Memory Consolidation for Contextual and Auditory Fear Conditioning Is Dependent on Protein Synthesis, PKA, and MAP Kinase (original) (raw)
Abstract
Fear conditioning has received extensive experimental attention. However, little is known about the molecular mechanisms that underlie fear memory consolidation. Previous studies have shown that long-term potentiation (LTP) exists in pathways known to be relevant to fear conditioning and that fear conditioning modifies neural processing in these pathways in a manner similar to LTP induction. The present experiments examined whether inhibition of protein synthesis, PKA, and MAP kinase activity, treatments that block LTP, also interfere with the consolidation of fear conditioning. Rats were injected intraventricularly with Anisomycin (100 or 300 μg), Rp-cAMPS (90 or 180 μg), or PD098059 (1 or 3 μg) prior to conditioning and assessed for retention of contextual and auditory fear memory both within an hour and 24 hr later. Results indicated that injection of these compounds selectively interfered with long-term memory for contextual and auditory fear, while leaving short-term memory intact. Additional control groups indicated that this effect was likely due to impaired memory consolidation rather than to nonspecific effects of the drugs on fear expression. Results suggest that fear conditioning and LTP may share common molecular mechanisms.
Classically conditioned fear is a behavioral paradigm in which animals learn to fear an initially neutral stimulus (CS; conditioned stimulus) that has been paired or followed by presentation of a noxious unconditioned stimulus (US), such as foot shock (Bouton and Bolles 1980; Davis 1992; LeDoux 1992). The learning is rapid and is extremely robust and enduring (LeDoux et al. 1989), characteristics that make fear conditioning well suited for the study of the neural mechanisms of learning and memory in the mammalian brain.
Whereas the neuroanatomical pathways and synaptic events underlying conditioned fear have been well characterized (see, e.g., Davis 1992; LeDoux 1992, 1995; Maren and Fanselow 1996), relatively little is known about the molecular mechanisms that underlie fear memory. In contrast, considerable progress has been made in elucidating the molecular changes underlying long-term potentiation (LTP), the leading cellular model of memory consolidation in the mammalian brain (see, e.g., Alberini et al. 1995; Kandel 1997; Milner et al. 1998). It is thus of interest that LTP has been demonstrated in pathways known to be relevant to fear conditioning (Chapman et al. 1990; Clugnet and LeDoux 1990; Rogan and LeDoux 1995; Rogan et al. 1997; Huang and Kandel 1998). Further, neural activity in the brain is modified similarly during fear conditioning and LTP induction (Rogan et al. 1997; McKernan and Shinnick-Gallagher 1997). Collectively, these results suggest that LTP and fear memory consolidation may share common molecular mechanisms.
Several forms of LTP have been extensively characterized using both in vitro brain slice preparations and in vivo preparations, especially in the hippocampus (see, e.g., Bliss and Lømo 1973; Madison et al. 1991; Barnes et al. 1995). Although the synaptic and molecular events underlying the induction of these various forms of LTP appear to differ (Madison et al. 1991), each has recently been shown to be characterized by two distinct temporal phases. The “early” phase (E-LTP), lasting from 1 to 3 hr, appears to involve covalent modification of existing proteins and does not require protein or RNA synthesis (Frey et al. 1993; Huang et al. 1994; Nguyen and Kandel 1996). The “late” phase (L-LTP), lasting from hours to days, is dependent upon de novo RNA and protein synthesis and appears to involve both the cAMP-dependent (PKA) and mitogen-activated (MAP) protein kinase signaling pathways (Frey et al. 1993; Huang et al. 1994; Nguyen and Kandel 1996; English and Sweatt 1997; Atkins et al. 1998; Impey et al. 1998a). These intracellular signaling pathways are thought to transduce the activity-dependent changes characteristic of shorter forms of synaptic plasticity into long-term structural and functional change by engaging activators of transcription in the nucleus (Alberini et al. 1995; Kandel 1997; Milner et al. 1998a). In support of this hypothesis, application of RNA or protein synthesis inhibitors or selective inhibitors of PKA to hippocampal slices prior to tetanization of the perforant or Schaffer collateral pathways has been shown to prevent the induction of L-LTP, while having no effect on that of E-LTP (Frey et al. 1993; Huang et al. 1994; Nguyen and Kandel 1996). Similarly, application of inhibitors to MAP kinase has been shown to prevent the induction of L-LTP in the Schaffer collateral pathway (English and Sweatt 1997; Atkins et al. 1998; Impey et al. 1998), while having little effect on E-LTP (Impey et al. 1998). Finally, stimuli that generate L-LTP in hippocampus have been shown to induce the transcription of cAMP response element (CRE)-mediated genes, an effect that is prevented by inhibitors of PKA or MAP kinase (MAPK) (Impey et al. 1996, 1998a). Collectively, results suggest that signal transduction involving both PKA and MAPK are necessary for the long-term protein synthesis-dependent synaptic plasticity believed to underlie memory consolidation (see, e.g., Kandel 1997; Kornhauser and Greenberg 1997, Abel et al. 1998; Milner et al. 1998).
The following experiments are part of a series of investigations we are conducting in an attempt to define the role of protein synthesis and intracellular signaling pathways in the acquisition and retention of classically conditioned fear to auditory and contextual stimuli. Although significant progress has been made in implicating specific circuits in fear conditioning (Davis 1992; LeDoux 1992, 1995; Maren and Fanselow 1997), in this initial study we have chosen to target these circuits broadly using intraventricular injections of drugs. Specifically, rats were injected intraventricularly with Anisomycin (a protein synthesis inhibitor), Rp-cAMPS (a PKA inhibitor), or PD098059 (a MAPK inhibitor) prior to conditioning and tested for fear memory retention both within 1 hr (short-term memory, STM) and 24 hr later (long-term memory, LTM). Each of these compounds has been shown in previous studies to impair L-LTP, while having little effect on E-LTP (Huang et al. 1994; Nguyen and Kandel 1996; English and Sweatt 1997; Impey et al. 1998). Consistent with the LTP literature, our results indicate that administration of these compounds dose dependently disrupted long-term, but not short-term, memory for fear. Additional control groups determined that this effect was specific to memory and not secondary to effects of the drugs on normal behavioral expression. Collectively, results suggest that fear memory consolidation and LTP may involve similar molecular mechanisms.
Materials and Methods
SUBJECTS
Subjects were adult male Sprague-Dawley rats obtained from Hilltop Labs, Scottdale, PA. Rats were housed individually in plastic Nalgene cages and maintained on a 12:12 hr light/dark cycle. Food and water were provided ad libitum throughout the experiment.
SURGERY
Under Nembutal anesthesia (45 mg/kg), rats were implanted unilaterally with a 26-gauge stainless steel cannula into the left lateral ventricle. Coordinates, taken from Paxinos and Watson (1986) and adjusted according to pilot data, were 0.4 mm posterior to bregma, 1.3 mm lateral to the midline, and 4.2 mm ventral to the skull surface. The cannula was anchored to the skull with stainless steel screws and a mixture of acrylic and dental cement. A 33-gauge dummy cannula was inserted to prevent clogging. Following surgery, rats were given 0.2 mg/kg buprenorphine HCl as an analgesic. Rats were given at least 4 days to recover prior to experimental procedures.
DRUGS
Rats were injected with either Anisomycin (Sigma, cat. no. A9789), Rp-adenosine 3′,5′-cyclic monophosphothioate triethylamine (Rp-cAMPS; RBI, cat. no. A-165), or PD098059 (RBI, cat. no. P-215). Anisomycin was dissolved in equimolar HCl, diluted with artificial cerebrospinal fluid (ACSF), and adjusted to pH 7.4 with NaOH. Rp-cAMPS was dissolved in ACSF. PD098059 was dissolved in 100% DMSO. Anisomycin is a potent inhibitor of mRNA translation via interference with transpeptidation and has been used successfully in a number of behavioral paradigms (see, e.g., Davis and Squire 1984). Rp-cAMPS inhibits the activation of cAMP-dependent protein kinases I and II by cAMP (Rothermel and Parker-Botelho 1988). PD098059 is a specific inhibitor of the activation of MAPK kinase (MEK), an upstream regulator of MAPK (Alessi et al. 1995).
INTRAVENTRICULAR INJECTIONS
For drug injections, rats were held gently in the experimenter’s lap. The dummy cannula was removed and replaced with a 33-gauge injector cannula attached to a 5.0 μl Hamilton syringe via 20-gauge polyurethane tubing. The tubing was back-filled with sesame oil to provide adequate pressure for drug injection. A small air bubble separated the oil from the drug solution. Drugs were infused slowly via infusion pump into the lateral ventricle at a rate of 0.25 μl/min. Following drug infusion, cannulas were left in place for an additional minute to allow diffusion of the drug away from the cannula tip. Dummy cannulas were then replaced and the rat was returned to its home cage.
APPARATUS
Conditioning and tone testing were conducted in two distinct chambers. For conditioning, rats were placed in a Plexiglas rodent conditioning chamber (chamber A) with a metal grid floor (Model E10-10, Coulbourn Instruments, Lehigh Valley, PA) that was enclosed within a sound attenuating chamber (model E10-20). The chamber was dimly illuminated by a single house light. For tone testing, rats were placed in a distinct Plexiglas chamber to minimize generalization from the conditioning environment (ENV-001, MedAssociates, Inc., Georgia, VT). The tone testing chamber (chamber B) was brightly lit with three house lights and contained a flat black formica floor that had been washed with a peppermint soap. A microvideo camera was mounted at the top of the chamber so that rats could be videotaped during testing.
HISTOLOGY
To verify the location of the cannula tip within the lateral ventricle, rats were anesthetized with an overdose of chloral hydrate (250 mg/kg; i.p.) and injected manually with 5.0 μl of a 0.5% solution of Cresyl violet into the ventricle. Rats were then decapitated and brains were removed and postfixed in 10% buffered formalin in 30% sucrose. Brains were then cut into 5-mm blocks and examined for dye in the ventricles. Blocks containing the cannula track were then sectioned on a cryostat at 50 μm thickness and stained for Nissl using Thionine. Sections were examined with light microscopy for cannula penetration into the lateral ventricle.
GENERAL BEHAVIORAL PROCEDURES
HABITUATION AND CONDITIONING
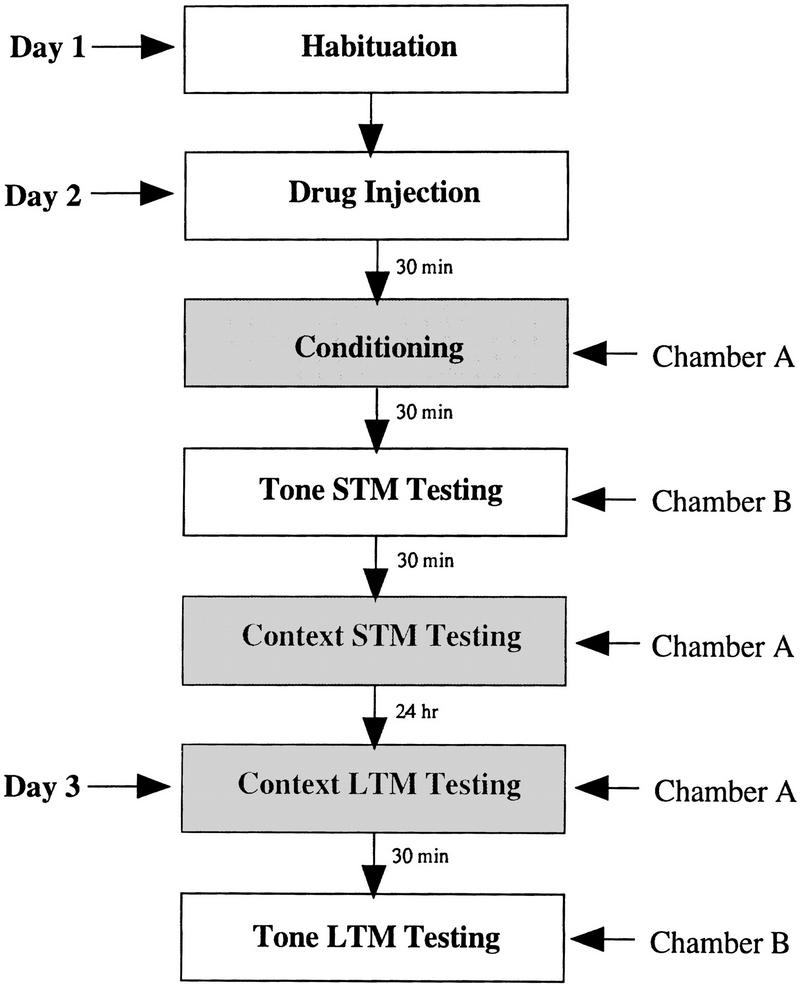
Figure 1 outlines the general behavioral procedures used in each experiment. On the day prior to conditioning (day 1), rats were habituated for 15 min to chambers A and B. On the conditioning day (day 2), separate groups of rats were injected with either Anisomycin (100 or 300 μg; 5 μl), Rp-cAMPS (90 or 180 μg; 5 μl), PD098059 (1 or 3 μg; 3 μl), or an equivalent volume of vehicle (ACSF; DMSO) 30 min prior to conditioning. The doses of Anisomycin were chosen based on recently published data showing that i.c.v. administration of 200 μg in the rat inhibits protein synthesis in the hippocampus by >90% within 20 min of injection (Meiri and Rosenblum 1998). Doses of Rp-cAMPS and PD098059 were chosen based on their effectiveness at blocking fear memory in pilot experiments. Following a 5 min acclimation period to the conditioning chamber, paired rats received a single conditioning trial consisting of a 30-sec presentation of a 5-kHz, 70-dB tone (CS) that coterminated with a 1.5-mA foot shock (US) delivered through the grid floor during the last 1.0 sec of the tone. Unpaired controls were injected with vehicle (ACSF; DMSO) and also received tones and shocks, but in a noncontingent, explicitly unpaired fashion. For these groups, the US shock preceded the CS tone (5 kHz, 70 dB, 30 sec) by 60 sec. Following conditioning, all rats were returned to their home cages.
Figure 1.
Outline of general behavioral procedures.
SHORT-TERM MEMORY TESTING
STM to the tone was evaluated 30 min after conditioning. For this test, rats were placed in chamber B and given 2 exposures to the CS tone (30 sec, 5 kHz, 70 dB) with an average intertrial interval (ITI) of 100 sec. Rats were videotaped during CS presentations for subsequent quantification of behavior. Time spent “freezing” during the presentation of the tone CS was measured during each CS presentation as well as during a 30-sec baseline period prior to the first tone trial (see, e.g., Bouton and Bolles 1980; LeDoux et al. 1990a for details). This latter measure served as an assay for both unconditioned effects of the drugs on general activity levels and for fear generalization between the conditioning and tone-testing chambers. Following tone testing, rats were returned to their home cages. To evaluate STM to the context, rats were again placed in the conditioning chamber (chamber A) 30 min following the tone test (for a total of 60 min following conditioning). Rats were allowed to explore for 5 min, after which freezing to the context was assessed every other 30 sec for an additional 5 min (for a total of five 30-sec observations).
LONG-TERM MEMORY TESTING
LTM for both the context and the tone were evaluated the following day (day 3; ∼24 hr following conditioning). For the context test, rats were placed in chamber A and allowed to explore for 5 min, after which freezing to the context was assessed every other 30 sec for an additional 5 min. For the tone test, rats were again placed in chamber B and presented with 5 tones (30 sec, 5 kHz, 70 dB, ITI = 100 sec). As for STM testing, freezing was evaluated during each presentation of the tone CS and during the 30-sec baseline period prior to the first tone trial. Following the memory tests, rats were returned to their home cages and to the colony.
RECONDITIONING
To evaluate whether the injection of these compounds resulted in long-term inability to express fear or to associate tones and shocks, rats were reconditioned drug free ∼1 week after LTM testing. As before, rats received a single pairing of a 30-sec, 5-kHz, 70-dB tone that coterminated with a 1-sec 1.5-mA foot shock. Twenty-four hours later, rats were evaluated for long-term contextual and auditory fear memory as described above.
Results and Discussion
INTRAVENTRICULAR INJECTION OF ANISOMYCIN, Rp-cAMPS, AND PD098059 DOSE-DEPENDENTLY IMPAIRED THE CONSOLIDATION OF CONTEXTUAL AND AUDITORY FEAR MEMORY
Histological observations revealed that most rats had successful cannula placements in the lateral ventricle. Only those rats with observable dye in the ventricle were included in the data analysis. A photograph of a representative cannula placement in the lateral ventricle can be found in Figure 2.
Figure 2.
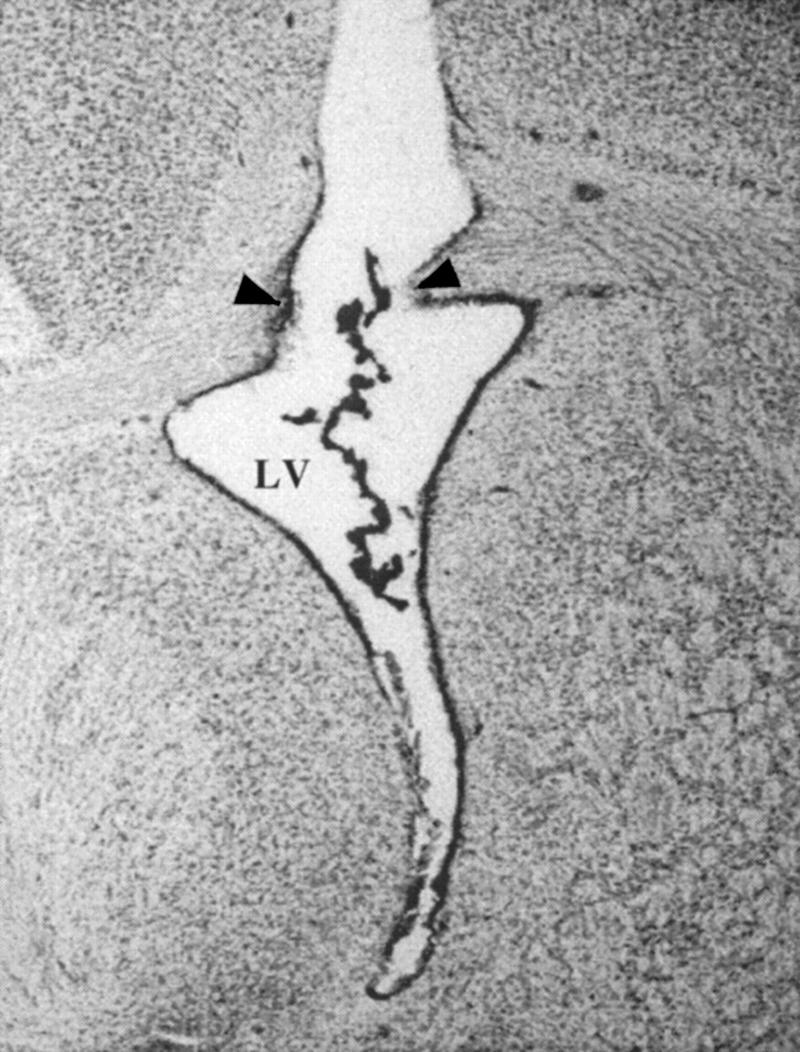
Representative cannula placement in the lateral ventricle (LV). (Arrows) Point of entry. Cresyl violet dye can be seen lining the ventricle ependyma.
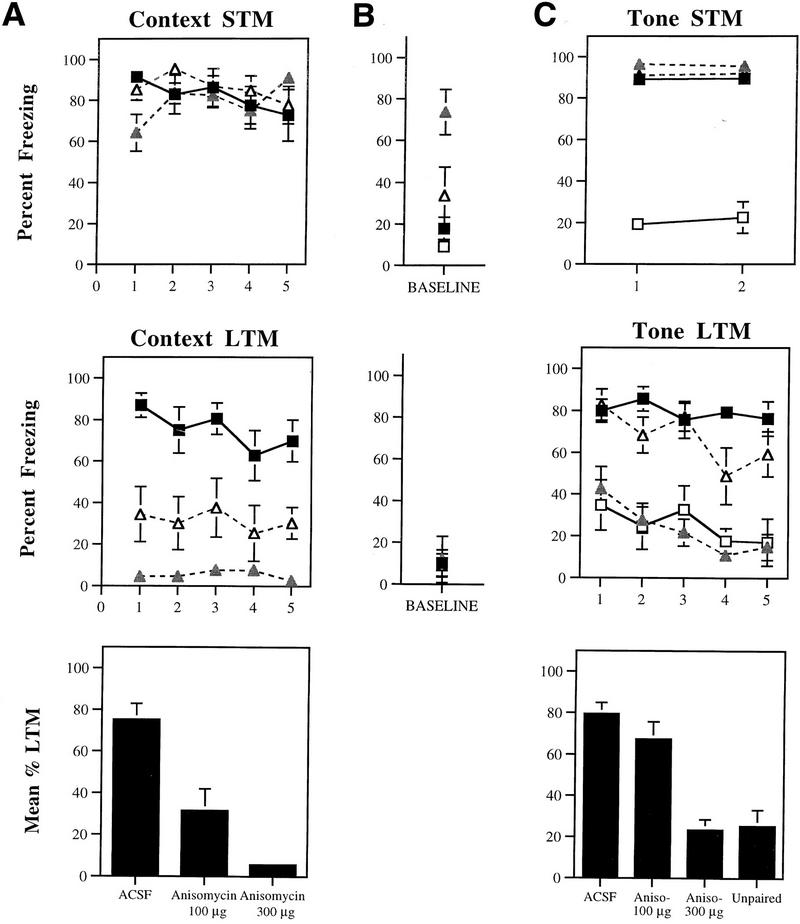
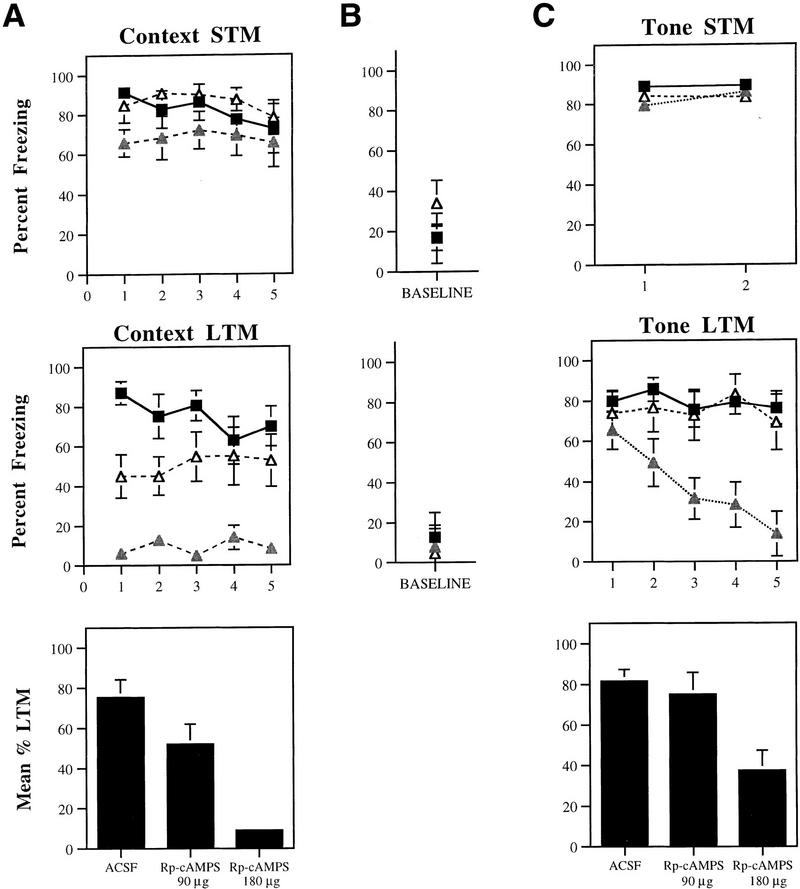
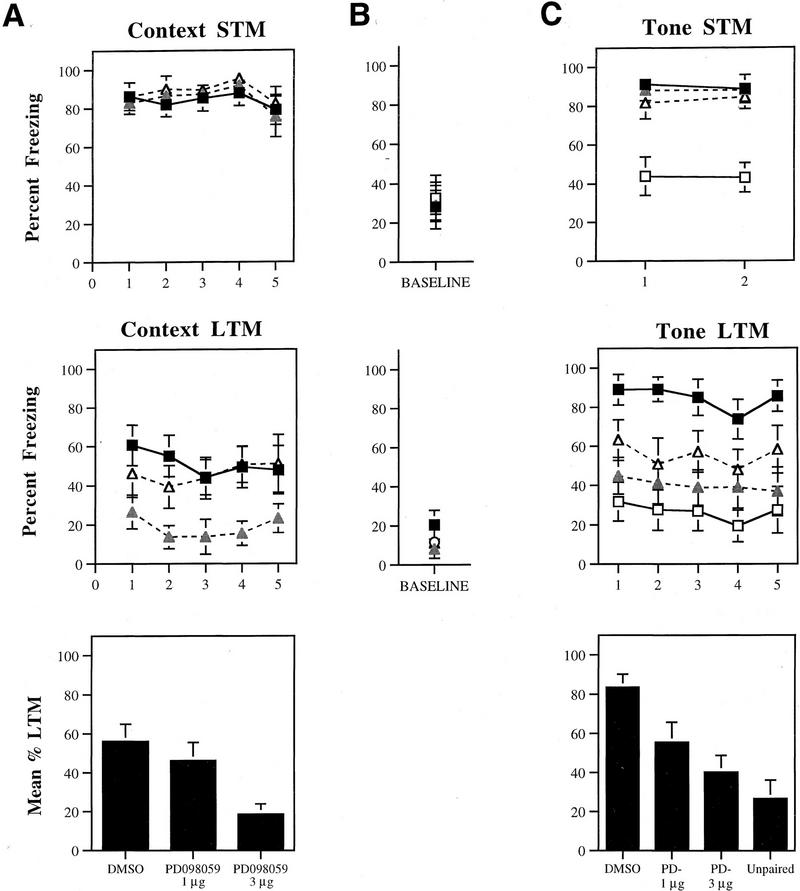
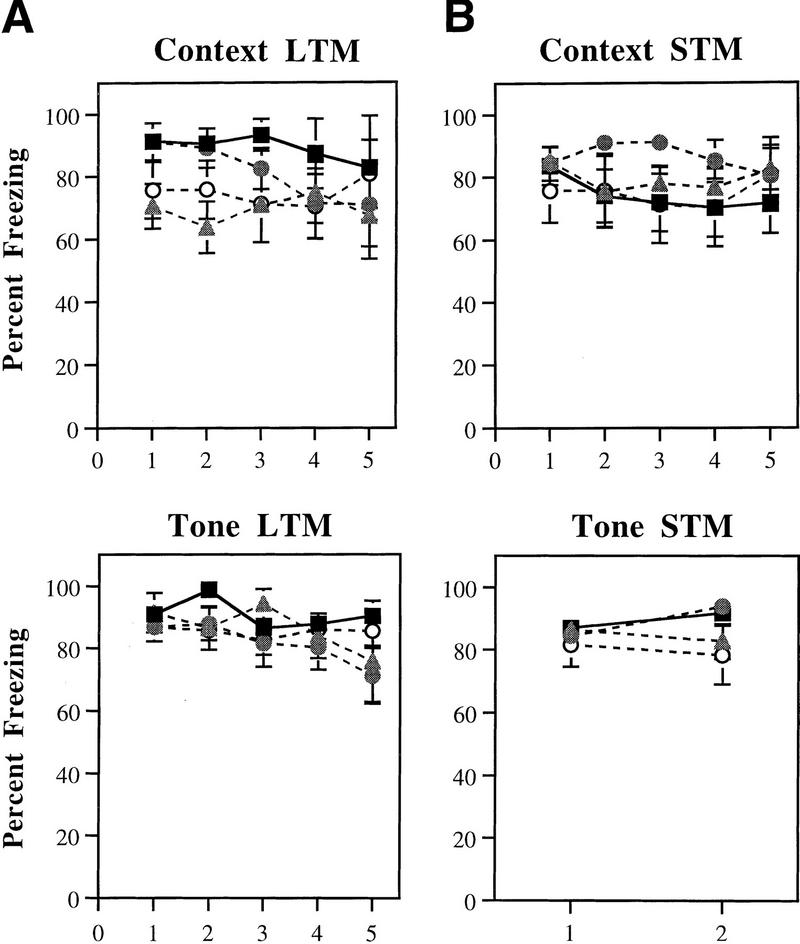
Figures 3, 4, and 5 depict mean percent freezing for contextual (A) and auditory (C) fear memory for rats injected with Anisomycin, Rp-cAMPS, PD098059, or vehicle. Baseline levels of freezing prior to the first trial in the tone memory tests (STM, LTM) can be found in B. The top and middle panels in each figure represent absolute scores for STM and LTM trials, respectively. The bottom panels represent mean percent LTM. For this latter score, LTM scores for both context and tone were averaged for each rat. All data were analyzed using analysis of variance (ANOVA) and Duncan’s multiple range _t_-tests.
Figure 3.
(A) (Top) Mean (±s.e.) percent freezing for context STM in rats injected with ACSF (█; n = 10), 300 μg Anisomycin (shaded triangles; n = 9), or 100 μg Anisomycin (▵; n = 7). (Middle) Mean (±s.e.) freezing for context LTM in the same rats. (Bottom) Mean (±s.e.) percent context LTM for ACSF- and Anisomycin-injected rats. (B) (Top) Mean (±s.e.) percent freezing during the baseline period prior to the first trial in the STM tone test. (Middle) Mean (±s.e.) percent freezing during the baseline period prior to the first trial in the LTM tone test (C) (Top) Mean (±s.e.) percent freezing for tone STM in unpaired controls (□; n = 8), and rats injected with ACSF (█), 300 μg Anisomycin (shaded triangles), or 100 μg Anisomycin (▵). (Middle) Mean (±s.e.) freezing for tone LTM in the same rats. (Bottom) Mean (±s.e.) percent tone LTM for ACSF- and Anisomycin-injected rats, and unpaired controls.
Figure 4.
(A) (Top) Mean (±s.e.) percent freezing for context STM in rats injected with ACSF (█; n = 9), 180 μg Rp-cAMPS (shaded triangles; n = 7), or 90 μg Rp-cAMPS (▵; n = 8). (Middle) Mean (±s.e.) freezing for context LTM in the same rats. (Bottom) Mean (±s.e.) percent context LTM for ACSF- and Rp-cAMPS-injected rats. (B) (Top) Mean (±s.e.) percent freezing during the baseline period prior to the first trial in the STM tone test. (Middle) Mean (±s.e.) percent freezing during the baseline period prior to the first trial in the LTM tone test. (C) (Top) Mean (±s.e.) percent freezing for tone STM in rats injected with ACSF (█), 180 μg Rp-cAMPS (shaded triangles), or 90 μg Rp-cAMPS (▵). (Middle) Mean (±s.e.) freezing for tone LTM in the same rats. (Bottom) Mean (±s.e.) percent tone LTM for ACSF- and Rp-cAMPS-injected rats.
Figure 5.
(A) (Top) Mean (±s.e.) percent freezing for context STM in rats injected with DMSO (█; n = 11), 3 μg PD098059 (shaded triangles; n = 9), or 1 μg PD098059 (▵; n = 8). (Middle) Mean (±s.e.) freezing for context LTM in the same rats. (Bottom) Mean (±s.e.) percent context LTM for DMSO- and PD098059-injected rats. (B) (Top) Mean (±s.e.) percent freezing during the baseline period prior to the first trial in the STM tone test. (Middle) Mean (±s.e.) percent freezing during the baseline period prior to the first tone trial in the LTM tone test. (C) (Top) Mean (±s.e.) percent freezing for tone STM in unpaired rats (□; n = 8), and rats injected with DMSO (█), 3 μg PD098059 (shaded triangles), or 1 μg PD098059 (▵). (Middle) Mean (±s.e.) freezing for tone LTM in the same rats. (Bottom) Mean (±s.e.) percent tone LTM for DMSO- and PD098059-injected rats and unpaired controls.
ANISOMYCIN
Relative to ACSF-injected controls, rats injected with either dose of Anisomycin displayed intact fear to the context when tested 60 min after conditioning (Fig. 3A). The ANOVA for context STM scores revealed no differences (P > 0.05). Twenty-four hours later, however, both Anisomycin groups showed substantial memory impairment. The ANOVA (group by trials) for context LTM scores showed a significant effect for group (ACSF vs. 100 μg vs. 300 μg), [F(2, 23) = 26.49, P < 0.01]. The effect for trials and the interaction did not achieve significance (_P_ > 0.05). Post-hoc _t_-tests revealed that freezing scores for both Anisomycin groups differed from the ACSF group on every trial (P < 0.05). In contrast, the high-dose group differed from the low-dose group only on trials one and four (P < 0.05). This same pattern of results is reflected in the mean context LTM scores [F(2, 23) = 26.49, P < 0.01], where memory impairment following either dose of Anisomycin was found to differ from the ACSF group (P < 0.05). The two drug groups were also found to differ (P < 0.05).
The results for tone memory showed a similar pattern (Fig. 3C). The ANOVA for tone STM scores revealed a significant effect for group (ACSF vs. 100 μg vs. 300 μg vs. unpaired) [F(3, 29) = 90.66, P < 0.01], a nonsignificant effect for trials, and a nonsignificant group by trials interaction. Both drug groups showed strong, intact STM for the tone, and they were not found to differ on either trial from each other or from the ACSF group (_P_ > 0.05). Both drug groups and ACSF controls, however, were found to differ significantly from the unpaired group on each tone trial (P < 0.05), indicating that STM to the tone was associative.
Twenty-four hours later, however, rats injected with the highest dose of Anisomycin (300 μg) showed little fear retention. The ANOVA for tone LTM scores revealed a significant effect for group [F(3, 29) = 20.20, P < 0.01], a significant effect for trials [_F_(4, 116) = 8.69, _P_ < 0.01], and a nonsignificant group by trials interaction. Post-hoc _t_-tests revealed that the high-dose group differed from both ACSF controls and the low-dose group on every trial (_P_ < 0.05). In fact, LTM scores for this group did not differ significantly from those of unpaired rats at any point (_P_ > 0.05). In contrast, the low-dose group was found to differ from ACSF controls only on the fourth tone trial (P < 0.05). This same pattern of results is reflected in the mean LTM scores [F(3, 29) = 20.20, P < 0.01], where memory impairment following the high dose of Anisomycin was found to differ from the other two groups (P < 0.05). Overall, no significant difference was detected between the ACSF and the low-dose group. Additionally, no difference was detected between the unpaired group and the group receiving the highest dose of Anisomycin.
Although tone STM scores for rats injected with the high dose of Anisomycin were not found to differ from those of the other groups on either trial, it should be noted that baseline levels of freezing for this group were found to be significantly higher than the other groups (Fig. 3B; P < 0.05). At this dose (300 μg), Anisomycin apparently produced either motor deficits or a general state of malaise characterized by general inactivity. These observations are in keeping with previous reports of the toxicity of protein synthesis inhibitors, including reports of suppressed activity within 1 hr following treatment with a high systemic dose (150 mg/kg) of Anisomycin (Davis and Squire 1984). However, although the behavioral inactivity in the high-dose group complicates the interpretation of the STM results for both context and tone, rats in this group nonetheless displayed significantly more freezing to both STM tone presentations than in the baseline period (_P_ < 0.05). This suggests that they did in fact have intact STM for the tone. Further, baseline freezing scores in the low-dose group were not different from those in ACSF or unpaired controls (_P_ > 0.05), despite substantial memory impairment for the low dose in the context LTM test. It thus appears likely that the pronounced LTM deficits seen in the high-dose group for both context and tone may be attributed to memory impairment rather than to unconditioned effects of the drug.
Rp-cAMPS
The pattern of results for Rp-cAMPS was similar to those for Anisomycin (Fig. 4A). Relative to ACSF-injected controls, rats injected with either dose of Rp-cAMPS displayed intact context STM on each trial (P > 0.05). Twenty-four hours later, however, rats receiving the highest dose (180 μg) showed little contextual fear, and those injected with the low dose (90 μg) showed attenuated fear relative to ACSF controls. The ANOVA (group by trials) for context LTM scores showed a significant effect for group (ACSF vs. 90 μg vs. 180 μg) [F(2, 21) = 16.15, P < 0.01], a nonsignificant effect for trials (_P_ > 0.05), and a significant group by trials interaction [F(8, 84) = 2.21, P < 0.05]. Post hoc _t_-tests revealed that this latter effect was due to differences between ACSF controls and the low-dose group in the first two trials. No differences were detected between these two groups in the last three trials. Importantly, freezing scores in rats receiving the highest dose of Rp-cAMPS (180 μg) were found to be different from the other two groups on every trial. This same pattern of results is evident in the mean context LTM scores [F(2, 21) = 16.15, P < 0.01], where memory impairment following the 180 μg dose of Rp-cAMPS was found to differ from that of the other groups (P < 0.05). Overall, no significant differences were detected between the ACSF and 90 μg groups, although a clear trend was noted (P = 0.05).
Rp-cAMPS had no effect on tone STM (Fig. 4C). The ANOVA for tone STM scores revealed no significant effects (P > 0.05). Additionally, no differences were detected between groups for the baseline period (P > 0.05) (Fig. 4B). Twenty-four hours later, however, rats injected with the highest dose of Rp-cAMPS showed deficits in LTM for the tone. The ANOVA for tone LTM scores revealed a significant effect for group [F(2, 21) = 6.94, P < 0.01], a significant effect for trials [F(4, 84) = 7.77, P < 0.01], and a significant group by trials interaction [F(8, 84) = 4.94, P < 0.01]. Post hoc _t_-tests revealed that there were no differences between groups for either the baseline period or for the first tone trial. However, rats injected with the high dose of Rp-cAMPS were found to differ from ACSF controls on trials 2–5 (P < 0.05) and from the group injected with the lower dose of Rp-cAMPS on trials 3–5 (P < 0.05). No differences were detected between ACSF controls and rats injected with the low dose of Rp-cAMPS on any trial. This same pattern of results is reflected in the mean tone LTM scores [F(2, 21) = 6.94, P < 0.01], where memory impairment following the high dose of Rp-cAMPS was found to differ from the other two groups (P < 0.05). Overall, no significant differences were detected between the ACSF and low-dose groups.
Although we did not run unpaired controls associated with the rats injected with Rp-cAMPS, the overall tone LTM for the high-dose group was found to be about 40% of STM, which is not significantly different from that found for rats injected with the highest dose of Anisomycin (P > 0.05). Additionally, it can be seen in Figure 4C that rats injected with the highest dose of Rp-cAMPS had equivalent freezing scores to vehicle controls on the first tone trial. Thus, it appears that some memory to the tone remained intact following treatment with this dose. We were not able to examine the effects of higher doses, however, as doses above 200 μg produced seizures in pilot experiments.
PD098059
Context STM memory for rats injected with either dose of PD098059 was not found to differ from that of DMSO controls (P > 0.05) (Fig. 5A). Twenty-four hours later, however, rats injected with the highest dose of PD098059 showed evidence of memory impairment. The ANOVA for context LTM scores showed a significant effect for group (DMSO vs. 1 μg vs. 3 μg), [F(2, 25) = 5.86, P < 0.01], a nonsignificant effect for trials, and a nonsignificant interaction. Freezing scores for rats injected with the highest dose of PD098059 were found to be different from DMSO controls on all but the fifth trial. No differences were detected on any trial between DMSO controls and the low-dose group. This same pattern of results is evident in the mean context LTM scores [F(2, 25) = 5.86, P < 0.01], where the high-dose group was found to differ from both DMSO controls and the low-dose group (P < 0.05). No difference was detected between DMSO controls and the low-dose group.
As before, tone STM was intact for all groups relative to unpaired controls (Fig. 5C). The ANOVA for tone STM scores revealed a significant effect for group (DMSO vs. 1 μg vs. 3 μg vs. unpaired) [F(3, 32) = 13.35, P < 0.01], a nonsignificant effect for trials, and a nonsignificant group by trials interaction. Additionally, no differences between groups were detected for the baseline period (Fig. 5B). Post-hoc _t_-tests revealed no differences between DMSO controls and PD098059-injected groups for either tone trial (_P_ > 0.05). The unpaired group, however, was found to be different from the other groups on both trials (P < 0.05), indicating that freezing to the tone shortly after conditioning was associative.
As with the other drugs, rats injected with PD098059 showed impaired tone memory when tested 24 hr later, and this effect was most pronounced for the highest dose of the drug. The ANOVA for tone LTM scores revealed a significant effect for group [F(3, 32) = 8.65, P < 0.01], a nonsignificant effect for trials, and a nonsignificant group by trials interaction. Additionally, no differences in freezing scores existed between groups for the baseline period. However, post-hoc tests revealed that both unpaired controls and rats injected with the highest dose of PD098059 differed from DMSO controls on every tone trial. No differences were detected between the two doses of PD098059, or between these doses and the unpaired group (_P_ > 0.05). This same pattern of results was seen in the mean tone LTM scores [F(3, 32) = 8.65, P < 0.01], where both doses of PD098059 were shown to produce memory impairment relative to DMSO (P < 0.05). Overall, no significant difference was detected between the two doses of PD098059.
INJECTION OF ANISOMYCIN, Rp-cAMPS, OR PD098059 DID NOT PREVENT NORMAL ACQUISITION TO THE CONTEXT OR TONE FOLLOWING RECONDITIONING 1 WEEK LATER
Figure 6A depicts mean percent freezing to the context and tone following reconditioning. It is evident in the figure that rats in each group were able to reacquire and express fear to both cues. An ANOVA for both context and tone memory revealed no effects (P > 0.05). Thus, the memory impairment produced by these compounds appears to be transient (<1 week), and the drugs do not appear to result in permanent inability to express fear or to associate tones and shocks.
Figure 6.
(A) Mean (±s.e.) percent freezing for context (top) and tone (bottom) LTM following re-conditioning in rats injected with ACSF (█), 300 μg Anisomycin (shaded triangles), 180 μg Rp-cAMPS (shaded circles), or 3 μg PD098059 (○). Rats were tested for context and tone memory 24 hr after reconditioning. (B) Mean (±s.e.) percent freezing for context (top) and tone (bottom) STM in rats injected with ACSF (█; n = 8), 300 μg Anisomycin (shaded triangles; n = 8), 180 μg Rp-cAMPS (shaded circles; n = 8), or 3 μg PD098059 (○; n = 8) 24 hr prior to conditioning. Rats were evaluated for tone and context memory either 30 or 60 min following conditioning, respectively.
INJECTION OF ANISOMYCIN, Rp-cAMPS, OR PD098059 24 HR PRIOR TO CONDITIONING HAS NO EFFECT ON FEAR ACQUISITION OR EXPRESSION
In the previous experiments, rats were injected with Anisomycin, Rp-cAMPS, or PD098059 30 min prior to conditioning and tested for LTM approximately 24 hr later. Although each of these groups displayed intact STM and was able to be reconditioned approximately 1 week later, the possibility remains that the failure to display significant amounts of freezing on test day represents a nonspecific effect of the drugs on normal behavioral expression 24 hr following injection. To evaluate this possibility, additional groups of rats were injected with the highest doses of Anisomycin (300 μg), Rp-cAMPS (180 μg), or PD098059 (3 μg) 24 hr prior to conditioning. The following day, rats were conditioned and tested for STM as described above. Thus, each group was subjected to STM tests for fear at approximately the same time as rats in the previous experiments were subjected to LTM tests. Freezing scores for each group can found in Figure 6B. It is evident in the figure that rats in each group displayed normal amounts of freezing behavior to both the context and tone and that no differences existed between groups. An ANOVA confirmed this (P > 0.05). Thus, it is unlikely that the memory deficits seen in rats injected with these compounds 30 min prior to conditioning are due to nonspecific effects of the drugs on behavioral expression 24 hr after injection.
General Discussion
Previous studies have shown that LTP exists in pathways known to be relevant to fear conditioning and that fear conditioning modifies neural activity in these pathways in the same manner that LTP does (Chapman et al. 1990; Clugnet and LeDoux 1990; Rogan and LeDoux 1995; Rogan et al. 1997; Huang and Kandel 1998; McKernan and Shinnick-Gallagher 1997). These studies suggest that fear conditioning and LTP may share similar molecular mechanisms. The present experiments examined whether pharmacological inhibition of protein synthesis, PKA, and MAPK activity, treatments that block LTP, also interfere with memory consolidation for fear conditioning. Results indicated that interference with these pathways selectively and dose dependently interfered with LTM for contextual and auditory fear, while leaving STM intact. This pattern of selective interference with LTM is similar to that observed in the LTP literature in which interference with these pathways has been shown to block L-LTP, while having little effect on E-LTP (Frey et al. 1993; Huang et al. 1994; Nguyen and Kandel 1996; Impey et al. 1998a). Collectively, results favor the conclusion that fear memory consolidation and LTP share common molecular mechanisms.
The present results are in agreement with recent reports showing that pharmacological inhibition of protein synthesis and PKA in mice blocks consolidation of contextual fear memory (Bourtchouladze et al. 1998) and that systemic inhibition of the MAPK signaling pathway blocks both contextual and auditory fear in rats (Atkins et al. 1998). The former study, however, did not evaluate fear memory to the tone, and the latter did not evaluate STM to either the tone or the context. In contrast to these previous reports, the present studies evaluated both STM and LTM to both context and tone. Additionally, we provide three sets of evidence that indicate that the behavioral effects of Anisomycin, Rp-cAMPS, and PD098059 are likely to be the result of impaired memory consolidation rather than a nonspecific effect on fear acquisition or expression. First, rats injected with these compounds displayed intact and comparable STM relative to vehicle-injected controls to both the context and the tone shortly after conditioning. Thus, rats were able to perceive the tone, form a representation of the context, and acquire normal fear to each stimulus when paired with foot shock while under the influence of the drugs. Second, rats were able to be reconditioned 1 week later and to reacquire normal fear to both the context and the tone, indicating that injection of these compounds did not result in permanent inability of the rats to express fear or associate tones and shocks. Third, rats were able to acquire and express normal amounts of fear to both the tone and the context when injected with these compounds 24 hr prior to conditioning and STM testing, indicating that the memory impairment observed during the LTM tests was not due to nonspecific effects on fear expression on the day after injection. When considered together with other recent reports (Atkins 1998; Bourtchouladze et al. 1998), our results strongly favor the conclusion that both the cAMP and MAPK signaling pathways are necessary for the long-term protein-synthesis dependent changes underlying fear memory consolidation.
Because rats in our experiments were injected prior to conditioning and thus trained under the influence of drugs, it might be argued that the effects that we observed on LTM were the result of state-dependent learning rather than impaired memory consolidation. We believe, however, that this is an unlikely possibility. First, a recent paper by Kandel and colleagues employing administration of Anisomycin and Rp-cAMPS in mice found equivalent effects on contextual fear memory with both pre- and immediate post-training injections (Bourtchouladze et al. 1998). Second, Atkins et al. (1998) found that both pre- and immediate post-training systemic administration of SL327, a MEK inhibitor similar functionally to PD098059, impairs memory consolidation of contextual and auditory fear. Because impaired memory was demonstrated following both pre- and post-training administration of each of these compounds, it cannot be concluded that memory impairment on the day following conditioning is due to state-dependent learning. Indeed, an effect of these compounds on memory following post-training administration adds further evidence to the argument that they are exerting their effects on memory consolidation rather than on sensory processing or some other nonspecific factor.
Studies utilizing invertebrate and in vitro cell culture preparations have provided a number of suggestions regarding the mechanisms whereby the cAMP and MAPK signaling pathways promote long-term synaptic plasticity. In Aplysia, for example, facilitation of the gill-withdrawal reflex also appears to be characterized by two temporal phases, the latter of which is dependent on de novo RNA and protein synthesis, cAMP, and MAPK signaling pathways (see, e.g., Alberini et al. 1995; Kandel 1997; Milner et al. 1998). Application of RNA or protein synthesis inhibitors to cocultured Aplysia sensory and motor neurons, for example, selectively blocks long-term facilitation (LTF) while leaving short-term facilitation (STF) intact (Montarolo et al. 1986). Similarly, a mutation in the nuclear phosphorylation site of PKA or inhibition of MAPK activity by anti-MAPK antibodies or PD098059 selectively interferes with LTF, while having no effect on STF (Kaang et al. 1993; Martin et al. 1997). Finally, stimulation that leads to LTF has been shown to be accompanied by translocation of both the catalytic subunit of PKA and MAPK to the sensory neuron nucleus where each of these pathways is thought to engage activators of transcription (Bacskai et al. 1993; Martin et al. 1997). These findings are in parallel to those of the LTP literature and suggest that the mechanisms underlying long-term synaptic plasticity are conserved across species and preparations.
A number of studies have suggested that the cAMP-response element binding protein (CREB) is the nuclear target where both PKA and MAPK exert their effects on LTF and LTP (see, e.g., Frank and Greenberg 1994; Stevens 1994; Yin and Tully 1996; Kandel 1997; Abel et al. 1998; Silva et al. 1998). CREB is a constituitively expressed nuclear transcription factor that consists of several functionally distinct isoforms (Kandel 1997; Abel et al. 1998; Silva et al. 1998). In Aplysia, for example, ApCREB1 appears to activate transcription by binding to CRE sites following phosphorylation by either PKA or calcium/calmodulin-dependent protein kinase (CaMK) (Dash et al. 1990; Kaang et al. 1993; Alberini et al. 1995). ApCREB2 contains a phosphorylation site for a MAPK (Gonzalez et al. 1991) and is thought to act as a repressor isoform (Abel et al. 1998). In support of this hypothesis, injection of oligonucleotides to CRE binding sites in to Aplysia sensory neurons effectively blocks LTF, while leaving STF intact (Dash et al. 1990). Conversely, injection of anti-ApCREB2 antibodies results in LTF (>1 day) following stimulation that normally produces only STF (<1 hr) (Bartsch et al. 1995). Finally, recent studies have shown that stimuli that generate LTP in hippocampus promote CRE-mediated gene transcription, an effect that is blocked by inhibitors of either PKA or MAPK (Impey et al. 1996, 1998a). Thus, CREB is a mechanism whereby intracellular signaling pathways may regulate the switch from short- to long-term plasticity and memory by acting to either promote or repress the synthesis of new proteins.
Consistent with the Aplysia model, a number of studies have implicated CREB in a variety of forms of learning and memory spanning a number of different species. Yin and colleagues (1994, 1995), for example, demonstrated that induced overexpression of a CREB activator isoform selectively enhanced the long-term retention of a classical conditioning task in Drosophila. Conversely, induced expression of a dominant-negative (repressor) isoform blocked LTM (Yin et al. 1994, 1995). In the rat, injection of antisense oligonucleotides to CREB into hippocampus or amygdala has been shown to selectively affect LTM for spatial and taste aversion learning, respectively (Guzowski and McGaugh 1997; Lamprecht et al. 1997). Finally, transgenic mice lacking the α and δ isoforms of CREB have been shown to have impaired LTM, but not STM, on a variety of tasks, including social transmission of food preferences (Kogan et al. 1997) and spatial learning (Bourtchuladze et al. 1994). Consistent with the present results, CREB-deficient mice have also been shown to have impaired LTM, but not STM, for contextual and auditory fear conditioning (Bourtchuladze et al. 1994). Collectively, results strongly favor the hypothesis that CREB is a molecular switch underlying memory consolidation, including fear memory consolidation. Further, the findings of the present studies suggest that fear memory consolidation in the rat may involve convergence of PKA and MAPK onto nuclear targets such as CREB. Additional studies will be necessary to evaluate this hypothesis.
Where in the brain are Anisomycin, Rp-cAMPS, and PD098059 acting to disrupt the long-term plastic changes underlying fear memory consolidation? Although several regions are likely candidates, evidence would favor the hippocampus and the lateral (LA) and basal nuclei of the amygdala (see, e.g., Davis 1992; LeDoux 1992; Maren and Fanselow 1996). Lesions of the hippocampus, for example, have been shown to disrupt contextual fear conditioning, whereas those of the amygdala have been shown to disrupt both auditory and contextual fear (LeDoux et al. 1990a; Phillips and LeDoux 1992). It is generally assumed that the role of hippocampus in fear conditioning is to provide the amygdala with a representation of the context in which conditioning occurs, and that the amygdala is the site of CS–US convergence or plasticity for both auditory and contextual fear conditioning (LeDoux 1992, 1995).
In support of the hypothesis that the amygdala is a key site of plasticity, the LA has been shown to receive convergent auditory and somatosensory inputs from the medial geniculate body, particularly from the medial division (MGm) and the posterior intralaminar nucleus (PIN) (LeDoux et al. 1984, 1985, 1990b; Romanski et al. 1993). The basal nucleus receives input from the hippocampus via the subiculum (Canteras and Swanson 1992). Importantly, LTP has been demonstrated in each of these pathways (Clugnet and LeDoux 1990; Maren and Fanselow 1995; Rogan and LeDoux 1995), and auditory fear conditioning has been shown to modify neural activity in the LA in the same manner that LTP does (Rogan et al. 1997; McKernan and Shinnick-Gallagher 1997). Thus, both the LA and the basal nucleus contain both the relevant inputs and the potential cellular mechanism whereby fear memory consolidation may occur. Alternatively, it may be the case that both the hippocampus and the amygdala undergo plastic changes necessary for contextual fear conditioning, whereas only the amygdala is necessary for tone conditioning (Phillips and LeDoux 1992). This interpretation would be consistent with studies demonstrating both synaptic plasticity and increases in the phosphorylation of MAPK, protein kinase C, and α-CaMKII in the hippocampus following fear conditioning (Doyere et al. 1995; Atkins et al. 1998). It would also account for the relatively pronounced effects observed for contextual fear conditioning in the present studies because the drugs would have had multiple sites at which to act. Further experiments will be necessary to evaluate this hypothesis. Nonetheless, it is clear that the amygdala is one important site of CS–US convergence and plasticity. Consistent with this hypothesis, CRE-mediated gene transcription has recently been shown to increase in the amygdala following contextual fear conditioning (Impey et al. 1998b) and overexpression of CREB in the amygdala has been shown to facilitate long-term memory for fear-potentiated startle (S.A. Josselyn, W.A. Carlezon, C. Shi, R.L. Neve, E.J. Nestler, and M. Davis, unpubl.). Further, injection of Rp-cAMPS into the amygdala has been shown to attenuate fear-potentiated startle (C. Ding, Y.-L. Lee, and M. Davis, unpubl.). Together with the LTP data, these studies suggest that molecular processes necessary for fear memory consolidation may be present in the amygdala. Additional studies employing selective cellular manipulations of this region will be necessary to further evaluate this hypothesis.
Acknowledgments
This research was supported in part by National Institute of Memtal Health grants RO1 MH 46516, KO2 MH00956, R37 MH 39774, and MH 11902-01A1. The work was also supported by a grant from the W.M. Keck Foundation to New York University. We thank Andrew Farnum for technical assistance. We also thank Hugh T. Blair, Yadin Dudai, Karim Nader, Marta Moita, and Marc Weisskopf for helpful comments about this manuscript.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: Inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Ghirardi M, Huang Y, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann NY Acad Sci. 1995;758:261–286. doi: 10.1111/j.1749-6632.1995.tb24833.x. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAP kinase cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Erikson CA, Davis S, McNaughton BL. Hippocampal synaptic enhancement as a basis for learning and memory: A selected review of current evidence from behaving animals. In: McGaugh JL, Weinberger NM, Lynch GL, editors. Brain and memory: Modulation and mediation of neuroplasticity. New York, NY: Oxford University Press; 1995. pp. 259–276. [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: Relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioff D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn & Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Conditioned fear assessed by freezing and by the suppression of three different baselines. Anim Learn Behav. 1980;8:429–434. [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: A PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Chapman PF, Kairiss EW, Keenan CL, Brown TH. Long-term synaptic potentiation in the amygdala. Synapse. 1990;6:271–278. doi: 10.1002/syn.890060306. [DOI] [PubMed] [Google Scholar]
- Clugnet M, LeDoux JE. Synaptic plasticity in fear conditioning circuits: Induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10:2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned fear. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss, Inc.; 1992. pp. 255–306. [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Doyere V, Redini-Del Negro C, Dutrieux G, Le Floch G, Davis S, Laroche S. Potentiation or depression of synaptic efficacy in the dentate gyrus is determined by the relationship between the conditioned and unconditioned stimulus in a classical conditioning paradigm in rats. Behav Brain Res. 1995;70:15–29. doi: 10.1016/0166-4328(94)00179-j. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long-term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP stimulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Frank DA, Greenberg ME. CREB: A mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Raden DL, Davis RJ. Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J Biol Chem. 1991;266:22159–22163. [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li X, Kandel ER. cAMP contributes to mossy fiber LTP initiating both a covalently mediated early phase and macromolecular synthesis-dependent phase late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Kaang BK, Kandel ER, Grant SGN. Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron. 1993;10:427–435. doi: 10.1016/0896-6273(93)90331-k. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Genes, synapses, and long-term memory. J Cell Physiol. 1997;173:124–125. doi: 10.1002/(SICI)1097-4652(199711)173:2<124::AID-JCP6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Greenberg ME. A kinase to remember: Dual roles for MAP kinase in long-term memory. Neuron. 1997;18:839–842. doi: 10.1016/s0896-6273(00)80322-0. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998a;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998b;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- ————— Emotion: Clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned by acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggerio DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Romanski LM, Xagoraris AE. Indelibility of subcortical emotional memories. J Cog Neurosci. 1989;1:238–243. doi: 10.1162/jocn.1989.1.3.238. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagorias A, Romanski LM. The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. J Neurosci. 1990a;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in acoustic thalamus that project to the amygdala. J Neurosci. 1990b;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Malenka RC, Nicoll RA. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— The amygdala and fear conditioning: Has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Meiri N, Rosenblum K. Lateral ventricle injection of the protein synthesis inhibitor anisomycin impairs long-term memory in a spatial memory task. Brain Res. 1998;789:48–55. doi: 10.1016/s0006-8993(97)01528-x. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in sterotaxic coordinates. 2nd ed. Orlando, FL: Academic Press; 1986. [Google Scholar]
- Phillips R, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet M, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Rothermel JD, Parker Botelho LH. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′,5′-(cyclic) phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J. 1988;251:757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Ann Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Stevens CF. CREB and memory consolidation. Neuron. 1994;13:769–770. doi: 10.1016/0896-6273(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Zhuo H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yin JCP, Del Vecchio M, Zhuo H, Tully T. CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]