Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association (original) (raw)
. Author manuscript; available in PMC: 2019 Sep 4.
Abstract
Cardiovascular disease (CVD) remains the leading cause of mortality in women, yet many people perceive breast cancer to be the number one threat to women’s health. CVD and breast cancer have several overlapping risk factors, such as obesity and smoking. Additionally, current breast cancer treatments can have a negative impact on cardiovascular health (eg, left ventricular dysfunction, accelerated CVD), and for women with pre-existing CVD, this might influence cancer treatment decisions by both the patient and the provider. Improvements in early detection and treatment of breast cancer have led to an increasing number of breast cancer survivors who are at risk of long-term cardiac complications from cancer treatments. For older women, CVD poses a greater mortality threat than breast cancer itself. This is the first scientific statement from the American Heart Association on CVD and breast cancer. This document will provide a comprehensive overview of the prevalence of these diseases, shared risk factors, the cardiotoxic effects of therapy, and the prevention and treatment of CVD in breast cancer patients.
Keywords: AHA Scientific Statement, breast cancer, cardiotoxicity, cardiovascular disease, oncology, prevention, risk factors
The number one cause of mortality in US women is cardiovascular disease (CVD),1 yet the general public awareness of this remains suboptimal despite large-scale public education campaigns. Awareness is particularly low in racial and ethnic minority communities.2,3 CVD and breast cancer have individually received significant publicity with media campaigns (such as the Red Dress and Pink Ribbon campaigns); however, there is inadequate public awareness of the coexistence of common risk factors associated with these 2 conditions.
Although cardiology and oncology are often considered separate medical fields, they are frequently intertwined. Multidisciplinary care is critical in the management of cancer patients. Cancer outcomes can be influenced by cardiovascular health: antecedent cardiovascular health can affect cancer treatment selection, and furthermore, cancer care can result in cardiovascular toxicities that could impact ongoing cancer treatment. Finally, latent effects of CVD from cancer treatment can alter cancer survivorship. Much of the intersection between CVD and breast cancer pertains to similarities in predisposing risk factors such as age, tobacco use, diet, obesity, and sedentary lifestyle. CVD risk factors are increased in long-term cancer survivors; however, discussion of CVD prevention and modification of these risk factors during and after cancer treatment is limited.4 The risk of CVD (heart failure [HF], myocardial ischemia, hypertension) is high, and development of CVD risk factors (obesity and dyslipidemia) is higher in older breast cancer survivors than the risk of tumor recurrence. In addition, with advancements in cancer care, survivors could develop latent cardiac effects secondary to the cancer treatment, which can include chemotherapy, radiotherapy, and targeted therapy (eg, trastuzumab).5–7
The field of cardio-oncology has emerged in response to the need to provide the best cancer care without compromising cardiovascular health. Societal committees and new organizations have rapidly emerged to address patients’ needs, including clinical care, research, and education.8,9 Recent societal publications include a consensus statement regarding the training of future cardio-oncologists and guidelines regarding the monitoring and prevention of left ventricular (LV) dysfunction in adult cancer survivors.10,11 Hospitals have been developing and marketing collaborative cardio-oncologic programs to meet rising clinical demands in the field. Lack of formal clinical training programs, funding, and cardiovascular guidelines are some of the unmet needs that will need to be addressed over time to further advance CVD care of cancer patients.8 This scientific statement is the American Heart Association’s first on the connection between CVD and breast cancer. It aims to provide a comprehensive overview of the prevalence of these diseases, shared risk factors, and the cardiotoxic effects of cancer therapy, as well as review the prevention and treatment of CVD in breast cancer patients. Although treatment of cardiotoxicity is not within the scope of this statement, it is clearly an important aspect of cardio-oncology, and it too warrants further research and guideline development.
SCOPE OF THE PROBLEM
CVD and breast cancer are significant causes of morbidity and mortality in the United States. CVD affects ≈47.8 million women,1 and breast cancer affects ≈3.32 million women.12 On the basis of 2014 Centers for Disease Control and Prevention attributable mortality data in women, 1 in 3.3 deaths was attributed to CVD and 1 in 8.3 deaths was attributed to coronary heart disease (CHD), whereas 1 in 31.5 deaths was attributed to breast cancer.1 Mortality rates for CVD and breast cancer have decreased, with an average decline in CVD deaths (both sexes) by 6.7% per year from 2004 to 2014 and in female breast cancer deaths by 1.8% per year from 2005 to 2014.1,12 Direct annual medical costs in the United States are high: ≈$272.5 billion for CVD, 124.57billionforallcancers,and124.57 billion for all cancers, and 124.57billionforallcancers,and16.5 billion for breast cancer; moreover, these costs are expected to continue to rise.13,14
The lifetime risk of developing breast cancer in women is ≈12.4% based on 2012 to 2014 data. The rate of new female breast cancer cases was 124.9 per 100 000 women per year, with a death rate of 21.2 per 100 000 women per year.12 Nearly 90% of breast cancer patients survive at least 5 years after their initial diagnosis.12 Currently, there are ≈3 million breast cancer survivors in the United States15; however, older women are more likely to die of diseases other than breast cancer, and CVD is the most frequent cause.16,17 In older, postmenopausal women, the risk of mortality attributable to CVD is higher in breast cancer survivors than in women without a history of breast cancer. This greater risk manifests itself ≈7 years after the diagnosis of breast cancer, which highlights the need to reduce the additional burden of CVD during this time frame with early recognition and treatment of CVD risk factors.18
Influence of Race and Age
Age-adjusted death rates are higher for CHD and stroke than for breast cancer in non-Hispanic white and black females; however, when race was examined, the rates of CHD, stroke, and breast cancer were higher in non-Hispanic black women than in non-Hispanic white women and Hispanic women (Figure 1).1 There are similar racial-ethnic differences concerning the incidence and mortality rates of breast cancer, with non-Hispanic black women having the highest mortality.5 Non-Hispanic white women 60 to 84 years of age have a higher incidence of breast cancer than black women; however, in black women, the incidence of breast cancer is higher in those <45 years of age, and the mortality rate for breast cancer is higher at all ages.19 Furthermore, American Indian/Alaska Native, Hispanic, and Asian/Pacific Islander women have lower breast cancer incidence and mortality rates than non-Hispanic white and non-Hispanic black women. Additionally, compared with non-Hispanic white women, Hispanic and non-Hispanic black women are more likely to present with later stages of breast cancer,20 which in turn can have a negative impact on clinical outcomes.
Figure 1. Rates of cardiovascular disease and breast cancer in women.
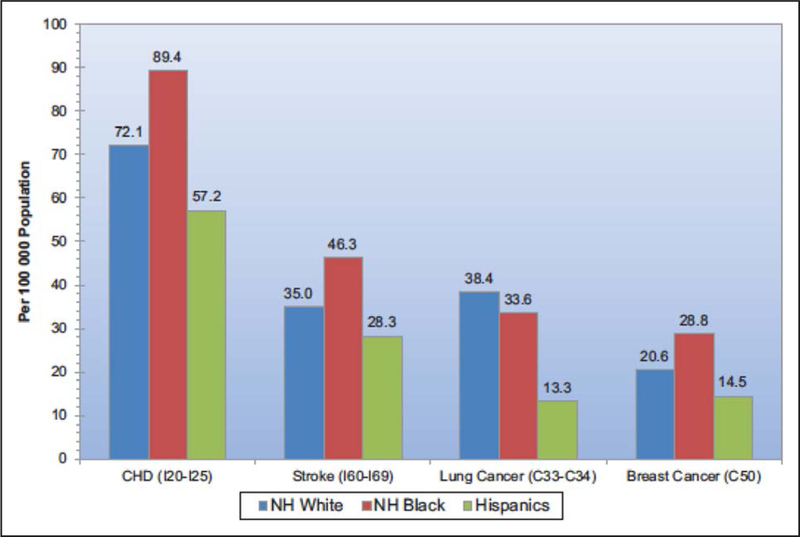
Age-adjusted mortality rates of coronary heart disease (CHD) and stroke are higher than that of breast cancer. Death rates are higher in NH black women than in NH white and Hispanic women for CHD, stroke, and breast cancer.1 NH indicates non-Hispanic. Reprinted from Benjamin et al.1 Copyright © 2017, American Heart Association, Inc.
The improved success of screening and treating breast cancer has contributed to the growing number of survivors, more than half of whom are >65 years of age.21 The prognosis of older breast cancer patients is affected by the effective management of preexisting comorbid conditions such as diabetes mellitus and hypertension.22 In older women diagnosed with breast cancer, CVD is the leading cause of mortality, and breast cancer is the second most common cause.23 Not surprisingly, comorbidities, including CVD, in older breast cancer patients have been associated with decreased overall survival.24 The identification and management of cardiovascular risk factors23 in this population is important because CVD, if not recognized and treated, can pose a greater health risk than the cancer itself.22 The expanding role of primary care physicians, oncologists, cardiologists, and allied healthcare providers in survivorship programs is essential to optimize the management of comorbidities to realize the gains seen in breast cancer treatment.25–28
COMMON RISK FACTORS
Breast cancer and CVD share a number of common risk factors (Figure 2). Cardiovascular clinical care and research have focused on risk factors for >60 years, because it is believed that ≈80% of CVD can be prevented through risk factor modifications such as promoting a healthy diet, physical activity, and a healthy weight; abstinence from tobacco; blood pressure control; diabetes mellitus management; and a good lipid profile.32 Adherence to a larger number of ideal cardiovascular health behaviors or factors from the American Heart Association’s Life’s Simple 7 health campaign1 is associated with a trend toward a lower incidence of breast cancer (P for trend=0.11).33 The Cardiovascular Lifetime Risk Polling Project, involving 18 cohort studies, found that at age 45 years, individuals with optimal risk factor profiles have a significantly lower lifetime risk of CVD events than those with even 1 major risk factor (4.1% versus 20.2% among women). Having ≥2 risk factors further increased lifetime CVD risk to 30.7% in these women.34 Aggressive management of these cardiovascular risk factors can also substantially reduce the lifetime risk of developing cancer.35 Risk factors for breast cancer have only been discussed more recently, but there is growing awareness that through risk factor modification, some cases of breast cancer might be prevented.36,37
Figure 2. Risk factors for cardiovascular disease (CVD) and breast cancer.
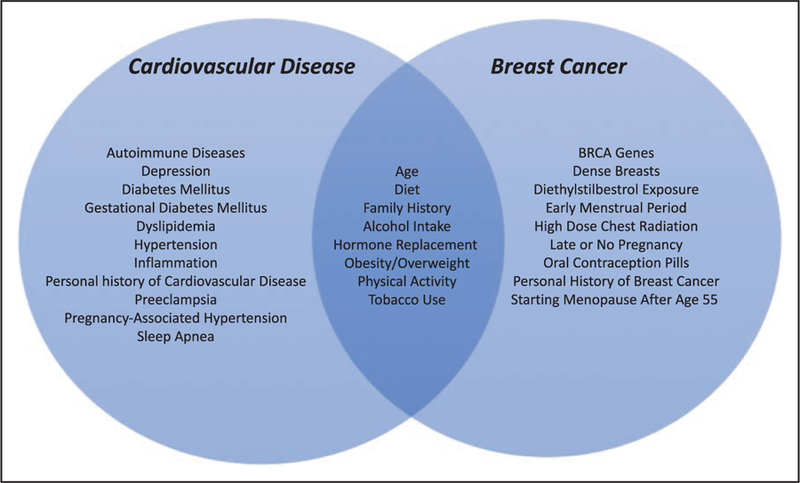
CVD and breast cancer have shared and separate risk factors.19,29–31
Dietary Patterns
Studies of the association between dietary patterns, CVD, and breast cancer typically compare a prudent or healthy dietary pattern (high in vegetables and fruits, poultry, fish, low-fat dairy products, and whole grains) with the Western diet or so-called unhealthy diet (red or processed meats, refined grains, sweets, and highfat dairy products).38 The American Heart Association’s 2020 Impact Goals include a definition of cardiovascular health with a healthy diet pattern that is appropriate in energy balance and consistent with a Dietary Approaches to Stop Hypertension–type eating plan.39 Dietary habits also affect multiple established cardiovascular risk factors, including blood pressure, cholesterol levels, glucose levels, and obesity.32,40 The NHS (Nurses’ Health Study) found that a dietary pattern consisting of higher intake of vegetables, fruits, fish, poultry, and whole grains (prudent diet) was associated with a 28% lower cardiovascular mortality (relative risk [RR], 0.72; 95% confidence interval [CI], 0.60–0.87), whereas a dietary pattern consisting of higher intake of processed or red meats, refined grains, and sweets (Western diet) was associated with a 22% higher cardiovascular mortality (RR, 1.22; 95% CI, 1.01–1.48).41
In contrast, epidemiological data regarding dietary patterns are less consistent in breast cancer. Some reports support prudent dietary patterns as being inversely associated with risk of breast cancer,42–45 whereas others found no association.46–48 The link between breast cancer and a healthy or prudent diet might depend on the tumor type.49 Among 86 261 women in the NHS, a diet high in fruits and vegetables, such as one represented by the Dietary Approaches to Stop Hypertension diet score, was associated with a lower risk of estrogen receptor (ER)–negative breast cancer. Furthermore, a diet high in plant protein and fat and moderate in carbohydrate content was associated with a lower risk of ER-negative breast cancer.36
Studies of the link between the Western diet and breast cancer have shown positive,50 negative,51 or no associations.46 The relationship between the frequency of consumption of energy-dense foods and fast foods and breast cancer risk was examined in black and white women. Higher consumption of energy-dense foods and fast foods was associated with increased breast cancer risk in both racial groups, with some differences by menopausal and ER status of the tumor.52 The positive association with frequency of fast food intake was stronger among premenopausal black women and postmenopausal white women, for whom a positive trend was also observed with frequent intake of energy-dense foods and sugary drinks. Adjustment for total energy intake attenuated odds ratios (ORs) in black women but strengthened risk estimates in white women. The Western dietary pattern was associated with higher breast cancer risk (OR, for top versus bottom quartile, 1.46; 95% CI, 1.06–2.01), especially in premenopausal women (OR, 1.75; 95% CI, 1.14–2.67).53
Dietary Fat
Dietary fat is one of the most intensively studied dietary factors related to breast cancer and CVD risk; however, epidemiological studies are difficult to interpret because of the heterogeneity in fat composition. Studies investigating animal and saturated fats, polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids, and trans fatty acids have reported that different types of fats differentially affect breast cancer development. Of the subtypes of dietary fat, n-3 PUFA is among the most studied. The Japan Collaborative Cohort Study reported an inverse association between n-3 PUFA intake and breast cancer risk.54 Several case-control studies have found that n-3 PUFA, measured as either dietary intake or with tissue biomarkers, is inversely associated with breast cancer risk.55,56 Other observational studies have found no association between n-3 PUFA and breast cancer risk.57–60 A more recent meta-analysis found that marine n-3 PUFA was associated with a 14% reduction of risk of breast cancer (RR for highest compared with lowest category, 0.86; 95% CI, 0.78–0.94), and the RR remained similar whether marine n-3 PUFA was measured as dietary intake or as tissue biomarkers.61
In the WHI (Women’s Health Initiative) dietary modification clinical trial of 48 835 women, reduction of total fat consumption from 37.8% of energy at baseline to 24.3% at 1 year and 28.8% at 6 years had no effect on incidence of major CHD (RR, 0.98; 95% CI, 0.88–1.09) or total CVD (RR, 0.98; 95% CI, 0.92–1.05) over a mean of 8 years.62 The WHI dietary modification trial of a low-fat, high fruit and vegetable diet also failed to provide a definitive answer as to whether fat intake modifies breast cancer risk (hazard ratio [HR], 0.91; 95% CI, 0.83–1.01 for intervention versus control group after 8 years of follow-up).63 Another randomized trial of reduced fat intake and increased carbohydrate intake in women who were followed for 10 years found no effect of fat intake but an increased risk of ER-positive breast cancer with lower carbohydrate intake.64 In a 20-year follow-up of the NHS, there was no association between fats and breast cancer risk, even when subtypes of fats were considered.65 However, the RR for breast cancer in premenopausal women in the NHS was 1.35 in the highest quartile of dietary fat intake compared with the lowest quartile (OR, 1.35; 95% CI, 1.00, 1.81).66
Blood plasma lipid levels are influenced by diet and weight as well and have been associated with breast cancer risk. In a cohort of 4670 women with increased mammographic density, higher levels of serum highdensity lipoprotein cholesterol (HDL-C) and apolipoprotein A1 were associated with increased risk of developing breast cancer (23% and 28%, respectively), whereas higher levels of non-HDL-C and apolipoprotein B were associated with a lower risk of developing breast cancer (19% and 22%, respectively), when compared with the reference group.67 These results demonstrated a possible link between lipids and breast cancer risk, specifically in women with increased mammographic density. The impact of cardiovascular risk reduction strategies on HDL-C and on breast cancer risk needs further study.
Alcohol Consumption
Meta-analyses of epidemiological studies have shown both beneficial and detrimental associations between alcohol intake and all-cause mortality in women, which is a function of the level of consumption and age.68 Observational data from 34 cohort studies demonstrated an association of moderate alcohol intake with reduced all-cause mortality, and in this report, alcohol intake of 5 g/d (a standard alcoholic drink contains 14 g of alcohol) was associated with an 18% reduction in all-cause mortality.69 However, the relationship between alcohol consumption and ischemic heart disease (IHD) is complex. In the NHS, light to moderate alcohol consumption (5 g/d) was associated with a lower risk of total stroke than for nondrinkers (RR, 0.83; 95% CI, 0.75–0.92).70 Several meta-analyses of observational studies show evidence for a cardioprotective association of average alcohol intake on IHD risk.71,72 More recently, a systematic review of 44 observational studies found that in women, a steep J-curve was observed for IHD mortality and morbidity, with the lowest risk shown at 11 g of alcohol per day and no cardioprotection at 14 g/d.73 Data from 8 prospective studies revealed an inverse relationship between alcohol intake and CHD. There was a significantly lower risk of CHD among women (mean age, 50 years) with an alcohol intake of up to 30 g/d (RR, 0.58; 95% CI, 0.49–0.68) compared with nondrinkers; however, higher levels of daily alcohol intake did not confer additional CVD benefit.74 However, no beneficial effect on IHD was seen with episodic and chronic heavy alcohol drinking.75
Although low to moderate intake of alcohol might decrease the risk for CHD, there are no cancer-related benefits of modest drinking.35 Alcohol consumption is a well-established, modifiable risk factor for breast cancer.76,77 There is considerable variability on how alcohol intake is defined, and this makes direct comparisons between studies difficult. Epidemiological studies, including the WHI,78 support the positive relationship between alcohol and breast cancer risk.79–82 Meta-analyses suggest a 5% to 9% increase in risk per drink per day, or ≤12.5 g/d.83–87 Consuming ≥2 alcoholic drinks per day for 5 years is associated with an 82% increased breast cancer risk compared with no alcohol intake.88 In the NHSII (Nurses’ Health Study II), the risk of breast cancer was 34% higher (RR, 1.34; 95% CI, 1.00–1.80) among women 24 to 44 years of age with alcohol intake of ≥15 g/d (≈1.5 drinks per day) compared with women who abstained from alcohol before first pregnancy.89 There was a dose-response relationship between alcohol intake before first pregnancy and breast cancer risk, with an RR of 1.11 (95% CI, 1.00–1.23) for each additional 10-g/d intake; there was no such relationship for alcohol consumption and breast cancer after the first pregnancy. Furthermore, in the NHS, the risk of breast cancer was increased by 21% in adult binge drinkers compared with nondrinkers after controlling for cumulative alcohol intake.82
Breast cancer is a heterogeneous disease with many subtypes defined by hormone receptor status and histological type. The elevated levels of intracellular estrogens resulting from alcohol intake might act through the ER to promote breast cancer.76 Epidemiological studies have shown that the relationship between alcohol intake and hormone receptor–positive breast tumors was stronger than with other types of breast cancer.85 Compared with never-drinkers, HRs for postmenopausal women who consumed ≥7 drinks per week were as follows: for ER-positive breast cancer, 1.48 (95% CI, 1.19–1.83); for progesterone receptor (PR)–positive cancer, 1.64 (95% CI 1.31–2.06); and for mixed/ductal/lobular cancer, 2.51 (95% CI, 1.20–5.24).90 The risks for ER-positive/PR-positive and ER-positive/PR-negative breast cancer increased by 8% (95% CI, 2%–15%) and 12% (95% CI, 0%–25%), respectively, per drink per day among postmenopausal women,91 which is comparable to the 12% increase in risk of ER-positive tumors per 10 g/d of alcohol intake reported in a meta-analysis of prospective and case-control studies.85
Meat Consumption
The association between meat consumption and breast cancer is not well understood. Among a large cohort of women in the United Kingdom, increased consumption of total and nonprocessed meat was associated with a higher risk of premenopausal breast cancer, and total processed meat and red meat consumption was positively associated with postmenopausal breast cancer.92 A case-control study found that processed meat intake, even just 1 or 2 times per week, was associated with a 2.7-fold (OR, 2.65; 95% CI, 1.36–5.14; _P_=0.004) higher likelihood of developing breast cancer, but red, white, and grilled meat intake was not.93 A study of 4684 French women found that processed meat intake was associated with higher breast cancer risk (HR, 1.45; 95% CI, 0.92–2.27), and this association was stronger when cooked ham was excluded and manifested only in women who were not taking antioxidant supplements.94 Among the 44 231 participants of the NHSII cohort, greater consumption of red meat in adolescence was significantly associated with higher premenopausal breast cancer risk (highest versus lowest quintiles: RR, 1.43; 95% CI, 1.05–1.94) but not postmenopausal breast cancer.95 Others have found weak positive associations between meat consumption and breast cancer.96,97 Several studies have linked red and processed meat intake with increased mortality98 and acute coronary events.99
Collectively, the evidence for the influence of diet on breast cancer and CVD risk is mixed. The results of epidemiological studies must be interpreted cautiously, because these studies cannot determine causation. The sample size, measurement error in nutrients and in outcome variables, and individual variation in human genetics and lifestyle factors limit the ability to measure the effect of diet on breast cancer.100 This is largely because of uncontrolled confounding factors and differences in study designs and measurement of end points. It appears that dietary factors are particularly important in determining premenopausal breast cancer risk. Likewise, there is increasing evidence to suggest the importance of alcohol consumption in the development of breast cancer. Understanding the impact of diet on breast cancer risk will require examination of gene-environment interactions and long-term epigenetic mechanisms. Some promising work has found that gut microbiota is less diverse and compositionally different in postmenopausal women with breast cancer than in similar women without breast cancer.101 Breast cancer risk can be affected by obscure early-life effects that are transmitted through the maternal line.102 It is plausible that the infant’s gut microbiota composition could influence breast cancer risk in adulthood.
Physical Activity
About 150 minutes of moderate-intensity physical activity per week is recommended for all Americans.103 Only 17.6% of American women meet these physical activity guidelines.32 Strong evidence supports the elevated risk that inactivity (<150 min/wk) poses for both CVD and breast cancer.32,104 Globally, physical inactivity is believed to be responsible for 12.2% of the burden of myocardial infarction after accounting for other CVD risk factors including cigarette smoking, hypertension, obesity, dyslipidemia, alcohol, and psychosocial factors.105 A meta-analysis of longitudinal studies of women found that the RRs of incident CHD were 0.83 (95% CI, 0.69–0.99), 0.77 (95% CI, 0.64–0.92), 0.72 (95% CI, 0.59–0.87), and 0.57 (95% CI, 0.41–0.79) across increasing quintiles of physical activity compared with the lowest quintile.106 Women in the CARDIA study (Coronary Artery Risk Development in Young Adults) who maintained high physical activity through young adulthood gained about 6 fewer kilograms of weight and about 3.8 fewer centimeters in waist circumference in middle age than those with lower activity.107 Similarly, among US women, every additional daily hour of television watching was associated with 0.3 lb of greater weight gain every 4 years, whereas every hour per day of less television watching was associated with a similar amount of relative weight loss.108 A study of >1 million women followed up over 9 years found that moderate physical activity but not strenuous physical activity was associated with lower risk of CHD or a cardiovascular event.109 Conversely, in a population-based study in Australia, among adults followed up for 6 years and reporting any moderate to vigorous physical activity, the proportion of vigorous activity showed an inverse doseresponse relationship with all-cause mortality compared with those reporting no vigorous activity.110
Studies have consistently shown that moderate to vigorous physical activity is associated with a decreased breast cancer risk among both premenopausal and postmenopausal women who are more active versus those who are less active.111 Several mechanisms have been proposed for how physical activity potentially reduces breast cancer risk, including reduced exposure to estrogen and androgens, insulin-related factors, adipokines, and inflammation.112,113 A meta-analysis of 29 observational studies found a significant reduction in breast cancer risk among the most physically active compared with the least active women.114 A more recent meta-analysis of 22 studies involving 123 574 participants found an inverse relationship between physical activity and breast cancer events and deaths.115 Compared with those who reported low or no lifetime recreational prediagnosis physical activity, those who reported high lifetime recreational physical activity had a significantly lower risk of breast cancer–related death (HR, 0.73; 95% CI, 0.54–0.98). Additionally, significant risk reduction for breast cancer–related death was demonstrated in women who had engaged in recreational physical activity more recently before diagnosis (HR, 0.84; 95% CI, 0.73–0.97). In NHSII, data showed that physical activity at ages 14 to 17 years was associated with a 15% lower risk of premenopausal breast cancer.116
Sedentary Lifestyle
Sedentary behavior is defined as activities performed in a sitting or reclining posture with an energy expenditure typically in the range of 1.0 to 1.5 times the basal metabolic rate.117 Sedentary behavior is also characterized by prolonged sitting or lying down and absence of whole-body movement, such as watching television or other forms of screen-based entertainment and car driving.118,119 Previous research suggests that sedentary behavior has been associated with CVD and breast cancer.120,121 In the WHI observational study involving 71 018 women, sitting for ≥10 hours each day compared with <5 hours each day was associated with increased CVD risk (HR, 1.18) in multivariable models that included physical activity. Low physical activity was also linked with higher CVD risk.122
Sedentary behavior has also been associated with high breast density, which has been shown to be a strong, independent risk factor for breast cancer, with a 4- to 6-fold increased risk compared with the least dense breasts.123,124 A case-control study found that for women in the highest quartile of physical activity compared with those in the lowest quartile, time spent in moderate to vigorous activity (measured by accelerometer) was inversely associated with risk of developing breast cancer after adjustment for known risk factors, sedentary behavior, and time spent wearing the accelerometer. They also found that sedentary behavior was positively associated with breast cancer, independent of moderate to vigorous activity (OR, 1.81; 95% CI, 0.27–0.56; _P_trend<0.001).125 In the Southern Community Cohort Study, sedentary behavior for ≥12 h/d compared with <5.5 h/d was associated with increased odds of breast cancer among white women (OR, 1.94; 95% CI, 1.01–3.70) but not black women (OR, 1.23; 95% CI, 0.82–1.83) after adjustment for physical activity (measured as metabolic equivalent hours per day).126 The racial differences noted in this study might be explained by variation in determining physical activity and underlying biological mechanisms that impact breast cancer risk. Independent of one another, sedentary behavior and physical inactivity are risk factors for breast cancer among white women. Reducing sedentary behavior and increasing physical activity are potentially independent targets for breast cancer prevention interventions.
Obesity, Overweight, and Body Mass Index
Overweight and obesity (body mass index [BMI] of ≥25 and ≥30 kg/m2, respectively) are major risk factors for CVD127; furthermore lifetime obesity and physical inactivity increase the risk of CVD. Severe obesity (class III, body mass index ≥40 kg/m2) may pose an even greater CVD risk than class I or class II obesity.128 Among women in the WHI (n=156 775) with severe obesity compared with normal BMI, HRs (95% CIs) for mortality were on the order of 1.5 to 2.6 across black, Hispanic, and white women. For CHD, HRs were 2- to nearly 3-fold higher. However, CHD risk was strongly related to CVD risk factors across BMI categories even in severe obesity, and CHD incidence was similar by race/ethnicity when adjusted for differences in BMI and CVD risk factors.128 A meta-analysis of white adults over a mean follow-up of 10 years reported that among women, overweight and obese status (and perhaps underweight) based on BMI correlated with elevated all-cause death.129
Obesity is also a factor that is associated with breast cancer risk specifically in postmenopausal women.130,131 The relationship between obesity and breast cancer is complex. A dose-response meta-analysis found that each 5-kg increase in adult weight gain was associated with an 11% increased risk of postmenopausal breast cancer among nonusers of hormone replacement therapy (HRT; RR, 1.11; 95% CI, 1.08–1.13); there was no evidence of a linear relationship for premenopausal breast cancer (RR, 0.99; 95% CI, 0.95–1.03).132 Furthermore, higher BMI (overweight-to-obese category) among women at age 18 years was associated with a 24% lower risk of breast cancer in premenopausal women compared with low BMI (95% CI, 0.60, 0.96, _P_trend=0.09).133 A BMI in the morbidly obese category, compared with normal- to low-weight BMI, was associated with a 19% reduced breast cancer risk (95% CI, 0.61–1.06; _P_trend=0.05) in premenopausal women. A meta-analysis of case-control and cohort studies of premenopausal women found an inverse relationship between BMI and breast cancer risk. There was a 5% reduced risk for developing premenopausal breast cancer with a 5-kg/m2 increase in BMI.134 Stratified by ethnicity, the inverse association remained for black (RR, 0.95; 95% CI, 0.91–0.98) and white women (RR, 0.93; 95% CI, 0.91–0.95) but not for Asian women. However, for waist-hip ratio, the dose response showed a statistically significant increase in RR of 8% for each 0.1 increment, with the largest association detected in Asian women (19%; RR, 1.19; 95% CI, 1.15–1.24).134
Investigators of the NHS and NHSII observed that childhood and adolescent body fatness was significantly associated with a 20% to 50% decreased breast cancer risk throughout life, regardless of menopausal status.135 More recently, investigators of the NHS evaluated the link between recent weight change and total premenopausal and postmenopausal invasive breast cancer and hormone receptor status subtypes while controlling for average BMI before and after menopause.136 Short-term weight change was significantly associated with breast cancer risk (RR, 1.20; 95% CI, 1.09–1.33) for a 4-year weight gain of ≥15 lb versus no change (≤15 lb). The association was stronger for premenopausal women (RR, 1.38; 95% CI, 1.13–1.69) than for postmenopausal women (RR, 1.10; 95% CI, 0.97–1.25). Premenopausal short-term weight gain had a stronger association for ER-positive/PR-negative (RR per 25-lb weight gain, 2.19; 95% CI, 1.33–3.61) and ER-negative/PR-negative breast cancer (RR per 25-lb weight gain, 1.61; 95% CI, 1.09–2.38) than for ER-positive/PR-positive breast cancer (RR per 25-lb weight gain, 1.13; 95% CI, 0.89–1.43). This study supported the deleterious effects of short-term weight gain, particularly before menopause, even after controlling for average BMI before and after menopause.136 Regardless of levels of adolescent physical activity, early-life body leanness of 74 723 women in the NHSII was significantly associated with higher breast cancer risk; the association was slightly attenuated among those who were active during adolescence compared with those who were inactive.137 Increased breast cancer risk among postmenopausal women who had adolescent leanness was confined to those who gained excess weight during adulthood.138
Tobacco Use
Although cigarette smoking is a major risk factor for CVD and stroke, the link between cigarette smoking and breast cancer remains inconclusive. A meta-analysis comparing pooled data of ≈2.4 million smokers and nonsmokers found that the RR for smokers compared with nonsmokers for developing CHD was 25% higher in women than in men (95% CI, 1.12–1.39).139 The association between smoking and breast cancer risk has been evaluated extensively in epidemiological studies, with an emerging consensus for a weak, positive association.140 One large Canadian study found a non-significant trend toward increased breast cancer risk in premenopausal women who were active smokers or began smoking before their first pregnancy.141 Moreover, several studies reported an increased breast cancer risk associated with smoking for long durations and initiation early in life.142–146 The association between passive or sidestream smoke and breast cancer is also mixed. Some reports have found an association,147,148 whereas others have not.149 Genetic variants in enzymes involved in the metabolisms of carcinogens are postulated to modify the association between smoking and breast cancer risk.150,151 More recent meta-analyses report a significant interaction between smoking and carcinogen-metabolizing genotype variants (eg, NAT2); smokers carrying the slow acetylator variant NAT2 genotypes had an increased breast cancer risk,152 and among women with high pack-years of smoking exposure, the NAT2 slow acetylator variant genotypes were associated with an increased breast cancer risk.153
Age
The incidence of breast cancer increases with advancing age, doubling approximately every 10 years until menopause is reached, at which time the rate of increase slows.154 The incidence of CVD increases steadily with advancing age, but the rate of increase becomes steeper at menopause, rather than slowing.155 Age is obviously not changeable, so its role in prevention is as a risk predictor rather than a modifiable risk factor.
Age at Menarche and Menopause
In addition to age in general, age of menarche and menopause is an additional age-related risk factor for both breast cancer and CVD. Women who start menstruating earlier in life (early menarche) have an increased risk of developing breast cancer.154 These women also have a higher risk for developing CVD, particularly in nonsmoking populations.156,157 In terms of menopause, women who undergo menopause earlier in life (early menopause) have an increased risk of developing CVD158 but a decreased risk of developing breast cancer. Women undergoing menopause after the age of 55 years are twice as likely to develop breast cancer as women who undergo menopause before the age of 45.154
Postmenopausal HRT
Several studies have shown an association between postmenopausal hormone use and risk of breast cancer. The NHS study followed >100 000 healthy women 30 to 50 years of age at the time of enrollment and found that the risk of developing breast cancer was 1.2 to 2 times greater for women who reported ≥5 years of current use of HRTs than for those who never used HRT.159 Long-term cumulative follow-up data of postmenopausal women with hysterectomy from the WHI demonstrated a 45% lower risk of breast cancer–related mortality in those randomized to estrogen-only therapy than in those given placebo. However, postmenopausal women with an intact uterus who were randomized to combination therapy of estrogen plus progestin had a 44% increase in breast cancer mortality compared with those in the placebo arm, although this result did not meet statistical significance.160
Several studies have now also reported that HRT is associated with an increased risk of CVD in older postmenopausal women and women with CHD.161,162 The largest of these studies is the WHI, which randomized >16 000 postmenopausal women (average age 63.3±7.1 years) with no history of coronary artery disease to receive either estrogen alone, estrogen combined with progesterone, or placebo and then followed them for an average of 5.6 years. The estrogen plus progestin arm was prematurely terminated (after 5.2 years of follow-up), and at the time of study discontinuation, there was an increased risk of CHD with HRT (HR, 1.29; nominal 95% CI, 1.02–1.63).162 These data confirm that postmenopausal HRT is associated with both breast cancer and CVD (stroke, thromboembolic events, CHD), and this is a potentially modifiable risk factor for both diseases.159,162,163
Genetics
Clinical and population-based studies have demonstrated that genetic factors play important roles in both breast cancer and CVD, and genes involved in the development of breast cancer and CVD have been identified. Two breast cancer susceptibility genes (BRCA1 and BRCA2) are thought to account for between 5% and 10% of all breast cancer cases.164 Genetics plays a role in therapies as well. Therapies have been developed to target genes shown to be important in progression of breast cancer, such as the HER2 (c-erbB-2) oncogene.165 These discoveries have greatly enhanced our ability to detect and treat breast cancer. Although genes involved in the development of CVD have also been identified,166–168 we have not been able to identify a single gene responsible for a substantial proportion of cases, such as with the relationship of BRCA1 and BRCA2 to breast cancer. Animal studies have demonstrated an important role of the BRCA1 and BRCA2 genes in cardiac injury responses and an increased susceptibility of mice harboring the cardiomyocyte-specific BRCA mutation to develop HF after anthracycline exposure.169 However, human studies in women who are BRCA mutation carriers did not identify a higher risk of myocardial dysfunction after breast cancer treatment with doxorubicin.170
Although most of the risk factors that are common to both CVD and breast cancer move risk in the same direction (ie, decrease or increase risk in both diseases), a few risk factors move risk in opposite directions (Figure 3). Decreased risk for developing CVD but increased risk for developing breast cancer was associated with low to moderate alcohol intake, whereas increased risk for CVD but decreased risk for breast cancer was associated with early menopause and premenopausal obesity. Knowledge and understanding of the risk factors for CVD and breast cancer will contribute to a better understanding of how best to prevent both diseases.
Figure 3. Factors associated with developing CVD and breast cancer.
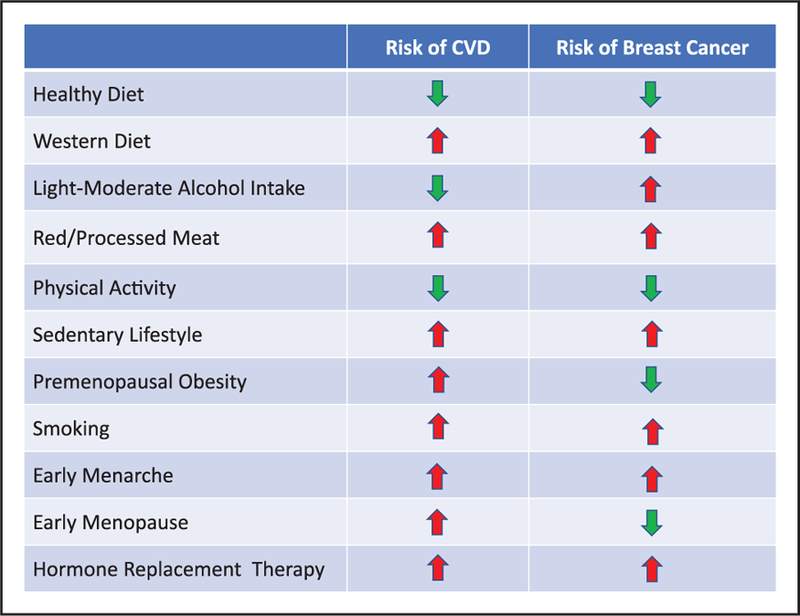
Impact of these factors in a positive (green downward arrow, reduced risk) or negative (red upward arrow, increased risk) on developing CVD or breast cancer.19,29–31 CVD indicates cardiovascular disease.
CARDIOVASCULAR EFFECTS OF CANCER THERAPY
Cancer treatment can result in early or delayed cardiotoxicity that can vary from LV dysfunction to overt HF, hypertension, arrhythmias, myocardial ischemia, valvular disease, thromboembolic disease, pulmonary hypertension, and pericarditis. The most commonly reported and monitored side effect of chemotherapy is LV systolic dysfunction.171 Arrhythmias, independent of other concurrent cardiac disease, can occur from breast cancer treatment, including chemotherapy and radiation therapy (RT).172 Several chemotherapeutic agents can also prolong QT intervals.173 The absence of a clear consensus in definitions of cardiotoxicity makes it difficult to compare results of cardiac end points between clinical trials and furthermore makes the applicability of these findings in the real world challenging at best. This section will briefly review the pathophysiological mechanisms and resultant adverse cardiovascular effects of common therapies for breast cancer (Table 1).
Table 1.
Cancer Treatment and Cardiovascular Adverse Effects
| Cancer Treatment | Cardiovascular Adverse Effects |
|---|---|
| Anthracyclines (eg, doxorubicin, epirubicin) | Left ventricular dysfunction, heart failure, myocarditis, pericarditis, atrial fibrillation, ventricular tachycardia, ventricular fibrillation |
| Alkylating agents (eg, cisplatin, cyclophosphamide) | Left ventricular dysfunction, heart failure, myocarditis, pericarditis, arterial thrombosis, bradycardia, atrial fibrillation, supraventricular tachycardia |
| Taxanes (eg, paclitaxel) | Bradycardia, heart block, ventricular ectopy |
| Antimetabolites (eg, 5-fluorouracil, capecitabine) | Coronary thrombosis, coronary artery spasm, atrial fibrillation, ventricular tachycardia, ventricular fibrillation |
| Endocrine therapy (eg, tamoxifen, anastrozole, letrozole) | Venous thrombosis, thromboembolism, peripheral atherosclerosis, dysrhythmia, valvular dysfunction, pericarditis, heart failure |
| HER-2–directed therapies (eg, trastuzumab, pertuzumab) | Left ventricular dysfunction, heart failure |
| Cyclin-dependent kinase 4/6 inhibitors (eg, palbociclib, ribociclib) | QTc prolongation |
| Radiation therapy | Coronary artery disease, cardiomyopathy, valvular disease, pericardial disease, arrhythmias |
Chemotherapeutic Agents
Anthracyclines
Anthracyclines, such as doxorubicin, have been used successfully in the treatment of various neoplasms such as breast cancer since the 1970s, but their use can result in significant irreversible LV dysfunction. Doxorubicin interacts with DNA, intercalating and inhibiting macro-molecular biosynthesis and inhibiting the progression of the topoisomerase IIβ within cardiac myocytes. By intercalating into the DNA, the anthracyclines bind to topoisomerase IIβ and disrupt replication, causing myocyte cell death.174,175 Another central mechanism that has been postulated is the generation of reactive oxygen species, which damage DNA, proteins, and lipids, including the mitochondrial membrane, and accelerate myocyte death.176 Anthracyclines also form complexes with intracellular iron, which in turn generate oxygen radicals. Interestingly, individuals with higher iron stores exhibit increased anthracycline toxicity.177
The myocardial toxicity of the anthracyclines can manifest early or late after exposure. The early manifestations could be related to inflammation resulting in a pericarditis-myocarditis syndrome,178 whereas the late manifestations are related to actual myocyte damage that results in the clinical syndrome of HF. Cell death has been confirmed by rising troponin levels.179 The risk of HF increases with increasing cumulative doses of anthracyclines; for instance, with doxorubicin, there is an ≈5% risk at a dose of 400 mg/m2, a 26% risk at a dose of 550 mg/m2, and up to a 48% risk at a cumulative dose of 700 mg/m2.
However, there is no “safe dose” threshold, because individuals exposed to lower doses of anthracycline (<400 mg/m2), particularly those with underlying cardiovascular risk factors, are also at risk of experiencing cardiotoxicity.180–182 Most at risk for anthracycline-mediated cardiotoxicity are older patients, those with a prior cardiac pathology, and those who have been exposed to concomitant chemotherapy or RT (Table 2).11,183–185 Detailed cardiovascular phenotyping has suggested that on average, there are modest but persistent declines in LV ejection fraction (LVEF) of ≈4% even at 3 years after anthracycline exposure.186 LV functional recovery and reduction in cardiac events might be possible with early detection and prompt treatment of LV dysfunction; however, complete LVEF recovery was not seen in patients treated >6 months after completion of chemotherapy.187
Table 2.
Risk for Developing Cardiac Dysfunction
| At-risk therapies including any of the following: |
|---|
| High-dose anthracycline therapy: doxorubicin ≥250 mg/m2 or epirubicin ≥600 mg/m2 |
| High-dose radiation therapy when heart is in the field of treatment: radiotherapy ≥30 Gy |
| Sequential treatment: lower-dose anthracycline therapy (doxorubicin <250 mg/m2 or epirubicin <600 mg/m2) and then subsequent treatment with trastuzumab |
| Combination therapy: lower-dose anthracycline (doxorubicin <250 mg/m2 or epirubicin <600 mg/m2) combined with lower-dose radiation therapy when heart is in the field of treatment (<30 Gy) |
| Presence of any of the following risk factors in addition to treatment with lower-dose anthracycline or trastuzumab alone: |
| Older age at time of cancer treatment (≥60 y) |
| ≥2 CVD risk factors during or after cancer treatment: diabetes mellitus, dyslipidemia, hypertension, obesity, smoking |
| History of myocardial infarction, moderate valvular disease, or low-normal left ventricular function (50%–55%) before or during cancer treatment |
Doxorubicin-based adjuvant chemotherapy for breast cancer has been found to cause arrhythmias and conduction abnormalities in 2.6% of patients versus 1% of patients who did not receive doxorubicin.188 Anthracyclines are associated with atrial fibrillation (2%–10%), which can occur acutely during or after chemotherapy.189 An individualized patient-centered approach to decisions regarding the use of antithrombotic agents in the management of atrial fibrillation is recommended, with careful consideration of the benefits and risks of treatment.171,190 Ventricular tachycardia and ventricular fibrillation are less likely to be associated with anthracycline treatment.189 The burden of arrhythmias detected on defibrillators in patients with anthracycline-induced cardiomyopathy is similar to those with other forms of cardiomyopathy (non-anthracycline chemotherapy or IHD), with nonsustained ventricular tachycardia as the most common arrhythmia, followed by atrial fibrillation and then sustained ventricular tachycardia/ventricular fibrillation.191 A small study of long-term survivors with childhood cancers treated with anthracyclines, especially with concomitant chest radiation, found that prior anthracycline therapy was associated with a prolonged corrected QT interval of 0.43 or higher, but no torsade de pointes was documented.188
Structural analogues of doxorubicin, such as epirubicin (commonly used in Europe and Canada), were suggested to have lower clinical cardiotoxicity than doxorubicin (OR, 0.39; 95% CI, 0.2–0.78).192 However, a recent Cochrane database review of 5 randomized trials (1036 patients) comparing doxorubicin and epirubicin did not find a statistically significant difference in HF incidence between the 2 regimens (RR, 0.36; 95% CI, 0.12–1.11),193 and therefore, epirubicin should not be perceived as cardioprotective or less cardiotoxic.
Alkylating Agents
Alkylating agents, including cisplatin and cyclophosphamide, can also damage DNA, resulting in cytotoxicity and myocyte death. Histopathology shows interstitial hemorrhage, edema, and necrosis. Cyclophosphamide has been the alkylating agent most commonly used in the treatment of breast cancer. Clinical cardiotoxicity with cyclophosphamide is extremely rare, can occur within 10 days of administration, and appears to be related to prior anthracycline therapy or mediastinal radiation.194–196 Myocarditis and pericarditis have also been described but are rare.197,198 Bradycardia, supraventricular tachycardia, and atrial fibrillation have also been reported in patients receiving systemic alkylating agents.171,189,199–201
Taxanes
Taxanes, such as paclitaxel, act as microtubule poisons and block mitotic cycle progression by affecting microtubule processes and producing abnormal spindles and by disrupting mitosis, which results in apoptosis.202 Taxanes are important agents in the treatment of early and advanced breast cancer. Taxanes can be given sequentially or in combination with anthracyclines in early-stage breast cancer. Sequencing of taxanes alters the pharmacokinetics of anthracyclines but is not known to directly result in HF.203
Administration of paclitaxel has been associated with bradycardia; 29% of patients in the phase II paclitaxel clinical trials experienced heart rates <40 bpm.204 Other rhythm disturbances noted in patients with rhythm monitoring were asymptomatic left bundle-branch block and nonsustained ventricular tachycardia, which was also noted when paclitaxel was combined with cisplatin. It is unclear whether the rhythm disturbances observed with paclitaxel might have been caused by the formulation vehicle, polyethoxylated castor oil, or premedication with H1 and H2 antagonists to prevent hypersensitivity reactions.204
Antimetabolite Drugs
The antimetabolite drugs, in particular 5-fluorouracil (5-FU) and capecitabine (a prodrug that is enzymatically converted to fluorouracil in the tumor), are pyrimidine analogues that disrupt DNA and RNA synthesis.205 These agents have been used as first-line therapy (capecitabine alone or intravenous 5-FU in combination with other cytotoxic agents) for metastatic breast cancer and in combination with anthracyclines (5-FU, epirubicin, cyclophosphamide) in early-stage breast cancer. The most commonly described cardiac side effect is chest pain; however, myocardial infarction, HF, and arrhythmias have been reported, albeit rarely.206–208 Mechanisms that have been attributed to the chest pain include thrombosis or coronary arterial vasospasm.209 These symptoms usually resolve by stopping the drug infusion.210,211 Patients without coronary artery disease can also develop ischemia but at a much lower incidence than in those with pretreatment coronary artery disease (1.1% versus 4.5%).212 Cardiotoxicity incidence varies in the literature, ranging between 1% and 68% with 5-FU, and can occur early after treatment because of high, continuous-infusion doses, which is rare in the treatment of breast cancer.206–208,212–215 Cardiotoxicity (vasospasm) with 5-FU can occur in the acute setting (during intravenous infusion) or can be delayed (2–5 days after administration). Long-term cardiotoxicity with 5-FU is uncommon.216 Other reported risk factors for cardiotoxicity with 5-FU include previous mediastinal RT, antecedent history of CVD, and concurrent use of combination chemotherapeutic agents.206,212,215 Arrhythmias induced by 5-FU and capecitabine, including ventricular tachycardia and ventricular fibrillation, are mostly ischemic in origin and usually occur in the context of coronary artery spasm; however, there are also reported cases of atrial fibrillation, atrial/ventricular ectopy, and QT prolongation.217–221
Endocrine Therapy
Endocrine therapy has an important role in the treatment of patients with breast cancer expressing ER or PR. In early-stage hormone receptor–positive breast cancer, use of adjuvant tamoxifen or aromatase inhibitors (AIs) reduces cancer recurrence rates, improves overall survival, and is recommended by the current clinical practice guidelines.222 Hormonal therapies act by interrupting the cellular processes through which estrogen promotes growth of normal and cancerous tissue, and importantly, the differences in their molecular targets determines their cardiovascular and overall side-effect profile. Two main strategies are interference with estrogen signaling by competitive binding to ER, represented by tamoxifen, and inhibition of endogenous estrogen production, as in the case of AIs. In the adjuvant setting, endocrine therapy is prescribed for an extended period, often ≥5 years, which emphasizes the importance of detailed evaluation of its overall toxicity and cardiovascular side-effect profile. Tamoxifen is the endocrine therapy of choice for premenopausal women, whereas strategies in postmenopausal women can include tamoxifen, AIs, or a sequential combination, with careful weighing of benefits and management of toxicity risks.222
Tamoxifen
Tamoxifen, a hormonal agent approved for treatment of early breast cancer >30 years ago, is a selective ER modulator that interferes with estrogen binding to ER and in turn alters the expression of genes downstream from ER.223 The effects of the selective ER modulator/ER nuclear receptor transcription complex on gene transcription are determined by the presence and preferential binding of corepressors and coactivators in different tissues and account for their tissue-specific estrogen agonist and estrogen antagonist actions. In breast tissue, tamoxifen exhibits estrogen antagonist activity, and the competitive binding of tamoxifen to ER leads to the inhibition of estrogen-dependent tumor growth. ER antagonism is also the mechanism of some adverse effects of tamoxifen, including hot flashes and mood disturbances. However, in most organs, including the cardiovascular system, bone, and uterus, tamoxifen has estrogen agonistic activity, which can result in beneficial or detrimental effects, depending on the affected tissue. Tamoxifen has a favorable effect on the lipid profile, with reductions in total serum cholesterol (in the range of 10% to 15%) and low-density lipoprotein cholesterol (reductions ranging from 15% to 22%), but no significant changes in HDL-C.224–227 Smaller studies reported increases in triglyceride levels in Asian patients treated with tamoxifen and raised concerns about hypertriglyceridemia,228,229 but the broader clinical relevance of these findings remains unknown.
Despite observed favorable effects and the reductions in low-density lipoprotein cholesterol and total cholesterol levels, long-term data from clinical trials have failed to demonstrate a protective effect of tamoxifen with regard to hard cardiovascular end points. In the Early Breast Cancer Trialists’ Collaborative Group overview,230 which included 55 trials and 37 000 women, there was no difference in cardiac or vascular death between adjuvant and placebo groups. This was similar to the findings in the National Surgical Adjuvant Breast and Bowel Project B14,231 which reported only a small number of cardiac or vascular deaths, with no statistically significant difference between tamoxifen and placebo groups. These results indicate the need for cautious interpretation of surrogate end points, such as lipid profile, which did not translate into a clinically relevant benefit of prevention of CVD death. Interesting comparisons have been made between findings in tamoxifen trials and the WHI study, which used HRT in postmenopausal women. Despite the lowering of low-density lipoprotein cholesterol and the increasing of HDL-C levels (but also increased triglyceride levels) in the WHI, HRT did not confer cardiovascular protection.162,232
In contrast to its protective effects on lipid metabolism, the estrogen-agonistic actions of tamoxifen result in a detrimental increase in thrombogenicity and increased risk of venous thrombosis and thromboembolism.233 Later studies that compared AI to tamoxifen demonstrated a reduced risk of thrombosis with AIs, presumably because of their lack of proestrogen effects. A meta-analysis of randomized adjuvant endocrine trials reported the incidence of thrombosis at 2.8% in the tamoxifen group compared with 1.6% in the AI group.234 Venous thromboembolism is associated with mortality and significant morbidity related to deep venous thrombosis, pulmonary embolism, and long-term sequelae such as pulmonary hypertension, which highlights the need to consider other risk factors (eg, history of stroke) when selecting endocrine therapy for women with ER-positive breast cancer.235
Aromatase Inhibitors
AIs act by inhibiting the aromatase enzyme and depleting estrogen levels in postmenopausal women. Circulating estrogen is made in peripheral tissues from the conversion of androstenedione to estradiol via the enzyme aromatase in postmenopausal women.236 The 3 AIs currently in clinical use (anastrozole, letrozole, and exemestane) are third-generation agents characterized by high specificity and suppression of the aromatase enzyme, and their side-effect profiles mostly reflect the effects of estrogen depletion.237 AIs are recommended either as primary therapy or after 2 to 3 years of tamoxifen therapy (total duration, 5 years) in postmenopausal women with hormone receptor–positive breast cancer, to lower the risk of breast cancer recurrence.222 This recommendation is based on randomized controlled trials that continue to demonstrate reduced cancer recurrence rates and disease-free survival in postmenopausal women treated with AIs compared with tamoxifen.222,238–240 AIs have also been shown to be effective for primary prevention of breast cancer in high-risk postmenopausal women.241
Because AIs deplete endogenous estrogen production, it has been hypothesized that they might increase the risk of CVD, and most large adjuvant trials that have compared AIs to tamoxifen have included cardiovascular end points. Only a few large studies assessed hypercholesterolemia, and these yielded inconclusive results. The ATAC trial (Arimidex, Tamoxifen, Alone or in Combination)242 and the BIG (Breast International Group) I-98 trial239 reported a higher incidence of hypercholesterolemia with anastrozole and letrozole, respectively, than with tamoxifen, whereas the MA.17 trial did not find significant differences with letrozole.243 However, a meta-analysis of adjuvant AI trials that accounted for the duration of AI treatment and differences in treatment approach demonstrated that longer duration of AI use was associated with a statistically significant 2.3- fold increase in the OR for hypercholesterolemia compared with tamoxifen.234 The effect was most apparent when upfront AI use was compared with tamoxifen and least evident when switching from tamoxifen to AI was compared with tamoxifen.
In the same meta-analyses of adjuvant trials, a similar pattern of risk for CVD was observed: longer durations of AI use were associated with increased risk for CVD compared with tamoxifen use, with the effect being the strongest in the trials that evaluated upfront AIs versus upfront tamoxifen.234 In a pooled analysis, the OR for development of CVD with the use of AI compared with tamoxifen was 1.26 (CI=1.10–1.43; P<0.001), with an increase in absolute risk of 0.8% (4.2% versus 3.4% risk, respectively). Despite the small absolute risk, the clinical relevance of these findings could be high in specific populations at risk. In the ATAC trial, for example, in women with preexisting heart disease, the incidence of cardiovascular events was 17% in the anastrozole arm compared with 10% in the tamoxifen arm, which led to the US Food and Drug Administration (FDA) label recommendation that the risks and benefits of anastrozole be considered in this group of patients.244 Various adverse CVD effects have been demonstrated in trials comparing AI with tamoxifen245; however, a randomized controlled trial comparing AI and placebo after 5 years of tamoxifen did not demonstrate any difference in CVD end points in early-stage breast cancer.246
The results of population-based studies that investigated cardiovascular risk in postmenopausal women treated with AIs versus tamoxifen have also been mixed,247,248 but large studies have traditionally been limited by a lack of information about cardiovascular risk factors and medications.247 A recent analysis of 13 273 postmenopausal women with endocrine-positive breast cancer in the Kaiser Permanente managed care system showed no association between AI use and risk of ischemia and stroke. Patients receiving AIs had a significantly higher risk of other forms of CVD (dysrhythmia, valvular dysfunction, pericarditis, HF, or cardiomyopathy), the significance of which requires further investigation.249 In a recent meta-analysis of AIs and tamoxifen in the adjuvant and extended adjuvant setting, significant CVD risk reduction was seen in patients treated with tamoxifen compared with placebo or no treatment in the adjuvant setting, and in the extended adjuvant setting, there was not an increased risk with AIs compared with placebo. These data suggest that the increased risk of cardiovascular effects of AIs compared with tamoxifen in randomized controlled trials might be secondary to the cardioprotective effects seen with tamoxifen.250
Ovarian Suppression Therapy
Premenopausal women with stage II or stage III breast cancers who would ordinarily be advised to receive adjuvant chemotherapy should receive ovarian suppression in addition to endocrine therapy.251 In clinical studies, ovarian suppression was associated with a substantial increase in menopausal symptoms, sexual dysfunction, and diminished quality of life compared with tamoxifen alone but not cardiovascular specific side effects, which likely reflects the younger age and overall low cardiovascular risk profile of these patients. Longer follow-up will be needed to assess the effect of early menopause on cardiovascular risk, morbidity, and mortality in these patients.
HER2-Targeted Therapies
Monoclonal Antibodies
Trastuzumab and pertuzumab are the 2 current FDA-approved monoclonal antibodies that inhibit the signaling of HER2 (human epidermal growth factor receptor 2). LV dysfunction associated with targeted therapies has been most extensively evaluated in the breast cancer population treated with trastuzumab.252 Trastuzumab binds to the extracellular domain of the ErbB2 (erb-b2 receptor tyrosine kinase 2)/HER2 and leads to reduced ErbB2 signaling via several mechanisms. It has been speculated that the cardiac dysfunction associated with trastuzumab is a direct consequence of ErbB2 inhibition in cardiomyocytes.253 In contrast to anthracycline-induced cardiotoxicity, trastuzumab exposure can result in LV dysfunction and HF that is mostly reversible.254 At highest risk for cardiotoxicity from trastuzumab exposure are those >50 years of age, patients with underlying heart disease or hypertension, those with baseline LVEF between 50% and 55% or lower, and those who have also received anthracycline therapy (Table 2).183,255 The introduction of adjuvant trastuzumab (with chemotherapy) for patients with HER2-positive early-stage breast cancer has reduced the risk of breast cancer recurrence by 50% and mortality by 33%.256 However, in the 5 major adjuvant trastuzumab trials,257–260 symptomatic, severe HF or cardiac events, with an incidence ranging from 0% to 3.9%, were observed with the addition of trastuzumab to traditional chemotherapy.261–263 Long-term follow-up data (median 8 years) after adjuvant chemotherapy in the international, multicenter, open-label, randomized HERA trial (HERceptin Adjuvant) of 5102 women demonstrated low overall cardiotoxicity, with a greater cumulative incidence of cardiotoxicity (4.1% versus 7.2% with New York Heart Association functional class I or II and significant drop in LVEF ≥10 points with absolute LVEF <50%) and no additional benefit in those treated with 2 years versus 1 year of trastuzumab therapy.259 Population-based studies, particularly in older individuals (women >65 years old), have suggested higher rates of cardiotoxicity. In a Surveillance, Epidemiology, and End Results program–based analysis of 9535 women (median age, 71 years) with early-stage breast cancer, of whom 2203 (23.1%) received trastuzumab, the rate of HF was 29.4% compared with 18.9% in nonusers of trastuzumab (P<0.001). Older age (age >80 years; HR, 1.53; 95% CI, 1.16–2.10), coronary artery disease (HR, 1.82; 95% CI, 1.34–2.48), hypertension (HR, 1.24; 95% CI, 1.02–1.50), and weekly trastuzumab administration (HR, 1.33; 95% CI, 1.05–1.68) increased the risk of HF.264
Current clinical trials in early breast cancer are taking advantage of the role of dual HER2 blockade, including the synergistic activity of pertuzumab and trastuzumab. Two neoadjuvant studies (NeoSphere and TRYPHAENA) demonstrated higher pathological complete response rates in women with HER2-positive breast cancer treated with chemotherapy and dual HER2 blockade (pertuzumab, trastuzumab) than in those treated with chemotherapy and trastuzumab therapy alone. In the TRYPHAENA study, the primary end point of cardiac safety was met, with a low incidence of symptomatic and asymptomatic LV systolic dysfunction across all arms.265 To date, there have not been any additional cardiac safety concerns when those agents were combined.266,267 In a recently published large, prospective, randomized trial (APHINITY trial) exploring trastuzumab with or without pertuzumab with adjuvant chemotherapy in the treatment of women with early-stage HER2-positive breast cancer, there was a low incidence of HF (0.7% in the pertuzumab group versus 0.3% in the placebo group, with HF defined as New York Heart Association functional class III or IV and a significant drop in LVEF ≥10 points with absolute LVEF <50%) and cardiac death (2 cardiac deaths in each arm).268
Small-Molecule Tyrosine Kinase Inhibitors
An alternative approach to inhibit HER2-mediated signaling is the use of small-molecule tyrosine kinase inhibitors that target the HER2 intracellular kinase domain. Lapatinib is a reversible small-molecule tyrosine kinase inhibitor that is frequently administered in combination with capecitabine or with endocrine therapy and is approved for the treatment of women with HER2-positive metastatic breast cancer.269,270 A phase III trial comparing lapatinib monotherapy versus combination therapy with trastuzumab demonstrated a 4.5-month survival advantage with combination therapy but also reported a higher incidence of serious adverse cardiac events of systolic dysfunction with combination therapy (6.7% versus 2%).271 Subsequently, the ALTTO trial (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization) of 8381 women with early-stage breast cancer demonstrated no significant improvement in disease-free survival with adjuvant treatment that included lapatinib compared with trastuzumab monotherapy. Those treated with lapatinib experienced more noncardiac side effects (diarrhea, cutaneous rash, and hepatic toxicity); however, in all treatment arms, the incidence of cardiotoxicity was low (2%–3% for HF, 2%–5% for any cardiac event).272
Emerging Therapies
Cyclin-dependent kinase (CDK) 4/6 inhibitors are being developed to overcome endocrine resistance based on cross talk between ER pathways. CDK 4/6 is a group of serine/threonine kinases that accomplish their action by forming a complex with cyclin D. In turn, this complex phosphorylates the retinoblastoma protein and deactivates it, which results in gene transcription and progression of cell replication in the transition between the G1 and S process.273–275 In cancer, CDK 4/6 are upregulated and participate in tumor growth by blocking tumor suppression and apoptosis. Blocking the formation of CDK 4/6 cyclin D complex causes cell cycle arrest.276,277
The CDK 4/6 inhibitors that have been evaluated in the treatment of women with metastatic breast cancer are palbociclib, ribociclib (both approved by the FDA), and abemaciclib (pending approval by the FDA). All of these agents have been tested in phase II and III clinical trials and are administered in combination with endocrine therapy (eg, AI, fulvestrant). The clinical benefit of CDK 4/6 inhibitors in the treatment of women with metastatic breast cancer is beyond the scope of this review. The most common adverse events reported with these agents are bone marrow suppression (neutropenia, anemia, and thrombocytopenia), fatigue, vomiting, and diarrhea.277,278 Ribociclib is the only oral CDK 4/6 inhibitor for which there are cardiovascular concerns: prolongation of the corrected QT interval was seen in ≈9% of patients at doses of 600 mg/d (current approved dose in clinical practice) and in ≈33% at doses of >600 mg/d.279,280 This has led to the product label stipulating that ribociclib should be initiated only when the corrected QT interval is <450 ms and that electrocardiograms must be performed at baseline, on day 14, before starting cycle 2 (day 28), and then as clinically indicated.281 Healthcare professionals and patients should be educated about the importance of avoiding concomitant use of other drugs that could further prolong the QT interval.
Radiation Therapy
The biological mechanisms of RT cardiotoxicity remain incompletely understood but are most likely secondary to alterations in multiple pathways, including myocyte ischemia/injury, inflammation, fibrosis, oxidative stress, and microvascular dysfunction. Animal models of RT cardiotoxicity indicate that an inflammatory response is elicited within cardiac and endothelial cells, with increased adhesion molecule and cytokine activity.282 RT also results in increased reactive oxygen species in cardiac tissues.283 In the rodent heart, radiation causes microvascular endothelial damage, which leads to lymphocyte adhesion and extravasation. This is followed by thrombus formation and capillary loss. Progressive reduction in capillary patency eventually results in ischemia, myocardial cell death, and fibrosis. In vitro and in vivo studies demonstrate that RT has significant effects on the macrovasculature and microvasculature, causing myocardial ischemia and injury.284 In large blood vessels, such as the coronary and carotid arteries, RT causes inflammation and oxidative damage, which, in the presence of high cholesterol, leads to lipid peroxidation and the formation of foam cells that initiate the atherosclerotic process. RT results in accelerated atherosclerosis, with thickened and fibrotic media/adventitia.285 Myocyte fibrosis is a consequence of ischemia and has been observed in cardiac histopathological studies in animals with RT.285 Intriguing, emerging data suggest a cardioprotective role for mast cells in cardiac RT injury, through the kallikrein-kinin pathway.286–288
Thoracic RT carries a significant risk of CVD toxicity that results in increased morbidity and mortality, which limits the critical gains in cancer control and survival.289–291 Cardiovascular effects secondary to coronary atherosclerosis and accelerated endothelial injury can occur as early as 5 years after exposure among breast cancer survivors who receive left-sided thoracic RT, and persistence of risk remains for up to 30 years.292 In addition to macrovascular disease, patients can also develop microvascular dysfunction that results clinically in impaired coronary flow reserve, myocardial ischemia, and myocardial fibrosis.292 A recent population-based case-control study demonstrated an increased risk in HF with preserved ejection fraction among women treated with contemporary breast cancer RT, which correlated with mean cardiac radiation dose.293 RT also increases the risk of anthracycline-induced cardiotoxicity.294 Acute and chronic pericarditis, valvular regurgitation and stenosis, and conduction abnormalities and sudden death are also well described, especially with chest radiation doses >30 Gy.295 Autonomic dysfunction is an underreported cardiovascular effect of RT that has an increased prevalence with higher radiation doses, and the associated impaired heart rate recovery significantly affects all-cause mortality.296
In a study of 2168 women with breast cancer, each 1-Gy increase in mean heart radiation dose was associated with a 7.4% increase in coronary events.5 In a meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group of >23 000 women, there was an excess of non–breast cancer deaths after 5 years among patients receiving RT, principally attributable to CVD and lung cancer.297 Multiple epidemiological studies support these results.6,298,299 Mortality ratios defined by laterality of breast cancer in 558 871 women from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program suggest that left-sided breast cancer patients treated with RT have a 1.19 to 1.90 increased risk of CVD mortality compared with those with right-sided breast cancer.299 Although some studies suggest that the CVD risks might be decreasing over time given improvements in treatment techniques, there is an important need for long-term data, particularly in the era of proton therapy.6 However, even with the use of modern techniques, including computed tomography planning and deep inspiration breath hold, incidental irradiation to smaller volumes of the heart results in cardiac perfusion defects.300–302
MONITORING FOR CARDIOVASCULAR TOXICITY
Early Identification of Risk
LVEF obtained by echocardiography, multigated acquisition scan, or cardiac magnetic resonance imaging (MRI) is used to identify patients with cardiotoxicity; however, a change in LVEF could constitute a late manifestation of myocardial damage and might represent irreversible cardiac damage, thus emphasizing the need to predict cardiac damage before its occurrence. In addition, the limited accuracy and variability of 2-dimensional echocardiography (on the order of 10% in non–core laboratory quantitation settings) for LVEF assessment,303 coupled with a lack of consensus of what constitutes a clinically significant reduction in cardiac function, suggest a critical need for a consensus for diagnostic and prognostic strategies. The variability in LVEF assessment can be of the same magnitude used to define cardiotoxicity in some settings. Furthermore, serial LVEF monitoring, although accepted, has never been validated in terms of outcomes. It is noteworthy that the sensitivity of 2-dimensional echocardiography for detection of small changes in LV function is low and is affected by changes in loading conditions associated with chemotherapy, which can then affect the LVEF value. As such, the development of strategies for early detection of cardiotoxicity has been the focus of more recent research efforts. In the clinical setting, these strategies thus far have fallen largely into 3 main categories: myocardial strain imaging, cardiac biomarkers, or a combination of imaging and biomarkers.
Myocardial Strain Imaging for Risk Identification
Speckle-tracking echocardiography for detection of LV myocardial strain is a newer cardiac imaging technique. It measures LV regional and global deformation in response to force as a marker of contractility and elasticity. Change in LV strain measures precedes change in LVEF,304 and strain has been shown to be a predictor of cardiac dysfunction in breast cancer patients receiving chemotherapy.305
One study showed that an absolute longitudinal strain value of ≤17.2% obtained 6 months after anthracycline (doxorubicin or epirubicin) therapy was predictive of abnormal longitudinal strain at 1-year follow-up, with a sensitivity of 100% and specificity of 80%.306 Strain alterations were associated with higher doses of anthracyclines, although within the dose range considered to have an acceptable toxicity profile. Global longitudinal strain has also been shown to be an early predictor of subsequent LV systolic dysfunction in patients treated with trastuzumab.307 Studies by others have also demonstrated that longitudinal and circumferential strain have diagnostic and prognostic relevance in female breast cancer patients undergoing treatment with doxorubicin or trastuzumab.308
Myocardial strain has excellent interobserver and intraobserver variability compared with other cardiac imaging modalities309 and is highly correlated with the relatively expensive and much less available cardiac MRI in the assessment of LV dysfunction.310 In addition, strain imaging as obtained with echocardiography is less expensive and less time consuming, does not confer radiation risk to the patient, and is not nephrotoxic compared with other cardiac imaging modalities for LVEF assessment; however, echocardiography image acquisition can be significantly limited in the setting of obesity and lung disease.
Biomarkers as Risk Markers of Chemotherapy-Induced Cardiac Disease
The role of biomarkers in this setting lies in the prediction of cardiomyopathy or detection of subclinical cardiomyopathy. Studies have evaluated the role of cardiac biomarkers, particularly brain natriuretic peptide (BNP) and troponin I, which accurately reflect myocardial injury, in the evaluation of cardiotoxicity associated with breast cancer therapy.311,312
Troponin I
Troponin I is a well-established, highly sensitive and specific marker of myocardial injury with a wide diagnostic window and is a robust chemical assay.313 A rise in troponin-I within ≈3 days of high-dose chemotherapy has been shown to predict a reduction in LVEF.314 Troponin I has an excellent negative predictive value for not developing cardiotoxicity during and immediately after treatment. Additional studies have shown similar findings, with a negative predictive value of 90% for ruling out the possibility of doxorubicin-induced systolic dysfunction with troponin I.305 Troponin I has also been successful in differentiating reversible and irreversible LV dysfunction associated with trastuzumab,315 as well as in the identification of cardiovascular outcomes (defined as death resulting from a cardiac cause, acute pulmonary edema, overt HF, asymptomatic LVEF reduction [≥25% from baseline], or life-threatening arrhythmias) in patients receiving various types and combinations of high-dose chemotherapy. In contrast, it has also been demonstrated that troponin I increases were common in breast cancer patients receiving both trastuzumab and lapatinib but were not associated with subsequent LV dysfunction according to multigated acquisition scans.316
Natriuretic Peptides
There is a lack of consensus regarding the role of N-terminal pro-BNP (NT-proBNP) in breast cancer cardiotoxicity diagnosis and prognosis. Although some studies have shown that NT-proBNP did not predict cardiotoxicity in adults with breast cancer,316,317 others have supported the role of proBNP in the identification of these patients.318,319 Of note, in a group of patients in which most had received chemotherapy, BNP showed an area under the curve of 0.86 in the detection of asymptomatic LV systolic dysfunction.320 One study showed NT-proBNP to be more useful than troponin in the detection of subclinical LV dysfunction,321 and another showed that it predicted mortality at 1-year follow-up.322 Pitfalls associated with the use of natriuretic peptides include biological variation and variability with age, weight, renal function, and BMI. This calls for caution in the interpretation of data obtained using these serum markers.
Novel Biomarkers in Risk Identification
Data are limited on associations between novel biomarkers and cancer therapy–related cardiac dysfunction323–329; however, there are limited data in breast cancer patients. A small study in 78 patients with breast cancer undergoing doxorubicin and trastuzumab chemotherapy found that early changes in high-sensitivity troponin I and myeloperoxidase improved risk prediction of the first cardiotoxic event.330 Consideration was given to every cardiotoxic event and biomarker measure over the entire 15-month follow-up period, and myeloperoxidase, placental growth factor, and growth differentiation factor 15 were significantly associated with a risk of cardiotoxicity at the same and subsequent visits.331 Another study showed only NT-proBNP as a predictor of subclinical cardiotoxicity after treatment with anthracyclines.332
Imaging Combined With Biomarkers
Because both imaging and biomarkers are used as end points of the same pathological process, there could be value to using these methods conjointly to detect cardiotoxicity. In one study,317 a change in longitudinal strain and troponin I at 3 months after doxorubicin chemotherapy predicted cardiotoxicity at the 6-month follow-up. Cardiotoxicity was also predicted by the number of segments with change in longitudinal strain at 3 months. In another seminal article by the same group,305 the combination of a 10% decrease in longitudinal strain with elevation in troponin I predicted cardiotoxicity after doxorubicin therapy with a specificity of 97%, whereas either marker alone showed a sensitivity of 89% and a negative predictive value of 97% in detecting cardiotoxicity. The data therefore suggest possible benefit to the use of combinations of risk markers (strain imaging modality plus biomarkers in this case) in the identification of people at risk for cardiotoxicity after chemotherapy.
Biomarkers in the Identification of Radiation-Induced Cardiotoxicity
A few studies have evaluated biomarkers in patients undergoing RT with conflicting results. One early study of 50 women with breast cancer did not find any change in serum troponin after a total dose of 45 to 46 Gy of RT.333 Conversely, a more recent study of lung and breast cancer patients found both troponin and BNP increased significantly with radiation, although the absolute and mean values remained at a relatively low level.334 In another recent study of 58 patients receiving RT for left-sided breast cancer, high-sensitivity troponin T increased during RT from baseline in 12 patients (21%). In that study, those patients with higher high-sensitivity troponin T values had received significantly higher radiation doses for the whole heart and LV than those with stable high-sensitivity troponin T values.335 Hence, cardiac biomarkers might be useful for evaluation of radiation-induced cardiotoxicity, but this requires further research before widespread implementation.
In recent times, the role of cardiac MRI in the detection of cardiotoxicity associated with cancer therapy has been studied. In addition to detecting an early subclinical decline in LVEF, cardiac MRI also has the ability to detect subtle changes in cardiac structure and to help identify the specific cause of abnormalities in LVEF.171 Thus, this is an imaging technique with a possible important role in detection of cardiotoxicity associated with cancer therapy; however, its specific role in this process is limited by associated costs and limited availability.
ONCOLOGIC STRATEGIES TO MITIGATE CARDIOTOXICITY
The multidisciplinary approach to the care of cancer patients exposed to multimodality cancer therapy has resulted in an increased understanding of the mechanisms of cancer therapy–related cardiotoxicity. This has led to the investigation and adoption of clinical approaches to mitigate the impact of cancer treatments on cardiovascular health.
Dexrazoxane
Dexrazoxane has been used as a chelating agent that binds to intracellular iron, thus decreasing free radical formation and reducing cardiomyocyte apoptosis. Similar cardioprotection has not been achieved with other iron-binding agents, however, and more recent work suggests that dexrazoxane changes the conformation of topoisomerase IIβ and interferes with anthracycline binding.336
Multiple trials conducted in breast cancer patients undergoing chemotherapy with doxorubicin or epirubicin showed significant reductions in the combined end point of decrease in LVEF or development of HF with the addition of dexrazoxane and follow-up intervals that ranged from 15 to 43 months.337 A meta-analysis of 7 trials (1000 patients) estimated an overall reduction in cardiac events of 65% (RR, 0.35; 95% CI, 0.27–0.45) with dexrazoxane versus placebo.338 Others estimated a 79% reduction in clinical HF when dexrazoxane was included in the treatment regimen (RR, 0.21; 95% CI, 0.13–0.33).192 The most recent Cochrane database concluded on the basis of 8 trials that dexrazoxane use was associated with an 82% reduction in HF (RR, 0.18; 95% CI, 0.1–0.32), with no impact on progression-free survival, overall survival, or cancer response rates (RR, 0.89; 95% CI, 0.78–1.02, _P_=0.08).339 Because of persistent uncertainty about the risk of secondary malignancies (primarily based on long-term follow-up of pediatric patients) and possibly reduced tumor response rates, the FDA indication for dexrazoxane is quite narrow in breast cancer patients. Dexrazoxane is “indicated for reducing the incidence and severity of cardiomyopathy associated with doxorubicin administration in women with metastatic breast cancer who have received a cumulative doxorubicin dose of 300 mg/m2 and who will continue to receive doxorubicin therapy to maintain tumor control. It is not recommended for use with the initiation of doxorubicin therapy.”340 The American Society of Clinical Oncology has issued similar guidance.341 The European Medicines Agency has expanded the usage to include patients who have a received a minimum cumulative epirubicin dose of 540 mg/m2.342,343
Doxorubicin Administration
Although total cumulative dose administered appears to be the strongest predictor of subsequent cardiotoxicity,294 rate of infusion (presumably representing peak plasma level) also has a significant impact. Administration of doxorubicin via infusion as opposed to bolus administration is associated with a significant decrease in the risk of symptomatic cardiotoxicity without loss of antitumor efficacy (OR, 4.13; 95% CI, 1.75–9.72 for bolus administration versus infusion).192 A Cochrane database meta-analysis of 6 trials involving 735 patients showed a 73% reduction in clinical HF with infusion duration of ≥6 hours compared with shorter duration of infusion (RR, 0.27; 95% CI, 0.09–0.81; _P_=0.02) without significant heterogeneity among trials.344 Only 1 of these studies reported on LVEF and demonstrated lesser declines with the prolonged infusion at cumulative doses of 300 and 400 mg/m2.345 Clinical HF rates and survival did not differ in 2 studies that compared peak doses of ≥60 mg/m2 with peak doses of <60 mg/m2.344 Doxorubicin administered via continuous infusion (up to 48–96 hours) is not widely used because of practical issues requiring hospital admission.
Liposomal (pegylated or nonpegylated) formulations of doxorubicin permit delivery of a higher cumulative dose with preserved efficacy but a lower rate of cardiac side effects (OR, 0.18; 95% CI, 0.08–0.38).192 This favorable ratio of benefit and adverse effect is attributed to ready penetration through the vasculature within the tumor tissue, whereas the liposomes tend to remain in the intravascular space in other organs, including the heart.346 In the United States, liposomal preparations do not carry an indication for breast cancer, but they are approved for secondary therapy in ovarian cancer, AIDS-related Kaposi sarcoma, and multiple myeloma.248
Radiation Techniques
Newer radiation techniques (proton therapy, deep-inspiration breath holding, respiratory gating, lateral decubitus position, or use of modern 3-dimensional planning) have been devised that limit radiation dose overall and dose per fraction and limit the volume of heart exposed to radiation.347–349 These newer techniques are likely to be associated with lower complication rates, but long-term follow-up data are not available at this time.
PREVENTIVE THERAPIES
The risk-benefit profile of each patient must be taken into consideration when choosing chemotherapy that can potentially cause cardiotoxicity, particularly in patients who, a priori, are considered to be at high risk for myocardial damage. Close collaboration between the oncologist and cardiologist is essential (Figure 4 provides a simplified approach to the detection, prevention, and treatment of HF in breast cancer patients). There are no definitive guidelines specific to CVD prevention in breast cancer patients, because most of these studies were small and had variable end point definitions. Nevertheless, the next section will review CVD medications that could be used from a preventive standpoint for CVD or breast cancer, the role of exercise in the breast cancer patient, and CVD prevention in breast cancer survivorship.
Figure 4. Simplified depiction of detection, prevention, and treatment of left ventricular systolic dysfunction in breast cancer.
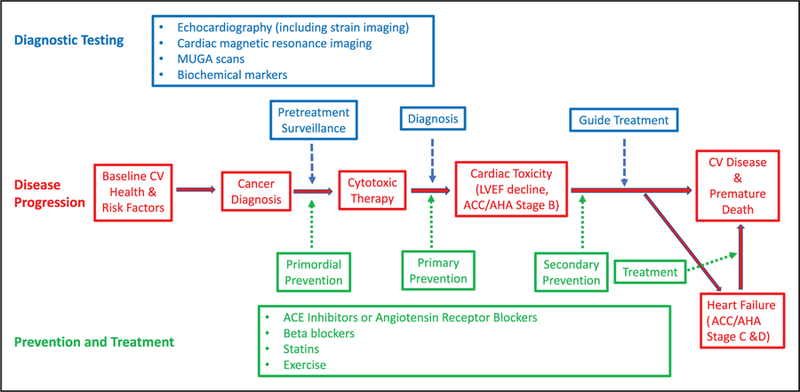
Schematic of the continuum of breast cancer treatment in relation to subsequent cardiac toxicity and heart failure. ACC indicates American College of Cardiology; ACE, angiotensin-converting enzyme; AHA, American Heart Association; CV, cardiovascular; LVEF, left ventricular ejection fraction; and MUGA, multigated acquisition. Modified from Khouri et al.350 Copyright © 2012, American Heart Association, Inc.
β-Blockers and Renin-Angiotensin-Aldosterone System Blockade
Small randomized, placebo-controlled trials have shown benefit of prophylactic β-blocker (BB) therapy in breast cancer patients. Two trials, one predominately of breast cancer patients and the other solely of breast cancer patients, showed less of a decline in LV function at 6 months with prophylactic BB therapy (carvedilol or nebivolol) than with placebo before the initiation of anthracycline-based chemotherapy.351,352 Another study found that breast cancer patients with structurally normal hearts at baseline who were treated with anthracycline or trastuzumab and who were taking a BB during treatment had a lower incidence of new HF events.353 Conversely, a retrospective study of 179 patients receiving adjuvant trastuzumab for early breast cancer found that BB and angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) use did not alter the risk of trastuzumab-induced cardiotoxicity.354 In balance, the data are mixed about whether and which type of prophylactic BB should be started in patients before the initiation of breast cancer therapy involving anthracycline or trastuzumab. PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) was a 2×2 factorial randomized, double-blind, placebo-controlled trial in patients with early breast cancer who had surgery and were scheduled for adjuvant chemotherapy (5-FU, epirubicin, and cyclophosphamide). Patients received a combination of candesartan, metoprolol succinate, and placebo. A modest reduction in LV function was attenuated with candesartan (0.8% versus 2.6% in the placebo group, _P_=0.026), but metoprolol succinate showed no effect on overall LVEF decline.355 The more recently published MANTI-CORE (Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research) 101–Breast trial was a randomized, placebo-controlled, double-blind trial in patients with HER2-positive early breast cancer who were being treated with trastuzumab adjuvant therapy. During the entire duration of adjuvant therapy, they also received treatment with either perindopril, bisoprolol, or placebo (1:1:1). There was a small decline in LVEF in the placebo group (5%), whereas the decline in LVEF was attenuated to 3% in the ACE inhibitor group and 1% in the BB group.356 However, neither pharmacotherapy prevented the primary end point of trastuzumab-mediated LV remodeling, which was defined as the change over time in indexed LV end-diastolic volume on cardiac MRI. Neither of these studies reported on HF outcomes, perhaps because of insufficient statistical power.
During treatment, early initiation of HF therapy for anthracycline-induced cardiomyopathy is beneficial. In a study of 201 patients with anthracycline-induced cardiomyopathy regardless of HF symptoms, 74% received maximally tolerated doses of enalapril and carvedilol, whereas the rest tolerated only enalapril. The majority of patients had breast cancer. A decreased time to HF treatment and low New York Heart Association functional class were independent predictors of LVEF recovery.187 This group went on to show, in a cohort of 2625 cancer patients, that ≈9% developed anthracycline-induced cardiomyopathy, 98% of whom developed a decline in LVEF within 1 year of treatment (median, 3 months). Early detection of anthracycline-induced cardiotoxicity and treatment with HF therapy was associated with LVEF improvement.357 In a smaller study of 25 patients with doxorubicin-induced cardiomyopathy, the majority of whom had breast cancer, those treated with a combination of an ACE inhibitor and a BB had significant improvement in LVEF, which was not seen with ACE inhibitor treatment alone.358
BBs have been associated with recovery of LV function in trastuzumab-induced cardiomyopathy. In a retrospective study of 499 women receiving adjuvant trastuzumab for early-stage breast cancer, the combined use of an ACE inhibitor and a BB was independently associated with hypertension and a significant decline in LVEF from baseline at 3 months. However, this association is confounded by indications for antihypertensive therapy, and those on combined therapy were significantly older, with worse baseline clinical profile (higher rates of history of hypertension and dyslipidemia). Interestingly, the combined use of an ACE inhibitor and a BB was also associated with LVEF recovery from 3 to 12 months, which suggests that these drugs are beneficial in cardiac recovery.359 In a study of 38 women with suspected trastuzumab-induced LV dysfunction, once HF symptoms and LVEF were stable after treatment with trastuzumab ended, 25 women who were given maximally tolerated doses of ACE inhibitor and BB were rechallenged with trastuzumab, and 22 (88%) had a stable LVEF without HF symptoms in follow-up.254 It is not clear how many women were taking an ACE inhibitor or BB at baseline; however, this series also suggests the combined use of an ACE inhibitor and BB can lead to LV systolic recovery. In 247 consecutive patients, the majority with breast cancer, interventions such as the initiation of cardiac medications, which included BBs in 80% of cases, were associated with a significant improvement in mean LVEF but not a return back to baseline.360 For trastuzumab cardiotoxicity, the European Society of Medical Oncology and the Canadian Trastuzumab Working Group recommend initiation of an ACE inhibitor and BB in patients with HF and LVEF <40%; they also recommend an ACE inhibitor in all asymptomatic patients with LVEF <40%, and recommend that a BB should be considered in asymptomatic patients with LVEF <40%.261,361 The Canadian Cardiovascular Society’s guidelines recommend ACE inhibitor/ARB and BB combination therapy in cancer patients with an LVEF <40%, and these therapies should also be considered in those with an asymptomatic decline in LVEF (<10% decrease from baseline or LVEF <53%).362
Observational studies suggest that BB use can influence breast cancer mortality or recurrence.363 A retrospective study of 466 female patients with operable breast cancer and follow-up >10 years showed that patients taking a BB had a significant reduction in the development of metastasis and tumor recurrence and had longer disease-free survival.364 A meta-analysis of 46 265 breast cancer patients showed significant breast cancer–specific survival in patients treated with a BB.365 A similar observational study demonstrated that BB use was associated with a lower risk of breast cancer recurrence and cause-specific mortality.366 Another retrospective study of 1413 breast cancer patients showed BB use was associated with significantly better relapsefree survival, including in patients with triple-negative breast cancer.367 Other studies have shown that breast cancer patients treated with propranolol are significantly less likely to present with advanced disease and have a significantly lower cumulative probability of breast cancer–specific mortality compared with matched non-users.368 Conversely, a cohort study of 4126 women with early-stage breast cancer in an integrated health plan showed BB use carried an increased risk of recurrence, whereas a nationwide study of Danish breast cancer survivors showed BBs did not attenuate recurrence risk.369,370 However, there are confounding factors in all of these studies, and thus, there is a need for a large randomized controlled trial to elucidate the potential benefit of BBs, ACE inhibitors, and ARBs in breast cancer survivorship.
ACE inhibitors have been the agents best studied to prevent or treat LV dysfunction, whereas ARBs are less well studied in this area. With the increased role of the mineralocorticoid antagonists in HF, including HF with preserved ejection fraction, and its potential to diminish fibrosis, spironolactone would be a logical protective agent. At least 1 study has shown that when spironolactone is used concomitantly with an anthracycline drug in breast cancer patients, the decline in ejection fraction was attenuated in addition to diastolic stabilization.371 This drug could have tremendous potential, and further studies are required. Although data from large randomized controlled trials of BB, ACE inhibitor/ARB, and spironolactone use in breast cancer patients are not yet available, it seems reasonable to treat anthracycline or trastuzumab-induced cardiomyopathy in accordance with the American College of Cardiology/American Heart Association HF guidelines.372,373
Aspirin
A number of observational studies have suggested a possible benefit of regular aspirin use in the prevention of breast cancer. A 2008 study found a strong protective effect of regular aspirin use and breast cancer. In that study, the investigators reported that taking low-dose aspirin ≥4 d/wk over 10 years decreased the risk of breast cancer by 35% (HR, 0.65; CI, 0.43–0.97).374 A 2011 study analyzed data from 26 580 postmenopausal women 59 to 77 years of age, 1581 of whom were diagnosed with breast cancer. These investigators found that women who regularly took aspirin had a 20% lower risk of developing breast cancer (RR, 0.80; 95% CI, 0.71–0.90). They further found that the risk decreased with increasing frequency of use, with a risk reduction of 29% in women taking aspirin ≥6 times per week compared with those who never took aspirin (RR, 0.71; P for trend=0.00001).375
Other trials, however have found less or no protective effect of regular aspirin use and prevention of breast cancer. A 1996 report using data from the NHS found no protective effect against breast cancer for those taking regular aspirin. These investigators further analyzed aspirin use in 2414 cases of invasive breast cancer and again, in this subset of patients, found that regular aspirin use was not associated with prevention of breast cancer.376
A few studies have suggested an increased risk of breast cancer among women taking regular aspirin. Data from a Danish cohort demonstrated regular aspirin use actually increased risk of breast cancer by 38% (RR, 1.38; 95% CI, 1.12–1.69).377 They found a nonsignificant increase in risk of recurrence of breast cancer in women taking aspirin.378 A large study on the role of aspirin in breast cancer prevention was the Women’s Health Study, published in 2005.379 This was a randomized, placebo-controlled trial of 39 876 women ≥45 years old who were randomly assigned to take either 100 mg of aspirin every other day or placebo. After 10 years of follow-up, no protective effect was seen in breast cancer (RR, 0.98; 95% CI, 0.87–1.09; _P_=0.68).
Although these individual studies do not provide strong support for aspirin use to prevent breast cancer, several meta-analyses did find a reduction in breast cancer incidence with regular aspirin use. One of the most important meta-analyses included data from 38 studies including 2 788 715 women and reported that regular aspirin use reduced risk of breast cancer by 13% (RR, 0.87; 95% CI, 0.82–0.92).380 There is clearly equipoise about the question of whether regular aspirin use prevents incident or recurrent breast cancer, and an ongoing placebo-controlled, randomized trial381 is expected to answer the latter question.
Statins
An early statin trial, the CARE trial (Cholesterol and Recurrent Events)382 reported the unexpected finding of an increased incidence of breast cancer in those assigned to statin therapy. However, these findings were thought to be spurious and an anomaly owing in part to a very low incidence of breast cancer cases in the placebo group and an imbalance in risk factors for breast cancer at baseline. No other statin trials have found an association or an increased risk,383 and other investigators had surmised that statins might exert a beneficial effect and a reduction of cancer incidence, perhaps via inhibition of cell proliferation by systemic cholesterol reduction. However, a meta-analysis of 7 randomized controlled trials and 9 observational studies, with a total of 7858 breast cancer cases, found no association between statin use and breast cancer incidence.384 Additionally, data from 24 observational studies with 76 759 total breast cancer cases,385 showed no association between statin use and breast cancer incidence (RR, 0.99; 95% CI, 0.94–1.04). Finally, the WHI program studied 154 587 postmenopausal women 50 to 79 years of age and identified 7430 pathologically confirmed cases of breast cancer over an average follow-up of 10.8 years.386 They also found no association between statin use and breast cancer risk; the annualized rate of breast cancer was 0.42% among statin users and 0.42% among nonusers, and the multivariable adjusted HR of breast cancer for users versus nonusers was 0.94 (95% CI, 0.83–1.06).
Despite no evidence for an effect of statins on breast cancer incidence, there is some evidence that statins might favorably impact breast cancer prognosis. One such study was conducted on patients ≈2 years after a breast cancer diagnosis who were then followed up for 5 years.387 Using data on medication use from the pharmacy records of the Kaiser Permanente Health System, the adjusted HR for developing a recurrence of breast cancer in statin users versus nonusers was 0.67 (95% CI, 0.39–1.13). Another larger cohort study of Danish breast cancer patients found similar results with lipophilic statins.388 Interestingly, there are also ongoing studies evaluating a potential cardioprotective effect of statin therapy on anthracycline-induced cardiomyopathy and HF.389 Clearly, more data are needed on the potential protective effects of statin therapy.
Exercise
The vast majority of studies investigating exercise prevention of cancer treatment–induced cardiovascular toxicity are in rodent models, but there are limited data in humans.390 A single-arm study investigated the effects of 4 months of exercise training in 17 breast cancer survivors receiving adjuvant trastuzumab therapy and found that despite exercise training, trastuzumab was associated with LV dilatation and reduced LVEF.391 However, the exercise training intensity might have been insufficient, because participants attended only 59% of prescribed sessions. In a multicenter randomized controlled trial in Canada conducted between 2003 and 2005, 242 breast cancer patients initiating anthracycline-based adjuvant chemotherapy were randomized to usual care, supervised resistance exercise, or supervised aerobic exercise for the duration of their chemotherapy (median, 17 weeks; 95% CI, 9–24 weeks). Although adherence to prescribed exercise was high (70.2%) and the study demonstrated improvement in self-esteem, reduced percent body fat with aerobic exercise, and improved muscular strength, lean body mass, and chemotherapy completion rate with resistance training, no cardiovascular end points were assessed.392
An analysis of 2 large-scale prospective cohort studies in 2973 women with nonmetastatic breast cancer demonstrated a strong, graded inverse relationship between exercise intensity and incidence of cardiovascular events in general, as well as for the incidence of coronary artery disease and HF in particular. The benefit emerged in those with an exercise level of ≥10 metabolic equivalent h/wk, which matches the national exercise guidelines for adult patients with cancer (≥9 metabolic equivalent h/wk), and resulted in a 23% lower adjusted risk of cardiovascular events, 26% lower risk of coronary artery disease, and 29% lower HF risk in the study cohort.393 Further clinical research is needed to determine whether exercise during cancer therapy is a feasible and effective method for the reduction of cardiovascular morbidity and mortality in breast cancer survivors. There are 2 ongoing trials examining the role of exercise in breast cancer patients receiving chemotherapy. TITAN (Multidisciplinary Team Intervention in Cardio-Oncology) is a randomized controlled trial that will compare the effects of intensive multidisciplinary team interventions (nutritional counseling, exercise training, etc) with usual care for prevention of LV remodeling in patients with newly diagnosed early breast cancer or lymphoma who are scheduled for anthracycline-, trastuzumab-, or anthracycline and trastuzumab–based chemotherapy.394 OptiTrain is a randomized controlled trial that will assess the effects of different exercises (aerobic training, combined resistance and aerobic exercise training) versus usual care on the physical and mental health of breast cancer patients undergoing chemotherapy.395
Survivorship Programs
Improvements in cancer treatment have resulted in an increasing number of long-term cancer survivors who could have their survival and quality of life affected by concomitant CVD. Cardiovascular comorbidity can be preexistent or secondary to toxicity related to cancer therapy. Secondary prevention in the context of breast cancer relates to the management of preexisting cardiovascular risk factors and monitoring for cardiotoxicity during the survivorship period after breast cancer treatment has been completed.396
An initial step in deciding the medical approach in the survivorship stage is to determine risk of CVD. Breast cancer survivors at higher risk for CVD include patients with any cardiovascular abnormality noted in follow-up or patients who received doxorubicin >240 mg/m2, radiation >30 Gy, radiation plus anthracycline, or high-dose cyclophosphamide, particularly if strenuous activity or pregnancy is planned.396 Medical treatment of left ventricular dysfunction in patients with cancer generally follows the American Heart Association/American College of Cardiology HF guidelines372,373 with some modifications adapted for patients with the high-risk factors mentioned above. Importantly, treatment recommendations will need to be individualized to the overall survival prognosis of the breast cancer patients.396,397
Traditional cardiovascular risk factors, namely, hypertension, diabetes mellitus, dyslipidemia, and lifestyle, among others, will need to be assessed in survivorship programs. Management of blood pressure, glucose, and hypercholesterolemia and treatment of tobacco abuse should follow current American Heart Association Guidelines.398,399 The use of statins in patients with breast cancer includes the same indications as in primary prevention of CVD.397–399
In women with breast cancer, treatment and the disease itself contribute to weight gain and to decreases in physical activity.400–402 Furthermore, it has been reported that cardiopulmonary function in patients with breast cancer improves with exercise, and regular exercise results in an improvement in quality of life.397,403–406 Although the usefulness of exercise to prevent CVD in patients with breast cancer has not been evaluated in randomized trials, it is reasonable to follow the American Heart Association recommendations for physical activity, that is, moderate-intensity aerobic physical activity of ≥30 minutes 5 days each week.407 In addition, a recent meta-analysis of studies that examined all the effects of physical activity on the risk of breast cancer recurrence and mortality found that prediagnosis and postdiagnosis physical activity were both associated with a lower mortality and cancer recurrence rate, which supports the idea that physical activity reduces these events among breast cancer survivors.115
Posttreatment Cardiac Imaging Surveillance
Long-term surveillance aims at monitoring those with preexisting and ongoing CVD and those at risk for late cardiotoxicity, particularly those who received anthracyclines and mediastinal RT.396,397 Longer-term data suggest modest but persistent declines in LVEF with exposure to common cancer therapies, as well as an increase in measures of arterial and chamber stiffness,186 possibly suggestive of HF with preserved ejection fraction. Accordingly, most guidelines or expert consensus documents recommend surveillance imaging during breast cancer imaging and possibly posttreatment depending on agent and dose administered. A consensus document for multimodality imaging in adult cancer patients by the American Society of Echocardiography and the European Association of Cardiovascular Imaging recommends the evaluation of cardiac function (typically echocardiography and, if suboptimal, then cardiac MRI) at baseline, at completion of therapy, and at 6-month follow-up in patients receiving anthracycline therapy at cumulative doses <240 mg/m2; those with higher cumulative doses should have repeat LVEF measurement before each additional dose of 50 mg/m2, and any patient with LVEF <53% is recommended to have a cardiology consultation. Those receiving HER2-targeted therapies are recommended to undergo a baseline evaluation of LVEF, and then an LVEF evaluation every 3 months during therapy; however, those who were previously treated with an anthracycline should have LVEF reassessed 6 months after completion of HER2-targeted therapies (Figure 5). Additionally, after completion of breast cancer treatment, it was recommended that patients undergo annual cardiovascular assessments by a provider; however, cardiac imaging should be at the discretion of the provider, based on the history and physical examination.408
Figure 5. Echocardiographic surveillance during and after treatment with anthracycline therapy or HER2-directed therapy.
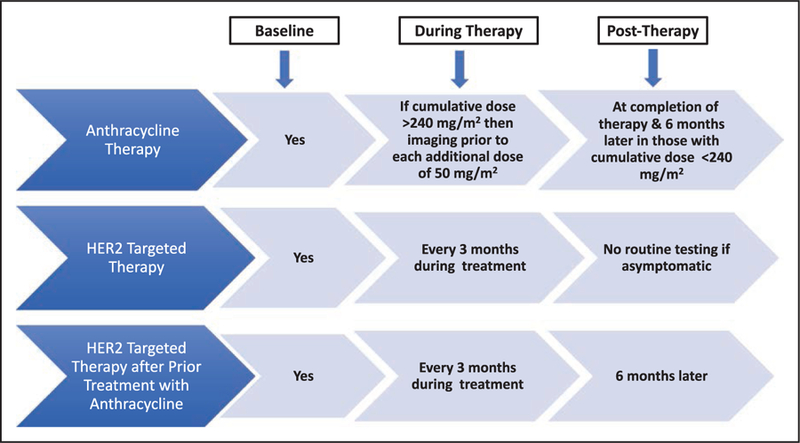
At any time during the course of surveillance imaging, if the ejection fraction is <53%, then cardiology consultation is recommended. If cardiac imaging is suboptimal, then cardiac magnetic resonance imaging should be considered. After completion of therapy, patients should undergo annual cardiovascular assessments by a provider; however, cardiac imaging should be at the discretion of the provider based on the history and physical examination. HER2 indicates human epidermal growth factor receptor 2. This Figure is based on recommendations from the consensus document for multimodality imaging in adult cancer patients prepared by the American Society of Echocardiography and the European Association of Cardiovascular Imaging.408
A subsequent expert consensus publication proposed that in low-risk patients, a transthoracic echocardiogram with strain be performed at the completion of therapy, with no need for further follow-ups if the test result is normal and the patient remains asymptomatic. In contrast, for patients at medium to high risk, a transthoracic echocardiogram with strain is recommended (1) only at completion of therapy for patients with cardiotoxicity as seen with agents such as trastuzumab, or (2) at completion of therapy and then at 6 to 12 months or 12 and 18 months after treatment among patients receiving anthracyclines alone or in combination with trastuzumab and RT; however further surveillance recommendations were not established.409 The American Society of Clinical Oncology practice guidelines for prevention and monitoring of LV dysfunction in adult cancer patients propose that all patients formerly treated with potentially cardiotoxic therapies undergo a detailed history and physical examination, and if there are any signs or symptoms of LV dysfunction, then further testing, including imaging assessment with echocardiography (or cardiac MRI or multigated acquisition), is recommended. Asymptomatic patients with a presumed elevated risk of potentially developing LV dysfunction may undergo imaging 6 to 12 months after completion of cancer therapy; however, if results are normal, there are no current recommendations regarding frequency of subsequent surveillance in these patients.11 These 3 different approaches reflect the knowledge gaps and uncertainty around the progression and natural course of these complications. Although more data are needed, it is reasonable to consider long-term imaging surveillance, and the decision should be a clinical decision (taking into account the history and physical examination), with the risks and benefits of further testing to be weighed by the clinician.408
FUTURE DIRECTIONS
As the population ages, there will likely be more women with breast cancer, CVD, or both. Current cancer treatments might increase the risk of both short- and long-term cardiotoxicity, and women who are breast cancer survivors could be at higher risk of death caused by CVD than women without a history of breast cancer. More research is needed in terms of optimal surveillance of cardiotoxicity, preventive therapies, and treatment of cardiotoxicity. There are gaps in care and in priorities that would help bridge care of the patient (Table 3). Improved risk assessment and development of personalized preventive strategies in cancer survivorship programs are imperative for improved outcomes and reductions in CVD mortality in the breast cancer patient. Over the past decade, the number of cardio-oncology programs has increased, but many are centered on the treatment of CVD secondary to cancer therapies, and less emphasis has been placed on prevention before development of cardiotoxicity. Clearly, there are common risk factors between breast cancer and CVD. The care of these patients extends beyond the silos of cardiology and oncology and should be interdisciplinary, with vigilance with regard to the primary prevention of CVD along with the secondary prevention of CVD.
Table 3.
Future Directions in Care of Breast Cancer and CVD Patients
| Gaps in care |
|---|
| What are more the effective predictors of cardiotoxicity? |
| Are there genetic or genomic variabilities that impact the risk of developing cardiotoxicity? |
| What are the determinants of racial disparities in outcomes? |
| With advancements in radiotherapy, what are radiation thresholds and alternative techniques to avoid the development of CVD toxicity? |
| Do newer breast cancer therapies substantially affect CVD risk? |
| What are the effects of risk factor modification on CVD outcomes in cancer patients? |
| What are pharmacological mechanisms to prevent or reverse cardiotoxicities of cancer therapy? |
| Priorities to improve outcomes |
| Improved screening and risk factor assessment in oncology patients |
| Development of targeted therapies with limited CVD adverse impact |
| Better understanding of combined chemotherapy and radiotherapy adverse effects on CVD |
| Earlier detection of CVD effects: imaging, biomarker, or clinical predictive models |
| Consistency in terminology of cardiovascular effects and then incorporation in trials as end point measures for CVD outcome |
| Formal guidelines for primary and secondary prevention of cardiotoxicity |
| Reduction in racial disparities in CVD outcomes with increase access to care |
| Need for national or international database on cardiovascular and oncologic outcomes |
Currently, several organizations, such as the American College of Cardiology (Cardio-Oncology Section), Canadian Cardiac Oncology Network, European Society of Cardiology, European Society of Medical Oncology, and the International Cardio-Oncology Society, are working on initiatives to promote collaboration of healthcare professionals from oncology and cardiology disciplines and to improve the care of cancer patients who are treated with potentially cardiotoxic therapies. Additionally, there must be standardization of nomenclature regarding cardiotoxicity, as well as training of the future generation of care providers.
CONCLUSIONS
Ideal breast cancer outcomes are reliant on coexisting cardiovascular health along the entire journey of breast cancer treatment. At the time of initial presentation, cardiac risk factors and preceding CVD can impact cancer treatment options. During breast cancer treatment, surveillance, prevention, and secondary management of cardiotoxicity are crucial; thereafter, long-term posttreatment monitoring for late cardiotoxicity and even non–treatment-related development of CVD is essential. With the evolving intersection of the cardiovascular and oncologic fields, comprehensive care is an essential element in the management of cancer patients to maximize gains in cancer treatment while minimizing the potential deleterious impact on cardiovascular health.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2017 update: a report from the American Heart Association [published corrections appear in Circulation. 2017;135:e646 and Circulation. 2017;136:e196]. Circulation 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosca L, Jones WK, King KB, Ouyang P, Redberg RF, Hill MN. Awareness, perception, and knowledge of heart disease risk and prevention among women in the United States: American Heart Association Women’s Heart Disease and Stroke Campaign Task Force. Arch Fam Med 2000;9: 506–515. [DOI] [PubMed] [Google Scholar]
- 3.Mosca L, Hammond G, Mochari-Greenberger H, Towfighi A, Albert MA; on behalf of the American Heart Association Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, Council on High Blood Pressure Research, and Council on Nutrition, Physical Activity and Metabolism. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation 2013;127:1254–1263, e1–e29. doi: 10.1161/CIR.0b013e318287cf2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver LC, Jessup A, Mayer DK. Cancer survivorship care: implications for primary care advanced practice nurses. Nurse Pract 2013;38:1–11. doi: 10.1097/01.NPR.0000435784.40143.62. [DOI] [PubMed] [Google Scholar]
- 5.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 6.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 7.EBCTC (Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials [published correction appears in Lancet. 2014;384:1848]. Lancet 2014;383:2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barac A, Murtagh G, Carver JR, Chen MH, Freeman AM, Herrmann J, Iliescu C, Ky B, Mayer EL, Okwuosa TM, Plana JC, Ryan TD, Rzeszut AK, Douglas PS. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. J Am Coll Cardiol 2015;65:2739–2746. doi: 10.1016/j.jacc.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenihan DJ, Cardinale D, Cipolla CM. The compelling need for a cardiology and oncology partnership and the birth of the International CardiOncology Society. Prog Cardiovasc Dis 2010;53:88–93. doi: 10.1016/j.pcad.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Lenihan DJ, Hartlage G, DeCara J, Blaes A, Finet JE, Lyon AR, Cornell RF, Moslehi J, Oliveira GH, Murtagh G, Fisch M, Zeevi G, Iakobishvili Z, Witteles R, Patel A, Harrison E, Fradley M, Curigliano G, Lenneman CG, Magalhaes A, Krone R, Porter C, Parasher S, Dent S, Douglas P, Carver J. Cardio-oncology training: a proposal from the International Cardioncology Society and Canadian Cardiac Oncology Network for a new multidisciplinary specialty. J Card Fail 2016;22:465–471. doi: 10.1016/j.cardfail.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 12.Cancer stat facts: female breast cancer. Surveillance, Epidemiology, and End Results Program websitehttps://seer.cancer.gov/statfacts/html/breast.html. Accessed June 17, 2017.
- 13.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; on behalf of the American Heart Association Advocacy Coordinating Committee, Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 14.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, Forsythe L, Scoppa S, Hachey M, Rowland JH. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev 2013;22:561–570. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman JA, Meng D, Shepherd L, Parulekar W, Ingle JN, Muss HB, Palmer M, Yu C, Goss PE. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst 2008;100:252–260. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, Valero V. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol 2007;25:4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 18.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology 2016;27:6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breast Cancer Facts & Figures 2015–2016https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2015-2016.pdf. Accessed March 5, 2017.
- 20.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54:78–93. [DOI] [PubMed] [Google Scholar]
- 21.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haque R, Prout M, Geiger AM, Kamineni A, Thwin SS, Avila C, Silliman RA, Quinn V, Yood MU. Comorbidities and cardiovascular disease risk in older breast cancer survivors. Am J Manag Care 2014;20:86–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 2011;103:1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunfeld E Cancer survivorship: a challenge for primary care physicians. Br J Gen Pract 2005;55:741–742. [PMC free article] [PubMed] [Google Scholar]
- 26.McCabe MS, Partridge AH, Grunfeld E, Hudson MM. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Semin Oncol 2013;40:804–812. doi: 10.1053/j.seminoncol.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin G, Berendsen A, Crawford SM, Dommett R, Earle C, Emery J, Fahey T, Grassi L, Grunfeld E, Gupta S, Hamilton W, Hiom S, Hunter D, Lyratzopoulos G, Macleod U, Mason R, Mitchell G, Neal RD, Peake M, Roland M, Seifert B, Sisler J, Sussman J, Taplin S, Vedsted P, Voruganti T, Walter F, Wardle J, Watson E, Weller D, Wender R, Whelan J, Whitlock J, Wilkinson C, de Wit N, Zimmermann C. The expanding role of primary care in cancer control. Lancet Oncol 2015;16:1231–1272. doi: 10.1016/S1470-2045(15)00205-3. [DOI] [PubMed] [Google Scholar]
- 28.Hong S, Nekhlyudov L, Didwania A, Olopade O, Ganschow P. Cancer survivorship care: exploring the role of the general internist. J Gen Intern Med 2009;24(suppl 2):S495–S500. doi: 10.1007/s11606-009-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK; on behalf of the American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 30.McSweeney JC, Rosenfeld AG, Abel WM, Braun LT, Burke LE, Daugherty SL, Fletcher GF, Gulati M, Mehta LS, Pettey C, Reckelhoff JF; on behalf of the American Heart Association Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Hypertension, Council on Lifestyle and Cardiometabolic Health, and Council on Quality of Care and Outcomes Research. Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American Heart Association. Circulation 2016;133:1302–1331. doi: 10.1161/CIR.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.What are risk factors for breast cancer? Centers for Disease Control and Prevention websitehttps://www.cdc.gov/cancer/breast/basic_info/risk_factors.htm. Accessed March 5, 2017.
- 32.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2016 update: a report from the American Heart Association [published correction appears in Circulation. 2016;133:e599]. Circulation 2015;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T; American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 36.Howell A, Anderson AS, Clarke RB, Duffy SW, Evans DG, Garcia-Closas M, Gescher AJ, Key TJ, Saxton JM, Harvie MN. Risk determination and prevention of breast cancer. Breast Cancer Res 2014;16:446. doi: 10.1186/s13058-014-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dartois L, Fagherazzi G, Baglietto L, Boutron-Ruault MC, Delaloge S, Mesrine S, Clavel-Chapelon F. Proportion of premenopausal and postmenopausal breast cancers attributable to known risk factors: estimates from the E3N-EPIC cohort. Int J Cancer 2016;138:2415–2427. doi: 10.1002/ijc.29987. [DOI] [PubMed] [Google Scholar]
- 38.Fung TT, Hu FB, Hankinson SE, Willett WC, Holmes MD. Low-carbohydrate diets, dietary approaches to stop hypertension-style diets, and the risk of postmenopausal breast cancer. Am J Epidemiol 2011;174:652–660. doi: 10.1093/aje/kwr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 40.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM; for the OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 41.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008;118:230–237. doi: 10.1161/CIRCULATIONAHA.108.771881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velie EM, Schairer C, Flood A, He JP, Khattree R, Schatzkin A. Empirically derived dietary patterns and risk of postmenopausal breast cancer in a large prospective cohort study. Am J Clin Nutr 2005;82:1308–1319. [DOI] [PubMed] [Google Scholar]
- 43.Brennan SF, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV. Dietary patterns and breast cancer risk: a systematic review and meta-analysis. Am J Clin Nutr 2010;91:1294–1302. doi: 10.3945/ajcn.2009.28796. [DOI] [PubMed] [Google Scholar]
- 44.Masala G, Assedi M, Bendinelli B, Ermini I, Sieri S, Grioni S, Sacerdote C, Ricceri F, Panico S, Mattiello A, Tumino R, Giurdanella MC, Berrino F, Saieva C, Palli D. Fruit and vegetables consumption and breast cancer risk: the EPIC Italy study. Breast Cancer Res Treat 2012;132:1127–1136. doi: 10.1007/s10549-011-1939-7. [DOI] [PubMed] [Google Scholar]
- 45.Catsburg C, Kim RS, Kirsh VA, Soskolne CL, Kreiger N, Rohan TE. Dietary patterns and breast cancer risk: a study in 2 cohorts. Am J Clin Nutr 2015;101:817–823. doi: 10.3945/ajcn.114.097659. [DOI] [PubMed] [Google Scholar]
- 46.Adebamowo CA, Hu FB, Cho E, Spiegelman D, Holmes MD, Willett WC. Dietary patterns and the risk of breast cancer. Ann Epidemiol 2005;15:789–795. doi: 10.1016/j.annepidem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez CA, Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer 2010;46:2555–2562. doi: 10.1016/j.ejca.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 48.van Gils CH, Peeters PH, Bueno-de-Mesquita HB, Boshuizen HC, Lahmann PH, Clavel-Chapelon F, Thiébaut A, Kesse E, Sieri S, Palli D, Tumino R, Panico S, Vineis P, Gonzalez CA, Ardanaz E, Sánchez MJ, Amiano P, Navarro C, Quirós JR, Key TJ, Allen N, Khaw KT, Bingham SA, Psaltopoulou T, Koliva M, Trichopoulou A, Nagel G, Linseisen J, Boeing H, Berglund G, Wirfält E, Hallmans G, Lenner P, Overvad K, Tjønneland A, Olsen A, Lund E, Engeset D, Alsaker E, Norat T, Kaaks R, Slimani N, Riboli E. Consumption of vegetables and fruits and risk of breast cancer. JAMA 2005;293:183–193. doi: 10.1001/jama.293.2.183. [DOI] [PubMed] [Google Scholar]
- 49.Lissowska J, Gaudet MM, Brinton LA, Peplonska B, Sherman M, Szeszenia-Dabrowska N, Zatonski W, Garcia-Closas M. Intake of fruits, and vegetables in relation to breast cancer risk by hormone receptor status. Breast Cancer Res Treat 2008;107:113–117. doi: 10.1007/s10549-007-9524-9. [DOI] [PubMed] [Google Scholar]
- 50.Edefonti V, Decarli A, La Vecchia C, Bosetti C, Randi G, Franceschi S, Dal Maso L, Ferraroni M. Nutrient dietary patterns and the risk of breast and ovarian cancers. Int J Cancer 2008;122:609–613. doi: 10.1002/ijc.23064. [DOI] [PubMed] [Google Scholar]
- 51.Murtaugh MA, Sweeney C, Giuliano AR, Herrick JS, Hines L, Byers T, Baumgartner KB, Slattery ML. Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: the Four-Corners Breast Cancer Study. Am J Clin Nutr 2008;87:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandran U, McCann SE, Zirpoli G, Gong Z, Lin Y, Hong CC, Ciupak G, Pawlish K, Ambrosone CB, Bandera EV. Intake of energy-dense foods, fast foods, sugary drinks, and breast cancer risk in African American and European American women. Nutr Cancer 2014;66:1187–1199. doi: 10.1080/01635581.2014.951737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castelló A, Pollán M, Buijsse B, Ruiz A, Casas AM, Baena-Cañada JM, Lope V, Antolín S, Ramos M, Muñoz M, Lluch A, de Juan-Ferré A, Jara C, Jimeno MA, Rosado P, Díaz E, Guillem V, Carrasco E, Pérez-Gómez B, Vioque J, Boeing H, Martín M; on behalf of the GEICAM researchers. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer 2014;111:1454–1462. doi: 10.1038/bjc.2014.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakai K, Tamakoshi K, Date C, Fukui M, Suzuki S, Lin Y, Niwa Y, Nishio K, Yatsuya H, Kondo T, Tokudome S, Yamamoto A, Toyoshima H, Tamakoshi A; for the JACC Study Group. Dietary intakes of fat and fatty acids and risk of breast cancer: a prospective study in Japan. Cancer Sci 2005;96:590–599. doi: 10.1111/j.1349-7006.2005.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Lim SY, Shin A, Sung MK, Ro J, Kang HS, Lee KS, Kim SW, Lee ES. Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: a case-control study. BMC Cancer 2009;9:216. doi: 10.1186/1471-2407-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maillard V, Bougnoux P, Ferrari P, Jourdan ML, Pinault M, Lavillonnière F, Body G, Le Floch O, Chajès V. N-3 and N-6 fatty acids in breast adipose tissue and relative risk of breast cancer in a case-control study in Tours, France. Int J Cancer 2002;98:78–83. [DOI] [PubMed] [Google Scholar]
- 57.Cho E, Spiegelman D, Hunter DJ, Chen WY, Stampfer MJ, Colditz GA, Willett WC. Premenopausal fat intake and risk of breast cancer. J Natl Cancer Inst 2003;95:1079–1085. [DOI] [PubMed] [Google Scholar]
- 58.Chajès V, Thiébaut AC, Rotival M, Gauthier E, Maillard V, Boutron-Ruault MC, Joulin V, Lenoir GM, Clavel-Chapelon F. Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC Study. Am J Epidemiol 2008;167:1312–1320. doi: 10.1093/aje/kwn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiébaut AC, Chajès V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, Berrino F, Riboli E, Bénichou J, Clavel-Chapelon F. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer 2009;124:924–931. doi: 10.1002/ijc.23980. [DOI] [PubMed] [Google Scholar]
- 60.Witt PM, Christensen JH, Schmidt EB, Dethlefsen C, Tjønneland A, Overvad K, Ewertz M. Marine n-3 polyunsaturated fatty acids in adipose tissue and breast cancer risk: a case-cohort study from Denmark. Cancer Causes Control 2009;20:1715–1721. doi: 10.1007/s10552-009-9423-y. [DOI] [PubMed] [Google Scholar]
- 61.Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013;346:f3706. [DOI] [PubMed] [Google Scholar]
- 62.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 63.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 64.Martin LJ, Li Q, Melnichouk O, Greenberg C, Minkin S, Hislop G, Boyd NF. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res 2011;71:123–133. doi: 10.1158/0008-5472.CAN-10-1436. [DOI] [PubMed] [Google Scholar]
- 65.Kim EH, Willett WC, Colditz GA, Hankinson SE, Stampfer MJ, Hunter DJ, Rosner B, Holmes MD. Dietary fat and risk of postmenopausal breast cancer in a 20-year follow-up. Am J Epidemiol 2006;164:990–997. doi: 10.1093/aje/kwj309. [DOI] [PubMed] [Google Scholar]
- 66.Linos E, Willett WC, Cho E, Frazier L. Adolescent diet in relation to breast cancer risk among premenopausal women. Cancer Epidemiol Biomarkers Prev 2010;19:689–696. doi: 10.1158/1055-9965.EPI-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin LJ, Melnichouk O, Huszti E, Connelly PW, Greenberg CV, Minkin S, Boyd NF. Serum lipids, lipoproteins, and risk of breast cancer: a nested case-control study using multiple time points. J Natl Cancer Inst 2015;107:djv032. doi: 10.1093/jnci/djv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knott CS, Coombs N, Stamatakis E, Biddulph JP. All cause mortality and the case for age specific alcohol consumption guidelines: pooled analyses of up to 10 population based cohorts. BMJ 2015;350:h384. doi: 10.1136/bmj.h384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 70.Jimenez M, Chiuve SE, Glynn RJ, Stampfer MJ, Camargo CA Jr, Willett WC, Manson JE, Rexrode KM. Alcohol consumption and risk of stroke in women. Stroke 2012;43:939–945. doi: 10.1161/STROKEAHA.111.639435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2010;55:1339–1347. doi: 10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roerecke M, Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: a systematic review and meta-analysis. Addiction 2012;107:1246–1260. doi: 10.1111/j.1360-0443.2012.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hvidtfeldt UA, Tolstrup JS, Jakobsen MU, Heitmann BL, Grønbaek M, O’Reilly E, Bälter K, Goldbourt U, Hallmans G, Knekt P, Liu S, Pereira M, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Rimm EB, Ascherio A. Alcohol intake and risk of coronary heart disease in younger, middle-aged, and older adults. Circulation 2010;121:1589–1597. doi: 10.1161/CIRCULATIONAHA.109.887513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med 2014;12:182. doi: 10.1186/s12916-014-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Nguyen N, Colditz GA. Links between alcohol consumption and breast cancer: a look at the evidence. Womens Health (Lond) 2015;11:65–77. doi: 10.2217/whe.14.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens, part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033–1034. [DOI] [PubMed] [Google Scholar]
- 78.Kabat GC, Kim M, Shikany JM, Rodgers AK, Wactawski-Wende J, Lane D, Powell L, Stefanick ML, Freiberg MS, Kazlauskaite R, Chlebowski RT, Wassertheil-Smoller S, Rohan TE. Alcohol consumption and risk of ductal carcinoma in situ of the breast in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev 2010;19:2066–2072. doi: 10.1158/1055-9965.EPI-10-0388. [DOI] [PubMed] [Google Scholar]
- 79.Engeset D, Dyachenko A, Ciampi A, Lund E. Dietary patterns and risk of cancer of various sites in the Norwegian European Prospective Investigation into Cancer and Nutrition cohort: the Norwegian Women and Cancer study. Eur J Cancer Prev 2009;18:69–75. doi: 10.1097/CEJ.0b013e328305a091. [DOI] [PubMed] [Google Scholar]
- 80.Zhang SM, Lee IM, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the Women’s Health Study. Am J Epidemiol 2007;165:667–676. doi: 10.1093/aje/kwk054. [DOI] [PubMed] [Google Scholar]
- 81.Newcomb PA, Nichols HB, Beasley JM, Egan K, Titus-Ernstoff L, Hampton JM, Trentham-Dietz A. No difference between red wine or white wine consumption and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2009;18:1007–1010. doi: 10.1158/1055-9965.EPI-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collaborative Group on Hormonal Factors in Breast Cancer. Alcohol, tobacco and breast cancer: collaborative reanalysis of individual data from 53 epidemiological studies, including 58 515 women with breast cancer and 95 067 women without the disease. Br J Cancer 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015;112:580–593. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki R, Orsini N, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Int J Cancer 2008;122:1832–1841. doi: 10.1002/ijc.23184. [DOI] [PubMed] [Google Scholar]
- 86.Key J, Hodgson S, Omar RZ, Jensen TK, Thompson SG, Boobis AR, Davies DS, Elliott P. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control 2006;17:759–770. doi: 10.1007/s10552-006-0011-0. [DOI] [PubMed] [Google Scholar]
- 87.Brooks PJ, Zakhari S. Moderate alcohol consumption and breast cancer in women: from epidemiology to mechanisms and interventions. Alcohol Clin Exp Res 2013;37:23–30. doi: 10.1111/j.1530-0277.2012.01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berstad P, Ma H, Bernstein L, Ursin G. Alcohol intake and breast cancer risk among young women. Breast Cancer Res Treat 2008;108:113–120. doi: 10.1007/s10549-007-9578-8. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, Colditz GA, Rosner B, Berkey CS, Collins LC, Schnitt SJ, Connolly JL, Chen WY, Willett WC, Tamimi RM. Alcohol intake between menarche and first pregnancy: a prospective study of breast cancer risk. J Natl Cancer Inst 2013;105:1571–1578. doi: 10.1093/jnci/djt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falk RT, Maas P, Schairer C, Chatterjee N, Mabie JE, Cunningham C, Buys SS, Isaacs C, Ziegler RG. Alcohol and risk of breast cancer in postmenopausal women: an analysis of etiological heterogeneity by multiple tumor characteristics. Am J Epidemiol 2014;180:705–717. doi: 10.1093/aje/kwu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, Lessin L, O’Sullivan MJ, Wactawski-Wende J, Yasmeen S, Prentice R. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the Women’s Health Initiative observational study. J Natl Cancer Inst 2010;102:1422–1431. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor EF, Burley VJ, Greenwood DC, Cade JE. Meat consumption and risk of breast cancer in the UK Women’s Cohort Study. Br J Cancer 2007;96:1139–1146. doi: 10.1038/sj.bjc.6603689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mourouti N, Kontogianni MD, Papavagelis C, Plytzanopoulou P, Vassilakou T, Psaltopoulou T, Malamos N, Linos A, Panagiotakos DB. Meat consumption and breast cancer: a case-control study in women. Meat Sci 2015;100:195–201. doi: 10.1016/j.meatsci.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 94.Pouchieu C, Deschasaux M, Hercberg S, Druesne-Pecollo N, Latino-Martel P, Touvier M. Prospective association between red and processed meat intakes and breast cancer risk: modulation by an antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Int J Epidemiol 2014;43:1583–1592. doi: 10.1093/ije/dyu134. [DOI] [PubMed] [Google Scholar]
- 95.Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Adolescent meat intake and breast cancer risk. Int J Cancer 2015;136:1909–1920. doi: 10.1002/ijc.29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alexander DD, Morimoto LM, Mink PJ, Cushing CA. A review and meta-analysis of red and processed meat consumption and breast cancer. Nutr Res Rev 2010;23:349–365. doi: 10.1017/S0954422410000235. [DOI] [PubMed] [Google Scholar]
- 97.Genkinger JM, Makambi KH, Palmer JR, Rosenberg L, Adams-Campbell LL. Consumption of dairy and meat in relation to breast cancer risk in the Black Women’s Health Study. Cancer Causes Control 2013;24:675–684. doi: 10.1007/s10552-013-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larsson SC, Orsini N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol 2014;179:282–289. doi: 10.1093/aje/kwt261. [DOI] [PubMed] [Google Scholar]
- 99.Kontogianni MD, Panagiotakos DB, Pitsavos C, Chrysohoou C, Stefanadis C. Relationship between meat intake and the development of acute coronary syndromes: the CARDIO2000 case-control study. Eur J Clin Nutr 2008;62:171–177. doi: 10.1038/sj.ejcn.1602713. [DOI] [PubMed] [Google Scholar]
- 100.Teegarden D, Romieu I, Lelièvre SA. Redefining the impact of nutrition on breast cancer incidence: is epigenetics involved? Nutr Res Rev 2012;25:68–95. doi: 10.1017/S0954422411000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, Falk RT, Gail MH, Shi J, Ravel J, Feigelson HS. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed]
- 102.Weinberg CR, Shi M, DeRoo LA, Taylor JA, Sandler DP, Umbach DM. Asymmetry in family history implicates nonstandard genetic mechanisms: application to the genetics of breast cancer. PLoS Genet 2014;10:e1004174. doi: 10.1371/journal.pgen.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Physical activity guidelines for Americans. Okla Nurse 2008;53:25. [PubMed] [Google Scholar]
- 104.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT; for the Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 106.Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med 2004;26:407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 107.Hankinson AL, Daviglus ML, Bouchard C, Carnethon M, Lewis CE, Schreiner PJ, Liu K, Sidney S. Maintaining a high physical activity level over 20 years and weight gain. JAMA 2010;304:2603–2610. doi: 10.1001/jama.2010.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Armstrong ME, Green J, Reeves GK, Beral V, Cairns BJ; on behalf of the Million Women Study Collaborators. Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation 2015;131: 721–729. doi: 10.1161/CIRCULATIONAHA.114.010296. [DOI] [PubMed] [Google Scholar]
- 110.Gebel K, Ding D, Chey T, Stamatakis E, Brown WJ, Bauman AE. Effect of moderate to vigorous physical activity on all-cause mortality in middle-aged and older Australians. JAMA Intern Med 2015;175:970–977. doi: 10.1001/jamainternmed.2015.0541. [DOI] [PubMed] [Google Scholar]
- 111.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 112.Monninkhof EM, Velthuis MJ, Peeters PH, Twisk JW, Schuit AJ. Effect of exercise on postmenopausal sex hormone levels and role of body fat: a randomized controlled trial. J Clin Oncol 2009;27:4492–4499. doi: 10.1200/JCO.2008.19.7459. [DOI] [PubMed] [Google Scholar]
- 113.McTiernan A, Tworoger SS, Rajan KB, Yasui Y, Sorenson B, Ulrich CM, Chubak J, Stanczyk FZ, Bowen D, Irwin ML, Rudolph RE, Potter JD, Schwartz RS. Effect of exercise on serum androgens in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2004;13:1099–1105. [PubMed] [Google Scholar]
- 114.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 115.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol 2015;54:635–654. doi: 10.3109/0284186X.2014.998275. [DOI] [PubMed] [Google Scholar]
- 116.Boeke CE, Eliassen AH, Oh H, Spiegelman D, Willett WC, Tamimi RM. Adolescent physical activity in relation to breast cancer risk. Breast Cancer Res Treat 2014;145:715–724. doi: 10.1007/s10549-014-2919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 118.Pate RR, O’Neill JR, Lobelo F. The evolving definition of “sedentary.” Exerc Sport Sci Rev 2008;36:173–178. doi: 10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- 119.Owen N, Sparling PB, Healy GN, Dunstan DW, Matthews CE. Sedentary behavior: emerging evidence for a new health risk. Mayo Clinic Proc 2010;85:1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 121.Zhou Y, Zhao H, Peng C. Association of sedentary behavior with the risk of breast cancer in women: update meta-analysis of observational studies. Ann Epidemiol 2015;25:687–697. doi: 10.1016/j.annepidem.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 122.Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, Garcia L, Allison MA, Sims ST, LaMonte MJ, Johnson KC, Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. J Am Coll Cardiol 2013;61:2346–2354. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2007;99:1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 124.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 125.Dallal CM, Brinton LA, Matthews CE, Lissowska J, Peplonska B, Hartman TJ, Gierach GL. Accelerometer-based measures of active and sedentary behavior in relation to breast cancer risk. Breast Cancer Res Treat 2012;134:1279–1290. doi: 10.1007/s10549-012-2129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cohen SS, Matthews CE, Bradshaw PT, Lipworth L, Buchowski MS, Signorello LB, Blot WJ. Sedentary behavior, physical activity, and likelihood of breast cancer among Black and White women: a report from the Southern Community Cohort Study. Cancer Prev Res (Phila) 2013;6:566–576. doi: 10.1158/1940-6207.CAPR-13-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 128.McTigue KM, Chang YF, Eaton C, Garcia L, Johnson KC, Lewis CE, Liu S, Mackey RH, Robinson J, Rosal MC, Snetselaar L, Valoski A, Kuller LH. Severe obesity, heart disease, and death among white, African American, and Hispanic postmenopausal women. Obesity (Silver Spring) 2014;22:801–810. doi: 10.1002/oby.20224. [DOI] [PubMed] [Google Scholar]
- 129.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults [published correction appears in N Engl J Med. 2011;365:869]. N Engl J Med 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 131.Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat 2010;123:641–649. doi: 10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- 132.Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 2015;107:djv088. doi: 10.1093/jnci/djv088. [DOI] [PubMed] [Google Scholar]
- 133.Berstad P, Coates RJ, Bernstein L, Folger SG, Malone KE, Marchbanks PA, Weiss LK, Liff JM, McDonald JA, Strom BL, Simon MS, Deapen D, Press MF, Burkman RT, Spirtas R, Ursin G. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev 2010;19:1532–1544. doi: 10.1158/1055-9965.EPI-10-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, Hainaut P. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev 2013;14:665–678. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- 135.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol 2010;171:1183–1194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosner B, Eliassen AH, Toriola AT, Hankinson SE, Willett WC, Natarajan L, Colditz GA. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res Treat 2015;150:643–653. doi: 10.1007/s10549-015-3344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oh H, Boeke CE, Tamimi RM, Smith-Warner SA, Wang M, Willett WC, Eliassen AH. The interaction between early-life body size and physical activity on risk of breast cancer. Int J Cancer 2015;137:571–581. doi: 10.1002/ijc.29272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Florath I, Sarink D, Saunders C, Heyworth J, Fritschi L. Breast cancer risk and the interaction between adolescent body size and weight gain in later life: a case-control study. Cancer Epidemiol 2016;45:135–144. doi: 10.1016/j.canep.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 139.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 140.US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress: A Report of the Surgeon General Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 141.Cotterchio M, Mirea L, Ozcelik H, Kreiger N. Active cigarette smoking, variants in carcinogen metabolism genes and breast cancer risk among pre- and postmenopausal women in Ontario, Canada. Breast J 2014;20:468–480. doi: 10.1111/tbj.12304. [DOI] [PubMed] [Google Scholar]
- 142.Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med 2011;171:125–133. doi: 10.1001/archinternmed.2010.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gram IT, Braaten T, Terry PD, Sasco AJ, Adami HO, Lund E, Weiderpass E. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev 2005;14:61–66. [PubMed] [Google Scholar]
- 144.Reynolds P, Hurley S, Goldberg DE, Anton-Culver H, Bernstein L, Deapen D, Horn-Ross PL, Peel D, Pinder R, Ross RK, West D, Wright WE, Ziogas A. Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J Natl Cancer Inst 2004;96:29–37. [DOI] [PubMed] [Google Scholar]
- 145.Cui Y, Miller AB, Rohan TE. Cigarette smoking and breast cancer risk: update of a prospective cohort study. Breast Cancer Res Treat 2006;100:293–299. doi: 10.1007/s10549-006-9255-3. [DOI] [PubMed] [Google Scholar]
- 146.Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, Salmon AG, Cantor KP, Miller MD, Boyd NF, Millar J, Turcotte F. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009). Tob Control 2011;20:e2. doi: 10.1136/tc.2010.035931. [DOI] [PubMed] [Google Scholar]
- 147.Reynolds P, Goldberg D, Hurley S, Nelson DO, Largent J, Henderson KD, Bernstein L. Passive smoking and risk of breast cancer in the California teachers study. Cancer Epidemiol Biomarkers Prev 2009;18:3389–3398. doi: 10.1158/1055-9965.EPI-09-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johnson KC. Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer 2005;117:619–628. doi: 10.1002/ijc.21150. [DOI] [PubMed] [Google Scholar]
- 149.Yang Y, Zhang F, Skrip L, Wang Y, Liu S. Lack of an association between passive smoking and incidence of female breast cancer in non-smokers: evidence from 10 prospective cohort studies. PLoS One 2013;8:e77029. doi: 10.1371/journal.pone.0077029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Terry PD, Goodman M. Is the association between cigarette smoking and breast cancer modified by genotype? A review of epidemiologic studies and meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:602–611. doi: 10.1158/1055-9965.EPI-05-0853. [DOI] [PubMed] [Google Scholar]
- 151.Tang LY, Chen LJ, Qi ML, Su Y, Su FX, Lin Y, Wang KP, Jia WH, Zhuang ZX, Ren ZF. Effects of passive smoking on breast cancer risk in pre/postmenopausal women as modified by polymorphisms of PARP1 and ESR1. Gene 2013;524:84–89. doi: 10.1016/j.gene.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 152.Ambrosone CB, Kropp S, Yang J, Yao S, Shields PG, Chang-Claude J. Cigarette smoking, N-acetyltransferase 2 genotypes, and breast cancer risk: pooled analysis and meta-analysis. Cancer Epidemiol Biomarkers Prev 2008;17:15–26. doi: 10.1158/1055-9965.EPI-07-0598. [DOI] [PubMed] [Google Scholar]
- 153.Zhang J, Qiu LX, Wang ZH, Wang JL, He SS, Hu XC. NAT2 polymorphisms combining with smoking associated with breast cancer susceptibility: a meta-analysis. Breast Cancer Res Treat 2010;123:877–883. doi: 10.1007/s10549-010-0807-1. [DOI] [PubMed] [Google Scholar]
- 154.McPherson K, Steel CM, Dixon JM. ABC of breast diseases: breast cancer-epidemiology, risk factors, and genetics. BMJ 2000;321:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J 1986;111:383–390. [DOI] [PubMed] [Google Scholar]
- 156.Charalampopoulos D, McLoughlin A, Elks CE, Ong KK. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol 2014;180:29–40. doi: 10.1093/aje/kwu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mueller NT, Odegaard AO, Gross MD, Koh WP, Yuan JM, Pereira MA. Age at menarche and cardiovascular disease mortality in Singaporean Chinese women: the Singapore Chinese Health Study. Ann Epidemiol 2012;22:717–722. doi: 10.1016/j.annepidem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, Hennekens C, Rosner B, Speizer FE. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 160.Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Chlebowski RT, Howard BV, Thomson CA, Margolis KL, Lewis CE, Stefanick ML, Jackson RD, Johnson KC, Martin LW, Shumaker SA, Espeland MA, Wactawski-Wende J; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA 2017;318:927–938. doi: 10.1001/jama.2017.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N; for the HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288:1064]. JAMA 2002;288:49–57. [DOI] [PubMed] [Google Scholar]
- 162.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 163.Moyer VA; on behalf of the US Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;158:47–54. doi: 10.7326/0003-4819-158-1-201301010-00553. [DOI] [PubMed] [Google Scholar]
- 164.Martin AM, Weber BL. Genetic and hormonal risk factors in breast cancer. J Natl Cancer Inst 2000;92:1126–1135. [DOI] [PubMed] [Google Scholar]
- 165.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 166.Ganesh SK, Arnett DK, Assimes TL, Assimes TL, Basson CT, Chakravarti A, Ellinor PT, Engler MB, Goldmuntz E, Herrington DM, Hershberger RE, Hong Y, Johnson JA, Kittner SJ, McDermott DA, Meschia JF, Mestroni L, O’Donnell CJ, Psaty BM, Vasan RS, Ruel M, Shen WK, Terzic A, Waldman SA; on behalf of the American Heart Association Council on Functional Genomics and Translational Biology, American Heart Association Council on Epidemiology and Prevention, American Heart Association Council on Basic Cardiovascular Sciences, American Heart Association Council on Cardiovascular Disease in the Young, American Heart Association Council on Cardiovascular and Stroke Nursing, American Heart Association Stroke Council. Genetics and genomics for the prevention and treatment of cardiovascular disease: update: a scientific statement from the American Heart Association. Circulation 2013;128:2813–2851. doi: 10.1161/01.cir.0000437913.98912.1d. [DOI] [PubMed] [Google Scholar]
- 167.Fatkin D, Seidman CE, Seidman JG. Genetics and disease of ventricular muscle. Cold Spring Harb Perspect Med 2014;4:a021063. doi: 10.1101/cshperspect.a021063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McPherson R, Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res 2016;118:564–578. doi: 10.1161/CIRCRESAHA.115.306566. [DOI] [PubMed] [Google Scholar]
- 169.Shukla PC, Singh KK, Quan A, Al-Omran M, Teoh H, Lovren F, Cao L, Rovira II, Pan Y, Brezden-Masley C, Yanagawa B, Gupta A, Deng CX, Coles JG, Leong-Poi H, Stanford WL, Parker TG, Schneider MD, Finkel T, Verma S. BRCA1 is an essential regulator of heart function and survival following myocardial infarction. Nat Commun 2011;2:593. doi: 10.1038/ncomms1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Barac A, Lynce F, Smith KL, Mete M, Shara NM, Asch FM, Nardacci MP, Wray L, Herbolsheimer P, Nunes RA, Swain SM, Warren R, Peshkin BN, Isaacs C. Cardiac function in BRCA½ mutation carriers with history of breast cancer treated with anthracyclines. Breast Cancer Res Treat 2016;155:285–293. doi: 10.1007/s10549-016-3678-2. [DOI] [PubMed] [Google Scholar]
- 171.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 172.Schmitz KH, Prosnitz RG, Schwartz AL, Carver JR. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer 2012;118(suppl):2270–2276. doi: 10.1002/cncr.27462. [DOI] [PubMed] [Google Scholar]
- 173.Bagnes C, Panchuk PN, Recondo G. Antineoplastic chemotherapy induced QTc prolongation. Curr Drug Saf 2010;5:93–96. [DOI] [PubMed] [Google Scholar]
- 174.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lenneman CG, Sawyer DB. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res 2016;118:1008–1020. doi: 10.1161/CIRCRESAHA.115.303633. [DOI] [PubMed] [Google Scholar]
- 176.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res 2002;62:4592–4598. [PubMed] [Google Scholar]
- 177.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 2014;64:938–945. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 178.Shakir DK, Rasul KI. Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res 2009;1:8–12. doi: 10.4021/jocmr2009.02.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, Sacks DB, Ottlinger ME. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 1997;96:2641–2648. [DOI] [PubMed] [Google Scholar]
- 180.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 181.van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Petersen EJ, Raemaekers JM, Kok WE, Aleman BM, van Leeuwen FE. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 182.Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, Nathan PC, Tissing WJ, Shankar S, Sieswerda E, Skinner R, Steinberger J, van Dalen EC, van der Pal H, Wallace WH, Levitt G, Kremer LC. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle JN, Winer EP, Gelmon KA, Gersh BJ, Jaffe AS, Rodeheffer RJ. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J 2013;34:1102–1111. doi: 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 185.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 186.Narayan HK, Finkelman B, French B, Plappert T, Hyman D, Smith AM, Margulies KB, Ky B. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation 2017;135:1397–1412. doi: 10.1161/CIRCULATIONAHA.116.023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracyclineinduced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 188.Hochster H, Wasserheit C, Speyer J. Cardiotoxicity and cardioprotection during chemotherapy. Curr Opin Oncol 1995;7:304–309. [DOI] [PubMed] [Google Scholar]
- 189.Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: chemotherapy-induced arrhythmia. Europace 2009;11:1579–1586. doi: 10.1093/europace/eup300. [DOI] [PubMed] [Google Scholar]
- 190.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol 2014;63:945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 191.Mazur M, Wang F, Hodge DO, Siontis BL, Beinborn DS, Villarraga HR, Lerman A, Friedman PA, Herrmann J. Burden of cardiac arrhythmias in patients with anthracycline-related cardiomyopathy. JACC Clin Electrophysiol 2017;3:139–150. doi: 10.1016/j.jacep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 192.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev 2010;(5):CD005006. doi: 10.1002/14651858.CD005006.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bovelli D, Plataniotis G, Roila F; on behalf of the ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol 2010;21(suppl 5):v277–v282. doi: 10.1093/annonc/mdq200. [DOI] [PubMed] [Google Scholar]
- 195.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 196.Goldberg MA, Antin JH, Guinan EC, Rappeport JM. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood 1986;68: 1114–1118. [PubMed] [Google Scholar]
- 197.Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol 1991;9:1215–1223. doi: 10.1200/JCO.1991.9.7.1215. [DOI] [PubMed] [Google Scholar]
- 198.Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth A, Ziegler J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med 1981;141:758–763. [PubMed] [Google Scholar]
- 199.Altundağ O, Celik I, Kars A. Recurrent asymptomatic bradycardia episodes after cisplatin infusion. Ann Pharmacother 2001;35:641–642. doi: 10.1345/aph.10180. [DOI] [PubMed] [Google Scholar]
- 200.Lara PN Jr, Mack PC, Synold T, Frankel P, Longmate J, Gumerlock PH, Doroshow JH, Gandara DR. The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: a California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin Cancer Res 2005;11:4444–4450. doi: 10.1158/1078-0432.CCR-04-2602. [DOI] [PubMed] [Google Scholar]
- 201.Hashimi LA, Khalyl MF, Salem PA. Supraventricular tachycardia: a probable complication of platinum treatment. Oncology 1984;41: 174–175. [DOI] [PubMed] [Google Scholar]
- 202.Shek TW, Luk IS, Ma L, Cheung KL. Paclitaxel-induced cardiotoxicity: an ultrastructural study. Arch Pathol Lab Med 1996;120:89–91. [PubMed] [Google Scholar]
- 203.Gollerkeri A, Harrold L, Rose M, Jain D, Burtness BA. Use of paclitaxel in patients with pre-existing cardiomyopathy: a review of our experience. Int J Cancer 2001;93:139–141. [DOI] [PubMed] [Google Scholar]
- 204.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol 1993;20(suppl 3):1–15. [PubMed] [Google Scholar]
- 205.Alter P, Herzum M, Soufi M, Schaefer JR, Maisch B. Cardiotoxicity of 5-fluorouracil. Cardiovasc Hematol Agents Med Chem 2006;4:1–5. [DOI] [PubMed] [Google Scholar]
- 206.Meyer CC, Calis KA, Burke LB, Walawander CA, Grasela TH. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy 1997;17:729–736. [PubMed] [Google Scholar]
- 207.Van Cutsem E, Hoff PM, Blum JL, Abt M, Osterwalder B. Incidence of cardiotoxicity with the oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil. Ann Oncol 2002;13:484–485. [DOI] [PubMed] [Google Scholar]
- 208.Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, Karabelis A, Tsavaris N. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol 2008;134:75–82. doi: 10.1007/s00432-007-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Südhoff T, Enderle MD, Pahlke M, Petz C, Teschendorf C, Graeven U, Schmiegel W. 5-Fluorouracil induces arterial vasocontractions. Ann Oncol 2004;15:661–664. [DOI] [PubMed] [Google Scholar]
- 210.Cardinale D, Colombo A, Colombo N. Acute coronary syndrome induced by oral capecitabine. Can J Cardiol 2006;22:251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Akhtar SS, Salim KP, Bano ZA. Symptomatic cardiotoxicity with high-dose 5-fluorouracil infusion: a prospective study. Oncology 1993;50:441–444. [DOI] [PubMed] [Google Scholar]
- 212.Labianca R, Beretta G, Clerici M, Fraschini P, Luporini G. Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori 1982;68:505–510. [DOI] [PubMed] [Google Scholar]
- 213.Robben NC, Pippas AW, Moore JO. The syndrome of 5-fluorouracil cardiotoxicity: an elusive cardiopathy. Cancer 1993;71:493–509. [DOI] [PubMed] [Google Scholar]
- 214.de Forni M, Malet-Martino MC, Jaillais P, Shubinski RE, Bachaud JM, Lemaire L, Canal P, Chevreau C, Carrié D, Soulié P. Cardiotoxicity of highdose continuous infusion fluorouracil: a prospective clinical study. J Clin Oncol 1992;10:1795–1801. doi: 10.1200/JCO.1992.10.11.1795. [DOI] [PubMed] [Google Scholar]
- 215.Jensen SA, Sørensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol 2006;58:487–493. doi: 10.1007/s00280-005-0178-1. [DOI] [PubMed] [Google Scholar]
- 216.Layoun ME, Wickramasinghe CD, Peralta MV, Yang EH. Fluoropyrimidine-induced cardiotoxicity: manifestations, mechanisms, and management. Curr Oncol Rep 2016;18:35. doi: 10.1007/s11912-016-0521-1. [DOI] [PubMed] [Google Scholar]
- 217.Rezkalla S, Kloner RA, Ensley J, al-Sarraf M, Revels S, Olivenstein A, Bhasin S, Kerpel-Fronious S, Turi ZG. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol 1989;7:509–514. doi: 10.1200/JCO.1989.7.4.509. [DOI] [PubMed] [Google Scholar]
- 218.Yilmaz U, Oztop I, Ciloglu A, Okan T, Tekin U, Yaren A, Somali I, Alacacioglu A, Kirimli O. 5-Fluorouracil increases the number and complexity of premature complexes in the heart: a prospective study using ambulatory ECG monitoring. Int J Clin Pract 2007;61:795–801. doi: 10.1111/j.1742-1241.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- 219.Eskilsson J, Albertsson M, Mercke C. Adverse cardiac effects during induction chemotherapy treatment with cis-platin and 5-fluorouracil. Radiother Oncol 1988;13:41–46. [DOI] [PubMed] [Google Scholar]
- 220.Ng M, Cunningham D, Norman AR. The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer (CRC). Eur J Cancer 2005;41:1542–1546. doi: 10.1016/j.ejca.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 221.Keefe DL, Roistacher N, Pierri MK. Clinical cardiotoxicity of 5-fluorouracil. J Clin Pharmacol 1993;33:1060–1070. [DOI] [PubMed] [Google Scholar]
- 222.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res 2004;64:1522–1533. [DOI] [PubMed] [Google Scholar]
- 224.Dewar JA, Horobin JM, Preece PE, Tavendale R, Tunstall-Pedoe H, Wood RA. Long term effects of tamoxifen on blood lipid values in breast cancer. BMJ 1992;305:225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Esteva FJ, Hortobagyi GN. Comparative assessment of lipid effects of endocrine therapy for breast cancer: implications for cardiovascular disease prevention in postmenopausal women. Breast 2006;15:301–312. doi: 10.1016/j.breast.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 226.Grey AB, Stapleton JP, Evans MC, Reid IR. The effect of the anti-estrogen tamoxifen on cardiovascular risk factors in normal postmenopausal women. J Clin Endocrinol Metab 1995;80:3191–3195. doi: 10.1210/jcem.80.11.7593425. [DOI] [PubMed] [Google Scholar]
- 227.Morales M, Santana N, Soria A, Mosquera A, Ordovás J, Nóvoa J, Betancor P, Valerón PF, Díaz-Chico B, Chirino R. Effects of tamoxifen on serum lipid and apolipoprotein levels in postmenopausal patients with breast cancer. Breast Cancer Res Treat 1996;40:265–270. [DOI] [PubMed] [Google Scholar]
- 228.Hozumi Y, Kawano M, Saito T, Miyata M. Effect of tamoxifen on serum lipid metabolism. J Clin Endocrinol Metab 1998;83:1633–1635. doi: 10.1210/jcem.83.5.4753. [DOI] [PubMed] [Google Scholar]
- 229.Liu CL, Yang TL. Sequential changes in serum triglyceride levels during adjuvant tamoxifen therapy in breast cancer patients and the effect of dose reduction. Breast Cancer Res Treat 2003;79:11–16. [DOI] [PubMed] [Google Scholar]
- 230.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998;351:1451–1467. [PubMed] [Google Scholar]
- 231.Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 2001;93:684–690. [DOI] [PubMed] [Google Scholar]
- 232.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M; Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 233.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J Clin Oncol 1991;9:286–294. doi: 10.1200/JCO.1991.9.2.286. [DOI] [PubMed] [Google Scholar]
- 234.Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 2011;103:1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 235.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer 2010;102(suppl 1):S2–S9. doi: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract 2007;61:2051–2063. doi: 10.1111/j.1742-1241.2007.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 238.Boccardo F, Guglielmini P, Bordonaro R, Fini A, Massidda B, Porpiglia M, Roagna R, Serra P, Orzalesi L, Ucci G, Rubagotti A. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: long term results of the Italian Tamoxifen Anastrozole trial. Eur J Cancer 2013;49:1546–1554. doi: 10.1016/j.ejca.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 239.Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Láng I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 240.Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for earlystage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 2008;9:45–53. [DOI] [PubMed] [Google Scholar]
- 241.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, Saunders C, Roche N, Mansel RE, von Minckwitz G, Bonanni B, Palva T, Howell A; on behalf of the IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014;383:1040]. Lancet 2014;383:1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 242.Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 2006;7:633–643. [DOI] [PubMed] [Google Scholar]
- 243.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 244.Arimidex® (anastrozole) [prescribing information] Wilmington, DE: AstraZeneca Pharmaceuticals; 2014. [Google Scholar]
- 245.Breast International Group (BIG) 1–98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 246.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 247.Ligibel JA, James O’Malley A, Fisher M, Daniel GW, Winer EP, Keating NL. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat 2012;131:589–597. doi: 10.1007/s10549-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 248.Obi N, Gornyk D, Heinz J, Vrieling A, Seibold P, Chang-Claude J, Flesch-Janys D. Determinants of newly diagnosed comorbidities among breast cancer survivors. J Cancer Surviv 2014;8:384–393. doi: 10.1007/s11764-013-0338-y. [DOI] [PubMed] [Google Scholar]
- 249.Haque R, Shi J, Schottinger JE, Chung J, Avila C, Amundsen B, Xu X, Barac A, Chlebowski RT. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol 2016;2:1590–1597. doi: 10.1001/jamaoncol.2016.0429. [DOI] [PubMed] [Google Scholar]
- 250.Khosrow-Khavar F, Filion KB, Al-Qurashi S, Torabi N, Bouganim N, Suissa S, Azoulay L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 2017;28:487–496. doi: 10.1093/annonc/mdw673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol 2016;34:1689–1701. doi: 10.1200/JCO.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 252.Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin 2016;66:309–325. doi: 10.3322/caac.21341. [DOI] [PubMed] [Google Scholar]
- 253.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc 2014;3:e000665. doi: 10.1161/JAHA.113.000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 255.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sánchez Rovira P, Piccart-Gebhart MJ; for the HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 256.Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, Ageev FT, Hitre E, Groetz J, Iwata H, Knap M, Gnant M, Muehlbauer S, Spence A, Gelber RD, Piccart-Gebhart MJ. Trastuzumab-associated cardiac adverse effects in the Herceptin adjuvant trial. J Clin Oncol 2007;25:3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 257.Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA, Wolmark N. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J; for the Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, Jackisch C, Cameron D, Weber HA, Heinzmann D, Dal Lago L, McFadden E, Dowsett M, Untch M, Gianni L, Bell R, Köhne CH, Vindevoghel A, Andersson M, Brunt AM, Otero-Reyes D, Song S, Smith I, Leyland-Jones B, Baselga J; for the Herceptin Adjuvant (HERA) Trial Study Team. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013;382:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 260.Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist 2006;11(suppl 1):4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 261.Mackey JR, Clemons M, Côté MA, Delgado D, Dent S, Paterson A, Provencher L, Sawyer MB, Verma S. Cardiac management during adjuvant trastuzumab therapy: recommendations of the Canadian Trastuzumab Working Group. Curr Oncol 2008;15:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 263.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD; for the Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 264.Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, Smith BD, Hortobagyi GN, Giordano SH. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol 2013;31:4222–4228. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sevcikova K, Vertakova-Krakovska B, Spanik S. Neoadjuvant treatment in patients with HER2-positive breast cancer. ISRN Oncol 2013;2013:362467. doi: 10.1155/2013/362467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lenihan D, Suter T, Brammer M, Neate C, Ross G, Baselga J. Pooled analysis of cardiac safety in patients with cancer treated with pertuzumab. Ann Oncol 2012;23:791–800. doi: 10.1093/annonc/mdr294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 268.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, Baselga J; for the APHINITY Steering Committee and Investigators. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 270.TYKERB (lapatinib) [prescribing information] Research Triangle Park, NC: GlaxoSmithKline; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022059s3s6lbl.pdf. Accessed June 30, 2017. [Google Scholar]
- 271.Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 272.Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, Zujewski JA, Goldhirsch A, Armour A, Pritchard KI, McCullough AE, Dolci S, McFadden E, Holmes AP, Tonghua L, Eidtmann H, Dinh P, Di Cosimo S, Harbeck N, Tjulandin S, Im YH, Huang CS, Diéras V, Hillman DW, Wolff AC, Jackisch C, Lang I, Untch M, Smith I, Boyle F, Xu B, Gomez H, Suter T, Gelber RD, Perez EA. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Choi YJ, Anders L. Signaling through cyclin D-dependent kinases. Oncogene 2014;33:1890–1903. doi: 10.1038/onc.2013.137. [DOI] [PubMed] [Google Scholar]
- 274.Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res 2014;20:3379–3383. doi: 10.1158/1078-0432.CCR-13-1551. [DOI] [PubMed] [Google Scholar]
- 275.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427–1438. [PubMed] [Google Scholar]
- 276.Pojer F, Ferrer JL, Richard SB, Nagegowda DA, Chye ML, Bach TJ, Noel JP. Structural basis for the design of potent and species-specific inhibitors of 3-hydroxy-3-methylglutaryl CoA synthases. Proc Natl Acad Sci U S A 2006;103:11491–11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Vidula N, Rugo HS. Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: a review of preclinical and clinical data. Clin Breast Cancer 2016;16:8–17. doi: 10.1016/j.clbc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 278.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptorpositive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 279.Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, Chakraborty A, Matano A, Dobson JR, Crystal AS, Parasuraman S, Shapiro GI. A Phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res 2016;22:5696–5705. doi: 10.1158/1078-0432.CCR-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Barroso-Sousa R, Shapiro GI, Tolaney SM. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care (Basel) 2016;11:167–173. doi: 10.1159/000447284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.KISQALI® (ribociclib) [US prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corp; 2017. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf. Accessed July 14, 2017. [Google Scholar]
- 282.Shukla J, Khan NM, Thakur VS, Poduval TB. L-arginine mitigates radiation-induced early changes in cardiac dysfunction: the role of inflammatory pathways. Radiat Res 2011;176:158–169. [DOI] [PubMed] [Google Scholar]
- 283.Azimzadeh O, Scherthan H, Sarioglu H, Barjaktarovic Z, Conrad M, Vogt A, Calzada-Wack J, Neff F, Aubele M, Buske C, Atkinson MJ, Tapio S. Rapid proteomic remodeling of cardiac tissue caused by total body ionizing radiation. Proteomics 2011;11:3299–3311. doi: 10.1002/pmic.201100178. [DOI] [PubMed] [Google Scholar]
- 284.Lee MO, Song SH, Jung S, Hur S, Asahara T, Kim H, Kwon SM, Cha HJ. Effect of ionizing radiation induced damage of endothelial progenitor cells in vascular regeneration. Arterioscler Thromb Vasc Biol 2012;32:343–352. doi: 10.1161/ATVBAHA.111.237651. [DOI] [PubMed] [Google Scholar]
- 285.Berry GJ, Jorden M. Pathology of radiation and anthracycline cardiotoxicity. Pediatr Blood Cancer 2005;44:630–637. doi: 10.1002/pbc.20346. [DOI] [PubMed] [Google Scholar]
- 286.Boerma M Experimental radiation-induced heart disease: past, present, and future. Radiat Res 2012;178:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Boerma M, Wang J, Wondergem J, Joseph J, Qiu X, Kennedy RH, Hauer-Jensen M. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res 2005;65:3100–3107. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- 288.Boerma M, Zurcher C, Esveldt I, Schutte-Bart CI, Wondergem J. Histopathology of ventricles, coronary arteries and mast cell accumulation in transverse and longitudinal sections of the rat heart after irradiation. Oncol Rep 2004;12:213–219. [DOI] [PubMed] [Google Scholar]
- 289.Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD, Buchholz TA, Hortobagyi GN, Giordano SH. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol 2005;23:7475–7482. doi: 10.1200/JCO.2005.13.755. [DOI] [PubMed] [Google Scholar]
- 290.Bouillon K, Haddy N, Delaloge S, Garbay JR, Garsi JP, Brindel P, Mousannif A, Lê MG, Labbe M, Arriagada R, Jougla E, Chavaudra J, Diallo I, Rubino C, de Vathaire F. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol 2011;57:445–452. doi: 10.1016/j.jacc.2010.08.638. [DOI] [PubMed] [Google Scholar]
- 291.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 2005;97:419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Iliescu C, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas K, Leesar MA, Marmagkiolis K. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory. Catheter Cardiovasc Interv 2016;87:895–899. doi: 10.1002/ccd.26375. [DOI] [PubMed] [Google Scholar]
- 293.Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation 2017;135:1388–1396. doi: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D’Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 295.Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol 2013;61:2319–2328. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 296.Groarke JD, Tanguturi VK, Hainer J, Klein J, Moslehi JJ, Ng A, Forman DE, Di Carli MF, Nohria A. Abnormal exercise response in long-term survivors of hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol 2015;65:573–583. doi: 10.1016/j.jacc.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 297.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 298.Harris EE. Cardiac mortality and morbidity after breast cancer treatment. Cancer Control 2008;15:120–129. [DOI] [PubMed] [Google Scholar]
- 299.Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer 2013;108:179–182. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zellars R, Bravo PE, Tryggestad E, Hopfer K, Myers L, Tahari A, Asrari F, Ziessman H, Garrett-Mayer E. SPECT analysis of cardiac perfusion changes after whole-breast/chest wall radiation therapy with or without active breathing coordinator: results of a randomized phase 3 trial. Int J Radiat Oncol Biol Phys 2014;88:778–785. doi: 10.1016/j.ijrobp.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 301.Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, Blazing M, Hollis D, Lind P, Tisch A, Wong TZ, Borges-Neto S. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 302.Hardenbergh PH, Munley MT, Bentel GC, Kedem R, Borges-Neto S, Hollis D, Prosnitz LR, Marks LB. Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results. Int J Radiat Oncol Biol Phys 2001;49:1023–1028. [DOI] [PubMed] [Google Scholar]
- 303.Otterstad JE, Froeland G, John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J 1997;18:507–513. [DOI] [PubMed] [Google Scholar]
- 304.Neilan TG, Jassal DS, Perez-Sanz TM, Raher MJ, Pradhan AD, Buys ES, Ichinose F, Bayne DB, Halpern EF, Weyman AE, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J 2006;27:1868–1875. doi: 10.1093/eurheartj/ehl013. [DOI] [PubMed] [Google Scholar]
- 305.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Stoodley PW, Richards DA, Boyd A, Hui R, Harnett PR, Meikle SR, Byth K, Stuart K, Clarke JL, Thomas L. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: a comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur J Cancer 2013;49:3396–3403. doi: 10.1016/j.ejca.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 307.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013;26:493–498. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 308.Narayan HK, French B, Khan AM, Plappert T, Hyman D, Bajulaiye A, Domchek S, DeMichele A, Clark A, Matro J, Bradbury A, Fox K, Carver JR, Ky B. Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics-related cardiac dysfunction. JACC Cardiovasc Imaging 2016;9:1131–1141. doi: 10.1016/j.jcmg.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J 2009;158:294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 310.Brown J, Jenkins C, Marwick TH. Use of myocardial strain to assess global left ventricular function: a comparison with cardiac magnetic resonance and 3-dimensional echocardiography. Am Heart J 2009;157:102.e1–102.e5. doi: 10.1016/j.ahj.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 311.Goel S, Simes RJ, Beith JM. Exploratory analysis of cardiac biomarkers in women with normal cardiac function receiving trastuzumab for breast cancer. Asia Pac J Clin Oncol 2011;7:276–280. doi: 10.1111/j.1743-7563.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- 312.D’Errico MP, Grimaldi L, Petruzzelli MF, Gianicolo EA, Tramacere F, Monetti A, Placella R, Pili G, Andreassi MG, Sicari R, Picano E, Portaluri M. N-terminal pro-B-type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left-sided breast cancer. Int J Radiat Oncol Biol Phys 2012;82:e239–e246. doi: 10.1016/j.ijrobp.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 313.O’Brien PJ. Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology 2008;245:206–218. doi: 10.1016/j.tox.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 314.Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM, Fiorentini C. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000;36:517–522. [DOI] [PubMed] [Google Scholar]
- 315.Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nolè F, Veglia F, Cipolla CM. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 316.Morris PG, Chen C, Steingart R, Fleisher M, Lin N, Moy B, Come S, Sugarman S, Abbruzzi A, Lehman R, Patil S, Dickler M, McArthur HL, Winer E, Norton L, Hudis CA, Dang CT. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res 2011;17:3490–3499. doi: 10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 317.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Feola M, Garrone O, Occelli M, Francini A, Biggi A, Visconti G, Albrile F, Bobbio M, Merlano M. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol 2011;148:194–198. doi: 10.1016/j.ijcard.2009.09.564. [DOI] [PubMed] [Google Scholar]
- 319.Pichon MF, Cvitkovic F, Hacene K, Delaunay J, Lokiec F, Collignon MA, Pecking AP. Drug-induced cardiotoxicity studied by longitudinal B-type natriuretic peptide assays and radionuclide ventriculography. In Vivo 2005;19:567–576. [PubMed] [Google Scholar]
- 320.Tarantini L, Cioffi G, Di Lenarda A, Valle R, Pulignano G, Del Sindaco D, Frigo G, Soravia G, Tessier R, Catania G. Screening for asymptomatic left ventricular systolic dysfunction in high-risk patients. Preliminary experience with a program based on the use of ECG and natriuretic peptide [in Italian]. G Ital Cardiol (Rome) 2008;9:835–843. [PubMed] [Google Scholar]
- 321.Caram MEV, Guo C, Leja M, Smerage J, Henry NL, Giacherio D, Rubenfire M, Schott A, Davis M, Hayes DF, Van Poznak C, Cooney KA, Hertz DL, Banerjee M, Griggs JJ. Doxorubicin-induced cardiac dysfunction in unselected patients with a history of early-stage breast cancer. Breast Cancer Res Treat 2015;152:163–172. doi: 10.1007/s10549-015-3454-8. [DOI] [PubMed] [Google Scholar]
- 322.De Iuliis F, Salerno G, Taglieri L, De Biase L, Lanza R, Cardelli P, Scarpa S. Serum biomarkers evaluation to predict chemotherapy-induced cardiotoxicity in breast cancer patients. Tumour Biol 2016;37:3379–3387. doi: 10.1007/s13277-015-4183-7. [DOI] [PubMed] [Google Scholar]
- 323.Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG, Hejna M, Köstler W, Zöchbauer-Müller S, Marosi C, Kornek G, Auerbach L, Schneider S, Parschalk B, Scheithauer W, Pirker R, Drach J, Zielinski C, Pacher R. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 324.Horacek JM, Tichy M, Jebavy L, Pudil R, Ulrychova M, Maly J. Use of multiple biomarkers for evaluation of anthracycline-induced cardiotoxicity in patients with acute myeloid leukemia. Exp Oncol 2008;30:157–159. [PubMed] [Google Scholar]
- 325.Horacek JM, Tichy M, Pudil R, Jebavy L. Glycogen phosphorylase BB could be a new circulating biomarker for detection of anthracycline cardiotoxicity. Ann Oncol 2008;19:1656–1657. doi: 10.1093/annonc/mdn414. [DOI] [PubMed] [Google Scholar]
- 326.Horacek JM, Vasatova M, Pudil R, Tichy M, Zak P, Jakl M, Jebavy L, Maly J. Biomarkers for the early detection of anthracycline-induced cardiotoxicity: current status. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014;158:511–517. doi: 10.5507/bp.2014.004. [DOI] [PubMed] [Google Scholar]
- 327.Soker M, Kervancioglu M. Plasma concentrations of NT-pro-BNP and cardiac troponin-I in relation to doxorubicin-induced cardiomyopathy and cardiac function in childhood malignancy. Saudi Med J 2005;26:1197–1202. [PubMed] [Google Scholar]
- 328.Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol 2008;97:318–326. doi: 10.1007/s00392-007-0633-6. [DOI] [PubMed] [Google Scholar]
- 329.Armenian SH, Gelehrter SK, Vase T, Venkatramani R, Landier W, Wilson KD, Herrera C, Reichman L, Menteer JD, Mascarenhas L, Freyer DR, Venkataraman K, Bhatia S. Screening for cardiac dysfunction in anthracycline-exposed childhood cancer survivors. Clin Cancer Res 2014;20:6314–6323. doi: 10.1158/1078-0432.CCR-13-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, Picard MH, Carver JR, Halpern EF, Kuter I, Passeri J, Cohen V, Banchs J, Martin RP, Gerszten RE, Scherrer-Crosbie M, Ky B. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem 2015;61:1164–1172. doi: 10.1373/clinchem.2015.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.van Boxtel W, Bulten BF, Mavinkurve-Groothuis AM, Bellersen L, Mandigers CM, Joosten LA, Kapusta L, de Geus-Oei LF, van Laarhoven HW. New biomarkers for early detection of cardiotoxicity after treatment with docetaxel, doxorubicin and cyclophosphamide. Biomarkers 2015;20:143–148. doi: 10.3109/1354750X.2015.1040839. [DOI] [PubMed] [Google Scholar]
- 333.Hughes-Davies L, Sacks D, Rescigno J, Howard S, Harris J. Serum cardiac troponin T levels during treatment of early-stage breast cancer. J Clin Oncol 1995;13:2582–2584. doi: 10.1200/JCO.1995.13.10.2582. [DOI] [PubMed] [Google Scholar]
- 334.Nellessen U, Zingel M, Hecker H, Bahnsen J, Borschke D. Effects of radiation therapy on myocardial cell integrity and pump function: which role for cardiac biomarkers? Chemotherapy 2010;56:147–152. doi: 10.1159/000313528. [DOI] [PubMed] [Google Scholar]
- 335.Skyttä T, Tuohinen S, Boman E, Virtanen V, Raatikainen P, Kellokumpu-Lehtinen PL. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol 2015;10:141. doi: 10.1186/s13014-015-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, Liu LF. Topoisomerase IIβ–mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 2007;67:8839–8846. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 337.Bhave M, Shah AN, Akhter N, Rosen ST. An update on the risk prediction and prevention of anticancer therapy-induced cardiotoxicity. Curr Opin Oncol 2014;26:590–599. doi: 10.1097/CCO.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 338.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer 2013;49:2900–2909. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 339.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev 2011;(2):CD003917. [DOI] [PubMed] [Google Scholar]
- 340.ZINECARD® (dexrazoxane for injection) [US package insert] New York, NY: Pfizer; 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020212s013lbl.pdf. Accessed March 2, 2017. [Google Scholar]
- 341.Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A 3rd, von Hoff D, Schuchter LM. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 2009;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 342.European Medicines Agency recommends restricting the use of dexrazoxane-containing medicines [press release] London, United Kingdom; European Medicines Agency; June 23, 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2011/06/WC500108013.pdf. Accessed September 16, 2017. [Google Scholar]
- 343.FDA statement on dexrazoxanehttp://web.archive.org/web/20170118092643/http://www.fda.gov/Drugs/DrugSafety/ucm263729.htm. Accessed January 18, 2018.
- 344.van Dalen EC, van der Pal HJ, Caron HN, Kremer LC. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev 2009;(4):CD005008. [DOI] [PubMed] [Google Scholar]
- 345.Shapira J, Gotfried M, Lishner M, Ravid M. Reduced cardiotoxicity of doxorubicin by a 6-hour infusion regimen: a prospective randomized evaluation. Cancer 1990;65:870–873. [DOI] [PubMed] [Google Scholar]
- 346.Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res 2001;7:223–225. [PubMed] [Google Scholar]
- 347.Taylor CW, Wang Z, Macaulay E, Jagsi R, Duane F, Darby SC. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys 2015;93:845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 348.Zagar TM, Kaidar-Person O, Tang X, Jones EE, Matney J, Das SK, Green RL, Sheikh A, Khandani AH, McCartney WH, Oldan JD, Wong TZ, Marks LB. Utility of deep inspiration breath hold for left-sided breast radiation therapy in preventing early cardiac perfusion defects: a prospective study. Int J Radiat Oncol Biol Phys 2017;97:903–909. doi: 10.1016/j.ijrobp.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 349.Chang JS, Chen J, Weinberg VK, Fowble B, Sethi RA. Evaluation of heart dose for left-sided breast cancer patients over an 11-year period spanning the transition from 2-dimensional to 3-dimensional planning. Clin Breast Cancer 2016;16:396–401. doi: 10.1016/j.clbc.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 350.Khouri MG, Douglas PS, Mackey JR, Martin M, Scott JM, Scherrer-Crosbie M, Jones LW. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation 2012;126:2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Inanc T, Oguzhan A, Eryol NK, Topsakal R, Ergin A. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 352.Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, Akpek M, Kalay N, Dikilitas M, Yarlioglues M, Karaca H, Berk V, Ardic I, Ergin A, Lam YY. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol 2013;167:2306–2310. doi: 10.1016/j.ijcard.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 353.Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH. Cardioprotective effect of β-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure. Circ Heart Fail 2013;6:420–426. doi: 10.1161/CIRCHEARTFAILURE.112.000055. [DOI] [PubMed] [Google Scholar]
- 354.Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, Sarti S, Cecconetto L, Pietri E, Ferrario C, Fedeli A, Faedi M, Nanni O, Frassineti GL, Amadori D, Rocca A. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart 2013;99:634–639. doi: 10.1136/heartjnl-2012-303151. [DOI] [PubMed] [Google Scholar]
- 355.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland Å, Storås TH, Hagve TA, Røsjø H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, Ghosh S, Oudit GY, Ezekowitz JA, Paterson DI. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol 2017;35:870–877. doi: 10.1200/JCO.2016.68.7830. [DOI] [PubMed] [Google Scholar]
- 357.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 358.Tallaj JA, Franco V, Rayburn BK, Pinderski L, Benza RL, Pamboukian S, Foley B, Bourge RC. Response of doxorubicin-induced cardiomyopathy to the current management strategy of heart failure. J Heart Lung Transplant 2005;24:2196–2201. doi: 10.1016/j.healun.2004.12.108. [DOI] [PubMed] [Google Scholar]
- 359.Oliva S, Cioffi G, Frattini S, Simoncini EL, Faggiano P, Boccardi L, Pulignano G, Fioretti AM, Giotta F, Lestuzzi C, Maurea N, Sabatini S, Tarantini L; on behalf of the Italian Cardio-Oncological Network. Administration of angiotensin-converting enzyme inhibitors and β-blockers during adjuvant trastuzumab chemotherapy for nonmetastatic breast cancer: marker of risk or cardioprotection in the real world? Oncologist 2012;17:917–924. doi: 10.1634/theoncologist.2011-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Thakur A, Witteles RM. Cancer therapy-induced left ventricular dysfunction: interventions and prognosis. J Card Fail 2014;20:155–158. doi: 10.1016/j.cardfail.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 361.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F; on behalf of the ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol 2012;23 Suppl 7:vii155–vii16. [DOI] [PubMed] [Google Scholar]
- 362.Virani SA, Dent S, Brezden-Masley C, Clarke B, Davis MK, Jassal DS, Johnson C, Lemieux J, Paterson I, Sebag IA, Simmons C, Sulpher J, Thain K, Thavendiranathan P, Wentzell JR, Wurtele N, Côté MA, Fine NM, Haddad H, Hayley BD, Hopkins S, Joy AA, Rayson D, Stadnick E, Straatman L. Canadian Cardiovascular Society guidelines for evaluation and management of cardiovascular complications of cancer therapy. Can J Cardiol 2016;32:831–841. doi: 10.1016/j.cjca.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 363.Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, Zanelotti A, Adamoli L, Colleoni M, Viale G, Goldhirsch A, Gandini S. Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat 2013;140:567–575. doi: 10.1007/s10549-013-2654-3. [DOI] [PubMed] [Google Scholar]
- 364.Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010;1:628–638. doi: 10.18632/oncotarget.101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Raimondi S, Botteri E, Munzone E, Cipolla C, Rotmensz N, DeCensi A, Gandini S. Use of beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: systematic review and meta-analysis. Int J Cancer 2016;139:212–219. doi: 10.1002/ijc.30062. [DOI] [PubMed] [Google Scholar]
- 366.Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat 2011;129:549–556. doi: 10.1007/s10549-011-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 369.Boudreau DM, Yu O, Chubak J, Wirtz HS, Bowles EJ, Fujii M, Buist DS. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat 2014;144:405–416. doi: 10.1007/s10549-014-2870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sørensen GV, Ganz PA, Cole SW, Pedersen LA, Sørensen HT, Cronin-Fenton DP, Garne JP, Christiansen PM, Lash TL, Ahern TP. Use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: a Danish nationwide prospective cohort study. J Clin Oncol 2013;31:2265–2272. doi: 10.1200/JCO.2012.43.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Akpek M, Ozdogru I, Sahin O, Inanc M, Dogan A, Yazici C, Berk V, Karaca H, Kalay N, Oguzhan A, Ergin A. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail 2015;17:81–89. doi: 10.1002/ejhf.196. [DOI] [PubMed] [Google Scholar]
- 372.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 373.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 374.Ready A, Velicer CM, McTiernan A, White E. NSAID use and breast cancer risk in the VITAL cohort. Breast Cancer Res Treat 2008;109:533–543. doi: 10.1007/s10549-007-9665-x. [DOI] [PubMed] [Google Scholar]
- 375.Bardia A, Olson JE, Vachon CM, Lazovich D, Vierkant RA, Wang AH, Limburg PJ, Anderson KE, Cerhan JR. Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat 2011;126:149–155. doi: 10.1007/s10549-010-1074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Egan KM, Stampfer MJ, Giovannucci E, Rosner BA, Colditz GA. Prospective study of regular aspirin use and the risk of breast cancer. J Natl Cancer Inst 1996;88:988–993. [DOI] [PubMed] [Google Scholar]
- 377.Friis S, Thomassen L, Sørensen HT, Tjønneland A, Overvad K, Cronin-Fenton DP, Vogel U, McLaughlin JK, Blot WJ, Olsen JH. Nonsteroidal anti-inflammatory drug use and breast cancer risk: a Danish cohort study. Eur J Cancer Prev 2008;17:88–96. doi: 10.1097/CEJ.0b013e3282b6fd55. [DOI] [PubMed] [Google Scholar]
- 378.Kwan ML, Habel LA, Slattery ML, Caan B. NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control 2007;18:613–620. doi: 10.1007/s10552-007-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 380.Takkouche B, Regueira-Méndez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst 2008;100:1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 381.Aspirin in preventing recurrence of cancer in patients with node positive HER2 negative stage II-III breast cancer after chemotherapy, surgery, and/or radiation therapyhttps://clinicaltrials.gov/ct2/show/NCT02927249?term=NCT02927249&rank=1. Accessed September 12, 2017.
- 382.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 383.Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 2005;23:8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- 385.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat 2012;135:261–269. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- 386.Desai P, Chlebowski R, Cauley JA, Manson JE, Wu C, Martin LW, Jay A, Bock C, Cote M, Petrucelli N, Rosenberg CA, Peters U, Agalliu I, Budrys N, Abdul-Hussein M, Lane D, Luo J, Park HL, Thomas F, Wactawski-Wende J, Simon MS. Prospective analysis of association between statin use and breast cancer risk in the women’s health initiative. Cancer Epidemiol Biomarkers Prev 2013;22:1868–1876. doi: 10.1158/1055-9965.EPI-13-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat 2008;109:573–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sørensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst 2011;103:1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Preventing Anthracycline Cardiovascular Toxicity With Statins (PREVENT)https://clinicaltrials.gov/ct2/show/NCT01988571. Accessed June 26, 2017.
- 390.Kirkham AA, Davis MK. Exercise prevention of cardiovascular disease in breast cancer survivors. J Oncol 2015;2015:917606. doi: 10.1155/2015/917606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res 2009;15:4963–4967. doi: 10.1158/1078-0432.CCR-09-0628. [DOI] [PubMed] [Google Scholar]
- 392.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, McKenzie DC. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 393.Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, Kwan ML, Quesenberry CP Jr, Scott J, Sternfeld B, Yu A, Kushi LH, Caan BJ. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol 2016;34:2743–2749. doi: 10.1200/JCO.2015.65.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Pituskin E, Haykowsky M, McNeely M, Mackey J, Chua N, Paterson I. Rationale and design of the multidisciplinary team IntervenTion in cArdio-oNcology study (TITAN). BMC Cancer 2016;16:733. doi: 10.1186/s12885-016-2761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Wengström Y, Bolam KA, Mijwel S, Sundberg CJ, Backman M, Browall M, Norrbom J, Rundqvist H. OptiTrain: a randomised controlled exercise trial for women with breast cancer undergoing chemotherapy. BMC Cancer 2017;17:100. doi: 10.1186/s12885-017-3079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc 2014;89:1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Xie Y, Collins WJ, Audeh MW, Shiao SL, Gottlieb RA, Goodman MT, Merz CN, Mehta PK. Breast cancer survivorship and cardiovascular disease: emerging approaches in cardio-oncology. Curr Treat Options Cardiovasc Med 2015;17:60. doi: 10.1007/s11936-015-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(suppl 2):S74–S75]. Circulation 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 399.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PWF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S46–S48 and Circulation. 2015;132:e396]. Circulation 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 400.Rock CL, Flatt SW, Newman V, Caan BJ, Haan MN, Stefanick ML, Faerber S, Pierce JP. Factors associated with weight gain in women after diagnosis of breast cancer. Women’s Healthy Eating and Living Study Group. J Am Diet Assoc 1999;99:1212–1221. [DOI] [PubMed] [Google Scholar]
- 401.Koelwyn GJ, Khouri M, Mackey JR, Douglas PS, Jones LW. Running on empty: cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol 2012;30:4458–4461. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev 2012;(8):CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Clin Otolaryngol 2012;37:390–392. doi: 10.1111/coa.12015. [DOI] [PubMed] [Google Scholar]
- 405.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev 2012;(8):CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Giallauria F, Maresca L, Vitelli A, Santucci de Magistris M, Chiodini P, Mattiello A, Gentile M, Mancini M, Grieco A, Russo A, Lucci R, Torella G, Berrino F, Panico S, Vigorito C. Exercise training improves heart rate recovery in women with breast cancer. Springerplus 2015;4:388. doi: 10.1186/s40064-015-1179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 408.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Hamo CE, Bloom MW, Cardinale D, Ky B, Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR, Butler J. Cancer therapy-related cardiac dysfunction and heart failure: part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail 2016;9:e002843. doi: 10.1161/CIRCHEARTFAILURE.115.002843. [DOI] [PMC free article] [PubMed] [Google Scholar]