Hemoglobin β93 cysteine is not required for export of nitric oxide bioactivity from the red blood cell (original) (raw)
. Author manuscript; available in PMC: 2020 Jun 4.
Abstract
Background:
Nitrosation of a conserved cysteine residue at position 93 in the hemoglobin β-chain (β93C) to form S-nitroso hemoglobin (SNO-Hb) is claimed to be essential for export of NO bioactivity by the red blood cell (RBC) to mediate hypoxic vasodilation and cardioprotection.
Methods:
To test this hypothesis we used RBCs from mice where the β93 cysteine had been replaced with alanine (β93A) in a number of ex vivo and in vivo models suitable for studying export of NO bioactivity.
Results:
In an ex vivo model of cardiac ischemia reperfusion (IR) injury, perfusion of a mouse heart with control RBCs (β93C) pre-treated with an arginase inhibitor to facilitate export of RBC NO bioactivity, improved cardiac recovery after IR injury and the response was similar with β93A RBCs. Next, when human platelets were co-incubated with RBCs and then deoxygenated in the presence of nitrite, export of NO bioactivity was detected as inhibition of ADP-induced platelet activation. This effect was the same in β93C and β93A RBCs. Moreover, vascular reactivity was tested in rodent aortas in the presence of RBCs pre-treated with S-nitrosocysteine, or with hemolysates or purified Hb treated with authentic NO to form nitrosyl(FeII)-Hb, the proposed precursor of SNO-Hb. SNO-RBCs or NO-treated Hb induced vasorelaxation, with no differences between β93C and β93A RBCs. Finally, hypoxic microvascular vasodilation was studied in vivo using a murine dorsal skin fold window model. Exposure to acute systemic hypoxia caused vasodilatation and the response was similar in β93C and β93A mice.
Conclusions:
RBCs clearly have the fascinating ability to export NO bioactivity but this occurs independently of SNO formation at β93 cysteine of Hb.
Keywords: Nitric oxide, nitrite, S-nitrosothiol, nitrosation, nitrosylation, hemoglobin
Introduction
The idea that red blood cells (RBCs) release nitric oxide (NO) bioactivity to control vascular function and blood flow is intriguing and has gained much interest among vascular biologists since originally proposed in 1996.1 According to this concept, NO present in the RBC first binds to the heme-iron in deoxygenated hemoglobin (Hb) thereby forming nitrosyl-Hb. Then, when Hb is oxygenated as it transits through the lungs, an NO+ moiety is transferred to a highly conserved cysteine residue on Hb (β93C) to form SNO-Hb. In turn, upon subsequent deoxygenation, SNO-Hb exports NO bioactivity from the RBC ultimately causing vasorelaxation.2–4 The elegance of this hypothesis is the tight coupling between RBC-induced vasorelaxation and blood deoxygenation thereby enabling exact matching of blood flow and tissue oxygen demand. With these findings the general concept of RBC physiology was expanded beyond oxygen and carbon dioxide transport to also include NO gas and therefore termed “a three gas system”.5
One general critique of the RBC/NO-bioactivity theory has been the assumed inability of NO to escape RBCs given the huge concentrations of Hb in these cells thereby effectively scavenging any NO produced. Oxygenated Hb reacts with NO extremely rapidly (with a bimolecular rate constant of 107 to 108 M−1s−1) to form methemoglobin and nitrate.6, 7 However, this shortcoming in the theory can be overcome by the fact that NO does not travel as free NO, but rather through transfer between small (and more stable) S-nitrosothiols.8 Indeed, this has been proposed and demonstrated by several groups. Moreover, recent studies do give strong support to the general ability of RBCs to export NO bioactivity, including the finding of an active endothelial NO synthase (eNOS) in the RBC9 capable of inducing NO-like effects when incubated in an isolated heart or vessel preparation10–12, and RBC-dependent inhibition of platelet activation by nitrite.13, 14
Essential to the theory described above is the presence of a highly conserved cysteine at position 93 of the beta chain of Hb (β93C), as discussed. The recent development of a humanized mouse model expressing wild-type human Hb (β93C) or human Hb in which the cysteine residue has been replaced with alanine (β93A) has provided additional insight, but results using this mouse model have been conflicting.15–17 Isbell and colleagues developed the mouse and in their study it surprisingly lacked a phenotype suggestive of a deficient hypoxic vasodilation or gross cardiovascular abnormalities.15 This study was criticized where it was noted that, among other things, vascular responses were mainly done in pulmonary vessels which respond differently to hypoxia.18 Later the same group published results suggestive of a severe cardiovascular phenotype in the β93A mice in response to hypoxia, but in that study actual NO export was not studied.16 Recently, several methods for robust detection of RBC NO-bioactivity export have been developed. These include stimulation of RBC NO generation and export by the use of arginase inhibitors19 or addition of nitrite to deoxygenated RBCs.13, 14 Here we decided to use RBCs and Hb from β93C and β93A mice in an attempt to further elucidate the role of this conserved residue in RBC-dependent stimulation of NO-signaling utilizing these novel approaches. Moreover, hypoxic vasodilation was directly studied in an extrapulmonary microvascular bed in an in vivo model using the same mice.
Material and methods
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Animals
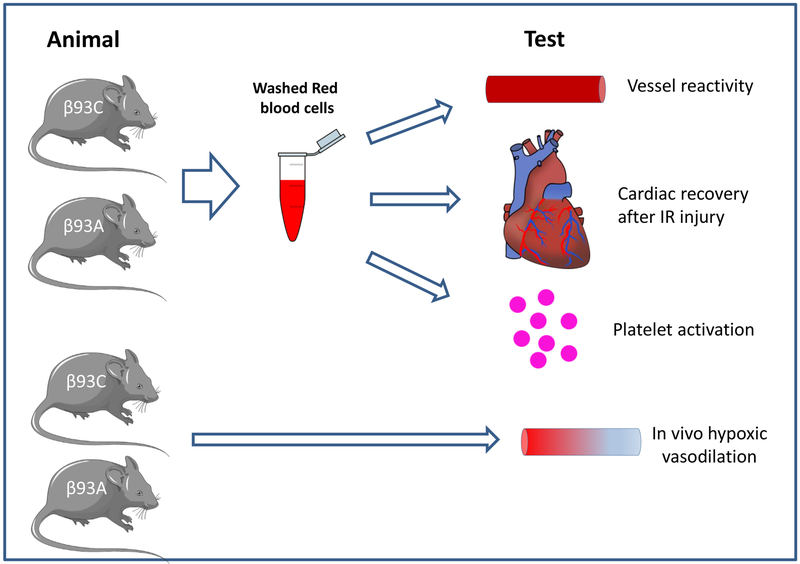
All animal studies were approved by the regional ethics committees. Male Sprague–Dawley rats (200–250 g) were purchased from Harlan (Indianapolis, IN). Transgenic mice with RBCs containing human Hb carrying an amino acid substitution (Cysteine to Alanine) at position 93 on the β-subunit and the corresponding wild type mice were developed as previously described15 and used at times when all gamma Hb has been replaced by beta Hb. All mice were allowed at least 10 days acclimation prior to any experiment. Wild type mice (C57Bl/6) for Langendorff heart and vessel preparations were housed at the animal facility at Karolinska Institutet. Animals were kept in ordinary cages, housed in a temperature and humidity-controlled room with 12/12 h light/dark cycle and feed with standard rodent chow and water ad libitum. The experimental settings for the different in vitro/ex vivo/in vivo approaches described below are displayed in Fig 1.
Figure 1. Scheme of approaches used to study export of NO bioactivity by red blood cells.
Washed red blood cells (RBCs) from control mice (β93C) or mutant mice (β93A) lacking the cysteine-93 on the β-chain of hemoglobin were used in 3 model systems known to respond to export of RBC NO bioactivity: i) isolated aortic dilation or contraction in response to NO- or S-nitrosothiol treated RBCs, ii) isolated mouse hearts perfused with RBCs prior to ischemia reperfusion injury and measuring cardiac recovery, iii) RBC and nitrite-dependent inhibition of platelets activation, iv) in vivo assessment of hypoxic vasodilation.
Isolated heart perfusion experiments
Mouse hearts (C57Bl/6 WT) were isolated and perfused in a Langendorff system as described previously.20, 21 Briefly, the ascending aorta was cannulated, and retrogradely perfused with gassed (5% CO2 and 95% O2) Krebs-Henseleit (KH) buffer at a constant pressure (75 mmHg) at 37°C. A balloon connected to a pressure transducer was inserted into the left ventricle through the left atrium for recording of isovolumetric left ventricular developed pressure (LVDP). Global ischemia was induced by clamping the inflow tubing for 40 min followed by reperfusion which was maintained for 60 min. Vehicle and nor-NOHA were diluted in RBCs suspension from β93C or β93A mice described below and incubated at 37 °C for 25 min before the RBCs suspension was injected into the coronary circulation of the isolated and perfused hearts at the start of ischemia. The investigator was blinded to the genotype of the mouse used for preparation of the RBCs suspension.
RBCs were isolated as previously described in detail.11 Briefly, blood was collected from the thoracic cavity after removal of the heart of anaesthetized mice. RBCs were isolated by removing the plasma and buffy coat from whole blood after centrifugation. The RBCs were washed 3 times in KH buffer and centrifuged and the RBCs were then diluted 1:1 with KH buffer.
Platelet activation experiments
Blood was collected from healthy volunteers after obtaining informed consent under an internal review group approved protocol at Wake Forest University. Platelet rich plasma (PRP) was prepared from citrated blood. Whole blood was centrifuged at 120 x g for 10 minutes and PRP was removed.
Whole blood, from β93C or β93A mice was collected and shipped overnight, on ice to Wake Forest University. RBCs were removed from mouse blood by centrifugation at 300 x g for five minutes. Plasma was discarded and erythrocytes were washed five times with PBS pH 7.4 (10 ml per wash) by centrifugation at 300 x g for five minutes. Erythrocytes were diluted with PBS to obtain 20% hematocrit and then deoxygenated under nitrogen at room temperature for one hour with gentle rocking.
PRP was diluted with PBS and RBCs giving a final hematocrit of 10% and PRP that was four-fold diluted compared to whole blood. To this cell suspension, 10 μM nitrite (final concentration) in deoxygenated PBS was added. The reaction mixture was incubated for five minutes at 37 °C. After five minutes of incubation, ADP was added in each reaction mixture (10 μM final ADP concentration) and was further incubated for six minutes at 37 °C. After six minutes of platelet activation monoclonal antibodies against CD 61 and PAC-1 were added. The assay mixture with antibodies was further incubated for fifteen minutes in the dark at room temperature. After fifteen minutes cells were fixed with 1% buffered formalin and kept in the refrigerator for analysis using flow cytometry
Platelet activation was analyzed in a BD FACS Calibur flow cytometer. Data collection and analysis were performed by Cell Quest Pro software.
Vascular reactivity in isolated vessels
Mouse vessels:
The vasoreactivity studies were performed as described.22 Briefly, aortas were isolated and mounted as ring preparations in a myograph (Danish MyoTech, Aarhus, Denmark). Vessels were allowed to stabilize and then contracted with KCl solution (120 mM) to confirm viability. After preconstriction of aortic rings, using PE (3 μM) and in some cases L-NAME (1 mM), cumulative doses of NO-treated hemolysate preparations from control and mutant mice, Hb or Hb(NO)4 (described below) were added to the aortas.
Hb(NO)4 preparation:
Different Hb(NO)4 preparations were prepared either from β93C-RBC hemolysate, β93A-RBC hemolysate or dithionite-reduced human Hb (Sigma-Aldrich, H7379). The Hb solutions (10ml; 1mM in heme; pH7.4) were deoxygenated in a vacuum/N2-gas system for 1 hr, then treated under agitation with pure NO-gas (1 atm; 5 min) and further evacuated (5 min) to remove dissolved but unbound NO. The electron paramagnetic resonance (EPR) spectra of the Hb(NO)4 solutions were recorded at 77K using an X-band spectrometer MS5000 (Magnettech, Berlin). The EPR analysis of Hb(NO)4 preparations showed that under these conditions >95% of the Hb heme groups were nitrosylated. The Hb(NO)4 solutions were frozen/kept in liquid nitrogen until used.
Rat vessels:
Thoracic aortas were isolated from male Sprague-Dawley rats (200–250g) and used as described previously.23 After two rounds of KCl-induced contractions followed by washing and 30 min equilibration, vessels were equilibrated with 21% or 1% oxygen in KH buffer at 37°C in the presence of 5% CO2. Vessels were pretreated with indomethacin (5 μM) and N-monomethyl-L-arginine (L-NMMA; 100μM) and precontracted with phenylephrine (200 nM at 21% oxygen tension and 400 nM at 1% oxygen tension) before the addition of S-nitrosated RBCs (SNO-RBC).
SNO-RBC preparation:
RBCs from β93C and β93A mice were collected by cardiac puncture in sodium citrate coated tubes, pelleted and washed 3 times with PBS containing DTPA (100 μM) at 4°C. All subsequent procedures were performed in low-light environment to prevent photolysis of SNO’s. S-nitrosocysteine (SNOC) was prepared fresh by mixing a solution of L-cysteine (in water) with sodium nitrite (150mM each final concentrations). After vortex mixing (30 sec), the reaction mixture was filtered using a sephadex G-25 pre-equilabrated with PBS + DTPA (100 μM). The concentration of eluted SNOC was measured using ε337nm = 900 M−1 cm−1 and then used immediately for S-nitrosating RBCs. Hemoglobin concentration was determined in packed RBCs and then RBCs diluted to 2% Hct or 400 μM heme in PBS + DTPA (100 μM) and SNOC added at a 1:1 ratio with heme. The reaction mixture was placed on a rocker for gentle mixing for 5 min, room temperature and then RBC pelleted (1000 × g, 3 min, 4°C) and washed twice with ice-cold PBS + DTPA (100 μM). RBC were then used to assess vasodilation of rat aortic rings as described above. In parallel aliquots, RBCs were lysed and high and low MWt fractions prepared by gel-filtration chromatography using Sephadex G-25 as previously described.15 The concentration of S-nitrosothiols was measured using the Saville assay and normalized to heme concentration as previously described.23, 24
Dorsal skin fold window model for assessment of hypoxic vasodilation in vivo
Window chamber preparation:
The complete surgical technique for this preparation has been previously described in detail and allows for the non-invasive study of the microcirculation in an intact subcutaneous tissue and a retractor muscle.25, 26 The window chamber implantation was performed seven to ten days prior to the study. Animals were reanesthetized two to four days prior to the experiment and their right carotid artery cannulated (PE-10 tubing). The catheter was tunneled under the skin and then exteriorized at the base of the window chamber.
Systemic Parameters.
Mean arterial pressure (MAP) and heart rate (HR) were monitored via the carotid catheter connected to a pressure transducer system (MP 150, Biopac System; Santa Barbara, CA). Systemic hematocrit and Hb were measured using a microhematocrit centrifuge and a handheld photometer (Hemocue, Sweden). Arterial blood was collected from the carotid artery catheter in heparinized glass capillaries (0.05 ml) and immediately analyzed for arterial PO2 (Blood Chemistry Analyzer 248, Siemens, Germany).
Microvessel Diameter:
Video image-shearing was used to measure vessel diameter (Image Shearing Monitor, Vista Electronics, San Diego, CA)27. Changes in arteriolar diameter from baseline were used as indicators of changes in vascular tone. Arterioles (3 – 10 each animal) were chosen for study by their size (diameter between 40 and 60 μm) and the visual sharpness of their edges. Vessels chosen for study at baseline were followed throughout the experiment; thus allowing for pairwise comparisons.
Experimental Design:
Awake mice were restrained in a plexiglas tube during the experiment. Prewarmed gas was gently blown (flow rate 2.6 l/minute) into the face of the animal through a diffuser connected at the front of the tube. The tube containing the conscious animal was then affixed onto a microscopic stage of an intravital microscope (BX51WI, Olympus, New York, 40X objective, n.a. 0.7 SW). The tissue image was projected onto a CCD camera (4815–2000, COHU, San Diego) and viewed on a monitor.
Experimental Protocol:
Animals were placed into a restraining tube which was then positioned on the microscope stage and allowed at least a 20 min adjustment period to become accustomed to the tube prior to measuring baseline and systemic parameters. The protocol steps were as follows: 1) Baseline (BL). Time = 0 min. Systemic (MAP, HR and blood gases) and arteriolar vessel diameter measurements were performed with the animal exposed to room air (FiO2 = 0.21). 2) Hypoxia (H). Time = 0 min. Animal was exposed to FiO2 = 0.10, balanced with N2. After 5 min for stabilization of the mean arterial pressure, the systemic and microvascular parameters were assessed. Arterial blood gases and chemistry were assessed to confirm the animal was hypoxic. Animals were considered hypoxic when arterial PO2 < 40 mmHg.
Statistical Analysis
Data is presented as mean ± SEM. Recovery from IR heart injury (i.e. Langendorff preparation) and isolated vascular responses (i.e. myograph) was analyzed by two-way repeated ANOVA, followed by appropriate post hoc analysis. Data on platelet activation was analysed by a two tailed paired t-test. For in vivo microvascular measurements, the diameter from 3–10 vessels were measured individually and then averaged to compute a single per mouse replicate. Differences within groups were first tested with one-way ANOVA for repeated measures and if significance was obtained then multiple comparisons between groups were performed using Tukey’s multiple comparison. Paired t-test was used to determine if hypoxic vasodilation was induced as a result of the reduction of FiO2. n denotes the number of animals studied. Changes were considered statistically significant if p<0.05. Statistics were performed using Prism version 6.0 for Windows (GraphPad, San Diego, CA).
Results
RBC-mediated cardioprotection ex vivo occurs independently of β93C
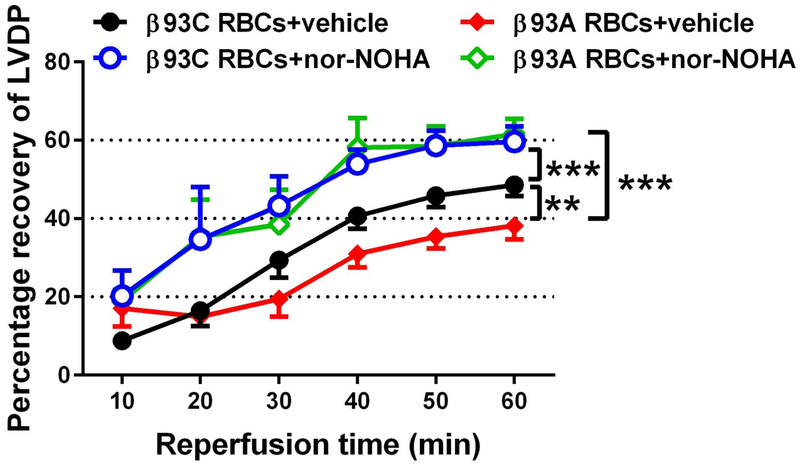
We have previously showed that inhibition of arginase in RBCs increases eNOS-dependent transfer of NO bioactivity to the ischemic heart, resulting in NO-dependent cardioprotection.10 Here we used this approach to test if β93C was of importance for such NO bioactivity export. Hearts from WT mice were used throughout in the Langendorff preparation. The recovery of post-ischemic LVDP hearts given β93A RBCs was slightly lower compared to that of hearts given β93C RBCs (Fig. 2). Pre-incubation of RBCs from both β93C and β93A mice with the arginase inhibitor nor-NOHA resulted in marked improvement in the recovery of post-ischemic LVDP (Fig. 2).
Figure 2. Effects of β93C and β93A RBCs on the recovery of cardiac function following ischemia-reperfusion.
Hearts from wild type mice were given red blood cells (RBCs) from β93C mice incubated with vehicle (n=6) or nor-NOHA (n=5) or RBCs from β93A mice incubated with vehicle (n=5) or nor-NOHA (n=8). Nor-NOHA is an arginase inhibitor that induces export of NO bioactivity from RBCs. Data are shown as mean ± SEM. Significant differences between groups were analyzed using two-way ANOVA; **P < 0.01, ***P < 0.001. LVDP= Left Ventricular Developed Pressure.
RBC-induced inhibition of platelet activation occurs independently of β93C
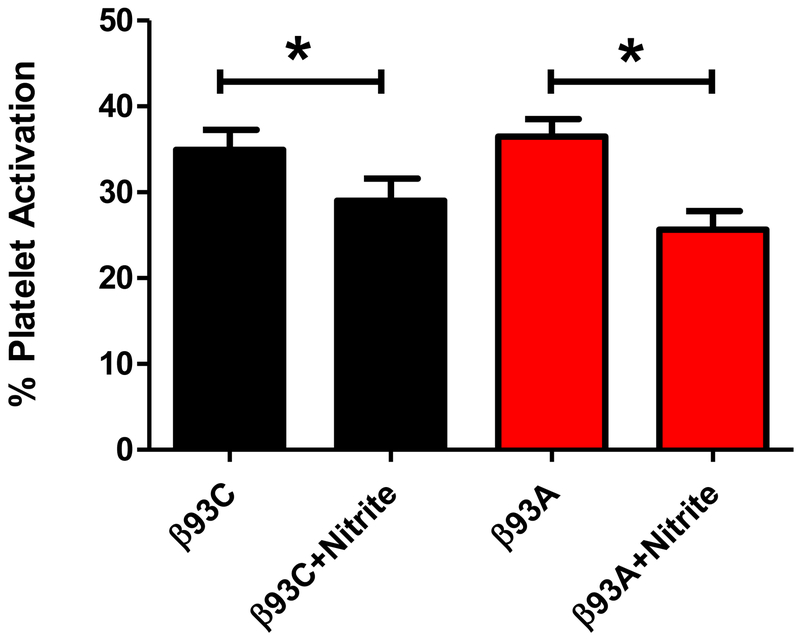
Several reports have shown that nitrite inhibits platelet activation in the presence, but not absence, of deoxygenated RBCs and that this action can be inhibited by an NO scavenger.28, 29 In order to assess the role of Hb β93C in nitrite-mediated inhibition of platelet activation by RBCs, we compared the ability of murine RBCs containing human β93C to those containing β93A. In the presence of nitrite, RBCs from both β93C and β93A mice significantly inhibited platelet activation, with the degree of inhibition being similar with each RBC. (Fig. 3). These data show that RBC-mediated bioactivation of nitrite does not require β93C.
Figure 3. β93C is not required for RBC-mediated inhibition of platelet activation by nitrite.
Murine red blood cells (RBCs) containing human hemoglobin β93C and mutant of β93C to β93A were used in the platelet activation assay at 10% hematocrit under partially deoxygenated conditions. Data show mean ± SEM, n=4. P values (*) were calculated using a paired t test: p< 0.03 between β93C with and without nitrite and p< 0.03 between β93A with and without nitrite.
NO-treated hemoglobin or SNOC-treated RBCs dilate vessels independently of β93C
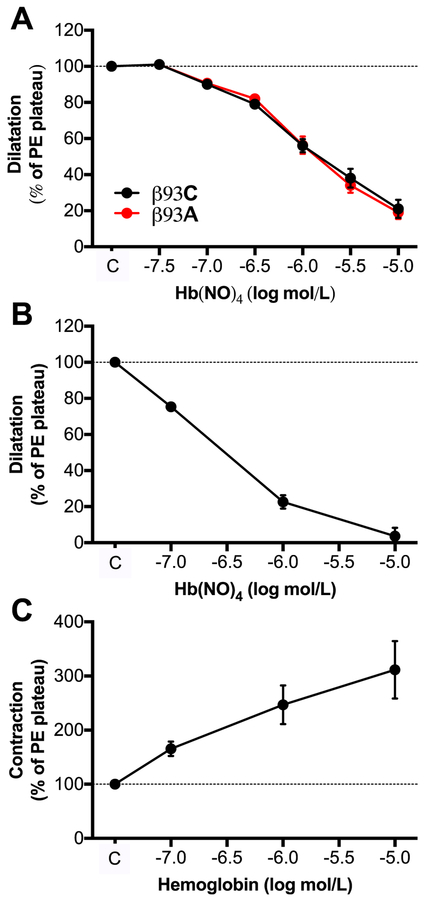
In an attempt to look closer into the processes within the Hb molecule that govern the transfer and release of NO bioactivity we used RBC hemolysates from control (β93C) and mutant (β93A) mice as well as purified human Hb. These preparations were treated with pure NO gas in the absence of oxygen in order to generate nitrosyl-heme, which was subsequently applied to PE-preconstricted mouse aortic segments to study vasodilator responses. NO-treated lysed RBCs dose-dependently dilated these vessels and there was clearly no difference between controls and mutant hemolysates (Fig. 4A). NO-treated purified Hb (i.e. Hb(NO)4) dilated vessels (Fig. 4B) while native free Hb constricted the vessels (Fig. 4C).
Figure 4. Responses of mouse aortae to hemolysates from of β93C and β93A RBCs or purified hemoglobin treated with NO gas.
Hemolysates from red blood cells (RBCs) of β93C or β93A mice (A) or purified human ferrohemoglobin (B) were treated with NO gas in the absence of oxygen and then tested on phenylephrine (PE) and L-NAME (1 mM, used only in A) preconstricted mouse aortae. Non-treated human ferrohemoglobin (C) was used as control. Data show mean ± SEM. n=3–6.
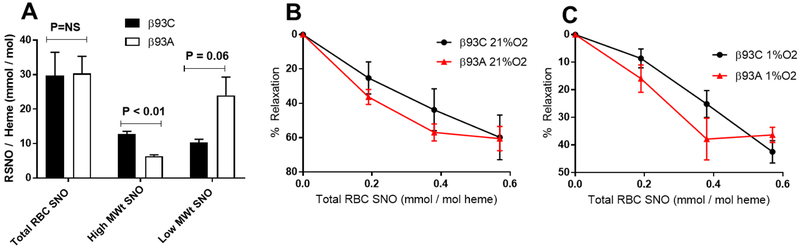
To evaluate RBC SNO-dependent dependent vasodilation, we treated RBC with S-nitroso cysteine (SNOC) to increase SNO concentrations. Fig 5A shows that SNOC treatment increased total S-nitrosothiol levels to similar extents in both RBCs. However, the distribution of SNO was different with high MWt SNO’s being decreased and low MWt SNO’s increased (both by approx. 2-fold) in β93A RBCs compared to β93C RBCs. SNO RBCs expressing β93C or β93A induced vasodilation in a dose-dependent manner at both high (Fig 5B) and low (Fig 5C) oxygen tensions, without any differences between the control and mutant RBCs.
Figure 5: Effects of β93C and β93A on SNO-RBC dependent vasodilation.
Panel A: S-nitrosothiol (SNO) concentrations normalized to heme in β93C and β93A red blood cells (RBCs) after reaction with S-nitroso cysteine. Data show total levels and levels after fractionating RBCs into high and low molecular weight (MWt) components. Data are mean ± SEM (n=3). P-values shown were calculated by unpaired t-test. Panel B and C: Rat aortic rings were incubated at 21% or 1% O2 respectively, and vasodilation assessed in response to increasing concentrations of SNO-β93C RBCs and SNO-β93A RBCs. Data are mean ± SEM (n=3).
Hypoxic vasodilation in skeletal muscle in vivo occurs independently of β93C
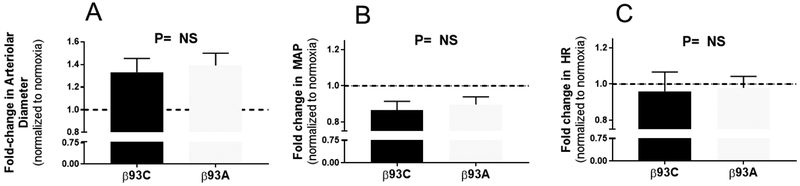
In a final series of experiments, the responses in skeletal muscle blood flow to global hypoxia were tested in the dorsal skin fold window model. This model allows interrogation of hypoxic vasodilation in awake mice without the influence of anesthesia. Hypoxia significantly increased arteriolar diameter in β93C or β93A mice, respectively (p<0.001, Fig. 6A); the magnitude of the hypoxic vasodilatory response was between 30–40% and not significantly different between β93C or β93A mice. Moreover, hypoxia significantly decreased MAP in both mice (p<0.001, Fig. 6B), with no differences between β93C and β93A mice noted. Finally, no significant effect of hypoxia on heart rate was observed in either β93C or β93A mice (Fig. 6C).
Figure 6: In vivo effects of hypoxia on microvascular and hemodynamic parameters in β93C and β93A mice.
β93C or β93A mice were exposed to global hypoxia and changes in arteriolar diameter (Panel A), mean arterial pressure (MAP, Panel B) and heart rate (HR, Panel C) were measured. Data show fold changes relative to normoxia and mean ± SEM (n=13–14). P value was not significant (NS) by unpaired t-test. Changes in MAP and Arterial diameter in response to hypoxia were significant in both groups of mice (p<0.001, students t test).
Discussion
Using multiple approaches we here confirm the fascinating ability of RBCs to export NO bioactivity. However, our results clearly suggest that the release of this NO bioactivity occurs independently of the presence of β93C in Hb. Thus, NO bioactivity measured as ex vivo cardioprotection, inhibition of platelet activation, or vessel relaxation, was entirely intact when using RBCs or hemolysates from mice lacking the β93C in Hb. Moreover, acute hypoxia-induced vasodilation observed in vivo was similar in β93A and β93C mice. In all three ex vivo models used here, the dependency of NO for the responses has been previously verified. As an example, the cardioprotective effects of RBCs in the ex vivo Langendorff preparation is lost when using RBCs from eNOS-deficient mice.10 A strength of the present study is that several different vascular beds were studied (coronary circulation, isolated aortae, skeletal muscle microcirculation) and in each case we found evidence for RBC-mediated release of NO bioactivity under deoxygenated conditions.
In the presence of nitrite, murine RBCs containing human β93A inhibited platelet activation to an extent similar to that observed when using RBCs containing human β93C. We and others have previously shown that nitrite-mediated inhibition of platelet activation requires the presence of RBCs (in the absence of RBCs nitrite has no effect), and this action is abrogated by an NO scavenger.28–30 These data strongly support the notion that RBCs can export NO bioactivity and that β93C is not required in this process. Our data contrast to those of another report that suggested that nitrite may induce export of RBC NO bioactivity by acting as a substrate for S-nitrosation of Hb at β93C.31 It should, however, be noted that our platelet activation studies do not subject the RBCs to oxygen tension cycling that was proposed in the original theory of SNO-Hb-mediated NO export by RBCs.1 Our samples were partially deoxygenated and the addition of nitrite forms nitrosyl-Hb, but the samples remained largely deoxygenated throughout the experiment. Therefore, looking at the results from this experiment alone, one cannot exclude the additional presence of RBC-mediated NO bioactivity involving also S-nitrosation of Hb at β93C. We can only conclude that RBC-mediated bioactivation of nitrite, as measured by inhibition of platelet activation, can proceed without the formation of SNO-Hb and without cycling of oxygen tension.
For the experiments using exogenous nitrite, non-physiological amounts of NO gas or treatment with SNOC, one could argue that thiols other than β93C on the Hb molecule are overwhelmingly S-nitrosated to further transduce NO bioactivity. However, these limitations cannot apply to the isolated heart experiments involving arginase inhibition as with this approach endogenously formed NO from RBC eNOS is simply allowed to proceed freely. The RBCs were present in the coronary circulation during the ischemia which should create optimal deoxygenated conditions for the release of NO bioactivity from the β93C according to the original hypothesis.1 Even in this setting however, export of NO bioactivity from the RBC was intact in the absence of β93C.
We also tested the ex vivo vascular responses of mouse aortae following exposure to purified human Hb pretreated with NO to form nitrosyl-Hb. NO-treated Hb dose-dependently dilated vessels whereas addition of untreated Hb caused strong vasoconstriction. Again, one could argue that in our experimental models cysteines other than β93 on Hb are first nitrosated and then transduce NO bioactivity via transnitrosation reactions to low molecular weight thiols. However, this seems unlikely given the fact that horse myoglobin, which contains no cysteine residues, also caused dilatations if pretreated with NO (unpublished data). Notably, this in vitro approach does not represent physiologically relevant conditions in terms of NO amounts, hematocrit etc. It should rather be viewed as a mechanistic experiment demonstrating that NO-treated hemoglobin indeed dilates vessels even in the absence of β93C.
What is then causing the release of NO bioactivity in the experiments above? It could occur through the release of the entire NO-heme complex as recently suggested32 or in the experiments using RBCs, through direct S-nitrosation of small molecular thiols without intermediate SNO-Hb formation. In support of the former are early observations by Ignarro showing activation of sGC involving NO-heme exchange.33 To further explore and specifically test SNO-dependent vasodilation, RBCs were treated with SNOC to increase S-nitrosothiol levels before being tested for vasodilating properties. Despite high MWt SNO’s being lower in β93A RBCs, no differences between SNO-RBC dependent vasodilation were observed under normoxic or hypoxic conditions.
Cortese-Krott and colleagues recently reported the identification of an intact sGC signaling pathway in human RBCs.34 Thus, another possibility regarding the nature of the NO bioactivity exported from RBCs is that cGMP or other downstream mediators of NO signaling are directly released from these cells rather than an NO related species. Further studies are needed to explore this possibility but theoretically, the phenomena studied here could be caused by an NO dependent activation of sGC within the RBC.
The development of the mouse lacking β93C has been a helpful tool when trying to elucidate the role of this amino acid residue in RBC physiology. However, the results published have been conflicting. Isbell and colleagues developed the mouse model and first reported that it lacked a phenotype suggestive of impaired vascular homeostasis.15 Here we expand these data using the intact murine dorsal skin fold window model. As expected, acute hypoxia induced vasodilation which was the same in mice expressing or lacking the β93C suggesting that SNO-Hb is not required for hypoxic dilation of arterioles in the skeletal muscle microvasculature.
Zhang et al recently demonstrated severe cardiac dysfunction and premature death in the same mutant mice and attributed these pathologies to the lack of NO bioactivity exported from the RBC16. The same group also looked at skeletal muscle blood flow in response to systemic hypoxia and found that blood flow was better maintained during hypoxia in the β93C expressing mice compared to the β93A mutant mice17 implying a defect hypoxic vasodilation in the latter. The authors used change in blood flow as indicator of vessel tension, but actual dilatation (i.e. change in vessel diameter) was not measured. This is problematic since blood flow is determined primarily by vessel diameter (resistance) and blood pressure difference (here blood flow = ΔP/Resistance). Therefore, changes in blood flow cannot be solely attributed to changes in resistance. With this in mind and when looking further at the data presented by Zhang and colleagues, it is apparent that blood pressure and cardiac output were increased in their control mice at the same inspired oxygen concentrations (FiO2) as in our study (i.e. 0.10) while muscle blood flow was unchanged. In the β93A mice, blood pressure and cardiac output decreased during the graded hypoxia as did skeletal muscle blood flow. This suggests contraction in control vessels (increased resistance) rather than vasodilatation in response to the global hypoxia. In aggregate, the poor blood flow in skeletal muscle reported by Zhang and colleagues in β93A mice during global hypoxia is likely primarily driven by an impaired cardiac contractility rather than an altered hypoxic vasodilation in peripheral tissues. Indeed, phenotypically this is exactly what the authors have seen in their published studies using these mice.16, 17 In contrast to Zhang et al. we fail to see acute signs of cardiodepression when the β93A animals breathe 10% oxygen. We are unsure why the results differ but one possibility is the fact that we used awake animals whereas Zhang used anesthetized ones. Thus, if the mice already have borderline cardiac failure, the additional stress imparted by anesthesia (Avertin used by Zhang et al. is a known cardio-depressant) may tip them over so that they cannot uphold a normal cardiac output, explaining the reduced blood flow observed.
Further studies are clearly needed to pinpoint the reason for the proposed cardiac failure in mice lacking the β93-cysteine of Hb. At this stage one can only speculate on such alternative mechanisms not involving SNO-Hb formation. We have noted that RBCs lacking β93C are more susceptible to oxidative damage indexed by higher concentration of ferryl-Hb and protein radicals after exposure to H2O2 ex vivo, and increased protein radicals and carbonyls in RBCs at baseline and after LPS challenge in vivo.35 Other studies have also identified the β93Cys as a redox ‘hot-spot’ important in dissipating oxidizing equivalents formed in the RBC.36 In fact, studies from the 1970s, ie 20 years before the SNO-Hb theory was released already suggested that the hemoglobin β−93 cysteine plays a protective role for the heme iron against oxidation.37, 38 It is possible that loss of an endogenous antioxidant residue has effects on cardiac function independently of SNO formation. Consistent with this hypothesis, β93A mice had a greater extent of acute lung injury and oxidative damage in the lungs after LPS exposure, compared to β93C mice.35 Moreover, the concept of reduced capacity to neutralize reactive oxygen species by RBCs leading to endothelial and cardiac dysfunction was recently supported by studies where RBCs from diabetic patients induced vascular dysfunction when co-incubated with human vessels in vitro12 and aggravated cardiac dysfunction when administered to an ischemic rat heart ex vivo.11
In conclusion, this study reinforces the fascinating ability of RBCs to release NO-bioactivity but clearly suggest that such export occurs independently of the presence of β93C in Hb.
Clinical Perspective.
- What is new?
- The red blood cell can export NO bioactivity under deoxygenated conditions and such export can be stimulated either by arginase inhibitors or by inorganic nitrite.
- This export however occurs independently of the conserved cysteine-93 of the hemoglobin β-chain
- In vivo hypoxic vasodilation in skeletal muscle microcirculation is also independent of the β93 cysteine.
- What are the clinical implications?
- The fascinating ability of RBCs to export NO bioactivity might be harnessed therapeutically e.g in protection against myocardial ischemia-reperfusion injury.
Acknowledgements:
Contribution: JOL, DBK-S and RPP provided conceptualization; JY did the Langendorff isolated hearts, AK and ZZ did preparation of NO-Hb and vessel reactivity studies, NW did the platelet activity studies, PC and AT performed hypoxic vasodilation in the microvasculature measurements, TSI performed SNO-RBC and vessel reactivity studies, CWS, JO and TT developed, maintained and performed genotyping and biochemical characterization on mouse models and mouse RBCs. MC and JP provided supervision and participated in conceptualization; JOL, RPP and DBK-S wrote the original draft; All authors performed review and editing of the manuscript.
Funding Sources:
University of Alabama at Birmingham Stem Cell Institute (CS, TT), NIH (HL092624 to RPP; HL058091 and HL098032 to DBK-S), Swedish Research Council, Swedish Heart and Lung Foundation (JOL, MC, JP).
Footnotes
Conflict of Interest Disclosures:
JOL is a named co-inventor of patents and patent applications related to the medical uses of inorganic nitrate and nitrite. RPP and DBK-S are co-inventors on a patent for use of nitrite salts for the treatment of cardiovascular conditions. The other co-authors report no conflict of interest.
References
- 1.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. [DOI] [PubMed] [Google Scholar]
- 2.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. [DOI] [PubMed] [Google Scholar]
- 3.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. [DOI] [PubMed] [Google Scholar]
- 4.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by s-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. [DOI] [PubMed] [Google Scholar]
- 5.Doctor A, Stamler JS. Nitric oxide transport in blood: A third gas in the respiratory cycle. Comp Physiol. 2011;1:541–568. [DOI] [PubMed] [Google Scholar]
- 6.Eich RF, Li TS, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Olson JS. Mechanism of no-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. [DOI] [PubMed] [Google Scholar]
- 7.Huang KT, Huang Z, Kim-Shapiro DB. Nitric oxide red blood cell membrane permeability at high and low oxygen tension. Nitric Oxide-Biol Chem. 2007;16:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: Role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. [DOI] [PubMed] [Google Scholar]
- 9.Cortese-Krott MM, Rodriguez-Mateos A, Sansone R, Kuhnle GG, Thasian-Sivarajah S, Krenz T, Horn P, Krisp C, Wolters D, Heiss C, Kroncke KD, Hogg N, Feelisch M, Kelm M. Human red blood cells at work: Identification and visualization of erythrocytic enos activity in health and disease. Blood. 2012;120:4229–4237. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Gonon AT, Sjoquist P-O, Lundberg JO, Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci USA. 2013;110:15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Zheng X, Mahdi A, Zhou Z, Tratsiakovich Y, Jiao T, Kiss A, Kovamees O, Alvarsson M, Catrina SB, Lundberg JO, Brismar K, Pernow J. Red blood cells in type 2 diabetes impair cardiac post-ischemic recovery through an arginase-dependent modulation of nitric oxide synthase and reactive oxygen species. JACC. Basic transl sci. 2018;3:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z, Mahdi A, Tratsiakovich Y, Zahoran S, Kovamees O, Nordin F, Uribe Gonzalez AE, Alvarsson M, Ostenson CG, Andersson DC, Hedin U, Hermesz E, Lundberg JO, Yang J, Pernow J. Erythrocytes from patients with type 2 diabetes induce endothelial dysfunction via arginase i. J Am Coll Cardiol. 2018;72:769–780. [DOI] [PubMed] [Google Scholar]
- 13.Srihirun S, Sriwantana T, Unchern S, Kittikool D, Noulsri E, Pattanapanyasat K, Fucharoen S, Piknova B, Schechter AN, Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PloS one. 2012;7:e30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Wajih N, Liu X, Basu S, Janes J, Marvel M, Keggi C, Helms CC, Lee AN, Belanger AM, Diz DI, Laurienti PJ, Caudell DL, Wang J, Gladwin MT, Kim-Shapiro DB. Mechanisms of human erythrocytic bioactivation of nitrite. J Biol Chem. 2015;290:1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Sno-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med. 2008;14:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang R, Hess DT, Reynolds JD, Stamler JS. Hemoglobin s-nitrosylation plays an essential role in cardioprotection. J Clin Invest. 2016;126:4654–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, Chaube R, Reynolds JD, Stamler JS. Hemoglobin betacys93 is essential for cardiovascular function and integrated response to hypoxia. Proc Natl Acad Sci USA. 2015;112:6425–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamler JS, Singel DJ, Piantadosi CA. SNO-hemoglobin and hypoxic vasodilation. Nat Med. 2008;14:1008–1009. [DOI] [PubMed] [Google Scholar]
- 19.Yang JN, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci USA. 2013;110:15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JN, Tiselius C, Dare E, Johansson B, Valen G, Fredholm BB. Sex differences in mouse heart rate and body temperature and in their regulation by adenosine a1 receptors. Acta Physiol (Oxf). 2007;190:63–75. [DOI] [PubMed] [Google Scholar]
- 21.Tahepold P, Vaage J, Starkopf J, Valen G. Hyperoxia elicits myocardial protection through a nuclear factor kappab-dependent mechanism in the rat heart. J Thorac Cardiovasc Surg. 2003;125:650–660. [DOI] [PubMed] [Google Scholar]
- 22.Zhuge Z, Paulo LL, Jahandideh A, Brandao MCR, Athayde-Filho PF, Lundberg JO, Braga VA, Carlstrom M, Montenegro MF. Synthesis and characterization of a novel organic nitrate ndhp: Role of xanthine oxidoreductase-mediated nitric oxide formation. Redox Biol. 2017;13:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Transduction of no-bioactivity by the red blood cell in sepsis: Novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104:1375–1382. [DOI] [PubMed] [Google Scholar]
- 24.Patel RP, Hogg N, Spencer NY, Kalyanaraman B, Matalon S, Darley-Usmar VM. Biochemical characterization of human s-nitrosohemoglobin. Effects on oxygen binding and transnitrosation. J Biol Chem. 1999;274:15487–15492. [DOI] [PubMed] [Google Scholar]
- 25.Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993;143:1055–1062. [PMC free article] [PubMed] [Google Scholar]
- 26.Cabrales P, Tsai AG, Frangos JA, Intaglietta M. Role of endothelial nitric oxide in microvascular oxygen delivery and consumption. Free Radic Biol Med. 2005;39:1229–1237. [DOI] [PubMed] [Google Scholar]
- 27.Intaglietta M, Tompkins WR. On-line measurement of microvascular dimensions by television microscopy. J Appl Physiol. 1972;32:546–551. [DOI] [PubMed] [Google Scholar]
- 28.Wajih N, Liu X, Shetty P, Basu S, Wu H, Hogg N, Patel RP, Furdui CM, Kim-Shapiro DB. The role of red blood cell s-nitrosation in nitrite bioactivation and its modulation by leucine and glucose. Redox Biol. 2016;8:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srihirun S, Sriwantana T, Unchern S, Kittikool D, Noulsri E, Pattanapanyasat K, Fucharoen S, Piknova B, Schechter AN, Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLOS One. 2012;7:e30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wajih N, Basu S, Jailwala A, Kim HW, Ostrowski D, Perlegas A, Bolden CA, Buechler NL, Gladwin MT, Caudell DL, Rahbar E, Alexander-Miller MA, Vachharajani V, Kim-Shapiro DB. Potential therapeutic action of nitrite in sickle cell disease. Redox Biol. 2017;12:1026–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angelo M, Singel DJ, Stamler JS. An s-nitrosothiol (sno) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci USA. 2006;103:8366–8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleschyov AL. The no-heme signaling hypothesis. Free Radic Biol Med. 2017;112:544–552. [DOI] [PubMed] [Google Scholar]
- 33.Ignarro LJ, Adams JB, Horwitz PM, Wood KS. Activation of soluble guanylate cyclase by no-hemoproteins involves no-heme exchange. Comparison of heme-containing and heme-deficient enzyme forms. J Biol Chem. 1986;261:4997–5002. [PubMed] [Google Scholar]
- 34.Cortese-Krott MM, Mergia E, Kramer CM, Luckstadt W, Yang J, Wolff G, Panknin C, Bracht T, Sitek B, Pernow J, Stasch JP, Feelisch M, Koesling D, Kelm M. Identification of a soluble guanylate cyclase in rbcs: Preserved activity in patients with coronary artery disease. Redox Biol. 2018;14:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitturi DA, Sun CW, Harper VM, Thrash-Williams B, Cantu-Medellin N, Chacko BK, Peng N, Dai Y, Wyss JM, Townes T, Patel RP. Antioxidant functions for the hemoglobin beta93 cysteine residue in erythrocytes and in the vascular compartment in vivo. Free Radic Biol Med. 2013;55:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassa T, Strader MB, Nakagawa A, Zapol WM, Alayash AI. Targeting betacys93 in hemoglobin s with an antisickling agent possessing dual allosteric and antioxidant effects. Metallomics : Integr Biomet Sci. 2017;9:1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansouri A Oxidation of human hemoglobin by sodium nitrite--effect of beta-93 thiol groups. Biochem Biophys Res Commun. 1979;89:441–447. [DOI] [PubMed] [Google Scholar]
- 38.Winterbourn CC, Carrell RW. Oxidation of human haemoglobin by copper. Mechanism and suggested role of the thiol group of residue beta-93. Biochem J. 1977;165:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]