Tau-targeting therapies for Alzheimer disease (original) (raw)
. Author manuscript; available in PMC: 2019 Jul 1.
Published in final edited form as: Nat Rev Neurol. 2018 Jul;14(7):399–415. doi: 10.1038/s41582-018-0013-z
Abstract
Alzheimer disease (AD) is the most common form of dementia. Pathologically, AD is characterized by amyloid plaques and neurofibrillary tangles in the brain, with associated loss of synapses and neurons, resulting in cognitive deficits and eventually dementia. Amyloid-β (Aβ) peptide and tau protein are the primary components of the plaques and tangles, respectively. In the decades since Aβ and tau were identified, development of therapies for AD has primarily focused on Aβ, but tau has received more attention in recent years, in part because of the failure of various Aβ-targeting treatments in clinical trials. In this article, we review the current status of tau-targeting therapies for AD. Initially, potential anti-tau therapies were based mainly on inhibition of kinases or tau aggregation, or on stabilization of microtubules, but most of these approaches have been discontinued because of toxicity and/or lack of efficacy. Currently, the majority of tau-targeting therapies in clinical trials are immunotherapies, which have shown promise in numerous preclinical studies. Given that tau pathology correlates better with cognitive impairments than do Aβ lesions, targeting of tau is expected to be more effective than Aβ clearance once the clinical symptoms are evident. With future improvements in diagnostics, these two hallmarks of the disease might be targeted prophylactically.
Introduction
Alzheimer disease (AD) represents a major health crisis, with an estimated 5.4 million people currently affected in the USA alone1. With an ageing population in the USA and elsewhere, the problem will only grow worse. The search for disease-modifying therapies for AD has centred on the two main hallmarks of the disease: the extracellular plaques composed primarily of amyloid-β (Aβ), and the intraneuronal neurofibrillary tangles (NFTs), the main constituent of which is the tau protein (Fig. 1). Unfortunately, attempts to alter the disease course by removing Aβ or reducing its production have been largely unsuccessful. Thus, interest in tau-targeting strategies is increasing.
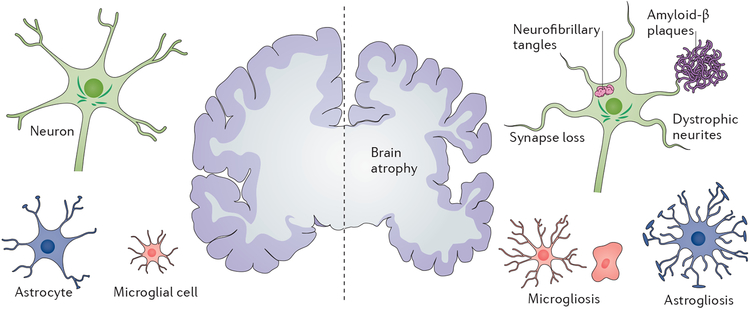
Figure 1 |. The defining pathological hallmarks of Alzheimer disease.
At the gross anatomical level, the disease is characterized by brain atrophy associated with loss of synapses and neurons. At the microscopic level, deposition of extracellular amyloid-β plaques and intraneuronal neurofibrillary tangles is observed, in association with dystrophic neurites and loss of synapses, as well as microgliosis and astrogliosis.
Under normal conditions, tau provides microtubule stability and contributes to the regulation of intracellular trafficking2,3. However, in AD and a range of other conditions, including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Pick disease, frontotemporal dementia (FTD), traumatic brain injury (TBI), stroke and ischaemia, the normal function of tau is disrupted, which ultimately leads to the development of NFT pathology. Although tau lesions are present in each of these conditions, many aspects of tau pathology, including the initial location, progression, associated symptoms, cell types affected, and even the ultrastructure of the tau filaments, are disease-dependent.
The development of tau pathology is a complex multifactorial process, presenting multiple points where therapeutic intervention is possible. In this Review, we will discuss some of the mechanisms that promote tau pathology and are being targeted in clinical trials, including post-translational modifications, cytoskeletal disruption and impairments in protein degradation mechanisms. We focus on AD, given that it is the most common tauopathy and has been the main focus of research and clinical development to date. Current and discontinued efforts to address tau pathology are discussed, and an overview of the results of clinical trials is provided.
Pathological processes in tauopathies
Pathological tau can be seen in the brain decades before the onset of symptoms, with the development of phosphorylated pre-tangles and neuropil threads4–6. NFTs, which can be visualized with the Gallyas silver stain, appear at a later stage. Even before the formation of tangles, tau undergoes a series of post-translational modifications, including hyperphosphorylation7, acetylation8 _N_-glycosylation9 and truncation10, which differentiate it from the normal tau that is seen in healthy brains (see Fig. 2 for a depiction of tau pathology in AD). In AD, deposition of tau aggregates follows a highly stereotyped pattern, beginning in the entorhinal cortex and hippocampus before spreading to other regions11,12. However, hippocampal-sparing and limbic-predominant subtypes are thought to constitute ~25% of AD cases13. Another potential pre-AD tau pathology subtype, termed primary age-related tauopathy (PART), has also been characterized14. People with PART have limited if any Aβ deposition, and many do not show cognitive impairments.
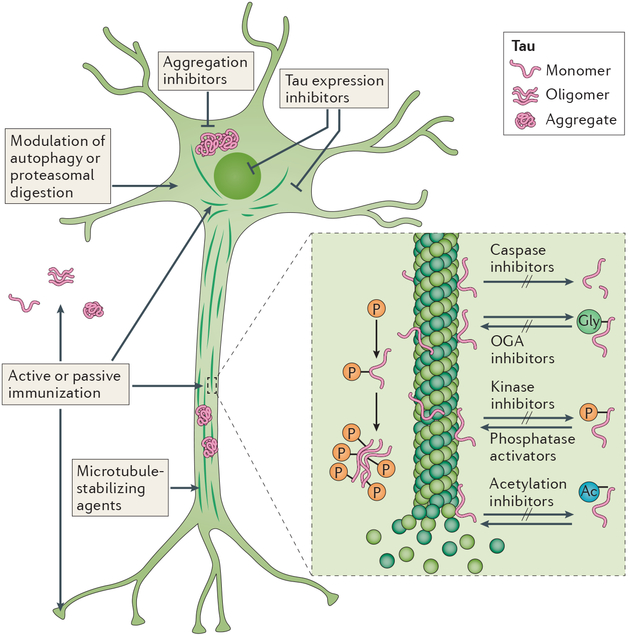
Figure 2 |. Tau-related therapeutic targets.
Drugs in preclinical or clinical development include active and passive immunotherapies; inhibitors of _O_-deglycosylation, aggregation, kinases, acetylation, caspases or tau expression; phosphatase activators; microtubule stabilizers; and modulators of autophagy or proteosomal degradation. Ac, acetyl group, Gly, glycosyl group; OGA, O-GlcNAcase; P, phosphate. Figure adapted with permission from ref. 266, Springer Nature.
The polymers that constitute tau lesions are a heterogeneous mixture, the composition of which depends on the disease and stage. In AD, tau exists as monomers, small oligomeric species, paired helical filaments (PHFs) and straight filaments15–17. NFTs in familial tauopathy can contain fibrils with a twisted ribbon morphology that is distinct from the typical PHFs, whereas straight filaments predominate in Pick disease18. The isoform composition of tau also differs between the various tauopathies. Neurons in the adult CNS express six tau isoforms, which differ in the inclusion or exclusion of two amino-terminal exons, and contain three or four amino acid repeat sequences in the microtubule-binding domain19,20. In AD and PART, the tau aggregates are a mixture of three-repeat and four-repeat tau isoforms, whereas four-repeat sequences dominate in PSP and CBD and three-repeat sequences predominate in Pick disease14,18,19,21.
Targetable pathological events
Post-translational modifications
Post-translational modifications of tau — with the exception of _O_-GlcNAcylation (see below) —interfere with tau–microtubule binding and promote tau misfolding. Thus, targeting of any of these modifications, alone or in combination, has the potential to prevent tau aggregation and restore normal function to the protein (Fig. 2). As these processes begin long before symptoms appear, development of further pathology might be prevented if treatment can be administered in the prodromal phase.
Hyperphosphorylation.
Concurrently with the identification of tau as the primary component of AD-associated NFTs15, the aggregated tau was discovered to be hyperphosphorylated7. Previous reports had shown that phosphorylation of the tau protein affects its ability to bind tubulin and promote microtubule assembly22. Since the initial reports, multiple phosphorylation sites and kinases have been identified. In AD the pattern of phosphorylation changes as the disease progresses. Early phosphorylation events disrupt the association of tau with microtubules and promote relocation of tau to the somatodendritic compartment. Phosphorylation at sites such as Ser199, Ser202/205, Thr231 and Ser262 seems to be associated with pre-tangles in the neuronal processes23,24. Subsequently, levels of somatic tau increase and additional epitopes such as phospho-Ser422 (pSer422) become evident. At both of these stages, phosphorylation can precede cleavage at Asp421, which renders the tau protein prone to aggregation, although phosphorylation at Ser422 inhibits this cleavage step25. Phosphorylation at other sites, such as Ser396, is more prominent later in the disease24,26. Adding to the complexity of this process, phosphorylation at some sites might prime other sites, leading to the formation of large multisite epitopes or promoting conformational changes21,27. The pattern of phosphorylation differs between tauopathies21, and in the case of familial tauopathies, the causative mutations might induce conformational changes that make tau a more favourable substrate for certain kinases.
Hyperphosphorylation of tau is one of the earliest events in the development of AD, and the degree of phosphorylation reflects abnormal activity of both protein kinases and phosphatases. Changes in the levels of active kinases in the brains of individuals with AD can be the result of upregulation of the kinase itself or disruption of its regulation. For example, evidence from post-mortem tissue from the brains of patients with AD indicates that levels of active cyclin-dependent-like kinase 5 (CDK5) and cyclin-dependent kinase 5 activator 1, p25 — a truncated form of a CDK5 regulator — are increased in disease28. Similarly, active glycogen synthase kinase-3β (GSK3β)29 and its regulator c-JUN N-terminal kinase (JNK)30 are associated with neurofibrillary pathology and are upregulated in AD. Because each of these kinases is responsible for phosphorylating different sites, the emergence of specific phospho-epitopes might indicate the stages at which increased activity is most pronounced.
In addition to their role in tau phosphorylation, aberrantly activated kinases can promote neurodegeneration through other mechanisms. CDK5 activity is implicated in the deposition of Aβ, indirect reduction of nerve growth factor (NGF), exacerbation of oxidative stress, promotion of aberrant cell cycle re-entry, and activation of JNK (reviewed elsewhere31,32). Overactive GSK3β induces inflammation via nuclear factor κB (NFκB), promoting apoptosis and impairment of axonal transport. These findings demonstrate that enhanced kinase activity can contribute to pathology in multiple ways, making these enzymes an attractive target for intervention.
Although many tau kinases have been identified, protein phosphatase 2A (PP2A) is one of only a few tau phosphatases, and is responsible for over 70% of the total tau phosphatase activity33. Expression of PP2A and its activators is significantly reduced in the brains of individuals with AD compared with age-matched controls, whereas PP2A inhibitors are upregulated34. Interestingly, PP2A also regulates GSK3β, CDK5 and JNK, providing an additional route to influence tau phosphorylation34.
Acetylation.
In addition to being hyperphosphorylated, tau from patients with AD and other tauopathies is more heavily acetylated than in the brains of cognitively normal individuals8. Like phosphorylation, tau acetylation can arise through multiple mechanisms, including histone acetyltransferase p300 (p300 HAT), cAMP-responsive element-binding protein (CREB)-binding protein, or auto-acetylation, with sirtuin 1 and histone deacetylase 6 acting to deacetylate tau35. Dysregulation of this process produces dysfunction in multiple systems, thereby contributing to neurodegeneration. Acetylation also contributes to tau pathology by inducing tau cleavage, preventing ubiquitin binding and impeding tau turnover8,35. Thus, tau becomes cytosolic, more aggregation-prone, and more difficult for the cell to remove.
Carboxy-terminal truncation.
During the characterization of PHFs isolated from the brains of individuals with AD, it was discovered that tau had undergone carboxy-terminal truncation by caspase-336,37. A similar phenomenon was observed in other tauopathies37. In the case of AD, Aβ promotes caspase activation, thereby providing a link between the two main pathologies36. Although cleavage of tau at Asp421 by caspase 3 is the most studied mechanism, tau has several other caspase cleavage sites in the amino terminus36 (caspase 6) and at Asp31438 (caspase 2), and activated caspase 3, 6 and 9 colocalize with tau pathology. In addition, tau can be cleaved by other proteases, including calpains and cathepsins.
Cleaved tau is also present in PSP, FTD, CBD and Pick disease, although the pattern is different from that observed in AD. A 35 kDa tau fragment is seen in brain extracts from individuals with PSP, FTD or CBD, but is not present in Pick disease or AD. In addition, a greater number of tau fragment sizes in the 35–64 kDa range is observed in non-AD tauopathies than in AD25. Like phosphorylation and acetylation, truncation inhibits the ability of tau to bind to microtubules, and also promotes tau aggregation36,37, mitochondrial dysfunction37 and synaptic deficits39.
O-GlcNAcylation and N-glycosylation.
Unlike other tau post-translational modifications, _O_-GlcNAcylation, a type of _O_-glycosylation, seems to be protective against tauopathies. In brain tissue from patients with AD, compared with healthy elderly controls, levels of _O_-GlcNAcylated tau are reduced40. Conversely, _N_-glycosylation of tau, which is thought to promote phosphorylation and pathological conformational changes, is increased in AD9,35.
Cytoskeletal dysfunction
Disruption of the tau-microtubule association can potentially affect microtubule dynamics and transport. Indeed, cytoskeletal dysfunction is a common feature of multiple neurodegenerative disorders, including AD. In AD, brain neurons show a reduction in the number and length of microtubules41, reduced levels of acetylated tubulin42,43 (a marker of stable microtubules), and axonal swellings containing vesicles and organelles44. Furthermore, tau influences the movement of kinesin and dynamin along the axon2,3, and has been shown to inhibit the transport of organelles and amyloid precursor protein (APP)45. Data from cell and animal models show that tau is also a mediator of Aβ-induced toxicity46–48.
Tau aggregation
Post-translational modifications and loss of microtubule binding lead to elevated levels of cytosolic tau, thereby increasing the potential for tau-tau interactions and polymerization. In AD and other tauopathies, the brain tissue contains a variety of tau multimer species, including the classic PHFs, straight filaments, twisted ribbons and small oligomeric aggregates15–17. The variations in conformation can be attributed to mutations, differing patterns of post-translational modification between diseases, and the presence of different polymerization inducers. Overall, in humans, tau aggregation and the presence of NFTs correlate more closely with symptom severity and neuron loss than do Aβ lesions49.
Large fibrils might contribute to cell dysfunction via molecular crowding and effects on cell metabolism50,51. Neurons containing tangles also have fewer synapses and reduced levels of synaptophysin mRNA compared with tangle-free neurons52,53. However, the mature filaments are unlikely to be the primary toxic species: current evidence suggests that small soluble species are more detrimental to cells (reviewed previously54,55). Indeed, larger aggregates might exert beneficial effects by sequestering misfolded monomers and soluble aggregates. This phenomenon could account for the seemingly paradoxical findings regarding tau levels in the cerebrospinal fluid (CSF) of patients with AD. Although CSF tau levels are higher in AD than in controls, the levels decrease as the disease progresses, despite the accrual of NFTs56. These observations might be explained by decreased tau secretion and/or release, possibly resulting from assembly of tau into larger, more stable filaments within the neurons, as well as from loss of synapses and neurons. CSF tau levels are not increased in non-AD tauopathies57–60, suggesting that extracellular tau is not a primary target in these conditions. These well-established findings should be taken into account in biomarker and therapeutic studies. For example, the documented decrease in CSF tau with pathology progression in patients with AD might impede the detection of drug-induced reductions in CSF tau. It also raises concerns about the validity of targeting extracellular tau in the later stages of the disease. Furthermore, detailed information on tau fragments obtained by mass spectroscopy could provide additional insight into therapy-related changes in CSF tau levels that are not detected with current enzyme-linked immunosorbent assays. This information will also guide which epitopes can be targeted extracellularly.
Oligomeric tau has emerged as the probable candidate for the most toxic species in tauopathies, and is present in the brain at early stages of mild cognitive impairment (MCI) and AD54,55,61. Tau oligomers promote toxicity in cell models to a greater extent than filamentous tau, and are linked to neurodegeneration and cognitive phenotypes in vivo54,55 Soluble tau aggregates might also affect the integrity of membranes62,63. In addition, tau oligomers are implicated in the spreading of tau pathology. For example, in cell models, soluble oligomeric tau can induce seeding of native tau54,64, and small tau aggregates isolated from patients or animals with AD can induce tau pathology in mice54,64,65. Thus, compounds that prevent or reverse tau aggregation have the potential to improve cell health and prevent the spread of tau pathology to other brain regions.
Protein degradation pathway impairment
In addition to changes to the tau protein itself, other factors can promote the development of tau pathology (Fig. 2). Impairment of protein degradation pathways, including autophagy (reviewed previously66–68), is found in a host of neurodegenerative diseases. In MCI and AD, the pattern of kinase activation in the mechanistic target of rapamycin (mTOR) pathway is altered69–71 and expression of other key autophagy-related proteins is reduced72 in the brain. Disruption of autophagic flux and a failure of autophagosomes to fuse with lysosomes leads to a build-up of vesicles, which can be seen in dystrophic neurites and cell bodies in AD, CBD and PSP, as well as in tauopathy models73–76. Impaired flux also directly contributes to AD pathology. Autophagosomes contain APP, presenilin and γ-secretase, and disruption of autophagosome processing in neurons can lead to increased production of Aβ77, which can in turn further inhibit protein digestion78,79. As tau is a target of autophagy80, any deficiencies in processing could lead to increased levels of intracellular tau, including misfolded, damaged or aggregated protein.
The ubiquitin-proteasome system is also dysfunctional in AD, leading to a build-up of ubiquitinated proteins including tau81,82. Alterations in proteasome efficiency also affect learning and memory via the CREB pathway. Digestion of the regulatory subunit of cAMP-dependent protein kinase A (PKA) in the proteasome results in increased PKA activity and phosphorylation of CREB83. Tau pathology and cognitive deficits can be either exacerbated84 or reduced85,86 in model animals through the use of inhibitors or enhancers of proteasome function. Interestingly, as with autophagic degradation, tau inhibits proteasome activity87 suggesting that tau pathology can become self-perpetuating once aggregates are present in neurons.
Tau-targeting drugs
Each stage in the development of tau pathology, from the expression of tau itself to post-translational modifications, aggregation and impairments in clearance, presents opportunities for intervention. In addition to AD, some of the candidate treatments have been examined in other tauopathies such as PSP, FTD and CBD, which involve similar processes. Thus, treatments that prove efficacious in one condition could have wider applications. Aβ is not a confounding variable in the non-AD tauopathies and, as rare conditions, their therapies qualify for orphan drug status (treatments for conditions affecting ≤200,000 people) and fast tracking for FDA approval. In this section, we review the diverse potential tau-targeting therapies that have reached the clinical trial stage for AD and other tauopathies (Table 1).
Table 1 |.
Clinical trials of tau-targeting small molecule therapies for Alzheimer disease and other tauopathies
| Drug | Clinical trial identifier | Dates | Trial description | Trial status |
|---|---|---|---|---|
| PP2A activators | ||||
| Memantine | NCT00097916 | 2004–2006 | Phase III randomized, double-blind, placebo-controlled, interventional study in moderate to severe AD (n = 34) | Completed |
| NCT00187525 | 2004–2006 | Phase IV non-randomized, open-label, interventional study in frontotemporal lobar degeneration | Completed | |
| NCT00120874 | 2005–2011 | Phase IV randomized, single-blind, parallel assignment, interventional study in AD | Completed | |
| NCT00235716 | 2005–2012 | Phase III randomized, quadruple-blind, placebo-controlled, interventional study in AD (n = 613) | Completed | |
| NCT00255086 | 2005–2009 | Phase III randomized, quadruple-blind, placebo-controlled interventional study in AD (n = 17) | Completed | |
| NCT00322153 | 2006–2008 | Phase III randomized, double-blind, placebo-controlled interventional study in moderate to severe AD (n = 677) | Completed | |
| NCT00334906 | 2006–2008 | Phase IV non-randomized, open-label interventional study in moderate AD (n = 75) | Completed | |
| NCT00401167 | 2006–2010 | Phase IV non-randomized, open-label, interventional study in severe AD (n = 32) | Completed | |
| NCT00469456 | 2007–2009 | Phase IV randomized, double-blind, placebo-controlled interventional study in AD (n = 265) | Completed | |
| NCT00476008 | 2007–2012 | Phase IV randomized, double-blind, parallel-assessment interventional study in mild AD (n = 60) | Completed | |
| NCT00505167 | 2007–2008 | Phase IV randomized, single-blind, memantine versus donepezil in mild to moderate AD (n = 64) | Completed | |
| NCT00545974 | 2007–2012 | Phase IV randomized, double-blind, placebo-controlled interventional study in FTD (n = 81) | Completed | |
| NCT00594737 | 2008–2012 | Phase III open-label interventional pilot study in FTD (n = 17) | Completed | |
| NCT00857233 | 2009–2012 | Phase III open-label extension study on efficacy and safety in moderate to severe AD (n = 297) | Terminated | |
| NCT00857649 | 2009–2013 | Phase III randomized, double-blind, parallel-assignment interventional study in moderate to severe AD (n = 369) | Terminated | |
| NCT00862940 | 2009–2012 | Phase IV randomized, double-blind, parallel assessment intervention study in AD (n = 277) | Completed | |
| NCT00933608 | 2009–2014 | Phase IV randomized, parallel-assessment intervention study in patients at risk of AD (n = 17) | Completed | |
| NCT01409694 | 2011–2016 | Phase III randomized, parallel, quadruple masking intervention study in moderate AD (n = 90) | Completed | |
| NCT02553928 | 2015–2016 | Phase IV randomized, double-blind interventional study in AD (n = 62) | Completed | |
| NCT02854917 | 2016–2018 | Case–control retrospective study (n = 20) | Completed | |
| NCT03168997 | 2017–2018 | Phase IV open-label, parallel-group interventional study (n = 222) | Not yet recruiting | |
| Sodium selenate | ACTRN12611001200976 | 2011–2012 | Phase IIa randomized, double-blind, placebo-controlled safety and efficacy study in mild to moderate AD (n = 20) | Completed |
| ACTRN12613000170729 | 2012–2013 | Open-label extension study (n = 20) | Completed | |
| GSK3β inhibitors | ||||
| Tideglusib | NCT00948259 | 2008–2009 | Phase I randomized, double-blind, placebo-controlled, dose escalation study in mild to moderate AD (n = 30) | Completed |
| NCT01049399 | 2009–2011 | Phase II randomized, double-blind, parallel assessment intervention study in mild to moderate PSP (n = 146) | Completed | |
| 2010-023322-21 | 2013–2015 | Phase II randomized, double-blind, placebo-controlled, four- arm efficacy study in mild to moderate AD (n = 306) | Completed | |
| Lithium chloride | NCT00088387 | 2004–2005 | Phase II assessment of the effects of lithium and divalproex on cerebrospinal fluid tau in AD (n = 35) | Completed |
| NCT00703677 | 2008–2010 | Phase I/II non-randomized, single-group efficacy study in PSP and CBD (n = 17) | Completed | |
| NCT02601859 | 2016 | Open-label, single-group efficacy study in MCI (n = 20) | Not yet recruiting | |
| NCT02862210 | 2016–2020 | Phase II randomized, double-blind, placebo-controlled low dose efficacy study in FTDP (n = 60) | Not yet recruiting | |
| Acetylation inhibitors | ||||
| Salsalate | NCT02422485 | 2015–2017 | Phase I open-label pilot in PSP (n = 10) | Active, not Recruiting |
| OGA inhibitors | ||||
| MK-8719 | Not registered | 2016 | Phase I single ascending dose safety and tolerability study in healthy individuals (n = 16) | Completed |
| Aggregation inhibitors | ||||
| LMTX | NCT01626391 | 2012–2013 | Phase II randomized, double-blind, placebo-controlled, safety and tolerability study in patients with mild to moderate AD already taking medication (n = 9) | Terminated |
| NCT01689233 | 2012–2016 | Phase III randomized, double-blind, placebo-controlled, parallel-group, single-dose efficacy study in mild AD (n = 800) | Completed | |
| NCT01689246 | 2013–2015 | Phase III randomized, double-blind, placebo-controlled, parallel-group, single-dose efficacy study in mild to moderate AD (n = 891) | Completed | |
| NCT02245568 | 2014–2018 | Phase III open-label extension study for patients in previous trials (n = 1,000) | Terminated | |
| Curcumin | NCT00099710 | 2003–2007 | Phase II randomized, double-blind, placebo-controlled, parallel-assessment safety and tolerability study in mild to moderate AD (n = 33) | Completed |
| NCT00164749 | 2004–2006 | Phase II randomized, double-blind, parallel-assessment pilot study in AD (n = 36) | Completed | |
| NCT00595582 | 2005–2008 | Single-group, open-label, dietary supplement in MCI (n = 10) | Terminated | |
| NCT01383161 | 2012–2017 | Phase II randomized, double-blind, placebo-controlled, parallel-assessment efficacy study in MCI (n = 132) | Active not recruiting | |
| NCT01811381 | 2014–2018 | Phase II randomized, double-blind, placebo-controlled, factorial-assignment prevention study in MCI (n = 80) | Recruiting | |
| Microtubule stabilizers | ||||
| Epithilone D | NCT01492374 | 2012–2013 | Phase I randomized, double-blind, placebo-controlled trial in mild AD (n = 40) | Completed |
| NAP | NCT00422981 | 2007–2008 | Phase II randomized, double-blind, placebo-controlled, parallel-assignment efficacy study in MCI (n = 144) | Completed |
| NCT01110720 | 2010–2012 | Phase II/III randomized, double-blind, placebo- controlled interventional study in PSP (n = 313) | Completed | |
| NCT01056965 | 2010–2017 | Phase II randomized, double-blind, placebo-controlled, interventional study in FTDP, PSP, CBD and Niemann–Pick type A (n = 12) | Active, not recruiting | |
| TPI 287 | NCT01966666 | 2013–2017 | Phase I randomized, double-blind, placebo-controlled, sequential-cohort dose-ranging study in mild AD (n = 33) | Active, not recruiting |
| NCT02133846 | 2014–2017 | Phase I randomized, double-blind, placebo-controlled, sequential-cohort dose-ranging study in CBD and PSP (n = 44) | Active, not recruiting | |
| PDE4 inhibitors | ||||
| BPN14770 | NCT02648672 | 2015–2016 | Phase I randomized, double-blind, placebo-controlled, single ascending dose study in healthy individuals (n = 32) | Completed |
| NCT02840279 | 2016 | Phase I randomized, double-blind, placebo-controlled, multiple ascending dose study in healthy elderly individuals (n = 77) | Completed |
Reducing tau expression
As tau is not only directly toxic to cells but is also a mediator of Aβ toxicity88, reducing tau levels would seem to be a logical therapeutic approach for AD. In mouse models, tau knockout has few adverse effects89, presumably because other microtubule-associated proteins can, to a large extent, compensate for loss of the tau protein. If the level of tau monomers in cells decreases, the equilibrium that governs aggregate formation dictates that the tau assemblies will depolymerize, leading to reductions in oligomeric tau and larger aggregates such as PHFs. Tau expression can be reduced with small interfering RNA (siRNA) or antisense oligonucleotides (ASOs). In cell and animal models, siRNA was found to reduce tau pathology and associated functional impairments37. Although this approach has not yet been tested in clinical trials for AD or other tauopathies, it has been used for other conditions, including cancer90,91. ASOs were a popular experimental approach about 25 years ago but fell out of favour because of adverse effects. However, the recent success of this approach in attenuating the progression of spinal muscular atrophy is likely to lead to a resurgence of this type of therapy for various conditions, including tauopathies92,93.
Targeting tau protein modifications
Phosphatase modifiers.
Memantine was first used as an agent to lower blood sugar levels, but was later discovered to function as an _N_-methyl-D-aspartate receptor antagonist94,95. In addition, memantine enhances PP2A activity by blocking inhibitor 296 (also known as I2PP2A). In early clinical trials, memantine produced some improvements in patients who were in a coma97,98. In patients with moderate to severe AD, memantine promoted small short-term improvements in cognition99,100, and it also seemed to provide modest benefits in FTD101. The drug might be more effective if administered in combination with cholinesterase inhibitors102.
Sodium selenate increases PP2A activity via activation of the regulatory B subunit, and reduces phosphorylation of tau in models of AD103–105, epilepsy106,107 and TBI108. In a phase IIa clinical trial in patients with mild to moderate AD, sodium selenate showed some benefits on diffusion MRI but not on other measures, including CSF levels of tau and Aβ, cognition, volumetric MRI and PET imaging109.
Kinase inhibitors.
Some of the earliest work to develop CDK5 inhibitors came from the cancer field, leading to the identification of compounds such as flavopiridol (alvocidib)110 and roscovitine (seliciclib)111. Both of these compounds compete with ATP for binding to CDK5, resulting in reduced activation of this kinase112. These inhibitors prevent cell death in various models113, and roscovitine prevents tau phosphorylation in animal models of AD and encephalitis114. Both compounds cross the blood-brain barrier and have been tested in various cancer trials, but not yet for AD or other neurodegenerative diseases114,115.
Tideglusib is an irreversible inhibitor of GSK3β that does not compete with ATP binding116,117. In animal models of AD, tideglusib reduces tau phosphorylation, Aβ plaque burden, memory deficits, cell death and astrocytosis118,119. In a pilot clinical study120, patients with AD who received 1,000 mg tideglusib daily showed significant improvements in cognition compared with placebo-treated patients121. In a larger phase II study, tideglusib treatment was associated with significant cognitive improvement and a reduction in CSF levels of β-secretase in a subgroup of patients with mild AD122. However, when the entire study cohort was analysed, tideglusib was found to be well tolerated but produced no significant improvements122. Another phase II trial of this drug123 was carried out in patients with PSP over 52 weeks, and no clinical improvements were observed124.
Lithium chloride (or ‘lithium’) was widely known as a treatment for bipolar disorder long before its identification as an inhibitor of GSK3β125,126. In cultured cells, lithium can prevent Aβ-induced toxicity and tau phosphorylation127. Transgenic animals treated with lithium show reduced phospho-tau levels, and also Aβ reduction in some studies127. Clinical studies of lithium treatment in patients with MCI or AD have been small, but have produced some positive results. Preliminary testing has shown few adverse effects in elderly patients with AD127. In short-term trials, increased serum levels of brain-derived neurotrophic factor and CSF levels of glial cell line-derived neurotrophic factor, both of which act as neuroprotectants, were observed, in addition to cognitive improvements127. In patients with MCI, lithium treatment significantly reduced phospho-tau levels in CSF and improved cognitive performance127,128. In a recent study129, patients with AD were given very low doses of lithium (300 μg daily) for 15 months. This treatment resulted in a stabilization of cognitive symptoms, whereas placebo-treated patients showed cognitive decline. Additional trials are planned in MCI130 and FTD131.
Inhibiting tau acetylation.
Salsalate is a small-molecule NSAID that inhibits acetylation of tau at Lys174 by p300 HAT132. In preclinical testing, salsalate reduced p300 HAT activity, leading to reduced tau pathology, preserved hippocampal volume and improved cognition in transgenic mice. Phase I testing of salsalate in patients with PSP was expected to be completed by March 2017, but the results have yet to be published133. The trial involves a 6-month open-label course of salsalate, and assessments of brain volume, motor functioning, CSF biomarkers and cognition.
Inhibiting tau deglycosylation.
MK-8719 is a small-molecule inhibitor of the _O_-GlcNAcase (OGA) enzyme. The human dosage was determined in preclinical studies in various species, using intravenous and oral administration134. Subsequently, safety, pharmacokinetics and pharmacodynamics were evaluated in healthy individuals, who received ascending oral doses of the drug. Efficacy was also assessed by measuring _O_-GlcNAcylated protein levels in peripheral blood mononuclear cells, which increased in a dose-dependent manner. The drug was well tolerated and the findings support further development134. In 2016, the FDA granted MK-8719 orphan drug status, and plans are underway to develop the drug for the treatment of PSP135.
Inhibiting tau truncation.
Broad caspase inhibitors have been used to reduce pathology in models of Huntington disease, ischaemia and amyotrophic lateral sclerosis (ALS)136. Although no specific caspase inhibitors are in clinical trials for tauopathies, several of these agents that have been used in vitro and in vivo have advanced to the clinical trial stage in ALS and Parkinson disease136.
Tau aggregation inhibitors
Methylene blue blocks the polymerization of tau in vitro by trapping the tau monomers in an aggregation-incompetent conformation137,138. This agent was effective at reducing tau pathology and improving cognitive phenotypes in transgenic mouse models of tauopathy137. Methylene blue crosses the blood–brain barrier and has a long history of use in humans, so it would seem to be a promising drug candidate.
Initial safety data for methylene blue in the context of AD were collected in healthy individuals139 before phase II testing140. Three different doses of an oxidized form of the molecule were administered to patients with AD for an initial period of 24 weeks141, which was later extended to 50 weeks142. Although some notable treatment effects were observed in initial testing, in two phase III clinical trials of the methylene blue derivative LMTX (also known as LMTM or TRx0237)143,144, none of the treatment groups experienced significant benefits. Although the authors claimed benefits in patients with mild or moderate AD who received LMTX in the absence of other AD medications145, these results were widely called into question146 owing to concerns over use of statistics and unsuitable controls. Data from the second completed phase III trial is expected to be forthcoming, although the first results presented at the Clinical Trials on Alzheimer’s Disease (CTAD) Congress in 2016 did not show slowing of disease progression with LMTX treatment147. An additional clinical trial was recently terminated that was recruiting individuals with AD and FTD who had already completed a previous trial, and was assessing the long-term safety of the drug148.
Curcumin is a natural product of the Curcuma longa plant, and has been used in cooking and herbal medicine for centuries. Its antioxidant and anti-inflammatory properties, as well as its history of safety in humans, made it an attractive candidate for clinical development. In addition to these properties, it can bind to proteins in β-sheet conformation and prevent aggregation149,150. In animal models, it can reduce tau and Aβ pathology and ameliorate cognitive deficits149,150.
In two phase II trials151,152 in patients with AD, curcumin had no effect on cognition or CSF biomarkers149, and a third study in patients with MCI was terminated153. A larger phase II study, in which patients with MCI will receive one of three doses of curcumin for 18 months, is ongoing154, and an additional trial of a combined course of curcumin and exercise is currently recruiting155. In both trials, patients will be assessed for biomarkers of AD, and for changes on PET and MRI scans.
Microtubule stabilizers
Epithilone D belongs to a class of molecules that were originally identified as antifungal agents, but were later discovered to also stabilize microtubules156. In preclinical testing, epithilone D increased microtubule numbers and reduced the number of axons with abnormal morphology in young157 and aged158 tau transgenic mice. In other mouse models of tauopathy, the drug improved cognition, and reduced tau pathology and tau-related changes in microtubule dynamics159. A phase I clinical trial160 was initiated to assess the safety, efficacy and pharmacodynamics of epithilone D in patients with AD. However, this trial was discontinued, presumably due to adverse effects.
Davunetide, also known as NAP, is an eight-amino-acid fragment of activity-dependent neuroprotective peptide. Both the full-length peptide and NAP have been shown to improve cognitive performance in wild-type animals and to protect against damage in a mouse model of TBI. In transgenic AD models, NAP reduced tau and amyloid pathology and improved cognition and axonal transport161. In a phase II trial, patients with MCI were treated with an ascending dose of NAP for 12 weeks. The drug was well tolerated, but no significant differences relative to placebo were seen at either dose, although a trend towards improvements in working memory and attention was observed. In the latest phase II/III study162, patients with PSP were treated with NAP for 52 weeks, but no differences were found between placebo-treated and NAP-treated patients163.
Two clinical trials of the microtubule-stabilizing compound TPI 287 (abeotaxane)164,165 are ongoing. TPI 287 has been shown to cross the blood–brain barrier in a mouse model of cancer metastasis166, and it has some efficacy in stabilization of tumours in the human brain167,168. This drug is currently being trialled in patients with mild to moderate AD, PSP or CBD. Patients received one of three doses of TPI 287 for 9 weeks with the option to continue treatment for a further 6 weeks164,165. The treatment is expected to be completed in December 2018. Outcome measures include CSF levels of TPI 287, tau and Aβ, cognitive testing and MRI.
Modulating protein degradation
Rolipram, a phosphodiesterase E4 (PDE4) inhibitor, was initially developed as an antidepressant169, but early human trials were discontinued owing to a narrow therapeutic window and gastrointestinal adverse effects. However, the drug has been shown to improve cognition and reduce pathology in Aβ and tauopathy mice, and in rats treated with exogenous Aβ, suggesting its potential utility for treating AD85. The APP/PS1 mouse model of AD develops long-term potentiation (LTP) deficits, but rolipram-treated APP/PS1 mice showed comparable LTP induction to wild-type animals85. In addition, increased phosphorylation of PKA — a mediator of proteasome function — and proteasome subunits was seen after rolipram treatment, indicating enhancement of the proteasome system170.
To apply these results in the clinical setting, BPN14770, a related PDE4 inhibitor with fewer adverse effects, was developed. To assess the safety of this agent, two phase I trials using three different doses were carried out in healthy individuals171,172. At the low and mid-range doses, improvements in two measures of working memory were reported. Although the tests were conducted in a small number of healthy individuals, the results were promising enough to plan phase II testing in patients with AD (trial not yet registered).
Tau immunotherapies
To date, Aβ immunotherapies have been largely unsuccessful, possibly because they were only administered once the symptoms of AD had become apparent173–178. Although there is still some hope that patients at earlier stages of AD might benefit from this approach, tau immunotherapies could be the most prudent course of action once the symptomatic phase is underway.
Some data indicate that Aβ immunotherapy has noteworthy but modest effects on tau pathology. In the first active immunization trial, the AN-1792 vaccine seemed to show some ability to clear Aβ plaque-associated tau lesions through plaque removal179–181, and in phase II trials, the anti-Aβ antibody bapineuzumab reduced CSF phospho-tau levels in patients with AD182,183. Unfortunately, these results did not extend to phase III trials, in which bapineuzumab had no effect on tau pathology184. Likewise, solanezumab did not alter tau levels in treated individuals in any of the trials. These data indicate that any clearance of Aβ during active or passive immunization cannot reduce tau levels sufficiently to alter the course of disease, and direct targeting of tau is the next logical step.
The first reports of successful active and passive immunization against tau in a tauopathy mouse model, using a phospho-tau peptide comprising the pSer396/404 epitope or the PHF1 antibody against this epitope185–188, came from our group. Immunization with these agents reduced pathological tau levels and attenuated the behavioural phenotypes associated with the tauopathy, demonstrating the feasibility of these related interventions. Subsequently, the efficacy of these approaches has been confirmed and extended by multiple groups, as detailed below.
Active tau immunization has been shown to reduce tau pathology by targeting single185, 187, 189–193 or multiple194 phospho-epitopes, the amino terminus195, full-length normal and mutant tau196, 197, or aggregated tau198. Reductions in pathological tau are achieved with few reported adverse effects, and the long-lasting immune response makes active immunization a promising option. However, elicitation of antibodies against a native protein always carries the risk of adverse immune reactions and detrimental targeting of the normal protein. In mice, tau vaccination has been reported to cause toxicity when administered in conjunction with strong T-helper 1-inducing adjuvants199,200, which are not approved for use in humans. Similar tau immunogens administered with a milder adjuvant or fewer immunizations do not produce such adverse reactions194,196, emphasizing the need to use mild adjuvants with tau immunogens, as we have always advocated185.
Passive immunization offers a potential solution to the safety concerns that arise from active strategies. Patients will not develop their own antibodies, and the effects of immunization are likely to be transient, which reduces the risk of immunological adverse effects. Passive immunization also offers greater specificity for the epitope that is being targeted. Because the epitope profile changes over the course of the disease, once diagnostics advance sufficiently, treatments could be tailored to the individual according to the disease stage. As mentioned above, the pSer396/404 region was the first to be studied and has since been targeted by several groups186, 188,201–208. In addition, monoclonal antibodies against the amino-terminal or repeat domain (306–320) region209–215, oligomeric tau216,217, misfolded tau201,218,219, pSer202220,221, pThr231208 pSer409222, pSer413223 and pSer422224 have proved to be efficacious in tauopathy models. Moreover, antibody fragments have been shown to reduce tau levels in vivo225,226.
The fact that NFTs are intracellular could potentially limit the utility of anti-tau antibodies as therapeutic agents. However, several groups have reported that antibodies, including anti-tau antibodies, can enter neurons, and that extracellular tau might be important for the spread of tau pathology227–229. Our group and others have shown that anti-tau antibodies can cross into the brain and enter neurons, where they primarily colocalize with endosomal and lysosomal markers185,202–204,206,219,224,230. The entry of these antibodies into neurons is mainly receptor-mediated but can also occur via bulk endocytosis to some extent. Fc receptors are expressed on neurons231,232 and seem to be the main route of entry for anti-tau antibodies: blockade of low-affinity FcγII/III receptors largely prevents antibody uptake202,219. Of note, binding of a tau–antibody complex to a cytosolic Fc receptor, E3 ubiquitin-protein ligase TRIM21, promotes proteosomal degradation of the complex, thereby inhibiting intracellular seeded tau aggregation233.
Antibodies could also modify disease progression by blocking the spread of tau pathology. Researchers have known for decades that tau lesions begin in specific brain regions, before spreading to other areas11,12. Tau-expressing cells secrete normal and pathological tau234, which can be taken up by other cells and induce seeding of tau pathology54,64,228. In animal models that express tau only in certain brain regions, or that are injected with exogenous tau, spreading of tau pathology to anatomically connected regions occurs over time54,64,228. This type of spreading mechanism is thought to govern disease propagation in all amyloid diseases and related proteinopathies235. Furthermore, tau is now known to be present in the interstitial space both in tauopathy animal models234 and in the human brain236. This phenomenon can also be seen in cultured cells: even healthy cells can release tau into the culture medium, where it can be endocytosed234,237. Multiple groups have employed antibodies in cell and animal models to block this spreading by targeting extracellular tau238. Tau exocytosis provides a pool of pathologically relevant tau that can be targeted without requiring internalization. Indeed, several reports are available of antibodies that are capable of reducing tau levels and preventing spreading without apparently entering neurons216,218,239. However, the most effective antibodies will probably be those that can also target tau inside the cell, where most of the pathological protein is located (Fig. 3 illustrates the potential intracellular and extracellular mechanisms).
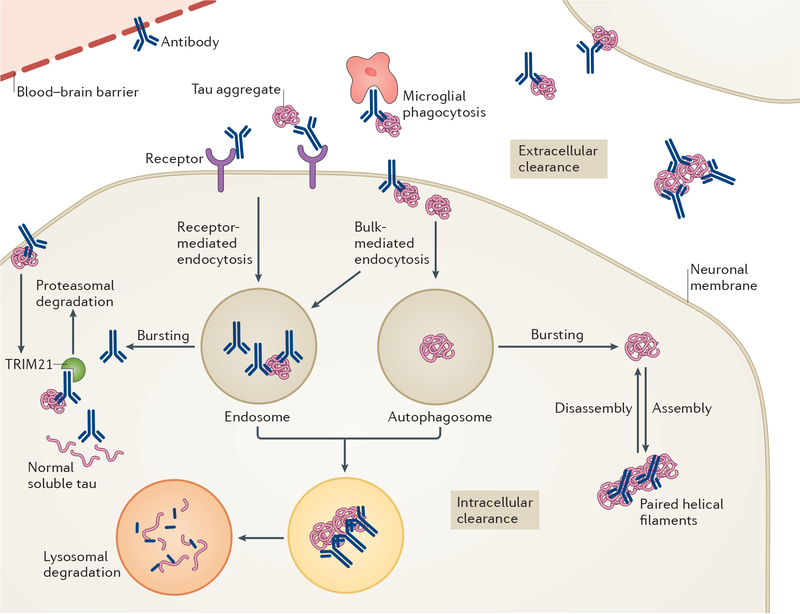
Figure 3 |. Proposed modes of action of anti-tau antibodies.
Antibodies can target tau both extracellularly and intracellularly. Pathological tau mostly resides within neurons but in certain individuals and/or tauopathies, it is also evident in other cell types, primarily glia (in particular, astrocytes and oligodendrocytes). A much smaller pool of tau aggregates is found extracellularly, in the form of small aggregates that are not easily detected or as remnants of neurofibrillary tangles following the death of the neuron. Some anti-tau antibodies are not readily taken up into neurons, presumably because of their unfavourable charge and, therefore, they work principally in the extracellular compartment. Within this compartment, antibodies might sequester tau aggregates, interfere with their assembly and promote microglial phagocytosis, with the overall effect of blocking the spread of tau pathology between neurons. Other antibodies are easily detected within neurons, in association with tau, and have been shown to work both intracellularly and extracellularly. Within the cells, these antibodies could bind to tau aggregates within the endosomal–lysosomal system and promote their disassembly, leading to enhanced access of lysosomal enzymes to degrade the aggregates; sequester tau assemblies in the cytosol and prevent their release from the neuron; or promote proteosomal degradation via E3 ubiquitin–protein ligase TRIM21 binding. The most efficacious antibodies are likely to target more than one pathway and/or pool of tau.
The promising results in animal models have prompted many different groups to move tau vaccines and antibodies into clinical development. In the sections that follow, we will discuss the antibodies that had reached clinical trials at the time of writing (Table 2).
Table 2 |.
Clinical trials of tau-targeting immunotherapies for Alzheimer disease
| Agent | Clinical trial identifier | Dates | Trial description | Trial status |
|---|---|---|---|---|
| Active immunotherapy | ||||
| AADvac1 | NCT01850238 | 2013–2015 | Phase I randomized, double-blind, placebo-controlled safety and tolerability study in mild to moderate AD (n =30) | Completed |
| NCT02031198 | 2014–2016 | Phase I unmasked 18-month follow-up for patients in previous study (n = 25) | Completed | |
| NCT02579252 | 2016–2019 | Phase II randomized, double-blind, placebo-controlled, safety and efficacy study in mild AD (n = 185) | Recruiting | |
| ACI-35 | ISRCTN13033912 | Started 2013 | Phase I randomized, double-blind, placebo-controlled safety, tolerability and immunogenicity study in mild to moderate AD (n = 24) | Completed |
| Passive immunotherapy | ||||
| RG7345 | NCT02281786 | 2015 | Phase I randomized, double-blind single ascending dose safety study in healthy individuals (n = 48) | Completed |
| BMS-986168 | NCT02294851 | 2014–2016 | Phase I randomized double-blind, placebo-controlled safety and tolerability study in healthy individuals (n = 65) | Completed |
| NCT02460094 | 2015–2017 | Phase I randomized, double-blind, placebo-controlled, multiple ascending dose study in PSP (n =48) | Completed | |
| NCT02658916 | 2016–2019 | Phase I extension study for participants in previous trial (n = 48) | Enrolling by invitation | |
| NCT03068468 | 2017–2020 | Phase II randomized, double-blind, placebo-controlled, parallel-group efficacy study in PSP (n =396) | Active, not enrolling | |
| C2N-8E12 | NCT02494024 | 2015–2016 | Phase I randomized, double-blind, placebo-controlled, single ascending dose safety and tolerability study in PSP (n = 32) | Active, not recruiting |
| NCT02985879 | 2016–2019 | Phase II randomized, double-blind, placebo-controlled, multiple-dose safety and efficacy study in PSP (n = 330) | Recruiting | |
| NCT02880956 | 2016–2020 | Phase II randomized, double-blind, placebo-controlled efficacy and safety study in early AD (n = 400) | Recruiting | |
| RO 7105705 | NCT02820896 | 2016–2017 | Phase I randomized, double-blind, placebo-controlled, single or multiple ascending dose safety and efficacy study in healthy individuals and patients with mild to moderate AD (n = 74) | Completed |
| LY3303560 | NCT02754830 | 2016–2017 | Phase I randomized, double-blind, placebo-controlled, single-dose escalation study to assess safety in healthy individuals and patients with mild to moderate AD (n = 90) | Active, not recruiting |
| NCT03019536 | 2017–2020 | Phase I randomized, parallel-assignment, multiple-dose escalation safety and efficacy study in mild cognitive impairment and mild to moderate AD (n = 132) | Recruiting | |
| JNJ-63733657 | NCT03375697 | Started 2017 | Phase I randomized safety and tolerability trial in healthy individuals and patients with prodromal or mild AD (n = 64) | Recruiting |
| UCB0107 | NCT03464227 | Started 2018 | Phase I randomized safety and tolerability trial in healthy individuals (n = 52) | Recruiting |
Active immunization
AADvac1.
The epitope for development of the tau vaccine AADvac1 was selected on the basis of in vitro experiments using a monoclonal antibody, DC8E8, which prevents tau oligomerization240. Epitope mapping revealed that DC8E8 binds a six-amino-acid sequence, HXPGGG, which is found in each of the microtubule-binding repeats of tau. Immunization of transgenic tauopathy rats with a vaccine based on this sequence (KLH-linked Tau294–305) elicited tau antibodies that bound to tau in the brain, reduced the levels of pathological tau and improved behaviour, compared with animals that received alum adjuvant only197. Adverse effects were limited to inflammation at the injection site, and the treatment did not produce any deleterious immunological responses.
On the basis of these results, human trials were initiated. In phase I testing, patients with mild to moderate AD were given three doses of AADvac1 in alum adjuvant over a 12-week period241,242. Almost all of the participants (29 of 30) developed an immunoglobulin G response, and no instances of encephalitis or oedema were reported. However, two patients withdrew owing to adverse reactions (seizure and viral infection), which might have been treatment-related, and an episode of microhaemorrhage was reported in one patient with a history of this condition. A follow-up trial to monitor these patients for additional 18 months and to provide booster vaccination is ongoing243.
A larger phase II trial of AADvac1 in patients with mild AD is underway244,245. Patients in the treatment group will be given six doses of AADvac1 over 6 months, with two additional booster injections. Assessments for safety, changes in clinical symptoms and AD biomarkers in blood and CSF will be conducted.
ACI-35.
ACI-35 targets the pSer396/404 epitope of tau and is delivered in a liposome-based adjuvant. This vaccine was tested in Tau.P301L mice — a transgenic tauopathy model that develops progressive motor impairment190. Serum samples from Tau.P301L mice that were given two doses of ACI-35 contained antibodies that selectively bound pSer396/404 over the non-phosphorylated version of the epitope and could detect tau pathology when used in immunostaining experiments. The vaccinated animals showed a delay in the emergence of the motor phenotype, and also displayed a significant reduction in soluble tau phosphorylated at Ser396, but not at other amino acid residues. There was no evidence of an astrocyte response or significant differences in body weight in the vaccinated mice compared with vehicle-treated animals. Phase I testing is currently being carried out in patients with mild to moderate AD to assess the tolerability and immunogenicity of ACI-35246.
Passive immunization
RG7345.
RG7345 is a humanized antibody that recognizes tau phosphorylated at Ser422, and was originally derived from a rabbit monoclonal antibody. In transgenic mice, RG7345 was shown to enter neurons and reduce tau pathology224. The antibody was administered to healthy young individuals in a phase I trial to assess safety247. However, the results of this trial have not been released and the drug has been discontinued by Roche, presumably because of an unfavourable pharmacokinetic profile, because no safety or efficacy concerns seem to have been raised during the trial.
BMS-986168.
The developers of BMS-986168 observed that neurons derived from stem cells of patients with familial AD secreted amino-terminal tau fragments, termed e-tau215. When added to the media of cultured neurons, these fragments induced hyperactivity and increased Aβ production, and these effects were blocked by the application of an antibody recognizing residues 9–18 of e-tau. The ability of the antibody to enter neurons was not assessed. In animals, administration of BMS-986168 significantly reduced the levels of tau in the interstitial space and soluble Aβ1–40 in the brain.
Four clinical trials of BMS-986168 have been registered. A phase I trial in healthy individuals248 and patients with PSP249 has been completed. This trial used a single ascending dose methodology, and was designed to assess tolerability, with effects on CSF tau as a secondary outcome. A second phase I trial in patients with PSP, involving a multiple ascending dose paradigm, is underway at several centres in the USA249, with follow-up studies planned for those who participated in the earlier trial250. A phase II trial aiming to study the clinical efficacy of BMS-986168 in 400 patients with PSP began recruiting in March 2017. In April 2017, BMS-986168 was licensed by Biogen, with plans to proceed with phase II testing251.
C2N-8E12.
C2N-8E12 recognizes amino acids 25–30 of the tau protein and, similar to BMS-986168, it was intended to work extracellularly. In cell culture, C2N-8E12 prevented pathological tau seeding caused by exogenous tau aggregates212. Infusion of this antibody into the brain in a transgenic mouse model of tauopathy reduced the levels of aggregated and hyperphosphorylated tau, and also improved cognition213. Similar results were seen when a higher dose was delivered systemically239. No adverse immune reactions were seen; in fact, microglial activation was reduced in the treated animals213,239.
Phase I testing of C2N-8E12 in patients with PSP did not show any major adverse effects or safety issues compared with placebo252. In 2016, two phase II trials were initiated, one in 330 patients with PSP253 and the other in 400 patients with early stage AD254. The studies are expected to run until 2019 and 2020, respectively, with patients being assessed on multiple cognitive and behavioural measures. The trial descriptions do not indicate whether CSF analysis and/or brain imaging will be included. In view of its potential utility in PSP, the FDA has granted fast-track status to C2N-8E12.
RO 7105705.
The epitope of RO 7105705 has not been disclosed, but this antibody probably targets pSer409 on the tau protein222. Phase I safety assessments of RO 7105705 are being conducted in healthy individuals and patients with AD255. The participants will receive either a single dose or multiple doses of the antibody to assess tolerability, and the antibody concentration in serum will be measured to determine the pharmacokinetics. In addition, patients with AD will be assessed using the Clinical Dementia Rating scale. As an early-stage trial, the number of participants is insufficient for efficacy assessment. Early reports from the Alzheimer’s Association International Conference in 2017 showed that the antibody was well tolerated in patients at doses up to 16.8 g, and the antibody was detectable in the CSF256.
LY3303560.
Eli Lilly initiated two phase I trials to study the safety and pharmacokinetics of the anti-tau antibody LY3303560, one in healthy individuals and patients with AD257, and the other in patients with MCI or AD258. LY3303560 possibly targets a conformational epitope, although this information has not been officially disclosed259. In the first trial, individuals will be given a single dose of LY3303560, and the concentrations of the antibody in serum and CSF will be monitored. In the second trial, patients with MCI or AD will receive multiple doses of LY3303560, and levels of the antibody in serum and CSF will be assessed, as will the uptake ratio of the Aβ-binding compound 18F-florbetapir in the brain. These studies are expected to end in 2017 and 2020, respectively.
Future prospects
Newly initiated and forthcoming trials.
Recently, it was announced that two new antibodies were entering human testing. Janssen Pharmaceuticals has begun a phase I clinical trial of passive immunization with JNJ-63733657260. The trial is recruiting healthy individuals and patients with AD to assess the safety, tolerability and pharmacological profile of this anti-tau antibody. We are not aware of any preclinical data on this antibody, but it seems to bind to the middle region of tau and has been designed to prevent tau seeding and spreading261. Also advancing into human trials is UCB0107, a tau antibody developed by UCB Biopharma262. Preclinical testing showed that this antibody binds to amino acids 235–246 in the proline-rich region of tau, and that the antibody was effective in preventing seeding and spreading of pathological tau261. The trial is currently recruiting healthy individuals.
Several additional promising tau immunotherapies are set to enter clinical trials in the near future227,238,263. For example, Lundbeck is currently developing a broad portfolio of anti-tau antibodies, targeting both total tau and pathological hyperphosphorylated PHF-tau. The first of these antibodies, a humanized mouse monoclonal antibody that specifically targets pathological hyperphosphorylated tau, has entered clinical development for the treatment of AD (Jan T. Pedersen, H. Lundbeck A/S, personal communication). Developments in the field of tau immunotherapy are occurring at a rapid pace, and several additional trials are likely to start in the near future.
Additional considerations.
Various factors govern the efficacy of anti-tau antibodies, the most important of which are probably the epitope (normal or primarily pathological) and the site of action (extracellular and intracellular, or extracellular only). Although the pathological epitopes seen in AD are common to most if not all of the tauopathies, the pathogenesis of these conditions is likely to differ. Of note, CSF tau levels are increased in AD but not in the other tauopathies57–60, suggesting that extracellular spread of tau pathology is not a prominent feature of non-AD tauopathies. In addition, mass spectroscopy studies indicate that CSF tau predominantly consists of tau fragments spanning amino acids 150–250 of the tau protein, with the amino and carboxyl termini presumably having been digested264,265. Therefore, anti-tau antibodies that primarily work extracellularly should probably target amino acids 150–250, and might only work in AD. The most efficacious antibodies are likely to be those that can target all pools of pathological tau protein, both intracellularly and extracellularly. Finally, it is unclear how closely tau seeding and spread are linked to tau toxicity. Hence, an antibody selected to prevent tau seeding and spread may not necessarily block tau toxicity.
Conclusions
In the search for disease-modifying therapies for AD and other tauopathies, multiple avenues have been and continue to be explored (Fig. 4, Table 1 and Table 2 show ongoing, completed and discontinued trials). Adding to the complexity is the fact that many of the compounds under investigation might exert effects through more than one pathway. On numerous occasions, preclinical success in animal models has failed to translate into benefits in humans. For example, despite encouraging results in preclinical studies, the clinical data on methylene blue derivatives are not very promising. Furthermore, curcumin is limited by its bioavailability, and has yet to demonstrate efficacy in humans with tauopathy.
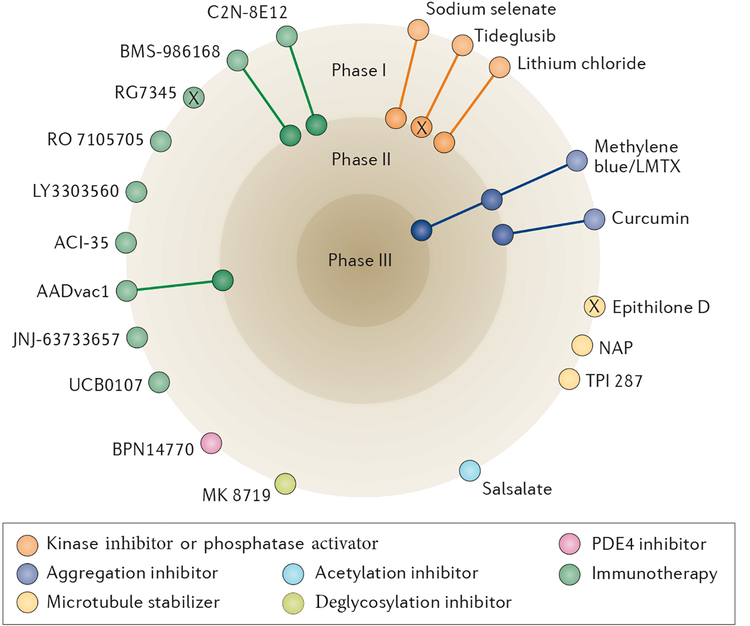
Figure 4 |. Current status of clinical trials of drugs that target tau pathology.
At the time of writing, the most active field is tau immunotherapy, with two active vaccines (AADvac1 and ACI-35) and six antibodies (LY3303560, RO 7105705, BMS-986168, C2N-8E12, JNJ-63733657 and UCB0107) in clinical trials, although most of these therapies are still in the early stages of development. Several of the other compounds in trials have complex or incompletely defined mechanisms of action; in this diagram, these compounds are categorized according to their presumed tau-related mode of action. ‘X’ indicates trials that, to our knowledge, have been halted or terminated, as detailed in the main text, although their current status is difficult to determine. PDE4, phosphodiesterase E4.
With many trials ongoing or awaiting analysis, the next few years will provide a clearer picture of which, if any, of the various tau-targeting approaches are the most viable. In initial tests in humans, MK-8719 was able to increase the levels of _O_-GlcNAcylated proteins, and the results of efficacy studies are eagerly awaited. In the area of immunotherapy, we expect to see the results of multiple active and passive immunization trials in the near future. In general, both strategies have been well tolerated and have not produced the types of adverse effects that led to trial termination or dosing adjustments in some of the Aβ immunotherapy trials. In addition, multiple groups have tau antibodies in development, but not yet in clinical trials. Antibody engineering and combined targeting of tau and Aβ might also improve treatment efficacy in the future.
A shift in the timing of intervention to the MCI stage or the very early stages of AD has been a feature of many recent trials, and as diagnostic methods continue to improve, treatment before symptom onset might be feasible. To some extent, this strategy is already possible, as has been shown in Aβ-targeting clinical trials involving carriers of mutations associated with familial AD, and in control individuals with PET evidence of Aβ accumulation. However, research to develop therapies that are effective once symptoms have progressed will always be needed. In addition, the continuation of basic research into the underlying causes of AD and the mechanisms of tau dysfunction is essential to identify new targets for intervention.
Key points.
- Therapies for Alzheimer disease in clinical trials are gradually shifting from amyloid-β (Aβ)-targeting to tau-targeting approaches.
- Early anti-tau therapies were based mainly on inhibition of kinases or tau aggregation, or on stabilization of microtubules, but most of these approaches have been discontinued because of toxicity and/or lack of efficacy.
- Most of the tau-targeting approaches that are currently in clinical trials are immunotherapies.
- Tau is likely to be a better target than Aβ once cognitive deficits manifest, because the tau burden correlates better with clinical impairments than does the Aβ burden.
- Eventually, with continued improvements in diagnostics, both Aβ and tau are likely to be targeted prophylactically for clearance.
Acknowledgements
Einar M. Sigurdsson was supported by NIH grants R01 NS077239 and R01 AG032611, and a pilot grant from the Michael J. Fox Foundation. Erin E. Congdon was supported by a grant from the Alzheimer’s Association.
Footnotes
Competing interests statement
E.M.S. is an inventor on various patents on immunotherapies and related diagnostics that are assigned to New York University. Some of those focusing on the tau protein are licensed to and are being co-developed with H. Lundbeck A/S. E.E.C. declares no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 12, 459–509 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Dixit R, Ross JL, Goldman YE & Holzbaur EL Differential regulation of dynein and kinesin motor proteins by tau. Science 319, 1086–1089 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vershinin M, Carter BC, Razafsky DS, King SJ & Gross SP Multiple-motor based transport and its regulation by Tau. Proc. Natl Acad. Sci. USA 104, 87–92 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H & Del Tredici K Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braak H, Thal DR, Ghebremedhin E & Del Tredici K Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70, 960–969 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Wharton SB et al. Epidemiological pathology of Tau in the ageing brain: application of staging for neuropil threads (BrainNet Europe protocol) to the MRC cognitive function and ageing brain study. Acta Neuropathol. Commun 4, 11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundke-Iqbal I et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl Acad. Sci. USA 83, 4913–4917 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min SW et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JZ, Grundke-Iqbal I & Iqbal K Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer’s disease. Nat. Med 2, 871–875 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Mena R, Edwards PC, Harrington CR, Mukaetova-Ladinska EB & Wischik CM Staging the pathological assembly of truncated tau protein into paired helical filaments in Alzheimer’s disease. Acta Neuropathol. 91, 633–641 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Braak H & Braak E Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Braak H & Braak E Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging 18, 351–357 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Murray ME et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 10, 785–796 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crary JF et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundke-Iqbal I et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem 261, 6084–6089 (1986). [PubMed] [Google Scholar]
- 16.Kidd M Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 197, 192–193 (1963). [DOI] [PubMed] [Google Scholar]
- 17.Meraz-Rios MA, Lira-De Leon KI, Campos-Pena V, De Anda-Hernandez MA & Mena-Lopez R Tau oligomers and aggregation in Alzheimer’s disease. J. Neurochem 112, 1353–1367 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Arima K Ultrastructural characteristics of tau filaments in tauopathies: immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology 26, 475–483 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Goedert M, Spillantini MG, Jakes R, Rutherford D & Crowther RA Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3, 519–526 (1989). [DOI] [PubMed] [Google Scholar]
- 20.Himmler A, Drechsel D, Kirschner MW & Martin DW Jr. Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol. Cell. Biol 9, 1381–1388 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble W, Hanger DP, Miller CC & Lovestone S The importance of tau phosphorylation for neurodegenerative diseases. Front. Neurol 4, 83 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindwall G & Cole RD Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem 259, 5301–5305 (1984). [PubMed] [Google Scholar]
- 23.Luna-Muñoz J, Chávez-Macías L, García-Sierra F & Mena R Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J. Alzheimers Dis. 12, 365–375 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Augustinack JC, Schneider A, Mandelkow EM & Hyman BT Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 103, 26–35 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Hanger DP & Wray S Tau cleavage and tau aggregation in neurodegenerative disease. Biochem. Soc. Trans 38, 1016–1020 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Kimura T et al. Sequential changes of tau-site-specific phosphorylation during development of paired helical filaments. Dementia 7, 177–181 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H & Goedert M Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38δ or JNK2 in the presence of heparin generates the AT100 epitope. J. Neurochem 99, 154–164 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Shukla V, Skuntz S & Pant HC Deregulated Cdk5 activity is involved in inducing Alzheimer’s disease. Arch. Med. Res 43, 655–662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tell V & Hilgeroth A Recent developments of protein kinase inhibitors as potential AD therapeutics. Front. Cell. Neurosci 7, 189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarza R, Vela S, Solas M & Ramirez MJ c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease. Front. Pharmacol 6, 321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu SL et al. The role of Cdk5 in Alzheimer’s disease. Mol. Neurobiol 53, 4328–4342 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Wilkaniec A, Czapski GA & Adamczyk A Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: facts and hypotheses. J. Neurochem 136, 222–233 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Grundke-Iqbal I, Iqbal K & Gong CX Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci 22, 1942–1950 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Sontag JM & Sontag E Protein phosphatase 2A dysfunction in Alzheimer’s disease. Front. Mol. Neurosci 7, 16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y & Mandelkow E Tau in physiology and pathology. Nat. Rev. Neurosci 17, 5–21 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Cotman CW, Poon WW, Rissman RA & Blurton-Jones M The role of caspase cleavage of tau in Alzheimer disease neuropathology. J. Neuropathol. Exp. Neurol 64, 104–112 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Guo T, Noble W & Hanger DP Roles of tau protein in health and disease. Acta Neuropathol. 133 665–704, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X et al. Caspase-2 cleavage of tau reversibly impairs memory. Nat. Med 22, 1268–1276 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Jadhav S et al. Truncated tau deregulates synaptic markers in rat model for human tauopathy. Front. Cell. Neurosci 9, 24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain 132, 1820–1832 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cash AD et al. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am. J. Pathol 162, 1623–1627 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hempen B & Brion JP Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer’s disease. J. Neuropathol. Exp. Neurol 55, 964–972 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Zhang F et al. Posttranslational modifications of α-tubulin in Alzheimer disease. Transl. Neurodegener 4, 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stokin GB et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307, 1282–1288 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Stamer K, Vogel R, Thies E, Mandelkow E & Mandelkow EM Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 156, 1051–1063 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rapoport M, Dawson HN, Binder LI, Vitek MP & Ferreira A Tau is essential to beta -amyloid-induced neurotoxicity. Proc. Natl Acad. Sci. USA 99, 6364–6369 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vossel KA et al. Tau reduction prevents Aβ-induced defects in axonal transport. Science 330, 198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberson ED et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Nelson PT et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J. Neuropathol. Exp. Neurol 66, 1136–1146 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vazquez A Metabolic states following accumulation of intracellular aggregates: implications for neurodegenerative diseases. PLoS ONE 8, e63822 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandelkow EM, Stamer K, Vogel R, Thies E & Mandelkow E Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging 24, 1079–1085 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Callahan LM, Vaules WA & Coleman PD Quantitative decrease in synaptophysin message expression and increase in cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. J. Neuropathol. Exp. Neurol 58, 275–287 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Ginsberg SD, Hemby SE, Lee VM, Eberwine JH & Trojanowski JQ Expression profile of transcripts in Alzheimer’s disease tangle-bearing CA1 neurons. Ann. Neurol 48, 77–87 (2000). [PubMed] [Google Scholar]
- 54.Shafiei SS, Guerrero-Munoz MJ & Castillo-Carranza DL Tau oligomers: cytotoxicity, propagation, and mitochondrial damage. Front. Aging Neurosci 9, 83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cárdenas-Aguayo Mdel C, Gómez-Virgilio L, DeRosa S & Meraz-Ríos MA The role of tau oligomers in the onset of Alzheimer’s disease neuropathology. ACS Chem. Neurosci 5, 1178–1191 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Fagan AM et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci. Transl. Med 6, 226ra230 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall S et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol 69, 1445–1452 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Hu WT, Trojanowski JQ & Shaw LM Biomarkers in frontotemporal lobar degenerations — progress and challenges. Prog. Neurobiol 95, 636–648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olsson B et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 15, 673–684 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Wagshal D et al. Divergent CSF tau alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 86, 244–250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mufson EJ, Ward S & Binder L Prefibrillar tau oligomers in mild cognitive impairment and Alzheimer’s disease. Neurodegener. Dis 13, 151–153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flach K et al. Tau oligomers impair artificial membrane integrity and cellular viability. J. Biol. Chem 287, 43223–43233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lasagna-Reeves CA et al. The formation of tau pore-like structures is prevalent and cell specific: possible implications for the disease phenotypes. Acta Neuropathol. Comm 2, 56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goedert M & Spillantini MG Propagation of Tau aggregates. Mol Brain 10, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Usenovic M et al. Internalized tau oligomers cause neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. J. Neurosci 35, 14234–14250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whyte LS, Lau AA, Hemsley KM, Hopwood JJ & Sargeant TJ Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer’s disease? J. Neurochem 140, 703–717 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Zare-Shahabadi A, Masliah E, Johnson GV & Rezaei N Autophagy in Alzheimer’s disease. Rev. Neurosci 26, 385–395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nixon RA The role of autophagy in neurodegenerative disease. Nat. Med 19, 983–997 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Tramutola A et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J. Neurochem 133, 739–749 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Li X, Alafuzoff I, Soininen H, Winblad B & Pei JJ Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. FEBS J 272, 4211–4220 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Lafay-Chebassier C et al. mTOR/p70S6k signalling alteration by Aβ exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J. Neurochem 94, 215–225 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Pickford F et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Invest 118, 2190–2199 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boland B et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J. Neurosci 28, 6926–6937 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez-Varo R et al. Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. Acta Neuropathol 123, 53–70 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bordi M et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 12, 2467–2483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piras A, Collin L, Gruninger F, Graff C & Ronnback A Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. Comm 4, 22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu WH et al. Macroautophagy — a novel β-amyloid peptide-generating pathway activated in Alzheimer’s disease. J. Cell Biol 171, 87–98 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin CL et al. Amyloid-β suppresses AMP-activated protein kinase (AMPK) signaling and contributes to α-synuclein-induced cytotoxicity. Exp. Neurol 275, 84–98 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Park H et al. Neuropathogenic role of adenylate kinase-1 in Aβ-mediated tau phosphorylation via AMPK and GSK3β. Hum. Mol. Genet 21, 2725–2737 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Wang Y & Mandelkow E Degradation of tau protein by autophagy and proteasomal pathways. Biochem. Soc. Trans 40, 644–652 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Mori H, Kondo J & Ihara Y Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science 235, 1641–1644 (1987). [DOI] [PubMed] [Google Scholar]
- 82.Perry G, Friedman R, Shaw G & Chau V Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc. Natl Acad. Sci. USA 84, 3033–3036 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gong B, Radulovic M, Figueiredo-Pereira ME & Cardozo C The ubiquitin–proteasome system: potential therapeutic targets for Alzheimer’s disease and spinal cord injury. Front. Mol. Neurosci 9, 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morawe T, Hiebel C, Kern A & Behl C Protein homeostasis, aging and Alzheimer’s disease. Mol. Neurobiol 46, 41–54 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurney ME, D’Amato EC & Burgin AB Phosphodiesterase-4 (PDE4) molecular pharmacology and Alzheimer’s disease. Neurotherapeutics 12, 49–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sweeney P et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl. Neurodegener 6, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deger JM, Gerson JE & Kayed R The interrelationship of proteasome impairment and oligomeric intermediates in neurodegeneration. Aging Cell 14, 715–724 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ittner LM & Götz J Amyloid-β and tau — a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci 12, 65–72 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Ke YD et al. Lessons from tau-deficient mice. Int. J. Alzheimers Dis 2012, 873270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wittrup A & Lieberman J Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet 16, 543–552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuckerman JE & Davis ME Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nature Rev. Drug Disc 14, 843–856 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Corey DR Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nat. Neurosci 20, 497–499 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Finkel RS et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388, 3017–3026 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Bormann J Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. Eur. J. Pharmacol 166, 591–592 (1989). [DOI] [PubMed] [Google Scholar]
- 95.Kornhuber J, Bormann J, Retz W, Hubers M & Riederer P Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur. J. Pharmacol 166, 589–590 (1989). [DOI] [PubMed] [Google Scholar]
- 96.Chohan MO, Khatoon S, Iqbal IG & Iqbal K Involvement of I2PP2A in the abnormal hyperphosphorylation of tau and its reversal by memantine. FEBS Lett 580, 3973–3979 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Miltner FO Use of symptomatic therapy with memantine in cerebral coma. II. Development of stretch synergisms in coma with brain stem symptoms. Arzneimittelforschung 32, 1271–1273 (1982). [PubMed] [Google Scholar]
- 98.Miltner FO Use of symptomatic therapy with memantine in cerebral coma. I. Correlation of coma stages and EEG spectral paters. Arzneimittelforschung 32, 1268–1270 (1982). [PubMed] [Google Scholar]
- 99.Jiang J & Jiang H Efficacy and adverse effects of memantine treatment for Alzheimer’s disease from randomized controlled trials. Neurol. Sci 36, 1633–1641 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Matsunaga S, Kishi T & Iwata N Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PloS ONE 10, e0123289 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kishi T, Matsunaga S & Iwata N Memantine for the treatment of frontotemporal dementia: a meta-analysis. Neuropsychiatr. Dis. Treat 11, 2883–2885 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsoi KK et al. Combination therapy showed limited superiority over monotherapy for Alzheimer disease: a meta-analysis of 14 randomized trials. J. Am. Med. Dir. Assoc 17, 863.e1–863.e8 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Corcoran NM et al. Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. J. Clin. Neurosci 17, 1025–1033 (2010). [DOI] [PubMed] [Google Scholar]
- 104.Rueli RH et al. Selenprotein S reduces endoplasmic reticulum stress-induced phosphorylation of tau: potential selenate mitigation of tau pathology. J. Alzheimers Dis 55, 749–762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Eersel J et al. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc. Natl Acad. Sci. USA 107, 13888–13893 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones NC et al. Targeting hyperphosphorylated tau with sodium selenate suppresses seizures in rodent models. Neurobiol. Dis 45, 897–901 (2012). [DOI] [PubMed] [Google Scholar]
- 107.Liu SJ et al. Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. Brain 139, 1919–1938 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Shultz SR et al. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 138, 1297–1313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malpas CB et al. A phase Iia randomized control trial of VEL (sodium selenate) in mild–moderate Alzheimer’s disease. J. Alzheimers Dis 54, 223–232 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Carlson BA, Dubay MM, Sausville EA, Brizuela L & Worland PJ Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res 56, 2973–2978 (1996). [PubMed] [Google Scholar]
- 111.Meijer L et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem 243, 527–536 (1997). [DOI] [PubMed] [Google Scholar]
- 112.Mapelli M et al. Mechanism of CDK5/p25 binding by CDK inhibitors. J. Med. Chem 48, 671–679 (2005). [DOI] [PubMed] [Google Scholar]
- 113.Wu J, Stoica BA & Faden AI Cell cycle activation and spinal cord injury. Neurotherapeutics 8, 221–228 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khalil HS, Mitev V, Vlaykova T, Cavicchi L & Zhelev N Discovery and development of Seliciclib. How systems biology approaches can lead to better drug performance. J. Biotechnol 202, 40–49 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Asghar U, Witkiewicz AK, Turner NC & Knudsen ES The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov 14, 130–146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dominguez JM et al. Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib. J. Biol. Chem 287, 893–904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martinez A, Alonso M, Castro A, Perez C & Moreno FJ First non-ATP competitive glycogen synthase kinase 3 β (GSK-3β) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J. Med. Chem 45, 1292–1299 (2002). [DOI] [PubMed] [Google Scholar]
- 118.DaRocha-Souto B et al. Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol. Dis 45, 425–437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sereno L et al. A novel GSK-3β inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis 35, 359–367 (2009). [DOI] [PubMed] [Google Scholar]
- 120.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00948259 (2009).
- 121.del Ser T et al. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. J. Alzheimers Dis 33, 205–215 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Lovestone S et al. A phase II trial of tideglusib in Alzheimer’s disease. J. Alzheimers Dis 45, 75–88 (2015). [DOI] [PubMed] [Google Scholar]
- 123.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01049399 (2012).
- 124.Tolosa E et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov. Disord 29, 470–478 (2014). [DOI] [PubMed] [Google Scholar]
- 125.Stambolic V, Ruel L & Woodgett JR Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol 6, 1664–1668 (1996). [DOI] [PubMed] [Google Scholar]
- 126.Klein PS & Melton DA A molecular mechanism for the effect of lithium on development. Proc. Natl Acad. Sci. USA 93, 8455–8459 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Forlenza OV, De-Paula VJ & Diniz BS Neuroprotective effects of lithium: implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem. Neurosci 5, 443–450 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Forlenza OV et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br. J. Psychiatry 198, 351–356 (2011). [DOI] [PubMed] [Google Scholar]
- 129.Nunes MA, Viel TA & Buck HS Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr. Alzheimer Res 10, 104–107 (2013). [DOI] [PubMed] [Google Scholar]
- 130.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02601859 (2015).
- 131.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02862210 (2017).
- 132.Min SW et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med 21, 1154–1162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02422485 (2017).
- 134.Sandhu P et al. Pharmacokinetics and pharmacodynamics to support clinical studies of MK-8719: an O-GlcNAcase inhibitor for progressive supranuclear palsy. Alzheimers Dement 12 (Suppl.), P1028 (2016). [Google Scholar]
- 135.[No authors listed] Alectos Therapeutics announces FDA orphan drug designation for MK-8719: an investigational small-molecule OGA inhibitor for treatment of progressive supranuclear palsy. Alectoshttp://alectos.com/content/alectos-therapeutics-announces-fda-orphan-drug-designation-mk-8719-investigational-small-molecule-oga-inhibitor-treatment-progressive-supranuclear-palsy/ (2016). [Google Scholar]
- 136.Rohn TT The role of caspases in Alzheimer’s disease; potential novel therapeutic opportunities. Apoptosis 15, 1403–1409 (2010). [DOI] [PubMed] [Google Scholar]
- 137.Panza F et al. Tau-centric targets and drugs in clinical development for the treatment of Alzheimer’s disease. Biomed Res. Int 2016, 3245935 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wischik CM, Edwards PC, Lai RY, Roth M & Harrington CR Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl Acad. Sci. USA 93, 11213–11218 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01253122 (2010).
- 140.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT00515333 (2008). [DOI] [PubMed]
- 141.Wischik CM et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J. Alzheimers Dis 44, 705–720 (2015). [DOI] [PubMed] [Google Scholar]
- 142.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT00684944 (2012).
- 143.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01689233 (2018).
- 144.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01689246 (2018).
- 145.Gauthier S et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388, 2873–2884 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fagan T In first phase 3 trial, the tau drug LMTM did not work. Period. Alzforumhttp://www.alzforum.org/news/conference-coverage/first-phase-3-trial-tau-drug-lmtm-did-not-work-period#show-more (2016). [Google Scholar]
- 147.Fagan T Tau inhibitor fails again — subgroup analysis irks clinicians at CTAD. Alzforumhttp://www.alzforum.org/news/conference-coverage/tau-inhibitor-fails-again-subgroup-analysis-irks-clinicians-ctad (2016). [Google Scholar]
- 148.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02245568 (2018).
- 149.Hu S et al. Clinical development of curcumin in neurodegenerative disease. Expert Rev. Neurother 15, 629–637 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hamaguchi T, Ono K & Yamada M Review: Curcumin and Alzheimer’s disease. CNS Neurosci. Ther 16, 285–297 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT00164749 (2008). [DOI] [PubMed]
- 152.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT00099710 (2009).
- 153.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT00595582 (2012).
- 154.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01383161 (2017).
- 155.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01811381 (2018).
- 156.Bollag DM et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res 55, 2325–2333 (1995). [PubMed] [Google Scholar]
- 157.Brunden KR et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci 30, 13861–13866 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang B et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci 32, 3601–3611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barten DM et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J. Neurosci 32, 7137–7145 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01492374 (2014).
- 161.Magen I & Gozes I Microtubule-stabilizing peptides and small molecules protecting axonal transport and brain function: focus on davunetide (NAP). Neuropeptides 47, 489–495 (2013). [DOI] [PubMed] [Google Scholar]
- 162.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01110720 (2013).
- 163.Boxer AL et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol 13, 676–685 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01966666 (2013).
- 165.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02133846. (2016).
- 166.Fitzgerald DP et al. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol. Cancer Ther 11, 1959–1967 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McQuade JL et al. A phase I study of TPI 287 in combination with temozolomide for patients with metastatic melanoma. Melanoma Res 26, 604–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mitchell D et al. A phase 1 trial of TPI 287 as a single agent and in combination with temozolomide in patients with refractory or recurrent neuroblastoma or medulloblastoma. Pediatr. Blood Cancer 63, 39–46 (2016). [DOI] [PubMed] [Google Scholar]
- 169.Wachtel H Potential antidepressant activity of rolipram and other selective cyclic adenosine 3’,5’-monophosphate phosphodiesterase inhibitors. Neuropharmacology 22, 267–272 (1983). [DOI] [PubMed] [Google Scholar]
- 170.Myeku N et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP–PKA signaling. Nat. Med 22, 46–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02648672 (2017).
- 172.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02840279 (2017).
- 173.Lobello K, Ryan JM, Liu E, Rippon G & Black R Targeting beta amyloid: a clinical review of immunotherapeutic approaches in Alzheimer’s disease. Int. J. Alzheimers Dis 2012, 628070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Valera E, Spencer B & Masliah E Immunotherapeutic approaches targeting amyloid-β, α-synuclein, and tau for the treatment of neurodegenerative disorders. Neurotherapeutics 13, 179–189, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Abushouk AI et al. Bapineuzumab for mild to moderate Alzheimer’s disease: a meta-analysis of randomized controlled trials. BMC Neurol 17, 66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Penninkilampi R, Brothers HM & Eslick GD Safety and efficacy of anti-amyloid-β immunotherapy in Alzheimer’s disease: a systematic review and meta-analysis. J. Neuroimmune Pharmacol 12, 194–203 (2017). [DOI] [PubMed] [Google Scholar]
- 177.Wisniewski T & Drummond E Developing therapeutic vaccines against Alzheimer’s disease. Expert Rev. Vaccines 15, 401–415 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Panza F, Solfrizzi V, Imbimbo BP & Logroscino G Amyloid-directed monoclonal antibodies for the treatment of Alzheimer’s disease: the point of no return? Expert Opin. Biol. Ther 14, 1465–1476 (2014). [DOI] [PubMed] [Google Scholar]
- 179.Serrano-Pozo A et al. Beneficial effect of human anti-amyloid-β active immunization on neurite morphology and tau pathology. Brain 133, 1312–1327 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boche D, Denham N, Holmes C & Nicoll JA Neuropathology after active Aβ42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol 120, 369–384 (2010). [DOI] [PubMed] [Google Scholar]
- 181.Boche D et al. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Aβ42 immunisation in Alzheimer’s disease. Acta Neuropathol 120, 13–20 (2010). [DOI] [PubMed] [Google Scholar]
- 182.Blennow K et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch. Neurol 69, 1002–1010 (2012). [DOI] [PubMed] [Google Scholar]
- 183.Salloway S et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73, 2061–2070 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vandenberghe R et al. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res. Ther 8, 18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Asuni AA, Boutajangout A, Quartermain D & Sigurdsson EM Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci 27, 9115–9129 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Boutajangout A, Ingadottir J, Davies P & Sigurdsson EM Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem 118, 658–667 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Asuni AA, Quartermain D, Sigurdsson EM Tau-based immunotherapy for dementia. Alzheimer Dement 2, S40–S41 (2006). [Google Scholar]
- 188.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM Passive tau immunotherapy diminishes functional decline and clears tau aggregates in a mouse model of tauopathy. Alzheimers Dement 6 (Suppl.), S578 (2010). [Google Scholar]
- 189.Bi M, Ittner A, Ke YD, Gotz J & Ittner LM Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PloS ONE 6, e26860 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Theunis C et al. Efficacy and safety of a liposome-based vaccine against protein tau, assessed in tau.P301L mice that model tauopathy. PloS ONE 8, e72301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Boutajangout A, Quartermain D & Sigurdsson EM Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J. Neurosci 30, 16559–16566, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Troquier L et al. Targeting phospho-Ser422 by active tau immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr. Alzheimer Res 9, 397–405 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rajamohamedsait H, Rasool S, Rajamohamedsait W, Lin Y, & Sigurdsson EM Prophylactic active tau immunization leads to sustained reduction in both tau and amyloid-b pathologies in 3xTg mice. Sci. Rep 7, 17034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Boimel M et al. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp. Neurol 224, 472–485 (2010). [DOI] [PubMed] [Google Scholar]
- 195.Davtyan H et al. MultiTEP platform-based DNA epitope vaccine targeting N-terminus of tau induces strong immune responses and reduces tau pathology in THY-Tau22 mice. Vaccine 35, 2015–2024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Selenica ML et al. Epitope analysis following active immunization with tau proteins reveals immunogens implicated in tau pathogenesis. J. Neuroinflammation 11, 152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kontsekova E, Zilka N, Kovacech B, Novak P & Novak M First-in-man tau vaccine targeting structural determinants essential for pathological tau–tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res. Ther 6, 44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ando K et al. Vaccination with Sarkosyl insoluble PHF-tau decrease neurofibrillary tangles formation in aged tau transgenic mouse model: a pilot study. J. Alzheimers Dis 40 (Suppl. 1), S135–S145 (2014). [DOI] [PubMed] [Google Scholar]
- 199.Rosenmann H et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch. Neurol 63, 1459–1467 (2006). [DOI] [PubMed] [Google Scholar]
- 200.Rozenstein-Tsalkovich L et al. Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. Exp. Neurol 248, 451–456 (2013). [DOI] [PubMed] [Google Scholar]
- 201.Chai X et al. Passive immunization with anti-tau antibodies in two transgenic models: reduction of tau pathology and delay of disease progression. J. Biol. Chem 286, 34457–34467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Congdon EE, Gu J, Sait HB & Sigurdsson EM Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance. J. Biol. Chem 288, 35452–35465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Congdon EE et al. Affinity of tau antibodies for solubilized pathological tau species but not their immunogen or insoluble Tau aggregates predicts in vivo and ex vivo efficacy. Mol. Neurodegener 11, 62–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gu J, Congdon EE & Sigurdsson EM Two novel tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce tau protein pathology. J. Biol. Chem 288, 33081–33095 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ittner A et al. Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice. J. Neurochem 132, 135–145 (2015). [DOI] [PubMed] [Google Scholar]
- 206.Krishnamurthy PK, Deng Y & Sigurdsson EM Mechanistic studies of antibody-mediated clearance of tau aggregates using an ex vivo brain slice model. Front. Psychiatry 2, 59 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu W et al. Vectored intracerebral immunization with the anti-tau monoclonal antibody PHF1 markedly reduces tau pathology in mutant tau transgenic mice. J. Neurosci 36, 12425–12435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sankaranarayanan S et al. Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PloS ONE 10, e0125614 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Subramanian S, Savanur G & Madhavadas S Passive immunization targeting the N-terminal region of phosphorylated tau (residues 68–71) improves spatial memory in okadaic acid induced tauopathy model rats. Biochem. Biophys. Res. Commun 483, 585–589 (2017). [DOI] [PubMed] [Google Scholar]
- 210.Dai CL et al. Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies. J. Neural Transm. (Vienna) 122, 607–617 (2015). [DOI] [PubMed] [Google Scholar]
- 211.Yanamandra K et al. Anti-tau antibody administration increases plasma tau in transgenic mice and patients with tauopathy. Sci. Transl. Med 9, eaal2029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kfoury N, Holmes BB, Jiang H, Holtzman DM & Diamond MI Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem 287, 19440–19451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yanamandra K et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80, 402–414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dai CL, Tung YC, Liu F, Gong CX & Iqbal K Tau passive immunization inhibits not only tau but also Aβ pathology. Alzheimers Res. Ther 9, 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bright J et al. Human secreted tau increases amyloid-beta production. Neurobiol. Aging 36, 693–709 (2015). [DOI] [PubMed] [Google Scholar]
- 216.Castillo-Carranza DL et al. Passive immunization with tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J. Neurosci 34, 4260–4272 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Castillo-Carranza DL et al. Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J. Alzheimers Dis 40 (Suppl. 1), S97–S111 (2014). [DOI] [PubMed] [Google Scholar]
- 218.d’Abramo C, Acker CM, Jimenez HT & Davies P Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PloS ONE 8, e62402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kondo A et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523, 431–436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.d’Abramo C, Acker CM, Jimenez H, & Davies P Passive immunization in JNPL3 transgenic mice using an array of phospho-tau specific antibodies. PLoS ONE 10, e0135774 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Walls KC, et al. p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD mice. Neurosci. Lett 575, 96–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee SH et al. Antibody-mediated targeting of tau in vivo does not require effector function and microglial engagement. Cell Rep 16, 1690–1700 (2016). [DOI] [PubMed] [Google Scholar]
- 223.Umeda T et al. Passive immunotherapy of tauopathy targeting pSer413-tau: a pilot study in mice. Ann. Clin. Transl. Neurol 2, 241–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Collin L et al. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain 137, 2834–2846 (2014). [DOI] [PubMed] [Google Scholar]
- 225.Nisbet RM et al. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain 140, 1220–1330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ising C et al. AAV-mediated expression of anti-tau scFvs decreases tau accumulation in a mouse model of tauopathy. J. Exp. Med 214, 1227–1238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pedersen JT & Sigurdsson EM Tau immunotherapy for Alzheimer’s disease. Trends Mol. Med 21, 394–402 (2015). [DOI] [PubMed] [Google Scholar]
- 228.Walker LC, Diamond MI, Duff KE & Hyman BT Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol 70, 304–310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sigurdsson EM Immunotherapy targeting pathological tau protein in Alzheimer’s disease and related tauopathies. J. Alzheimers Dis 15, 157–168 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Krishnaswamy S et al. Antibody-derived in vivo imaging of tau pathology. J. Neurosci 34, 16835–16850 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fuller JP, Stavenhagen JB & Teeling JL New roles for Fc receptors in neurodegeneration — the impact on immunotherapy for Alzheimer’s disease. Front. Neurosci 8, 235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.van der Kleij H et al. Evidence for neuronal expression of functional Fc (ε and γ) receptors. J. Allergy Clin. Immunol 125, 757–760 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.McEwan WA et al. Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. Proc. Natl Acad. Sci. USA 114, 574–579 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Medina M & Avila J The role of extracellular tau in the spreading of neurofibrillary pathology. Front. Cell. Neurosci 8, 113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sigurdsson EM, Wisniewski T & Frangione B Infectivity of amyloid diseases. Trends Mol. Med 8, 411–413 (2002). [DOI] [PubMed] [Google Scholar]
- 236.Herukka SK et al. Amyloid-β and tau dynamics in human brain interstitial fluid in patients with suspected normal pressure hydrocephalus. J. Alzheimers Dis 46, 261–269 (2015). [DOI] [PubMed] [Google Scholar]
- 237.Croft CL et al. Membrane association and release of wild-type and pathological tau from organotypic brain slice cultures. Cell Death Dis 8, e2671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sigurdsson EM Tau immunotherapy. Neurodegener. Dis 16, 34–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yanamandra K et al. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann. Clin. Transl. Neurol 2, 278–288 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kontsekova E, Zilka N, Kovacech B, Skrabana R & Novak M Identification of structural determinants on tau protein essential for its pathological function: novel therapeutic target for tau immunotherapy in Alzheimer’s disease. Alzheimers Res. Ther 6, 45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT01850238 (2015).
- 242.Novak P et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol 16, 123–134 (2017). [DOI] [PubMed] [Google Scholar]
- 243.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02031198 (2017).
- 244.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02579252 (2017).
- 245.Ondrus M & Novak P Design of the phase II clinical study of the tau vaccine AADvac1 in patients with mild Alzheimer’s disease. Neurobiol. Aging 39 (Suppl. 1), S26 (2016). [Google Scholar]
- 246.International Clincal Trials Registry Platform. ISRCTN.comhttp://www.isrctn.com/ISRCTN13033912 (2013).
- 247.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02281786 (2016).
- 248.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02294851 (2014).
- 249.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02460094. (2017).
- 250.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02658916 (2018).
- 251.[No authors listed] Biogen licenses phase 2 anti-tau antibody from Bristol-Myers Squibb. Biogenhttp://media.biogen.com/press-release/corporate/biogen-licenses-phase-2-anti-tau-antibody-bristol-myers-squibb (2017). [Google Scholar]
- 252.West T et al. Safety, tolerability and pharmacokinetics of ABBV-8E12, a humanized anti-tau monoclonal antibody, in a Phase I, single ascending dose, placebo-controlled study in subjects with progressive supranuclear palsy. J. Prev. Alzheimers Dis 3, 285 (2016). [Google Scholar]
- 253.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02985879 (2018).
- 254.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02880956 (2018).
- 255.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02820896 (2017).
- 256.Rogers MB Treating tau: finally, clinical candidates are stepping into the ring. Alzforumhttp://www.alzforum.org/news/conference-coverage/treating-tau-finally-clinical-candidates-are-stepping-ring (2017). [Google Scholar]
- 257.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02754830 (2018).
- 258.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT03019536 (2018).
- 259.Hayashi ML, Lu J, Driver D, Alvarado A Antibodies to tau and uses thereof US Patent Application publication no. US20160251420 A1 (2016).
- 260.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03375697 (2018).
- 261.Rogers MB To block tau’s proteopathic spread, antibody must attack its mid-region. Alzforumhttps://www.alzforum.org/news/conference-coverage/block-taus-proteopathic-spread-antibody-must-attack-its-mid-region (2018). [Google Scholar]
- 262.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03464227 (2018).
- 263.Congdon EE, Krishnaswamy S & Sigurdsson EM Harnessing the immune system for treatment and detection of tau pathology. J. Alzheimers Dis 40 (Suppl. 1), S113–S121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Barthelemy NR et al. Differential mass spectrometry profiles of tau protein in the cerebrospinal fluid of patients with Alzheimer’s disease, progressive supranuclear palsy, and dementia with lewy bodies. J. Alzheimers Dis 51, 1033–1043 (2016). [DOI] [PubMed] [Google Scholar]
- 265.Barthelemy NR et al. Tau protein quantification in human cerebrospinal fluid by targeted mass spectrometry at high sequence coverage provides insights into its primary structure heterogeneity. J. Proteome Res 15, 667–676 (2016). [DOI] [PubMed] [Google Scholar]
- 266.Giacobini E & Gold G Alzheimer disease therapy — moving from amyloid-β to tau. Nat Rev. Neurol 9, 677–686 (2013). [DOI] [PubMed] [Google Scholar]