Insulin action and resistance in obesity and type 2 diabetes (original) (raw)
. Author manuscript; available in PMC: 2018 Jul 17.
Published in final edited form as: Nat Med. 2017 Jul 11;23(7):804–814. doi: 10.1038/nm.4350
Abstract
Nutritional excess is a major forerunner of type 2 diabetes and enhances the secretion of insulin but attenuates its metabolic actions in the liver, skeletal muscle and adipose tissue. However, conflicting evidence indicates a lack of knowledge of the timing of these events during the development of obesity and diabetes and is a key gap in our understanding of metabolic disease. This Perspective reviews alternate viewpoints and recent results on the temporal and mechanistic connections between hyperinsulinemia, obesity and insulin resistance. Although much attention has addressed early steps in the insulin signaling cascade, insulin resistance in obesity appears to be largely elicited downstream of these steps. New findings also connect insulin resistance to extensive metabolic crosstalk between liver, adipose, pancreas and skeletal muscle. These and other advances over the last 5 years offer both exciting opportunities and daunting challenges in developing new therapeutic strategies for the treatment of type 2 diabetes.
The term “insulin resistance” refers to a decrease in a target cell’s metabolic response to insulin or, at the whole organism level, an impaired blood glucose lowering effect of circulating or injected insulin on blood glucose (see Box 1 for overview of insulin signaling)1. It is a hallmark of obesity and sedentary behavior, and is a forerunner of type 2 diabetes which affects a remarkable 9% of the US population2. Significant co-morbidities are associated with diabetes, including kidney failure, neuropathy, retinopathy as well as vascular morbidities that lead to ischemic heart disease and to nearly 75,000 amputations per year2.
Box 1.
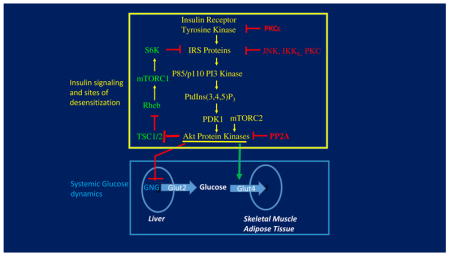
Glucose homeostasis is maintained by coordinating the production of glucose in the liver through the pathways of glycogenolysis and gluconeogenesis in times of fasting with the disposal of glucose into skeletal muscle through glycogen synthesis and glucose metabolism and to a much lesser extent adipose tissue during feeding (blue box in the Figure). The hormone insulin, secreted by the beta cells of the pancreas in times of nutrient uptake, inhibits hepatic glucose output while enhancing glucose uptake into muscle and adipose tissue. Glucose is released through the glucose transporter Glut2 in liver, while the insulin-sensitive Glut4 mediates glucose uptake in muscle and fat. The major canonical insulin signaling cascade required for this maintenance of blood glucose concentrations activates a key protein kinase denoted as Akt (upper box in the Figure below)10. This Akt protein kinase (three isoforms are known) is required for insulin regulation of the pathways that control systemic glucose homeostasis, including glucose transport in adipocytes and muscle164–166, inhibition of hepatic gluconeogenesis17,22 as well as cell autonomous activation of hepatic lipogenesis17,167.
Insulin binding to its receptor protein activates its intrinsic tyrosine kinase activity that phosphorylates Insulin Receptor Substrate (IRS) Proteins on tyrosine residues that then serve as anchoring sites for the p85 regulatory subunits of p85/p110 PI-3kinase at the cell membrane10. This generates the formation of the phospholipid phosphatidyl 3,4,5 phosphate (PtdIns3,4,5P3) from PtdIns 4,5 P2 in the membrane, which facilitates recruitment and interaction between the protein kinases PDK1 and Akt, leading to phosphorylation (and activation) of the latter on threonine 308. Full activation of Akt occurs upon its phosphorylation by a second protein kinase, mTORC2. Interestingly, through an Akt-mediated pathway that activates a related complex denoted as mTORC1, homologous desensitization is accomplished by phosphorylation of IRS proteins on serine residues which attenuates their tyrosine phosphorylation by the receptor.
Much attention in the field has been focused on heterologous desensitization mechanisms associated with obesity that attenuate insulin signaling to Akt (red elements on right side of yellow box in the Figure)10,52,89–92. Such mechanisms are often claimed to be the cause of systemic insulin resistance in obesity. A major thesis of this Perspective is that the attenuated systemic metabolic responses to insulin observed in obesity and under HFD conditions in rodents and humans largely occur either downstream or independent of insulin signaling to Akt.
The factors involved in development of metabolic disease are complex however since many obese individuals with a preponderance of subcutaneous rather than visceral adipose tissue appear to be protected from insulin resistance and adverse metabolic responses3. Nonetheless, numerous findings over many decades of work have solidified a strong overall paradigm4,5 that over-nutrition in prone individuals causes peripheral tissue resistance to insulin’s actions, raising blood glucose levels, which then stimulates islet beta cell insulin secretion. However, based on frequent unexpected observations, counter arguments have appeared over the years that propose reversing this scenario, claiming that hyperinsulinemia may actually be the primary disruption in obesity that drives the insulin resistance6–9. In this latter model, insulin circulating at higher than normal levels under both fasting and fed conditions is itself complicit in the metabolic dysregulations that occur in obesity.
This article examines these opposing hypotheses in light of recent research on the timing and molecular basis of insulin resistance during development of obesity and type 2 diabetes in mouse models and humans. The intent in this Perspective is not to survey the entire field and summarize all the exciting ongoing work, but rather to highlight a few key issues that are central to the large gaps in our knowledge in this field. Following presentation of the conceptual framework for the models that insulin resistance versus hyperinsulinemia are primary, the early timeline of their appearances after initiation of nutrient overload are evaluated. Physiological consequences of hyperinsulinemia are discussed in light of recent studies using genetic and chemical manipulation of insulin levels in obesity. Finally, the underlying molecular mechanisms of insulin resistance are reviewed in the context of whether hyperinsulinemia may be a major causative factor.
Hyperinsulinemia and Insulin Resistance
Viewpoint: insulin resistance is primary, causing hyperinsulinemia
Experimental induction of insulin resistance in mice by disruption of insulin signaling in liver, skeletal muscle or adipose tissue causes hyperinsulinemia and can lead to diabetes10. Similarly, elegant studies on human subjects with monogenic mutations in insulin signaling components resulting in insulin resistance show similar high circulating insulin and consequent diabetes11. These data point towards the concept that both monogenic and common forms of obesity also initially cause insulin resistance, which secondarily causes hyperinsulinemia, promoting fatty liver and hypertriglyceridemia (Figure 1).
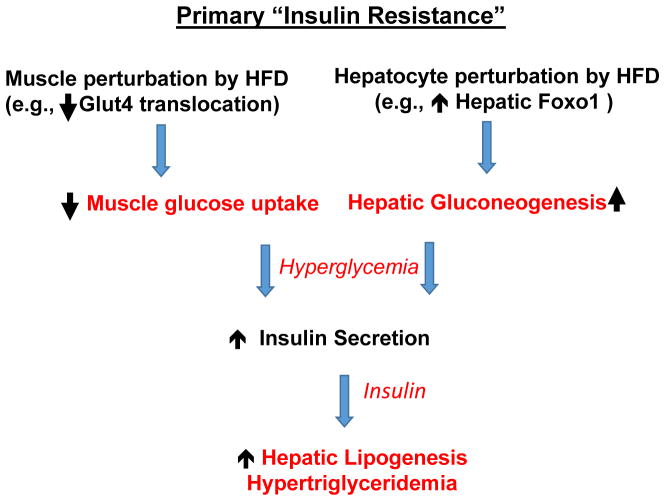
Figure 1. Plausible pathways whereby insulin resistance is the initiating response to high fat diet feeding and obesity to cause hyperglycemia and hyperlipidemia.
High fat diets and overfeeding, either directly or indirectly through gut perturbations, disrupt downstream hepatocyte regulators of gluconeogenesis (e.g., increasing nuclear actions of the transcription factor Foxo1), causing increased hepatic glucose output, and disrupt Glut4 glucose transporter response to insulin. Inhibition of adipose insulin responsiveness to insulin also occurs (not shown). These disruptions cause hyperglycemia, which stimulates islet beta cells to secrete insulin, leading to hyperinsulinemia, which in turn activates hepatic lipogenesis and increased secretion of VLDL (hyperlipidemia).
Proposed initiating mechanisms that impair insulin’s ability to lower blood glucose levels include activation of the transcription factor Foxo1 in the liver12 and disruption of Glut4 glucose transporter translocation to the surface membrane in skeletal muscle13,14. Foxo1 is a transcription factor that increases the expression of key enzymes of gluconeogenesis, hence its upregulation results in the increased conversion of incoming substrates to the liver to glucose. A decrease in Glut4 levels at the surface membrane in muscle would reduce glucose uptake from the circulation. In the liver, insulin normally causes phosphorylation and suppression of Foxo1 function through the action of the protein kinase Akt, causing Foxo1 to be retained in the cytoplasm where it is inactive15,16 (Figure 2). However, in obese mice Foxo1 expression is upregulated and the protein apparently modified to become insensitive to insulin regulation17,18. How that disruption of Foxo1 regulation is caused by over-nutrition is still being investigated19,20, but recent studies in mice pinpoint it as a key step in a feed forward loop of unrestrained gluconeogenesis in obesity17,21,22. The hypothesis is that hyperglycemia caused by Foxo1 uncoupling from suppression by insulin and the resulting chronic hyperinsulinemia in obese mice may dampen insulin’s inhibitory action on adipose tissue lipolysis (Figure 2)17. This unrestrained lipolysis in visceral adipocytes in turn increases delivery to liver of its products--free fatty acids, which promote gluconeogenesis through allosteric mechanisms during their metabolism, and the gluconeogenesis substrate glycerol. This unrestrained lipolysis concept was proposed in earlier studies in dogs23,24, and in more recent work showing that in mice lacking the adipocyte lipase ATGL hepatic gluconeogenesis is attenuated and glucose intolerance is attenuated25. Thus, under HFD conditions, the stimulated hepatic glucose output by upregulated Foxo1 is further enhanced by unrestrained adipocyte lipolysis.
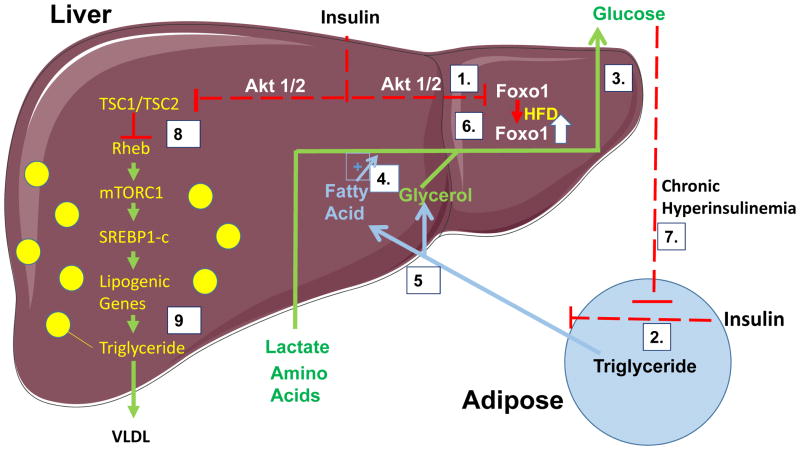
Figure 2. A deleterious cycle between adipose tissue and liver whereby release of free fatty acids from adipocytes promote hepatic gluconeogenesis under high fat feeding/obesity conditions.
Under normal fed conditions, Insulin suppresses gluconeogenesis through Akt dependent pathways (boxed 1) which phosphorylate and retain Foxo1 in the cytoplasm. Insulin also suppresses (boxed 2) adipocyte lipolysis (boxed 5), thereby limiting the flow to liver of glycerol, which is a substrate along with lactate and amino acids for gluconeogenesis (boxed 3). According to this model, HFD/obesity mediates a primary effect to upregulate Foxo1 (boxed 6) and disrupt its suppression by insulin signaling to protein kinase Akt, causing increased glucose output. The resultant hyperglycemia/chronic hyperinsulinemia is hypothesized to disrupt the normal acute insulin suppression of adipocyte lipolysis (boxed 7). Increased fatty acid delivery and metabolism in liver promotes gluconeogenesis through allosteric regulation of fatty acid oxidation products such as Acetyl CoA (boxed 4). Thus, under conditions of HFD/obesity, gluconeogenesis is promoted by both directed upregulation and deregulation of Foxo1 and by products of adipose lipolysis, while under normal diet/lean conditions, the direct action of insulin on Foxo1 (boxed 1) is sufficient to suppress gluconeogenesis. Insulin is also required for lipogenesis through an insulin receptor-dependent, Akt-dependent pathway (boxed 8) that activates mTOR1, promoting SREBP1 and stimulates expression of enzymes in the de novo lipogenesis pathway (boxed 9). This model requires Akt to have significant activity, even under HFD and obesity conditions.
In addition to the impaired insulin responsiveness in adipocytes, lipolysis might also be promoted in obesity by the decreased expression of adipocyte lipid droplet proteins such as perilipin26 and Cide proteins27. These molecules promote triglyceride retention in unilocular droplets in mature adipocytes through inhibition of lipolysis, and humans or mice lacking perilipin28 and Cidec29,30 have lipodystrophy and insulin resistance.
The decreased capacity of adipocytes to store and retain triglyceride in obesity, causing ectopic fat accumulation and “lipotoxicity” in liver and muscle as a cause of insulin resistance has received much support31. Experiments also show that transplants of relatively small amounts of adipose tissue from lean mice can cause weight loss and correct the insulin resistance in obese mice32. Such small transplants wouldn’t seem to have the capacity to store much triglyceride, suggesting there may also be therapeutic value derived from secreted factors33. In any case, the resultant blood glucose increase in response to the primary insulin resistance caused by “lipotoxicity” or by disruption of beneficial factors secreted from adipocytes is postulated to trigger insulin secretion, causing hyperinsulinemia.
The above scenario also explains how hypertriglyceridemia may occur in obesity. Insulin signaling through Akt in the liver (left dashed line in Figure 2) activates fatty acid synthesis from glucose and amino acids, a pathway termed de novo lipogenesis (DNL), that culminates in triglyceride packaging into VLDL lipoprotein for export and uptake into peripheral tissues21,34. Thus, hyperinsulinemia may amplify the usual stimulation of this lipogenic pathway under conditions of nutrition excess sustaining the obese state, and leading to overproduction of lipid. How insulin resistance could be selectively imposed on gluconeogenesis while leaving its actions on lipogenesis intact35 is likely explained by the divergence of insulin signaling downstream of Akt. While Foxo1 inactivation by Akt controls gluconeogenesis, Akt activation of the mTORC1 protein kinase complex and transcription factor SREBP-1c enhances lipid synthesis36. Under HFD feeding conditions, the blunted Akt activation by insulin is unable to suppress the modified, dysregulated hepatic Foxo1 and adipocyte lipolysis, but remains sufficient to activate mTORC1 and the lipogenic pathway. The availability of additional substrate for triglyceride synthesis in liver also accompanies over-nutrition, and amino acids may further activate mTORC137. Thus, lipogenesis and VLDL synthesis and export are brisk in obesity.
The model described above would be exaggerated in type 2 diabetes whereby hyperglycemia develops even during fasting, and beta cell deficiency fails to secrete enough insulin to overcome the insulin insensitivity of Foxo138,39. But whether the deregulation of Foxo1 is mediated by dietary or gut factors, or chronic high circulating insulin is extremely difficult to decisively validate experimentally since insulin resistance and hyperinsulinemia are so tightly linked5. As noted, inducing insulin resistance experimentally does indeed cause hyperinsulinemia, but induced hyperinsulinemia in turn causes insulin resistance7 and perhaps other maladies40.
Viewpoint: hyperinsulinemia causes insulin resistance
In mildly glucose intolerant obese, non-diabetic human subjects, fasting hyperinsulinemia occurs without detectable increases in blood glucose that would theoretically be required to stimulate beta cells to secrete additional insulin. This is also true with the apparently identical increases in blood glucose concentrations that occur in such hyperinsulinemic subjects upon ingestion of glucose. Such apparent “uncoupling” of circulating insulin levels from glucose levels is also observed in obese human subjects after bariatric surgery8.
The above confounding considerations gave rise to the hypothesis (Figure 3) that hyperinsulinemia is the initial, primary effect caused by HFD feeding and obesity6–9, induced by stimulation of beta cell insulin secretion41,42 and suppression of insulin degradation43. According to this viewpoint, primary hyperinsulinemia is what initially causes insulin resistance in target tissues such as liver, at least under conditions of nutrient excess. The mechanisms involved may include downregulation of insulin signaling to Akt, but other, indirect pathways are likely even more important. For example, enhanced skeletal muscle glucose conversion to lactate in response to hyperinsulinemia in obese and mildly diabetic subjects is predicted to provide increased substrate for gluconeogenesis and hepatic glucose output8. Bariatric surgery in such obese human subjects markedly reduces circulating lactate in conjunction with bringing insulin levels to within the normal range through decreased lactate-driven gluconeogenesis8. Additionally, hyperinsulinemia in both rats44 and humans45–51 enhances activation of inflammatory pathways, which in turn can impair insulin responsiveness in target tissues52. Even relatively acute infusions of insulin in human subjects causes elevated circulating cytokines53. Moreover, attenuation of the hyperinsulinemia in genetically obese mice by treatment with streptozotocin or diazoxide reduces adipose tissue inflammation and increases insulin responsiveness54. Similar improvement in glucose tolerance is seen by reducing hyperinsulinemia in a mouse knockout model that impairs beta cell insulin secretion55.
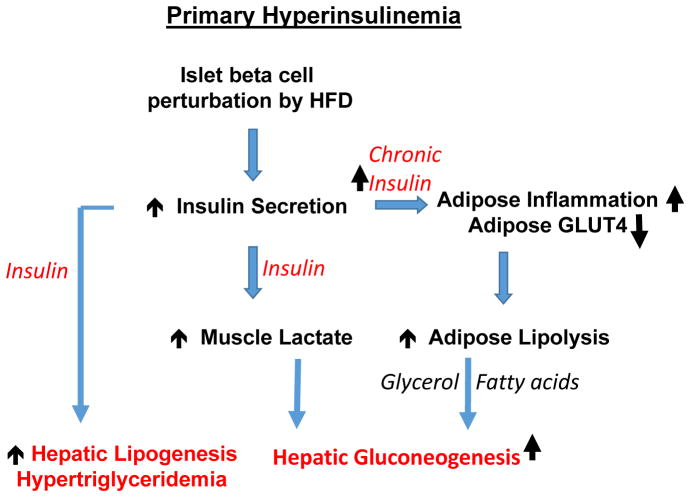
Figure 3. Plausible pathways whereby nutrient-induced hyperinsulinemia is the initiating response to high fat diet feeding and obesity to cause insulin resistance, hyperglycemia and hyperlipidemia.
High fat diets and overfeeding, either directly or indirectly through gut secretions, cause increased insulin release from islet beta cells and primary hyperinsulinemia. Insulin increases muscle glycolysis and lactate formation, which is released to the circulation as a substrate to increase gluconeogenesis in liver. The hyperinsulinemia also activates hepatic lipogenesis and increased secretion of VLDL, causing hyperlipidemia. In the adipose tissue, hyperinsulinemia activates an inflammatory response, which through cytokine action on adipocytes compromises their lipogenic capacity and increases lipolysis. Fatty acid flow (from overnutrition and decreased lipid storage and increased lipolysis in adipocytes) to the liver promotes gluconeogenesis through metabolic allosteric regulation.
HFD feeding can cause primary hyperinsulinemia through direct stimulation of islet beta cells to produce insulin in the absence of insulin resistance or increased blood glucose levels. Potential mediators of increased insulin secretion are the elevated circulating free fatty acids that sometimes occur in obesity. Experimentally raising circulating free fatty acids levels in human subjects under hyperglycemic conditions increases insulin secretion rates, confirmed by assessing concentrations of C-peptide, which is also released into the circulation upon its cleavage from proinsulin in beta cells to produce insulin56. Such direct effects on the pancreas are supported by data in other species57. Preservatives such as monoacylglycerides or other substances in the food supply may also be a cause of heightened insulin secretion41. Intracellular mediators that may potentiate glucose-induced insulin secretion include reactive oxygen species and long chain acyl-CoA, which are increased in beta cells exposed to fatty acids58. Thus, insulin secretion in response to glucose may be directly amplified by agents supplied by over-nutrition.
The effects of blocking hyperinsulinemia
Genetic manipulation of one or both of the mouse insulin genes (Ins1 and Ins2) have produced important insights into the effects of hyperinsulinemia under HFD conditions59–61. The mouse Ins2 gene is most highly expressed in pancreatic beta cells but also is expressed at low levels in other tissues including the brain, similar to the single human INS gene. The expression of the mouse Ins1 gene appears restricted to the beta cells, and it also contributes to secreted insulin. Mice lacking only Ins2 show normal insulin levels on control diets and respond to HFD with beta cell expansion and fasting hyperinsulinemia at all ages, as do wild type mice60. Deletion of a single Ins1 allele in mice lacking Ins2 results in initial hyperinsulinemia at 5 to 8 weeks of HFD, but insulin levels return to normal at 50 weeks. Surprisingly, at this later time, mice lacking Ins2 and with only one copy of Ins1 maintain the same glucose tolerance as the hyperinsulinemic mice lacking Ins2 only, indicating that high insulin levels in these mice do not enhance glucose tolerance. Importantly, the hyperinsulinemic mice lacking only Ins2 gain more weight on a HFD compared to control diet fed mice, as expected, whereas the Ins1 deficient mice do not gain weight on a HFD, despite no difference in food intake between the two types of mice60 (Figure 4). A second mouse model of genetic insulin deficiency in which mice missing both alleles of Ins1 and one allele of Ins2 also displayed less weight gain on HFD59 than Ins 1 deficient mice with both alleles of Ins2 intact. Thus, hyperinsulinemia in response to a HFD regimen is required for the increased weight gain that is the result of adipose tissue expansion in these mice. Taken together, these results are reminiscent of the remarkable weight gains of the first human subjects with type 1 diabetes to receive insulin, and the oft observed type 2 diabetics who gain weight on insulin therapy.
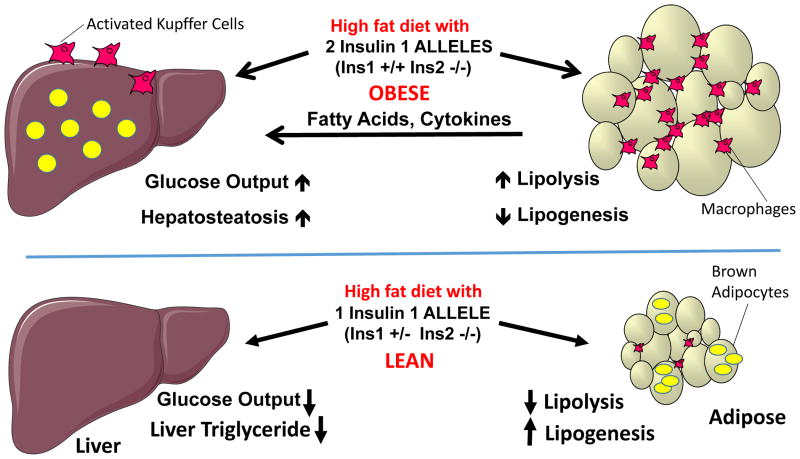
Figure 4. Genetically-induced Insulin deficiency enhances energy expenditure and adipose browning, while preventing adipose expansion, glucose intolerance and hepatosteatosis in mice on a high fat diet.
The Figure compares the effects of HFD on genetic mouse models that display either a normal hyperinsulinemic response to the diet (Top panel) versus a hypoinsulinemic response due to partial genetic deletion of insulin alleles(Bottom panel). Top panel: A mouse model that responds to HFD with hyperinsulinemia displays similar effects as the response of wild type mice to HFD60: adipose expansion and inflammation, glucose intolerance, hepatic steatosis and hyperlipidemia. Expanded, inflamed adipose is thought to secondarily promote hepatic steatosis and gluconeogenesis. Bottom panel: A mouse model with one less insulin allele than that described in the top panel, which does not display hyperinsulinemia in response to HFD, shows little or no adipose expansion, glucose intolerance or hepatic steatosis under HFD conditions. In addition, adipose browning occurs with upregulation of uncoupling protein 1 and energy expenditure is increased under HFD feeding in this mouse model.
Increased energy expenditure would explain the reduced fat deposition in insulin deficient mice, assuming no increased caloric loss through excretion, and indeed, oxygen consumption is increased in Ins2 deficient mice with one Ins1 allele intact compared to Ins2 deficient mice with both Ins1 alleles60. This increased energy expenditure was associated with the appearance of multilocular adipocytes and increased uncoupling protein UCP1 expression in white adipose tissue compared to the hyperinsulinemic mice (Figure 4). These are features of brown or “beige” adipocytes, which display high rates of fatty acid oxidation and heat production, and promote enhanced glucose tolerance62. Consistent with increased fatty acid oxidation, these mice failed to develop fatty liver on a HFD. How hyperinsulinemia suppresses adipocyte UCP1 in this model is not known, but its ability to attenuate the cAMP pathway that activates lipolysis and causes adipose browning through mTORC163 may play a role. This explanation suggests that mTORC1 stimulation by the cAMP pathway has different downstream outputs compared to stimulation by insulin.
The above experiments also revealed that low insulin levels equivalent to those observed on a normal diet lowered expression of the macrophage marker Emr1 and the cytokine TNF-α in white adipose tissue, indicating hyperinsulinemia promotes adipose inflammation60, consistent with the model in Figure 3. Furthermore, the finding that hyperinsulinemia is required for obesity to occur in HFD mice complements demonstrations that hyperinsulinemia induced by genetic manipulation64 or insulin infusion44 causes systemic insulin resistance. Nonetheless, these results do not establish whether hyperinsulinemia initiates the dysfunction in HFD mice. It remains possible that a signal emanating from insulin resistant tissues, glucose or other factor, is required to cause the hyperinsulinemia which then promotes obesity and amplifies the insulin resistance. In addition, in syndromes of known primary hyperinsulinemia, in which hyperinsulinemia is known to occur before other symptoms, such as insulinoma, insulin resistance is evident, but marked hypoglycemia is also observed65. Similarly, mutations in TBC1D4, which causes insulin resistance in skeletal muscle and extreme hyperinsulinemia during feeding, is not associated with hyperlipidemia66 in Inuit populations of Greenland. Thus, hyperinsulinemia alone may not be able to induce sufficient insulin resistance to cause glucose intolerance nor fatty liver, but may require severe over-nutrition and the obese state to maximally promote these consequences.
A timeline of metabolic changes upon overfeeding
A major technical problem in assessing the roles of hyperinsulinemia and insulin resistance in established obesity is that measurements of blood glucose and insulin concentrations may not be sufficiently precise to dissect cause and effect, in a manner analogous to the difficulty in measuring temperature changes within the limits set by a thermostat. Additionally, it should be noted that the two hypotheses illustrated in Figures 1 and 3 are not mutually exclusive and probably act in parallel since hyperinsulinemia initially induced by insulin resistance, as shown in Figure 1, further exaggerates the insulin resistance through mechanisms depicted in Figure 3. Other important complications are the heterogeneity of insulin resistance in various mouse strains studied and whether liver or skeletal muscle or both are affected by insulin resistance67.
One approach to the question of whether hyperinsulinemia or insulin resistance is the initiating factor in the development of diabetes is to dissect the sequence of events that occur at very early time points after the start of a HFD or overfeeding. Results from many such studies in mice and rats68–76 and humans77–83 are summarized in Table 1. In one study68, HFD feeding to mice caused increased adipose mass and fasting hyperinsulinemia after only 1 day without a change in fasting blood glucose levels. In 5 studies out of 6, rodents fed a HFD for 3–4 days exhibited no change in fasting blood glucose, while fasting insulin levels were already elevated in 4 of these studies69,70,74,75. At this 3–4 day point of HFD feeding in rats and mice, most studies also revealed an increase in body weight or adipose tissue mass and glucose intolerance or hepatic or systemic insulin resistance. At 7 days of HFD feeding, most studies also failed to detect a change in fasting blood glucose and all studies showed a statistically significant or strong trend toward fasting hyperinsulinemia. Of seven reports on human subjects presented in Table 177–83, all but one83 demonstrated fasting hyperinsulinemia at the earliest stages of overfeeding or a HFD in subjects while most did not detect increases in fasting blood glucose concentrations (Table 1). Although a few reports in the Table indicated either no change or an increase in both parameters at early times after overfeeding, none found fasting hyperglycemia occurring first.
Table 1. Progression of metabolic parameters upon initiating HFD or overfeeding in mice (left columns) and human subjects (right columns) shows initial fasting hyperinsulinemia without fasting hyperglycemia in multiple studies (data obtained from references68–83).
NC, no change; 2x, approximately 2 fold increase; Asterisks denote strongly increased values that did not reach statistical significance. References to the studies are provided in parentheses.
| Days of High Fat Diet or Overfeeding | ||||||
|---|---|---|---|---|---|---|
| Mouse Studies | Human Studies | |||||
| Metabolic Parameter | 1day | 3–4days | 7days | 2days | 3–4days | 7days |
| Fasting Glucose | NC (68) | NC (69) | NC (69) | NC (77) | Increase (78) | NC (77) |
| NC (70) | NC (71) | NC (83) | NC (79) | |||
| NC (72) | Increase (80) | |||||
| NC (73) | NC (81) | |||||
| Increase (74) | Increase (74) | NC (82) | ||||
| NC (75) | NC (76) | NC (83) | ||||
| Fasting Plasma Insulin | Increase 2x (68) | Increase 2x# (69) | Increase 3x (69) | Increase 2x # (77) | Increase # (78) | Increase 2x # (77) |
| Increase 2x (70) | Increase 2x (71) | NC (83) | Increase (82) | |||
| NC (72) | Increase 2x (79) | |||||
| NC (73) | Increase (80) | |||||
| Increase (74) | Increase (74) | Increase (81) | ||||
| Increase 2x # (75) | Increase # (76) | |||||
| Body Weight | Increase (68) | NC (69) | Increase (71) | Increase (77) | NC (78) | Increase (79) |
| Increase (70) | Increase (80) | |||||
| Increase (72) | Increase (81) | |||||
| Increase (73) | Increase (82) | |||||
| Increase (74) | Increase (74) | NC (83) | ||||
| NC (75) | ||||||
| WATg mass | Increase (68) | Increase (69) | Increase (71) | |||
| Increase (74) | Increase (74) | |||||
| Fasting NEFA | NC (69) | NC (69) | NC (77) | Decrease (78) | NC (77) | |
| Increase (70) | NC (71) | Decrease (79) | ||||
| NC (76) | ||||||
| GTT | Intolerance (69) | Intolerance (69) | Intolerance (83) | Intolerance (79) | ||
| Intolerance (73) | ||||||
| ITT | Decrease (73) | Decrease (71) | ||||
| HOMAR-IR | Increase (71) | Increase # (77) | Increase (82) | Increase (77) | ||
| Increase (79) | ||||||
| Increase (81) | ||||||
| Hepatic Glucose Output | Increase (69) | Increase (69) | Increase (78) | |||
| Increase (74) | Increase (74) | |||||
| Increase (75) | Less Suppression (76) |
Together, the experimental findings summarized in Table 1 indicate that the first measurable change that occurs in HFD feeding regimens in both murine and human subjects is usually an elevated fasting level of circulating insulin, not glucose, consistent with hyperinsulinemia being a key initiating cause of insulin resistance. It is also generally recognized that some human subjects with long established obesity display fasting hyperinsulinemia without detectable elevations in blood glucose concentrations that would theoretically be required to stimulate insulin secretion38,39. A caveat to these conclusions is the difficulty in measuring the very small changes in blood glucose concentrations that may be sufficient to be sensed by beta cells. It is also possible that postprandial increases in blood glucose concentrations may influence insulin secretion even during subsequent fasting periods or that portal vein glucose concentrations are higher than peripheral levels. Nonetheless, signals within the diet, or emanating from the gut84,85, the brain85 or peripheral tissues86, that may stimulate or potentiate beta cells to chronically secrete insulin in the early stages of HFD feeding will be important to identify and characterize in future studies.
Interestingly, impaired insulin responsiveness of hepatocyte glucose output occurs prior to defective insulin-stimulated muscle glucose uptake during the initial course of HFD feeding in mice and rats69,87. Perhaps this is because portal vein insulin levels are much higher than circulating levels, thereby affecting liver more than muscle. This is an important difference compared to insulin resistant human pre-diabetic subjects who present with skeletal muscle insulin resistance as the earliest abnormality88. In any case, chronic hyperinsulinemia may be a factor in the HFD-mediated disruption of Foxo1 depicted in Figure 2 or the deregulated TBC1D4 (and the related TBC1D1) required for full Glut4 translocation in skeletal muscle (Figure 1), which could be tested in future experiments.
Cellular and molecular causes of impaired insulin responsiveness
Whether hyperinsulinemia or dietary factors cause insulin resistance in high fat feeding can also be addressed by defining the molecular mechanisms that cause defective intracellular signaling and metabolic pathways.
Akt-independent mechanisms of insulin resistance
Much elegant work has decisively demonstrated that human monogenic mutations in insulin receptor, PI3-kinase and Akt cause severe insulin resistance11. Most studies on common forms of obesity have therefore also concentrated on deficiencies in insulin receptor signaling to Akt, which is required for the major metabolic effects of insulin (see Box 1). Much of this work10,52,89,90 has attributed the cause of insulin resistance to inhibitory serine/threonine phosphorylations of the insulin receptor tyrosine kinase91 or its obligatory substrate IRS proteins90 mediated by diacylglycerol89, or dephosphorylation of Akt by phosphatase activity in response to ceramides92. These concepts continue to be explored and debated, and conflicting data is common among different laboratory groups93. However, careful examination of the available data indicate that upstream and downstream pathways of insulin responsiveness, including modulation of metabolic flux17,22, transcriptional regulation94,95 and other pathways96–98 may be even more important than the phosphorylations mentioned above in the majority of obese type 2 diabetics.
For example, provocative findings in mice show that skeletal muscle resistance to insulin in obesity is likely due to a defect downstream of the insulin receptor and IRS proteins99. In these studies, mice with ectopic expression of PDGF receptors in their skeletal muscle were able to respond to the growth factor PDGF in a manner analogous to responding to insulin, such that PDGF signaling causes increased glucose transport in the muscle. HFD feeding of these PDGF receptor-expressing transgenic mice caused resistance to both PDGF and insulin action on glucose transport, even though PDGF receptor signaling doesn’t involve IRS proteins. Even more striking, at 17 days of HFD feeding the impaired glucose transport responsiveness was not accompanied by decreased phosphorylation of Akt or its substrate TBC1D499, which is linked to GLUT4 glucose transporter regulation100. Similarly, marked glucose intolerance at 7 days of HFD feeding of wild type mice can be observed without changes in insulin stimulated Akt phosphorylation in liver, adipose tissue and skeletal muscle in spite of marked impaired insulin responsiveness in the former two tissues69. Only at longer times of HFD feeding do decreases in phospho-Akt become detectable, even though the insulin resistance has not further increased. These data reinforce the point that resistance to the actions of insulin on metabolism can be strongly promoted by pathways downstream of insulin signaling to Akt.
Upstream of Akt
Even when Akt activity is compromised in obesity models, the primary sites of signaling disruption may be far removed from insulin receptor signaling to this protein kinase. Factors upstream of the insulin receptor that may impair insulin action on adipocytes101, skeletal muscle102–104 and liver105 in obesity include extracellular matrix signaling as well as reduced capillary recruitment and blood flow that could limit access of insulin and glucose to the myotubes and perhaps other tissues. Enhanced expression of collagens and other extracellular matrix proteins and their integrin receptors that are in direct contact with skeletal muscle capillaries promote insulin resistance in mice106. The pseudokinase Integrin Linked Kinase (ILK), which binds within a complex to the intracellular domain of β integrins, is required for optimal HFD-induced glucose intolerance and insulin resistance of skeletal muscle glucose disposal107. Mice without ILK in skeletal muscle have increased capillarization and presumed blood flow to the muscle due to the lack of negative regulation from stress kinases such as JNK, P38 and ERK107. Interestingly, accumulation of extracellular matrix proteins and fibrosis96,108 promote insulin resistance in adipose tissue, where capillary formation and expansion are critical for normal adipose function33,109. A fragment of collagen VI has also been reported to confer metabolic dysfunction in adipose tissue101. Since IGF-1 is a potent stimulator of collagen expression, perhaps high insulin levels stimulate the IGF-1 receptor or cause IGF binding protein degradation110 to strongly promote collagen synthesis in fibroblasts of adipose tissue. Thus, this pathway could represent another mechanism through which hyperinsulinemia causes insulin resistance.
Downstream of Akt
Downstream of insulin signaling to Akt, GLUT4-mediated glucose transport is relatively rate limiting for glucose utilization under normal glucose and insulin concentrations in skeletal muscle111–113, and insulin-stimulated GLUT4 glucose transporter translocation to the plasma membrane is impaired in obesity13. However, conflicting data has been reported on whether the amount of free intracellular glucose increases14,114 or not115–118 in skeletal muscle of insulin resistant human subjects. Increased intracellular glucose would reflect decreased activity of glucose metabolism, as is predicted to be the case in response to the decreased glycogen synthesis in muscle observed38. Especially at the high concentrations of circulating glucose and insulin observed in fed obese mice and human subjects, both glucose transport and metabolism may be impaired in skeletal muscle. Increasing glucose and fatty acid utilization by increasing mitochondrial respiration via uncoupling of electron transport from ATP production in obese mice has the beneficial effect of ameliorating fatty liver and insulin resistance119, although a causative role for mitochondria dysfunction in insulin resistance is still debated120,121. The extent to which chronic hyperinsulinemia may play a role in inducing these skeletal muscle abnormalities in glucose metabolism is unknown.
In some studies in mice adipocytes during short term HFD feeding, downstream pathways of glucose metabolism122, Glut4 protein expression123 and insulin signaling to Akt124,125 are already impaired as they are in long term obesity. However, a much less than maximal activation of Akt by insulin is needed to obtain a maximal stimulation of adipocyte glucose transport126,127. Thus even marked inhibition of Akt would not diminish the effect of a high insulin concentration to maximally stimulation glucose metabolism, but in fact adipocytes from obese rats are resistant to even very high insulin concentrations122. Perhaps some of the insulin resistance is a reflection of some specificity in the disruption in Akt-mediated phosphorylation, for example at the level of TBC1D4 or downstream in the insulin signaling pathway127. Remarkably, siRNA-based depletion of levels of Akt protein by 80% does not affect TBC1D4 phosphorylation even through glucose transport is markedly reduced, indicating TBC1D4 may not be the major driver of GLUT4 translocation127. Furthermore, when adipocytes from obese rats are stimulated with insulin at very low glucose concentrations in which intracellular enzymes are not saturated, insulin stimulation is robust, although these adipocytes are considered to be insulin resistant122. Also, glucose uptake into adipose tissue is markedly reduced without a decrease in insulin-stimulated Akt phosphorylation at early times after HFD feeding69. These results suggest that modest inhibitions of insulin signaling to Akt even in long term obesity is not the major cause of insulin resistance in adipocytes that result in decreased glucose utilization. Rather, decreased activity in the pathways of glucose uptake and metabolism are the primary cause of decreased utilization.
On the other hand, overexpression of adipocyte GLUT4 rescues the systemic insulin resistance of mice on a HFD, indicating that increasing the numbers of glucose transporters can still enhance glucose uptake under insulin resistant conditions123. Activating insulin signaling to Akt in adipocytes in mice by deleting the negative regulator PTEN exclusively in adipocytes also enhances glucose tolerance and greatly lowers circulating insulin levels in such lean and obese mice128. Thus, even though disruptions occur downstream of Akt in obesity, experimentally enhancing insulin signaling and glucose uptake in adipocytes can overcome these downstream defects, providing multiple opportunities for therapeutic approaches. Interestingly, chronic insulin stimulation of cultured adipocytes in vitro also decreases GLUT4 expression129, indicating hyperinsulinemia may indeed drive this major adipocyte dysfunction to cause insulin resistance.
The pathway of adipocyte glucose metabolism downstream of Akt that is very rapidly and most dramatically depressed by obesity is de novo fatty acid synthesis (DNL), reflecting greatly decreased expression of the enzymes acetyl CoA carboxylase, fatty acid synthase130–134 (Figure 5) and ATP citrate lyase133 (not shown in Figure 5). These effects derive from decreased activity of the lipogenic transcription factors ChREBPα and ChREBPβ potentially caused by the depressed levels of GLUT4, as they are responsive to intermediates of glucose metabolism132,135. Fatty acid synthase deletion in adipose tissue can prevent insulin resistance in mice, possibly through generation of bio-active lipids136, although other work suggest beneficial lipids are actually derived from DNL137,138. DNL may regulate adipocyte biology through the multiple signaling pathways that it controls (Figure 5, within rectangle at right), including the potential to regulate neuronal innervation and sympathetic nerve activity within adipose tissue139. Acetyl CoA is a substrate for protein acetylation reactions, most notably acetylation of histones that modulate their DNA binding activities to regulate transcription140–144. Many of the adipocyte genes that are downregulated in obesity are specifically upregulated during adipocyte differentiation and controlled by the major regulator of adipogenesis, PPARg94,145,146, which is also regulated by acetylation. These include genes encoding components of insulin signaling pathways, lipid droplet and lipolytic regulators, and mitochondrial proteins94,147–149. Thus, adipocytes become less capable during onset of obesity in their crucial functions such as lipid storage that indirectly maintain normal hepatocyte and skeletal muscle glucose handling. Recent results exploring effects of HFD in mice on the global DNA site binding and transcriptional activity of PPARγ also show how environmental cues can modulate the epigenome and alter adipocyte function150.
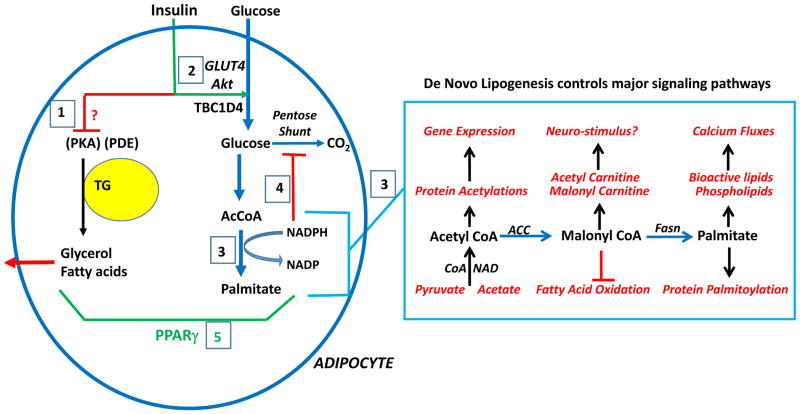
Figure 5. Adipocyte pathways downstream of insulin signaling to Akt disrupted by HFD/obesity that may contribute to systemic insulin resistance in mice and humans.
Contained within the circle at left are pathways of glucose and lipid metabolism that become impaired in their response to insulin in obesity. Insulin inhibits hydrolysis of triglyceride (TG) to glycerol and fatty acids (boxed 1) by a mechanism independent of phosphodiesterase (PDE) phosphorylation by Akt that remains a major unknown and key challenge in the field. Also unknown is how disruption of this anti-lipolytic effect of insulin in obesity is impaired. Insulin enhances glucose uptake into adipocytes through Akt-dependent translocation of Glut4 glucose transporters to the plasma membrane (boxed 2). Adipocyte Glut4 expression is decreased in obesity, and this is mimicked by chronic insulin stimulation in vitro, suggesting that hyperinsulinemia may contribute to this effect in vivo. Adipocyte de novo lipogenesis (DNL) (boxed 3) is markedly inhibited in HFD/obesity by mechanisms that are not defined. According to this model, DNL inhibition through feedback inhibition by NADPH decreases glucose flux through the pentose shunt (boxed 4), contributing to increased free glucose and decreased glucose uptake. PPARγ (boxed 5) is a master regulator of adipocyte genes, thus attenuation of its activity in obesity has the potential to cause adipocyte dysfunction and insulin resistance. Large rectangle at right illustrates the many ways that intermediates of DNL may act as signaling molecules to regulate gene expression and many other cellular functions, for example through histone and other protein acetylations, allosteric modulation, protein palmitoylation and generation of bio-active lipids.
Akt independent regulation of adipocyte lipolysis
Finally, insulin’s control of adipocyte lipolysis is a critical mode by which adipocytes influence hepatic gluconeogenesis and overall systemic glucose tolerance in HFD/obesity (Figure 2)17,22,25,151. Much is known about adipocyte lipid droplets and the components that mediate activation of the lipases that cause hydrolysis of triglyceride in response to activation of the cAMP pathway26,152–154. Circumstantial data initially suggested that phosphorylation and activation of cAMP phosphodiesterase by Akt could explain insulin’s inhibition of lipolysis155–157. However, recent results unexpectedly undermine this concept, demonstrating that inhibiting Akt phosphorylation of phosphodiesterase does not eliminate this insulin action158–160. The mechanism of insulin action on adipocyte lipolysis thus remains a premier unsolved question in the field, and is further complicated by an indirect action of insulin on lipolysis mediated through the brain161. How these anti-lipolytic actions of insulin may be blunted by hyperglycemia, hyperinsulinemia or other factors under certain HFD conditions also remains a mystery17. Taken together, the disruptions in obesity that occur in many of the pathways of adipocyte metabolism downstream of insulin-activated Akt (Figure 5) mirror the situation in liver. The influences of these downstream pathways in adipocytes and liver on systemic glucose and lipid metabolism, and the extent to which chronic stimulation by insulin itself modulates these pathways offer fertile territory for future research in this field.
Conclusions and perspectives for future studies
Deterioration of systemic insulin responses related to glucose handling, referred to as insulin resistance, is a serious syndrome associated with obesity and sedentary behavior. It promotes glucose intolerance and type 2 diabetes with associated comorbidities, as well as increasing the risk of cancer162. Yet the etiology of insulin resistance is complicated and multifaceted, involving both cell autonomous mechanisms and inter-organ communications (Figure 2). Careful investigation has revealed that many disruptions causing systemic insulin resistance actually occur downstream or independent of insulin signaling to the protein kinase Akt69,122,126,127,130,139, even though the Akt pathway is often also effected. We are still unable to precisely define the mechanisms that cause most of these basic disruptions, partly because there is considerable disagreement among the many laboratories in the field. For example, what goes awry with the hepatic gluconeogenesis regulator Foxo1 downstream of Akt in obesity? How is adipocyte Glut4 expression decreased and how is adipocyte and skeletal muscle Glut4 translocation to the plasma membrane attenuated in obesity? What mediates the blockade of adipocyte fatty acid synthesis under HFD/obesity conditions, and does this metabolic pathway in adipocytes control systemic glucose tolerance? How does insulin suppress adipocyte lipolysis and what disconnects insulin signaling from adipocyte lipolysis under HFD feeding conditions? It is striking that such fundamental questions remain elusive.
Have we learned enough in the last few years to suggest novel therapeutic strategies to approach type 2 diabetes? The striking beneficial effects of implanting relatively small amounts of mouse subcutaneous32 or human “Beige” adipocytes33 into insulin resistant mouse models offer the possibility that as yet undiscovered factors in adipocytes are potent enhancers of systemic glucose tolerance. Such adipocyte factors may also connect to neuronal control of metabolism33,139. Enhanced inhibition of adipocyte lipolysis under feeding conditions or potentiating insulin’s anti-lipolytic action in obesity would also seem useful (Figure 2). But one huge challenge to this idea could be the need to make such a therapeutic selective for adipocytes since inhibition of lipolysis in other tissues such as heart may lead to toxicity163. This issue of tissue selectivity is a major hurdle for exploiting many potential targets that have been uncovered in recent years. For example, enhancing adipose DNL may prove beneficial, but not if hepatic lipogenesis is also activated to produce hyperlipidemia and fatty liver. These considerations suggest that a steep challenge for future success in diabetes therapies (and therapeutics in general) is development of tissue specific delivery modalities for therapeutic agents.
The fact that insulin resistance triggers hyperinsulinemia, and that hyperinsulinemia in turn causes insulin resistance, makes the above conundrums even more interesting. Mechanisms whereby insulin secretion is enhanced in obesity need further exploration. Adipocytes may signal directly to beta cells to regulate insulin secretion86, and therefore could drive hyperinsulinemia independent of blood glucose levels. Experimental blockade of hyperinsulinemia in mice prevents obesity while increasing energy expenditure and adipose browning, showing that insulin itself plays both beneficial and deleterious roles in the obese, insulin resistant syndrome and possibly in promoting the onset of type 2 diabetes. These insights raise the possibility that pancreatic islets are the direct or indirect target of HFD feeding and that hyperinsulinemia is the primary driving force in eliciting insulin resistance. More likely, hyperinsulinemia is one of a combination of factors in HFD feeding and obesity that significantly contributes to the malady. Thus, rather than searching for therapeutic modalities that enhance insulin secretion, perhaps discovery of mild suppressors of insulin secretion specifically in response to overfeeding may prove to be of value in certain cases of diabetes. In any case, opportunities abound for further exploration of the molecular mechanisms whereby chronic hyperinsulinemia modulates pathways that may lead to insulin resistance, such as adipose whitening and inflammation.
Acknowledgments
I thank Drs. Morris Birnbaum, Steven O’Rahilly, Silvia Corvera, Joseph Virbasius, Adilson Guilherme and David Pedersen for critical reading of the manuscript and helpful comments. I also thank our laboratory group members for stimulating discussions on these topics and Lisa Smith for contributions to formatting and editing of the manuscript. Apologies to the many colleagues whose citations had to be omitted during editing due to space constraints. The work cited from our laboratory was funded by NIH Grants DK 085753 and DK 030898, and the Isadore and Fannie Foxman endowed professorship in medical science.
References
- 1.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 2.National Diabetes Statistics Report. 2014 www.CDC.gov.
- 3.Kloting N, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Reaven GM. Insulin resistance and hyperinsulinemia: you can’t have one without the other. Diabetes Care. 2008;31:1433–1438. doi: 10.2337/dc08-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 7.Shanik MH, et al. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–268. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 8.Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care. 2012;35:2438–2442. doi: 10.2337/dc12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes. 2012;61:4–13. doi: 10.2337/db11-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker VE, Savage DB, O’Rahilly S, Semple RK. Mechanistic insights into insulin resistance in the genetic era. Diabet Med. 2011;28:1476–1486. doi: 10.1111/j.1464-5491.2011.03463.x. [DOI] [PubMed] [Google Scholar]
- 12.Klotz LO, et al. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryder JW, Gilbert M, Zierath JR. Skeletal muscle and insulin sensitivity: pathophysiological alterations. Front Biosci. 2001;6:D154–163. doi: 10.2741/ryder. [DOI] [PubMed] [Google Scholar]
- 14.Pendergrass M, et al. Muscle glucose transport and phosphorylation in type 2 diabetic, obese nondiabetic, and genetically predisposed individuals. Am J Physiol Endocrinol Metab. 2007;292:E92–100. doi: 10.1152/ajpendo.00617.2005. [DOI] [PubMed] [Google Scholar]
- 15.Nakae J, Barr V, Accili D. Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J. 2000;19:989–996. doi: 10.1093/emboj/19.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 17.Titchenell PM, et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell Metab. 2016;23:1154–1166. doi: 10.1016/j.cmet.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu S, et al. Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006;147:5641–5652. doi: 10.1210/en.2006-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozcan L, et al. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 2012;15:739–751. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banks AS, et al. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metab. 2011;14:587–597. doi: 10.1016/j.cmet.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 22.Perry RJ, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherrington AD, Edgerton D, Sindelar DK. The direct and indirect effects of insulin on hepatic glucose production in vivo. Diabetologia. 1998;41:987–996. doi: 10.1007/s001250051021. [DOI] [PubMed] [Google Scholar]
- 24.Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest. 1996;98:741–749. doi: 10.1172/JCI118846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoiswohl G, et al. Impact of Reduced ATGL-Mediated Adipocyte Lipolysis on Obesity-Associated Insulin Resistance and Inflammation in Male Mice. Endocrinology. 2015;156:3610–3624. doi: 10.1210/en.2015-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmel AR, Sztalryd C. The Perilipins: Major Cytosolic Lipid Droplet-Associated Proteins and Their Roles in Cellular Lipid Storage, Mobilization, and Systemic Homeostasis. Annu Rev Nutr. 2016;36:471–509. doi: 10.1146/annurev-nutr-071813-105410. [DOI] [PubMed] [Google Scholar]
- 27.Puri V, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci U S A. 2008;105:7833–7838. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandotra S, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364:740–748. doi: 10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubio-Cabezas O, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1:280–287. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, et al. Insulin resistance and white adipose tissue inflammation are uncoupled in energetically challenged Fsp27-deficient mice. Nat Commun. 2015;6:5949. doi: 10.1038/ncomms6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotta LA, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49:17–26. doi: 10.1038/ng.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min SY, et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med. 2016;22:312–318. doi: 10.1038/nm.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biddinger SB, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Caron A, Richard D, Laplante M. The Roles of mTOR Complexes in Lipid Metabolism. Annu Rev Nutr. 2015;35:321–348. doi: 10.1146/annurev-nutr-071714-034355. [DOI] [PubMed] [Google Scholar]
- 37.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24:400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck-Nielsen H. The role of glycogen synthase in the development of hyperglycemia in type 2 diabetes: ‘To store or not to store glucose, that’s the question’. Diabetes Metab Res Rev. 2012;28:635–644. doi: 10.1002/dmrr.2337. [DOI] [PubMed] [Google Scholar]
- 39.Beck-Nielsen H, Henriksen JE, Vaag A, Hother-Nielsen O. Pathophysiology of non-insulin-dependent diabetes mellitus (NIDDM) Diabetes Res Clin Pract. 1995;28(Suppl):S13–25. doi: 10.1016/0168-8227(95)01082-o. [DOI] [PubMed] [Google Scholar]
- 40.Nolan CJ, Ruderman NB, Kahn SE, Pedersen O, Prentki M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. 2015;64:673–686. doi: 10.2337/db14-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corkey BE. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care. 2012;35:2432–2437. doi: 10.2337/dc12-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT. Chronic Exposure to Excess Nutrients Left-shifts the Concentration Dependence of Glucose-stimulated Insulin Secretion in Pancreatic beta-Cells. J Biol Chem. 2015;290:16191–16201. doi: 10.1074/jbc.M114.620351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim MK, Reaven GM, Chen YD, Kim E, Kim SH. Hyperinsulinemia in individuals with obesity: Role of insulin clearance. Obesity (Silver Spring) 2015;23:2430–2434. doi: 10.1002/oby.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi M, Olefsky JM. Effect of experimental hyperinsulinemia on insulin binding and glucose transport in isolated rat adipocytes. Am J Physiol. 1978;235:E53–62. doi: 10.1152/ajpendo.1978.235.1.E53. [DOI] [PubMed] [Google Scholar]
- 45.Soop M, et al. Euglycemic hyperinsulinemia augments the cytokine and endocrine responses to endotoxin in humans. Am J Physiol Endocrinol Metab. 2002;282:E1276–1285. doi: 10.1152/ajpendo.00535.2001. [DOI] [PubMed] [Google Scholar]
- 46.Siklova-Vitkova M, et al. Effect of hyperinsulinemia and very-low-calorie diet on interstitial cytokine levels in subcutaneous adipose tissue of obese women. Am J Physiol Endocrinol Metab. 2009;297:E1154–1161. doi: 10.1152/ajpendo.00086.2009. [DOI] [PubMed] [Google Scholar]
- 47.Murdolo G, et al. Monocyte chemoattractant protein-1 in subcutaneous abdominal adipose tissue: characterization of interstitial concentration and regulation of gene expression by insulin. J Clin Endocrinol Metab. 2007;92:2688–2695. doi: 10.1210/jc.2006-2814. [DOI] [PubMed] [Google Scholar]
- 48.Westerbacka J, et al. Acute in vivo effects of insulin on gene expression in adipose tissue in insulin-resistant and insulin-sensitive subjects. Diabetologia. 2006;49:132–140. doi: 10.1007/s00125-005-0075-5. [DOI] [PubMed] [Google Scholar]
- 49.Westerbacka J, et al. Insulin regulation of MCP-1 in human adipose tissue of obese and lean women. Am J Physiol Endocrinol Metab. 2008;294:E841–845. doi: 10.1152/ajpendo.00653.2006. [DOI] [PubMed] [Google Scholar]
- 50.Krogh-Madsen R, Plomgaard P, Keller P, Keller C, Pedersen BK. Insulin stimulates interleukin-6 and tumor necrosis factor-alpha gene expression in human subcutaneous adipose tissue. Am J Physiol Endocrinol Metab. 2004;286:E234–238. doi: 10.1152/ajpendo.00274.2003. [DOI] [PubMed] [Google Scholar]
- 51.Jansen HJ, et al. Start of insulin therapy in patients with type 2 diabetes mellitus promotes the influx of macrophages into subcutaneous adipose tissue. Diabetologia. 2013;56:2573–2581. doi: 10.1007/s00125-013-3018-6. [DOI] [PubMed] [Google Scholar]
- 52.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12:15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 53.Tsiotra PC, Boutati E, Dimitriadis G, Raptis SA. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. Biomed Res Int. 2013;2013:487081. doi: 10.1155/2013/487081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedersen DJ, et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol Metab. 2015;4:507–518. doi: 10.1016/j.molmet.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roth Flach RJ, et al. Protein Kinase Mitogen-activated Protein Kinase Kinase Kinase Kinase 4 (MAP4K4) Promotes Obesity-induced Hyperinsulinemia. J Biol Chem. 2016;291:16221–16230. doi: 10.1074/jbc.M116.718932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44:1239–1242. doi: 10.2337/diab.44.10.1239. [DOI] [PubMed] [Google Scholar]
- 57.Stein DT, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deeney JT, et al. Acute stimulation with long chain acyl-CoA enhances exocytosis in insulin-secreting cells (HIT T-15 and NMRI beta-cells) J Biol Chem. 2000;275:9363–9368. doi: 10.1074/jbc.275.13.9363. [DOI] [PubMed] [Google Scholar]
- 59.Templeman NM, Clee SM, Johnson JD. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia. 2015;58:2392–2402. doi: 10.1007/s00125-015-3676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehran AE, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16:723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 61.D’Souza AM, Johnson JD, Clee SM, Kieffer TJ. Suppressing hyperinsulinemia prevents obesity but causes rapid onset of diabetes in leptin-deficient Lepob/ob mice. Mol Metab. 2016;5:1103–1112. doi: 10.1016/j.molmet.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Liu D, et al. Activation of mTORC1 is essential for beta-adrenergic stimulation of adipose browning. J Clin Invest. 2016;126:1704–1716. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marban SLRJ. In: Transgenic hyperinsulinemia: a mouse model of insulin resistance and glucose intolerance without obesity. Shafrir E, editor. Boston: Birkhauser; 1996. [Google Scholar]
- 65.Pontiroli AE, Alberetto M, Capra F, Pozza G. The glucose clamp technique for the study of patients with hypoglycemia: insulin resistance as a feature of insulinoma. J Endocrinol Invest. 1990;13:241–245. doi: 10.1007/BF03349549. [DOI] [PubMed] [Google Scholar]
- 66.Manousaki D, et al. Toward Precision Medicine: TBC1D4 Disruption Is Common Among the Inuit and Leads to Underdiagnosis of Type 2 Diabetes. Diabetes Care. 2016 doi: 10.2337/dc16-0769. [DOI] [PubMed] [Google Scholar]
- 67.Chen DL, et al. Phenotypic Characterization of Insulin-Resistant and Insulin-Sensitive Obesity. J Clin Endocrinol Metab. 2015;100:4082–4091. doi: 10.1210/jc.2015-2712. [DOI] [PubMed] [Google Scholar]
- 68.Waise TM, et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun. 2015;464:1157–1162. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- 69.Turner N, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56:1638–1648. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 70.Scherer T, et al. Short term voluntary overfeeding disrupts brain insulin control of adipose tissue lipolysis. J Biol Chem. 2012;287:33061–33069. doi: 10.1074/jbc.M111.307348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paglialunga S, Ludzki A, Root-McCaig J, Holloway GP. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia. 2015;58:1071–1080. doi: 10.1007/s00125-015-3531-x. [DOI] [PubMed] [Google Scholar]
- 72.Barzel B, et al. Short term fat feeding rapidly increases plasma insulin but does not result in dyslipidaemia. Front Physiol. 2014;5:469. doi: 10.3389/fphys.2014.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji Y, et al. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem. 2012;287:24378–24386. doi: 10.1074/jbc.M112.371807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee YS, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ben-Shlomo S, et al. Perinephric and epididymal fat affect hepatic metabolism in rats. Obesity (Silver Spring) 2012;20:151–156. doi: 10.1038/oby.2011.261. [DOI] [PubMed] [Google Scholar]
- 76.Commerford SR, et al. Diets enriched in sucrose or fat increase gluconeogenesis and G-6-Pase but not basal glucose production in rats. Am J Physiol Endocrinol Metab. 2002;283:E545–555. doi: 10.1152/ajpendo.00120.2002. [DOI] [PubMed] [Google Scholar]
- 77.Boden G, et al. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci Transl Med. 2015;7:304re307. doi: 10.1126/scitranslmed.aac4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brons C, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol. 2009;587:2387–2397. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lagerpusch M, Bosy-Westphal A, Kehden B, Peters A, Muller MJ. Effects of brief perturbations in energy balance on indices of glucose homeostasis in healthy lean men. Int J Obes (Lond) 2012;36:1094–1101. doi: 10.1038/ijo.2011.211. [DOI] [PubMed] [Google Scholar]
- 80.Olefsky J, Crapo PA, Ginsberg H, Reaven GM. Metabolic effects of increased caloric intake in man. Metabolism. 1975;24:495–503. doi: 10.1016/0026-0495(75)90074-8. [DOI] [PubMed] [Google Scholar]
- 81.Wadden D, et al. Serum acylated ghrelin concentrations in response to short-term overfeeding in normal weight, overweight, and obese men. PLoS One. 2012;7:e45748. doi: 10.1371/journal.pone.0045748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cahill F, Shea JL, Randell E, Vasdev S, Sun G. Serum peptide YY in response to short-term overfeeding in young men. Am J Clin Nutr. 2011;93:741–747. doi: 10.3945/ajcn.110.003624. [DOI] [PubMed] [Google Scholar]
- 83.Numao S, et al. Effects of a single bout of aerobic exercise on short-term low-carbohydrate/high-fat intake-induced postprandial glucose metabolism during an oral glucose tolerance test. Metabolism. 2013;62:1406–1415. doi: 10.1016/j.metabol.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Drucker DJ. Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes. 2015;64:317–326. doi: 10.2337/db14-1514. [DOI] [PubMed] [Google Scholar]
- 85.Perry RJ, et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lo JC, et al. Adipsin is an adipokine that improves beta cell function in diabetes. Cell. 2014;158:41–53. doi: 10.1016/j.cell.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kraegen EW, et al. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 88.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55:1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 89.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petersen MC, et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J Clin Invest. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaurasia B, Summers SA. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol Metab. 2015;26:538–550. doi: 10.1016/j.tem.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Zhang C, et al. Inhibited insulin signaling in mouse hepatocytes is associated with increased phosphatidic acid but not diacylglycerol. J Biol Chem. 2015;290:3519–3528. doi: 10.1074/jbc.M114.602789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang S, Tsai LT, Rosen ED. Nuclear Mechanisms of Insulin Resistance. Trends Cell Biol. 2016;26:341–351. doi: 10.1016/j.tcb.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15:639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 97.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sabio G, Davis RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci. 2010;35:490–496. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoehn KL, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miinea CP, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun K, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011;301:E252–263. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee WL, Klip A. Endothelial Transcytosis of Insulin: Does It Contribute to Insulin Resistance? Physiology (Bethesda) 2016;31:336–345. doi: 10.1152/physiol.00010.2016. [DOI] [PubMed] [Google Scholar]
- 104.Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26:357–366. doi: 10.1016/j.tem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams AS, et al. Integrin alpha1-null mice exhibit improved fatty liver when fed a high fat diet despite severe hepatic insulin resistance. J Biol Chem. 2015;290:6546–6557. doi: 10.1074/jbc.M114.615716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang L, et al. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes. 2011;60:416–426. doi: 10.2337/db10-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang L, et al. Integrin-Linked Kinase in Muscle Is Necessary for the Development of Insulin Resistance in Diet-Induced Obese Mice. Diabetes. 2016;65:1590–1600. doi: 10.2337/db15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdennour M, et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99:898–907. doi: 10.1210/jc.2013-3253. [DOI] [PubMed] [Google Scholar]
- 109.Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta. 2014;1842:463–472. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gealekman O, et al. Control of adipose tissue expandability in response to high fat diet by the insulin-like growth factor-binding protein-4. J Biol Chem. 2014;289:18327–18338. doi: 10.1074/jbc.M113.545798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furler SM, Jenkins AB, Storlien LH, Kraegen EW. In vivo location of the rate-limiting step of hexose uptake in muscle and brain tissue of rats. Am J Physiol. 1991;261:E337–347. doi: 10.1152/ajpendo.1991.261.3.E337. [DOI] [PubMed] [Google Scholar]
- 112.Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action in skeletal muscle from patients with NIDDM. Mol Cell Biochem. 1998;182:153–160. [PubMed] [Google Scholar]
- 113.Turner N, Cooney GJ, Kraegen EW, Bruce CR. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol. 2014;220:T61–79. doi: 10.1530/JOE-13-0397. [DOI] [PubMed] [Google Scholar]
- 114.Bonadonna RC, et al. Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes. 1996;45:915–925. doi: 10.2337/diab.45.7.915. [DOI] [PubMed] [Google Scholar]
- 115.Cline GW, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 116.Petersen KF, Shulman GI. Cellular mechanism of insulin resistance in skeletal muscle. J R Soc Med. 2002;95(Suppl 42):8–13. [PMC free article] [PubMed] [Google Scholar]
- 117.Kelley DE, et al. The effect of non-insulin-dependent diabetes mellitus and obesity on glucose transport and phosphorylation in skeletal muscle. J Clin Invest. 1996;97:2705–2713. doi: 10.1172/JCI118724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams KV, Price JC, Kelley DE. Interactions of impaired glucose transport and phosphorylation in skeletal muscle insulin resistance: a dose-response assessment using positron emission tomography. Diabetes. 2001;50:2069–2079. doi: 10.2337/diabetes.50.9.2069. [DOI] [PubMed] [Google Scholar]
- 119.Perry RJ, Zhang D, Zhang XM, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science. 2015;347:1253–1256. doi: 10.1126/science.aaa0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4:R1–r15. doi: 10.1530/EC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–395. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Czech MP. Cellular basis of insulin insensitivity in large rat adipocytes. J Clin Invest. 1976;57:1523–1532. doi: 10.1172/JCI108422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kahn BB. Lilly lecture 1995. Glucose transport: pivotal step in insulin action. Diabetes. 1996;45:1644–1654. doi: 10.2337/diab.45.11.1644. [DOI] [PubMed] [Google Scholar]
- 124.Gonzalez E, Flier E, Molle D, Accili D, McGraw TE. Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc Natl Acad Sci U S A. 2011;108:10162–10167. doi: 10.1073/pnas.1019268108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan SX, et al. Amplification and demultiplexing in insulin-regulated Akt protein kinase pathway in adipocytes. J Biol Chem. 2012;287:6128–6138. doi: 10.1074/jbc.M111.318238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tan SX, et al. Selective insulin resistance in adipocytes. J Biol Chem. 2015;290:11337–11348. doi: 10.1074/jbc.M114.623686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morley TS, Xia JY, Scherer PE. Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements. Nat Commun. 2015;6:7906. doi: 10.1038/ncomms8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomson MJ, Williams MG, Frost SC. Development of insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 1997;272:7759–7764. doi: 10.1074/jbc.272.12.7759. [DOI] [PubMed] [Google Scholar]
- 130.Richardson DK, Czech MP. Primary role of decreased fatty acid synthesis in insulin resistance of large rat adipocytes. Am J Physiol. 1978;234:E182–189. doi: 10.1152/ajpendo.1978.234.2.E182. [DOI] [PubMed] [Google Scholar]
- 131.Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia. 2013;56:949–964. doi: 10.1007/s00125-013-2869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Herman MA, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang Y, et al. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat Commun. 2016;7:11365. doi: 10.1038/ncomms11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Solinas G, Boren J, Dulloo AG. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol Metab. 2015;4:367–377. doi: 10.1016/j.molmet.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baraille F, Planchais J, Dentin R, Guilmeau S, Postic C. Integration of ChREBP-Mediated Glucose Sensing into Whole Body Metabolism. Physiology (Bethesda) 2015;30:428–437. doi: 10.1152/physiol.00016.2015. [DOI] [PubMed] [Google Scholar]
- 136.Lodhi IJ, et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yore MM, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159:318–332. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. 2016;280:465–475. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guilherme A, et al. Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Mol Metab. 2017 doi: 10.1016/j.molmet.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dutta A, Abmayr SM, Workman JL. Diverse Activities of Histone Acylations Connect Metabolism to Chromatin Function. Mol Cell. 2016;63:547–552. doi: 10.1016/j.molcel.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- 142.Londono Gentile T, et al. DNMT1 is regulated by ATP-citrate lyase and maintains methylation patterns during adipocyte differentiation. Mol Cell Biol. 2013;33:3864–3878. doi: 10.1128/MCB.01495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McDonnell E, et al. Lipids Reprogram Metabolism to Become a Major Carbon Source for Histone Acetylation. Cell Rep. 2016;17:1463–1472. doi: 10.1016/j.celrep.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25:293–302. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sugii S, Evans RM. Epigenetic codes of PPARgamma in metabolic disease. FEBS Lett. 2011;585:2121–2128. [Google Scholar]
- 147.Wilson-Fritch L, et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Choi JH, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Soccio RE, et al. Genetic Variation Determines PPARgamma Function and Anti-diabetic Drug Response In Vivo. Cell. 2015;162:33–44. doi: 10.1016/j.cell.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Soccio RE, et al. Targeting PPARgamma in the epigenome rescues genetic metabolic defects in mice. J Clin Invest. 2017;127:1451–1462. doi: 10.1172/JCI91211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kienesberger PC, et al. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J Biol Chem. 2009;284:30218–30229. doi: 10.1074/jbc.M109.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zechner R. FAT FLUX: enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO Mol Med. 2015;7:359–362. doi: 10.15252/emmm.201404846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kory N, Farese RV, Jr, Walther TC. Targeting Fat: Mechanisms of Protein Localization to Lipid Droplets. Trends Cell Biol. 2016;26:535–546. doi: 10.1016/j.tcb.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krahmer N, Farese RV, Jr, Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol Med. 2013;5:973–983. doi: 10.1002/emmm.201100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Degerman E, et al. Phosphorylation and activation of hormone-sensitive adipocyte phosphodiesterase type 3B. Methods. 1998;14:43–53. doi: 10.1006/meth.1997.0564. [DOI] [PubMed] [Google Scholar]
- 156.Choi YH, et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116:3240–3251. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kitamura T, et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol Cell Biol. 1999;19:6286–6296. doi: 10.1128/mcb.19.9.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Choi SM, et al. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol. 2010;30:5009–5020. doi: 10.1128/MCB.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koren S, et al. The role of mouse Akt2 in insulin-dependent suppression of adipocyte lipolysis in vivo. Diabetologia. 2015;58:1063–1070. doi: 10.1007/s00125-015-3532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.DiPilato LM, et al. The Role of PDE3B Phosphorylation in the Inhibition of Lipolysis by Insulin. Mol Cell Biol. 2015;35:2752–2760. doi: 10.1128/MCB.00422-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shin AC, et al. Insulin Receptor Signaling in Pomc, but not Agrp, Neurons Controls Adipose Tissue Insulin Action. Diabetes. 2017 doi: 10.2337/db16-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vigneri R, Goldfine ID, Frittitta L. Insulin, insulin receptors, and cancer. J Endocrinol Invest. 2016;39:1365–1376. doi: 10.1007/s40618-016-0508-7. [DOI] [PubMed] [Google Scholar]
- 163.Zierler KA, et al. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J Biol Chem. 2013;288:9892–9904. doi: 10.1074/jbc.M112.420620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 165.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 166.Jiang ZY, et al. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci U S A. 2003;100:7569–7574. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]