Impaired vasodilation by red blood cells in sickle cell disease (original) (raw)
Abstract
Red blood cells (RBCs) have been ascribed a unique role in dilating blood vessels, which requires O2-regulated binding and bioactivation of NO by Hb and transfer of NO equivalents to the RBC membrane. Vasoocclusion in hypoxic tissues is the hallmark of sickle cell anemia. Here we show that sickle cell Hb variant S (HbS) is deficient both in the intramolecular transfer of NO from heme iron (iron nitrosyl, FeNO) to cysteine thiol (_S_-nitrosothiol, SNO) that subserves bioactivation, and in transfer of the NO moiety from _S_-nitrosohemoglobin (SNO-HbS) to the RBC membrane. As a result, sickle RBCs are deficient in membrane SNO and impaired in their ability to mediate hypoxic vasodilation. Further, the magnitudes of these impairments correlate with the clinical severity of disease. Thus, our results suggest that abnormal RBC vasoactivity contributes to the vasoocclusive pathophysiology of sickle cell anemia, and that the phenotypic variation in expression of the sickle genotype may be explained, in part, by variable deficiency in RBC processing of NO. More generally, our findings raise the idea that defective NO processing may characterize a new class of hemoglobinopathy.
Keywords: nitric oxide, _S_-nitrosohemoglobin, _S_-nitrosothiols, hemoglobin, hemoglobinopathy
Hb is a tetrameric protein composed of two α-subunits and two β-subunits. One in 600 African-Americans is a Glu→Val homozygote at the sixth position of the β-chains (Hb variant S, HbS). HbS has a lower oxygen affinity and upon deoxygenation polymerizes, creating red blood cells (RBCs) that are distorted (sickled) and adherent (vasoocclusion) as well as fragile (hemolysis). Clinical sickle cell disease (SCD) is a chronic, compensated hemolytic anemia punctuated by recurrent vasoocclusion. The consequences are manifold and variable, and include bone pain, deep venous thrombosis, acute chest syndrome, and stroke. It is unclear why the phenotypic expression of SCD (homozygosity for a single-nucleotide transversion) can vary so appreciably among patients.
A recently discovered role of RBCs in hypoxic dilation of blood vessels, and inhibition of platelet activation has been attributed to release of NO bioactivity (1–6). It has been shown that NO groups can be transferred within Hb from hemes to highly conserved cysteine thiols (β-Cys-93) to form bioactive _S_-nitrosohemoglobin (SNO-Hb), and that efficient production of SNO-Hb requires selective processing of NO within the β-subunits (1, 7–9). The binding of O2 modulates the molecular and electronic structure of Hb to support preferential β-subunit reactivity. Hb, including bioactive SNO-Hb, associates with the RBC membrane primarily through interaction with Band 3, the transmembrane anion-exchanger 1 protein (AE1), via its N-terminal cytoplasmic domain (CDAE1). Upon deoxygenation [which may be accompanied by heme oxidation (6)], transfer of the NO group from β-Cys-93 of SNO-Hb to a cysteine thiol within CDAE1 subserves generation of at least a significant portion of RBC vasodilatory activity, although the mechanism through which NO/NO bioequivalents are subsequently transferred to the vessel wall remains poorly understood (3). Hypoxic vasodilation by RBCs may thus be impaired in SCD: the lower O2 affinity in vivo and altered redox properties of HbS would favor retention of NO on α-hemes, inhibiting SNO-Hb formation (1, 6, 9–11), and sickle RBCs exhibit increased levels of oxidized membrane thiols (12) that may inhibit transfer of NO groups to AE1 and the export of NO bioactivity.
Substantial evidence indicates that NO bioavailability is reduced in SCD (13–15), but the molecular basis of that defect remains enigmatic. It has been suggested that cell-free HbS (released hemolytically) sequesters NO (16). However, SCD patients characteristically are hypotensive both at steady-state (17) and during acute vasoocclusive crisis (18) rather than hypertensive as seen with NO deficiency. Hypotension, attributable to increased vascular NO production, is also seen in sickle cell mouse models (19). Further, basal blood flow (20) and plasma NOx (21) may be increased in SCD patients and vasodilatory responses to endogenous (endothelial-derived) and exogenous (nitroprusside) NO may be preserved or even enhanced (20, 22). Collectively, these observations suggest that the principal defect in NO signaling is unlikely to reside within the plasma or vessel wall. In the present study, we show that RBCs from patients with SCD are impaired in the generation of vasodilatory NO bioactivity, and that the magnitude of impairment is correlated with severity of disease. Thus, defective vasodilation by RBCs is a physiological correlate and possible cause of vasoocclusion.
Materials and Methods
Classification of SCD. Consensus criteria for SCD severity do not currently exist, and neither frequency of, nor hospital admissions for, painful crises is a reliable predictor of severity or outcome (23). Our criteria for severe SCD were based on multiple manifestations of damage to vital organ(s) and/or severe persistent anemia (see Table 1, which is published as supporting information on the PNAS web site). Patients homozygous for HbS but having none of these signs or symptoms were classified as mild SCD. Subjects receiving hydroxyurea, erythropoietin, or chronic transfusion therapy were not included in the study. Investigators were blinded to a patient's severity classification. The study was approved by the Duke Institutional Review Board.
Bioassay. Endothelium-intact rabbit thoracic aorta rings were suspended in Krebs-bicarbonate buffer at 37°C, gassed either with 21% O2/5% CO2/74% argon or with 5% CO2/95% argon [measured partial pressure of O2 (pO2) ≈ 5–7 mmHg (1mmHg = 133 Pa)] (1, 3). Resting tension was maintained at 2 gm, and active (phenylephrine-induced) tension did not differ significantly between normoxic (4.7 ± 0.3 g) and hypoxic (4.4 ± 0.3 g) rings. RBCs, in PBS at 50% hematocrit, were added to yield a bath hematocrit of 0.4%.
Photolysis-Chemiluminescence. Quantitation of FeNO and _S_-nitrosothiol (SNO) by photolysis-chemiluminescence was as described (3, 24). Washed RBCs were lysed in 5 volumes of morpholinepropane sulfonic acid (Mops) buffer (10 mM; pH 7.0). Membrane and cytosolic fractions were separated by centrifugation at 20,000 × g, and membranes were solubilized in 1% Triton X-100. SNO content was calculated by subtracting the chemiluminescence signal obtained from samples treated with HgCl2 from the signal obtained from untreated samples and was quantified by reference to standard curves generated with _S_-nitrosoglutathione ± HgCl2.
HbA and HbS. HbA was from Apex Bioscience, and HbS was from Sigma or was purified from RBCs of homozygous sickle cell patients (<5% HbF) by filtering hemolysate through Sephadex G-25 (Amersham Pharmacia). All Hb solutions were reduced with cyanoborohydride and desalted through Sephadex G-25 before use. There were no discernable functional differences between the two HbS preparations.
Inside-Out Vesicle (IOV) Preparation. IOVs were prepared from fresh normal and sickle RBCs as described (25). Buffers used for reductive preparation included 10 mM DTT and 10 mM β-mercaptoethanol. Reductants were removed from IOVs just before use by washing.
SNO-Hb-Sepharose. HbA or HbS was immobilized on Sepharose 4b (26), β-Cys-93 was selectively _S_-nitrosylated with _S_-nitrosocysteine (SNO-Cys) (27, 28), and Hb heme was reduced with cyanoborohydride before use. To evaluate NO group transfer, IOVs were incubated with immobilized SNO-HbA or SNO-HbS (50 nmol of SNO-Hb per mg of IOV protein, representing about a 10-fold molar excess over AE1) for 15 min at pH 7 and 37°C. IOVs were collected by centrifugation through a 0.45-μm filter, solubilized with Triton X-100, and analyzed by photolysis-chemiluminescence.
Two-Dimensional Electrophoresis. RBCs were lysed hypotonically (10 mM Mops, 4°C, pH 7.0; preparation at pH 7.0 preserves membrane-bound hemichromes). The membrane fraction was isolated by centrifugation and solubilized with 2% SDS. Membrane protein complexes were analyzed by two-dimensional (non-reducing → reducing) SDS/PAGE (3), followed by silver staining and analysis by scanning densitometry. The α- and β-subunits of Hb were distinguished on the basis of their differential mobility in the second-dimension gel. The position of AE1 was identified by Western blot analysis of gels run in parallel. The molar ratio of disulfide-linked HbS/AE1 was calculated on the basis of spectrophotometric quantitation of Hb content before gel electrophoresis, and on the assumption that AE1 comprised 25% by mass of RBC membrane protein.
AE1 Thiol Redox Status. RBC ghosts were prepared from fresh RBCs by hypotonic lysis as above, and solubilized in 1% SDS (without reductant)/10 mM Tris (pH 6.8). After determination of protein concentration, an aliquot was reduced with Tris[2-carboxyethyl]phosphine (TCEP) and assayed for thiol content with 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) (total thiol). The remaining sample was labeled with the thiol-specific fluorescent probe ThioGlo-1 (Calbiochem; ≈1:1 with total thiols) for 10 min, followed by quenching of unbound probe with l-cysteine for 10 min. Oxidized thiols were then reduced with TCEP (10-fold excess over total thiols) for 15 min, and proteins were separated by SDS/PAGE. Gels were negatively stained with Zn2+-imidazole (Bio-Rad), the region containing AE1 was excised, AE1 protein was electro-eluted, and protein concentration was determined. ThioGlo fluorescence associated with eluted AE1 was quantified by fluorimetry. Maximal AE1 fluorescence (five thiols per AE1) was determined by treating a TCEP-reduced aliquot of eluted AE1 with excess ThioGlo-1 (method confirmed by DTNB assay). The extent of AE1 thiol oxidation was calculated by multiplying times 5 the fluorescence signal ratio of reduced and nonreduced samples.
Statistical Analysis. All results were analyzed by comparing mean values for data sets derived from normal, mild SCD, or severe SCD subjects, employing Student's two-tailed t test.
Results
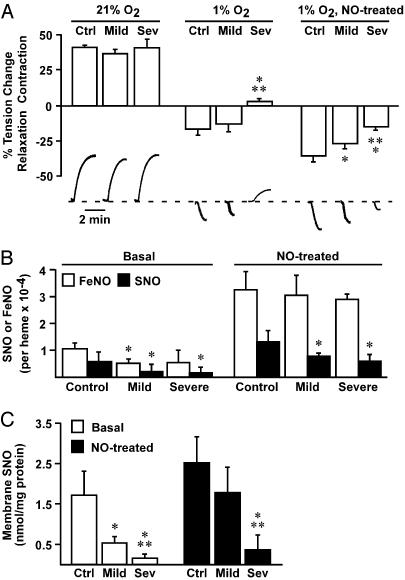
To examine the relationship between NO content and RBC vasoactivity, we used an aortic ring bioassay and RBCs obtained from normal subjects and from patients with mild or severe SCD. RBCs were studied in the basal state (obtained from venous blood and used 2–4 h after collection) or after exposure to aqueous NO (1:250 NO/heme; final bath concentration of SNO was 31–46 nM) (3). At 21% oxygen (pO2 ≈ 150 mmHg), RBCs from normal subjects or from patients elicited contractions (Fig. 1_A_), ascribed in significant part to scavenging of endothelial-derived NO (1, 3), and the magnitude of contractile responses did not differ between normal and patient groups. RBCs treated with NO also elicited contractions at 21% O2 (data not shown), which did not differ in magnitude from contractions elicited by untreated cells (Fig. 1 A). However, at ≈1% O2 (pO2 = 5–10 mmHg), to simulate precapillary arteriolar oxygen tension (6, 29), RBCs from normal subjects or from patients with mild SCD elicited vasorelaxation. Relaxations produced by normal cells were of greater magnitude than those produced by mild SCD cells, and strikingly, RBCs from patients with severe SCD remained vasoconstrictive (Fig. 1 A). In addition, hemolysis was not evident in our experiments, and thus could not account for the impaired responses to sickle RBCs, and vasodilation was preserved in endothelium-denuded vessels, and thus was not mediated by ATP that might be released from RBCs. As for other NO donors (6), vasodilation by RBCs was blocked by pharmacologic inhibition of guanylate cyclase (data not shown).
Fig. 1.
RBC vasoactivity and NO distribution in normal and sickle RBCs. (A) Oxygen-regulated vasoactivity in an aortic ring bioassay of RBCs from normal subjects (Ctrl) and from subjects with mild (Mild) or severe (Sev) SCD. All RBCs elicited vasoconstriction at 21% O2, consistent with scavenging of endothelial NO by R-state Hb. At tissue oxygen tension (1% O2, pO2 = 5–10 mmHg, favoring T-state Hb), normal and mild sickle RBCs evoked relaxation, but RBCs from severe disease subjects remained vasoconstrictive. Adjuvant NO amplified the hypoxic vasodilatory activity of control and mild RBCs, and transformed severe RBCs into vasodilators. Representative polygraph tracings of vasomotor activity are illustrated below. *, Significant with respect to control; **, significant with respect to mild SCD (n = 5–7 in each group; P < 0.05). (B and C) Both total SNO content (B) and membrane-associated SNO (C) were decreased in sickle vs. normal RBCs, but levels of membrane SNO differed significantly between mild and severe SCD and are thus more closely correlated with hypoxic vascular activity. Treatment with aqueous NO (1:250 NO/heme) increased and equalized FeNO in all groups, but total SNO content remained significantly lower in sickle vs. control RBCs (B), and membrane SNO content remained significantly lower in severe sickle RBCs (C). *, Significant with respect to control; **, significant with respect to mild SCD (n = 8–17 in each group; P < 0.05).
We have previously reported that RBCs form membrane SNO through transfer of NO groups from SNO-Hb to AE1, which generates vasorelaxant activity (3). Endogenous levels of both total RBC NO (SNO plus HbFeNO; Fig. 1_B_) as well as membrane-associated SNO (Fig. 1_C_) were lower in sickle RBCs than in normal RBCs. However, the level of endogenous membrane SNO was the better predictor of clinical status and of the differences between vasoactivity of mild and severe sickle RBCs (Fig. 1 A and C).
A decrease in endogenous SNO levels in sickle RBCs might result from a deficiency in endothelial NO production and/or from anomalous processing of NO by RBCs. To determine whether sickle RBCs are intrinsically deficient in NO processing, we supplemented normal and sickle RBCs with aqueous NO, and assessed the effects on NO content and distribution and on vasoactivity. NO treatment (1:250 NO/heme) increased and equalized FeNO in all RBC groups; total SNO content was also increased substantially, but significant differences remained between sickle and control cells (Fig. 1_B_). These differences increased when membrane SNO levels were examined separately, and much lower levels of membrane SNO in severe sickle RBCs indicated a significant deficiency in NO processing (Fig. 1_C_). Treatment with NO resulted in a 2-fold increase in the vasodilatory ability of normal and of mild sickle RBCs at 1% O2, and severe sickle RBCs were converted from vasoconstrictive to vasodilatory, generating relaxations similar to those obtained from untreated, normal RBCs (Fig. 1 A). The disproportional relationship between degree of SNO repletion and augmented vasodilatory activity may reflect, in part, creation of a pool of _S_-nitrosoglutathione, a known source of RBC vasodilatory activity that is in equilibrium with SNO-Hb (6, 27, 30–32). It has also been suggested that nitrite may augment the vasodilatory activity of RBCs, but the anaerobic conditions we used are not conducive to formation of SNO-Hb (6), and added nitrite had no effect in our RBC bioassays (data not shown). Higher levels of supplemental NO did not produce further increases in vasodilatory activity of any RBC group (data not shown), consistent with the known plateau in the amount of SNO-Hb that can be formed in normal RBCs (6, 11), and with an intrinsic deficit in the ability of sickle RBCs to generate SNO-Hb and membrane SNO. Thus, these findings demonstrate a significant interrelationship between RBC SNO content, hypoxic vasoactivity, and clinical severity of SCD.
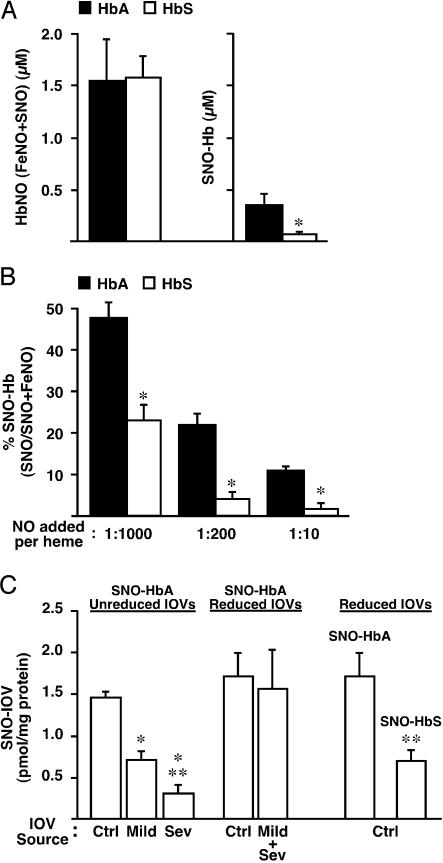
Reduced membrane SNO content in sickle RBCs could stem from impairment at one or more stages of NO processing, including decreased NO import, inefficient formation of SNO-HbS (mediated by the intramolecular transfer of NO groups from heme to thiol) (6, 11), decreased SNO-HbS stability, or defects in the intermolecular transfer of NO groups from SNO-HbS to AE1 at the RBC membrane. Evidently, uptake of NO by sickle RBCs is not significantly impaired, because total NO levels were closely comparable in sickle and normal RBCs after addition of supplemental NO (Fig. 1_B_), and equivalent vasoconstriction (NO scavenging) was elicited by normal and sickle RBCs at 21% O2 (Fig. 1 A). In addition, decreased stability of SNO-HbS was not observed in previous reports (10) or in our own preparations (see below). To test the fidelity of the intramolecular NO-transfer mechanism, aqueous NO (1:200 NO/heme) was added to cell-free deoxy HbA and deoxy HbS (100 μM tetramer) followed by reoxygenation, and the products measured by photolysis-chemiluminescence (3, 11). As in RBCs exposed to exogenous NO (Fig. 1_B_), total Hb-bound NO (FeNO + SNO) was similar for HbS and HbA (Fig. 2_A_). However, much less SNO-HbS than SNO-HbA was formed (Fig. 2 A and B). This disparity was maintained over a 100-fold range of initial NO/heme (Fig. 2_B_). [Note that, as described previously (11), SNO yield declines as the NO/heme ratio increases.] These results indicate that access and binding of NO to heme iron is unaffected in HbS but that NO does not transfer effectively from heme to thiol upon transition from T (deoxy)- to R (oxy)-state.
Fig. 2.
NO processing defects of sickle Hb and RBC membranes. (A) SNO-Hb formation by cell-free HbA and HbS (100 μM tetramer) after addition of aqueous NO (1:200 NO/heme). NO solution was added slowly to deoxygenated Hb followed by reoxygenation and filtration through Sephadex G-25. Quantitation of NO bound to heme (FeNO) and thiol (SNO) was carried out by photolysis-chemiluminescence. Total incorporation of NO (FeNO + SNO) was equivalent for HbA and HbS, but significantly less SNO-HbS was generated. *, Significant with respect to HbA (n = 4; P < 0.05). (_B_) SNO yield declines as the initial NO/heme ratio increases (absolute amount plateaus) (11), but the disparity between SNO formation by HbA vs. HbS is maintained. (_C_) Transfer of NO from immobilized SNO-HbA or SNO-HbS (generated from SNO-Cys and matched for SNO content) to IOVs prepared reductively (DTT/β-mercaptoethanol) or nonreductively from RBCs obtained from normal subjects (Ctrl) or from subjects with mild (Mild) or severe (Sev) SCD. Less NO was transferred from SNO-HbA to nonreduced sickle IOVs vs. nonreduced control IOVs (control > mild > severe), but transfer to control and sickle IOVs prepared reductively did not differ (Mild + Sev represents a mixture of IOVs from subjects with mild and severe disease). Note that NO group transfer from SNO-HbA to reduced vs. unreduced IOVs did not differ significantly. NO transfer from SNO-HbS to control IOVs (prepared reductively) was significantly decreased with respect to transfer from SNO-HbA. *, Significant with respect to nonreduced control IOV; **, significant with respect to nonreduced mild IOVs (left) or SNO-HbA (right) (n = 4–6 in each group; P < 0.05).
The formation of SNO-Hb from deoxy-Hb(Fe[II]NO) upon oxygenation requires not only a conformational change (T → R transition) but also the loss of an electron (6), and it has been shown that the transfer of NO groups from heme to thiol within Hb can be supported by heme iron redox chemistry (6, 7). Bonaventura et al. (10) have demonstrated that the decline in heme redox potential that accompanies the allosteric T → R transition is significantly attenuated in HbS vs. HbA, consistent with the observed failure of HbS to support _S_-nitrosylation. Conformational changes in Hb are also accompanied by characteristic changes in O2 affinity (P50) and thiol reactivity, and Hb can undergo oxidative modification (e.g., glutathionylation) under conditions of oxidative stress. We therefore examined and eliminated explanations for differences in SNO formation that were independent of heme iron redox, including oxidation of HbS thiol, differences in thiol reactivity between HbS and HbA, and aggregation of HbS at concentrations below the gelling threshold (see Fig. 5, which is published as supporting information on the PNAS web site): (i) electrospray mass spectroscopy revealed no differences between HbS and HbA that could be accounted for by oxidative thiol modification(s); (ii) formation of SNO-Hb by transnitrosylation from _S_-nitroso-cysteine yielded a stoichiometry of 2 SNO/Hb for both HbS and HbA, indicating that β-Cys-93 is available and equally capable of participating in NO+ transfer that is independent of heme iron redox; (iii) the kinetics of 5,5′-dithiobis-2-nitrobenzoic acid conversion were indistinguishable between HbS and HbA as assessed by stopflow analysis, indicating equal thiol reactivity; and (iv) analysis by dynamic laser light scattering indicated no differences in the degree of aggregation of HbS vs. HbA at the concentrations used in our experiments (100 μM). It is well known that O2-binding affinity, expressed as P50, does not differ significantly between HbS and HbA at concentrations below the gelling threshold. The disparity between the effects of the sickle mutation on processing of O2 vs. NO by heme iron apparently reflects the unique redox requirements for NO transfer. Thus, defective formation of SNO-HbS constitutes the result of an “experiment of nature,” which confirms the critical role of allosterically regulated heme redox properties in SNO formation by Hb under physiological conditions (6, 7).
It should be noted that, upon polymerization in vivo, the higher P50 of HbS vs. HbA (T-state predilection) may further accentuate the imbalance in SNO formation. This interpretation is supported by the finding (unpublished observations; ref. 33) that allosteric effectors that increase the P50 of HbA (CO2, inositol hexaphosphate, and high phosphate concentrations) inhibit formation of SNO-Hb, and by crystallographic results of Arnone and colleagues (34) that have more generally excluded the possibility of SNO production in T-structured tetramers.
Generation of vasodilatory NO bioactivity by RBCs involves transfer of NO groups from SNO-Hb to cysteine thiol in CDAE1 (3). Decreased formation of membrane SNO in sickle RBCs could therefore reflect alterations in the interaction of SNO-HbS (the NO donor) and CDAE1 (the NO acceptor), in addition to impairment of SNO-HbS formation. To evaluate these possibilities, we examined the transfer of NO from Sepharose-linked SNO-HbA or SNO-HbS to IOVs, which were prepared from normal or sickle RBCs in the presence (reductively) or absence (nonreductively) of DTT/β-mercaptoethanol. The amount of NO transferred from SNO-HbA to sickle IOVs prepared without reductants was decreased compared with normal IOVs and was correlated with clinical severity (Fig. 2_C_); these results were the same whether IOVs were prepared in room air or at <1% O2. There was no difference in the efficiency of NO group transfer from SNO-HbA to normal vs. sickle IOVs prepared with reductants (Fig. 2_C_). In addition (Fig. 2_C_), significantly less NO was transferred to normal IOVs (prepared reductively) from SNO-HbS vs. SNO-HbA (matched for SNO content; as shown in Fig. 5, HbA and HbS were equally efficient in forming β-Cys-93-NO from Cys-NO). Sepharose-immobilized SNO-Hb (HbA or HbS) was stable in the absence of IOVs, and all NO recovered was accounted for in the form of SNO-Hb plus SNO-IOV (data not shown). These results indicate that reduced formation of membrane SNO in sickle RBCs reflects both a defect in the molecular mechanism of NO transfer from SNO-Hb to AE1, and an alteration in the redox status of CDAE1 at the sickle RBC membrane.
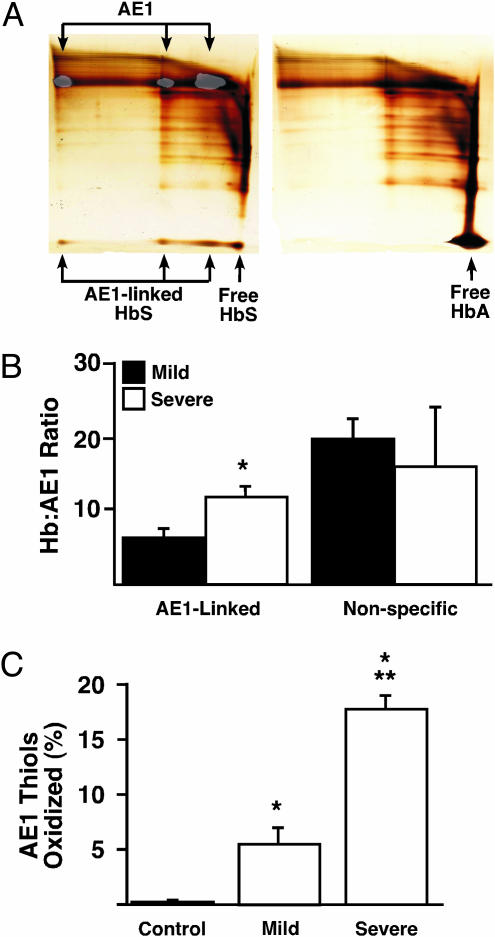
Previous studies have described impaired antioxidative mechanisms in sickle RBCs (35, 36) and oxidative membrane damage reflected in the presence of oxidized protein thiols (12), but affected proteins have not been identified. The transfer of NO from SNO-Hb to CDAE1 requires close apposition of a β-Cys-93 thiol to cysteine thiol within CDAE1, and bound AE1 and HbA (or SNO-HbA) can in fact be disulfide-linked by oxidative (Cu2+) catalysis (3). Thus, stable oxidative cross-linking of a vicinal thiol pair in HbS and CDAE1 might decrease the number of AE1 thiols otherwise available to serve as NO acceptors. To examine this possibility, we analyzed native sickle and normal RBC membranes by two-dimensional electrophoresis (nonreducing → reducing). Substantial quantities of HbS were disulfide-linked to AE1 in sickle RBC membranes, whereas crosslinking of HbA and AE1 was not detected in normal RBC membranes (Fig. 3 A and B); these results were the same whether membranes were prepared in room air or at <1% O2. Further, the calculated ratio of linked HbS/AE1 was notably higher in membranes from severe vs. mild sickle RBCs (Fig. 3_B_). The relative amounts of membrane-associated but unlinked Hb (reflecting predominantly nonspecific binding) were unrelated to disease severity (Fig. 3_B_). The obtained ratios (>1:1) of cross-linked HbS/AE1 may reflect the presence of bound hemichromes (denatured, disulfide and otherwise covalently cross-linked HbS complexes), which are known to associate with CDAE1 in 5–8:1 ratios (globin chain/AE1) (37–40), as well as chains of disulfide-linked HbS that may form via intermolecular β-Cys-93/β-Cys-93 coupling (40). Note that, whereas only the β-subunit of HbA is cross-linked to IOVs by oxidative catalysis (3), we observed that β- and α-subunits were associated in roughly equal proportions with native sickle RBC membranes, consistent with disulfide coupling of β-chains to both AE1 and additional Hb subunits. Formation in vivo of either hemichrome/CDAE1 complexes or chained Hb would predictably be facilitated by oxidative stress, as in SCD.
Fig. 3.
Hb cross-linking and AE1 thiol oxidation in native normal and sickle RBCs. (A) Two-dimensional gel analysis (nonreducing → reducing) of control (right) and sickle (left) RBC membranes. No disulfide-linked HbA was detected in association with membranes from normal RBCs. In contrast, HbS (lower arrows) was disulfide-linked to mono-, di-, and multimeric AE1 (upper arrows), identified by Western blot analysis of a gel run in parallel (low-intensity overlay). (Horizontal streaking represents dispersion in the first-dimension gel of cross-linked protein complexes, which did not differ between HbA and HbS.) (B) Association of HbS with sickle RBC membranes. The molar ratio of HbS disulfide-linked to AE1 was significantly greater in severe vs. mild SCD (11.19 vs. 6.34), whereas no difference was observed in the amount of membrane-associated but unlinked HbS. *, Significant with respect to mild SCD (n = 7–13 in each group; P < 0.05). (C) Analysis of AE1 thiol redox status, based on quantitation of free thiols with thiol-specific fluorescent labeling. AE1 thiol oxidation was more extensive in severe vs. mild sickle RBCs, whereas normal RBC AE1 thiols were essentially unoxidized. *, Significant with respect to normal; **, significant with respect to mild disease (n = 4–6 in each group; P < 0.05).
To determine the magnitude of AE1 thiol oxidation, we quantified free AE1 thiols by using thiol-specific fluorescent labeling. Thiol oxidation was approximately 3-fold greater in AE1 from severe vs. mild sickle RBCs, whereas essentially all normal RBC AE1 thiols were in the reduced state (Fig. 3_C_). We did not identify directly the cysteine thiols oxidized in AE1 from sickle RBCs. However, AE1 contains five cysteine residues, two of which are in CDAE1, and loss of ≈20% of free thiols as observed in severe SCD (Fig. 3_C_) thus suggests a stoichiometry of one oxidized thiol per AE1, consistent with disulfide linkage of β-Cys-93 of HbS to a vicinal thiol within CDAE1. In combination with the analysis of cross-linked HbS/AE1, these results suggest that oxidative coupling of HbS to CDAE1 can largely account for the loss of free AE1 thiols in sickle RBCs.
Discussion
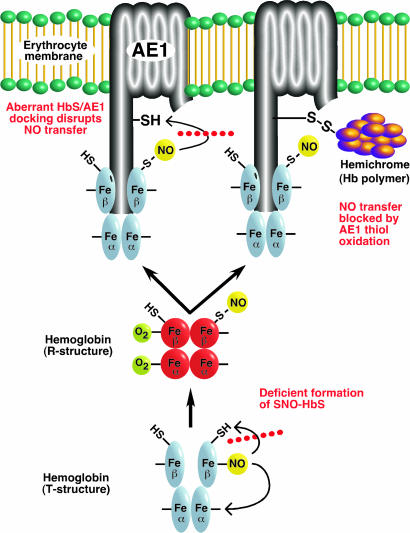
Recent genetic analysis in animals (30, 31) and clinical studies (4–6, 41) support a central role for SNOs in vascular homeostasis. Our findings identify in sickle cell patients a deficiency of SNO-Hb as well as intrinsic defects in the processing of NO by sickle RBCs, which impair SNO-mediated intercellular communication with the vascular wall. Both the production of SNO-HbS (intramolecular transfer of NO from heme to β-Cys-93) and the generation of vasoactive membrane SNO (intermolecular transfer of NO from SNO-HbS to CDAE1 thiol) are impaired in SCD (Fig. 4). Our results suggest further that these defects result at least in part from redox potential differences of heme iron within HbS vs. HbA (10), which inhibit the generation of SNO-Hb upon transition to R structure, and from differences in HbS vs. HbA binding to the RBC membrane. These impairments in NO processing may exemplify a new form of hemoglobinopathy.
Fig. 4.
Schematic summary of the proposed defects in NO trafficking in the sickle erythrocyte, which inhibit generation of vasodilatory NO bioactivity at the RBC membrane. As shown at the bottom, the allosterically (O2/redox) regulated intramolecular transfer of NO from heme to thiol is impaired in HbS. This defect is likely to represent differences between HbS vs. HbA in heme redox potential (and in P50 upon polymerization), and thus in the ability to support _S_-nitrosylation. [Note that some portion of NO groups bound to β-heme will redistribute to α-heme (“recapture”) rather than thiol, that one oxygen is omitted from R-state SNO-Hb to provide for the possibility of β-heme oxidation (Fe2+ → Fe3+) coupled to SNO-Hb formation (6, 7), and that O2/redox-regulation of Hb is represented as a simplified two-state (R/T) model (6).] In addition, transfer of NO groups from SNO-HbS to a cysteine thiol within the CDAE1 is deficient, shown on the left, as a result of aberrant binding of HbS and CDAE1. As shown on the right, oxidative (disulfide) coupling of HbS to CDAE1, which may be facilitated by aberrant binding, results in the formation of cross-linked AE1-hemichrome complexes that preclude NO group transfer from SNO-HbS to the RBC membrane.
Differences in membrane (IOV) binding of HbA vs. HbS have been reported at low pO2 (39), and there are differences between HbA and HbS in the configuration of the 2,3-diphosphoglycerate-binding pocket (β-cleft) (42). Interaction between the polyanionic N terminus of CDAE1 and residues within the β-cleft subserves specific binding of T-state Hb to the RBC membrane, which creates close apposition (vicinality) of β-Cys-93 thiol and a cysteine thiol within CDAE1 (3). Our results suggest that the precise geometry of that interaction may influence the probability of transnitrosylation vs. disulfide formation; the interaction of SNO-HbA and CDAE1 favors NO group transfer, whereas transnitrosylation by SNO-HbS is significantly less efficient, and disulfide cross-linking is more likely (perhaps facilitated by the oxidative milieu in sickle RBCs). HbS disulfide-linked to AE1 presents a barrier to the generation of vasodilatory NO bioactivity by sickle RBCs, and the extent of oxidative membrane-coupling of HbS (reflected in the deficiency of membrane SNO) is correlated with SCD severity.
Clinical heterogeneity is a hallmark of SCD, and predictors of severity are unfortunately lacking. Our results suggest that impaired hypoxic vasodilation by RBCs ex vivo is a physiological correlate of vasoocclusion in vivo, and more generally, that phenotypic variance in SCD may be explained at least in part by the magnitude of defects in the O2-regulated pathway that couples synthesis and export of NO bioactivity by RBCs. Low pO2 in the microcirculation drives the abnormal changes in sickle RBC rheology that contribute to vasoocclusion. Impaired vasodilation by sickle RBCs may thus contribute directly to the vasoocclusive process by promoting hypoxia-dependent sickling. In addition, because RBCs help regulate pulmonary as well as systemic blood pressure (6), our results suggest that the elevated pulmonary blood pressure characteristic of SCD may result in part from impaired vasodilation by RBCs. It is interesting to note that impaired NO processing and hypoxic vasodilation by RBCs has recently been reported in diabetes, another condition characterized by microvascular complications (4). Taken together, these findings point to a new aspect of vascular flow dysregulation that results from alterations in NO processing by RBCs. Supplemental NO can partially restore membrane SNO and vasodilation by sickle RBCs. However, a persistent defect in RBCs from patients with severe SCD emphasizes a requirement for reduction of oxidized thiols to normalize RBC bioactivity, and points to the potential of therapeutic modalities that incorporate both NO repletion and amelioration of oxidative defects.
Supplementary Material
Supporting Information
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants PO1-HL42444 (to J.S.S.) and HL-068721 (to J.R.P.) and by a Doris Duke Clinical Scientist Development Award (to J.R.P.).
Abbreviations: HbS, Hb variant S; RBC, red blood cell; SCD, sickle cell disease; SNO, _S_-nitrosothiol; SNO-Hb, _S_-nitrosohemoglobin; AE1, anion-exchanger 1; CDAE1, cytoplasmic domain of AE1; pO2, partial pressure of O2; IOV, inside-out vesicle.
References
- 1.McMahon, T. J., Moon, R. E., Luchsinger, B. P., Carraway, M. S., Stone, A. E., Stolp, B. W., Gow, A. J., Pawloski, J. R., Watke, P., Singel, D. J., et al. (2002) Nat. Med. 8**,** 711-717. [DOI] [PubMed] [Google Scholar]
- 2.Pawloski, J. R., Swaminathan, R. V. & Stamler, J. S. (1998) Circulation 97**,** 263-267. [DOI] [PubMed] [Google Scholar]
- 3.Pawloski, J. R., Hess, D. T. & Stamler, J. S. (2001) Nature 409**,** 622-626. [DOI] [PubMed] [Google Scholar]
- 4.James, P. E., Lang, D., Tufnell-Barrett, T., Milsom, A. B. & Frenneaux, M. P. (2004) Circ. Res. 94**,** 976-983. [DOI] [PubMed] [Google Scholar]
- 5.Datta, B., Tufnell-Barrett, T., Bleasdale, R. A., Jones, C. J., Beeton, I., Paul, V., Frenneaux, M. & James, P. (2004) Circulation 109**,** 1339-1342. [DOI] [PubMed] [Google Scholar]
- 6.Singel, D. J. & Stamler, J. S. (2005) Annu. Rev. Physiol. 67**,** in press. [DOI] [PubMed]
- 7.Luchsinger, B. P., Rich, E. N., Gow, A. J., Williams, E. M., Stamler, J. S. & Singel, D. J. (2003) Proc. Natl. Acad. Sci. USA 100**,** 461-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pezacki, J. P., Ship, N. J. & Kluger, R. (2001) J. Am. Chem. Soc. 123**,** 4615-4616. [DOI] [PubMed] [Google Scholar]
- 9.Yonetani, T., Tsuneshige, A., Zhou, Y. & Chen, X. (1998) J. Biol. Chem. 273**,** 20323-20333. [DOI] [PubMed] [Google Scholar]
- 10.Bonaventura, C., Taboy, C. H., Low, P. S., Stevens, R. D., Lafon, C. & Crumbliss, A. L. (2002) J. Biol. Chem. 277**,** 14557-14563. [DOI] [PubMed] [Google Scholar]
- 11.Gow, A. J. & Stamler, J. S. (1998) Nature 391**,** 169-173. [DOI] [PubMed] [Google Scholar]
- 12.Rank, B. H., Carlsson, J. & Hebbel, R. P. (1985) J. Clin. Invest. 75**,** 1531-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan, K. J., Kissoon, N., Duckworth, L. J., Sandler, E., Freeman, B., Bayne, E., Sylvester, J. E. & Lima, J. J. (2001) Am. J. Respir. Crit. Care Med. 164**,** 2186-2190. [DOI] [PubMed] [Google Scholar]
- 14.Morris, C. R., Kuypers, F. A., Larkin, S., Vichinsky, E. P. & Styles, L. A. (2000) J. Pediatr. Hematol. Oncol. 22**,** 515-520. [DOI] [PubMed] [Google Scholar]
- 15.Lopez, B. L., Barnett, J., Ballas, S. K., Christopher, T. A., Davis-Moon, L. & Ma, X. (1996) Acad. Emerg. Med. 3**,** 1098-1103. [DOI] [PubMed] [Google Scholar]
- 16.Reiter, C. D., Wang, X., Tamus-Santos, J. E., Hogg, N., Cannon, R. O., III, Schechter, A. N. & Gladwin, M. T. (2002) Nat. Med. 8**,** 1383-1389. [DOI] [PubMed] [Google Scholar]
- 17.Pegelow, C. H., Colangelo, L., Steinberg, M., Wright, E. C., Smith, J., Phillips, G. & Vichinsky, E. (1997) Am. J. Med. 102**,** 171-177. [DOI] [PubMed] [Google Scholar]
- 18.Ernst, A. A., Weiss, S. J., Johnson, W. D. & Takakuwa, K. M. (2000) South. Med. J. 93**,** 590-592. [PubMed] [Google Scholar]
- 19.Kaul, D. K., Liu, L., Fabry, M. E. & Nagel, R. L. (2000) Am. J. Physiol. 278**,** H1799-H1806. [DOI] [PubMed] [Google Scholar]
- 20.Belhassen, L., Pelle, G., Sediame, S., Bachir, D., Carville, C., Bucherer, C., Lacombe, C., Galacteros, F. & Adnot, S. (2001) Blood 97**,** 1584-1589. [DOI] [PubMed] [Google Scholar]
- 21.Rees, D. C., Cervi, P., Grimwade, D., O'Driscoll, A., Hamilton, M., Parker, N. E. & Porter, J. B. (1995) Br. J. Haematol. 91**,** 834-837. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardt, R. T., McMahon, L., Duffy, S. J., Steinberg, M. H., Perrine, S. P., Loscalzo, J., Coffman, J. D. & Vita, J. A. (2003) Am. J. Hematol. 74**,** 104-111. [DOI] [PubMed] [Google Scholar]
- 23.Schnog, J.-J. B., Lard, L. R., Rojer, R. A., Van der Dijs, F. P. L., Muskiet, F. A. J. & Duits, A. J. (1998) Am. J. Hematol. 58**,** 61-66. [DOI] [PubMed] [Google Scholar]
- 24.Stamler, J. S. & Feelisch, M. (1996) in Methods in Nitric Oxide Research, eds. Stamler, J. S. & Feelisch, M. (Wiley, Chichester, U.K.), pp. 521-539.
- 25.Bennett, V. (1983) Methods Enzymol. 96**,** 313-324. [DOI] [PubMed] [Google Scholar]
- 26.Kohn, J. & Wilchek, M. (1982) Biochem. Biophys. Res. Commun. 107**,** 878-884. [DOI] [PubMed] [Google Scholar]
- 27.Jia, L., Bonaventura, C., Bonaventura, J. & Stamler, J. S. (1996) Nature 380**,** 221-226. [DOI] [PubMed] [Google Scholar]
- 28.Stamler, J. S., Jia, L., Eu, J. P., McMahon, T. J., Demchenko, I. T., Bonaventura, J., Gernert, K. & Piantadosi, C. A. (1997) Science 276**,** 2034-2037. [DOI] [PubMed] [Google Scholar]
- 29.Gorczynski, R. J. & Duling, B. R. (1978) Am. J. Physiol. 235**,** H505-H515. [DOI] [PubMed] [Google Scholar]
- 30.Liu, L., Yan, Y., Zeng, M., Zhang, J., Hanes, M. A., Ahearn, G., McMahon, T. J., Dickfeld, T., Marshall, H. E., Que, L. G., et al. (2004) Cell 116**,** 617-628. [DOI] [PubMed] [Google Scholar]
- 31.Lipton, A. J., Johnson, M. A., Macdonald, T., Lieberman, M. W., Gozal, D. & Gaston, B. (2001) Nature 413**,** 171-174. [DOI] [PubMed] [Google Scholar]
- 32.Romeo, A. A., Capobianco, J. A. & English, A. M. (2003) J. Am. Chem. Soc. 125**,** 14370-14378. [DOI] [PubMed] [Google Scholar]
- 33.Gow, A. J., Luchsinger, B. P., Pawloski, J. R., Singel, D. J. & Stamler, J. S. (1999) Proc. Natl. Acad. Sci. USA 96**,** 9027-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan, N.-L., Kavanaugh, J. S., Rogers, P. H. & Arnone, A. (2004) Biochemistry 43**,** 118-132. [DOI] [PubMed] [Google Scholar]
- 35.Zerez, C. R., Lachant, N. A., Lee, S. J. & Tanaka, K. R. (1988) Blood 71**,** 512-515. [PubMed] [Google Scholar]
- 36.Lachant, N. A., Davidson, W. D. & Tanaka, K. R. (1983) Am. J. Hematol. 15**,** 1-13. [DOI] [PubMed] [Google Scholar]
- 37.Kannan, R., Labotka, R. J. & Low, P. S. (1988) J. Biol. Chem. 263**,** 13766-13773. [PubMed] [Google Scholar]
- 38.Waugh, S. M., Walder, J. A. & Low, P. S. (1987) Biochemistry 26**,** 1777-1783. [DOI] [PubMed] [Google Scholar]
- 39.Shaklai, N., Sharma, V. S. & Ranney, H. M. (1981) Proc. Natl. Acad. Sci. USA 78**,** 65-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Low, P. S. (1986) Biochim. Biophys. Acta 864**,** 145-167. [DOI] [PubMed] [Google Scholar]
- 41.Massy, Z. A., Fumeron, C., Borderie, D., Tuppin, P., Nguyen-Khoa, T., Benoit, M. O., Jacquot, C., Buisson, C., Drueke, T. B., Ekindjian, O. G., et al. (2004) J. Am. Soc. Nephrol. 15**,** 470-476. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch, R. E., Juszczak, L. J., Fataliev, N. A., Friedman, J. M. & Nagel, R. L. (1999) J. Biol. Chem. 274**,** 13777-13782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information