed] [Google Scholar]
- 98.Tsolia MN, Chapgier A, Taprantzi P, Servitzoglou M, Tassios I, Spyridis N, Papageorgiou F, Santos OF, Casanova JL, Spyridis P. Disseminated nontuberculous mycobacterial infection in a child with interferon-gamma receptor 1 deficiency. Eur J Pediatr. 2006;165(7):458–461. doi: 10.1007/s00431-006-0110-7. [DOI] [PubMed] [Google Scholar]
- 99.Wang Q, Xia W, Zhao D. Interferon-gamma receptor 1 deficiency in a 19-month-old child: case report and literature review. Zhonghua Er Ke Za Zhi. 2014;52(5):387–391. [PubMed] [Google Scholar]
- 100.de Groot R, van Dongen JJ, Neijens HJ, Hooijkaas H, Drexhage HA. Familial disseminated atypical mycobacterial infection in childhood. Lancet. 1995;345(8955):993. doi: 10.1016/s0140-6736(95)90744-0. [DOI] [PubMed] [Google Scholar]
- 101.Altare F, Jouanguy E, Lamhamedi S, Doffinger R, Fischer A, Casanova JL. Mendelian susceptibility to mycobacterial infection in man. Curr Opin Immunol. 1998;10(4):413–417. doi: 10.1016/s0952-7915(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 102.Feinberg J, Fieschi C, Doffinger R, Feinberg M, Leclerc T, Boisson-Dupuis S, Picard C, Bustamante J, Chapgier A, Filipe-Santos O, et al. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur J Immunol. 2004;34(11):3276–3284. doi: 10.1002/eji.200425221. [DOI] [PubMed] [Google Scholar]
- 103.Costa-Pereira AP, Hermanns HM, Is’harc H, Williams TM, Watling D, Arulampalam V, Newman SJ, Heinrich PC, Kerr IM. Signaling through a mutant IFN-gamma receptor. J Immunol. 2005;175(9):5958–5965. doi: 10.4049/jimmunol.175.9.5958. [DOI] [PubMed] [Google Scholar]
- 104.Fieschi C, Dupuis S, Picard C, Smith CI, Holland SM, Casanova JL. High levels of interferon gamma in the plasma of children with complete interferon gamma receptor deficiency. Pediatrics. 2001;107(4):E48. doi: 10.1542/peds.107.4.e48. [DOI] [PubMed] [Google Scholar]
- 105.Emile JF, Patey N, Altare F, Lamhamedi S, Jouanguy E, Boman F, Quillard J, Lecomte-Houcke M, Verola O, Mousnier JF, et al. Correlation of granuloma structure with clinical outcome defines two types of idiopathic disseminated BCG infection. J Pathol. 1997;181(1):25–30. doi: 10.1002/(SICI)1096-9896(199701)181:1<25::AID-PATH747>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 106.Ward CM, Jyonouchi H, Kotenko SV, Smirnov SV, Patel R, Aguila H, McSherry G, Dashefsky B, Holland SM. Adjunctive treatment of disseminated Mycobacterium avium complex infection with interferon alpha-2b in a patient with complete interferon-gamma receptor R1 deficiency. Eur J Pediatr. 2007;166(9):981–985. doi: 10.1007/s00431-006-0339-1. [DOI] [PubMed] [Google Scholar]
- 107.Bax HI, Freeman AF, Ding L, Hsu AP, Marciano B, Kristosturyan E, Jancel T, Spalding C, Pechacek J, Olivier KN, et al. Interferon alpha treatment of patients with impaired interferon gamma signaling. J Clin Immunol. 2013;33(5):991–1001. doi: 10.1007/s10875-013-9882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van de Wetering D, van Wengen A, Savage ND, van de Vosse E, van Dissel JT. IFN-alpha cannot substitute lack of IFN-gamma responsiveness in cells of an IFN-gammaR1 deficient patient. Clin Immunol. 2011;138(3):282–290. doi: 10.1016/j.clim.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Nguyen KB, Cousens LP, Doughty LA, Pien GC, Durbin JE, Biron CA. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat Immunol. 2000;1(1):70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 110.Bouchonnet F, Boechat N, Bonay M, Hance AJ. Alpha/beta interferon impairs the ability of human macrophages to control growth of Mycobacterium bovis BCG. Infect Immun. 2002;70(6):3020–3025. doi: 10.1128/IAI.70.6.3020-3025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roesler J, Horwitz ME, Picard C, Bordigoni P, Davies G, Koscielniak E, Levin M, Veys P, Reuter U, Schulz A, et al. Hematopoietic stem cell transplantation for complete IFN-gamma receptor 1 deficiency: a multi-institutional survey. J Pediatr. 2004;145(6):806–812. doi: 10.1016/j.jpeds.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 112.Horwitz ME, Uzel G, Linton GF, Miller JA, Brown MR, Malech HL, Holland SM. Persistent Mycobacterium avium infection following nonmyeloablative allogeneic peripheral blood stem cell transplantation for interferon-gamma receptor-1 deficiency. Blood. 2003;102(7):2692–2694. doi: 10.1182/blood-2003-04-1268. [DOI] [PubMed] [Google Scholar]
- 113.Reuter U, Roesler J, Thiede C, Schulz A, Classen CF, Oelschlagel U, Debatin KM, Friedrich W. Correction of complete interferon-gamma receptor 1 deficiency by bone marrow transplantation. Blood. 2002;100(12):4234–4235. doi: 10.1182/blood-2002-02-0433. [DOI] [PubMed] [Google Scholar]
- 114.Rottman M, Soudais C, Vogt G, Renia L, Emile JF, Decaluwe H, Gaillard JL, Casanova JL. IFN-gamma mediates the rejection of haematopoietic stem cells in IFN-gammaR1-deficient hosts. PLoS Med. 2008;5(1):e26. doi: 10.1371/journal.pmed.0050026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Remiszewski P, Roszkowska-Sliz B, Winek J, Chapgier A, Feinberg J, Langfort R, Bestry I, Augustynowicz-Kopec E, Ptak J, Casanova JL, et al. Disseminated Mycobacterium avium infection in a 20-year-old female with partial recessive IFNgammaR1 deficiency. Respiration. 2006;73(3):375–378. doi: 10.1159/000088682. [DOI] [PubMed] [Google Scholar]
- 116.Allende LM, Lopez-Goyanes A, Paz-Artal E, Corell A, Garcia-Perez MA, Varela P, Scarpellini A, Negreira S, Palenque E, Arnaiz-Villena A. A point mutation in a domain of gamma interferon receptor 1 provokes severe immunodeficiency. Clin Diagn Lab Immunol. 2001;8(1):133–137. doi: 10.1128/CDLI.8.1.133-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holland SM. Treatment of infections in the patient with Mendelian susceptibility to mycobacterial infection. Microbes Infect. 2000;2(13):1579–1590. doi: 10.1016/s1286-4579(00)01314-9. [DOI] [PubMed] [Google Scholar]
- 118.Kong XF, Vogt G, Chapgier A, Lamaze C, Bustamante J, Prando C, Fortin A, Puel A, Feinberg J, Zhang XX, et al. A novel form of cell type-specific partial IFN-gammaR1 deficiency caused by a germ line mutation of the IFNGR1 initiation codon. Hum Mol Genet. 2010;19(3):434–444. doi: 10.1093/hmg/ddp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moilanen P, Korppi M, Hovi L, Chapgier A, Feinberg J, Kong XF, Boisson-Dupuis S, Arola M, Casanova JL, Saarinen-Pihkala UM. Successful hematopoietic stem cell transplantation from an unrelated donor in a child with interferon gamma receptor deficiency. Pediatr Infect Dis J. 2009;28(7):658–660. doi: 10.1097/INF.0b013e318195092e. [DOI] [PubMed] [Google Scholar]
- 120.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche MC, Dupuis S, Doffinger R, Altare F, Girdlestone J, Emile JF, et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet. 1999;21(4):370–378. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 121.Okada S, Ishikawa N, Shirao K, Kawaguchi H, Tsumura M, Ohno Y, Yasunaga S, Ohtsubo M, Takihara Y, Kobayashi M. The novel IFNGR1 mutation 774del4 produces a truncated form of interferon-gamma receptor 1 and has a dominant-negative effect on interferon-gamma signal transduction. J Med Genet. 2007;44(8):485–491. doi: 10.1136/jmg.2007.049635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arend SM, Janssen R, Gosen JJ, Waanders H, de Boer T, Ottenhoff TH, van Dissel JT. Multifocal osteomyelitis caused by nontuberculous mycobacteria in patients with a genetic defect of the interferon-gamma receptor. Neth J Med. 2001;59(3):140–151. doi: 10.1016/s0300-2977(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 123.Raszka WV, Jr, Trinh TT, Zawadsky PM. Multifocal M. intracellulare osteomyelitis in an immunocompetent child. Clin Pediatr (Phila) 1994;33(10):611–616. doi: 10.1177/000992289403301007. [DOI] [PubMed] [Google Scholar]
- 124.Waibel KH, Regis DP, Uzel G, Rosenzweig SD, Holland SM. Fever and leg pain in a 42-month-old. Ann Allergy Asthma Immunol. 2002;89(3):239–243. doi: 10.1016/S1081-1206(10)61949-7. [DOI] [PubMed] [Google Scholar]
- 125.Villella A, Picard C, Jouanguy E, Dupuis S, Popko S, Abughali N, Meyerson H, Casanova JL, Hostoffer RW. Recurrent Mycobacterium avium osteomyelitis associated with a novel dominant interferon gamma receptor mutation. Pediatrics. 2001;107(4):E47. doi: 10.1542/peds.107.4.e47. [DOI] [PubMed] [Google Scholar]
- 126.Sasaki Y, Nomura A, Kusuhara K, Takada H, Ahmed S, Obinata K, Hamada K, Okimoto Y, Hara T. Genetic basis of patients with bacille Calmette-Guerin osteomyelitis in Japan: identification of dominant partial interferon-gamma receptor 1 deficiency as a predominant type. J Infect Dis. 2002;185(5):706–709. doi: 10.1086/339011. [DOI] [PubMed] [Google Scholar]
- 127.Hoshina T, Takada H, Sasaki-Mihara Y, Kusuhara K, Ohshima K, Okada S, Kobayashi M, Ohara O, Hara T. Clinical and host genetic characteristics of Mendelian susceptibility to mycobacterial diseases in Japan. J Clin Immunol. 2011;31(3):309–314. doi: 10.1007/s10875-010-9498-y. [DOI] [PubMed] [Google Scholar]
- 128.Obinata K, Lee T, Niizuma T, Kinoshita K, Shimizu T, Hoshina T, Sasaki Y, Hara T. Two cases of partial dominant interferon-gamma receptor 1 deficiency that presented with different clinical courses of bacille Calmette-Guerin multiple osteomyelitis. J Infect Chemother. 2013;19(4):757–760. doi: 10.1007/s10156-012-0493-5. [DOI] [PubMed] [Google Scholar]
- 129.Rose DM, Atkins J, Holland SM, Infante AJ. A novel mutation in IFN-gamma receptor 1 presenting as multisystem Mycobacterium intracellulare infection. J Allergy Clin Immunol. 2014;133(2):591–592. doi: 10.1016/j.jaci.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 130.Storgaard M, Varming K, Herlin T, Obel N. Novel mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64(2):137–139. doi: 10.1111/j.1365-3083.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- 131.Glosli H, Stray-Pedersen A, Brun AC, Holtmon LW, Tonjum T, Chapgier A, Casanova JL, Abrahamsen TG. Infections due to various atypical mycobacteria in a Norwegian multiplex family with dominant interferon-gamma receptor deficiency. Clin Infect Dis. 2008;46(3):e23–27. doi: 10.1086/525855. [DOI] [PubMed] [Google Scholar]
- 132.Pac M, Bustamante J, Buda P, Wiesteska-Klimczar A, Ziolkowki J, Krasinska M, Lipka B, Klaudel-Dreszler M, Casanova JL, Piatosa B, et al. Disseminated Mycobacterium tuberculosis complex infection in a girl with partial dominant IFN-g receptor 1 deficiency. Centr Eur J Immunol. 2012;37(4):378–381. [Google Scholar]
- 133.Han JY, Rosenzweig SD, Church JA, Holland SM, Ross LA. Variable presentation of disseminated nontuberculous mycobacterial infections in a family with an interferon-gamma receptor mutation. Clin Infect Dis. 2004;39(6):868–870. doi: 10.1086/423804. [DOI] [PubMed] [Google Scholar]
- 134.Takeda K, Kawai T, Nakazawa Y, Komuro H, Shoji K, Morita K, Katsuta T, Yamamoto M, Miyairi I, Ohya Y, et al. Augmentation of antitubercular therapy with IFNgamma in a patient with dominant partial IFNgamma receptor 1 deficiency. Clin Immunol. 2014;151(1):25–28. doi: 10.1016/j.clim.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 135.Auld B, Urquhart D, Walsh M, Nourse C, Harris MA. Blurring the lines in interferon {gamma} receptor deficiency: an infant with near-fatal airway disease. Pediatrics. 2011;127(5):e1352–1355. doi: 10.1542/peds.2010-0387. [DOI] [PubMed] [Google Scholar]
- 136.Janssen R, Van Wengen A, Verhard E, De Boer T, Zomerdijk T, Ottenhoff TH, Van Dissel JT. Divergent role for TNF-alpha in IFN-gamma-induced killing of Toxoplasma gondii and Salmonella typhimurium contributes to selective susceptibility of patients with partial IFN-gamma receptor 1 deficiency. J Immunol. 2002;169(7):3900–3907. doi: 10.4049/jimmunol.169.7.3900. [DOI] [PubMed] [Google Scholar]
- 137.Muszlak M, Chapgier A, Barry Harivelo R, Castella C, Cremades F, Goulois E, Laporte R, Casanova JL, Ranaivoarivony V, Hebert JC, et al. Multifocal infection due to Mycobacterium intracellulare: first case of interferon gamma receptor partial dominant deficiency in tropical French territory. Arch Pediatr. 2007;14(3):270–272. doi: 10.1016/j.arcped.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 138.Edgar JD, Smyth AE, Pritchard J, Lammas D, Jouanguy E, Hague R, Novelli V, Dempsey S, Sweeney L, Taggart AJ, et al. Interferon-gamma receptor deficiency mimicking Langerhans’ cell histiocytosis. J Pediatr. 2001;139(4):600–603. doi: 10.1067/mpd.2001.117068. [DOI] [PubMed] [Google Scholar]
- 139.Holland SM. Immunotherapy of mycobacterial infections. Semin Respir Infect. 2001;16(1):47–59. doi: 10.1053/srin.2001.22728. [DOI] [PubMed] [Google Scholar]
- 140.Vogt G, Bustamante J, Chapgier A, Feinberg J, Boisson Dupuis S, Picard C, Mahlaoui N, Gineau L, Alcais A, Lamaze C, et al. Complementation of a pathogenic IFNGR2 misfolding mutation with modifiers of N-glycosylation. J Exp Med. 2008;205(8):1729–1737. doi: 10.1084/jem.20071987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vogt G, Chapgier A, Yang K, Chuzhanova N, Feinberg J, Fieschi C, Boisson-Dupuis S, Alcais A, Filipe-Santos O, Bustamante J, et al. Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat Genet. 2005;37(7):692–700. doi: 10.1038/ng1581. [DOI] [PubMed] [Google Scholar]
- 142.Kong XF, Vogt G, Itan Y, Macura-Biegun A, Szaflarska A, Kowalczyk D, Chapgier A, Abhyankar A, Furthner D, Djambas Khayat C, et al. Haploinsufficiency at the human IFNGR2 locus contributes to mycobacterial disease. Hum Mol Genet. 2013;22(4):769–781. doi: 10.1093/hmg/dds484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rosenzweig SD, Dorman SE, Uzel G, Shaw S, Scurlock A, Brown MR, Buckley RH, Holland SM. A novel mutation in IFN-gamma receptor 2 with dominant negative activity: biological consequences of homozygous and heterozygous states. J Immunol. 2004;173(6):4000–4008. doi: 10.4049/jimmunol.173.6.4000. [DOI] [PubMed] [Google Scholar]
- 144.Furthner D, Mühleder J, Eitelberger F, Ritcher E, Engelhorn C, Belohradsky BH, Casanova JL. Neu beschriebener homozygoter Interferon-g-Rezeptor-2-Defkt. Chronisch multifokale Osteomyelitis durch Mycobacterium avium. Monatsschr Kinderheilkd. 2007;155:S58–S61. [Google Scholar]
- 145.Martinez-Barricarte R, Megged O, Stepensky P, Casimir P, Moncada-Velez M, Averbuch D, Assous MV, Abuzaitoun O, Kong XF, Pedergnana V, et al. Mycobacterium simiae infection in two unrelated patients with different forms of inherited IFN-gR2 deficiency. J Clin Immunol. 2014 doi: 10.1007/s10875-014-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vogt G, Vogt B, Chuzhanova N, Julenius K, Cooper DN, Casanova JL. Gain-of-glycosylation mutations. Curr Opin Genet Dev. 2007;17(3):245–251. doi: 10.1016/j.gde.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 147.Rosenzweig SD, Schwartz OM, Brown MR, Leto TL, Holland SM. Characterization of a dipeptide motif regulating IFN-gamma receptor 2 plasma membrane accumulation and IFN-gamma responsiveness. J Immunol. 2004;173(6):3991–3999. doi: 10.4049/jimmunol.173.6.3991. [DOI] [PubMed] [Google Scholar]
- 148.Kilic SS, van Wengen A, de Paus RA, Celebi S, Meziane B, Hafizoglu D, van Dissel JT, van de Vosse E. Severe disseminated mycobacterial infection in a boy with a novel mutation leading to IFN-gammaR2 deficiency. J Infect. 2012;65(6):568–572. doi: 10.1016/j.jinf.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 149.Moncada-Velez M, Martinez-Barricarte R, Bogunovic D, Kong XF, Blancas-Galicia L, Tirpan C, Aksu G, Vincent QB, Boisson B, Itan Y, et al. Partial IFN-gammaR2 deficiency is due to protein misfolding and can be rescued by inhibitors of glycosylation. Blood. 2013;122(14):2390–2401. doi: 10.1182/blood-2013-01-480814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Doffinger R, Jouanguy E, Dupuis S, Fondaneche MC, Stephan JL, Emile JF, Lamhamedi-Cherradi S, Altare F, Pallier A, Barcenas-Morales G, et al. Partial interferon-gamma receptor signaling chain deficiency in a patient with bacille Calmette-Guerin and Mycobacterium abscessus infection. J Infect Dis. 2000;181(1):379–384. doi: 10.1086/315197. [DOI] [PubMed] [Google Scholar]
- 151.de Paus RA, Kilic SS, van Dissel JT, van de Vosse E. Effect of amino acid substitutions in the human IFN-gammaR2 on IFN-gamma responsiveness. Genes Immun. 2011;12(2):136–144. doi: 10.1038/gene.2010.74. [DOI] [PubMed] [Google Scholar]
- 152.Kong XF, Bousfiha A, Rouissi A, Itan Y, Abhyankar A, Bryant V, Okada S, Ailal F, Bustamante J, Casanova JL, et al. A novel homozygous p. R1105X mutation of the AP4E1 gene in twins with hereditary spastic paraplegia and mycobacterial disease. PLoS One. 2013;8(3):e58286. doi: 10.1371/journal.pone.0058286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rieux-Laucat F, Casanova JL. Autoimmunity by haploinsufficiency. Science. 2014 doi: 10.1126/science.1260791. in press. [DOI] [PubMed] [Google Scholar]
- 154.Chapgier A, Wynn RF, Jouanguy E, Filipe-Santos O, Zhang S, Feinberg J, Hawkins K, Casanova JL, Arkwright PD. Human complete Stat-1 deficiency is associated with defective type I and II IFN responses in vitro but immunity to some low virulence viruses in vivo. J Immunol. 2006;176(8):5078–5083. doi: 10.4049/jimmunol.176.8.5078. [DOI] [PubMed] [Google Scholar]
- 155.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33(3):388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 156.Vairo D, Tassone L, Tabellini G, Tamassia N, Gasperini S, Bazzoni F, Plebani A, Porta F, Notarangelo LD, Parolini S, et al. Severe impairment of IFN-gamma and IFN-alpha responses in cells of a patient with a novel STAT1 splicing mutation. Blood. 2011;118(7):1806–1817. doi: 10.1182/blood-2011-01-330571. [DOI] [PubMed] [Google Scholar]
- 157.Chapgier A, Kong XF, Boisson-Dupuis S, Jouanguy E, Averbuch D, Feinberg J, Zhang SY, Bustamante J, Vogt G, Lejeune J, et al. A partial form of recessive STAT1 deficiency in humans. J Clin Invest. 2009;119(6):1502–1514. doi: 10.1172/JCI37083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Averbuch D, Chapgier A, Boisson-Dupuis S, Casanova JL, Engelhard D. The clinical spectrum of patients with deficiency of Signal Transducer and Activator of Transcription-1. Pediatr Infect Dis J. 2011;30(4):352–355. doi: 10.1097/INF.0b013e3181fdff4a. [DOI] [PubMed] [Google Scholar]
- 159.Kong XF, Ciancanelli M, Al-Hajjar S, Alsina L, Zumwalt T, Bustamante J, Feinberg J, Audry M, Prando C, Bryant V, et al. A novel form of human STAT1 deficiency impairing early but not late responses to interferons. Blood. 2010;116(26):5895–5906. doi: 10.1182/blood-2010-04-280586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kristensen IA, Veirum JE, Moller BK, Christiansen M. Novel STAT1 alleles in a patient with impaired resistance to mycobacteria. J Clin Immunol. 2011;31(2):265–271. doi: 10.1007/s10875-010-9480-8. [DOI] [PubMed] [Google Scholar]
- 161.Shamriz O, Engelhard D, Rajs AP, Kaidar-Shwartz H, Casanova JL, Averbuch D. Mycobacterium szulgai chronic multifocal osteomyelitis in an adolescent with inherited STAT1 deficiency. Pediatr Infect Dis J. 2013;32(12):1345–1347. doi: 10.1097/01.inf.0000437067.43859.4c. [DOI] [PubMed] [Google Scholar]
- 162.Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, Holland SM, Schreiber RD, Casanova JL. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 2001;293(5528):300–303. doi: 10.1126/science.1061154. [DOI] [PubMed] [Google Scholar]
- 163.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208(8):1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36(4):515–528. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365(1):54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 166.Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, Chandrasekaran P, Rosen LB, Carvalho DS, Ding L, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol. 2013;131(6):1624–1634. doi: 10.1016/j.jaci.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kumar N, Hanks ME, Chandrasekaran P, Davis BC, Hsu AP, Van Wagoner NJ, Merlin JS, Spalding C, La Hoz RM, Holland SM, et al. Gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation-related primary immunodeficiency is associated with disseminated mucormycosis. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee PP, Mao H, Yang W, Chan KW, Ho MH, Lee TL, Chan JF, Woo PC, Tu W, Lau YL. Penicillium marneffei infection and impaired IFN-gamma immunity in humans with autosomal-dominant gain-of-phosphorylation STAT1 mutations. J Allergy Clin Immunol. 2014;133(3):894–896. e895. doi: 10.1016/j.jaci.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 169.Hori T, Ohnishi H, Teramoto T, Tsubouchi K, Naiki T, Hirose Y, Ohara O, Seishima M, Kaneko H, Fukao T, et al. Autosomal-dominant chronic mucocutaneous candidiasis with STAT1-mutation can be complicated with chronic active hepatitis and hypothyroidism. J Clin Immunol. 2012;32(6):1213–1220. doi: 10.1007/s10875-012-9744-6. [DOI] [PubMed] [Google Scholar]
- 170.Toth B, Mehes L, Tasko S, Szalai Z, Tulassay Z, Cypowyj S, Casanova JL, Puel A, Marodi L. Herpes in STAT1 gain-of-function mutation [corrected] Lancet. 2012;379(9835):2500. doi: 10.1016/S0140-6736(12)60365-1. [DOI] [PubMed] [Google Scholar]
- 171.Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, Noel RJ, Verbsky JW, Freeman AF, Janssen E, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131(6):1611–1623. doi: 10.1016/j.jaci.2012.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Takezaki S, Yamada M, Kato M, Park MJ, Maruyama K, Yamazaki Y, Chida N, Ohara O, Kobayashi I, Ariga T. Chronic mucocutaneous candidiasis caused by a gain-of-function mutation in the STAT1 DNA-binding domain. J Immunol. 2012;189(3):1521–1526. doi: 10.4049/jimmunol.1200926. [DOI] [PubMed] [Google Scholar]
- 173.Aldave JC, Cachay E, Nunez L, Chunga A, Murillo S, Cypowyj S, Bustamante J, Puel A, Casanova JL, Koo A. A 1-year-old girl with a gain-of-function STAT1 mutation treated with hematopoietic stem cell transplantation. J Clin Immunol. 2013;33(8):1273–1275. doi: 10.1007/s10875-013-9947-5. [DOI] [PubMed] [Google Scholar]
- 174.Wang WB, Levy DE, Lee CK. STAT3 negatively regulates type I IFN-mediated antiviral response. J Immunol. 2013;187(5):2578–2585. doi: 10.4049/jimmunol.1004128. [DOI] [PubMed] [Google Scholar]
- 175.Soltesz B, Toth B, Shabashova N, Bondarenko A, Okada S, Cypowyj S, Abhyankar A, Csorba G, Tasko S, Sarkadi AK, et al. New and recurrent gain-of-function STAT1 mutations in patients with chronic mucocutaneous candidiasis from Eastern and Central Europe. J Med Genet. 2013;50(9):567–578. doi: 10.1136/jmedgenet-2013-101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mizoguchi Y, Tsumura M, Okada S, Hirata O, Minegishi S, Imai K, Hyakuna N, Muramatsu H, Kojima S, Ozaki Y, et al. Simple diagnosis of STAT1 gain-of-function alleles in patients with chronic mucocutaneous candidiasis. J Leukoc Biol. 2014;95(4):667–676. doi: 10.1189/jlb.0513250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Frans G, Moens L, Schaballie H, Van Eyck L, Borgers H, Wuyts M, Dillaerts D, Vermeulen E, Dooley J, Grimbacher B, et al. Gain-of-function mutations in signal transducer and activator of transcription 1 (STAT1): Chronic mucocutaneous candidiasis accompanied by enamel defects and delayed dental shedding. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wildbaum G, Shahar E, Katz R, Karin N, Etzioni A, Pollack S. Continuous G-CSF therapy for isolated chronic mucocutaneous candidiasis: complete clinical remission with restoration of IL-17 secretion. J Allergy Clin Immunol. 2013;132(3):761–764. doi: 10.1016/j.jaci.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 179.Smeekens SP, Plantinga TS, van de Veerdonk FL, Heinhuis B, Hoischen A, Joosten LA, Arkwright PD, Gennery A, Kullberg BJ, Veltman JA, et al. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS One. 2011;6(12):e29248. doi: 10.1371/journal.pone.0029248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Romberg N, Morbach H, Lawrence MG, Kim S, Kang I, Holland SM, Milner JD, Meffre E. Gain-of-function STAT1 mutations are associated with PD-L1 overexpression and a defect in B-cell survival. J Allergy Clin Immunol. 2013;131(6):1691–1693. doi: 10.1016/j.jaci.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mekki N, Ben-Mustapha I, Liu L, Boussofara L, Okada S, Cypowyj S, Ghariani N, Saidi W, Denguezli M, Casanova JL, et al. IL-17 T cells’ defective differentiation in vitro despite normal range ex vivo in chronic mucocutaneous candidiasis due to STAT1 mutation. J Invest Dermatol. 2014;134(4):1155–1157. doi: 10.1038/jid.2013.480. [DOI] [PubMed] [Google Scholar]
- 182.Al Rushood M, McCusker C, Mazer B, Alizadehfar R, Grimbacher B, Depner M, Ben-Shoshan M. Autosomal dominant cases of chronic mucocutaneous candidiasis segregates with mutations of signal transducer and activator of transcription 1, but not of Toll-like receptor 3. J Pediatr. 2013;163(1):277–279. doi: 10.1016/j.jpeds.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 183.Sharfe N, Nahum A, Newell A, Dadi H, Ngan B, Pereira SL, Herbrick JA, Roifman CM. Fatal combined immunodeficiency associated with heterozygous mutation in STAT1. J Allergy Clin Immunol. 2014;133(3):807–817. doi: 10.1016/j.jaci.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 184.Hirata O, Okada S, Tsumura M, Kagawa R, Miki M, Kawaguchi H, Nakamura K, Boisson-Dupuis S, Casanova JL, Takihara Y, et al. Heterozygosity for the Y701C STAT1 mutation in a multiplex kindred with multifocal osteomyelitis. Haematologica. 2013;98(10):1641–1649. doi: 10.3324/haematol.2013.083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tsumura M, Okada S, Sakai H, Yasunaga S, Ohtsubo M, Murata T, Obata H, Yasumi T, Kong XF, Abhyankar A, et al. Dominant-negative STAT1 SH2 domain mutations in unrelated patients with Mendelian susceptibility to mycobacterial disease. Hum Mutat. 2012;33(9):1377–1387. doi: 10.1002/humu.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sampaio EP, Bax HI, Hsu AP, Kristosturyan E, Pechacek J, Chandrasekaran P, Paulson ML, Dias DL, Spalding C, Uzel G, et al. A novel STAT1 mutation associated with disseminated mycobacterial disease. J Clin Immunol. 2012;32(4):681–689. doi: 10.1007/s10875-012-9659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Horvath CM, Darnell JE., Jr The antiviral state induced by alpha interferon and gamma interferon requires transcriptionally active Stat1 protein. J Virol. 1996;70(1):647–650. doi: 10.1128/jvi.70.1.647-650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 189.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280(5368):1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 190.Jirapongsananuruk O, Luangwedchakarn V, Niemela JE, Pacharn P, Visitsunthorn N, Thepthai C, Vichyanond P, Piboonpocanun S, Fleisher TA. Cryptococcal osteomyelitis in a child with a novel compound mutation of the IL12RB1 gene. Asian Pac J Allergy Immunol. 2012;30(1):79–82. [PubMed] [Google Scholar]
- 191.van de Vosse E, Haverkamp MH, Ramirez-Alejo N, Martinez-Gallo M, Blancas-Galicia L, Metin A, Garty BZ, Sun-Tan C, Broides A, de Paus RA, et al. IL-12Rbeta1 deficiency: mutation update and description of the IL12RB1 variation database. Hum Mutat. 2013;34(10):1329–1339. doi: 10.1002/humu.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pedraza-Sanchez S, Herrera-Barrios MT, Aldana-Vergara R, Neumann-Ordonez M, Gonzalez-Hernandez Y, Sada-Diaz E, de Beaucoudrey L, Casanova JL, Torres-Rojas M. Bacille Calmette-Guerin infection and disease with fatal outcome associated with a point mutation in the interleukin-12/interleukin-23 receptor beta-1 chain in two Mexican families. Int J Infect Dis. 2010;14 (Suppl 3):e256–260. doi: 10.1016/j.ijid.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 193.Schepers K, Schandene L, Bustamante J, Van Vooren JP, de Suremain M, Casanova JL, Yombi JC, Jacobs F, Mascart F, Goffard JC. IL-12Rbeta1 deficiency and disseminated Mycobacterium tilburgii disease. J Clin Immunol. 2013;33(8):1285–1288. doi: 10.1007/s10875-013-9941-y. [DOI] [PubMed] [Google Scholar]
- 194.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med. 2003;197(4):527–535. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fieschi C, Bosticardo M, de Beaucoudrey L, Boisson-Dupuis S, Feinberg J, Santos OF, Bustamante J, Levy J, Candotti F, Casanova JL. A novel form of complete IL-12/IL-23 receptor beta1 deficiency with cell surface-expressed nonfunctional receptors. Blood. 2004;104(7):2095–2101. doi: 10.1182/blood-2004-02-0584. [DOI] [PubMed] [Google Scholar]
- 196.van de Vosse E, Ottenhoff TH, de Paus RA, Verhard EM, de Boer T, van Dissel JT, Kuijpers TW. Mycobacterium bovis BCG-itis and cervical lymphadenitis due to Salmonella enteritidis in a patient with complete interleukin-12/-23 receptor beta1 deficiency. Infection. 2010;38(2):128–130. doi: 10.1007/s15010-009-9222-0. [DOI] [PubMed] [Google Scholar]
- 197.Yancoski J, Rocco C, Bernasconi A, Oleastro M, Bezrodnik L, Vratnica C, Haerynck F, Rosenzweig SD. A 475 years-old founder effect involving IL12RB1: a highly prevalent mutation conferring Mendelian Susceptibility to Mycobacterial Diseases in European descendants. Infect Genet Evol. 2009;9(4):574–580. doi: 10.1016/j.meegid.2009.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schejbel L, Rasmussen EM, Kemp HB, Lundstedt AC, Nielsen KR, Obel N, Marquart H, Andersen AB. Combined IL-12 receptor and IgA deficiency in an adult man intestinally infested by an unknown, non-cultivable mycobacterium. Scand J Immunol. 2011;74(6):548–553. doi: 10.1111/j.1365-3083.2011.02603.x. [DOI] [PubMed] [Google Scholar]
- 199.Haerynck F, Holland SM, Rosenzweig SD, Casanova JL, Schelstraete P, De Baets F. Disseminated Mycobacterium avium infection in a patient with a novel mutation in the interleukin-12 receptor-beta1 chain. J Pediatr. 2008;153(5):721–722. doi: 10.1016/j.jpeds.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 200.Casanova JL, Blanche S, Emile JF, Jouanguy E, Lamhamedi S, Altare F, Stephan JL, Bernaudin F, Bordigoni P, Turck D, et al. Idiopathic disseminated bacillus Calmette-Guerin infection: a French national retrospective study. Pediatrics. 1996;98(4 Pt 1):774–778. [PubMed] [Google Scholar]
- 201.Aksu G, Tirpan C, Cavusoglu C, Soydan S, Altare F, Casanova JL, Kutukculer N. Mycobacterium fortuitum-chelonae complex infection in a child with complete interleukin-12 receptor beta 1 deficiency. Pediatr Infect Dis J. 2001;20(5):551–553. doi: 10.1097/00006454-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 202.Filiz S, Kocacik Uygun DF, Verhard EM, van Dissel JT, Uygun V, Bassorgun C, Bingol A, Yegin O, van de Vosse E. Cutaneous leukocytoclastic vasculitis due to Salmonella enteritidis in a child with interleukin-12 receptor beta-1 deficiency. Pediatr Dermatol. 2014;31(2):236–240. doi: 10.1111/j.1525-1470.2012.01856.x. [DOI] [PubMed] [Google Scholar]
- 203.Staretz-Haham O, Melamed R, Lifshitz M, Porat N, Fieschi C, Casanova JL, Levy J. Interleukin-12 receptor beta1 deficiency presenting as recurrent Salmonella infection. Clin Infect Dis. 2003;37(1):137–140. doi: 10.1086/375229. [DOI] [PubMed] [Google Scholar]
- 204.Sakai T, Matsuoka M, Aoki M, Nosaka K, Mitsuya H. Missense mutation of the interleukin-12 receptor beta1 chain-encoding gene is associated with impaired immunity against Mycobacterium avium complex infection. Blood. 2001;97(9):2688–2694. doi: 10.1182/blood.v97.9.2688. [DOI] [PubMed] [Google Scholar]
- 205.Grant AV, Boisson-Dupuis S, Herquelot E, de Beaucoudrey L, Filipe-Santos O, Nolan DK, Feinberg J, Boland A, Al-Muhsen S, Sanal O, et al. Accounting for genetic heterogeneity in homozygosity mapping: application to Mendelian susceptibility to mycobacterial disease. J Med Genet. 2011;48(8):567–571. doi: 10.1136/jmg.2011.089128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gimenez-Sanchez F, Cobos-Carrascosa E, Sanchez-Forte M, Martinez-Lirola M, Lopez-Ruzafa E, Galera-Martinez R, Del Rosal-Babes T, Martinez-Gallo M. Different penetrance of disseminated infections caused by nontuberculous Mycobacteria in Mendelian susceptibility to mycobacterial disease associated with a novel mutation. Pediatr Infect Dis J. 2014;33(3):328–330. doi: 10.1097/INF.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 207.Sanal O, Turul T, De Boer T, Van de Vosse E, Yalcin I, Tezcan I, Sun C, Memis L, Ottenhoff TH, Ersoy F. Presentation of interleukin-12/-23 receptor beta1 deficiency with various clinical symptoms of Salmonella infections. J Clin Immunol. 2006;26(1):1–6. doi: 10.1007/s10875-006-7830-3. [DOI] [PubMed] [Google Scholar]
- 208.Senanayake MP, Kumararatne DS, Doffinger R, Barcenas-Morales G. Disseminated BCG in an infant with interleukin-12 receptor B1 (IL12RB1) deficiency. Paediatr Int Child Health. 2014 doi: 10.1179/2046905514Y.0000000129. 2046905514Y0000000129. [DOI] [PubMed] [Google Scholar]
- 209.Lichtenauer-Kaligis EG, de Boer T, Verreck FA, van Voorden S, Hoeve MA, van de Vosse E, Ersoy F, Tezcan I, van Dissel JT, Sanal O, et al. Severe Mycobacterium bovis BCG infections in a large series of novel IL-12 receptor beta1 deficient patients and evidence for the existence of partial IL-12 receptor beta1 deficiency. Eur J Immunol. 2003;33(1):59–69. doi: 10.1002/immu.200390008. [DOI] [PubMed] [Google Scholar]
- 210.Gruenberg DA, Anover-Sombke S, Gern JE, Holland SM, Rosenzweig SD, Torgerson TR, Seroogy CM. Atypical presentation of IL-12 receptor beta1 deficiency with pneumococcal sepsis and disseminated nontuberculous mycobacterial infection in a 19-month-old girl born to nonconsanguineous US residents. J Allergy Clin Immunol. 2010;125(1):264–265. doi: 10.1016/j.jaci.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ehlayel M, de Beaucoudrey L, Fike F, Nahas SA, Feinberg J, Casanova JL, Gatti RA. Simultaneous presentation of 2 rare hereditary immunodeficiencies: IL-12 receptor beta1 deficiency and ataxia-telangiectasia. J Allergy Clin Immunol. 2008;122(6):1217–1219. doi: 10.1016/j.jaci.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 212.Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, Tran MV, Blankenship D, Pascual V, Savino DA, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121(17):3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, Arkwright PD, Kreins AY, Averbuch D, Engelhard D, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119(17):3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tassone L, Carvalho AC, Calabresi A, Tortoli E, Apostoli A, Scomodon O, Spina C, Vairo D, Villanacci V, Matteelli A, et al. Disseminated Mycobacterium genavense infection after immunosuppressive therapy shows underlying new composite heterozygous mutations of beta1 subunit of IL-12 receptor gene. J Allergy Clin Immunol. 2013;131(2):607–610. doi: 10.1016/j.jaci.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 215.Darleguy A, Bost-Bru C, Pagnier A, Plantaz D, Piolat C, Nugues F, Picard C. Mendelian susceptibility to mycobacterial disease: a case report of disseminated infection due to Mycobacterium avium. Arch Pediatr. 2013;20(7):758–761. doi: 10.1016/j.arcped.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 216.Elloumi-Zghal H, Barbouche MR, Chemli J, Bejaoui M, Harbi A, Snoussi N, Abdelhak S, Dellagi K. Clinical and genetic heterogeneity of inherited autosomal recessive susceptibility to disseminated Mycobacterium bovis bacille calmette-guerin infection. J Infect Dis. 2002;185(10):1468–1475. doi: 10.1086/340510. [DOI] [PubMed] [Google Scholar]
- 217.Diniz LM, Guimaraes T, de Oliveira M, Pinto JA, de Miranda SS. Lymphadenitis caused by infection with an isoniazid- and rifampin-resistant strain of Mycobacterium bovis BCG in an infant with IFN-gamma/IL-12 pathway defect. J Bras Pneumol. 2014;40(2):188–192. doi: 10.1590/S1806-37132014000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tuerlinckx D, Vermylen C, Brichard B, Ninane J, Cornu G. Disseminated Mycobacterium avium infection in a child with decreased tumour necrosis factor production. Eur J Pediatr. 1997;156(3):204–206. doi: 10.1007/s004310050583. [DOI] [PubMed] [Google Scholar]
- 219.Asilsoy S, Bilgili G, Turul T, Dizdarer C, Kalkan S, Yasli H, Can D, Genel F, Sanal O. Interleukin-12/-23 receptor beta 1 deficiency in an infant with draining BCG lymphadenitis. Pediatr Int. 2009;51(2):310–312. doi: 10.1111/j.1442-200X.2009.02818.x. [DOI] [PubMed] [Google Scholar]
- 220.Kutukculer N, Genel F, Aksu G, Karapinar B, Ozturk C, Cavusoglu C, Casanova JL, Fieschi C. Cutaneous leukocytoclastic vasculitis in a child with interleukin-12 receptor beta-1 deficiency. J Pediatr. 2006;148(3):407–409. doi: 10.1016/j.jpeds.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 221.Scheuerman O, de Beaucoudrey L, Hoffer V, Feinberg J, Casanova JL, Garty BZ. Mycobacterial disease in a child with surface-expressed non-functional interleukin-12Rbeta1 chains. Isr Med Assoc J. 2007;9(7):560–561. [PubMed] [Google Scholar]
- 222.Tanir G, Dogu F, Tuygun N, Ikinciogullari A, Aytekin C, Aydemir C, Yuksek M, Boduroglu EC, de Beaucoudrey L, Fieschi C, et al. Complete deficiency of the IL-12 receptor beta1 chain: three unrelated Turkish children with unusual clinical features. Eur J Pediatr. 2006;165(6):415–417. doi: 10.1007/s00431-005-0078-8. [DOI] [PubMed] [Google Scholar]
- 223.Sanal O, Morgan G, Gocmen A, Novelli V, Klein N, Tezcan I, Ersoy F, Berkel AI, Yel L. Isolated cutaneous response to granulocyte-monocyte colony stimulating factor in fatal idiopathic disseminated Bacillus-Calmette-Guerin infection. Eur J Pediatr. 2000;159(3):149–152. doi: 10.1007/s004310050039. [DOI] [PubMed] [Google Scholar]
- 224.Rosenzweig SD, Yancoski J, Bernasconi A, Krasovec S, Marciano BE, Casimir L, Berberian G, Simboli N, Rousseau M, Calle G. Thirteen years of culture-positive M. bovis-BCG infection in an IL-12Rbeta1 deficient patient: treatment and outcome. J Infect. 2006;52(3):e69–72. doi: 10.1016/j.jinf.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 225.Guia S, Cognet C, de Beaucoudrey L, Tessmer MS, Jouanguy E, Berger C, Filipe-Santos O, Feinberg J, Camcioglu Y, Levy J, et al. A role for interleukin-12/23 in the maturation of human natural killer and CD56+ T cells in vivo. Blood. 2008;111(10):5008–5016. doi: 10.1182/blood-2007-11-122259. [DOI] [PubMed] [Google Scholar]
- 226.de Boer T, van Dissel JT, Kuijpers TW, Rimmelzwaan GF, Kroon FP, Ottenhoff TH. Influenza virus vaccination induces interleukin-12/23 receptor beta 1 (IL-12/23R beta 1)-independent production of gamma interferon (IFN-gamma) and humoral immunity in patients with genetic deficiencies in IL-12/23R beta 1 or IFN-gamma receptor I. Clin Vaccine Immunol. 2008;15(8):1171–1175. doi: 10.1128/CVI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Costa FF, Castro G, Andrade J, de Jesus AR, de Almeida RP, Nascimento-Carvalho CM. Resistant Mycobacterium bovis disseminated infection. Pediatr Infect Dis J. 2006;25(2):190. doi: 10.1097/01.inf.0000200103.69932.4c. [DOI] [PubMed] [Google Scholar]
- 228.Miro F, Nobile C, Blanchard N, Lind M, Filipe-Santos O, Fieschi C, Chapgier A, Vogt G, de Beaucoudrey L, Kumararatne DS, et al. T cell-dependent activation of dendritic cells requires IL-12 and IFN-gamma signaling in T cells. J Immunol. 2006;177(6):3625–3634. doi: 10.4049/jimmunol.177.6.3625. [DOI] [PubMed] [Google Scholar]
- 229.Cleary AM, Tu W, Enright A, Giffon T, Dewaal-Malefyt R, Gutierrez K, Lewis DB. Impaired accumulation and function of memory CD4 T cells in human IL-12 receptor beta 1 deficiency. J Immunol. 2003;170(1):597–603. doi: 10.4049/jimmunol.170.1.597. [DOI] [PubMed] [Google Scholar]
- 230.Verhagen CE, de Boer T, Smits HH, Verreck FA, Wierenga EA, Kurimoto M, Lammas DA, Kumararatne DS, Sanal O, Kroon FP, et al. Residual type 1 immunity in patients genetically deficient for interleukin 12 receptor beta1 (IL-12Rbeta1): evidence for an IL-12Rbeta1-independent pathway of IL-12 responsiveness in human T cells. J Exp Med. 2000;192(4):517–528. doi: 10.1084/jem.192.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Altare F, Ensser A, Breiman A, Reichenbach J, Baghdadi JE, Fischer A, Emile JF, Gaillard JL, Meinl E, Casanova JL. Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J Infect Dis. 2001;184(2):231–236. doi: 10.1086/321999. [DOI] [PubMed] [Google Scholar]
- 232.Shah P, El-Maaytah M, Jerjes W, Upile T, Ayliffe P. Interleukin 12 receptor beta1 chain deficiency in a child with disseminated tuberculosis: a case report. J Oral Maxillofac Surg. 2010;68(4):909–911. doi: 10.1016/j.joms.2009.04.116. [DOI] [PubMed] [Google Scholar]
- 233.Ailal F, Tazi A, Bustamante J, Picard C, Najib J, Casanova JL, Bousfiha A. Péricardite purulente et infiltration colique à Salmonella enteritidis compliquée d’invagination intestinale aigüe dans un cas de déficit en IL-12Rb1. Archives de Pédiatrie. 2014 doi: 10.1016/j.arcped.2014.09.009. in press. [DOI] [PubMed] [Google Scholar]
- 234.van de Vosse E, de Paus RA, van Dissel JT, Ottenhoff TH. Molecular complementation of IL-12Rbeta1 deficiency reveals functional differences between IL-12Rbeta1 alleles including partial IL-12Rbeta1 deficiency. Hum Mol Genet. 2005;14(24):3847–3855. doi: 10.1093/hmg/ddi409. [DOI] [PubMed] [Google Scholar]
- 235.Potjewijd J, de Paus RA, van Wengen A, Damoiseaux J, Verbon A, van de Vosse E. Disseminated Mycobacterium genavense infection in a patient with a novel partial interleukin-12/23 receptor beta1 deficiency. Clin Immunol. 2012;144(2):83–86. doi: 10.1016/j.clim.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 236.Strickler A, Perez A, Risco M, Gallo S. Enfermedad por bacilo de Calmette-Guerin (BCG) y deficiencia del receptor b1 de interleuquina 12. Experiencia clinica de dos casos en una familia y un caso aislado. Revista chilena de Infectologia. 2014 doi: 10.4067/S0716-10182014000400010. in press. [DOI] [PubMed] [Google Scholar]
- 237.Alangari AA, Al-Zamil F, Al-Mazrou A, Al-Muhsen S, Boisson-Dupuis S, Awadallah S, Kambal A, Casanova JL. Treatment of disseminated mycobacterial infection with high-dose IFN-gamma in a patient with IL-12Rbeta1 deficiency. Clin Dev Immunol. 2011;2011:691956. doi: 10.1155/2011/691956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Altare F, Lammas D, Revy P, Jouanguy E, Doffinger R, Lamhamedi S, Drysdale P, Scheel-Toellner D, Girdlestone J, Darbyshire P, et al. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J Clin Invest. 1998;102(12):2035–2040. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Salzer E, Kansu A, Sic H, Majek P, Ikinciogullari A, Dogu FE, Prengemann NK, Santos-Valente E, Pickl WF, Bilic I, et al. Early-onset inflammatory bowel disease and common variable immunodeficiency-like disease caused by IL-21 deficiency. J Allergy Clin Immunol. 2014;133(6):1651–1659. e1612. doi: 10.1016/j.jaci.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 241.Picard C, Casanova JL. Inherited disorders of cytokines. Curr Opin Pediatr. 2004;16(6):648–658. doi: 10.1097/01.mop.0000145919.92477.5f. [DOI] [PubMed] [Google Scholar]
- 242.Mansouri D, Adimi P, Mirsaeidi M, Mansouri N, Khalilzadeh S, Masjedi MR, Adimi P, Tabarsi P, Naderi M, Filipe-Santos O, et al. Inherited disorders of the IL-12-IFN-gamma axis in patients with disseminated BCG infection. Eur J Pediatr. 2005;164(12):753–757. doi: 10.1007/s00431-005-1689-9. [DOI] [PubMed] [Google Scholar]
- 243.Ben-Mustapha I, Ben-Ali M, Mekki N, Patin E, Harmant C, Bouguila J, Elloumi-Zghal H, Harbi A, Bejaoui M, Boughammoura L, et al. A 1,100-year-old founder effect mutation in IL12B gene is responsible for Mendelian susceptibility to mycobacterial disease in Tunisian patients. Immunogenetics. 2014;66(1):67–71. doi: 10.1007/s00251-013-0739-0. [DOI] [PubMed] [Google Scholar]
- 244.Pulickal AS, Hambleton S, Callaghan MJ, Moore CE, Goulding J, Goodsall A, Baretto R, Lammas DA, Anderson ST, Levin M, et al. Biliary cirrhosis in a child with inherited interleukin-12 deficiency. J Trop Pediatr. 2008;54(4):269–271. doi: 10.1093/tropej/fmm119. [DOI] [PubMed] [Google Scholar]
- 245.MacLennan C, Fieschi C, Lammas DA, Picard C, Dorman SE, Sanal O, MacLennan JM, Holland SM, Ottenhoff TH, Casanova JL, et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190(10):1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 246.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202(6):761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Marquis JF, LaCourse R, Ryan L, North RJ, Gros P. Disseminated and rapidly fatal tuberculosis in mice bearing a defective allele at IFN regulatory factor 8. J Immunol. 2009;182(5):3008–3015. doi: 10.4049/jimmunol.0800680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Turcotte K, Gauthier S, Tuite A, Mullick A, Malo D, Gros P. A mutation in the Icsbp1 gene causes susceptibility to infection and a chronic myeloid leukemia-like syndrome in BXH-2 mice. J Exp Med. 2005;201(6):881–890. doi: 10.1084/jem.20042170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Marquis JF, Kapoustina O, Langlais D, Ruddy R, Dufour CR, Kim BH, MacMicking JD, Giguere V, Gros P. Interferon regulatory factor 8 regulates pathways for antigen presentation in myeloid cells and during tuberculosis. PLoS Genet. 2011;7(6):e1002097. doi: 10.1371/journal.pgen.1002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 251.Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol. 2003;170(3):1131–1135. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- 252.Turcotte K, Gauthier S, Malo D, Tam M, Stevenson MM, Gros P. Icsbp1/IRF-8 is required for innate and adaptive immune responses against intracellular pathogens. J Immunol. 2007;179(4):2467–2476. doi: 10.4049/jimmunol.179.4.2467. [DOI] [PubMed] [Google Scholar]
- 253.Collin M, Bigley V, Haniffa M, Hambleton S. Human dendritic cell deficiency: the missing ID? Nat Rev Immunol. 2011;11(9):575–583. doi: 10.1038/nri3046. [DOI] [PubMed] [Google Scholar]
- 254.Conley ME, Casanova JL. Discovery of single-gene inborn errors of immunity by next generation sequencing. Curr Opin Immunol. 2014;30C:17–23. doi: 10.1016/j.coi.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bogunovic D, Boisson-Dupuis S, Casanova JL. ISG15: leading a double life as a secreted molecule. Exp Mol Med. 2013;45:e18. doi: 10.1038/emm.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.D’Cunha J, Knight E, Jr, Haas AL, Truitt RL, Borden EC. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc Natl Acad Sci U S A. 1996;93(1):211–215. doi: 10.1073/pnas.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.D’Cunha J, Ramanujam S, Wagner RJ, Witt PL, Knight E, Jr, Borden EC. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J Immunol. 1996;157(9):4100–4108. [PubMed] [Google Scholar]
- 258.Zhang X, Bogunovic D, Payelle-Brogard B, Francois-Newton V, Speer SD, Yuan C, Volpi S, Li Z, Sanal O, Mansouri D, et al. Intracellular human ISG15 is an IFN-a/b inducible negative regulator of IFN-a/b amplification and prevents IFN-a/b mediated auto-inflammation. Nature. 2014 in press. [Google Scholar]
- 259.Morales DJ, Lenschow DJ. The antiviral activities of ISG15. J Mol Biol. 2013;425(24):4995–5008. doi: 10.1016/j.jmb.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Crow YJ, Casanova JL. STING-associated vasculopathy with onset in infancy--a new interferonopathy. N Engl J Med. 2014;371(6):568–571. doi: 10.1056/NEJMe1407246. [DOI] [PubMed] [Google Scholar]
- 261.Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. 2011;1238:91–98. doi: 10.1111/j.1749-6632.2011.06220.x. [DOI] [PubMed] [Google Scholar]
- 262.Bustamante J, Picard C, Boisson-Dupuis S, Abel L, Casanova JL. Genetic lessons learned from X-linked Mendelian susceptibility to mycobacterial diseases. Ann N Y Acad Sci. 2011;1246:92–101. doi: 10.1111/j.1749-6632.2011.06273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27(3):277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 264.Smahi A, Courtois G, Rabia SH, Doffinger R, Bodemer C, Munnich A, Casanova JL, Israel A. The NF-kappaB signalling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11(20):2371–2375. doi: 10.1093/hmg/11.20.2371. [DOI] [PubMed] [Google Scholar]
- 265.Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, Israel A, Heiss NS, Klauck SM, Kioschis P, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium . Nature. 2000;405(6785):466–472. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 266.Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, Avcin T, de Boer M, Bustamante J, Condino-Neto A, et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update) Blood Cells Mol Dis. 2010;45(3):246–265. doi: 10.1016/j.bcmd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Holland SM. Chronic granulomatous disease. Hematol Oncol Clin North Am. 2013;27(1):89–99. viii. doi: 10.1016/j.hoc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hubeau M, Ngadjeua F, Puel A, Israel L, Feinberg J, Chrabieh M, Belani K, Bodemer C, Fabre I, Plebani A, et al. New mechanism of X-linked anhidrotic ectodermal dysplasia with immunodeficiency: impairment of ubiquitin binding despite normal folding of NEMO protein. Blood. 2011;118(4):926–935. doi: 10.1182/blood-2010-10-315234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Puel A, Reichenbach J, Bustamante J, Ku CL, Feinberg J, Doffinger R, Bonnet M, Filipe-Santos O, de Beaucoudrey L, Durandy A, et al. The NEMO mutation creating the most-upstream premature stop codon is hypomorphic because of a reinitiation of translation. Am J Hum Genet. 2006;78(4):691–701. doi: 10.1086/501532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2(3):223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 271.Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, Shapira SK, Farndon PA, Wara DW, Emmal SA, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67(6):1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Orange JS, Levy O, Brodeur SR, Krzewski K, Roy RM, Niemela JE, Fleisher TA, Bonilla FA, Geha RS. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114(3):650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 273.Grubisha O, Kaminska M, Duquerroy S, Fontan E, Cordier F, Haouz A, Raynal B, Chiaravalli J, Delepierre M, Israel A, et al. DARPin-assisted crystallography of the CC2-LZ domain of NEMO reveals a coupling between dimerization and ubiquitin binding. J Mol Biol. 2010;395(1):89–104. doi: 10.1016/j.jmb.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 274.Shu U, Kiniwa M, Wu CY, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25(4):1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 275.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kelsall BL, Stuber E, Neurath M, Strober W. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann N Y Acad Sci. 1996;795:116–126. doi: 10.1111/j.1749-6632.1996.tb52660.x. [DOI] [PubMed] [Google Scholar]
- 277.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183(2):693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Frucht DM, Holland SM. Defective monocyte costimulation for IFN-gamma production in familial disseminated Mycobacterium avium complex infection: abnormal IL-12 regulation. J Immunol. 1996;157(1):411–416. [PubMed] [Google Scholar]
- 279.Holland SM, Eisenstein EM, Kuhns DB, Turner ML, Fleisher TA, Strober W, Gallin JI. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med. 1994;330(19):1348–1355. doi: 10.1056/NEJM199405123301904. [DOI] [PubMed] [Google Scholar]
- 280.Frucht DM, Sandberg DI, Brown MR, Gerstberger SM, Holland SM. IL-12-Independent costimulation pathways for interferon-gamma production in familial disseminated Mycobacterium avium complex infection. Clin Immunol. 1999;91(2):234–241. doi: 10.1006/clim.1999.4688. [DOI] [PubMed] [Google Scholar]
- 281.Haverkamp MH, Marciano BE, Frucht DM, Jain A, van de Vosse E, Holland SM. Correlating interleukin-12 stimulated interferon-gamma production and the absence of ectodermal dysplasia and anhidrosis (EDA) in patients with mutations in NF-kappaB essential modulator (NEMO) J Clin Immunol. 2014;34(4):436–443. doi: 10.1007/s10875-014-9998-2. [DOI] [PubMed] [Google Scholar]
- 282.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19(5):641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 283.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5(7):521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 284.Nedorost ST, Elewski B, Tomford JW, Camisa C. Rosacea-like lesions due to familial Mycobacterium avium-intracellulare infection. Int J Dermatol. 1991;30(7):491–497. doi: 10.1111/j.1365-4362.1991.tb04869.x. [DOI] [PubMed] [Google Scholar]
- 285.Dinauer MC, Orkin SH. Chronic granulomatous disease. Annu Rev Med. 1992;43:117–124. doi: 10.1146/annurev.me.43.020192.001001. [DOI] [PubMed] [Google Scholar]
- 286.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. (Baltimore) 2000;79(3):170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 287.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. (Baltimore) 2000;79(3):155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 288.Bustamante J, Aksu G, Vogt G, de Beaucoudrey L, Genel F, Chapgier A, Filipe-Santos O, Feinberg J, Emile JF, Kutukculer N, et al. BCG-osis and tuberculosis in a child with chronic granulomatous disease. J Allergy Clin Immunol. 2007;120(1):32–38. doi: 10.1016/j.jaci.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 289.Baba LA, Ailal F, El Hafidi N, Hubeau M, Jabot-Hanin F, Benajiba N, Aadam Z, Conti F, Deswarte C, Jeddane L, et al. Chronic Granulomatous Disease in Morocco: Genetic, Immunological, and Clinical Features of 12 Patients from 10 Kindreds. J Clin Immunol. 2014 doi: 10.1007/s10875-014-9997-3. [DOI] [PubMed] [Google Scholar]
- 290.Lee PP, Chan KW, Jiang L, Chen T, Li C, Lee TL, Mak PH, Fok SF, Yang X, Lau YL. Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr Infect Dis J. 2008;27(3):224–230. doi: 10.1097/INF.0b013e31815b494c. [DOI] [PubMed] [Google Scholar]
- 291.Koker MY, Camcioglu Y, van Leeuwen K, Kilic SS, Barlan I, Yilmaz M, Metin A, de Boer M, Avcilar H, Patiroglu T, et al. Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol. 2013;132(5):1156–1163. e1155. doi: 10.1016/j.jaci.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 292.Fattahi F, Badalzadeh M, Sedighipour L, Movahedi M, Fazlollahi MR, Mansouri SD, Khotaei GT, Bemanian MH, Behmanesh F, Hamidieh AA, et al. Inheritance pattern and clinical aspects of 93 Iranian patients with chronic granulomatous disease. J Clin Immunol. 2011;31(5):792–801. doi: 10.1007/s10875-011-9567-x. [DOI] [PubMed] [Google Scholar]
- 293.Agudelo-Florez P, Prando-Andrade CC, Lopez JA, Costa-Carvalho BT, Quezada A, Espinosa FJ, de Souza Paiva MA, Roxo P, Jr, Grumach A, Jacob CA, et al. Chronic granulomatous disease in Latin American patients: clinical spectrum and molecular genetics. Pediatr Blood Cancer. 2006;46(2):243–252. doi: 10.1002/pbc.20455. [DOI] [PubMed] [Google Scholar]
- 294.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Espanol T, Fischer A, Kurenko-Deptuch M, Mouy R, et al. Chronic granulomatous disease: the European experience. PLoS One. 2009;4(4):e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Deffert C, Cachat J, Krause KH. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell Microbiol. 2014;16(8):1168–1178. doi: 10.1111/cmi.12322. [DOI] [PubMed] [Google Scholar]
- 296.Martire B, Rondelli R, Soresina A, Pignata C, Broccoletti T, Finocchi A, Rossi P, Gattorno M, Rabusin M, Azzari C, et al. Clinical features, long-term follow-up and outcome of a large cohort of patients with Chronic Granulomatous Disease: an Italian multicenter study. Clin Immunol. 2008;126(2):155–164. doi: 10.1016/j.clim.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 297.El Kares R, Barbouche MR, Elloumi-Zghal H, Bejaoui M, Chemli J, Mellouli F, Tebib N, Abdelmoula MS, Boukthir S, Fitouri Z, et al. Genetic and mutational heterogeneity of autosomal recessive chronic granulomatous disease in Tunisia. J Hum Genet. 2006;51(10):887–895. doi: 10.1007/s10038-006-0039-8. [DOI] [PubMed] [Google Scholar]
- 298.Lugo Reyes SO, Suarez F, Herbigneaux RM, Pacquement H, Reguerre Y, Riviere JP, de Suremain M, Rose Y, Feinberg J, Malahoui N, et al. Hodgkin lymphoma in 2 children with chronic granulomatous disease. J Allergy Clin Immunol. 2011;127(2):543–544. e541–543. doi: 10.1016/j.jaci.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Dogru D, Kiper N, Ozcelik U, Yalcin E, Tezcan I. Tuberculosis in children with congenital immunodeficiency syndromes. Tuberk Toraks. 2010;58(1):59–63. [PubMed] [Google Scholar]
- 300.Wolach B, Gavrieli R, de Boer M, Gottesman G, Ben-Ari J, Rottem M, Schlesinger Y, Grisaru-Soen G, Etzioni A, Roos D. Chronic granulomatous disease in Israel: clinical, functional and molecular studies of 38 patients. Clin Immunol. 2008;129(1):103–114. doi: 10.1016/j.clim.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 301.Bustamante J, Picard C, Fieschi C, Filipe-Santos O, Feinberg J, Perronne C, Chapgier A, de Beaucoudrey L, Vogt G, Sanlaville D, et al. A novel X-linked recessive form of Mendelian susceptibility to mycobaterial disease. J Med Genet. 2007;44(2):e65. doi: 10.1136/jmg.2006.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Conti F, Aragão-Filho WC, Prando C, Deswarte C, Hubeau M, Newburger PE, Casanova JL, Bustamante J, Condino-Neto A. Phagocyte NADPH oxidase activity in patients with inherited IFN-gR1 or IFN-gR2 deficiency. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.11.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181(2):713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Doffinger R, Jouanguy E, Altare F, Wood P, Shirakawa T, Novelli F, Lammas D, Kumararatne D, Casanova JL. Inheritable defects in interleukin-12- and interferon-gamma-mediated immunity and the TH1/TH2 paradigm in man. Allergy. 1999;54(5):409–412. doi: 10.1034/j.1398-9995.1999.00088.x. [DOI] [PubMed] [Google Scholar]
- 305.Wood PM, Fieschi C, Picard C, Ottenhoff TH, Casanova JL, Kumararatne DS. Inherited defects in the interferon-gamma receptor or interleukin-12 signalling pathways are not sufficient to cause allergic disease in children. Eur J Pediatr. 2005;164(12):741–747. doi: 10.1007/s00431-005-1745-5. [DOI] [PubMed] [Google Scholar]
- 306.Doffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, Espitia-Pinzon C, Barnes N, Bothamley G, Casanova JL, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38(1):e10–14. doi: 10.1086/380453. [DOI] [PubMed] [Google Scholar]
- 307.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2013;207(2):291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool K, Zaman R, Ding L, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367(8):725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Chi CY, Chu CC, Liu JP, Lin CH, Ho MW, Lo WJ, Lin PC, Chen HJ, Chou CH, Feng JY, et al. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood. 2013;121(8):1357–1366. doi: 10.1182/blood-2012-08-452482. [DOI] [PubMed] [Google Scholar]
- 310.Casanova JL, Abel L. Human genetics of infectious diseases: a unified theory. Embo J. 2007;26(4):915–922. doi: 10.1038/sj.emboj.7601558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317(5838):617–619. doi: 10.1126/science.1142963. [DOI] [PubMed] [Google Scholar]
- 312.Gaschignard J, Levy C, Chrabieh M, Boisson B, Bost-Bru C, Dauger S, Dubos F, Durand P, Gaudelus J, Gendrel D, et al. Invasive pneumococcal disease in children can reveal a primary immunodeficiency. Clin Infect Dis. 2014;59(2):244–251. doi: 10.1093/cid/ciu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Picard C, Casanova JL, Abel L. Mendelian traits that confer predisposition or resistance to specific infections in humans. Curr Opin Immunol. 2006;18(4):383–390. doi: 10.1016/j.coi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 314.Orth G. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr Top Microbiol Immunol. 2008;321:59–83. doi: 10.1007/978-3-540-75203-5_3. [DOI] [PubMed] [Google Scholar]
- 315.Zhang SY, Abel L, Casanova JL. Mendelian predisposition to herpes simplex encephalitis. Handb Clin Neurol. 2013;112:1091–1097. doi: 10.1016/B978-0-444-52910-7.00027-1. [DOI] [PubMed] [Google Scholar]
- 316.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616–622. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Byun M, Abhyankar A, Lelarge V, Plancoulaine S, Palanduz A, Telhan L, Boisson B, Picard C, Dewell S, Zhao C, et al. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med. 2010;207(11):2307–2312. doi: 10.1084/jem.20101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Byun M, Ma CS, Akcay A, Pedergnana V, Palendira U, Myoung J, Avery DT, Liu Y, Abhyankar A, Lorenzo L, et al. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med. 2013;210(9):1743–1759. doi: 10.1084/jem.20130592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39(4):676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bolze A, Mahlaoui N, Byun M, Turner B, Trede N, Ellis SR, Abhyankar A, Itan Y, Patin E, Brebner S, et al. Ribosomal protein SA haploinsufficiency in humans with isolated congenital asplenia. Science. 2013;340(6135):976–978. doi: 10.1126/science.1234864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Crequer A, Troeger A, Patin E, Ma CS, Picard C, Pedergnana V, Fieschi C, Lim A, Abhyankar A, Gineau L, et al. Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections. J Clin Invest. 2012;122(9):3239–3247. doi: 10.1172/JCI62949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Boisson B, Wang YD, Bosompem A, Ma CS, Lim A, Kochetkov T, Tangye SG, Casanova JL, Conley ME. A recurrent dominant negative E47 mutation causes agammaglobulinemia and BCR(−) B cells. J Clin Invest. 2013;123(11):4781–4785. doi: 10.1172/JCI71927. [DOI] [PMC free article] [PubMed] [Google Scholar]