Presynaptic active zones in invertebrates and vertebrates (original) (raw)
Abstract
The regulated release of neurotransmitter occurs via the fusion of synaptic vesicles (SVs) at specialized regions of the presynaptic membrane called active zones (AZs). These regions are defined by a cytoskeletal matrix assembled at AZs (CAZ), which functions to direct SVs toward docking and fusion sites and supports their maturation into the readily releasable pool. In addition, CAZ proteins localize voltage-gated Ca2+ channels at SV release sites, bringing the fusion machinery in close proximity to the calcium source. Proteins of the CAZ therefore ensure that vesicle fusion is temporally and spatially organized, allowing for the precise and reliable release of neurotransmitter. Importantly, AZs are highly dynamic structures, supporting presynaptic remodeling, changes in neurotransmitter release efficacy, and thus presynaptic forms of plasticity. In this review, we discuss recent advances in the study of active zones, highlighting how the CAZ molecularly defines sites of neurotransmitter release, endocytic zones, and the integrity of synapses.
Keywords: active zone, cytoskeletal matrix, fusion, release, synaptic vesicle
Introduction
Chemical synapses are asymmetric structures comprising a presynaptic bouton, designed for the rapid and regulated release of neurotransmitter, and a postsynaptic neurotransmitter reception apparatus (the postsynaptic density—PSD) separated by a small space known as the synaptic cleft. In general, synapse size and morphology are closely coupled to function and reliability 1,2. For example, reliable synapses, such as the neuromuscular junction (NMJ), tend to be very large, containing thousands of SVs and elaborate AZs and PSDs, thereby ensuring that muscle contraction occurs with high fidelity 3. Conversely, synapses of the central nervous system, such as hippocampal glutamatergic synapses, are generally small and less reliable, yet possess an enormous capacity to change their reliability/strength in a process called synaptic plasticity. Importantly, synaptic plasticity can involve changes on either side of the synapse, including alterations in neurotransmitter release probability (presynaptic) or in the number and activity of postsynaptic neurotransmitter receptors (postsynaptic). In the current review, we will highlight roles of CAZ proteins in regulating the fidelity, reliability, plasticity, and integrity of synapses, drawing upon advances at invertebrate and vertebrate synapses.
Invertebrate active zones
Active zones in Caenorhabditis elegans
Active zones of NMJ synapses in the worm Caenorhabditis elegans (C. elegans) comprise a broad surface of plasma membrane situated between electron-dense projections (DPs) and flanking cellular tight junctions 4. At these synapses, most SV fusion and endocytic events occur within 30–200 nm of the DPs, suggesting a role for these structures in the regulated release of neurotransmitter (Fig1) 1. One of the first components identified at these AZs was SYD-2, a multidomain scaffold protein that is structurally related to the vertebrate presynaptic protein Liprin-α 5-7. Loss-of-function mutations in Syd-2 cause AZs to become less compact and more elongated 5, and impair SV docking and synaptic transmission, indicating that SYD-2 organize the neurotransmitter release machinery 8,9. Consistent with these findings, SYD-2 and Liprin-α have been found to interact and colocalize with other CAZ proteins including ELKS-1, UNC-10/RIM, and UNC-13 at the C. elegans NMJ. These molecules are also required for the regulated docking and fusion of SVs at C. elegans NMJ synapses. For example, loss of UNC-10/RIM not only impairs synaptic transmission, but also causes a redistribution of docked vesicles away from the DPs 10. This finding suggests that UNC-10/RIM may position SVs close to sites of calcium entry via voltage-gated calcium channels (VGCCs) 8 (Table1).
Figure 1.
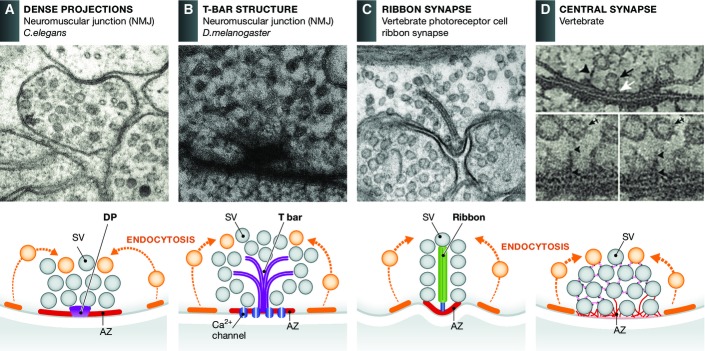
Active zones display different morphologies
Electron micrographs and schematic drawings reveal that AZs can be divided into morphologically distinct groups: those with elaborate electron-dense projections such as T-bars and ribbons and those with less prominent dense projections including C. elegans active zones and those present at most vertebrate central nervous system synapses. (A) AZ from a C. elegans NMJ. In general, these AZs are quite simple and generally characterized as a broad surface of plasma membrane situated between electron-dense projections (DPs) and flanking cellular tight junctions. (B) AZs present at the NMJ of the fly Drosophila melanogaster. These are more elaborate forming a platform consisting of a meshwork of filaments overlaying a pedestal which gave them the name T-bars. (C) Vertebrate photoreceptor cell ribbon synapse. These synapses are characterized by a large AZ with a specialized organelle, the synaptic ribbon, which tethers large numbers of SVs near the AZ, facilitating fast-sustained synaptic transmission. (D) AZs of vertebrate central synapses are less complex than at sensory synapses, exhibiting fine filamentous projections that connect proximal (docked vesicles) and more distally located SVs, up to 100 nm, to the plasma membrane holding them close to the release sites. In schematic drawing, active and endocytic (not shown in EM micrograph) zones are marked as red and orange, respectively. Reproduced with permission, from 4 (A); Hollmann and Sigrist (B); 155 (C); 11 (D).
Table 1.
Active zone proteins in invertebrates and vertebrates
| Protein | Function | References |
|---|---|---|
| C. elegans | ||
| SYD-2/Liprin-α | Synaptic scaffolding protein: active zone morphology; SV docking; synaptic transmission | 8,9 |
| UNC10 | Synaptic vesicle priming factor: SV docking,SV priming, calcium channel localization | 10,19 |
| Drosophila | ||
| Bruchpilot | Synaptic scaffolding protein: component of the T-bar structure; calcium channel clustering | 15-17 |
| dRBP | Synaptic scaffolding protein: calcium channel clustering | 20 |
| Fife | Synaptic scaffolding protein: active zone organization | 24 |
| Neurexin | Synaptic adhesion protein: synapse assembly, synapse growth | 26 |
| Neuroligin | Synaptic adhesion protein: synapse assembly | 25,27 |
| Intersectin | Endocytic protein: SV fusion, membrane retrieval | |
| Mice | ||
| RIM | Synaptic vesicle priming factor: SV docking, SV priming, calcium channel localization | 60-68,69,70 |
| Munc13 | Synaptic vesicle priming factor: SV priming, SV fusion | 66-70 |
| RBP | Synaptic scaffolding protein_:_ calcium channel clustering | 20 |
| Bassoon | Synaptic scaffolding protein: ribbon attachment, calcium channel clustering, synapse integrity | 46-51,151 |
| Piccolo | Synaptic scaffolding protein: actin assembly, synapse integrity | 119-121,151 |
| Piccolino | Synaptic scaffolding protein: ribbon shape | 52 |
Greater insight into the 3D organization of SV pools associated with C. elegans AZs has come with the development of high-pressure freeze fixation techniques (hpf-EM) combined with electron tomography 8,9. These unprecedented images reveal that SVs, clustered within NMJ boutons, are not freely floating but rather interconnected by a fine filamentous network 8 similar to that seen at vertebrate synapses (Fig1) 11,12. These filaments are also observed between SVs and the AZ plasma membrane 8. At present, little is known about the structural organization of vesicle clusters in C. elegans, or of the proteins building these filamentous networks, though SYD-2/Liprin-α and UNC-10/RIM are possible candidates. This latter concept is supported by experiments showing that in syd-2 and unc-10 loss-of-function mutants, the number of docked vesicles contacting DP filaments is reduced, as is evoked neurotransmission 8,9. Hpf-EM has also revealed that AZs in C. elegans are assembled through the polymerization of these DPs into 2D arrays that scale with SYD-2 expression levels and synaptic transmission, suggesting that SYD-2-dependent changes in AZ size may be part of a plasticity mechanism used to adjust the reliability of synaptic transmission in C. elegans 9. These features imply that neurotransmitter release sites in general are organized in modules that can be assembled into a diverse set of arrays with distinct functionalities, as will be discussed below.
Drosophila active zones
Active zones present at the Drosophila melanogaster NMJ are more elaborate than those observed in C. elegans 1,13. They have been given the name “T-bars”, reflecting their morphology as a meshwork of filaments overlying a pedestal (Fig1) 14. Initial studies identified the protein Bruchpilot (BRP) as a key component of the T-bar 15, as this structure is lacking in brp null mutants 16. Super-resolution microscopy further revealed that single BRP molecules adopt an elongated conformation, reaching from the AZ membrane into the cytoplasm of boutons, and thereby forming the T-bar structure (Fig1). In addition, BRP is essential for the clustering of Ca2+ channels beneath the T-bar at the center of the AZ 17, bringing the Ca2+ source close to the fusion machinery. This close association is created by two BRP isoforms that assemble in an alternating pattern, forming a circular array around the T-bars. This arrangement appears to create discrete adjacent slots for the Ca2+ channels and SV docking sites that are critical for efficient neurotransmission 18.
In addition to Bruchpilot, T-bars also contain RIM, Dunc13, RBP, and Fife. Rab3-interacting molecule (RIM) has long been appreciated for its role in synaptic transmission, working in concert with the Drosophila homolog of Unc13, Dunc13, to promote SV priming and docking 19. In Drosophila, RIM loss-of-function decreases synaptic transmission by reducing neurotransmitter release probability without affecting the morphology/integrity of AZs 19, an observation consistent with normal levels of BRP at synapses lacking RIM. Intriguingly, RIM loss-of-function also reduces the synaptic localization of cacophony, a VGCC, explaining in part the reduced release probability at synapses lacking RIM 19.
Coupling of VGCCs to AZs is also mediated by RIM-binding proteins (RBPs) of which there are three variants in mammals and one in Drosophila 20,21. As with RIM, RBP loss-of-function impairs synaptic transmission at the Drosophila NMJ and causes the mislocalization of cacophony 21. Here, RBP appears to perform a corralling function that surrounds and maintains VGCC beneath T-bars, a concept consistent with a reduction in electron density at the base of T-bars in synapses lacking RBP 21. Importantly, the tufts created by BRP still form without RBP or RIM, implying that as in C. elegans, the functional assembly of AZs is hierarchical 22 and requires specific associations between AZ proteins 23.
Nearly all CAZ proteins identified at invertebrate synapses are also present at mammalian synapses, suggesting a significant conservation of function throughout evolution 23 (Fig2). Two exceptions are Piccolo and Bassoon, which may have evolved to perform vertebrate-specific functions. However, studies by Bruckner et al 24 have recently identified a Piccolo homolog, aptly named Fife, which is selectively localized at Drosophila AZs. Structurally, Fife is a hybrid molecule with features from both Piccolo and RIM, including zinc finger (ZnF), PDZ, and C2 domains. Fife loss-of-function has profound effects on AZ organization and synaptic transmission, including a dramatic decrease in excitatory junctional potential amplitude and quantal content. Morphologically, boutons lacking Fife exhibit a 20% reduction in the number of SVs associated with T-bars, as well as floating T-bars and detachment of pre/postsynaptic membranes. Intriguingly, floating T-bars are occasionally seen at fly NMJs lacking RBP 21, suggesting that Fife and RBP both function to tether SVs at the AZ. Furthermore, the detached membrane phenotype implies a role in transsynaptic adhesion 24.
Figure 2.
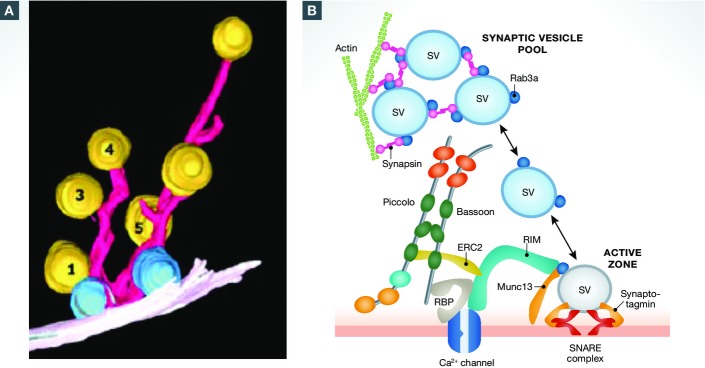
Cytoskeletal matrix proteins organize synaptic vesicle release sites at presynaptic active zones
(A) 3D reconstruction of filaments (pink) and SVs (yellow) tethered near the active zone from a vertebrate (rat) hippocampal synapse. Docked vesicles are in blue. Reproduced with permission from 11. (B) Schematic diagram of CAZ molecules mediating the capture (synapsin, actin), docking (SNARE complex), priming (RIM, Munc13, Rab3) and fusion (synaptotagmin) of SVs and VGCCs (Bassoon, RBP, RIM, ELKS) at presynaptic active zones. At present, the spatial relationship of these molecules within the cryo-fixed EM image in (A) is not well resolved.
Interestingly, several CAZ proteins have been identified to work in concert with the transsynaptic adhesion/signaling molecules Neurexin-1 (Nrx-1) and Neuroligin-1 (Nlg1) at the Drosophila NMJ, including Syd1, Syd2/DLiprinα, and Wrd (a regulatory subunit B’ of the phosphatase PP2A). As at mammalian synapses, Nrx-1 and Nlg1 facilitate synapse assembly at the Drosophila NMJ, and their loss-of-function causes severe synaptic assembly defects 25-27. Mechanistically, Nrx-1 appears to promote synapse assembly by directly interacting with presynaptic Syd-1. Syd-1 in turn interacts with and stabilizes nascent Syd2/DLiprin-α clusters critical for early synapse assembly 26 and BRP recruitment 28, linking transsynaptic adhesion and active zone scaffold assembly. Of note, the stabilization of these transsynaptic complexes and the inhibition of ectopic synapse assembly also require Wrd/PP2A through its association with DLiprin-α 29. At present, it is unclear whether this same ensemble of presynaptic proteins promotes synapse formation at vertebrate synapses discussed below. Another important question is how activity regulates the remodeling of AZs, and whether this process is fundamentally different from normal development.
Vertebrate active zones
Vertebrate AZs share many of the same features and core proteins of invertebrate synapses. One major difference is the molecular diversity of CAZ proteins, which arise from both gene duplications and alternative splicing. Why is so much diversity necessary? A likely answer is that molecular diversity allows vertebrates to build more elaborate AZs with greater functional diversity, permitting more nuanced modulation of synaptic responsiveness and plasticity. In exploring this concept, we will first discuss how the assembly of unitary release sites contributes to morphologically distinct AZs present at sensory and central synapses. We will then describe emerging roles of AZ proteins that have been uncovered in recent studies, which include coupling SV exocytosis with endocytosis, and regulating synapse plasticity and integrity. Open questions in these areas are highlighted in Sidebar A.
Sidebar A: In need of answers.
Tremendous progress has been made in the molecular and functional characterization of proteins that define presynaptic AZs. These analyses have revealed significant functional and structural conservation across species, allowing information garnered from studies of the invertebrate AZ to be applied to vertebrates and vice versa. However, many outstanding questions remain. For example
- Why are there so many additional isoforms for each AZ protein in vertebrates? Do they simply allow for functional diversity, or also for structural diversity? In order to answer this question, we will need a better understanding of the nano-domain organization of AZs.
- How are active and peri-active/endocytic zones created and maintained?
- Do presynaptic AZs contain functionally distinct micro-domains that support specific types of neurotransmitter release (i.e., spontaneous versus phasic release)? One attractive but untested idea is that transsynaptic adhesion molecules define these nano-domains by triggering the bidirectional assembly of subsets of pre- and postsynaptic proteins.
- What regulates the integrity of AZs? Recent studies have uncovered several exciting and unanticipated functions of CAZ proteins, such as regulating protein homeostasis mechanisms critical for synaptic health and integrity. It will be important to address whether and how the CAZ regulates the formation and trafficking of autophagic and endocytic structures. Clearly, this topic has fundamental importance for our understanding of neurodegenerative disease mechanisms.
- What mechanisms regulate fast synaptic transmission and plasticity? Live imaging studies reveal that AZs are highly dynamic structures that undergo continuous remodeling and turnover. However, our current understanding of the AZ is based primarily upon static images and the dynamics of just a few CAZ molecules, and is therefore unable to give us a complete picture of the molecular interactions that underlie fast synaptic signaling and plasticity. Thus, it is imperative for future studies to investigate the dynamics of CAZ proteins at high temporal and spatial resolution.
CNS synapses
The organization of AZs at vertebrate central nervous system (CNS) synapses resembles that seen in invertebrates. For instance, core AZ proteins involved in the regulated release of neurotransmitters (i.e., RIMs, Munc13, RBP, SNAREs, complexin, synaptotagmin, and Munc18) are also present at vertebrate synapses. Morphologically, AZs at CNS synapses are far less complex than those observed at sensory synapses discussed below. Initial studies using conventional EM revealed that AZs in the CNS were organized into pyramidal-shaped structures interconnected by 50- to 100-nm-spaced fibrils, forming slots for synaptic vesicles to dock and fuse 1,30. However, in cryo-fixed material examined by electron tomography 11, such lattices were not detected. Instead, numerous fine filaments were observed to project from the surface of the AZ, contacting docked synaptic vesicles and those up to ∼100 nm from the plasma membrane (Figs3) 8,11. These findings suggest that the pattern seen in aldehyde-fixed material may be an artifact of this fixation process, caused by protein deposition between docked synaptic vesicles. Intriguingly, the filamentous structures emanating from the AZ in these cryo-fixed preparations resemble those observed at invertebrate synapses (Figs1 and 2), suggesting that the molecular structures responsible for capturing and guiding SVs to their fusion sites are evolutionarily conserved. These images also indicate a role for CAZ proteins beyond the tethering of calcium channels or the priming of SVs (e.g., RBP, Munc13, and RIMs), a concept that will be discussed more fully below in the context of lessons learned at vertebrate NMJs.
Figure 3.
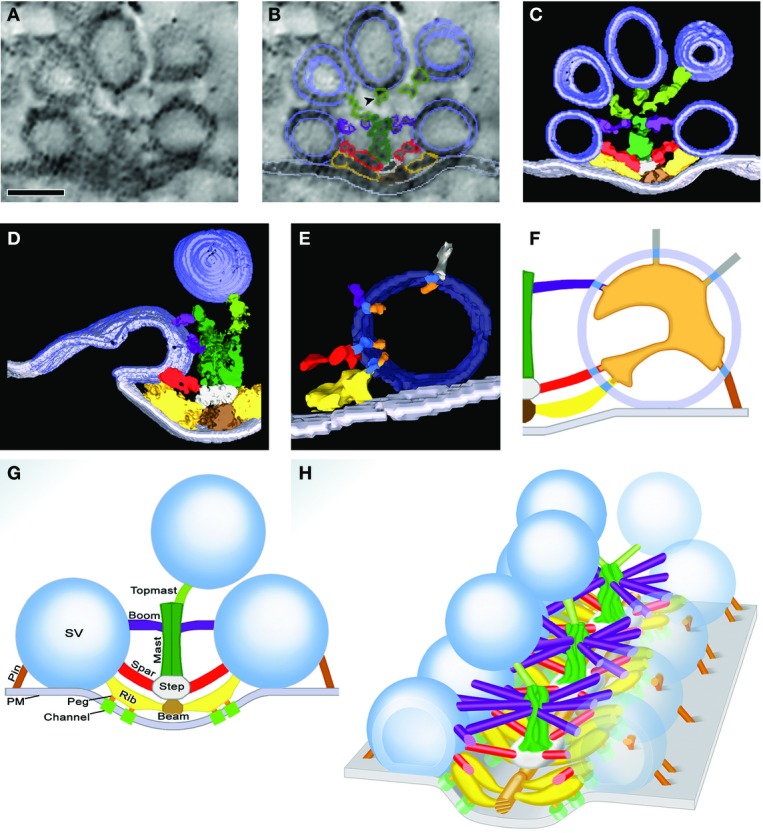
Macromolecular organization of the frog NMJ
At the NMJ of the frog, SVs are arranged in highly organized linear arrays. The precise alignment of the vesicles is achieved through different filamentous structures, which contact the vesicles. (A) Electron micrograph showing subdomain organization of SVs at the frog NMJ. Synaptic vesicles are held in place through filamentous structures. Scale bar, 50 nm. (B) Schematic representation of the electron micrograph in (A) with summed outlined of AZ material (AZM) macromolecules colored. (C) 10-nm-thick surface model of AZM and SVs shown in (A) and (B), derived from eight adjacent slices. Docked vesicles close to the plasma membrane are attached to several filaments contrary to vesicles further away that are only connected to one filament. (D) 25-nm-thick surface model of an AZ, capturing a former docked vesicle that has fused with the plasma membrane but remains in contact with ribs, spars, and boom implying that AZM participates in SV fusion. (E) Surface model, ∼10 nm thick, showing in 3D the nubs linked by transmembrane bands connected to rib, booms, and spars. (F) Schematic diagram of the luminal assembly of macromolecules within a docked SV that form contact with rib, boom, spar, and pin as well as non-AZM molecules. (G) Composition diagram of layers of AZM shown in transverse plane of the AZ at frog NMJ. The main body of AZM includes beams, ribs, and pegs; the intermediate layer: steps and spars; and the deep layer masts, booms, and topmasts. (H) Diagram of NMJ revealing the repetitive array of core AZ proteins centered around a mast (green) that contacts surrounding SV through ribs, spars booms and topmasts. This geometric arrangement allows the creation of tens of SV docking and fusion sites. Reproduced with permission from Szule and Harlow 3,39.
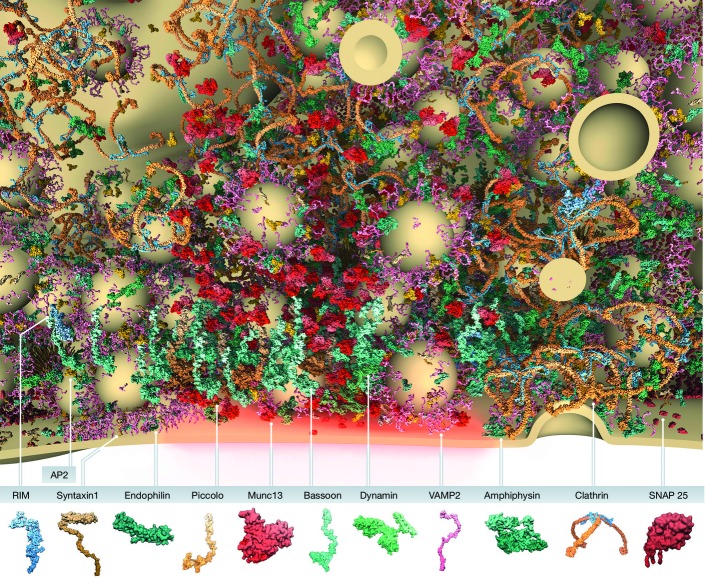
A major challenge facing the field is how to assign molecular identities to the filaments and structures observed in these images. Strategies being employed to resolve this issue include the use of super-resolution and immuno-electron microscopy. Super-resolution microscopy is emerging as a powerful approach to understand the spatial relationships between different presynaptic molecules, as well as the number of such molecules present within boutons 31-33. Of particular note is a visually stunning study performed by Wilhelm et al 33 designed to define the number and relative distributions of presynaptic molecules per bouton (Fig4). Such images nicely complement proteomic-based studies 34, and challenge the community to look beyond our favorite molecules as we try to understand the intricacies of synapse architecture and function. However, it is important to note that such studies also have their limitations. For example, many of the molecules associated with AZs are very large (> 100 kDa), complicating the assignment of their localization based on immunostaining with a single antibody. Furthermore, the information derived from these experiments is relative, for example, based on the distance to one or two other molecules co-labeled in a given experiment. Such limitations argue for caution in the acceptance of models based solely upon such data.
Figure 4.
Model of presynaptic bouton and active zone organization
A section through the active and endocytic zones of a vertebrate synapse indicating the spatial distribution and copy number of presynaptic proteins that help define the presynaptic AZ as the site of SV exocytosis and clathrin-mediated endocytosis. Panel at the bottom, a graphical legend of the predicted structures of presynaptic proteins included in the model. Displayed SVs have a diameter of 42 nm. The image was generously created by Burkhard Rammner in the Rizzoli Laboratory. See also 33.
A complementary approach, providing information about the molecular composition of the AZ, is immunogold labeling of cryo-fixed material combined with electron tomography. Siksou and colleagues have used this strategy to show that the SV-associated protein Synapsin is one component of the short filaments between SVs within the reserve pool. Their data also indicate that the longer, AZ-attached filaments might represent Bassoon tethered to the plasma membrane-associated AZ protein CAST/ERC 11. More recent immuno-EM studies indicate that RIM1α is a component of the fine filaments tethering SVs to the AZ plasma membrane 35. One downside of this approach is the technically demanding nature of experiments, highlighting the need for innovation in order to accelerate data acquisition in the realm of EM tomography.
Neuromuscular junctions
Two major limitations of CNS synapses with respect to AZ structure are their small size and their intrinsic variability. This was illustrated in studies by Schikorski and Stevens 36, who showed that the number and geometric relationship of release sites per bouton were highly variable in CNS synapses, a situation that hampers the assignment of molecules to specific filaments across AZs. Fortunately for synaptic biologists, nature created the neuromuscular junction (NMJ), a synapse formed between motor neurons in the ventral horn of the spinal cord and individual muscle fibers. AZs at this synapse are very long (∼1 μm) and have a very regular, stereotyped pattern. Detailed ultrastructural studies reveal that SVs docked at the NMJ are tethered to the AZ by four classes of macromolecular structures: ribs, pins, spars, and booms (Fig3) 37,38. More recently, these dense projections have been further divided into three sublayers, each defined by specific electron-dense macromolecules 3. The superficial layer, adjacent to the presynaptic membrane, consists of beams, ribs, and pegs; it is followed by the intermediate layer, defined by steps and spars, and finally the deepest layer, containing masts, booms, and topmasts (Fig3) 3.
These elegant findings will revolutionize our understanding of how CAZ proteins facilitate the efficient docking and fusion of SVs. For instance, one is struck by the regularity with which the mast, boom, and ribs are assembled. From each mast core, the booms, spars, and topmast extend radially to contact 4–5 SVs. This arrangement indicates that AZs of the NMJ are assembled by the sequential addition of these structures, creating a linear array of AZs. Intriguingly, these structures are highly reminiscent of the radial AZs created by BRP at the Drosophila NMJ (Fig1), implying a conservation of AZ core structures across evolution.
A second concept addressed by these morphometric studies is the function of filaments emanating from the AZ. On average, each SV docked at the AZ is connected to four ribs, four pins, two spars, and five booms (Fig3) 3. With each SV fusion step, SVs in the reserve pool replace docked SVs, forming contact with each of the four classes of CAZ molecules. Intriguingly, the number of contacts is inversely proportional to SV distance from the plasma membrane, suggesting that these filaments guide SVs to their docking sites, helping to position them near VGCCs.
At present, the identity of the proteins that define each class of filaments remains unclear. Yet given the almost crystalline arrangement of the masts, booms, and spars, one can see how this preparation is ideal for defining their composition and generating hypotheses regarding their functions. For example, given the proximity of ribs to the plasma membrane and their association with VGCCs, one could infer that they comprise proteins such as RIM, Munc13, and RBP, which are involved in SV priming and VGCC tethering. Consistent with this idea, these filaments remain associated with the SV membrane during fusion 3.
It is worth mentioning a third provocative observation arising from these studies. Specifically, using freeze substitution methods, Harlow et al 39 have found that the lumen of SVs is not only filled with neurotransmitter, but also contains an assembly of macromolecules organized into a shape that exhibits chirality and is similar from vesicle to vesicle. Moreover, this structure has arms that radiate from the center of each vesicle and connect via nubs to the surface of SVs (Fig3). These in turn contact the ribs, booms, spars, and pegs of the CAZ. Importantly, the relative orientation of this chiral SV assembly toward the CAZ is identical for all docked SVs (Fig3). This intriguing observation indicates that the CAZ plays an active role in orienting each SV with respect to the plasma membrane. It also suggests that the organization of SV proteins is not random and that AZs use these tethers to position proteins involved in fusion (i.e., VAMP-2, synaptotagmin-1) toward the plasma membrane. It will be interesting to see whether this chirality is preserved at other CNS synapses, and how its disruption alters SV release kinetics.
Ribbon synapses
Although AZs of the vertebrate NMJ are complex, sensory synapses of the retina and auditory system have taken AZ design to a yet higher level. Similar to the NMJ, AZs of retinal ribbon synapses are elongated structures, called “archiform densities”, with a row of docked SVs flanking the central axis. Attached to each archiform density is a specialized organelle, the synaptic ribbon, that is perpendicular to the plasma membrane (Fig1) and tethers large numbers of SVs near the AZ, an arrangement that facilitates rapid, sustained synaptic transmission 40,41. At present, the principles that organize the assembly of ribbon synapses are not known, though it is provocative to consider that ribbon synapses, like the NMJ, employ specific classes of scaffold proteins to (i) build the ribbon and (ii) tether it along a longitudinal array of AZs. Consistent with this concept, the molecular characterization of retinal ribbon synapse reveals that each compartment is defined by distinct groups or subfamilies of CAZ proteins. For example, major structural components of photoreceptor and bipolar cell ribbons include Ribeye, CtBP1, Piccolo, Kif3A, and RIM1 41-43,44. In contrast, the archiform density is comprised of proteins involved in synaptic vesicle fusion and calcium channel clustering (RIM2, ubMunc13-2, Liprins, CASTs/ERCs/ELKS, Bassoon) 41. Loss-of-function studies have identified several of these that dramatically affect the integrity of ribbon synapses. The most remarkable is the multifunctional CAZ protein Bassoon, which is physically anchored to the archiform density 45. In photoreceptor and hair cells of Bassoon mutant mice, ribbons are no longer attached to AZs and float freely in the cytoplasm 46-48, implying a crucial role for Bassoon in tethering ribbons to the archiform density. Loss of Bassoon also reduces the number of synaptic Ca2+ channels tethered at SV release sites 48. While less dramatic, the inactivation of CAST/ERC2 (a Bassoon/RIM binding partner) 49,50 leads to a concomitant decrease in AZ size and complement of VGCCs, without affecting the attachment of ribbons, implying a role for CAST/ERC2 in AZ organization 51. A third critical component of ribbons is a short isoform of Piccolo, Piccolino, which is predominantly expressed at sensory ribbon synapses of the eye and ear 44. At these sites, Piccolino loss-of-function is associated with a dramatic change in ribbon shape, leading to the presence of spherical rather than plate-shaped ribbons 52. Intriguingly, during ribbon biogenesis, precursor spheres are associated with the initial assembly of ribbons, maturing from spherical to plate-shaped ribbons during light adaptation 53,54. This transition is disrupted in the absence of Piccolino, implying a role for this scaffold protein in the formation of plate-shaped ribbons 52. This function is complementary to that performed by Ribeye, which promotes the formation of spheres 55, and to Bassoon, which anchors them to the archiform density 54.
Further progress in this field will require a better understanding of the molecular organization of these synapses and the dynamics of their assembly. Equally important will be the characterization of other sensory synapses such as those found in the cochlea 56, where intriguing similarities and differences are being uncovered. For example, while hair cell ribbons require Bassoon for their attachment, they use Otoferlin rather than Munc13 for SV exocytosis 57-59. Finally, it will be important to better understand the macromolecular principle of assembly and whether rules defined at simpler synapses are utilized wholly or in part at these highly specialized synapses.
Active zone and release probability
The reliability of synaptic transmission is dependent on many factors. Among the most important are the efficiency of SV docking and priming, and the proximity of Ca2+ channels to SV release sites. Several CAZ proteins are key regulators of these events. For example, RIM1 is essential for SV priming 60,61, and also regulates Ca2+-channel localization by directly binding to the C-terminal tails of N- and P/Q-type Ca2+ channels via its PDZ domain 61. Conditional RIM1/2 knockout mice exhibit a reduction in presynaptic Ca2+-channel density at SV release sites 62. A similar phenotype is seen in Drosophila, where RIM1 loss-of-function also leads to a reduced density of Ca2+ channels at NMJs 19. However, RIM is not the only AZ protein responsible for the subcellular localization of Ca2+ channels. For example, Bassoon loss-of-function also reduces the density of channel subunits (Cav2.1) at the AZ, through an interaction with RIM-binding protein (RBP) 63. A central role for RBP in calcium channel clusters has also been shown in Drosophila 21.
Synaptic vesicle priming, a process that activates SVs for exocytosis, is another important determinant of vesicular release probability (for a comprehensive review, see 64). Two AZ proteins critical for priming are RIM and Munc13. Munc13 plays an essential role in converting the SNARE complex protein Syntaxin-1 into an open conformation, allowing for formation of the full SNARE complex with VAMP-2 and SNAP25, and thus SV fusion 65-67. In contrast, RIMs are thought to play a structural role during SV priming, recruiting Munc13 to the AZ (through an interaction between their N-terminal Zn2+-finger (Znf) domains and the N-terminal C2A domain of Munc13) 68,69. However, more recently, this interaction was shown to functionally convert Munc13 from an inactive homodimer into an active RIM/Munc13 heterodimer, thereby activating its priming function 70. Consistent with these findings, studies in C. elegans show that interactions between RIM/Unc10 and Unc13 are important not only for efficient neurotransmitter release, but also for the precise localization of Unc13 within the AZ 71. For instance, in Unc13 mutants lacking their RIM-binding C2A domains, SV docking occurred distal to the AZ, potentially displacing SVs from their calcium source and thus reducing release probability 71. In addition, Unc13 paralogs and isoforms may contribute functionally to synaptic heterogeneity at vertebrate synapses. For example, while all Unc13 isoforms share a similar C-terminus, they differ significantly in their N-termini. On one hand, some isoforms lack the C2A domain important for RIM interaction, leading to less efficient and more asynchronous release in C. elegans 72. Even among the mammalian Munc13 isoforms (Munc13-1 and ubMunc13-2), both of which contain functional C2A domains, release probability and short-term plasticity characteristics differ significantly 73,74, indicating that Munc13 regulates release efficiency through several mechanisms.
Importantly, changes in SV release probability, and thus synaptic reliability, can be dynamically regulated. In general, synaptic strength/efficacy correlates with the number of readily releasable vesicles (N) docked at the AZ, which are proportional to the active zone area and their vesicular release probability (Pvr) 75,76. Thus, larger terminals typically have higher synaptic release probabilities 77,78. Changes in these parameters during synaptic plasticity can alter neurotransmitter release. For example, the size and number of active zones in Aplysia, as well as the number of vesicles close to the plasma membrane, change upon habituation or sensitization of the gill-withdrawal reflex 79. Similarly, facilitated NMJ synapses in the crayfish have more AZs and an increased number of readily releasable SVs 80. These observations imply a tight relationship between the functional properties of a synapse and the size of the AZ.
Recent studies have begun to elucidate the molecular underpinnings of this relationship. For example, at the Drosophila NMJ, changes in vesicular release probability and readily releasable vesicle pool size during synaptic plasticity correlate with AZ size, as well as with levels of the core AZ protein BRP 81. Intriguingly, these structural changes can be fast, occurring within minutes after plasticity induction, as well as long-lasting 81. Importantly, conditions that promote homeostatic changes in synaptic strength and release probability trigger changes not only in the composition of CAZ proteins, but also in key regulators of vesicular release probability such as synaptotagmin and P/Q-type calcium channels 82. Studies in Drosophilia suggest that one key regulator in this process is the AZ protein RIM 83.
These observations raise fundamental questions about how synaptic activity sculpts AZ size and vesicular release probability. One emerging theme is that bidirectional signaling, via a growing number of transsynaptic cell adhesion molecules 84-86, couples changes in postsynaptic responsiveness with presynaptic efficacy. The best-characterized of these is the Neurexin–Neuroligin (Nrx/Nlg) complex, whose postsynaptic activation can potently enhance presynaptic size and reliability 87,88, through a still-unknown mechanism. Studies in Drosophila suggest that Syd1 is a critical molecule in this signaling cascade, as its binding to the C-terminal tail of Nrx1 controls not only the number and stability of Nrx1/Nlg1 complexes, but also synaptic size and reliability 26.
A second emerging theme is bidirectional signaling between the presynaptic compartment and the nucleus. While transsynaptic signaling can promote real-time changes in synaptic strength, long-lasting changes and homeostatic plasticity are known to require changes in gene expression. The best-characterized mechanism involves the translocation of signaling molecules from the postsynaptic density to the nucleus 89. Studies by Ivanova and colleagues recently found that presynaptic AZs also participate in this conversation. Specifically, the Bassoon binding partner CtBP1 is a transcription factor that transits between presynaptic AZs and the nucleus in response to homeostatic changes in synaptic strength 90. How CtBP1-mediated signaling regulates synaptic size and function remains to be explored.
Exo-endocytosis coupling
After priming, readily releasable SVs fuse with the plasma membrane and release neurotransmitter upon Ca2+ influx. To maintain exocytosis, release sites must not only be cleared of excess synaptic proteins and membranes, but these proteins must also be retrieved and reassembled into functional SVs, necessitating tight coupling between exocytosis and endocytosis 91,92.
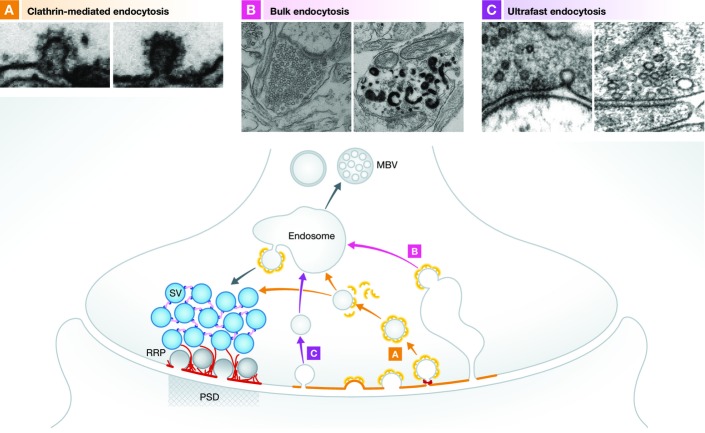
Emerging evidence points toward a role for CAZ scaffold proteins in this coupling, though many questions remain. Over the last decade, most efforts have focused on whether and how exo- and endocytosis are coupled. Relevant to this issue is the type of endocytosis being examined. Until recently, three forms of endocytosis have been described at synapses: kiss-and-run, clathrin-mediated, and bulk 93 (Fig5). Kiss-and-run, in which vesicles do not fully fuse with the membrane but transiently flicker open at a fusion pore, is the fastest of these, occurring within ∼1 s 94,95. Clathrin-mediated endocytosis (CME) is the most common form of endocytosis 96, facilitating retrieval of SV proteins in tens of seconds following full-collapse fusion of SVs. CME occurs in a spatially separate region of presynaptic plasma membrane referred to as the peri-active zone 93,97 (Fig5). Bulk endocytosis is a pathway triggered by strong stimulation, mediating the retrieval of SV proteins via large endocytic vacuoles. Clathrin-mediated budding is subsequently used to regenerate new SVs from these structures 98,99 (Fig5).
Figure 5.
Synaptic vesicle cycle at the presynaptic terminal
The presynaptic AZ functionally defines the space within boutons where upon calcium influx synaptic vesicle fusion and neurotransmission takes place (lower panel). It is the center of the SV life cycle. Vesicles are recruited from the vesicle cluster toward the AZ where they undergo maturation steps such as docking and priming and finally fuse with the plasma membrane upon action potential stimulation. After exocytosis, SV protein and membrane retrieval occurs through endocytosis in a region spatially adjacent to the active zone, the peri-active zone. It is an important compensatory reaction to recapture excess membrane and generate new SVs. Different endocytosis pathways are known and can be visualized by EM following evoke stimulation (top panels). Clathrin-mediated endocytosis (A) is a slow form of membrane retrieval. Synaptic vesicle proteins and membrane are taken up through the formation of clathrin-coated pits, which are later pinched off from the membrane through the GTPase dynamin. After uncoating, the newly formed vesicles either join the vesicle cluster or pass an additional sorting step through an early endosome. Bulk endocytosis (B) is a second form of endocytosis that mainly takes place during strong stimulation. Large membrane fractions are collected in big invaginations and pinched off from the plasma membrane. New vesicles are then formed via clathrin-mediated budding from these structures. Most recently, a new mode of endocytosis has been described, ultrafast endocytosis (C). It is a very fast retrieval mechanism as it can take place within 50–100 ms after stimulation. Vesicles are pinched off from the plasma membrane at the edge of the active zone. Free vesicles fuse with a sorting endosome from which new synaptic vesicles are formed in a clathrin-dependent manner. Top panels, EM micrographs of clathrin-mediated (A), bulk (B), and ultrafast (C) endocytosis. Reproduced with permission from Frauke Ackermann, Joshua A. Gregory, and Lennart Brodin (A); 156 (B); 100 (C).
Unfortunately, all three mechanisms are rather slow, making it difficult to explain how sufficient SV regeneration c an occur at high firing rates. In a groundbreaking set of studies, Watanabe et al have used a “flash-freeze” approach to capture an “ultrafast” form of endocytosis that occurs within 50 and 100 ms after stimulation 100,101 (Fig5). Importantly, these authors find that ultrafast endocytosis captures ∼ four vesicles at a time and occurs at the very edge of the AZ, implying that endocytic zones are functionally and perhaps physically linked to AZs. However, this linking mechanism remains unknown.
One obvious link between the AZ and endocytic zone is calcium, which plays fundamental roles in exocytosis 102. Studies at the frog NMJ provided early evidence that endocytosis requires calcium. Here, α-Latrotoxin, a spider toxin that triggers calcium-independent exocytosis of SVs, was used to show that low levels of extracellular calcium depress endocytosis following α-Latrotoxin-mediated SV exocytosis 103. This conclusion is also supported by calcium buffers such as BAPTA that decreases endocytosis rates at synapses by up to 1,000-fold 104. Consistently, calcium itself has been found to regulate endocytosis at synapses of different types 105-107,108,109.
A fundamental but unresolved question is where this calcium comes from. One concept, put forth by Yao and colleagues, is that SVs carry VGCCs, which are inserted into the plasma membrane following SV exocytosis, supplying the calcium necessary for endocytosis. One potential candidate, identified in Drosophila, is the vesicular protein Flower 110. However, while Flower appears to be a calcium channel, its conductance is small 110. Moreover, subsequent studies failed to find evidence that exocytosis transiently inserts calcium channels into the plasma membrane 111. Rather, these authors found that more traditional VGCCs (e.g., P/Q-type) provided the calcium essential to trigger both rapid and slow endocytosis at the presynaptic membrane of the Calyx of Held 111 (see also 92). As discussed above, several of these VGCCs are physically tethered to AZs by the scaffold protein RIM and RBP 17-61, allowing rapid coupling between action potential-mediated calcium influx and SV fusion. Yet as discussed below, endocytic zones lie in a peri-active zonal region 93, raising questions of whether calcium entry at the AZ remains at sufficiently high levels to facilitate endocytosis [see also 92,112]. Intriguingly, endocytosis still occurs at the Drosophila NMJ when the main AZ VGCC, cacophony, is absent 113, but can be inhibited by several different calcium channel blockers. These data suggest that exocytosis and endocytosis may use distinct calcium channels [113,114; see also 91], a concept supported by experiments at the calyx of Held 115, but raise new questions of how these channels are tethered near AZs.
Studies at both Drosophila NMJs and rat hippocampal synapses are beginning to provide answers to these questions. For example, super-resolution microscopy was recently used to show that several different endocytic proteins, for example, Intersectin, Stonin, and GIT1, exhibit a punctate peri-active zone-like pattern around T-bars and AZs labeled with BRP or Bassoon, respectively 116-118. These findings suggest that SV exocytosis sites are surrounded by an array of discrete endocytic zones or hot spots. This radial arrangement implies the presence of molecular tethers that link them to AZs.
At present, the identities of these tethers are unknown. Potential candidates include the AZ scaffold proteins Piccolo, Bassoon, ELKs/CAST, or RIM, as their large sizes could bridge multiple presynaptic micro-domains over distances of several hundred nanometers. Of these, Piccolo is the most intriguing, as it has been found to scaffold proteins critical for endocytosis. These include Profilin and Daam1, which together with Piccolo promote F-actin assembly within presynaptic boutons in an activity-dependent manner 119,120. Piccolo also binds Abp1 and GIT1 121,122. The former is an actin-binding protein that also interacts with dynamin, another key endocytic protein, while the latter is a G-protein-coupled receptor kinase-interacting (GIT) protein that directly associates with the endocytic adaptor protein Stonin2/StonedB 118. In synapses lacking GIT1, Stonin2/StonedB is displaced and SV recycling impaired, implying that GIT1 acts as a bridge between endocytic zones, defined by Stonin2/StonedB, and active zones 118. Whether the Piccolo-GIT1 association 122 is a further component of this bridge will require further investigation. Intriguingly, the dynamic assembly of F-actin is required for ultrafast endocytosis and bulk endocytosis 100-124. Given the capacity of Piccolo to act as a platform for F-actin assembly during activity-induced SV recycling 120, it will be important to explore its involvement in these endocytic events.
Intersectin is another up-and-coming candidate for spatial coupling of the exocytic and endocytic machinery. It is a multidomain endocytic protein existing in two vertebrate isoforms ITSN-S and ITSN-L 125. ITSN-S consists of two N-terminal EH domains, a coiled-coil region, and four or five SH3 domains. The longer isoform ITSN-L also possesses C-terminal DH, PH and C2 domains 126 providing it with guanine nucleotide exchange factor (GEF) properties for Cdc42 127. Intersectin interacts with several endocytic proteins including dynamin, AP2, epsins, and synaptojanin. Loss-of-function studies in C. elegans and Drosophila show mislocalization of dynamin, endophilin, and synaptojanin, as well as impaired endocytosis 128,129. In vertebrates, intersectin is important not only for endocytosis, but also for exocytosis 130, as ITSN1 loss-of-function impairs exocytosis in PC12 cells and chromaffin cells as well as at the Calyx of Held 116-132. Consistent with this concept, intersectin interacts with the t-SNAREs SNAP23 and SNAP25 133, and shuttles between active and peri-active zones in an activity-dependent manner 134,135. Future studies will need to focus on whether intersectin performs a bridge function linking exo- and endocytosis or is rather a multifunctional adaptor participating in these two aspects of SV recycling.
Synapse stability and integrity
Presynaptic AZs must have the capacity to undergo remodeling during periods of synaptic plasticity, but also to remain stable for long-term synaptic maintenance and information storage 136-138. At present, the mechanisms that control AZ remodeling and stability are poorly understood. Major contributors include cellular systems that control the dynamic exchange and turnover of synaptic proteins 138-140. These have shown that while most proteins (pre or post) exchange with synapses on the order of tens of minutes to hours, protein turnover is much slower, with most synaptic proteins having a half-life of 2–7 days 139. This arrangement seems to make sense, as it allows large neurons to simultaneously control the local dynamics of synapses while maintaining sufficient pools of proteins to support activities taking place at great distances from the cell soma. Importantly, while global degradation rates are low, local turnover, regulated by posttranslational modifications such as phosphorylation and ubiquitination that are sensitive to changes in synaptic activity, can be much higher 141,142. For instance, early proteomic studies demonstrated that synaptic activity could dramatically alter the phosphorylation state of many presynaptic proteins, including Bassoon 143,144, as well as their associations with AZs 145. Similarly, ubiquitination and the proteasome have been observed to underlie changes in AZ protein composition during homeostatic plasticity 82.
Increasing evidence also implicates degradative pathways, including the ubiquitin–proteasome system (UPS) and the autophagy–lysosomal system, in regulating AZ plasticity and integrity 137. For example, Liprinα, RIM1, and Munc13 have been shown to undergo degradation via the UPS, through specific ubiquitinating enzymes (anaphase-promoting complex (APC), Scrapper, and FboX45, respectively) that conjugate lysine 48-linked ubiquitin chains to these proteins, targeting them for proteasomal degradation [see 137]. Ubiquitination of RIM1 and Munc13 also modulates neurotransmitter release probability 146-148,149,150, suggesting that dynamic ubiquitination of these AZ proteins could be an important mechanism for regulating presynaptic plasticity.
Intriguingly, recent work implicates the two largest CAZ proteins, Piccolo and Bassoon, in regulating the local activity of the UPS and endo-lysosomal degradative pathways 151,152. Specifically, Piccolo/Bassoon loss-of-function triggers not only the loss of SV pools and synaptic junctions, but also the accumulation of ubiquitinated proteins, pleiomorphic vesicles, and multi-vesicular bodies within degenerating boutons. Importantly, these phenotypes require the activity of both the UPS and endo-lysosomal systems, as well as the E3 ubiquitin ligase Siah1, whose activity is inhibited by its association with the ZnF domains of Piccolo and Bassoon 151. At present, the function of the AZ-associated degradative system remains rather speculative. One attractive idea is that it performs a surveillance role to facilitate the removal of misfolded/nonfunctional or aging proteins. Alternatively, it could contribute to SV recycling during synaptic transmission. Finally, this system could regulate the growth or elimination of specific synapses during development or periods of synaptic strengthening or weakening. Intriguingly, the phenotypes seen in boutons lacking Piccolo and Bassoon are reminiscent of those seen in animal models of Alzheimer’s and Parkinson’s diseases 153,154, raising the question of whether local dysregulation of AZ-mediated degradative pathways contributes to the etiology and/or progression of neurodegenerative disease.
Summary
Active zones are highly specialized microdomains designed to regulate neurotransmitter release on a millisecond time scale. The development of super-resolution imaging and cryo-EM technologies is providing unprecedented insights into the nanoscale organization of AZs and their relationship to endocytic zones. These technologies, coupled with emerging strategies to tag-specific domains of synaptic proteins, should allow investigators to elucidate the structures and functions of individual CAZ molecules and define their precise roles in synaptic transmission. Recent work has also begun to shed light on the role of AZs in maintaining synapse integrity, by locally regulating the activity of surveillance systems that monitor protein and organelle health. This new area of investigation will certainly contribute to our understanding of both neurodevelopmental and neurodegenerative disorders.
Acknowledgments
We would like to give special thanks to Christian Rosenmund, Volker Hauke, Kang Shen, Noam Ziv, and Stephen Sigrist for helpful suggestions and comments. We would like to apologize to the many investigators whose work we were unable to cite due to space limitations. CCG and FA are supported by German Research Council grants (SFB665, SFB958), NeuroCure, German Center for Neurodegenerative Diseases (DZNE), and the US-Israel Binational Science Foundation. CLW is supported by Columbia University startup funds, the Brain Research Foundation, and National Institute of Health grant #NS080967.
Glossary
Abp
actin-binding protein 1
AP2
adapter protein 2
APC
anaphase-promoting complex
AZ
active zone
BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid
BRP
Bruchpilot
CAST
CAZ-associated structural protein
Cav2.1
P/Q voltage-dependent calcium channel
CAZ
cytomatrix at the active zone
Cdc42
cell division control protein 42
CME
clathrin-mediated endocytosis
CtBP
C-terminal-binding protein 1
Daam
Disheveled-associated activator of morphogenesis 1
DH
Dbl Homology
DP
dense projections
Dunc13
Drosophila homolog of unc13
EH
Eps15 homology
ELKS
protein rich in the amino acids E, L, K, and S
EM
electron microscopy
ERCs
ELKS-Rab6-interacting protein CAST
FboX45
F-box protein 45
GEF
guanine exchange factor
GIT1
ARF GTPase-activating protein 1
Hpf
high-pressure freezing
ITSN-L
intersectin-long
ITSN-S
intersectin-short
kDa
kilodalton
Kif3A
kinesin-like protein 3A
Nlg
neuroligin
NMJ
neuromuscular junction
Nrx
neurexin
PDZ
postsynaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein (zo-1)
PH
pleckstrin homology
PP2A
protein phosphatase 2A
PSD
postsynaptic density
Pvr
vesicular release probability
Rab3A
synaptic 21-kDa GTP-binding protein
RBP
RIM-binding protein
RIM
Rab3-interacting molecule
SH3
Src homology domain 3
Siah1
seven in absentia homolog 1
SNAP
synaptosomal-associated protein
SNARE
soluble _N_-ethylmaleimide-sensitive-factor attachment r_e_ceptor
SV
synaptic vesicle
Syd1
synaptic scaffold protein 1
Unc13
uncoordination mutant 13
UPS
ubiquitin–proteasome system
VAMP-2
synaptobrevin-2
VGCC
Voltage-gated calcium channel
Wrd
Well rounded
Zn
Zinc
ZnF
Zinc finger
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- Jin Y, Garner CC. Molecular mechanisms of presynaptic differentiation. Annu Rev Cell Dev Biol. 2008;24:237–262. doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- Szule JA, Harlow ML, Jung JH, De-Miguel FF, Marshall RM, McMahan UJ. Regulation of synaptic vesicle docking by different classes of macromolecules in active zone material. PLoS ONE. 2012;7:e33333. doi: 10.1371/journal.pone.0033333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Liu Q, Davis MW, Hollopeter G, Thomas N, Jorgensen NB, Jorgensen EM. Ultrafast endocytosis at Caenorhabditis elegans neuromuscular junctions. eLife. 2013;2:e00723. doi: 10.7554/eLife.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–375. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]
- Yeh E, Kawano T, Weimer RM, Bessereau JL, Zhen M. Identification of genes involved in synaptogenesis using a fluorescent active zone marker in Caenorhabditis elegans. J Neurosci. 2005;25:3833–3841. doi: 10.1523/JNEUROSCI.4978-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Na M, Kim S, Lee JR, Kim E. Interaction of the ERC family of RIM-binding proteins with the liprin-alpha family of multidomain proteins. J Biol Chem. 2003;278:42377–42385. doi: 10.1074/jbc.M307561200. [DOI] [PubMed] [Google Scholar]
- Stigloher C, Zhan H, Zhen M, Richmond J, Bessereau JL. The presynaptic dense projection of the Caenorhabditis elegans cholinergic neuromuscular junction localizes synaptic vesicles at the active zone through SYD-2/liprin and UNC-10/RIM-dependent interactions. J Neurosci. 2011;31:4388–4396. doi: 10.1523/JNEUROSCI.6164-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelmann M, Hegermann J, Goncharov A, Taru H, Ellisman MH, Richmond JE, Jin Y, Eimer S. Liprin-alpha/SYD-2 determines the size of dense projections in presynaptic active zones in C. elegans. J Cell Biol. 2013;203:849–863. doi: 10.1083/jcb.201302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer RM, Gracheva EO, Meyrignac O, Miller KG, Richmond JE, Bessereau JL. UNC-13 and UNC-10/rim localize synaptic vesicles to specific membrane domains. J Neurosci. 2006;26:8040–8047. doi: 10.1523/JNEUROSCI.2350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtova A, Kao HT, Greengard P, Gundelfinger ED, Triller A, et al. Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Harada A, Takeda S, Kobayashi K, Terada S, Noda T, Takahashi T, Hirokawa N. Synapsin I deficiency results in the structural change in the presynaptic terminals in the murine nervous system. J Cell Biol. 1995;131:1789–1800. doi: 10.1083/jcb.131.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Sigrist SJ. Assembling the presynaptic active zone. Curr Opin Neurobiol. 2009;19:311–318. doi: 10.1016/j.conb.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Contribution of active zone subpopulation of vesicles to evoked and spontaneous release. J Neurophysiol. 1999;81:1495–1505. doi: 10.1152/jn.1999.81.4.1495. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovic T, Siebert M, Knoche E, Depner H, Mertel S, Owald D, Schmidt M, Thomas U, Sickmann A, Kamin D, et al. The Bruchpilot cytomatrix determines the size of the readily releasable pool of synaptic vesicles. J Cell Biol. 2013;202:667–683. doi: 10.1083/jcb.201301072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Valakh V, Wright CM, Wu C, Liu Z, Zhang YQ, DiAntonio A. RIM promotes calcium channel accumulation at active zones of the Drosophila neuromuscular junction. J Neurosci. 2012;32:16586–16596. doi: 10.1523/JNEUROSCI.0965-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sugita S, Sudhof TC. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J Biol Chem. 2000;275:20033–20044. doi: 10.1074/jbc.M909008199. [DOI] [PubMed] [Google Scholar]
- Liu KS, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, Matkovic T, Muhammad K, Depner H, Mettke C, et al. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
- Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, Shen K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat Neurosci. 2006;9:1488–1498. doi: 10.1038/nn1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Schmitz D. Structural and functional plasticity of the cytoplasmic active zone. Curr Opin Neurobiol. 2011;21:144–150. doi: 10.1016/j.conb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Bruckner JJ, Gratz SJ, Slind JK, Geske RR, Cummings AM, Galindo SE, Donohue LK, O’Connor-Giles KM. Fife, a Drosophila Piccolo-RIM homolog, promotes active zone organization and neurotransmitter release. J Neurosci. 2012;32:17048–17058. doi: 10.1523/JNEUROSCI.3267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banovic D, Khorramshahi O, Owald D, Wichmann C, Riedt T, Fouquet W, Tian R, Sigrist SJ, Aberle H. Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron. 2010;66:724–738. doi: 10.1016/j.neuron.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Owald D, Khorramshahi O, Gupta VK, Banovic D, Depner H, Fouquet W, Wichmann C, Mertel S, Eimer S, Reynolds E, et al. Cooperation of Syd-1 with Neurexin synchronizes pre- with postsynaptic assembly. Nat Neurosci. 2012;15:1219–1226. doi: 10.1038/nn.3183. [DOI] [PubMed] [Google Scholar]
- Mozer BA, Sandstrom DJ. Drosophila neuroligin 1 regulates synaptic growth and function in response to activity and phosphoinositide-3-kinase. Mol Cell Neurosci. 2012;51:89–100. doi: 10.1016/j.mcn.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Fouquet W, Schmidt M, Wichmann C, Mertel S, Depner H, Christiansen F, Zube C, Quentin C, Korner J, et al. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J Cell Biol. 2010;188:565–579. doi: 10.1083/jcb.200908055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Tian X, Zhu M, Bulgari D, Bohme MA, Goettfert F, Wichmann C, Sigrist SJ, Levitan ES, Wu C. Drosophila Syd-1, liprin-alpha, and protein phosphatase 2A B’ subunit Wrd function in a linear pathway to prevent ectopic accumulation of synaptic materials in distal axons. J Neurosci. 2014;34:8474–8487. doi: 10.1523/JNEUROSCI.0409-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, et al. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann N, Sauer M, Kittel RJ. Super-resolution microscopy of the synaptic active zone. Front Cell Neurosci. 2015;9:7. doi: 10.3389/fncel.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm BG, Mandad S, Truckenbrodt S, Krohnert K, Schafer C, Rammner B, Koo SJ, Classen GA, Krauss M, Haucke V, et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science. 2014;344:1023–1028. doi: 10.1126/science.1252884. [DOI] [PubMed] [Google Scholar]
- Lassek M, Weingarten J, Volknandt W. The synaptic proteome. Cell Tissue Res. 2015;359:255–265. doi: 10.1007/s00441-014-1943-4. [DOI] [PubMed] [Google Scholar]
- Fernandez-Busnadiego R, Asano S, Oprisoreanu AM, Sakata E, Doengi M, Kochovski Z, Zurner M, Stein V, Schoch S, Baumeister W, et al. Cryo-electron tomography reveals a critical role of RIM1alpha in synaptic vesicle tethering. J Cell Biol. 2013;201:725–740. doi: 10.1083/jcb.201206063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog’s neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. J Comp Neurol. 2009;513:457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow ML, Szule JA, Xu J, Jung JH, Marshall RM, McMahan UJ. Alignment of synaptic vesicle macromolecules with the macromolecules in active zone material that direct vesicle docking. PLoS ONE. 2013;8:e69410. doi: 10.1371/journal.pone.0069410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi G, Matthews G. The molecular architecture of ribbon presynaptic terminals. Mol Neurobiol. 2009;39:130–148. doi: 10.1007/s12035-009-8058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regus-Leidig H, Brandstatter JH. Structure and function of a complex sensory synapse. Acta Physiol. 2012;204:479–486. doi: 10.1111/j.1748-1716.2011.02355.x. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtova A, Bracko O, Gundelfinger ED, Brandstatter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regus-Leidig H, Ott C, Lohner M, Atorf J, Fuchs M, Sedmak T, Kremers J, Fejtova A, Gundelfinger ED, Brandstatter JH. Identification and immunocytochemical characterization of Piccolino, a novel Piccolo splice variant selectively expressed at sensory ribbon synapses of the eye and ear. PLoS ONE. 2013;8:e70373. doi: 10.1371/journal.pone.0070373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstatter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wassle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangrsic T, Khimich D, Fejtova A, Gundelfinger ED, Liberman MC, Harke B, et al. Bassoon and the synaptic ribbon organize Ca(2)+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu X, Biederer T, Sudhof TC. A family of RIM-binding proteins regulated by alternative splicing: implications for the genesis of synaptic active zones. Proc Natl Acad Sci U S A. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi-Tawarada M, Inoue E, Takao-Rikitsu E, Inoue M, Kitajima I, Ohtsuka T, Takai Y. Active zone protein CAST is a component of conventional and ribbon synapses in mouse retina. J Comp Neurol. 2006;495:480–496. doi: 10.1002/cne.20893. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Specht D, Strenzke N, Hida Y, Krishnamoorthy V, Schmidt KF, Inoue E, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, et al. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J Neurosci. 2012;32:12192–12203. doi: 10.1523/JNEUROSCI.0752-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regus-Leidig H, Fuchs M, Lohner M, Leist SR, Leal-Ortiz S, Chiodo VA, Hauswirth WW, Garner CC, Brandstatter JH. In vivo knockdown of Piccolino disrupts presynaptic ribbon morphology in mouse photoreceptor synapses. Front Cell Neurosci. 2014;8:259. doi: 10.3389/fncel.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regus-Leidig H, Tom Dieck S, Specht D, Meyer L, Brandstatter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J Comp Neurol. 2009;512:814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- Regus-Leidig H, tom Dieck S, Brandstatter JH. Absence of functional active zone protein Bassoon affects assembly and transport of ribbon precursors during early steps of photoreceptor synaptogenesis. Eur J Cell Biol. 2010;89:468–475. doi: 10.1016/j.ejcb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J Neurosci. 2008;28:7954–7967. doi: 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AB, Rutherford MA, Gabrielaitis M, Pangrsic T, Gottfert F, Frank T, Michanski S, Hell S, Wolf F, Wichmann C, et al. Developmental refinement of hair cell synapses tightens the coupling of Ca2+ influx to exocytosis. EMBO J. 2014;33:247–264. doi: 10.1002/embj.201387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Z, Rutherford MA, Takago H, Frank T, Fejtova A, Khimich D, Moser T, Strenzke N. Disruption of the presynaptic cytomatrix protein bassoon degrades ribbon anchorage, multiquantal release, and sound encoding at the hair cell afferent synapse. J Neurosci. 2013;33:4456–4467. doi: 10.1523/JNEUROSCI.3491-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouvian R, Neef J, Bulankina AV, Reisinger E, Pangrsic T, Frank T, Sikorra S, Brose N, Binz T, Moser T. Exocytosis at the hair cell ribbon synapse apparently operates without neuronal SNARE proteins. Nat Neurosci. 2011;14:411–413. doi: 10.1038/nn.2774. [DOI] [PubMed] [Google Scholar]
- Vogl C, Cooper BH, Neef J, Wojcik SM, Reim K, Reisinger E, Brose N, Rhee JS, Moser T, Wichmann C. Unconventional molecular regulation of synaptic vesicle replenishment in cochlear inner hair cells. J Cell Sci. 2015;128:638–644. doi: 10.1242/jcs.162099. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM determines Ca(2)+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydova D, Marini C, King C, Klueva J, Bischof F, Romorini S, Montenegro-Venegas C, Heine M, Schneider R, Schroder MS, et al. Bassoon specifically controls presynaptic P/Q-type Ca(2+) channels via RIM-Binding Protein. Neuron. 2014;82:181–194. doi: 10.1016/j.neuron.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339:421–425. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Sudhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Machius M, Dulubova I, Dai H, Sudhof TC, Tomchick DR, Rizo J. Structural basis for a Munc13-1 homodimer to Munc13-1/RIM heterodimer switch. PLoS Biol. 2006;4:e192. doi: 10.1371/journal.pbio.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Kaeser PS, Xu W, Sudhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Stawicki TM, Goncharov A, Jin Y. Position of UNC-13 in the active zone regulates synaptic vesicle release probability and release kinetics. eLife. 2013;2:e01180. doi: 10.7554/eLife.01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Tong XJ, Kaplan JM. UNC-13L, UNC-13S, and Tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans. eLife. 2013;2:e00967. doi: 10.7554/eLife.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Rhee JS, Jahn O, Varoqueaux F, Spiess J, Waxham MN, Rosenmund C, Brose N. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, et al. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Branco T, Marra V, Staras K. Examining size-strength relationships at hippocampal synapses using an ultrastructural measurement of synaptic release probability. J Struct Biol. 2010;172:203–210. doi: 10.1016/j.jsb.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983;220:91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Marin L, Atwood HL. Activity-induced changes in synaptic release sites at the crayfish neuromuscular junction. J Neurosci. 1994;14:3688–3703. doi: 10.1523/JNEUROSCI.14-06-03688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyhersmuller A, Hallermann S, Wagner N, Eilers J. Rapid active zone remodeling during synaptic plasticity. J Neurosci. 2011;31:6041–6052. doi: 10.1523/JNEUROSCI.6698-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Schone C, Heine M, Gundelfinger ED, Fejtova A. Extensive remodeling of the presynaptic cytomatrix upon homeostatic adaptation to network activity silencing. J Neurosci. 2011;31:10189–10200. doi: 10.1523/JNEUROSCI.2088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Liu KS, Sigrist SJ, Davis GW. RIM controls homeostatic plasticity through modulation of the readily-releasable vesicle pool. J Neurosci. 2012;32:16574–16585. doi: 10.1523/JNEUROSCI.0981-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr Opin Neurobiol. 2011;21:132–143. doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K. Transsynaptic modulation of the synaptic vesicle cycle by cell-adhesion molecules. J Neurosci Res. 2008;86:223–232. doi: 10.1002/jnr.21484. [DOI] [PubMed] [Google Scholar]
- Arons MH, Thynne CJ, Grabrucker AM, Li D, Schoen M, Cheyne JE, Boeckers TM, Montgomery JM, Garner CC. Autism associated mutations in ProSAP2/Shank3 impair synaptic transmission and neurexin-neuroligin mediated transsynaptic signaling Journal. Neuroscience. 2012;32:14966–14978. doi: 10.1523/JNEUROSCI.2215-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera S, Kreutz MR. When synaptic proteins meet the genome: transcriptional regulation in cell death and plasticity by the synapto-nuclear messenger Jacob. Neuropsychopharmacology. 2014;39:245–246. doi: 10.1038/npp.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova D, Dirks A, Montenegro-Venegas C, Schone C, Altrock WD, Marini C, Frischknecht R, Schanze D, Zenker M, Gundelfinger ED, et al. Synaptic activity controls localization and function of CtBP1 via binding to Bassoon and Piccolo. EMBO J. 2015;34:1056–1077. doi: 10.15252/embj.201488796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 2011;12:127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- Wu LG, Hamid E, Shin W, Chiang HC. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol. 2014;76:301–331. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol. 2012;4:a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Bai J, Chapman ER. Two modes of exocytosis at hippocampal synapses revealed by rate of FM1-43 efflux from individual vesicles. J Cell Biol. 2005;168:929–939. doi: 10.1083/jcb.200407148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cao YQ, Tsien RW. Quantum dots provide an optical signal specific to full collapse fusion of synaptic vesicles. Proc Natl Acad Sci USA. 2007;104:17843–17848. doi: 10.1073/pnas.0706906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Cheung G, Jupp OJ, Cousin MA. Activity-dependent bulk endocytosis and clathrin-dependent endocytosis replenish specific synaptic vesicle pools in central nerve terminals. J Neurosci. 2010;30:8151–8161. doi: 10.1523/JNEUROSCI.0293-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Rost BR, Camacho-Perez M, Davis MW, Sohl-Kielczynski B, Rosenmund C, Jorgensen EM. Ultrafast endocytosis at mouse hippocampal synapses. Nature. 2013;504:242–247. doi: 10.1038/nature12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Trimbuch T, Camacho-Perez M, Rost BR, Brokowski B, Sohl-Kielczynski B, Felies A, Davis MW, Rosenmund C, Jorgensen EM. Clathrin regenerates synaptic vesicles from endosomes. Nature. 2014;515:228–233. doi: 10.1038/nature13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Armbruster M, Ryan TA. Calcium control of endocytic capacity at a CNS synapse. J Neurosci. 2008;28:6742–6749. doi: 10.1523/JNEUROSCI.1082-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Neves G, Gomis A, Lagnado L. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc Natl Acad Sci U S A. 2001;98:15282–15287. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci. 2005;25:11676–11683. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, Wensel TG, Bellen HJ. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–960. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Zhang Z, McNeil BD, Luo F, Wu XS, Sheng J, Shin W, Wu LG. Voltage-dependent calcium channels at the plasma membrane, but not vesicular channels, couple exocytosis to endocytosis. Cell Rep. 2012;1:632–638. doi: 10.1016/j.celrep.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T. Ca2+-dependent regulation of synaptic vesicle endocytosis. Neurosci Res. 2012;73:1–7. doi: 10.1016/j.neures.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Honda A, Kidokoro Y. Ca2+ influx through distinct routes controls exocytosis and endocytosis at drosophila presynaptic terminals. Neuron. 2004;41:101–111. doi: 10.1016/s0896-6273(03)00815-8. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Ueno K, Kidokoro Y. Two types of Ca channel linked to two endocytic pathways coordinately maintain synaptic transmission at the Drosophila synapse. Eur J Neurosci. 2010;32:335–346. doi: 10.1111/j.1460-9568.2010.07300.x. [DOI] [PubMed] [Google Scholar]
- Midorikawa M, Okamoto Y, Sakaba T. Developmental changes in Ca2+ channel subtypes regulating endocytosis at the calyx of Held. J Physiol. 2014;592:3495–3510. doi: 10.1113/jphysiol.2014.273243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Kononenko NL, Bacetic J, Pechstein A, Schmoranzer J, Yao L, Barth H, Shupliakov O, Kobler O, Aktories K, et al. Fast neurotransmitter release regulated by the endocytic scaffold intersectin. Proc Natl Acad Sci U S A. 2013;110:8266–8271. doi: 10.1073/pnas.1219234110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NL, Diril MK, Puchkov D, Kintscher M, Koo SJ, Pfuhl G, Winter Y, Wienisch M, Klingauf J, Breustedt J, et al. Compromised fidelity of endocytic synaptic vesicle protein sorting in the absence of stonin 2. Proc Natl Acad Sci USA. 2013;110:E526–E535. doi: 10.1073/pnas.1218432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podufall J, Tian R, Knoche E, Puchkov D, Walter AM, Rosa S, Quentin C, Vukoja A, Jung N, Lampe A, et al. A presynaptic role for the cytomatrix protein GIT in synaptic vesicle recycling. Cell Rep. 2014;7:1417–1425. doi: 10.1016/j.celrep.2014.04.051. [DOI] [PubMed] [Google Scholar]
- Waites CL, Leal-Ortiz SA, Andlauer TF, Sigrist SJ, Garner CC. Piccolo regulates the dynamic assembly of presynaptic f-actin. J Neurosci. 2011;31:14250–14263. doi: 10.1523/JNEUROSCI.1835-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh D, Terry-Lorenzo R, Waites CL, Leal-Ortiz SA, Maas C, Reimer RJ, Garner CC. Piccolo directs activity dependent F-actin assembly from presynaptic active zones via Daam1. PLoS ONE. 2015;10:e0120093. doi: 10.1371/journal.pone.0120093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster SD, Kessels MM, Qualmann B, Chung WJ, Nash J, Gundelfinger ED, Garner CC. Interactions between Piccolo and the actin/dynamin-binding protein Abp1 link vesicle endocytosis to presynaptic active zones. J Biol Chem. 2003;278:20268–20277. doi: 10.1074/jbc.M210792200. [DOI] [PubMed] [Google Scholar]
- Kim S, Ko J, Shin H, Lee JR, Lim C, Han JH, Altrock WD, Garner CC, Gundelfinger ED, Premont RT, et al. The GIT family of proteins forms multimers and associates with the presynaptic cytomatrix protein Piccolo. J Biol Chem. 2003;278:6291–6300. doi: 10.1074/jbc.M212287200. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Maucort G, Sullivan RK, Schenning M, Lavidis NA, McCluskey A, Robinson PJ, Meunier FA. Actin- and dynamin-dependent maturation of bulk endocytosis restores neurotransmission following synaptic depletion. PLoS ONE. 2012;7:e36913. doi: 10.1371/journal.pone.0036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbergenova Y, Bykhovskaia M. Enhancement of the endosomal endocytic pathway increases quantal size. Mol Cell Neurosci. 2009;40:199–206. doi: 10.1016/j.mcn.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guipponi M, Scott HS, Chen H, Schebesta A, Rossier C, Antonarakis SE. Two isoforms of a human intersectin (ITSN) protein are produced by brain-specific alternative splicing in a stop codon. Genomics. 1998;53:369–376. doi: 10.1006/geno.1998.5521. [DOI] [PubMed] [Google Scholar]
- Roos J, Kelly RB. Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with Drosophila dynamin. J Biol Chem. 1998;273:19108–19119. doi: 10.1074/jbc.273.30.19108. [DOI] [PubMed] [Google Scholar]
- Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N, et al. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chu PY, Bowser DN, Keating DJ, Dubach D, Harper I, Tkalcevic J, Finkelstein DI, Pritchard MA. Mice deficient for the chromosome 21 ortholog Itsn1 exhibit vesicle-trafficking abnormalities. Hum Mol Genet. 2008;17:3281–3290. doi: 10.1093/hmg/ddn224. [DOI] [PubMed] [Google Scholar]
- Malacombe M, Ceridono M, Calco V, Chasserot-Golaz S, McPherson PS, Bader MF, Gasman S. Intersectin-1L nucleotide exchange factor regulates secretory granule exocytosis by activating Cdc42. EMBO J. 2006;25:3494–3503. doi: 10.1038/sj.emboj.7601247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momboisse F, Ory S, Calco V, Malacombe M, Bader MF, Gasman S. Calcium-regulated exocytosis in neuroendocrine cells: intersectin-1L stimulates actin polymerization and exocytosis by activating Cdc42. Ann N Y Acad Sci. 2009;1152:209–214. doi: 10.1111/j.1749-6632.2008.03998.x. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Schoch S, Sudhof TC. EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J Biol Chem. 1999;274:18446–18454. doi: 10.1074/jbc.274.26.18446. [DOI] [PubMed] [Google Scholar]
- Evergren E, Benfenati F, Shupliakov O. The synapsin cycle: a view from the synaptic endocytic zone. J Neurosci Res. 2007;85:2648–2656. doi: 10.1002/jnr.21176. [DOI] [PubMed] [Google Scholar]
- Winther AM, Jiao W, Vorontsova O, Rees KA, Koh TW, Sopova E, Schulze KL, Bellen HJ, Shupliakov O. The dynamin-binding domains of Dap160/intersectin affect bulk membrane retrieval in synapses. J Cell Sci. 2013;126:1021–1031. doi: 10.1242/jcs.118968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- Waites CL, Garner CC. Presynaptic function in health and disease. Trends Neurosci. 2011;34:326–337. doi: 10.1016/j.tins.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Fisher-Lavie A. Presynaptic and postsynaptic scaffolds: dynamics fast and slow. Neuroscientist. 2014;20:439–452. doi: 10.1177/1073858414523321. [DOI] [PubMed] [Google Scholar]
- Cohen LD, Zuchman R, Sorokina O, Muller A, Dieterich DC, Armstrong JD, Ziv T, Ziv NE. Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PLoS ONE. 2013;8:e63191. doi: 10.1371/journal.pone.0063191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minerbi A, Kahana R, Goldfeld L, Kaufman M, Marom S, Ziv NE. Long-term relationships between synaptic tenacity, synaptic remodeling, and network activity. PLoS Biol. 2009;7:e1000136. doi: 10.1371/journal.pbio.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Lavie A, Zeidan A, Stern M, Garner CC, Ziv NE. Use dependence of presynaptic tenacity. J Neurosci. 2011;31:16770–16780. doi: 10.1523/JNEUROSCI.3384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Pothula S, Andres-Alonso M, Fejtova A. Molecular mechanisms driving homeostatic plasticity of neurotransmitter release. Front Cell Neurosci. 2013;7:244. doi: 10.3389/fncel.2013.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MO, Yu L, Coba MP, Husi H, Campuzano I, Blackstock WP, Choudhary JS, Grant SG. Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem. 2005;280:5972–5982. doi: 10.1074/jbc.M411220200. [DOI] [PubMed] [Google Scholar]
- Munton RP, Tweedie-Cullen R, Livingstone-Zatchej M, Weinandy F, Waidelich M, Longo D, Gehrig P, Potthast F, Rutishauser D, Gerrits B, et al. Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol Cell Proteomics. 2007;6:283–293. doi: 10.1074/mcp.M600046-MCP200. [DOI] [PubMed] [Google Scholar]
- Schroder MS, Stellmacher A, Romorini S, Marini C, Montenegro-Venegas C, Altrock WD, Gundelfinger ED, Fejtova A. Regulation of presynaptic anchoring of the scaffold protein Bassoon by phosphorylation-dependent interaction with 14-3-3 adaptor proteins. PLoS ONE. 2013;8:e58814. doi: 10.1371/journal.pone.0058814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese SD, Trotta N, Rodesch CK, Aravamudan B, Broadie K. The ubiquitin proteasome system acutely regulates presynaptic protein turnover and synaptic efficacy. Curr Biol. 2003;13:899–910. doi: 10.1016/s0960-9822(03)00338-5. [DOI] [PubMed] [Google Scholar]
- Yao I, Takagi H, Ageta H, Kahyo T, Sato S, Hatanaka K, Fukuda Y, Chiba T, Morone N, Yuasa S, et al. SCRAPPER-dependent ubiquitination of active zone protein RIM1 regulates synaptic vesicle release. Cell. 2007;130:943–957. doi: 10.1016/j.cell.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad CC, Feliu-Mojer MI, Spangler SA, Milstein AD, Dunah AW, Hung AY, Sheng M. Liprinalpha1 degradation by calcium/calmodulin-dependent protein kinase II regulates LAR receptor tyrosine phosphatase distribution and dendrite development. Dev Cell. 2007;12:587–602. doi: 10.1016/j.devcel.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Tada H, Okano HJ, Takagi H, Shibata S, Yao I, Matsumoto M, Saiga T, Nakayama KI, Kashima H, Takahashi T, et al. Fbxo45, a novel ubiquitin ligase, regulates synaptic activity. J Biol Chem. 2010;285:3840–3849. doi: 10.1074/jbc.M109.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Litkowski PE, Taylor AA, Lin Y, Snider BJ, Moulder KL. A role for the ubiquitin-proteasome system in activity-dependent presynaptic silencing. J Neurosci. 2010;30:1798–1809. doi: 10.1523/JNEUROSCI.4965-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites CL, Leal-Ortiz SA, Okerlund N, Dalke H, Fejtova A, Altrock WD, Gundelfinger ED, Garner CC. Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. EMBO J. 2013;32:954–969. doi: 10.1038/emboj.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko N, Pechstein A, Haucke V. Synaptic requiem: a duet for Piccolo and Bassoon. EMBO J. 2013;32:920–922. doi: 10.1038/emboj.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V, Ebert U. Is Alzheimer’s disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid. Rev Neurosci. 2009;20:1–12. doi: 10.1515/revneuro.2009.20.1.1. [DOI] [PubMed] [Google Scholar]
- Cheng F, Vivacqua G, Yu S. The role of alpha-synuclein in neurotransmission and synaptic plasticity. J Chem Neuroanat. 2011;42:242–248. doi: 10.1016/j.jchemneu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Schmitz F. Presynaptic [Ca(2+)] and GCAPs: aspects on the structure and function of photoreceptor ribbon synapses. Front Mol Neurosci. 2014;7:3. doi: 10.3389/fnmol.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, O’Toole ET, Girard M, Ritter B, Messa M, Liu X, McPherson PS, Ferguson SM, De Camilli P. A dynamin 1-, dynamin 3- and clathrin-independent pathway of synaptic vesicle recycling mediated by bulk endocytosis. eLife. 2014;3:e01621. doi: 10.7554/eLife.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]