Trp channels and itch (original) (raw)
. Author manuscript; available in PMC: 2017 May 1.
Published in final edited form as: Semin Immunopathol. 2015 Sep 18;38(3):293–307. doi: 10.1007/s00281-015-0530-4
Abstract
Itch is a unique sensation associated with the scratch reflex. Although the scratch reflex plays a protective role in daily life by removing irritants, chronic itch remains a clinical challenge. Despite urgent clinical need, itch has received relatively little research attention and its mechanisms have remained poorly understood until recently. The goal of the present review is to summarize our current understanding of the mechanisms of acute as well as chronic itch and classifications of the primary itch populations in relationship to transient receptor potential (Trp) channels, which play pivotal roles in multiple somatosensations. The convergent involvement of Trp channels in diverse itch signaling pathways suggests that Trp channels may serve as promising targets for chronic itch treatments.
Introduction
Itch, also known as pruritus, is defined as an “unpleasant sensation that elicits the desire or reflex to scratch”[1] and can be distinguished as acute and chronic forms with the latter lasting more than six weeks[2]. Itch can be caused by various stimuli including those that are mechanical, electrical and chemical, with exogenous and endogenous chemical stimuli ranging from amines, proteases, neuropeptides to inflammation mediators and certain drugs (see Table 1)[3]. Acute itch, such as that caused by a mosquito bite, is commonly experienced in daily life and has a protective role as to remove irritants and avoid future insults. The mechanical pain generated by scratching can usually suppress acute itch. However chronic itch conditions commonly accompanied by co-morbidities, such as depression and sleep disorders, can be debilitating and remain unmet clinical needs. Chronic itch conditions are generally divided into four categories: dermatological, systemic, neurological and psychogenic. Dermatological itch comes from skin conditions such as atopic dermatitis, psoriasis and urticaria. Systemic itch can be caused by pathology of other organs, for example liver cholestasis and kidney dialysis. Neurological itch is caused by direct damage to the nervous system either peripherally or centrally. Finally, psychogenic itch is associated with psychological and psychiatric disorders.
Table 1. Pruritogens and related itch transduction.
| Pruritogen | Receptor | Channel | Signaling | |
|---|---|---|---|---|
| G protein couple receptors | Histamine | H1R, H4R[25, 26] | TrpV1[19] | PLCβ3[19, 23], IP3[24], Ca2+[21, 180], PLA2/LO[21] |
| Chloroquine | MrgprA3[9] | TrpA1 [10] | Gβγ[10] | |
| BAM | MrgprC11[9] | TrpA1 [10] | PLC[10] | |
| Cathepsin S | MrgprC11[78] | |||
| Tryptase | PAR2, IL7α[79] | |||
| Serotonin | 5-HT2[45], 5-HT7[46] | TrpA1 [46] | PLCβ3[19], AC, Gβγ[46] | |
| 12(S)-HPETE | LTB4 receptor 2[181], 5-HT1, 5-HT2[182] | |||
| Endothelin | ETa[64] | TrpA1[183] | PKC, AC[65] | |
| Cathepsin E | ETa[67] | |||
| Bradykinin | B2R[32, 33] | TrpV1[21, 34], TrpA1[32–34] | PLA2/LO[21], PLC[106] | |
| Substance P | NK1R[27] | NO[36], LTB4[35] | ||
| Bile acids | TGR5[56] | TrpA1[57] | PKC, Gβγ[57] | |
| LPA | LPA1, LPA3[60] | Rho/ROCK[184] | ||
| LTB4 | LTB4 receptor 2[47] | TrpV1, TrpA1[47] | RO[47] | |
| LTD4 | Cysltr2[175] | |||
| TXA2 | TP receptor[185] | |||
| β-alanine | MrgprD[42] | |||
| Cytokine receptors | TSLP | TSLPR, IL7α[79] | TrpA1[79] | PLC[79] |
| IL31 | IL31RA[90], OSMR[90, 186] | TrpV1, TrpA1[90] | ERK[90] | |
| IL13 | TrpA1[91] | |||
| Toll like receptors | Imiquimod | TLR7?[96] | K2P, Kv1.1, Kv1.2[99] | |
| LPS (no direct itch) | TLR4[101] | |||
| Direct channel activation | Oxidants | TrpA1[107, 108] |
Itch sensation is initiated in the skin by peripheral afferents of primary sensory neurons, with cell bodies located in dorsal root ganglia (DRG) and trigeminal ganglia, then transmitted to the spinal dorsal horn and then further to the brain. Itch fibers are small unmyelinated C fibers and lightly myelinated Aδ fibers with slow conduction velocities and relatively small cell bodies, responding not only to itch but also to pain and other somatosensory stimuli.
Itch used to be regarded as a sub-modality of pain because of their similarities. The related theory is called intensity theory, stating that weak and strong activation of the same group of neurons generates itch and pain sensations respectively[4–6]. Another competing theory is called labeled line theory, which, in contrast, proposes mutually exclusive populations for the detection of itch and pain [7]. It is clear now that itch is a distinct sensation from pain. Loss of the gastrin releasing peptide receptor (Grpr) population in the spinal cord resulted in a profound defect of itch behaviors, but pain responses remained intact unequivocally demonstrating the existence of separable itch and pain pathways[8]. However periphery itch neurons are exclusively poly-modal. They respond to painful stimuli such as capsaicin or mustard oil in addition to itchy stimuli [9–11]. The coding puzzle of itch and pain is better explained by selectivity theory. When the itch population is selectively activated, an itchy sensation is generated, regardless of the stimuli. In contrast, when an algogen activates a larger population, including both pain and itch sensing neurons strong enough, itch is occluded by inhibition from pain neurons, just as scratching induced pain inhibits itch and therefore only pain sensation is perceived. Theoretically, itch and pain can be perceived at the same time when the amount of pain is not enough to mask itch. Although being two separate sensations, itch and pain share common downstream pathways. Recent evidence suggests Trp (transient receptor potential) channels play major roles in both itch and pain sensations.
Trp channels are a large family of six trans-membrane cation channels first identified in drosophila to be mediator of photo-transduction. There are 28 identified Trp family members in mice and 27 in humans falling into 6 subgroups based on sequence homology (TrpC, TrpV, TrpM, TrpA, TrpP and TrpML). Trp channels have been shown to play critical roles in various sensory functions including vision, olfaction, thermosensation, taste, mechanosensation and pain[12–14]. Itch is newly added to the list based on recent research and is thus the focus of this review.
Trp channels and GPCRs in itch
With the booming of itch research in recent years, a common theme of itch transduction has emerged: pruritogens sensed by G protein coupled receptors (GPCRs) with Trp channels downstream (see Figure 1). G protein coupled receptors are seven trans-membrane receptors capable of sensing various extracellular stimuli and triggering signaling inside cells. Ligand binding to GPCRs promotes exchange of GDP bound to the Gα subunit for GTP. The GTP bound g protein can then dissociate with the activated receptor. Both Gα and Gβγ are free to modulate downstream effectors including ion channels and enzymes[15].
Figure 1. Signal transductions of histamine and non-histamine itch.
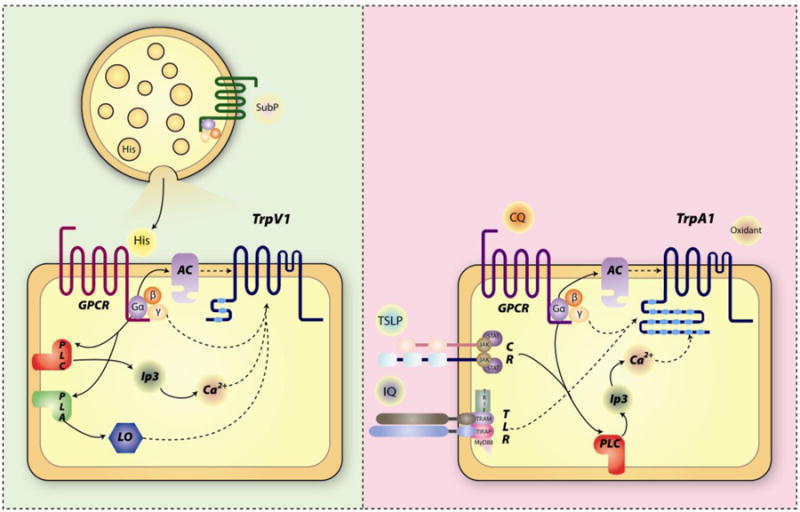
A) Histamine itch: Substance P liberates histamine from mast cells. Histamine activates G protein coupled receptor and triggers downstream signaling to open TrpV1. B) Non-histamine itch: examples of pruritogens work on GPCR, cytokine receptor, toll like receptor or directly on TrpA1. Arrows indicate signaling pathways, dashed arrow contribution to Trp channel activation. Abbreviations: AC, adenylate cyclase; PLC, phospholipase C; PLA, phospholipase A; LO, lipoxygenase; CR, cytokine receptor; TLR, toll like receptor; sub P, substance P; His, histamine; CQ, chloroquine; IQ, imiquimod.
Histamine receptors
The most studied pruritogen is histamine, which serves as a classical example. Histamine can produce itch associated with flare and wheal in human skin [16]. All four histamine receptors, from H1R to H4R are G protein coupled receptors. The H1R is presumed to be responsible for histamine induced itch[17, 18]. TrpV1 is reported to be downstream as implicated by the observation that TrpV1 knockout reduced most histamine induced itch[19]. Consistently, most histamine sensitive small diameter DRG neurons respond to capsaicin while only about 40% of them respond to mustard oil, a TrpA1 agonist[9, 20]. TrpV1 also plays a pivotal role in the detection of noxious heat, pain and the regulation of body temperature. TrpV1 can be activated by a wide variety of exogenous and endogenous stimuli including noxious heat (higher than 43°C), low pH, capsaicin and endocannabinoid anandamide. Both the PLCβ/PKC pathway and the PLA2/lipoxygenase (LO) pathway have been proposed to link the Gq coupled histamine receptor and TrpV1 (summarized in Figure 1a)[21–23].
However TrpV1 might not be the only Trp channel in histaminergic itch. In TrpV1 deficient mice neither neuronal calcium response or behavioral scratching was completely eliminated and at the mean time some histamine responsive neurons failed to respond to capsaicin[19, 22, 24]. The critical role of H4R in histamine itch has also recently been recognized[25, 26]. Knockout of H4R greatly reduced histamine induced itch and moreover H1R/H4R double knockout or H4R knockout with H1R antagonist completely abolished the itch response, begging the question whether there are other Trp channels also involved in histaminergic itch, potentially downstream of H4R. However detection of functional H4R expression in related brain regions raises the question of whether H4R mediates itch in the central nervous system. Tissue specific knockout of H4R is best suited to clarify the role of H4R.
Other classic pruritogens like bradykinin, substance P, compound 48/80, PGE2 and platelet activating factor (PAF) can induce histamine release from mast cells, basophils and others thus mediating itch largely in histamine dependent way[27–30]. Although there is report showing that high dose bradykinin elicited itch is not blocked by antihistamine, bradykinin itch in a largely histamine dependent[31]. Bradykinin works through the g protein coupled B2 receptor (B2R) and requires both TrpA1 and TrpV1 downstream[32–34]. Substance P induces histamine dependent itch in humans however substance P triggered scratching cannot be blocked by antihistamine in mice[35]. Substance P itch in mice requires neurokinin receptor 1 (NK1R), but not mast cells with nitric oxide (NO) signaling downstream[27, 35, 36]. In addition to potent histamine liberation effect, compound 48/80 can also generate itch in mast cell deficient mice potentially via direct activation of TrpV1 positive neurons[26, 37].
Mas-related G protein coupled receptors
Mas-related G protein coupled receptors (Mrgprs) is a class of novel histamine independent itch receptors with more than 50 members in mice[38, 39]. MrgprA3 is the receptor for the anti-malarial drug chloroquine, which causes severe anti-histamine resistant itch as a side effect and scratching in mice. Bovine adrenal medulla (BAM) 22, an endogenous opioid peptide produced from proenkephalin gene and especially its cleaved product BAM 8-22 can potently activate MrgprC11 and induce itch in both humans and mice[9, 40]. BAM 8-22 generates histamine independent itch not accompanied by flare and wheal when delivered to human skin. Both MrgprA3 and MrgprC11 are coupled to TrpA1 and are expressed in a small subset of largely overlap small diameter DRG population (about 5-8%) which also express histamine receptors as well as TrpV1[10, 41]. TrpA1 knockout greatly reduced scratching produced by both chloroquine and BAM. TrpA1 is the only member of the TrpA subgroup and a key player in pain and inflammation. TrpA1 can be activated by various reactive compounds such as mustard oil, cinnamaldehyde, formalin and hydrogen peroxide. Surprisingly, both Gq/11 coupled receptors in the largely overlapping population exhibit distinct signaling mechanisms. MrgprA3 is Gβγ dependent but not coupled to PLC while MrgprC11 signaling requires PLC but not Gβγ (see Figure1b).
MrgprD, another member from the family is a receptor for β-alanine, a muscle-building supplement with non-histamine itch as side effect[42]. β-alanine feeding and intradermal delivery evoked itch are MrgprD dependent. Interestingly MrprD defines a unique itch population of DRGs not responding to either histamine or chloroquine. The downstream signaling mechanisms of MrgprD and involvement of Trp channels remains elusive.
Serotonin receptors
Serotonin (5-HT) iontophoresis in human skin induces itch with wheal and flare, however only flare and itch (but not wheal) are antihistamine resistant, suggesting serotonin itch is histamine independent[31, 43]. All the serotonin receptors except 5-HT3 are g protein coupled receptors[44]. Serotonin induced itch was blocked by 5-HT1/2 receptor antagonist but not by 5-HT3 receptor antagonist[45].
However, Morita et al. have recently shown that 5-HT7 receptor mediates itch elicited by low dose of serotonin and 5-HT7 specific agonist LP44 which produce only itch but not pain in animals with TrpA1 channel downstream. LP44 activates about 8% of cultured DRG neurons. This LP44 sensitive population also respond to capsaicin, mustard oil and serotonin itself, but only about two thirds of them overlap with the chloroquine responsive population and just one third with histamine. This data suggests that serotonin defines a distinct TrpV1 and TrpA1 double positive population only partially overlaps with the histamine and chloroquine populations. The LP44 triggered calcium response was reduced in either TrpA1 knockout or 5-HT7 receptor knockout. Like MrgprA3, activation of TrpA1 by 5-HT7 is also Gβγ dependent but Gs coupled 5-HT7 utilizes adenylate cyclase (AC) instead of PLC downstream. TrpA1 knockout significantly attenuated low dose serotonin induced scratching as well as scratching mediated by intradermal selective serotonin reuptake inhibitors (SSRIs) which can increase local serotonin level[46]. Notably high dose serotonin response was not affected in 5-HT7 null mice. It will be interesting to determine whether high dose serotonin itch is instead mediated by other 5-HT receptors and if Trp channels are involved downstream.
Leukotriene B4 receptor 2
Leukotriene B4 (LTB4), an inflammation related leukotriene requires both TrpA1 and TrpV1 downstream of g protein coupled leukotriene B4 receptor 2 for itch response[47]. LTB4 is released from leukocytes as leukocyte chemoattractant. LTB4 level is up-regulated in various skin conditions including atopic dermatitis, prsoriasis and Sjögren-Larsson syndrome[48–50]. LTB4 is a downstream mediator of sphingosylphosphorylcholine (SPC) induced itch[51]. SPC is produced from sphingomyelin and is elevated in atopic dermatitis.
G protein coupled bile acid receptor 1
Cholestatic itch, a debilitating condition of elusive causes, is common with heptobiliary disorders[52]. Bile acid is considered an important pruritogen for cholestatic itch because it accumulates in tissues of cholestasis patients[53], intradermal delivery can trigger itch in humans[54] and clearance of bile acids can relieve itch symptoms[55]. Recent studies identified g protein coupled bile acid receptor 1 (TGR5) as a potential mediator of cholestatic itch. TGR5 expresses in about 8% of DRG neurons co-expressing TrpA1 and partially TrpV1. TGR5 knockout partially blocked bile acid generated scratching [56]. Interestingly blockade of TrpA1, either genetically or pharmacologically, totally abolished behavioral as well as neuronal response toward bile acid[57] suggesting its critical role. Pharmacological evidence indicates that TGR5 utilizes Gβγ and PKC downstream to activate TrpA1.
Another promising candidate as a cholestatic itch pruritogen is lysophosphatidic acid (LPA), a bioactive phospholipid with various functions in different cell types. Intradermal LPA can elicit itch in animals[58] and unlike bile acids, LPA level is only increased in cholestatic patients with pruritus. Moreover, the level of autotaxin (ATX), the enzyme that synthesizes LPA, correlates with severity of itch in patients and decreases following nasobiliary drainage that ameliorates pruritus[59]. The LPA induced itch was reduced by LPA1 and LPA3 receptor antagonist but not by LPA3 receptor specific antagonist in mice[60]. TrpV1 is potentially involved since LPA has been reported to activate TrpV1 directly[61].
Endothelin receptors
Endothelin is a vessel constricting peptide primarily secreted by endothelial cells. In human skin, endothelin triggers itch with wheal and flare, which is not accompanied by increased histamine [62]. Endothelin iontophoresis induced itch in humans was only partially blocked by a H1R antagonist, indicating the participation of non-histamine mechanisms. There are three endothelin isoforms, ET-1, ET-2 and ET-3. The ET-1 isoform, commonly used in itch studies, increases during chronic itch diseases[63]. The ETa receptor antagonist suppresses ET-1 induced itch while the ETb receptor antagonist increases ET-1 induced itch[64, 65]. TrpA1, but not TrpV1, is critically involved as shown in a recent report by Kido-Nakahara et al. that only TrpA1 knockout abolished the ET-1 itch response[19, 63]. However it remains controversial whether ET-1 works directly on DRGs or through indirect mechanisms. Endothelin receptors also express in non-neuronal cells. ET-1 elicited no or very weak calcium responses on DRGs and was not significantly affected by TrpA1 deficiency[63, 66]. Kido-Nakahara et al. also identified endothelin-converting enzyme 1 (ECE1), a neuronal peptidase mediating endothelin receptor recycling as a key modulator of ET-1 itch. Blocking ECE1 significantly boosted ET-1 induced scratching. In addition, a recent study suggests that endothelin is responsible for aspartic protease cathepsin E induced itch, as cathepsin E elevated the expression of endothelin and an ETa antagonist blocked the associated itch response[67].
Protease activated receptors
Protease activated receptors (PARs) are a family of g protein coupled receptors (PAR1 to PAR4) named after their ligands. PARs are distinguished from other GPCRs by their activation mechanism. The proteolytic cleave of the extracellular N terminus exposes the tethered ligand to activate the receptor itself[68]. The expression of proteases are elevated in multiple itch related diseases and cowhage, a tropical legume commonly used in studies of histamine independent itch contains the protease mucunain as its active component[69]. PARs, especially PAR2 can be activated by various endogenous and exogenous itch related proteases, including serine protease trypsin, tryptase and cysteine protease cathepsin S, mucunain[69–71]. Thus, PAR2 is proposed to mediate protease induced itch. Accordingly expression of PAR2 and its ligand tryptase increase in chronic itch conditions[72–75].
SLIGRL-NH2, the tethered ligand of PAR2 is commonly used as a receptor agonist to study protease itch in animals[76]. However, a recent study shows that SLIGRL-NH2 induced itch surprisingly remained unaffected in PAR2 mutant mice, while trypsin generated more scratch bouts. Instead, Mrgprs deletion, in particular that of MrgprC11, significantly reduced SLIGRL-NH2 mediated response. Consistent with this, a shorter peptide SLIGR, agonist of PAR2 but not of MrgprC11, induced only thermal hyperalgesia but not itch[77]. This study implicates MrgprC11 as a mediator of protease induced itch, but raises the question of whether MrgprC11 needs the PAR2 tethered ligand to trans-activate. This question is now addressed by a new study. Reddy et al. demonstrate that cysteine proteases, including cathepsin S and papain, directly activate MrgprC11 via proteolytic cleavage of the N terminus. Cathepsin S mediated itch was significantly reduced in Mrgpr-null mice, but not in PAR2 mutant mice[78]. Mutations of residuals critical for protease cleavage abolished cathepsin S and papain induced calcium response in heterologous cells showing the involvement of proteolytic cleavage. Interestingly, activation of MrgprC11 by protease does not involve tethered or diffusible ligands as neither of them served as agonist. When the N terminus of MrgprC11 is replaced by that from an unrelated GPCR β2-adrenergic receptor (β2AR) or a melanocortin-1 receptor (MC1R) also containing a cysteine protease cleavage site but cannot be activated by proteolysis, both cathepsin S and papain can surprisingly still trigger robust calcium increase in Hela cells expressing these hybrid receptors suggesting proteolysis of MrgprC11 N terminus results in direct conformation change that allows g protein signaling. Thus, MrgprC11 demonstrates a novel GPCR activation mechanism after proteolysis.
In contrast, serine protease, tryptase has recently been shown to generate itch through PAR2[79]. It will be interesting to determine whether another cysteine protease mucunain, the active component of cowhage, generates itch through PAR2 or MrgprC11. Although the precise role of Trp channels in protease itch is not yet clear, evidence strongly indicates involvement of Trp channels. PAR2 sensitizes both TrpV1 and TrpA1 to induce hyperalgesia[80, 81], but whether the same mechanism is utilized in itch remains unknown. MrgprC11 with another ligand BAM is coupled to TrpA1, making TrpA1 a likely downstream mediator of protease itch.
In addition to g protein coupled receptors a few other receptors can also mediate itch responses, including cytokine receptors and toll like receptors. Trp channels are commonly utilized in these scenarios as well.
Cytokine receptors and Trp channels
Cytokine receptors are typically single trans-membrane proteins with a conserved extracellular cytokine receptor homology domain. Cytokine binding can induce receptor multimerization to activate Janus kinases (JAKs). Activated JAKs phosphorylate both receptors and themselves to recruit signal transducers and activators of transcription (STATs) and adaptors that link receptors to downstream mediators. Phosphorylated STATs can also dimerize and translocate to the nucleus to function as transcription factors[82].
TSLP receptor
Thymic stromal lymphopoietin (TSLP) is an epithelial cell derived cytokine, that is critical for Th2 response related diseases such as atopic dermatitis[83]. Atopic dermatitis, also commonly known as atopic eczema, is a skin inflammation condition characterized by itchy, swollen and cracked skin. Intradermal application of TSLP can elicit itch but not pain in mice. TSLP over-expression in mouse skin resulted in atopic dermatitis like phenotypes, demonstrating that TSLP is capable of directly triggering allergic inflammation cascades[84]. TSLP signals through a heterodimer of TSLP receptor chain and IL7 receptor α chain[85]. Wilson et al. recently identified TSLP receptor expression in about 5-6% of DRGs, suggesting that TSLP acts directly on DRG neurons to generate itch sensation (Figure 1b). Consistently, mice lacking T cells, B cells or mast cells retained their normal TSLP induced itch response. TSLP evoked scratches were abolished in TrpA1 null mice and the PLC inhibitor could also block TSLP induced calcium increase in DRGs[79]. Thus TSLP receptors are coupled to TrpA1 via PLC, similar to MrgprC11. Notably, TSLP responsive DRG neurons are all TrpA1 and TrpV1 double positive, but do not respond to histamine or chloroquine. Therefore, TSLP marks a unique population of primary itch neurons. Interestingly, tryptase evoked itch was also attenuated in both PAR2 and IL7α knockout mice. In addition, both tryptase and the PAR2 tethered ligand SLIGRL can trigger calcium dependent TSLP release from keratinocytes. The ORAI1/NFAT calcium signaling pathway has also been identified as an essential regulator of TSLP release. These indicate that tryptase may cause itch via PAR2 mediated TSLP release from keratinocytes.
IL31 receptor
Interleukin 31 (IL31) is a cytokine preferentially produced from T helper type 2 cells. Levels of IL31 have been shown to increase in pruritic conditions such as atopic dermatitis and cutaneous T-cell lymphoma[86, 87]. Like TSLP, mice with IL31 over-expression developed atopic dermatitis like phenotypes. IL31 antibodies can also relieve itch and dermatitis symptoms in Nc/Nga mice which spontaneously develop dermatitis under conventional housing conditions[88, 89]. IL31 signals through the receptor complex of IL31 receptor A (IL31 RA) and oncostatin M receptor. IL31 RA expresses in about 4% DRG neurons. These neurons respond to both capsaicin and mustard oil, representing a subset of MrgprA3 population. Consistent with previous findings, a recent study also reported elevated IL31 receptor expression in patients with atopic dermatitis and psoriasis. In an animal model of repeated allergen challenge induced allergic dermatitis, IL31 level is also increased. Furthermore, both intradermal and intrathecal IL31 can directly produce itch in mice with ERK but not p38 phosphorylation downstream. Based on genetic deletion evidence, both TrpV1 and TrpA1 are required for IL31 induced itch response[90]. IL13, another Th2 cytokine mediating allergic diseases including asthma and dermatitis, has been reported to cause dermatitis with strong scratching when over expressed in mice. Interestingly, IL13 can up-regulate TrpA1, while the TrpA1 specific antagonist attenuates IL13 over expression associated itch[91]. Further studies are needed to elucidate the receptors and detailed signaling pathways involved in IL13 pruritogenesis.
Toll like receptors and Trp channels
Toll like receptors (TLRs) are a class of single membrane spanning receptors playing key roles in innate immune systems. Immune cells such as macrophages and dendritic cells usually express toll like receptors to detect microbe-derived molecules. Toll like receptors function as dimers and may depend on co-receptors. Activated toll like receptors recruit TIR domain containing adaptor proteins including MyD88, TRIF, TIRAP and TRAM to trigger downstream responses such as release of cytokines and chemokines (Figure 1b)[92].
TLR 3, 4 and 7 also express in dorsal root ganglia neurons[93]. Genetic deletions of TLR 3, 4 and 7 reduced itch responses toward multiple pruritogens. However, it remains unclear whether neuronal TLRs are required in itch transmission given the broad expression patterns of TLRs. Tissue-specifc knockout lines of TLRs are needed to confirm the role of neuronal TLRs in itch.
Imiquimod (IQ), a small molecule anti-viral anti-tumor immune modifier targeting TLR7, is used to treat genital warts which causes itch as a drug side effect[94, 95]. IQ directly activates TrpV1 expressing DRG neurons and induce itch independent of TrpV1 per se[96, 97]. However, the detailed mechanisms remain controversial. Liu et al. identified TLR7 as the mediator of IQ induced itch. All four non-histamine pruritogens tested in the paper, including CQ, ET-1 and SLIGRL, exhibited significantly reduced scratching responses in TLR7 null mice, suggesting that TLR7 plays an essential role in non-histamine itch, instead of being a specific receptor of certain pruritogen. In contrast, Kim et al. reported unchanged IQ itch response in TLR7 deficient mice. Indeed, although IQ has been implicated in antagonizing adenosine receptors and potassium channels to increase neuronal excitability, but the link between IQ and itch is still unclear[98, 99]. Interestingly, a recent paper by Park et al. reported that let 7b microRNA can directly activate DRG neurons and generate pain through TLR7. TrpA1 is a critical mediator in this TLR7 mediated pain, hinting at the potential role of TrpA1 in TLR7 itch responses[100].
TLR3 and TLR4 are also implicated in itch transmission[101, 102]. The synthetic TLR3 agonist PIC can induce action potentials directly in TrpV1 expressing DRG neurons and elicits scratching behavior in a TLR3 dependent way. Strikingly, compound 48/80, CQ, ET-1, serotonin, SLIGRL and trypsin mediated itch were all greatly reduced in TLR3 knockout mice along with formalin second phase pain and capsaicin induced secondary hyperalgesia. This effect appears to involve central mechanisms since CQ induced calcium responses remained intact in TLR3 null mice, while spinal LTP was totally abolished. The downstream mechanism likely also involves TrpV1, as TrpV1 expression in DRG central terminals, capsaicin induced sEPSC increase in substantia gelatinosa neurons and intrathecal capsaicin evoked licking behavior were all attenuated in TLR3 knockout[102]. TLR4 is also expressed in TrpV1 positive DRG neurons. Both histamine and CQ induced itch were significantly reduced in TLR4 knockout. LPS, a TLR4 agonist can activate TrpV1 positive neurons and sensitize TrpV1 both in TLR4 dependent ways[103, 104]. However, LPS fails to generate scratching responses itself, leaving the role of direct LPS activation unknown. The reduced histamine response appears to involve TrpV1, as TrpV1 activity but not TrpA1 activity was decreased in TLR4 knockout. In contrast, the expression level of MrgprA3 was reduced in TLR4 deficient mice, likely responsible for the reduced CQ response[101].
Pruritogens directly activate Trp channel
Oxidative stress contributes to the pathogenesis of multiple skin disorders, particularly those involving allergic reactions[105]. TrpA1 can be directly activated by a wide variety of irritants both exogenous and endogenous irritants, including oxidants[106]. Hydrogen peroxide and other oxidants can trigger calcium increase in DRG neurons and these responses are TrpA1 dependent[107]. Recent research shows that hydrogen peroxide and tert-butylhydroperoxide (tBHP) can induce dose dependent scratching behaviors not affected by antihistamine. Both hydrogen peroxide and tBHP induced itch can be reduced by either TrpA1 knockout or TrpA1 antagonist, revealing a novel itch mechanism where pruritogens directly target Trp channels (Figure 1b). TrpV1 expressing fiber, but not TrpV1 per se, is required for oxidant induced itch as TrpV1 chemical ablation (but not TrpV1 gene knockout) abolished scratching. Consistently, antioxidant effectively attenuated scratching evoked by hydrogen peroxide and tBHP[108].
For a full list of pruritogens and related itch transmission mechanisms, please refer to Table 1.
Trp channels and itch inhibition
By definition, itch can be inhibited by pain. Itch is an unpleasant sensation that elicits the desire to scratch thus generating moderate mechanical pain to inhibit itch. Two parallel reports point out there is tonic inhibition of itch pathways by pain even in the absence of pruritogen that deleting vGlut2, vesicular glutamate transporters in either TrpV1 positive or NaV1.8 positive nociceptors greatly reduced pain and not only boosted itch responses to multiple pruritogens but also elicited spontaneous scratching[109, 110].
Not just mechanical pain, but also other “counter stimuli”, such as noxious heat and cooling can effectively block itch[111, 112]. TrpM8 is the Trp channel that responds to non-noxious cooling temperature and compounds that produce cooling sensations, such as menthol and icilin. Agonists of thermal Trp channels such as TrpV1 agonist capsaicin, TrpA1 agonist mustard oil and TrpM8 agonists menthol, icilin are able to inhibit itch[113, 114], indicating involvement of thermal Trp channels. The inhibition of itch by pain can be directly observed in spinal cord. By scratching the receptive field of histamine responsive spinal projection neurons, the output of somatosensory information from spinal cord can inhibit histamine evoked activities, but not spontaneous or algogens evoked activities[115]. The spontaneous activities of dorsal horn neurons in the chronic itch model are also inhibited by scratching, pinching and noxious heat and this inhibition can be blocked by spinal GABA antagonists[116], thus indicating involvement of a spinal inhibitory population.
A neural specific transcription factor Bhlhb5 likely labels spinal interneurons which mediate itch inhibition. Deletion of Bhlhb5 resulted in loss of a population of inhibitory spinal interneurons (B5I for short) and spontaneous scratching[117]. The B5I population also expresses kappa opioid dynorphin and kappa opioid signaling potential contributes to the itch inhibition effect of this population consistent with previous finding that kappa opioid antagonists elicit strong scratching[118]. The B5I population receives direct synaptic inputs from TrpV1, TrpA1 and TrpM8 positive primary neurons transmitting “counter-stimuli” information from peripheral. Loss of Bhlhb5 interneurons abolished the inhibition effect of menthol on chloroquine induced itch[119] confirming that “counter-stimuli” sensed by Trp channels work through Bhlhb5 inhibitory interneurons to achieve itch inhibition.
Trp channels in chronic itch
Trp channels play an essential role in chronic itch conditions in addition to their critical involvement in acute itch. Increased Trp channel expressions have been reported in multiple itch related diseases and critical involvement of Trp channels demonstrated in related animal studies. Therefore Trp channels are receiving growing attention as potential drug targets for chronic itch diseases.
Increased Trp channel expression in chronic itch diseases
For example, TrpV1 is up-regulated in multiple chronic itch conditions including neuronal and dermal over-expression in prurigo nodularis[120], a skin disease characterized by itchy nodules on arms and legs, and in mast cells from mastocytosis[121], an itchy skin condition caused by too many mast cells and precursors. TrpV1 is also up-regulated in atopic dermatitis lesional skin[122]. TrpV1 activity increases in allergic rhinitis[123], which features itchy eyes and sneezing. Notably, TrpV1 is increased in all four subtypes of rosacea, a chronic inflammation condition on central facial regions, while other TrpVs are also increased in certain subtypes despite their elusive link to itch[124].
Another Trp channel with major role in itch transmission, TrpA1, is increased in nerve fibers, keratinocytes and tryptase positive mast cells from lesional skin of atopic dermatitis patients. Notably dermal cells in healthy skin have minimal expression of TrpA1[91]. In addition, expression of TrpV3 is also increased in atopic dermatitis lesional skin though its role in itch is not entirely clear[125]. Elevated TrpA1 expression is also detected in post-burn pruritus. Levels of TrpA1 and two other channels TrpV3 and TrpV4 are all higher in post-burn patients with pruritus comparing with post-burn patients without pruritus[126].
Unlike TrpV1 and TrpA1, TrpV3 expression is mainly detected in keratinocytes[127]. TrpV3 is a heat activated Trp channel with threshold around 33°C. Consistent with its expression in skin, TrpV3 plays an important role in skin physiology and pathophysiology. TrpV3 is a key regulator of skin barrier formation[128]. Activation of TrpV3 can trigger release of multiple factors including PGE2[129], ATP[130], nitric oxide[131] and NGF[132], contributing to the inflammation processes in dermatitis. TrpV3 Gly573 mutations are detected in DS-Nh mice (Gly573Ser) and WBN/Kob-Ht rats (Gly573Cys), both spontaneous hairless mutant strains. These animals develop spontaneous dermatitis phenotypes including increased keratinocytes and pruritus[133]. Transgenic mice carrying Gly573Ser mutation mimics dermatitis phenotypes from the two spontaneous mutant rodent strains confirming the causal role of this single amino acid mutation[132]. Recent studies also link this TrpV3 missense mutation to Olmsted Syndrome (OS), a rare congenital disorder featuring palmoplantar, periorificial keratoderma and severe itching[134, 135]. OS patients were identified to carry missense mutation in TrpV3 gene (in most cases Gly573Ser or Gly573Cys). Like TrpV3, TrpV4 is also found in keratinocytes and sensitive to warm temperature in addition to osmolarity change[136–139]. TrpV4 agonists can promote, while TrpV4 antagonists inhibit skin barrier recovery and intercellular junctions were impaired in TrpV4 knockout mice[128, 140]. However the role of TrpV4 in itch remains obscure.
Multiple TrpC channels have been implicated in keratinocyte differentiations. siRNA knockdown of TrpC1/TrpC4 prevents calcium induced keratinocyte differentiation[141] while TrpC6 activation promote differentiation and inhibit proliferation via increased cytoplasmic calcium[142]. All six TrpC channels are down-regulated in psoriasis, consistent with the excessive keratinocyte proliferation and impaired differentiation phenotypes of psoriasis[143]. In contrast, TrpC1 increases in Darier's disease, an autosomal dominant disorder caused by loss of function mutation of sarco(endo)plasmic reticulum Ca2 ATPase isoform 2b (SERCA2b)[144]. SERCA can transport calcium from cytosol to sarco(endo)plasmic reticulum at the expense of ATP. Darier's disease is characterized by dark crusty patches on the skin with intense itch and keratinocytes from Darier's disease patients show enhanced proliferation potentially caused by TrpC1 mediated apoptosis resistance[145].
Trp channels in chronic itch animal models
With the help of animal models, the functions of Trp channels in chronic itch conditions are now being more thoroughly investigated.
In addition to DS-Nh mice and WBN/Kob-Ht rats mentioned above, Nc/Nga is another mouse strain that spontaneously develops dermatitis with aging, and it is commonly used as a model for atopic dermatitis studies. In Nc/Nga mice, antagonism of TrpV1 attenuates itch symptoms induced by house dust mite allergens[146]. Further, dermatitis related itch in Nc/Nga mice is also reduced by serine protease inhibitor, PAR2 antibody[74], IL31 antibody[88] and NK1R blocker[147], thus suggesting multiple potential targets for treatment of dermatitis.
In addition to spontaneous mutants and genetically engineered animals over-expressing genes of interest (for instance IL31 and TSLP as mentioned above), repeated challenge of allergen is another common way of inducing allergic dermatitis symptoms in mice. However in oxazolone, haptens and urushiol (poison ivy allergen) challenged dermatitis models, genetic ablation or pharmacological blockade of TrpA1, but not TrpV1 effectively relieved scratching[148]. NK1R antagonists were also able to block this antihistamine resistant itch. Consistently, TrpA1 and HTR7 knockout mice both showed impaired scratching and dermatitis phenotypes in the model of topical vitamin D analog MC903 evoked atopic[46]. All the above evidence indicates a pivotal role of TrpA1 in chronic itch in addition to TrpV1. Although dermatitis related itch is widely regarded as antihistamine resistant, in the mouse model of contact dermatitis, L-histidine decarboxylase knockout (in which there is lost ability to produce histamine from histidine) completely abolished itch responses[121]. Consistently, H4R antagonism successfully reduced scratching with dermatitis model[149]. These results suggest the involvement of histamine in dermatitis and indicate the potential clinical value of H4R antagonists.
Dry skin (Xerosis), skin dehydration with constant itch is another chronic itch condition commonly modeled in animals. Repeated skin dehydration with acetone and ether can trigger spontaneous scratching and increase trans-epidermal water loss without infiltration of inflammatory cells mimicking symptoms of Xerosis[150, 151]. In animal model of dry skin, both TrpA1 and TrpV3 are required for induction of spontaneous itch[152–154]. Similar to dermatitis models, blocking serotonin signaling and PAR2 can also effectively reduce itch in dry skin models[155, 156]. Skin barriers play a critical role in preventing dehydration and entry of toxins, microbes and allergens. Damage of skin barrier integrity is thus commonly involved in both Xerosis and dermatitis pathogenesis[157]. Trp channels are important regulators of skin barrier functions. In addition to TrpV3 and TrpV4 mentioned above, TrpA1 agonist also promotes skin barrier recovery[158]. In contrast, TrpV1 blocks skin barrier recovery as TrpV1 antagonist mimicked the effect of TrpA1 agonist[122].
Trp channels and chronic itch treatments
Given the critical role of Trp channels in both chronic and acute itch, treatments targeting Trp channels are receiving more and more attention.
Many clinical studies target TrpV1 for itch relief, since it is the most studied Trp channel. TrpV1 antagonists have often been tested in clinical trials as analgesics, but side effects, such as hyperthermia due to disrupted body temperature regulation, have largely prevented their progression in clinical trials[159]. Recently, several TrpV1 antagonists have been tested for itch relief. PAC-14028, a TrpV1 antagonist effective in the mouse model of atopic dermatitis[146], went through phase I clinical trial with low hyperthermia potential and good tolerability[160]. There are two finished phase II clinical trials with PAC-14028 targeting dermal pruritus and rosacea respectively, though the results have yet to be published. Another TrpV1 antagonist SB-705498 was also tested as drug for itch related diseases and was well tolerated in clinical trials. Intranasal SB-705498 was tested in phase II clinical trials targeting allergic rhinitis and non-allergic rhinitis, while topical SB-705498 was used to block histamine and cowhage induced itch in phase I trial. Intranasal SB-705498 attenuated capsaicin driven nasal hyper-reactivity in non-allergic rhinitis[161], but had only limited effect in treating symptoms of allergic rhinitis symptoms[162]. Topical SB-705498, however, failed to demonstrate clinically relevant efficacy in relieving histamine or non-histamine itch[163].
In addition to TrpV1 antagonists, topical TrpV1 agonists (for instance capsaicin and resiniferatoxin) have also been considered as potential treatments for itch. Topical TrpV1 agonists have been used clinically for pain relief for many years, though the burning pain associated with this treatment hampers compliance[164]. Recently, topical TrpV1 agonists have shown efficacy in itch relief targeting multiple itch related diseases including prurigo nodularis, psoriasis, uremic itch with hemodialysis and idiopathic intractable itch despite occasional relapses after cessation of treatment[165–168]. Although these studies cannot provide convincing evidence for the use of TrpV1 agonist in treating pruritus due to methodological issues such as improper blinding and crossover designs[169], TrpV1 agonists demonstrate great potentials for clinical itch relief.
TrpA1 has received increasing attention in recent years as a potential drug target. A TrpA1 antagonist, GRC 17536, has finished phase II clinical trial and initial results are promising for treating diabetic neuropathy pain. As the major mediator for non-histamine itch, TrpA1 antagonists might have great potential for chronic itch conditions resistant to antihistamines. Cooling and compounds producing cooling sensation, as another “counter-stimuli” of itch, have also been considered as itch treatments. In placebo controlled clinical research, the TrpM8 agonist, menthol, significant reduced mustard gas exposure induced itch in placebo controlled clinical research[170]. There are also case reports confirming the role of menthol in reducing itch in conditions such as therapy-resistant pruritus in lichen amyloidosis[171] and hydroxyethyl starch-induced itch[172]. Another TrpM8 agonist icilin can also relieve vulva pruritus[113].
Primary itch populations and Trp channels
Acute itch can be broadly categorized as histamine itch and non-histamine itch. The two forms are quite distinct from each other in many aspects in addition to their differential sensitivities to antihistamines. Histamine and non-histamine itch produced by cowhage, activate non-overlapping populations of projection neurons in the spinal cord[173]. Furthermore, activity-dependent silencing of the histamine sensitive neurons with the lidocaine derivative QX-314 attenuates histamine but not chloroquine induced scratching and vice versa[20]. However, the chloroquine sensitive primary neurons also respond to histamine, indicating an overlap of histamine and non-histamine primary itch populations. Moreover, ablation of MrgprA3 population affects not only chloroquine but also histamine and other pruritogen mediated itch[41]. A plausible explanation for the discrepancy is that chloroquine responsive neurons are less sensitive to histamine than are the other histamine responding neurons, thus lower dose of histamine and non-histamine pruritogen can engage separable subsets of neurons. Indeed, high dose histamine applied with QX-314 can attenuate chloroquine itch. Spinal projection neurons responsive to both histamine and cowhage, though small percentage, were also identified when larger numbers of neurons were included[174].
Histamine itch is largely mediated by TrpV1 positive population as mentioned above, while non-histamine itch is usually mediated by TrpA1 and TrpV1 double positive neurons. MrgprA3 and C11 express in an overlapping population sensitive to both capsaicin and mustard oil. Bradykinin, IL31 and LTB4 induced itch require both TrpV1 and TrpA1, while most serotonin, TSLP, oxidant and bile acid sensitive neurons also respond to both capsaicin and mustard oil. IL31 population largely overlaps with MrgprA3 population[90], while 5-HT7 receptor expression only partially with histamine and chloroquine receptors[46]. Consistently, MrgprA3 ablation only partially reduce serotonin mediated itch. In contrast, TSLP defines a unique population that seldom responds to histamine or chloroquine[79]. Likewise, MrgprD marks another separate population of itch neurons, which are not sensitive to histamine or chloroquine[42].
A recent study by Usoskin et al. provides additional information regarding primary itch populations[175]. Single-cell RNA sequencings was done on 622 DRG neurons and the transcriptomes were used to categorize DRG neurons in an unbiased manner, independent of previous knowledge. Four neuronal clusters were identified. The Non-peptidergic (NP) cluster was strongly linked to itch processing. The NP cluster was further divided into three subgroups NP1 to NP3 representing distinct itch populations. MrgprA3 was exclusively detected in the NP2 group and was expressed in over 60% of NP2 group neurons. MrgprC11 as previously reported mainly expressed in a slightly smaller NP2 population than did MrgprA3. However a smaller subset of MrgprC11 positive cells was also found in the NP3 population.
The NP3 population harbors multiple itch receptors. Both IL31 receptor and OSMR were enriched in the NP3 population. 5HT1f receptor can serve as a good marker for the NP3 population as it is exclusively expressed in this population and covers 83% of NP3 making it a good candidate of receptor of serotonin itch. Another highly expressing serotonin receptor 5HT2a was detected in both the NP3 and the PEP2 (from the peptidergic cluster) populations. Interestingly, cysteine leukotriene receptor 2 is highly expressed in the NP3 population. Cysteine leukotrienes are potent pro-inflammatory mediators that play an essential role in diseases such as allergic rhinitis, asthma and atopic dermatitis. Cysteine leukotriene antagonists are effective in treating allergic rhinitis, atopic dermatitis, allergic urticaria and other itch related conditions in clinical studies[176–178]. However the role of cysteine leukotrienes in pruritogenesis is unclear. The expression of cysteine leukotriene receptor 2 strongly suggests that the NP3 group may mediate pruritus induced by cysteine leukotrienes. Direct intradermal injection of LTD4 indeed elicited scratching confirming the sequencing result. Further experiments are needed to fully elucidate the pruritogenesis of cysteine leukotrienes. Another good marker for NP3 is natriuretic polypeptide b (nppb). Nppb was exclusively found in NP3 and represents 83% of the NP3 population. A recent report showed nppb knockout greatly attenuated itch mediated by all six pruritogens tested including histamine, chloroquine, serotonin and SLIGRL[179]. Nppb is therefore proposed to be a neuropeptide required for itch perception in general and its expression in this versatile NP3 population largely matches this proposed role.
Histamine receptors, in contrast, were poorly detected. The H1R was only found at low level in the NP2 and the NP3 populations (and was only statistically significant in NP2). This could be caused by lower expression level of histamine receptors or limitation of detection methods. Nevertheless, histamine itch might be mediated by NP2 and NP3. Consistently, TrpV1 is strongly expressed in both NP2 and NP3 as well as in another peptidergic group PEP1. TrpA1, on the other hand, was highly detected in all three NP groups matching its pivotal role in itch. MrgprD, reported to label a unique itch population, can serve as a good marker for NP1 population as it covers 84% of NP1 neurons. Another itch related receptor LPA3 receptor is also exclusively expressed in NP1 and covers 66% of the NP1 population, thus making NP1 a good candidate mediating cholestatic itch. In addition to TrpA1, TrpC3 was also found in a high percentage of the NP1 cells making both of them good candidates mediating β-alanine and cholestatic itch.
Conclusions
With the rapid growth of our knowledge of the neuronal mechanisms of itch sensation in recent years, Trp channels downstream of receptors especially g protein coupled receptors emerge as a common theme for itch signaling. Histamine itch and non-histamine itch are mainly mediated by TrpV1 and TrpA1 respectively. In addition, Trp channels can be coupled to cytokine receptors, toll like receptors or may even be directly activated by pruritogens. The diversity of itch transmissions including receptors and downstream mediators not covered in this review, matches the diversity of exogenous and endogenous pruritogens and the complex etiology of itchy diseases involving not just neurons but dermal and immune cells as well. Trp channels also start to show greater and greater importance in chronic itch conditions. Further understandings of Trp channels in itch, particularly chronic itch, might provide invaluable knowledge for clinical itch treatments.
Acknowledgments
This work was supported by grants (DE022750 and NS054791) from the National Institutes of Health to X.D. X.D. is an investigator of the Howard Hughes Medical Institute. We thank Sarah L. Poynton for editing.
Footnotes
Conflict of Interest statement: The authors declare no conflict of interest.
References
- 1.Ikoma A, Steinhoff M, Ständer S, et al. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 2.Ständer S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87:291–294. doi: 10.2340/00015555-0305. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von frey M. Zur Physiologie der Juckempfindung. Arch Neerl Physiol. 1922:142–145. [Google Scholar]
- 5.Lewis T, Grant RT, Marvin HM. Vascular reactions of the skin to injury. Part X The intervention of a chemical stimulus illustrated especially by the flare. The response to faradism - Google Search. Heart. 1927:139–160. [Google Scholar]
- 6.Patel KN, Dong X. An itch to be scratched. Neuron. 2010;68:334–339. doi: 10.1016/j.neuron.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norrsell U, Finger S, Lajonchere C. Cutaneous sensory spots and the “law of specific nerve energies”: history and development of ideas. Brain Res Bull. 1999;48:457–465. doi: 10.1016/s0361-9230(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 8.Sun YG, Zhao ZQ, Meng XL, et al. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson SR, Gerhold KA, Bifolck-Fisher A, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmelz M, Schmidt R, Weidner C, et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 12.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 14.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 15.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 16.MacDermot HE. The Blood Vessels of the Human Skin and Their Responses. Can Med Assoc J. 1927;17:1574. [Google Scholar]
- 17.Greaves MW, Davies MG. Histamine receptors in human skin: indirect evidence. Br J Dermatol. 1982;107(Suppl 1):101–105. doi: 10.1111/j.1365-2133.1982.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 18.Inagaki N, Nakamura N, Nagao M, et al. Participation of histamine H1 and H2 receptors in passive cutaneous anaphylaxis-induced scratching behavior in ICR mice. Eur J Pharmacol. 1999;367:361–371. doi: 10.1016/s0014-2999(98)00974-1. [DOI] [PubMed] [Google Scholar]
- 19.Imamachi N, Park GH, Lee H, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberson DP, Gudes S, Sprague JM, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci. 2013;16:910–918. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett. 2004;361:159–162. doi: 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Shim WS, Tak MH, Lee MH, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Nicolson TA, Bevan S, Richards CD. Characterisation of the calcium responses to histamine in capsaicin-sensitive and capsaicin-insensitive sensory neurones. Neuroscience. 2002;110:329–338. doi: 10.1016/s0306-4522(01)00561-9. [DOI] [PubMed] [Google Scholar]
- 25.Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunford PJ, Williams KN, Desai PJ, et al. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther. 1998;286:1140–1145. [PubMed] [Google Scholar]
- 28.Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- 29.Cormia FE, Dougherty JW. Proteolytic activity in development of pain and itching. Cutaneous reactions to bradykinin and kallikrein. J Invest Dermatol. 1960;35:21–26. doi: 10.1038/jid.1960.78. [DOI] [PubMed] [Google Scholar]
- 30.Hägermark O. Studies on experimental itch induced by kallikrein and bradykinin. Acta Derm Venereol. 1974;54:397–400. [PubMed] [Google Scholar]
- 31.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Bandell M, Story GM, Hwang SW, et al. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Dai Y, Fukuoka T, et al. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- 34.Bautista DM, Jordt SE, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. J Invest Dermatol. 2001;117:1621–1626. doi: 10.1046/j.0022-202x.2001.01585.x. [DOI] [PubMed] [Google Scholar]
- 36.Andoh T, Kuraishi Y. Nitric oxide enhances substance P-induced itch-associated responses in mice. Br J Pharmacol. 2003;138:202–208. doi: 10.1038/sj.bjp.0705004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eglezos A, Lecci A, Santicioli P, et al. Activation of capsaicin-sensitive primary afferents in the rat urinary bladder by compound 48/80: a direct action on sensory nerves? Arch Int Pharmacodyn thérapie. 315:96–109. [PubMed] [Google Scholar]
- 38.Dong X, Han S, Zylka MJ, et al. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q, Dong X. The role of the Mrgpr receptor family in itch. Handb Exp Pharmacol. 2015;226:71–88. doi: 10.1007/978-3-662-44605-8_5. [DOI] [PubMed] [Google Scholar]
- 40.Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci. 2011;31:7563–7567. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q, Sikand P, Ma C, et al. Mechanisms of itch evoked by β-alanine. J Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weisshaar E, Ziethen B, Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res. 1997;46:412–416. doi: 10.1007/s000110050213. [DOI] [PubMed] [Google Scholar]
- 44.Bockaert J, Claeysen S, Bécamel C, et al. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 46.Morita T, McClain SP, Batia LM, et al. HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron. 2015;87:124–138. doi: 10.1016/j.neuron.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandes ES, Vong CT, Quek S, et al. Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B4-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J. 2013;27:1664–1673. doi: 10.1096/fj.12-221218. [DOI] [PubMed] [Google Scholar]
- 48.Ruzicka T, Simmet T, Peskar BA, Ring J. Skin levels of arachidonic acid-derived inflammatory mediators and histamine in atopic dermatitis and psoriasis. J Invest Dermatol. 1986;86:105–108. doi: 10.1111/1523-1747.ep12284061. [DOI] [PubMed] [Google Scholar]
- 49.Willemsen MA, Lutt MA, Steijlen PM, et al. Clinical and biochemical effects of zileuton in patients with the Sjögren-Larsson syndrome. Eur J Pediatr. 2001;160:711–717. doi: 10.1007/s004310100838. [DOI] [PubMed] [Google Scholar]
- 50.Brain S, Camp R, Dowd P, et al. The release of leukotriene B4-like material in biologically active amounts from the lesional skin of patients with psoriasis. J Invest Dermatol. 1984;83:70–73. doi: 10.1111/1523-1747.ep12261712. [DOI] [PubMed] [Google Scholar]
- 51.Andoh T, Saito A, Kuraishi Y. Leukotriene B(4) mediates sphingosylphosphorylcholine-induced itch-associated responses in mouse skin. J Invest Dermatol. 2009;129:2854–2860. doi: 10.1038/jid.2009.155. [DOI] [PubMed] [Google Scholar]
- 52.Bunchorntavakul C, Reddy KR. Pruritus in chronic cholestatic liver disease. Clin Liver Dis. 2012;16:331–346. doi: 10.1016/j.cld.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Ghent CN, Bloomer JR, Klatskin G. Elevations in skin tissue levels of bile acids in human cholestasis: relation to serum levels and topruritus. Gastroenterology. 1977;73:1125–1130. [PubMed] [Google Scholar]
- 54.Varadi DP. Pruritus induced by crude bile and purified bile acids. Experimental production of pruritus in human skin. Arch Dermatol. 1974;109:678–681. [PubMed] [Google Scholar]
- 55.Mela M, Mancuso A, Burroughs AK. Review article: pruritus in cholestatic and other liver diseases. Aliment Pharmacol Ther. 2003;17:857–870. doi: 10.1046/j.1365-2036.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 56.Alemi F, Kwon E, Poole DP, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lieu T, Jayaweera G, Zhao P, et al. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology. 2014;147:1417–1428. doi: 10.1053/j.gastro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashimoto T, Ohata H, Momose K. Itch-scratch responses induced by lysophosphatidic acid in mice. Pharmacology. 2004;72:51–56. doi: 10.1159/000078632. [DOI] [PubMed] [Google Scholar]
- 59.Kremer AE, Martens JJWW, Kulik W, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–1018, 1018.e1. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu Y, Morikawa Y, Okudaira S, et al. Potentials of the circulating pruritogenic mediator lysophosphatidic acid in development of allergic skin inflammation in mice: role of blood cell-associated lysophospholipase D activity of autotaxin. Am J Pathol. 2014;184:1593–1603. doi: 10.1016/j.ajpath.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 61.Nieto-Posadas A, Picazo-Juárez G, Llorente I, et al. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat Chem Biol. 2012;8:78–85. doi: 10.1038/nchembio.712. [DOI] [PubMed] [Google Scholar]
- 62.Katugampola R, Church MK, Clough GF. The neurogenic vasodilator response to endothelin-1: a study in human skin in vivo. Exp Physiol. 2000;85:839–846. [PubMed] [Google Scholar]
- 63.Kido-Nakahara M, Buddenkotte J, Kempkes C, et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1-induced pruritus. J Clin Invest. 2014;124:2683–2695. doi: 10.1172/JCI67323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trentin PG, Fernandes MB, D'Orléans-Juste P, Rae GA. Endothelin-1 causes pruritus in mice. Exp Biol Med (Maywood) 2006;231:1146–1151. [PubMed] [Google Scholar]
- 65.Liang J, Kawamata T, Ji W. Molecular signaling of pruritus induced by endothelin-1 in mice. Exp Biol Med (Maywood) 2010;235:1300–1305. doi: 10.1258/ebm.2010.010121. [DOI] [PubMed] [Google Scholar]
- 66.Vellani V, Prandini M, Giacomoni C, et al. Functional endothelin receptors are selectively expressed in isolectin B4-negative sensory neurons and are upregulated in isolectin B4-positive neurons by neurturin and glia-derived neurotropic factor. Brain Res. 2011;1381:31–37. doi: 10.1016/j.brainres.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 67.Andoh T, Yoshida T, Lee JB, Kuraishi Y. Cathepsin E induces itch-related response through the production of endothelin-1 in mice. Eur J Pharmacol. 2012;686:16–21. doi: 10.1016/j.ejphar.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 68.Macfarlane SR, Seatter MJ, Kanke T, et al. Proteinase-Activated Receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 69.Reddy VB, Iuga AO, Shimada SG, et al. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinhoff M, Vergnolle N, Young SH, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 71.Reddy VB, Shimada SG, Sikand P, et al. Cathepsin S elicits itch and signals via protease-activated receptors. J Invest Dermatol. 2010;130:1468–1470. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinhoff M, Corvera CU, Thoma MS, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 73.Steinhoff M, Neisius U, Ikoma A, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsujii K, Andoh T, Ui H, et al. Involvement of Tryptase and Proteinase-Activated Receptor-2 in Spontaneous Itch-Associated Response in Mice With Atopy-like Dermatitis. J Pharmacol Sci. 2009;109:388–395. doi: 10.1254/jphs.08332fp. [DOI] [PubMed] [Google Scholar]
- 75.Kim N, Bae KB, Kim MO, et al. Overexpression of cathepsin S induces chronic atopic dermatitis in mice. J Invest Dermatol. 2012;132:1169–1176. doi: 10.1038/jid.2011.404. [DOI] [PubMed] [Google Scholar]
- 76.Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol. 2006;530:281–283. doi: 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Liu Q, Weng HJ, Patel KN, et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. 2011;4:ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reddy VB, Sun S, Azimi E, et al. Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs. Nat Commun. 2015;6:7864. doi: 10.1038/ncomms8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson SR, Thé L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amadesi S, Nie J, Vergnolle N, et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai Y, Wang S, Tominaga M, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:3140–3140. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Sullivan LA, Liongue C, Lewis RS, et al. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Mol Immunol. 2007;44:2497–2506. doi: 10.1016/j.molimm.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 83.Ziegler SF, Roan F, Bell BD, et al. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He R, Geha RS. Thymic stromal lymphopoietin. Ann N Y Acad Sci. 2010;1183:13–24. doi: 10.1111/j.1749-6632.2009.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singer EM, Shin DB, Nattkemper LA, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133:2783–2785. doi: 10.1038/jid.2013.227. [DOI] [PubMed] [Google Scholar]
- 87.Boguniewicz M, Leung DYM. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grimstad O, Sawanobori Y, Vestergaard C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 89.Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 90.Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oh MH, Oh SY, Lu J, et al. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol. 2013;191:5371–5382. doi: 10.4049/jimmunol.1300300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 93.Barajon I, Serrao G, Arnaboldi F, et al. Toll-like Receptors 3, 4, and 7 Are Expressed in the Enteric Nervous System and Dorsal Root Ganglia. J Histochem Cytochem. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 95.Stern PL, van der Burg SH, Hampson IN, et al. Therapy of human papillomavirus-related disease. Vaccine. 2012;30(Suppl 5):F71–82. doi: 10.1016/j.vaccine.2012.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu T, Xu ZZ, Park CK, et al. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim SJ, Park GH, Kim D, et al. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc Natl Acad Sci U S A. 2011;108:3371–3376. doi: 10.1073/pnas.1019755108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schön MP, Schön M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 99.Lee J, Kim T, Hong J, et al. Imiquimod enhances excitability of dorsal root ganglion neurons by inhibiting background (K(2P)) and voltage-gated (K(v)1.1 and K(v)1.2) potassium channels. Mol Pain. 2012;8:2. doi: 10.1186/1744-8069-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park CK, Xu ZZ, Berta T, et al. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Min H, Lee H, Lim H, et al. TLR4 enhances histamine-mediated pruritus by potentiating TRPV1 activity. Mol Brain. 2014;7:59. doi: 10.1186/s13041-014-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu T, Berta T, Xu ZZ, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diogenes A, Ferraz CCR, Akopian AN, et al. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90:759–764. doi: 10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 104.Ferraz CCR, Henry MA, Hargreaves KM, Diogenes A. Lipopolysaccharide From Porphyromonas gingivalis Sensitizes Capsaicin-Sensitive Nociceptors. J Endod. 2011;37:45–48. doi: 10.1016/j.joen.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 106.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu T, Ji RR. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull. 2012;28:145–154. doi: 10.1007/s12264-012-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lagerström MC, Rogoz K, Abrahamsen B, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y, Abdel Samad O, Zhang L, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fruhstorfer H, Hermanns M, Latzke L. The effects of thermal stimulation on clinical and experimental itch. Pain. 1986;24:259–269. doi: 10.1016/0304-3959(86)90048-5. [DOI] [PubMed] [Google Scholar]
- 112.Bromm B, Scharein E, Darsow U, Ring J. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett. 1995;187:157–160. doi: 10.1016/0304-3940(95)11362-z. [DOI] [PubMed] [Google Scholar]
- 113.Han JH, Choi HK, Kim SJ. Topical TRPM8 agonist (icilin) relieved vulva pruritus originating from lichen sclerosus et atrophicus. Acta Derm Venereol. 2012;92:561–562. doi: 10.2340/00015555-1244. [DOI] [PubMed] [Google Scholar]
- 114.Patel T, Yosipovitch G. Therapy of pruritus. Expert Opin Pharmacother. 2010;11:1673–1682. doi: 10.1517/14656566.2010.484420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davidson S, Zhang X, Khasabov SG, et al. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One. 2011;6:e22665. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ross SE, Mardinly AR, McCord AE, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kamei J, Nagase H. Norbinaltorphimine, a selective kappa-opioid receptor antagonist, induces an itch-associated response in mice. Eur J Pharmacol. 2001;418:141–145. doi: 10.1016/s0014-2999(01)00941-4. [DOI] [PubMed] [Google Scholar]
- 119.Kardon AP, Polgár E, Hachisuka J, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ständer S, Moormann C, Schumacher M, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 121.Seike M, Ikeda M, Kodama H, et al. Inhibition of scratching behaviour caused by contact dermatitis in histidine decarboxylase gene knockout mice. Exp Dermatol. 2005;14:169–175. doi: 10.1111/j.0906-6705.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 122.Yun JW, Seo JA, Jang WH, et al. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J Invest Dermatol. 2011;131:1576–1579. doi: 10.1038/jid.2011.87. [DOI] [PubMed] [Google Scholar]
- 123.Alenmyr L, Högestätt ED, Zygmunt PM, Greiff L. TRPV1-mediated itch in seasonal allergic rhinitis. Allergy. 2009;64:807–810. doi: 10.1111/j.1398-9995.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- 124.Sulk M, Seeliger S, Aubert J, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012;132:1253–1262. doi: 10.1038/jid.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamamoto-Kasai E, Yasui K, Shichijo M, et al. Impact of TRPV3 on the development of allergic dermatitis as a dendritic cell modulator. Exp Dermatol. 2013;22:820–824. doi: 10.1111/exd.12273. [DOI] [PubMed] [Google Scholar]
- 126.Yang YS, Cho SI, Choi MG, et al. Increased expression of three types of transient receptor potential channels (TRPA1, TRPV4 and TRPV3) in burn scars with post-burn pruritus. Acta Derm Venereol. 2015;95:20–24. doi: 10.2340/00015555-1858. [DOI] [PubMed] [Google Scholar]
- 127.Peier AM, Reeve AJ, Andersson DA, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 128.Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol. 2007;127:654–659. doi: 10.1038/sj.jid.5700590. [DOI] [PubMed] [Google Scholar]
- 129.Huang SM, Lee H, Chung MK, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mandadi S, Sokabe T, Shibasaki K, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miyamoto T, Petrus MJ, Dubin AE, Patapoutian A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat Commun. 2011;2:369. doi: 10.1038/ncomms1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshioka T, Imura K, Asakawa M, et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009;129:714–722. doi: 10.1038/jid.2008.245. [DOI] [PubMed] [Google Scholar]
- 133.Asakawa M, Yoshioka T, Matsutani T, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006;126:2664–2672. doi: 10.1038/sj.jid.5700468. [DOI] [PubMed] [Google Scholar]
- 134.Lin Z, Chen Q, Lee M, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90:558–564. doi: 10.1016/j.ajhg.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lai-Cheong JE, Sethuraman G, Ramam M, et al. Recurrent heterozygous missense mutation, p.Gly573Ser, in the TRPV3 gene in an Indian boy with sporadic Olmsted syndrome. Br J Dermatol. 2012;167:440–442. doi: 10.1111/j.1365-2133.2012.11115.x. [DOI] [PubMed] [Google Scholar]
- 136.Strotmann R, Harteneck C, Nunnenmacher K, et al. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 137.Liedtke W, Choe Y, Martí-Renom MA, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- 139.Güler AD, Lee H, Iida T, et al. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sokabe T, Fukumi-Tominaga T, Yonemura S, et al. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010;285:18749–18758. doi: 10.1074/jbc.M110.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beck B, Lehen'kyi V, Roudbaraki M, et al. TRPC channels determine human keratinocyte differentiation: new insight into basal cell carcinoma. Cell Calcium. 2008;43:492–505. doi: 10.1016/j.ceca.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 142.Müller M, Essin K, Hill K, et al. Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation. J Biol Chem. 2008;283:33942–33954. doi: 10.1074/jbc.M801844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leuner K, Kraus M, Woelfle U, et al. Reduced TRPC channel expression in psoriatic keratinocytes is associated with impaired differentiation and enhanced proliferation. PLoS One. 2011;6:e14716. doi: 10.1371/journal.pone.0014716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barfield RL, Barrett KR, Moon CM, David-Bajar K. Pruritic linear papules on a 75-year-old woman: a case of localized Darier-White disease. Cutis. 2002;70:225–228. [PubMed] [Google Scholar]
- 145.Pani B, Cornatzer E, Cornatzer W, et al. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier's disease. Mol Biol Cell. 2006;17:4446–4458. doi: 10.1091/mbc.E06-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yun JW, Seo JA, Jang WH, et al. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J Invest Dermatol. 2011;131:1576–1579. doi: 10.1038/jid.2011.87. [DOI] [PubMed] [Google Scholar]
- 147.Ohmura T, Hayashi T, Satoh Y, et al. Involvement of substance P in scratching behaviour in an atopic dermatitis model. Eur J Pharmacol. 2004;491:191–194. doi: 10.1016/j.ejphar.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 148.Liu B, Escalera J, Balakrishna S, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cowden JM, Zhang M, Dunford PJ, Thurmond RL. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J Invest Dermatol. 2010;130:1023–1033. doi: 10.1038/jid.2009.358. [DOI] [PubMed] [Google Scholar]
- 150.Miyamoto T, Nojima H, Shinkado T, et al. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88:285–292. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- 151.Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neurosci Lett. 2010;484:62–65. doi: 10.1016/j.neulet.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilson SR, Nelson AM, Batia L, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamamoto-Kasai E, Imura K, Yasui K, et al. TRPV3 as a therapeutic target for itch. J Invest Dermatol. 2012;132:2109–2112. doi: 10.1038/jid.2012.97. [DOI] [PubMed] [Google Scholar]
- 154.Cheng X, Jin J, Hu L, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141:331–343. doi: 10.1016/j.cell.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda) 2011;26:286–292. doi: 10.1152/physiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320–322. doi: 10.1038/jid.2008.252. [DOI] [PubMed] [Google Scholar]
- 158.Denda M, Tsutsumi M, Goto M, et al. Topical application of TRPA1 agonists and brief cold exposure accelerate skin permeability barrier recovery. J Invest Dermatol. 2010;130:1942–1945. doi: 10.1038/jid.2010.32. [DOI] [PubMed] [Google Scholar]
- 159.Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 160.Lim KM, Park YH. Development of PAC-14028, a novel transient receptor potential vanilloid type 1 (TRPV1) channel antagonist as a new drug for refractory skin diseases. Arch Pharm Res. 2012;35:393–396. doi: 10.1007/s12272-012-0321-6. [DOI] [PubMed] [Google Scholar]
- 161.Holland C, van Drunen C, Denyer J, et al. Inhibition of capsaicin-driven nasal hyper-reactivity by SB-705498, a TRPV1 antagonist. Br J Clin Pharmacol. 2014;77:777–788. doi: 10.1111/bcp.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bareille P, Murdoch RD, Denyer J, et al. The effects of a TRPV1 antagonist, SB-705498, in the treatment of seasonal allergic rhinitis. Int J Clin Pharmacol Ther. 2013;51:576–584. doi: 10.5414/CP201890. [DOI] [PubMed] [Google Scholar]
- 163.Gibson RA, Robertson J, Mistry H, et al. A randomised trial evaluating the effects of the TRPV1 antagonist SB705498 on pruritus induced by histamine, and cowhage challenge in healthy volunteers. PLoS One. 2014;9:e100610. doi: 10.1371/journal.pone.0100610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin J Pain. 2008;24:142–154. doi: 10.1097/AJP.0b013e318158ed9e. [DOI] [PubMed] [Google Scholar]
- 165.Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471–478. doi: 10.1067/mjd.2001.110059. [DOI] [PubMed] [Google Scholar]
- 166.Ellis CN, Berberian B, Sulica VI, et al. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993;29:438–442. doi: 10.1016/0190-9622(93)70208-b. [DOI] [PubMed] [Google Scholar]
- 167.Lysy J, Sistiery-Ittah M, Israelit Y, et al. Topical capsaicin--a novel and effective treatment for idiopathic intractable pruritus ani: a randomised, placebo controlled, crossover study. Gut. 2003;52:1323–1326. doi: 10.1136/gut.52.9.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Makhlough A, Ala S, Haj-Heydari Z, et al. Topical capsaicin therapy for uremic pruritus in patients on hemodialysis. Iran J Kidney Dis. 2010;4:137–140. [PubMed] [Google Scholar]
- 169.Gooding SMD, Canter PH, Coelho HF, et al. Systematic review of topical capsaicin in the treatment of pruritus. Int J Dermatol. 2010;49:858–865. doi: 10.1111/j.1365-4632.2010.04537.x. [DOI] [PubMed] [Google Scholar]
- 170.Panahi Y, Davoodi SM, Khalili H, et al. Phenol and menthol in the treatment of chronic skin lesions following mustard gas exposure. Singapore Med J. 2007;48:392–395. [PubMed] [Google Scholar]
- 171.Frölich M, Enk A, Diepgen TL, Weisshaar E. Successful treatment of therapy-resistant pruritus in lichen amyloidosis with menthol. Acta Derm Venereol. 2009;89:524–526. doi: 10.2340/00015555-0725. [DOI] [PubMed] [Google Scholar]
- 172.Haught JM, Jukic DM, English JC. Hydroxyethyl starch-induced pruritus relieved by a combination of menthol and camphor. J Am Acad Dermatol. 2008;59:151–153. doi: 10.1016/j.jaad.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 173.Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Davidson S, Zhang X, Khasabov SG, et al. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2014;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 176.Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36:689–703. doi: 10.1111/j.1365-2222.2006.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nettis E, D'Erasmo M, Di Leo E, et al. The employment of leukotriene antagonists in cutaneous diseases belonging to allergological field. Mediators Inflamm. 2010 doi: 10.1155/2010/628171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Angelova-Fischer I, Tsankov N. Successful treatment of severe atopic dermatitis with cysteinyl leukotriene receptor antagonist montelukast. Acta dermatovenerologica Alpina, Pannonica, Adriat. 2005;14:115–119. [PubMed] [Google Scholar]
- 179.Mishra SK, Hoon MA. The Cells and Circuitry for Itch Responses in Mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. 80- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 181.Kim HJ, Kim DK, Kim H, et al. Involvement of the BLT2 receptor in the itch-associated scratching induced by 12-(S)-lipoxygenase products in ICR mice. Br J Pharmacol. 2008;154:1073–1078. doi: 10.1038/bjp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim DK, Kim HJ, Kim H, et al. Involvement of serotonin receptors 5-HT1 and 5-HT2 in 12(S)-HPETE-induced scratching in mice. Eur J Pharmacol. 2008;579:390–394. doi: 10.1016/j.ejphar.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 183.Gomes LO, Hara DB, Rae GA. Endothelin-1 induces itch and pain in the mouse cheek model. Life Sci. 2012;91:628–633. doi: 10.1016/j.lfs.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 184.Hashimoto T, Ohata H, Momose K. Itch-scratch responses induced by lysophosphatidic acid in mice. Pharmacology. 2004;72:51–56. doi: 10.1159/000078632. [DOI] [PubMed] [Google Scholar]
- 185.Andoh T, Nishikawa Y, Yamaguchi-Miyamoto T, et al. Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. J Invest Dermatol. 2007;127:2042–2047. doi: 10.1038/sj.jid.5700810. [DOI] [PubMed] [Google Scholar]
- 186.Kasraie S, Niebuhr M, Baumert K, Werfel T. Functional effects of interleukin 31 in human primary keratinocytes. Allergy. 2011;66:845–852. doi: 10.1111/j.1398-9995.2011.02545.x. [DOI] [PubMed] [Google Scholar]