Phosphorylation of p21 in G2/M Promotes Cyclin B-Cdc2 Kinase Activity (original) (raw)
Abstract
Little is known about the posttranslational control of the cyclin-dependent protein kinase (CDK) inhibitor p21. We describe here a transient phosphorylation of p21 in the G2/M phase. G2/M-phosphorylated p21 is short-lived relative to hypophosphorylated p21. p21 becomes nuclear during S phase, prior to its phosphorylation by CDK2. S126-phosphorylated cyclin B1 binds to T57-phosphorylated p21. Cdc2 kinase activation is delayed in p21-deficient cells due to delayed association between Cdc2 and cyclin B1. Cyclin B1-Cdc2 kinase activity and G2/M progression in p21−/− cells are restored after reexpression of wild-type but not T57A mutant p21. The cyclin B1 S126A mutant exhibits reduced Cdc2 binding and has low kinase activity. Phosphorylated p21 binds to cyclin B1 when Cdc2 is phosphorylated on Y15 and associates poorly with the complex. Dephosphorylation on Y15 and phosphorylation on T161 promotes Cdc2 binding to the p21-cyclin B1 complex, which becomes activated as a kinase. Thus, hyperphosphorylated p21 activates the Cdc2 kinase in the G2/M transition.
The cell cycle regulatory function of cyclin-dependent protein kinases (CDKs) is well known to depend on nuclear localization, subunit assembly of the holoenzymes, phosphorylation and dephosphorylation of the CDK subunit, and degradation of the regulatory cyclin subunits (48-50). Proper subcellular localization of regulatory proteins is also essential for control of cell division (55). A number of mitotic kinases regulate the cell division cycle and its checkpoints through phosphorylation of different substrates (51). In addition, two families of inhibitors negatively regulate cyclin-CDK-dependent progression of the cell cycle (65). p21 is an inhibitor whose expression is regulated by the p53 tumor suppressor gene (18). Other functions of p21 include a role in cellular differentiation, senescence, inhibition of apoptosis, and activation of cyclin D-CDK4 (16, 19, 47, 54, 65). Cyclin D-CDK4 assembly promotes CDK4 kinase activity in G1-phase progression under conditions of normal cellular proliferation, i.e., at low stoichiometry of p21:CDK4 in the absence of DNA damage or checkpoint activation (11, 42, 65, 75, 79).
Cyclins and CDKs bind p21 through the consensus site CRRL (p21 amino acids [aa] 18 to 21) at the N-terminal end, and this motif is required for inhibition of cell cycle progression (11, 12, 23, 46). Interestingly, there is a second, KRRL motif (aa 154 to 157) for CDK-cyclin binding near the C-terminal end of p21, which is absent in the other members of the CDK inhibitor family (11, 23). In addition to cyclin-CDK binding, p21 through aa 144 to 151 also associates with proliferating cell nuclear antigen (PCNA). PCNA stimulates the processivity of DNA polymerase δ and ɛ, which are required for high-fidelity DNA replication and excision repair (11, 62, 69). PCNA forms quaternary complexes in normal fibroblasts but appears to act independently of p21 in cancerous cells (22, 75, 76, 78). Furthermore, the full-length p21 protein or a peptide consisting of aa 144 to 151 of p21 can inhibit PCNA function in DNA synthesis in vitro (11, 27, 70).
The ability of p21 to regulate the cell cycle is dependent on its subcellular localization. Akt has been reported to phosphorylate p21 at T145 in HER-2/neu-overexpressing cells and to retain p21 in the cytoplasm (82). Cytoplasmic retention of p21 resulted in loss of cell cycle inhibition and gain of an apoptosis inhibitory activity (17, 82). It has also been reported that upon differentiation of U937 cells into monocytes, p21 translocates from the nucleus to the cytoplasm and rescues cell death (4). Thus, the cell cycle inhibitory activity of p21 correlates with its nuclear localization. Moreover, expression of a p21 mutant that lacks a nuclear localization sequence did not induce cell cycle arrest or monocytic differentiation (4). The apoptosis resistance phenotype of cytoplasmic p21 has been associated with inhibition of apoptosis signal-regulating kinase 1 function (4). Following exposure to genotoxic agents, p21 enters the nucleus, and in addition to promoting cell cycle arrest to prevent S-phase progression, p21 also colocalizes with PCNA to promote nucleotide excision repair (43, 45).
Immunoprecipitation analysis has shown that p21 interacts with cyclin D throughout the cell cycle, whereas interaction between p21 and cyclin A or cyclin B occurs in the later part of the cell cycle (44). It has also been reported for nontransformed fibroblast cells that p21 transiently colocalizes with cyclin A or cyclin B1 in the nucleus at G2/M (15). p21 associates with a number of cyclin-CDK complexes, including the G1/S-phase kinases. Primary embryonic fibroblasts cells from p21−/− mice have a significantly reduced number of premitotic cells with nuclear cyclin B1, suggesting that p21 may promote a transient pause late in G2 where it may play a role in late cell cycle checkpoint controls (15). p21 or 14-3-3σ null cells were also found to be unable to maintain G2/M arrest and die when exposed to DNA-damaging agents (5, 6, 9). Subsequently, studies on 14-3-3σ and p21 double-knockout cells established a complementary role of both proteins in G2/M arrest following DNA damage (9). The G2/M arrest following p21 overexpression apparently occurs even though p21 binds poorly to cyclin B1-Cdc2 (31).
In normal cell cycle progression, phosphorylation of Cdc2 by CDK activated kinase and dephosphorylation by Cdc25C on two conserved residues, Thr 14 and Tyr 15, activate the cyclin B1-Cdc2 kinase and trigger the cells to enter into M phase (48-50). Similarly, inactivation of both cyclin B-Cdc2 and cyclin A-CDK2 is required for completion of mitosis. Several reports demonstrated that p21 binds with cyclin A-CDK2 and cyclin B-Cdc2 (11, 31, 49, 76). However, whether p21 binding with these two kinases during normal cell cycle progression might affect the cell cycle during G2/M remains unclear.
Some previous studies have investigated the phosphorylation of p21 (33, 79). An in vitro kinase assay was performed with 35S-labeled p21 in unsynchronized sf9 cells (79). The cell lysates were incubated with preformed cyclin A-CDK2 kinase complexes followed by immunoprecipitation with either anti-cyclin A or anti-p21 antibody. Using autoradiography of the immunoprecipitated proteins, it was shown that p21 has a high-molecular-mass band along with the 21-kDa band. Similarly, two different mobility forms of the p21 protein were noted by immunoblotting when mouse embryonic fibroblasts (MEFs) arrested at G2/M and immunoprecipitated with cyclin A, cyclin B1, or p21 antibodies (15). An in vitro kinase experiment with either full-length p21 protein, human p21 expressed in sf9 cells, or a p21 C-terminal peptide showed that different amino acids within p21 can be phosphorylated by PKA and PKC, and this may modulate PCNA binding (62). p21 contains many consensus sites that may serve as substrates for different kinases, including CDK2, ATM/ATR, Akt, PKA, PKC, GSK3, CKI and CKII. However, there is no clear evidence of which sites can be phosphorylated in vivo and which kinase(s) are involved in this process, particularly at G2/M.
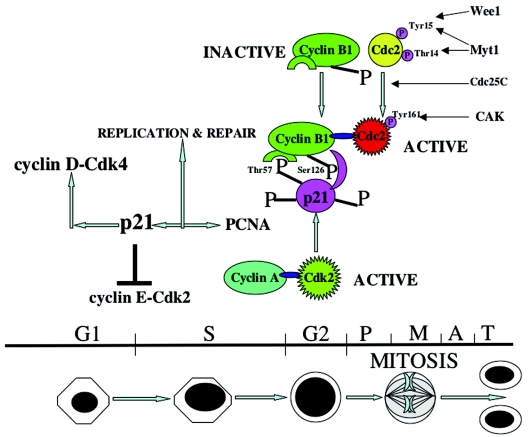
In the present study, we have established that p21 is hyperphosphorylated during G2/M, that CDK2 is likely the major kinase which hyperphosphorylates p21 on threonine 57, and that hyperphosphorylated p21 plays a positive role in promoting the G2-prophase transition. p21 enters the nucleus during S phase, becomes hyperphosphorylated by CDK2 at the S/G2 boundary, and subsequently interacts specifically with serine 126 of cyclin B1 prior to activation of Cdc2 kinase by Cdc25C. Threonine 57, when phosphorylated in p21, appears to be the critical residue for interaction with cyclin B1. There also appears to be a preferential association between cyclin B1 with G2/M-hyperphosphorylated p21 compared to hypophosphorylated p21, whereas cyclin A appears to associate similarly with either hyperphosphorylated or hypophosphorylated p21. Association between p21 and cyclin B1-Cdc2 appears to promote cyclin B1-Cdc2 kinase activity at G2/M. In support of the role of p21 in positively regulating normal G2/M progression, we show that p21-null cells have significantly delayed activation of cyclin B1-Cdc2 kinase activity compared to cells with wild-type p21. Introduction of wild-type but not threonine 57-mutated p21 restored efficient Cdc2 kinase activation. Although we show that either p21 or cyclin B1 interacts poorly with Y15-phosphorylated Cdc2, they appear to interact well with Thr-161-phosphorylated Cdc2. Our working model is that p21 becomes phosphorylated prior to Cdc25C activation. The phosphorylated p21 binds to cyclin B1 at a time when Cdc2 is phosphorylated on Y15 and associates poorly with the complex. Subsequent dephosphorylation of Cdc2 by Cdc25C promotes binding of Cdc2 to the p21-cyclin B1 complex, which can then become activated as a kinase. Our studies provide molecular insights into the events leading to the G2/M-specific hyperphosphorylation on p21 as well as a novel understanding of the role of p21 in control of Cdc2 activation during the early steps of G2/M progression.
MATERIALS AND METHODS
Cell lines, synchronization, and cell cycle analysis.
The human non-small-cell lung cancer cell line H460 was a gift from S. Baylin (Johns Hopkins University). HCT116, HCT116 p21−/−, DLD1 p21+/+, and DLD1 p21−/− colon cancer cells were a gift from B. Vogelstein (Johns Hopkins University). PA1 ovarian cancer cells and U2OS osteosarcoma cells were obtained from American Type Culture Collection.
In order to synchronize cells in quiescence, asynchronously growing cells were cultured in fetal bovine serum (FBS)-depleted (0%) medium for 32 h. For S-phase enrichment, cells were grown in FBS-depleted medium for 32 h followed by treatment with aphidicolin (2 μg/ml) for another 24 h. The cells were then released from aphidicolin block by washing with Hanks balanced salt solution and propagated for another 2 or 4 h in complete medium as previously described (38). To monitor aphidicolin release of cells into S phase and G2/M, they were grown for different time periods (see figures and legends) following aphidicolin release. Similarly, nocodazole (0.8 μg/ml, Sigma), vincristine (1.0 μg/ml; Sigma), colchicine (1.0 μg/ml, Sigma), colcimid (1.0 μg/ml, Sigma), or taxol (1.0 μg/ml, Sigma) was used to arrest cells at G2/M. When using nocodazole to block the cells, they were released by washing several times with Hanks balanced salt solution and medium and propagated with complete medium for different time points as described in legends to the figures.
For dual labeling with BrdU and propidium iodide, H460 cells were treated with nocodazole for different time points (see Fig. 3A). BrdU was added at 10 μM (final concentration) 30 min before harvesting. Using anti-BrdU and goat F(ab′)2 anti-mouse immunoglobulin G (IgG) (H+L)-fluorescein isothiocyanate (FITC) (Becton-Dickinson), cells were stained and analyzed by fluorescence-activated cell sorting (FACS).
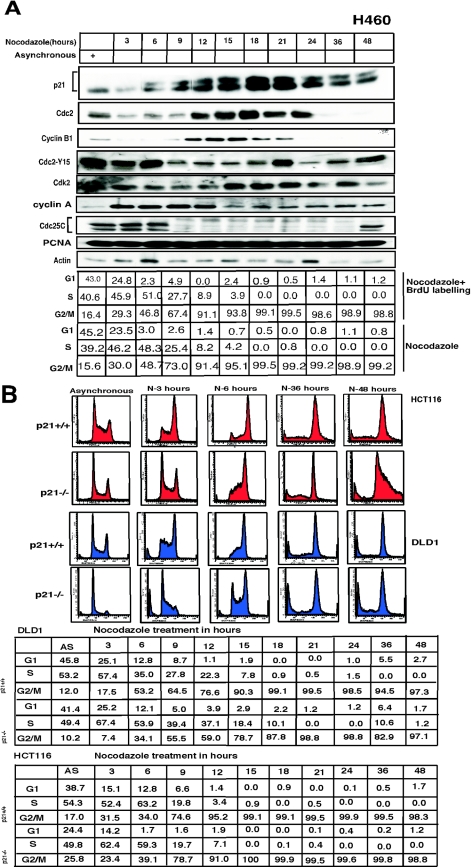
FIG. 3.
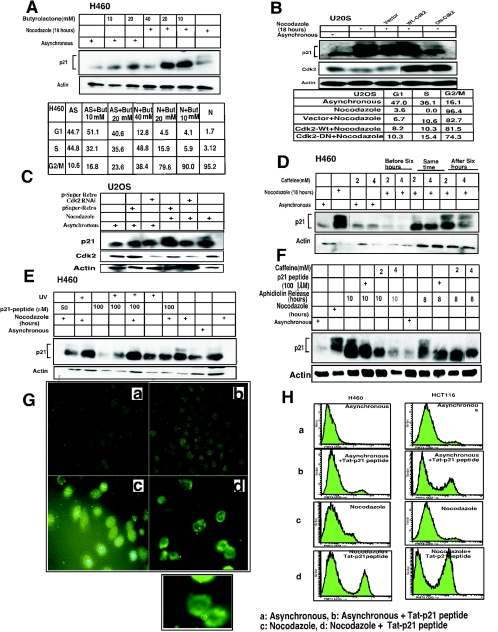
Phosphorylation of p21 precedes G2/M progression. (A) H460 cells were treated with nocodazole (0.8 μg/ml) for different time points (as indicated) and harvested. Samples were electrophoresed by using SDS-15% PAGE, and membranes were immunoblotted with different antibodies to detect p21, Cdc2, CDK2, cyclin B1, cyclin A, Cdc25C, PCNA, and actin (as indicated). Phosphorylation of p21 was observed after 6 h of nocodazole treatment, and this increased till 18 h, where equal intensities of hyperphosphorylated and hypophosphorylated bands were noted between 15 to 18 h. Gradually the hyperphosphorylated band intensity decreased at subsequent time points but persisted till 48 h. The Cdc2 and cyclin B1 band intensity became stronger after 12 h of nocodazole treatment and undetectable in the Western blots after 36 h of nocodazole treatment. The mitotic form of Cdc25C appeared after 9 h of nocodazole treatment and persisted till 36 h of nocodazole exposure. The level of CDK2 did not appear to change significantly with nocodazole treatment. PCNA expression also remained essentially unchanged throughout the prolonged period of nocodazole treatment. Actin was used as a loading control. Blots were probed with anti-Cdc2-Y15 antibodies in nocodazole-treated cells, and this revealed strong bands of inactive Y15-phosphorylated protein till 6 h after nocodazole exposure, and subsequently Cdc2 phosphorylated at Y15 diminished up to 36 h, with reappearance of a strong banding pattern by 48 h. Cell cycle distribution is depicted in the chart at the bottom of panel A. BrdU incorporation as an indicator of S phase, in nocodazole-treated H460 cells, showed complete loss of S phase with increase in G2/M-phase cells after 18 h. Similar results were observed in cells treated with nocodazole alone compared to those with nocodazole- plus BrdU-treated cells. (B) Delayed S-G2 and G2/M progression in p21−/− cells versus p21+/+ cells. Analysis of cell cycle profile after nocodazole treatment for HCT116 and DLD1 cells demonstrated that the change in profile from that of asynchronous cells took 3 h for p21+/+ cells, whereas for the p21−/− cells it took 6 h. FACS analysis also showed a higher number of cell deaths at longer time periods of nocodazole treatment for p21−/− cells than for p21+/+ cells. Cell cycle analysis is shown for the entire time course in the table below the histograms. AS refers to asynchronous cells. (C) Comparison of p21+/+ and p21−/− cells released from aphidicolin block at different time points showed appearance of p21 phosphorylation as early as 3 h and maximum phosphorylation at 6 to 8 h after release, coinciding well with an increased percentage of G2/M cells. Subsequently, the phosphorylated p21 disappeared as the cells progressed to the subsequent cell phase.
To confirm that p21 was present in G2/M cells, dual staining of p21 and propidium iodide was performed, followed by flow cytometric measurement using DLD1 p21+/+ and p21−/− cells, both asynchronous cells and cells synchronized at G1, S, or G2/M phases. To further confirm the submitotic phase shown in Fig. 3B, phosphoserine 10-histone H3 staining was performed with anti-histone H3 phosphorylation-specific antibody (Sigma) and anti-mouse IgG-FITC (Caltag), and cells were stained with propidium iodide to measure the DNA content.
For FACS analysis, cells were harvested by treatment with trypsin, washed in phosphate-buffered saline, and resuspended in 70% ethanol for storage at −80°C until further analysis. Propidium iodide (50 μg/ml; Sigma)-stained cells were analyzed using an Epics Elite sorter (Beckman-Coulter).
DNA damage, kinase inhibition, and peptide synthesis.
All tissue culture media and serum were purchased from GIBCO BRL. Both asynchronous and synchronized cells were treated either with or without UV light at 5 J/m2 and mitomycin C (MMC) (20 μg/ml) (Sigma) for the last 4 h (after 18 h of nocodazole treatment) before harvesting. Exponentially growing H460 cells were treated with nocodazole plus either butyrolactone (Affiniti Research Products Ltd.), caffeine (Sigma), wortmannin (Sigma), GSK3 inhibitor (Calbiochem), UCN-01 (a gift from Robert J. Schultz, National Institutes of Health), or a 24-amino-acid p21 peptide including human immunodeficiency virus (HIV) Tat and the C-terminal nuclear localization sequence (synthesized in the Protein Chemistry Laboratory, University of Pennsylvania Core; supported by grants DK-19525 and CA16520). The amino acid sequence of the peptide used included 11 amino acids of HIV Tat and 13 amino acids of the nuclear localization signal (NLS)/PCNA binding region of p21 (in bold), respectively: Y-G-R-K-K-R-R-Q-R-R-R-G-R-K-R-R-Q-T-S-M-T-D-F-Y. A 24-amino-acid region of the HIV Tat-Tat fusion peptide was used as a control.
Antibodies.
The following primary antibodies were used: WAF1 (Ab-1) mouse monoclonal from Oncogene Research Products, actin (C-2) mouse monoclonal, cyclin B1 (GNS1) mouse monoclonal, PCNA (PC-10) mouse monoclonal, Cdc2 p34 (clone17) mouse monoclonal, Cdc25C (C-20) rabbit polyclonal, CDK2 (M2) goat polyclonal, p27 (F-8) mouse monoclonal, cyclin A mouse monoclonal, cyclin D1 mouse monoclonal and cyclin D1 rabbit polyclonal from Santa Cruz Biotechnology, and phospho-Cdc2 (Tyr15) rabbit polyclonal (Cell Signaling Technology). Histone H1 antibody was purchased from Sigma.
Whole-cell extract preparation.
Cells were washed twice with PBS, scraped, and resuspended in 250 μl of lysis buffer. Cell lysis buffer contained 50 mM Tris (pH-8), 120 mM NaCl, 0.5% Nonidet P-40, 50 mM NaF, 1 mM sodium orthovanadate, 100 μg of polymethylsulfonyl fluoride/ml, 20 μg of aprotinin/ml, and 10 μg of leupeptin/ml. The lysates were kept on ice for 1 h, followed by centrifugation at 14,000 rpm (Beckman Coulter) for 10 min to remove the insoluble materials, and kept at −80°C until used.
Phosphatase treatment.
H460, HCT116, and PA1 cell extracts (prepared under nondenaturing conditions) were diluted with phosphatase buffer and 0.2 U of Lambda protein phosphatase (New England Biolabs)/ml and then incubated at 30°C for 1 h. Samples were separated by sodium dodecyl sulfate (SDS)-15% polyacrylamide gel electrophoresis (PAGE) and immunoblotted for expression of hyper- and hypophosphorylated p21 proteins.
Orthophosphate labeling and one- or two-dimensional (2D) gel electrophoresis.
H460 cells were washed with phosphate-free medium (Gibco/BRL) and treated with nocodazole (for 18 h) in T75 flasks, while labeling was done with 2 mCi of [32P]orthophosphate/flask in 5 ml of Dulbecco's modified Eagle's medium containing 10% FBS. Cells were collected and placed in lysis buffer (see “Whole-cell extract preparation”) and immunoprecipitated with p21 antibodies to deplete the p21 protein. The hyper- and hypophosphorylated p21 proteins were separated either by one-dimensional gel electrophoresis or according to their isoelectric points on a 2D Ettan gel electrophoresis apparatus (Amersham).
Expression of wild-type CDK2 and dominant-negative CDK2 (DN-CDK2) in U2OS cells.
U20S cells were transfected with either wild-type CDK2, DN-CDK2 (gifts from Greg Enders, University of Pennsylvania), or empty vector for 24 h and then treated with nocodazole to enrich the cells in prometaphase stage for 18 h. Cells were collected, placed in lysis buffer (see “Whole-cell extract preparation”), and electrophoresed using a 15% polyacrylamide gel to observe the phosphorylation status of p21.
Expression of siRNA directed against CDK2.
The CDK2 short interfering RNA (siRNA) vector was constructed by inserting the oligonucleotide sequence TCCGCCTGGACACTGAGAC (complementary to the CDK2 mRNA sequence from nucleotides 104 to 122 into the plasmid pSuperRetro (Oligoengine). The construct was confirmed by DNA sequencing. U2OS cells were transfected with either pSuperRetro vector or pSuperRetro CDK2 RNAi for 24 h and then treated with nocodazole for another 18 h. Asynchronous cells were kept for 48 h after transfection. Cells were collected for flow cytometric analysis or were analyzed using 15% PAGE to observe the phosphorylation status of p21 and CDK2 expression.
Annexin V-enhanced green fluorescent protein assays.
Asynchronous cells treated with nocodazole, the 24-amino-acid p21 peptide, or both were stained with annexin V-enhanced green fluorescent protein (Bio Vision Research Products, Palo Alto, Calif.) for analysis of phosphoserine inversion as recommended by the manufacturer.
Immunofluorescence.
Exponentially growing cells were plated on four-chamber glass slides (Nalge Nunc International, Naperville, Ill.) according to instructions provided by the manufacturer and synchronized following the cell synchronization procedure mentioned above. H460 cells were also treated with p21 peptide along with nocodazole or with p21 peptide alone for 18 h at 37°C. The cells were washed with PBS three times and fixed in Histochoice for 5 min. Cells were subjected to permeabilization with 0.5% Triton X-100 for 5 min and blocked with goat serum (GIBCO BRL) for 1 h at room temperature. All antibodies were diluted with goat serum. p21 primary monoclonal antibody (1:100) was added to the slides, which were kept at room temperature for 2 h, followed by three additional washes in PBS. Goat anti mouse-IgG conjugated with biotin (1:200) and avidin conjugated with FITC (1:200) were added to the slides, respectively, and incubated at room temperature for 1 h each. Finally, slides were washed three times in PBS and mounted with Vectashield (Vector Laboratories, Inc., Burlingame, Calif.). The slides were observed and photographed with a Nikon (Diaphot) microscope using ×20 and ×40 lenses.
To observe colocalization of p21 with other nuclear proteins, H460 cells were fixed in Histochoice, followed by permeabilization with 0.5% Triton X-100 for 5 min. Colocalization of p21 (Ab-5, rabbit polyclonal; Oncogene Research) with cyclin A (mouse monoclonal; Santa Cruz Biotechnology), cyclin D1 (rabbit polyclonal; Santa Cruz Biotechnology), or phosphoserine 10 H3-histone (Sigma) was performed. Similarly, either mouse monoclonal or rabbit polyclonal antibodies of p21 (Oncogene Research) with the respective rabbit polyclonal or mouse monoclonal anti-cyclin B1 (Santa Cruz Biotechnology) antibodies were used for 2 h at room temperature. All the antibodies were diluted in PBS without Ca2+ or Mg2+. The FITC-conjugated Affinipure goat anti-rabbit IgG (H+L) and Rhodamine Red-TM-X-conjugated Affinipure goat anti-mouse IgG (H+L) secondary antibodies were used for 1 h, also following the manufacturer's instructions (Jackson Immuno Research Laboratories, Inc.).
Time kinetic study of nocodazole-arrested cells.
Asynchronous cells were treated with nocodazole (0.8 μg/ml) and cultured for several hours to observe the initial time of p21 phosphorylation and entry of different cell lines into the mitotic phase. p21, Cdc2, CDK2 cyclin B1, PCNA, cyclin A, Cdc25C, and p27 were detected by Western blot analysis. Actin was used as a loading control. The cell cycle profile was analyzed to compare the pattern of G2/M cells for H460, DLD1, and HCT116 p21+/+ and p21−/− cells. Samples from different time periods were collected for Western analysis, kinase assays, and FACS analysis to study the phosphorylation status, kinase activity, and cell cycle profiles, respectively. Similarly, 18-h nocodazole-arrested H460 cells were treated with cycloheximide (Sigma) at a 10-μg/ml concentration for different time periods to block new protein synthesis and determine the relative half-lives of hyperphosphorylated and hypophosphorylated p21 proteins.
DLD1 p21+/+ or p21−/− cells were grown in chamber slides, followed by treatment with nocodazole for 18 h. Cells were released from nocodazole at different time points, and mitotic cells were counted following 4′,6′-diamidino-2-phenylindole (DAPI) staining.
Immunoprecipitation and immunoblotting.
One hundred micrograms of whole-cell extracts were loaded per lane on SDS-15% PAGE gels to observe the mobility shift of the p21 protein. Polyvinylidene difluoride membranes were probed with primary antibodies, including p21 diluted at 1:200 or others (Cdc2, CDK2, cyclin B1, p27, actin, etc.) diluted at 1:1,000, and kept at room temperature for 2 h. The secondary antibody was peroxidase-conjugated goat anti-mouse IgG (H+L; Pierce) diluted at 1:5,000 in 1% nonfat dry milk in Tris-buffered saline with Tween 20. Signals were developed using an ECL-plus kit (Amersham Biosciences).
For immunoprecipitations, precleared 0.5 to 1 mg of whole-cell lysates (resuspended in nondenaturing lysis buffer; see section on whole-cell extracts) were immunodepleted with antibody for 2 h. To this antibody complex, either protein A/G agarose or protein L agarose (Invitrogen, Inc.) beads were added for another hour and kept at 4°C in an end-to-end shaker. The beads were washed three times with lysis buffer without protease inhibitors. Finally, 2× Laemmli buffer was added to the beads, and the samples were boiled before analysis by use of SDS-PAGE.
In vitro kinase assay.
Precleared cell lysates, using 500 μg from nocodazole-treated H460, HCT116 p21+/+, or p21−/− cells, were immunodepleted with saturating amounts (4 μg) of either cyclin B1 or CDK2 antibody. Mock samples were incubated with protein G agarose beads for 2 h at 4°C. Twenty-five microliters of protein G agarose beads were added to the tube with end-to-end mixing for another hour, followed by three washes with lysis buffer without protease inhibitors (see section on whole-cell extracts). In vitro kinase assays were performed as described previously (79) at 30°C for 1 h with histone H1 as a substrate and using the kinase buffer (50 mM Tris HCl [pH 8.0], 10 mM MgCl2, 2.5 mM MnCl2, ATP [25 μM], [γ32p]ATP [10 μCi]). Similarly, adenovirus His-p21 was used to infect DLD1 p21−/− asynchronous cells for 24 h, and His-p21 protein was purified by using nickel-nitrilotriacetic acid agarose column. In vitro kinase assays were performed with His-p21 as a substrate in the presence of [γ32p]ATP (10 μCi), using the above-mentioned kinase buffer and nocodazole-treated G2/M-enriched DLD1 p21−/− cell extract.
Mutagenesis studies.
The plasmid constructs of cyclin B1 (wild type and four or double serine mutants of the NLS region) were a kind gift from Eisuke Nishida (Kyoto University, Kyoto, Japan) (68). However, we also generated the single serine mutants of cyclin B1 at S126A, S128A, S133A, and S147A, using the Quikchange site-directed mutagenesis kit (Stratagene). All constructs were sequenced to verify the authenticity of the sequences. The plasmids were transfected and immunodepleted with hemagglutinin (HA) antibody, and blots were developed with p21 monoclonal antibody to observe any specific interaction between cyclin B1 and p21. Similarly, HA constructs of p21 (wild-type and different CDK2 phosphorylation sites) were mutated (as described in the legend to Fig. 6). The plasmids were transfected using Lipofectamine 2000 (Invitrogen) for 24 h, followed by 18 h of nocodazole treatment. Cells were harvested with whole-cell extract lysis buffer, immunoprecipitated with HA antibody, and probed with cyclin B1 or p21 antibody. Wild-type or single or multiple mutants were made against the nuclear localization signal sequence of Flag-tagged p21 and transfected in U2OS cells. Immunofluorescence staining against the Flag epitope (Flag monoclonal antibody Cy3 conjugate; Sigma) was performed, and cells were analyzed for the localization of p21, using both asynchronous or nocodazole-treated cells.
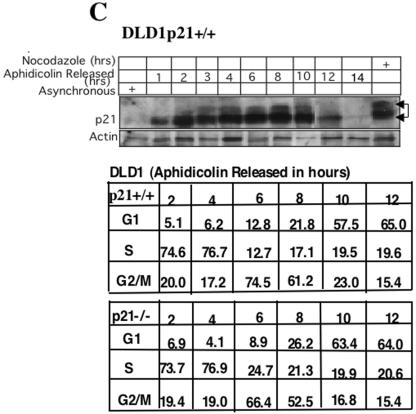
FIG.6.
Cyclin B1 interacts specifically with phosphorylated Thr 57 of p21. (A) H460 cell lysates treated with nocodazole plus a GSK3 inhibitor (10 μM) and immunodepleted with anti-cyclin B1 antibody showed hyperphosphorylated p21 on the immunoblot. (B) Caffeine- plus nocodazole-treated cell lysates showed hyperphosphorylated p21 after immunodepletion with anti-cyclin B1 antibodies. Even though caffeine inhibits phosphorylation of p21 (Fig. 2D and F), residual hyperphosphorylated p21 immunoprecipitates with cyclin B1. (C) UCN-01, a PKC and Chk1 kinase inhibitor, did not prevent anti-cyclin B1 from immunoprecipitating the hyperphosphorylated p21 band in nocodazole-treated G2/M-arrested H460 cells. (D) Butyrolactone, the CDK inhibitor, blocked the interaction between cyclin B1 and p21 in G2/M-enriched nocodazole-treated cells. (E) Transient transfection of HA-tagged p21HA-Wt, p21HA-T98A, S99A mutant, or p21HA-S130A mutant in U20S cells for 24 h and treatment with nocodazole for 18 h did not prevent interaction between phosphorylated p21 and cyclin B1. (F) p21HA-T57A or p21HA-T57A,T98A, S99A mutant, or p21HAT57A,S130A did not immunodeplete cyclin B1 after immunoprecipitation with anti-HA antibodies in nocodazole-treated cells. (G) Transfection of p21HA-T98A, S99A mutant, or p21HA-S130A and immunoprecipitation with anti-HA antibody showed in association with cyclin B1. (H) Transient transfection of p21HA-Wt or p21HA-T57A mutant constructs in U20S cells for 24 h and further treatment with nocodazole for 18 h followed by immunodepletion with anti-HA antibodies and immunoblotting with anti-CDK2 antibody showed CDK2 protein association. (I) p21HA-Wt or p21HA-T57A transient transfection in U20S cells treated with nocodazole for 18 h and immunodepleted with anti-HA antibody showed Cdc2 on the immunoblot. (J) p21HA-Wt or p21HA-T57A transfected in U20S cells and treatment with nocodazole for 18 h followed by immunodepletion with anti-HA antibodies and immunoblotted with cyclin A showed cyclin A association.
RESULTS
p21 is phosphorylated at G2/M.
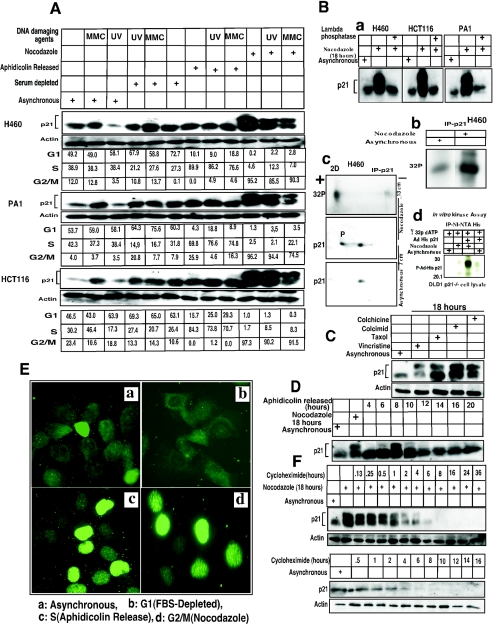
The role for p21 in the G1 checkpoint is well established (16, 18, 30). p21 has also been implicated in a G2/M checkpoint and is known to be a universal inhibitor of cell cycle kinases (15, 75). However, p21 does not inhibit the kinase activities of cyclin B1-Cdc2 as efficiently as CDK2, CDK4/6, and CDK3 (31). To explore the possibility that the p21 protein may be altered in G2/M to permit more-efficient inhibition or possibly activation of cyclin B1-Cdc2, we investigated p21 expression in various cell lines following cell synchronization. Western analysis showed that the expression of p21 was elevated in G1-arrested cells (H460, HCT116, and PA1) compared to the asynchronous control cells (Fig. 1A). Expression of p21 in S phase did not change significantly following aphidicolin release (Fig. 1A and D) as has been reported for other cell types (8). During G2/M, p21 immunoblots showed a higher-molecular-mass band of approximately 23 kDa in addition to the 21-kDa band (Fig. 1A, B, C, D, and F). To determine if the upper band in nocodazole-treated G2/M-arrested cells represents a hyperphosphorylated form of p21, cell lysates were treated with Lambda protein phosphatase (Fig. 1B). The higher-molecular-mass band disappeared in the phosphatase-treated lysates, confirming that this is a phosphorylated form of p21 (Fig. 1B-a). In addition, cells labeled with [32P]orthophosphate also showed evidence for p21 phosphorylation, as demonstrated by immunoprecipitation of p21 followed by autoradiography (Fig. 1B-b). The degree of p21 phosphorylation was found to be greater in nocodazole-treated G2/M-arrested cells than in asynchronous cells (Fig. 1B-b). To further confirm that both the lower-molecular-mass (21 kDa) and higher-molecular-mass (23 kDa) molecular mass bands at G2/M were phosphorylated in one or more residues, 32P-labeled H460 cell lysates were immunoprecipitated with p21 monoclonal antibodies and analyzed by two-dimensional gel electrophoresis (Fig. 1B-c). This experiment identified two species of 32P-labeled p21 that coincide with the two bands for p21 observed in G2/M, with a higher intensity of 32P labeling of the slower-migrating (23-kDa) p21 band (Fig. 1B-c). To further substantiate that p21 indeed gets phosphorylated at the G2/M phase, an in vitro kinase assay using purified His-tagged p21 and DLD1 p21−/− asynchronous or nocodazole-treated G2/M-enriched cell lysates were incubated at 30°C for 1 h in the presence of [γ-32P]ATP. The results showed high phosphorylation of His-p21 in G2/M-enriched cells (Fig. 1B-d) compared to the asynchronous cells, suggesting that p21 phosphorylation can occur in G2/M. In total, in addition to H460, PA1, and HCT116 cells, hyperphosphorylated p21 was found in nocodazole-treated G2/M-arrested U2OS, SW480, HeLa, and SAOS2 tumor cells and WI-38 normal human lung fibroblasts (Fig. 1; also data not shown).
FIG. 1.
p21 is phosphorylated at G2/M phase. (A) Expression of the p21 protein in asynchronous and synchronized cells at G1, S, or G2/M phase. Western blot using H460, PA1, or HCT116 cells with p21 monoclonal antibody showed a high-molecular-mass band of ∼23-kDa p21 protein at G2/M (nocodazole for 18 h; see Materials and Methods) but a reduced phosphorylated protein band postexposure to UV or MMC for anadditional 4 h. (B) (a) Phosphatase treatment of G2/M-enriched cells abolished the high-molecular-weight p21 band. (b) 32P-Orthophosphate-labeled asynchronous or nocodazole-arrested H460 cells showed phosphorylated p21 protein after immunoprecipitation with p21 antibody. (c) Nocodazole-treated plus 32P-orthophosphate-labeled cells showed hyper- and hypophosphorylated bands after 2D gel electrophoresis. Similarly, Western blotting also showed two bands separated according to their isoelectric points. Asynchronous cells showed a single p21 band after 2D-gel electrophoresis. (d) Asynchronous or nocodazole-treated DLD1 p21−/− cell extracts were incubated in vitro with purified His-tagged p21 in the presence of [γ-32P]ATP. High phosphorylation of His-p21 was observed after immunoprecipitation with nickel-nitrilotriacetic acid, using cell extract from nocodazole-treated DLD1 p21−/− cells. (C) Treatment of H460 cells with other spindle poisoning agents, including taxol, vincristine, colchicine, or colcimid (like nocodazole) enriched for cells with hyperphosphorylated p21. (D) H460 cells released from aphidicolin block had a peak of S-phase cells at 4 h and showed phosphorylation of p21 at 8 h (corresponding to G2/M-enriched cells). Hyperphosphorylated p21 disappeared at subsequent times with progression out of the G2/M phase. (E) (a) In asynchronous cells, p21 localized in the cytoplasm in the majority of H460 cells with some cells showing nuclear localization (approximately 20 to 25%). (b) In FBS-depleted G1-arrested cells, p21 showed only a cytoplasmic localization. (c) In aphidicolin-released cells, the p21 protein appeared to translocate to the nucleus, and similarly, in nocodazole-arrested G2/M cells, the p21 antibodies stained nuclear p21 (d). (F) 18-h nocodazole-treated G2/M-enriched H460 cells were exposed to cycloheximide (10 μg/ml) for different time periods. The half-life of the p21 phosphorylated band was less than that of the unphosphorylated band (upper panels). Cycloheximide-treated asynchronous cells also showed a pattern of protein elimination for the unphosphorylated p21 band similar to that observed with the nocodazole-treated cells (lower panels).
Treatment of cells with spindle-disturbing agents like taxol, vincristine, colchicine, or colcimid, which arrest cells in G2/M by different mechanisms, also resulted in the same higher-molecular-mass (23 kDa) phosphorylated band of p21 (Fig. 1C). To demonstrate that the presence of hyperphosphorylated p21 was not due to cell cycle arrest but rather was a consequence of passage through G2/M, aphidicolin release of H460 cells was used to analyze cells as they transited from S phase into G2/M (Fig. 1D). After 4 h of aphidicolin release, approximately 90% of the cells reached S phase and subsequently progressed to the G2/M stage by 6 to 8 h (Fig. 1D; also data not shown). The phosphorylated band appeared after 8 h of release and subsequently disappeared as cells exited G2/M, indicating that p21 phosphorylation is a transient G2/M event (Fig. 1D). Similarly, following nocodazole release of H460 cells, the p21 phosphorylated band disappeared after 1 to 2 h as cells reached the following G1 phase (data not shown). Further, to examine the impact of DNA-damaging agents on p21 hyperphosphorylation, cells arrested with nocodazole for 18 h were exposed to UV or MMC for another 4 h. Of note, there was no remarkable change in the expression of the p21 protein after coexposure, although a small decrease in the intensity of the p21 hyperphosphorylated band was observed (Fig. 1A). It is suggested that the hyperphosphorylation of p21 is cell cycle dependent, and probably kinases activated by DNA-damaging agents play a minor role in p21 phosphorylation (see below the section on CDK2 as the principal kinase that phosphorylates p21). However, DNA damage-dependent degradation of phosphorylated p21 protein cannot be ruled out as in the case of cyclin D1 in G1-phase checkpoint activation or cyclin A and cyclin B1 at G2/M phase (2, 40, 50).
To determine the localization of the p21 protein with respect to different cell cycle stages and its phosphorylation, H460 lung cancer cells were synchronized in G1, S, and G2/M phase, and p21 protein was detected by immunofluorescence (Fig. 1E). p21 protein localized to the cytoplasm in FBS-depleted, G0-G1-arrested cells compared to asynchronous cells (Fig. 1E-b versus 1E-a). However, 4 h after release from aphidicolin, almost 90% of the cells were in S phase and showed nuclear staining (Fig. 1E-c; also data not shown). Similarly, in nocodazole-arrested cells (18 h), p21 localized to the chromatin material (Fig. 1E-d). To further confirm that p21 localizes to chromatin, colocalization of p21 and phosphoserine 10 H3-histone was performed by immunofluorescence with H460 cells treated with nocodazole for 18 h. Both p21 and phosphoserine 10 H3-histone were colocalized with the DAPI-positive chromatin materials in prophase and prometaphase cells (see Fig. S1 in the supplemental material). Thus, comparing localization of the p21 protein in S and G2/M phases with Western analysis (Fig. 1A and E), it is clear that p21 is not hyperphosphorylated during S phase, but it is already in the nucleus. In cells released from nocodazole, within 2 h, p21 retranslocates to the cytoplasm (data not shown). However, at this time point Western analysis showed loss of hyperphosphorylation of p21. These results led us to hypothesize that during cell cycle progression, p21 enters the nucleus during S phase, where it may become phosphorylated, possibly serve a regulatory function, and then exit the nucleus as cells transit towards the next G1 phase. Below, we further examine candidate kinases and possible functions of hyperphosphorylated p21 in G2/M.
G2/M hyperphosphorylated p21 protein is short-lived compared to hypophosphorylated p21.
H460 cells arrested with nocodazole for 18 h were treated for different time periods with 10-μg/ml cycloheximide, an inhibitor of protein synthesis. After 6 h of cycloheximide exposure, the hyperphosphorylated p21 band disappeared while the hypophosphorylated band remained visible up to 8 h after cycloheximide treatment (Fig. 1F). This suggests that the hypophosphorylated form of p21 may have a longer half-life and may possibly be more stable than the hyperphosphorylated form. In cycloheximide-treated asynchronous cells, p21 has a half-life similar to that of the hypophosphorylated p21 in nocodazole-arrested cells (Fig. 1F). These results raise the interesting possibility that hyperphosphorylated p21 may be transiently expressed in cells during G2/M in order to perform one or more specific function(s). It is possible that by ubiquitination and subsequent degradation and/or dephosphorylation (as an alternative, not mutually exclusive explanation for reduced levels of hyperphosphorylated p21), levels of hyperphosphorylated p21 may be tightly controlled. Although it is formally possible that there may be interconversion between the hyper- and hypophosphorylated forms of p21, it is clear that in the absence of new protein synthesis the hyperphosphorylated p21 species is short-lived compared to the hypophosphorylated form of p21. In further support of this notion, we have observed prolonged expression of hyperphosphorylated p21 in cycloheximide-treated nocodazole-arrested cells treated with the proteasome inhibitor ALLN (unpublished observation).
CDK2 is likely the major kinase that phosphorylates p21 at G2/M.
CDK2 or Cdc2 are candidate kinases that may mediate p21 phosphorylation, since these two kinases are expressed and active at the S and G2/M phases of the cell cycle (26, 35, 57, 61). Although a role for CDK2 in G2/M cell cycle regulation has been proposed for both human and Xenopus cells, little is known about the substrates that might mediate subsequent events. Moreover, these kinases do not require DNA damage for their activation during S and G2/M phases. To determine which kinase(s) is involved in p21 phosphorylation, we initially tested several kinase inhibitors, including butyrolactone, wortmannin, caffeine, UCN-01, and GSK3 inhibitors (Fig. 2; also data not shown). With examination of the amino acid sequence of p21 and consensus sites for phosphorylation by different kinases, it is clear that there is no site for Cdc2, but there are at least three sites for CDK2. Thus, it is unlikely that Cdc2 phosphorylates p21. With this in mind, asynchronous H460 cells were treated with either butyrolactone (a CDK inhibitor) plus nocodazole, butyrolactone alone, or nocodazole alone. Western analysis (Fig. 2A) revealed that even with very low concentrations of butyrolactone, the hyperphosphorylation of p21 was inhibited. In contrast, there was no change in the protein level with two doses of nocodazole plus butyrolactone tested in the asynchronous cells. Analysis of cell cycle distribution shows that 90% of the nocodazole-exposed cells treated with the lowest dose of butyrolactone (10 μM) were in G2/M, suggesting that loss of p21 hyperphosphorylation was not due to a change in cell cycle position (Fig. 2A). To confirm further that CDK2 is the main kinase that phosphorylates p21 at G2/M, CDK2 or DN-CDK2 was ectopically expressed in U20S cells, followed by nocodazole treatment (Fig. 2B). DN-CDK2 blocked the hyperphosphorylation of p21 after nocodazole treatment compared with the wild-type CDK2 (Fig. 2B). Ectopic expression of DN-CDK2 partially blocked cells from entering into G2/M phase (by about 7.2%) compared to results with CDK2 (Fig. 2B). However, the inhibition of p21 phosphorylation by DN-CDK2 cannot be attributed to prevention of cells from entering G2/M, because 74.3% of the cells were found in G2/M. In a similar manner, targeting of CDK2 by siRNA, which reduced CDK2 expression dramatically compared to that with the vector control, inhibited p21 phosphorylation in nocodazole-treated G2/M-arrested U2OS cells (Fig. 2C). These results further support the conclusion that CDK2 might be the principal kinase responsible for p21 phosphorylation in G2/M-arrested cells. Previously, cyclin A-CDK2 has been shown to phosphorylate p21 in vitro at two different amino acid residues (33, 37, 79). However, it is possible that other kinases may also be involved in the phosphorylation of p21 at G2/M in vivo.
FIG.2.
CDK2 is the principal kinase that phosphorylates p21 at G2/M phase. (A) Ten, 20, and 40 μM concentrations of butyrolactone dissolved in dimethyl sulfoxide were added, along with nocodazole (0.8 μg/ml), to H460 cells and incubated at 37°C for 18 h with 5% CO2. Western analysis shows complete dephosphorylation of p21 even at the lowest concentrations of butyrolactone used. (B) Transient transfection of wild-type CDK2 or DN-CDK2 in U2OS cells showed reduced phosphorylation of p21 at Nocodazole-arrested G2/M phase. Cell cycle profile of wild-type CDK2 or DN-CDK2-transfected arrested U20S cells in G2/M phase after treatment with nocodazole for 18 h. (C) Transient transfection of pSuper Retro CDK2 RNAi in U2OS cells showed reduced CDK2 expression in asynchronous or nocodazole-treated cells and also inhibited phosphorylation of p21 in G2/M-enriched cells. However, no change in CDK2 levels or p21 phosphorylation was observed in control vector-transfected cells. (D) Caffeine, an ATM/ATR kinase inhibitor, inhibits p21 phosphorylation when added at 2 or 4 mM concentrations to the exponentially growing H460 cells at 6 h before or at the same time as nocodazole but not if added after 6 h of nocodazole treatment. (E) Two different concentrations (50 or 100 μM) of a 24-amino-acid p21 peptide including the NLS/PCNA region (see Materials and Methods for sequence) dissolved in water were added to asynchronous H460 cells without FBS for 3 h, followed by 0.8-μg/ml nocodazole with complete medium and incubation for another 18 h. Cells were harvested and dissolved in whole-cell lysis buffer, electrophoresed on SDS-PAGE, and immunoblotted with p21 antibodies. There was almost complete inhibition of p21 phosphorylation with or without UV exposure in nocodazole-treated cells at the higher dose, while less inhibition was observed at the lower dose tested. (F) H460 cells were FBS depleted for 30 h, followed by aphidicolin treatment for another 24 h and release from aphidicolin block for 8 or 10 h. p21 peptide was added after 4 h of aphidicolin release without FBS for 1 h to adsorb the peptide, with the cells subjected to 10% FBS added and incubated at 37°C for another 4 or 6 h before cells were harvested. Western analysis showed dephosphorylation of p21 protein after 8 or 10 h of release from aphidicolin with the 100 μM peptide-treated cells. (G) Immunofluorescence staining for p21 protein expression in H460 cells treated with p21 peptide with or without nocodazole. Nuclear p21 protein translocation was blocked by the p21 peptide with evidence cytoplasmic localization in p21- plus nocodazole-treated H460 cells (d) but not in nocodazole-treated cells in the absence of peptide (c). The asynchronous H460 cells showed cytoplasmic or nuclear localization of the protein (a). p21 protein was detected throughout the cells in p21 peptide-treated cells not exposed to nocodazole (b). Inset shows an enlarged view of cells from panel G-d. (H) Treatment with p21 peptide along with nocodazole in H460 and HCT116 cells resulted in a significant population of annexin V-positive cells in both cell lines (panels H-d) compared to the controls (panels H-a, H-b, and H-c).
Caffeine causes the disruption of DNA damage checkpoints, including the G2/M checkpoint, and sensitizes cells to ionizing radiation and other genotoxic agents (52). Caffeine inhibits both ATM and ATR kinase activities (29, 81). ATM and ATR are expressed in all cell cycle phases, and after ionizing or UV radiation they may phosphorylate Cdc25 proteins through their targets, Chk2 and Chk1, respectively, which contributes to the G2/M checkpoint (53). The p21 protein has one ATM/ATR consensus site at amino acids (134 to 138) for phosphorylation. In nocodazole-treated or aphidicolin-released cells treated with caffeine, there was a decrease in p21 phosphorylation if cells were treated with caffeine before or at the same time as nocodazole exposure or aphidicolin release but not if caffeine was introduced after 6 hours of nocodazole treatment or aphidicolin release (Fig. 2D and F). It cannot be ruled out that a longer time period of caffeine treatment might inhibit CDK2 kinase activity. Additional experiments with ATM−/− cells treated with nocodazole for 18 h showed p21 hyperphosphorylation, ruling out a requirement for ATM (data not shown).
Wortmannin inhibits the PI-3 kinases and downstream targets like Akt, ATM, DNA-PK and mTOR kinase activities (60). However, wortmannin does not inhibit the ATR kinase. Since p21 phosphorylation was found to be sensitive to caffeine, it is possible that ATR and not the ATM kinase may be responsible. Also, p21 phosphorylation by Akt at residue T145 has been reported (82). Wortmannin had no effect on hyperphosphorylation of p21 in G2/M (data not shown). UCN-01, a PKC as well as a Chk1 kinase inhibitor (3, 39), also did not affect p21 phosphorylation (data not shown). These results suggested that neither ATM nor PI-3Ks/Akt, DNA-PK, PKC, or Chk1 was responsible for p21 phosphorylation at G2/M. However, it could not be ruled out that T145 phosphorylation of p21 at the end of the mitotic phase may lead to cytoplasmic migration. However, nocodazole-treated cell lysates did not show p21 when probed with Akt substrate-specific antibodies, suggesting that Akt might not phosphorylate p21 at G2/M (data not shown). Thus, by comparing the phosphorylation of p21 after exposure to various checkpoint-signaling inhibitors, it is clear that ATM, Chk1, Akt, or PKC is unlikely to be the kinase involved in G2/M-dependent phosphorylation of p21. However, ATR involvement cannot specifically be ruled out, although we found no report in the literature to suggest ATR kinase activation in nocodazole-treated cells.
A 24-amino-acid Tat-p21 fusion peptide encompassing the nuclear localization signal and the PCNA-binding region of p21 blocks p21 phosphorylation and results in cell death.
Previously, small peptides have been used as powerful tools to probe regions of proteins mediating different biological activities and protein-protein interactions (27, 72, 73). Because nuclear translocation of p21 was found to precede its phosphorylation, we designed a short peptide to attempt to interfere with its nuclear translocation. We hypothesized that the region containing the NLS region of p21 (13 aa) as a Tat fusion (an additional 11 aa) may serve as an effective competitive inhibitor for p21 nuclear translocation and may possibly inhibit p21 hyperphosphorylation. We used two concentrations (50 and 100 μM) of the 24-amino-acid Tat-p21 fusion peptide with either nocodazole-treated or aphidicolin-released cells to examine effects on the subcellular localization of p21. Although at a 50 μM concentration of the Tat-p21 peptide there was a minimal effect on p21 hyperphosphorylation, we found that at 100 μM the synthetic Tat-p21 peptide completely blocked p21 hyperphosphorylation (Fig. 2E and F). UV exposure had no effect on either the hyperphosphorylation of p21 in the presence of nocodazole or the ability of the Tat-p21 fusion peptide to block this phosphorylation (Fig. 2E). If the cells were pretreated with the Tat-p21 fusion peptide, subsequent UV exposure prevented p21 translocation to the nucleus (data not shown).
To confirm that p21 must translocate to the nucleus in order to become hyperphosphorylated at G2/M, H460 cells were treated with Tat-p21 fusion peptide either alone or with nocodazole. By immunofluorescence, the Tat-p21 fusion peptide blocked p21 translocation to the nucleus in nocodazole-treated cells (Fig. 2G). Flow cytometric analysis at this time point showed that about 95% cells were at G2/M phase (not shown) with a cytoplasmic localization of p21 (Fig. 2G-d). In contrast, nocodazole-arrested cells showed only nuclear accumulation of p21 (Fig. 2G-c). In the asynchronous cells, the Tat-p21 fusion showed no specific effect on endogenous p21 localization (Fig. 2G-b), likely because a significant fraction of the cells already had nuclear p21. We performed control experiments showing that the Tat portion introduced as a 24-amino-acid Tat-Tat fusion does not block chromatin association of p21 or cyclin D1 in nocodazole-treated cells (see Fig. S2A in the supplemental material) and does not block p21 phosphorylation in nocodazole-exposed cells (see Fig. S2B in the supplemental material). We further show (supplemental Fig. S2C, middle panels) that the Tat-p21 fusion specifically blocks association between p21 and DAPI-positive nuclear materials, whereas cyclin D1 still localizes with DAPI-positive staining in nocodazole-treated cells. We further confirm that the Tat-Tat control peptide does not prevent association between either p21 or cyclin D1 with the DAPI-positive nuclear materials (see Fig. S2C, lower panels, in the supplemental material). Analysis of intracellular localization of p21 (wild type and different single or multiple mutant constructs of the NLS region of Flag-tagged p21) showed wild-type p21 localized to the DAPI-positive nuclear material after transfection in asynchronous or nocodazole-treated cells, while with the RKR-AAA140-142 mutant p21, about 85% of cells showed cytoplasmic staining (see Fig. S2D in the supplemental material). There was no G1 cell cycle arrest observed with these constructs after transfection. Blockade of p21 nuclear translocation leads to unphosphorylated p21 after exposure to the Tat-p21 peptide followed by nocodazole treatment, suggesting that for its phosphorylation the protein has to be translocated to the nucleus.
Interestingly, treatment of tumor cells with the Tat-p21 fusion peptide induced cell death to a much greater extent for nocodazole-treated cells than for asynchronous cells (Fig. 2H). However this result was not predictable, since p21-null cells are viable. It is possible that the lethality may have been caused by blockade of nuclear translocation of other proteins, nonspecific effects of the peptide, or other unknown targets, although it is of interest that the death was higher in nocodazole-treated cells than in asynchronous cells. We found no evidence that the Tat-p21 fusion peptide blocked chromatin association of cyclin D1 in nocodazole-treated cells. Although speculative, it is also possible that lethality may have been related to interference with endogenous p21 association with PCNA. Nonetheless, the cytotoxic effects of the Tat-p21 fusion peptide may be of interest for therapeutic purposes and may merit further study. There was no toxicity noted from the Tat-Tat control fusion peptide.
Phosphorylation of p21 at the S-G2 boundary precedes hyperphosphorylation of Cdc25C, dephosphorylation of Cdc2-Y15, and increased expression levels of cyclin B1.
We explored the kinetics of p21 phosphorylation with respect to known G2/M markers and events (Fig. 3). p21 protein hyperphosphorylation was evident after 6 h of treatment of H460 cells with nocodazole (Fig. 3A), whereas in HCT116 and DLD1 cells, it was evident by 3 h (see Fig. S3A and B in the supplemental material). Hyperphosphorylation of p21 preceded the hyperphosphorylation of Cdc25C, which was evident by 9 h in nocodazole-treated H460 cells (Fig. 3A), 9 h in HCT116 cells, or 12 h in DLD1 cells (see Fig. S3A and B in the supplemental material). In each case the hyperphosphorylation of Cdc25C occurred later than p21 hyperphosphorylation. Likewise, dephosphorylation of Cdc2 at Y15 occurred in H460 cells by 9 h, coincident with hyperphosphorylation of Cdc25C (Fig. 3A). p21 hyperphosphorylation began to occur in H460 cells while cyclin A levels were high and preceded increased expression of cyclin B1, which was first noted by 9 h and became maximal by 12 h of nocodazole exposure (Fig. 3A). Interestingly, expression of cyclin B1 and Cdc2 was found to be highest between 12 and 24 h and subsequently declined while the cells remained in G2/M (Fig. 3A). Expression of p27, another cyclin kinase inhibitor, was not detected by immunoblotting in nocodazole-treated cells (data not shown). Levels of CDK2 remained constant, while as expected, cyclin A levels decreased after 15 h of nocodazole exposure. In summary, hyperphosphorylation of p21 is an early G2 event and takes place before the appearance of hyperphosphorylated Cdc25C as well as the higher expression of the main mitotic markers, i.e., before the onset of mitosis.
Delayed G2/M accumulation in p21-null cells treated with nocodazole or those released from aphidicolin block.
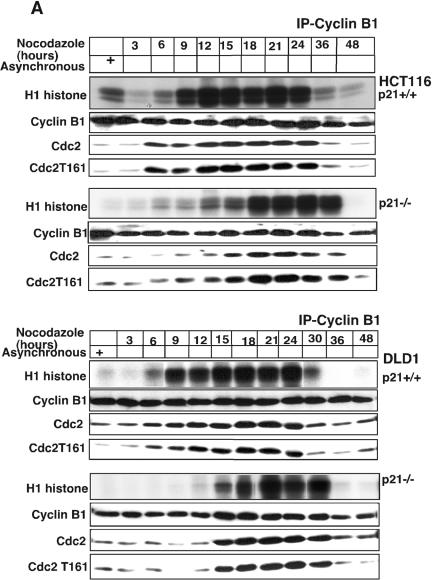
To begin to address a functional role for p21 in regulation of the G2/M transition, we examined cell cycle progression following exposure of wild-type and p21-null cells to nocodazole or release from aphidicolin blockade. To establish that p21 is present in S and G2/M cells, flow cytometric measurement of asynchronous or cells synchronized in G1, S, or G2/M phase, stained with anti-p21 monoclonal antibody and propidium iodide, was performed. There were no p21-positive cells among DLD1 p21−/− cells. However, there was a clear correlation of DNA content and the presence of p21-positive cells for asynchronous or S- and G2/M-enriched DLD1 p21+/+ cells (see Fig. S4A in the supplemental material). Figure 3B shows that p21-null HCT116 cells or p21-null DLD1 cells took a longer time to reach G2/M than the p21-proficient counterparts. For example, by 6 h of nocodazole exposure, HCT116 cells were primarily in G2/M, while there remained a significant fraction of the p21-null HCT116 cells in S phase (Fig. 3B, upper panels). In the p21-null DLD1 cells, there were more cells in G1 and S phase at 6 h compared to the wild-type p21-containing DLD1 cells (Fig. 3B, lower panels). Examination of the cell cycle profile of p21+/+ and p21−/− cells showed an increase in G2/M cells after 3 h of nocodazole treatment in HCT116 and DLD1 p21+/+ cells, but it took 6 h with p21−/− cells for the same pattern to appear (Fig. 3B). Interestingly, for HCT116 p21−/− cells, the cell cycle profile pattern showed 8N and 16N cells after 48 h (Fig. 3B). There was also increased cell death for the nocodazole-treated p21−/− cells, as reported previously (6, 71).
We also examined the entry into G2/M for wild-type and p21-null DLD1 cells released from aphidicolin blockade (Fig. 3C). The results revealed that by 3 h after release from aphidicolin blockade, wild-type p21-expressing DLD1 cells harbored detectable hyperphosphorylated p21, which persisted until 10 h after release from aphidicolin (Fig. 3C). Examination of the cell cycle profiles of wild-type p21-expressing DLD1 cells released from aphidicolin blockade revealed that 74.5 and 61.2% of the cells were in G2/M by 6 and 8 h, respectively (Fig. 3C). In contrast, the p21-null DLD1 cells showed delayed entry into G2/M after release from aphidicolin blockade, with 66.4 and 52.5% of the cells in G2/M by 6 and 8 h, respectively (Fig. 3C). There were twice as many p21-null cells (24.7%) still in S phase after release from aphidicolin blockade than with the wild-type p21-expressing released cells (12.7%). Thus, it is clear that the delayed accumulation of p21-null cells in G2/M is at least partly due to delayed entry into G2/M.
There was a similar delay in phosphoserine 10 H3-histone phosphorylation in nocodazole-treated p21−/− cells compared to results with p21+/+ cells (see Fig. S4B in the supplemental material). This indicates that the delayed G2 progression in p21−/− cells was also accompanied by a delay in entry into mitosis as marked by histone H3 phosphorylation. Our results reveal that the presence of p21 is critical for accumulation of cells in G2/M following nocodazole exposure. This suggests a role for hyperphosphorylation of p21 at the S-G2 transition as well as in the G2/M phase.
p21 promotes cyclin B1-Cdc2 kinase activity.
Because loss of p21 has a profound effect on cell cycle progression into G2/M (Fig. 3), we examined the effect of the presence or absence of p21 on CDK2 and Cdc2 kinase activities in human cells exposed to nocodazole. Using either HCT116 or DLD1 cells (Fig. 4A), we found that cyclin B1-associated kinase activity began to rise by 9 h after exposure to nocodazole and remained high for up to 24 h. In contrast, p21-null HCT116 or DLD1 cells showed delayed appearance of cyclin B1-associated kinase activity following nocodazole exposure, with maximal levels being evident by 18 to 24 h (Fig. 4A). The delayed activation of the cyclin B1-associated kinase in p21-deficient cells appears to be due to delayed association between cyclin B1 and Cdc2. Thus, although cyclin B1 is present in the immunoprecipitates at 9 and 12 h after nocodazole exposure of p21-null HCT116 or p21-null DLD1 cells, there was relatively much less associated Cdc2 or threonine 161-phosphorylated Cdc2 (Fig. 4A). Of note, Y15-phosphorylated Cdc2, which is present in total cellular extracts of nocodazole-treated cells (see Fig. S3 in the supplemental material), was not detectably associated with the cyclin B1 immunoprecipitated from the nocodazole-exposed cells (data not shown).
FIG. 4.
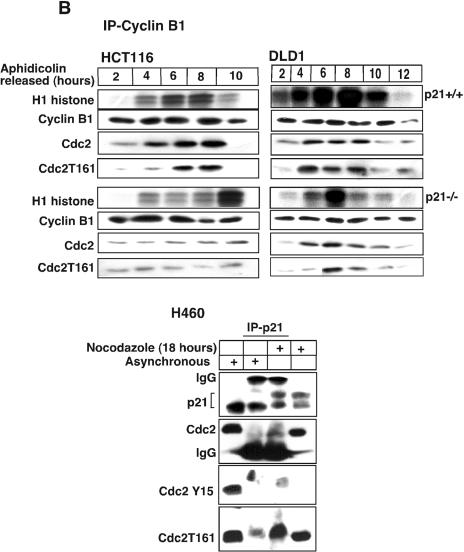
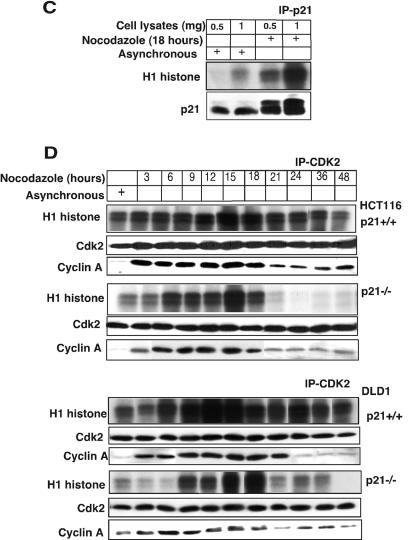
Absence of p21 in HCT116 and DLD1 cells delays cyclin B1-Cdc2 kinase activation. (A) Immnodepletion with cyclin B1 monoclonal antibodies in p21+/+ and p21−/− cell lysates collected after Nocodazole treatment at different time points was followed by an in vitro histone H1 kinase assay. The results demonstrate that the cyclin B1-associated kinase activity begins to rise at 6 h in HCT116 p21+/+ cells but is significantly delayed in p21−/− cells. In DLD1 p21+/+ cells, the H1 kinase activity also appears earlier than in p21−/− cells. Kinase activity was also observed to persist for 6 to 8 h longer following nocodazole exposure of HCT116 and DLD1 p21−/− cells than with the matched p21+/+ cells. Association between immunoprecipitated cyclin B1 and Cdc2 or T161-phosphorylated Cdc2 is shown. (B) Delayed histone H1 kinase activity was noted in aphidicolin-released cells immunodepleted with cyclin B1 antibody in p21−/− cells. Surprisingly, in DLD1 p21+/+ cells the kinase activity not only started at early time points but also progressed for longer time periods. However, the nocodazole exposure (A) or aphidicolin release (B) methods gave similar results with respect to the delay in cyclin B1-associated histone H1 kinase activation in the two different p21−/− human cell lines. Association between immunoprecipitated cyclin B1 and Cdc2- or T161-phosphorylated Cdc2 is shown. In the lower panels, association between p21- and T161-phosphorylated but not Y15-phosphorylated Cdc2 is shown. (C) Association of kinase activity with p21-containing immune complexes from cells enriched at G2/M. Nocodazole-treated G2/M-enriched H460 cells immunodepleted with p21 antibodies showed H1 histone phosphorylation. (D) CDK2-associated kinase activity is observed at all progressive time points following Nocodazole exposure with a peak at 15 to 18 h in p21+/+ cells (HCT116 and DLD1). After 18 h, the kinase activity returned to the basal levels in HCT116 and DLD1 p21−/− cells.
We next directly tested whether the catalytically inactive Y15-phosphorylated Cdc2 could interact with p21 in either asynchronous or nocodazole-treated cells. Using either asynchronous H460 cells or those treated for 18 h with nocodazole, we performed immunoprecipitation using an anti-p21 antibody and immunoblotting with antibody against p21, total Cdc2, Y15-phosphorylated Cdc2, or T161-phosphorylated Cdc2 (Fig. 4B lower panels). The results clearly indicate an association between p21 and T161-phosphorylated Cdc2 which is enriched in nocodazole-treated cells. We observed no detectable interaction between p21 and Y15-phosphorylated Cdc2 in either asynchronous or nocodazole-treated cells. However, by 18 h after nocodazole exposure, there was also no detectable Y15-phosphorylated Cdc2 in the total cell extracts.
To document the effect of p21 with a different synchronization method, we examined cyclin B1-associated kinase activity following release from aphidicolin block (Fig. 4B). After release from aphidicolin, there was a significant delay in reaching maximal cyclin B1-associated kinase activity in p21-null cells versus wild-type p21-expressing cells (Fig. 4B). We observed a delay in association between cyclin B1 and either Cdc2 or T161-phosphorylated Cdc2 in p21-null cells released from aphidicolin blockade (Fig. 4B, upper panels). These results suggest that the presence of p21 promotes cyclin B1-Cdc2 kinase assembly and activity, which may be important for cell cycle progression in the G2/M phase.
While it is clear there is some delay in S phase in p21-null cells released from aphidicolin blockade (Fig. 3C), the majority of the cells have reached G2/M by 6 h after release from aphidicolin. Thus, the delayed activation of cyclin B1-Cdc2 kinase cannot be explained entirely by an S-phase block in the p21-null cells but rather by delayed kinase activation once cells have reached G2/M. In further support of this conclusion, p21 immunoprecipitates from G2/M-enriched cell populations were found to have a much higher level of kinase activity against a histone H1 substrate than complexes derived from asynchronous cells (Fig. 4C). This result shows directly that in G2/M extracts there is significant kinase activity associated with p21 that is unexpected for a kinase inhibitor.
p21 deficiency is associated with early loss of CDK2 kinase activity in G2/M.
Because p21 immunoprecipitates in G2/M were associated with significant kinase activity, we wondered whether CDK2 kinase activity might be modulated by p21. Surprisingly, we found that following nocodazole exposure there was persistent CDK2-associated kinase activity up to 48 h, while in p21-null cells CDK2 kinase activity was dramatically reduced after 18 h of nocodazole exposure (Fig. 4D). We also examined CDK2 kinase activity in aphidicolin-released p21+/+ and p21−/− cells and found similar results (data not shown). Thus, CDK2 was active during G2/M phase in cells containing wild-type p21. The relationship between loss of CDK2 kinase activity and lack of p21 expression in p21-null cells remains unclear.
Preferential association of hyperphosphorylated p21 with Cdc2 or cyclin B1.
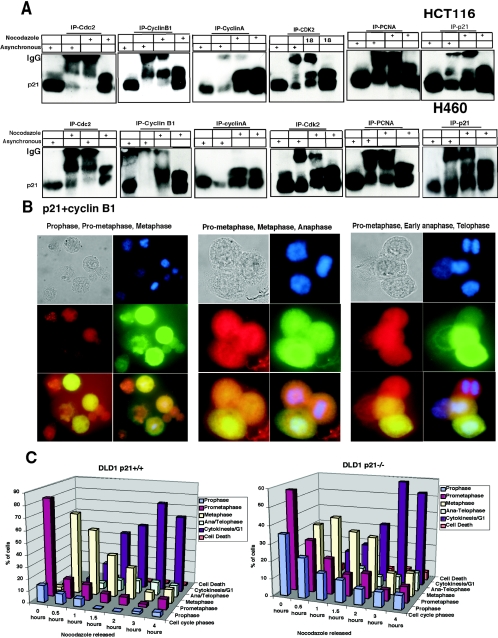
To begin to understand the role of p21, and specifically hyperphosphorylated p21, in regulation of cyclin B1-Cdc2 kinase activity, we examined the assembly of CDK complexes in nocodazole-treated HCT116 or H460 cells (Fig. 5). Immunoprecipitation of either Cdc2 or cyclin B1 showed preferential association to the hyperphosphorylated p21 band from HCT116 or H460 cells (Fig. 5A). In contrast, there was no preferential association between hyperphosphorylated p21 and PCNA, cyclin A, or CDK2 (Fig. 5A). These results suggest the possibility that phosphorylated p21 may specifically associate with cyclin B1-Cdc2 complexes and promote their activity. To confirm the association between p21 and cyclin B1 in human cells, we examined their colocalization by immunofluorescent staining. p21 and cyclin B1 colocalized in G2, prophase, prometaphase, and metaphase cells but not in replicating cells or anaphase/telophase cells (Fig. 5B). At the anaphase/telophase stage, p21 was still located in the nucleus but cyclin B1 was degraded and not detectable. Consistent with the predicted effect of p21 in associating with and promoting cyclin B1-Cdc2 kinase activity, we further found that there was greater accumulation of p21-null cells than of the wild-type p21-expressing DLD1 cells in prophase following release from nocodazole (Fig. 5C). We also show that cyclin A colocalizes with p21 in nocodazole-treated cells (see Fig. S5 in the supplemental material). These results further support the notion that p21 and its association with cyclin B1-Cdc2 may promote early events in progression from G2-prophase to prometaphase and metaphase, and they are consistent with earlier reports (15). However, the novel finding here is the preferential association between G2/M-specific hyperphosphorylated p21 and either cyclin B1 or Cdc2.
FIG.5.
Cyclin B1 interacts exclusively with hyperphosphorylated p21, whereas Cdc2 interacts preferentially with hyperphosphorylated p21 compared to hypo-phosphorylated p21. (A) Immunodepletion of asynchronous or G2/M-enriched H460 or HCT116 cells with various monoclonal antibodies (as indicated) and immunoblotting for p21 protein. Total cell extracts from asynchronous and nocodazole-treated cells are shown in each case to mark the migration of the hyper- and hypophosphorylated p21. Immunoprecipitation using anti-cyclin A, CDK2, or PCNA antibodies revealed similar association of these proteins with hyper- and hypo-phosphorylated p21. The p21 monoclonal antibody used for immunoprecipitation (rightmost panels) bound and precipitated both the hyper- and hypophosphorylated p21 proteins. (B) p21 colocalizes with cyclin B1 in the nucleus at G2/M. Eighteen-hour nocodazole-treated H460 cells released from microtubule inhibition for 0.5 h were fixed and simultaneously stained with human monoclonal p21 (red) and human cyclin B1 polyclonal antibodies (green). Prophase, prometaphase, and metaphase cells show milkish/pink granular structures over the chromatin material with nuclear p21 and cyclin B1 localization after cells were treated for 18 h with nocodazole or released from nocodazole for half an hour. In late anaphase or early telophase stage, only p21 was localized in the nucleoplasm (red), but cyclin B1 degraded. (C) Phosphorylated p21 acts as a positive regulator of the G2-prophase stage. Comparison of percentage of mitotic phases in nocodazole-treated or nocodazole-released cells at different time points using DLD1 p21−/− and p21+/+ cells is shown.
Cyclin B1 interacts with threonine 57-phosphorylated p21 at G2/M.
Our earlier studies (Fig. 2) suggested that CDK2 is a strong candidate mediator of p21 phosphorylation as cells enter the G2 phase. We also showed that cyclin B1 preferentially interacts with hyperphosphorylated p21 (Fig. 5). We further extended the experiments performed for Fig. 2 to determine the effects of various kinase inhibitors on the association between p21 and cyclin B1 in nocodazole-treated G2/M-arrested cells (Fig. 6A to D). Consistent with the results shown in Fig. 2, we found that only the CDK2 inhibitor butyrolactone was able to inhibit the association between p21 and cyclin B1 (Fig. 6D). Inhibitors of GSK3 (Fig. 6A), PKC or Chk1 (UCN-01; Fig. 6C), or the PI3K/Akt inhibitor wortmannin (data not shown) did not prevent association between p21 and cyclin B1. However, the inhibition of ATM/ATR by caffeine reduced apparent binding between p21 and cyclin B1 (Fig. 6B), consistent with the finding that caffeine inhibits G2/M-specific p21 hyperphosphorylation (Fig. 2D) and that p21 hyperphosphorylation is required for interaction between p21 and cyclin B1 (Fig. 5A). These results further support the idea that blockade of CDK2 kinase activity may prevent association between p21 and cyclin B1, possibly by inhibiting the G2/M-phosphorylated form of p21, which preferentially associates with cyclin B1. We then performed additional experiments to examine the role of specific CDK2 phosphorylation sites within p21 and the effect of their mutation on the association between p21 and cyclin B1.
p21 has three potential CDK2 consensus sites, located at Thr 57, Thr/Ser 98/99, and Ser 130. Thr 98 and Ser 99, as well as Ser 130, of p21 have been previously reported as targets for CDK2 phosphorylation in vitro or in experiments using baculovirus expression systems (33, 75). Thr 57 of p21 has been previously suggested to be a target for phosphorylation by GSK3 (58), but in the present study the GSK3 inhibitor did not prevent the phosphorylation of p21 at G2/M (data not shown) or the association between cyclin B1 and p21 (Fig. 6A). We note that at Thr 57 within p21 the peptide sequence TPLE does not represent a match to the consensus sequence for GSK3 (SXXXS) (20) but rather is a better match for a consensus for CDK2 (S/TPXX) (1). We generated HA-tagged mutants of p21 at candidate CDK2 phosphorylation sites, including the following single or multiple substitutions: T57A, TS98AA, S130A, TS98AA-S130A, T57A-TS98AA, and T57A-S130A. These CDK2-site p21 single or compound mutants were transfected individually into U2OS cells for 24 h, and this was followed by nocodazole treatment for another 18 h. Immunoprecipitation of transfected cells with HA antibody and immunoblotting with anti-cyclin B1 antibody demonstrated that Thr 57 of p21 is critical for interaction between p21 and cyclin B1 in G2/M-arrested cells (Fig. 6E to G). However, when p21 Thr 57 was replaced by glutamic acid, the interaction between p21 and cyclin B1 was detected (data not shown), confirming further that cyclin B1 interaction with the Thr 57-phosphorylated p21 is due to charge potential which may also change the conformation of p21. To demonstrate the specificity of phosphorylated Thr 57 on p21 for interaction with cyclin B1, we tested the interaction between the T57A p21 mutant and CDK2, Cdc2, or cyclin A (Fig. 6H to J). The results show clearly that transfection of the T57A p21 mutant resulted in detectable interaction between the transfected p21 and CDK2 (Fig. 6H) or cyclin A (Fig. 6J). The T57A mutant of p21 interacted poorly with Cdc2 in nocodazole-treated cells (Fig. 6I). This binding between the T57A mutant of p21 and Cdc2 suggests the possibility that CDK2-phosphorylated p21 may first bind with cyclin B1, followed by recruitment and binding of Cdc2. Another possibility is that dephosphorylation of p21 may allow release of cyclin B1 while p21 remains bound to Cdc2. It is clear from our earlier experiments that hypophosphorylated p21 can (although with reduced affinity compared to the hyperphosphorylated p21) bind with Cdc2 but not cyclin B1 (Fig. 5A).
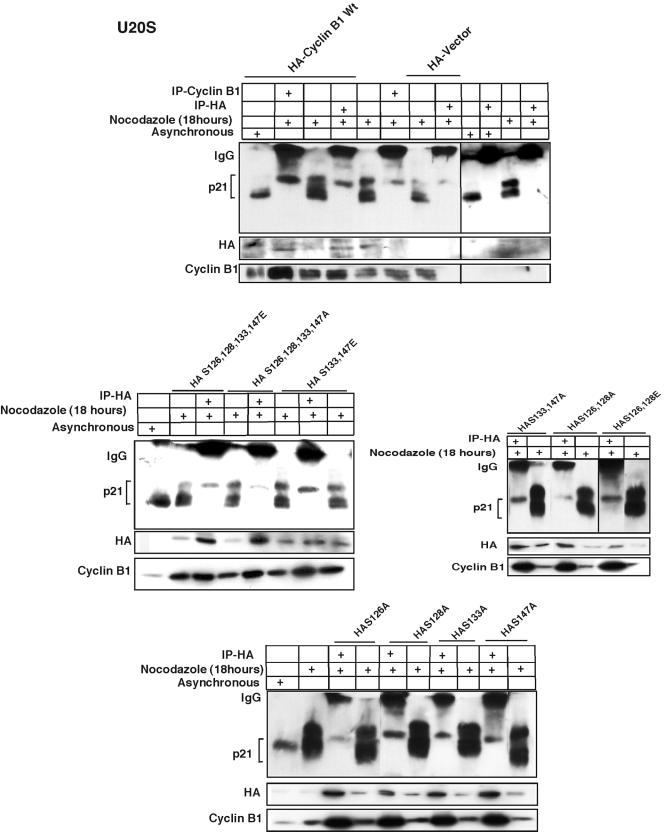
Phosphorylated p21 specifically binds within the nuclear localization signal sequence at phosphorylated serine 126 of cyclin B1.
Polo-like kinase-dependent phosphorylation on four-serine residues of cyclin B1 located within the nuclear localization signal of cyclin B1 helps entry of cyclin B1 into the nucleus at prophase (68). Our earlier experiments showed that cyclin B1 binds to phosphorylated p21 (Fig. 5A) and that phosphorylation of Thr 57 of p21 is critical for its association with cyclin B1 (Fig. 6). We therefore explored whether G2/M-dependent phosphorylation of cyclin B1 may also affect the interaction between cyclin B1 and p21 (Fig. 7). We generated individual HA-tagged cyclin B1 alanine substitution mutants at Ser 126, Ser 128, Ser 133, and Ser 147, and we obtained previously described (68) multiple mutants (Fig. 7). Immunoprecipitation of transfected U2OS cells with HA antibody and immunoblotting with anti-p21 antibody demonstrated that phosphorylated p21 binds poorly to mutants of cyclin B1 with alanine substitutions at Ser 126 (Fig. 7). This was observed with single S126A mutants or mutants containing alanine substitutions at other sites phosphorylated in G2/M, which also included the S126A substitution.
FIG. 7.
Phosphorylated p21 interacts specifically with serine 126 of cyclin B1. HA-tagged wild-type cyclin B1 constructs were transfected into U2OS cells for 24 h, and the cells were treated with nocodazole for another 18 h. Cells were then immunodepleted with HA monoclonal antibody and immunoblotted with p21 monoclonal antibody. Binding with hyperphosphorylated p21 protein was observed as with extracts immunodepleted with cyclin B1 antibody (Fig. 5A). Transfection of the HA-tagged cyclin B1 S126A S128A S133A S147A multiple serine-to-alanine substitution mutant very poorly bound with the phosphorylated p21, as did the S126A S128A mutant. There was no change in interaction with p21 with either the S126E S128E S133E S147E multiple serine-to-glutamic acid mutant or the S133A S147A, the S133E S147E, or the S126E S128E mutants. Phosphorylated p21 interacted very poorly with the cyclin B1 S126A mutant. However, the S128A, S133A, or S147A mutant construct interacted as normally as wild-type cyclin B1.
Phosphorylation of p21-T57 and cyclin B1-S126 is required for cyclin B1-associated kinase activation in G2/M.
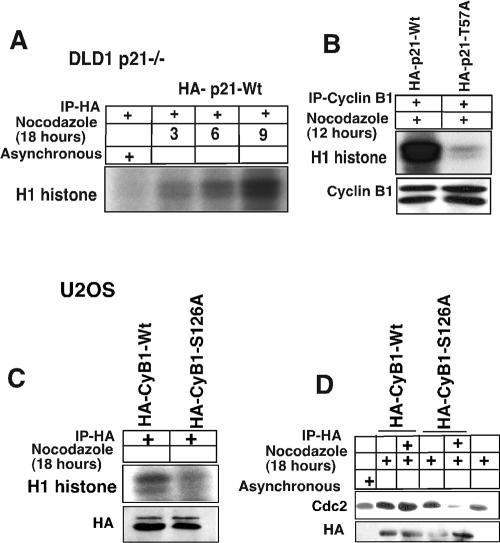
To further examine the role of p21 phosphorylation in activation of the cyclin B1-Cdc2 kinase, and in particular the role of Thr 57, we determined H1 kinase activity in cyclin B1 immunoprecipitates of p21-null DLD1 cells transfected with either wild-type or T57A-mutated p21 (Fig. 8). Wild-type p21-transfected p21-null DLD1 cells were treated with nocodazole to enrich for G2/M cells, and histone H1 kinase assays using p21 immunoprecipitates (using anti-HA to precipitate HA-tagged p21) were performed at successive time points after nocodazole exposure (Fig. 8A). It is clear that following nocodazole exposure, kinase activity was found to be associated with p21 immunoprecipitates (Fig. 8A). This is consistent with a model wherein p21 association with a kinase in G2/M phase is activating rather than inhibitory and together with our other results is consistent with an interpretation that p21 may help assemble the cyclin B1-Cdc2 kinase and also enhance its activity. To further examine the role of Thr 57 phosphorylation in activation of cyclin B1-associated kinase activity, we determined histone H1 kinase activity following cyclin B1 immunoprecipitation from nocodazole-treated p21-null cells transfected by either wild-type p21 or the T57A mutant of p21 (Fig. 8B). The results show clearly that the T57A mutant of p21 was incapable of stimulating cyclin B1-associated kinase activity at 12 h after nocodazole exposure, while wild-type p21 was very efficient (Fig. 8B).
FIG. 8.
Kinase activity in DLD1 p21−/− cells after introduction of wild-type but not T57A-mutated p21 or in U2OS cells after introduction of wild-type but not S126A-mutated cyclin B1. (A) DLD1 p21−/− cells were transfected with a HA-tagged p21-Wt plasmid and treated with nocodazole for another 18 h. Histone H1 kinase assays were performed on the HA antibody-immunodepleted lysates, which showed H1 histone phosphorylation as early as after 3 h of nocodazole treatment. See panel B for an essential negative control. (B) p21HA-Wt or p21HA-T57A mutant plasmids were transfected in DLD1 p21−/− cells for 24 h, and the cells were treated with nocodazole for 12 h. Cell lysates were immunodepleted with anti-cyclin B1 antibodies, and H1 histone kinase assays were performed. The p21Wt construct-transfected DLD1 cells showed significantly higher phosphorylation of histone H1 compared to the T57A mutant. In nocodazole-treated DLD1−/− cells not transfected by a p21 expression plasmid, there was virtually no detectable cyclin B1-associated kinase activity (see Fig. 4A). (C) Plasmids expressing wild-type or S126A-mutated cyclin B1 tagged with HA were transfected into U2OS cells for 24 h, and the cells were treated with nocodazole for another 18 h. Cell lysates were immunoprecipitated with anti-HA antibody, and histone H1 kinase activity was determined. (D) U2OS cells were transfected with wild-type or S126A-mutated cyclin B1 tagged with HA for 24 h, and the cells were treated with nocodazole for another 18 h. Cell lysates were immunoprecipitated with anti-HA antibody and immunoblotted with anti-Cdc2 antibody.
Transfection of HA-tagged wild-type cyclin B1 followed by HA immunoprecipitation resulted in detectable kinase activity from the transfected U2OS cells (Fig. 8C). In contrast, transfection of HA-tagged S126A mutant cyclin B1 followed by HA immunoprecipitation resulted in low to undetectable mutant cyclin B1-associated kinase activity (Fig. 8C). S126A-mutated cyclin B1 appeared to associate poorly with Cdc2 in coimmunoprecipitation compared to the HA-tagged wild-type control cyclin B1 (Fig. 8D). The data appear to support a model wherein p21-T57 and cyclin B1-S126 phosphorylation plays an important role in association between cyclin B1, Cdc2, and p21 and to support that this association allows for efficient cyclin B1-associated kinase activation.
DISCUSSION
Our results provide evidence for G2/M-specific phosphorylation of the cyclin-dependent kinase-interacting protein p21. G2/M-phosphorylated p21 appears to serve a previously unrecognized role in mediating the assembly and efficient activation of the cyclin B1-Cdc2 kinase. The results provide a detailed analysis of events leading up to the phosphorylation of p21 as well as important molecular details of the modifications that allow interaction between p21 and cyclin B1. Specifically, we demonstrate that p21 enters into the nucleus during S phase, where it may associate with and become phosphorylated by CDK2. We provide evidence that blockade of a number of cellular kinases with potential to target p21 for phosphorylation is unlikely to be involved in the G2/M-specific phosphorylation of p21. These include Akt, which was recently shown to directly phosphorylate p21 at Thr 146 and localize it in the cytoplasm in Her2-overexpressing breast tumors, as well as PKC or GSK3, which were also previously shown to phosphorylate p21. The evidence is strong that CDK2 is involved in p21 phosphorylation at G2/M, because it associates with p21 in vivo and is present in the form of cyclin A-CDK2 complexes as early as S phase and persists partly into M phase. Our data that dominant-negative CDK2 or the CDK inhibitor butyrolactone blocks phosphorylation of p21 in nocodazole-treated cells support the conclusion that CDK2 is the principal kinase responsible for p21 phosphorylation in likely G2/M cell extracts. That hyperphosphorylated p21 may serve a unique role in G2/M phase is suggested by the observation that it specifically and preferentially associates with cyclin B1 or Cdc2. In the case of Cdc2, hypophosphorylated p21 is also able to bind but at much lower affinity. We identify Thr 57 of p21 as critical for its interaction with cyclin B1 and the efficient activation of cyclin B1-associated kinase activity in G2/M phase. We further show that Ser 126 of cyclin B1 is important for association with hyperphosphorylated p21. Although we show that either p21 or cyclin B1 interacts poorly with Y15-phosphorylated Cdc2, they appear to interact well with Thr-161-phosphorylated Cdc2. Our results provide evidence that p21-null cells are profoundly delayed in gaining maximal cyclin B1-Cdc2 kinase activity and proceed more slowly to accumulate in G2/M following nocodazole exposure or release from aphidicolin. The data provided support a model (Fig. 9) wherein phosphorylation of p21 specifically at G2/M serves a previously unknown role in the assembly and activation of the cyclin B1-Cdc2 kinase, which is important for cell cycle progression.
FIG. 9.
Proposed model depicting the hyperphosphorylation of p21 by active cyclin A-CDK2 at G2 phase and interaction of phosphorylated p21 with cyclin B1 at G2/M phase to promote Cdc2 kinase activity. This model predicts a dual nature for p21 function depending on cell cycle phase. The novel finding here is that hyperphosphorylated p21 possesses a unique function in G2/M phase. In the G1 phase, in the absence of DNA damage, p21 serves to facilitate assembly of the cyclin D-CDK4 complex and promotes its kinase activity and G1-phase progression. However, during the G1 phase, p53-dependent p21 induction following cellular stress results in G1 cell cycle arrest through inhibition of both CDK4 and CDK2 kinases. In G2/M, hyperphosphorylation of p21 by cyclin A-CDK2 facilitates p21 binding to S126-phosphorylated cyclin B1 and assembly of the cyclin B1-Cdc2 kinase, thereby promoting kinase activation and G2/M progression. S126-phosphorylated cyclin B1 binds to T57-phosphorylated p21. Phosphorylated p21 binds to cyclin B1 when Cdc2 is phosphorylated on Y15 and associates poorly with the complex. Dephosphorylation on Y15 by Cdc25C and phosphorylation on T161 by CAK promotes Cdc2 binding to the p21-cyclin B1 complex that becomes activated as a kinase. Thus, hyperphosphorylated p21 assembles and activates the Cdc2 kinase in the G2/M transition. Cell cycle phases are shown at the bottom. P, M, A, and T refer to prophase, metaphase, anaphase, and telophase, respectively.
We believe the impact of this work lies in the fact that it changes our understanding of the function of the universal cell cycle inhibitor p21 in G2/M phase. We find that a switch from hypophosphorylated p21 to hyperphosphorylated p21 is associated with the early stages of progression through G2/M. This phosphorylation switch converts p21 from an inhibitory subunit to a positive regulatory factor that assists cyclin B1-Cdc2 activation. Moreover, we have documented preferred binding between phosphorylated p21 and cyclin B1-Cdc2, and we have delineated specific phosphorylation sites on p21, cyclin B1, and Cdc2 as essential for their observed interactions.
It is well established that CDK2 and Cdc2 kinases and their counterpart cyclins play an essential role in S and G2/M transitions, and this is regulated by ubiquitination of cyclins at the metaphase-anaphase transition prior to mitotic exit (25, 48-50). The report of Dulic et al. suggested that p21 might have a role at the G2/M transition because it colocalizes with cyclin B1 during the G2/M phase (15). The present study is the first report to link functionally posttranslational modification of p21 at G2/M with two regulatory mitotic kinases.
We conclude that CDK2 but not Cdc2 kinase is involved in phosphorylating p21. The first clue was that the p21 protein sequence contains three different CDK2 phosphorylation sites but contains no consensus phosphorylation sites for Cdc2. p21 has been shown previously to be a substrate for phosphorylation by cyclin A-CDK2 (33, 37, 79). p21 phosphorylation is observed in synchronized cells to precede activation of the cyclin B1-Cdc2 kinase and the appearance of hyperphosphorylated Cdc25C. Hypophosphorylated p21 binds very poorly to cyclin B1-Cdc2, whereas the hyperphosphorylated p21 binds strongly and appears to promote cyclin B1-Cdc2 kinase activity in G2/M. Based on these structural, kinetic, and binding studies, we conclude that CDK2 is the likely kinase that phosphorylates p21 at G2/M. Moreover, we think the data in particular exclude cyclin B1-Cdc2 as the kinase that phosphorylates p21. To further substantiate our results, we show clearly that dominant-negative CDK2 inhibits p21 phosphorylation while most of the cells are still at the G2/M stage. We argue that the blockade in phosphorylation is not due to failure of cells to reach G2/M but rather is a more direct effect of dominant-negative CDK2 inhibiting wild-type CDK2 from phosphorylating p21 even in the majority of the cells which have already reached G2/M phase. We present evidence that mutation of Thr 57 in p21 to alanine (one of the consensus sites for CDK2) prevents physical association between the mutant p21 and cyclin B1-Cdc2 but not cyclin A-CDK2. The kinase inhibitor studies are ancillary and are supportive of other experiments. Further, they are most helpful when they exclude specific kinases. For example, UCN-01, which blocks Chk1 and PKC, fails to inhibit p21 phosphorylation. Wortmannin, which inhibits PI3-kinases and ATM, fails to inhibit p21 phosphorylation. Because we observe phosphorylation of p21 in ATM-null cells, we can exclude ATM as a kinase for p21. However, because caffeine, which inhibits both ATM and ATR kinases, blocks p21 phosphorylation, we cannot exclude ATR as a potential kinase. Thus, the inhibitor studies are limited, but they do provide some useful information to guide the exclusion of potential kinases that may phosphorylate p21.
The finding that CDK2 phosphorylates p21 at the S-G2 boundary provides a functionally important substrate for CDK2 at G2. Cdc2 appears to be regulated by interaction with phosphorylated p21 through cyclin B1. Known substrates of cyclin E-CDK2, in addition to p21 and the Rb family, include Cdc25A in G1 phase (34), p220 (NPAT), which is required for S-phase entry (74, 77), the Cdc6 protein which binds origin recognition complexes (24), and HIRA, which is required for S-phase progression and can also be phosphorylated by cyclin A-CDK2 (28). Other CDK2 substrates include the mouse Mps1p-like kinase, which regulates centrosome duplication (21), the ubiquitin-conjugating enzyme hHR6A, whose phosphorylation peaks in G2/M (59), and Cdc25C (32), although Cdc25C can be phosphorylated in CDK2- and Cdc2-depleted mitotic extracts (36). Thus, there are few known substrates for cyclin A-CDK2 with the potential to regulate G2/M progression.
p21 interaction with cyclin A has been demonstrated to occur through the RxL motifs present within the N- and C-terminal domains of p21 and one on the middle of the protein, as occurs in other substrates, such as p27, p107, p130, E2F, and Rb for CDK2-dependent phosphorylation (56). Taken together with the present results, it is suggested that the interaction between cyclin A and p21 is followed by recruitment of the holoenzyme CDK2 and subsequent p21 hyperphosphorylation. However, unlike cyclin A, cyclin B1 binds only the hyperphosphorylated form of p21. In addition, there is some evidence that cyclin B1 also gets phosphorylated before it enters the nucleus (68), where it may then bind with hyperphosphorylated p21 protein. Thus, an increase in the negative charge potential on the surface of both proteins and/or a change in conformation after phosphorylation could facilitate interaction between p21 and cyclin B1. Further support for this notion is derived from our mutational analysis showing that Thr 57 of p21 and Ser 126 of cyclin B1 are required for interaction between the two proteins. In the future, X-ray crystallographic analysis of these two proteins may provide additional details of the specific interaction. Our study is consistent with prior models in suggesting that cyclins may recognize their substrates and subsequently recruit the binary complex for CDK kinase action (31, 41, 56, 63, 64).
p21 is a universal inhibitor of cyclin-dependent kinases as described initially (18, 30, 31, 75, 79), although p21-containing complexes can be catalytically active (42, 78, 79, 80). Similarly, it has become clear that p21 could promote the kinase activity of cyclin D1-CDK4, in addition to its role as a CDK inhibitor (13, 42). While the notion is well established that p21 inhibitor or activator function may depend on its expression level, we for the first time suggest that p21 modification may alter its inhibitory function. This is based on evidence that hypophosphorylated p21 can interact with Cdc2 in a presumably kinase-inactive complex, while hyperphosphorylated p21 interacts with cyclin B1 and appears to activate the Cdc2 kinase complex. It is also possible (although unproven in the present studies), because of the apparently short-lived nature of hyperphosphorylated p21, that its inhibitory activity may be negatively regulated through a degradative pathway following G2/M-specific phosphorylation.
Our results suggest that the kinase CDK2, which is efficiently inhibited in the cyclin E-CDK2 complex by p21 upregulation during G1-phase checkpoints, is capable of acting as a kinase for p21 phosphorylation at G2/M in vivo. One obvious difference is the partner cyclin A at the G2/M phase. Thus, in vivo at G2/M, the p21 protein not only is phosphorylated by the cyclin A-CDK2 complex but also assembles and promotes cyclin B1-Cdc2 kinase activity. These observations, along with the delayed G2/M progression phenotype of p21-null cells, provide independent indications of a positive rather than a negative effect of p21 on cell cycle progression during G2/M.
p21 nullizygous mice undergo normal development (14), but this does not eliminate the possibility that defects might exist after specific stimuli or in certain cell types. It is clear that p21-deficient human cells, compared with matched p21 wild-type human cells, show a remarkable delay in Cdc2 kinase activation (Fig. 4A) and increased cell death at longer time periods following nocodazole arrest (Fig. 3B). It is possible in the case of cyclin B1-Cdc2 assembly and activation, as in the case for cyclin D1-CDK4 assembly and activation in G1, that other factors ultimately allow cell cycle progression and viability of p21-null cells. In the cell lines examined here, we detected no expression of p27 in G2/M.
We show in Fig. 4 that there are no differences in cyclin B1 protein levels in immunoprecipitates of 500 μg of total cell extracts prepared from cells exposed to nocodazole for 3 to 6 h versus 12 to 24 h. However, at these time points we found significant differences in H1 histone kinase activity in the immunoprecipitates. First, we found that in the wild-type p21-containing HCT116 there was low kinase activity at the 3- to 6-h time window compared to the 12- to 24-h time window. We also observed a significant delay in the time to reach maximal H1 kinase activity in the p21−/− cells compared to that for the wild-type p21-containing HCT116 cells. Our observations were reproduced using release from aphidicolin block as an alternative method of synchronization.
These results suggest that the cyclin B1 present in the immunoprecipitates at the early time points following nocodazole exposure is associated with low H1 kinase activity. We further note that while we show in supplementary Fig. S2A and B that Cdc2 levels are similar among the wild-type and p21-null cells at the 3- to 6-h time window versus the 12- to 24-h time window after nocodazole exposure, in results shown in Fig. 4 we find that there is a highly significant difference between the association of Cdc2 with cyclin B1 between the wild-type and p21-null cells exposed to nocodazole. The association between Cdc2 and cyclin B1 appears to correlate very well with the observed kinase activity. We further find that Cdc2 phosphorylated on Y15 binds poorly with cyclin B1 in the nocodazole-exposed or aphidicolin-released cells. There is also apparently a reduction in the cellular content of Y15-phosphorylated Cdc2 over time following nocodazole exposure or aphidicolin release that appears to occur more in the wild-type cells than in p21-null cells. Although we show that either p21 or cyclin B1 interacts poorly with Y15-phosphorylated Cdc2, they appear to interact well with Thr-161-phosphorylated Cdc2. Our working model is that p21 becomes phosphorylated prior to Cdc25C activation. The phosphorylated p21 binds to cyclin B1 at a time when Cdc2 is phosphorylated on Y15 and associates poorly with the complex. Subsequent dephosphorylation of Cdc2 by Cdc25C promotes binding of Cdc2 to the p21-cyclin B1 complex, which can then become activated as a kinase.
A role for p21 in G2/M progression may possibly have been underestimated in p21-deficient mice (14). Following treatment with _N_-(phosphonacetyl)-l-aspartate (PALA), a specific inhibitor of de novo uridine biosynthesis, both p21−/− and p53−/− MEFs demonstrated a decrease in G1-arrested cells and an increase in the number of S-phase cells compared with wild-type MEFs. Interestingly, p21-null MEFs showed a significant increase in G2-phase cells following various doses of PALA, which was not observed in p53−/− MEFs (14). Deng et al. invoked a p53-dependent G2 arrest following PALA treatment in p21−/− cells. However, it can also be argued, based on the present data set, that p21 may have a positive regulatory role in the G2/M transition, and this may be independent of p53 function. Deng et al. also observed a preferential accumulation of 4N cells following Colcemid exposure of p21−/− cells, whereas p53−/− cells proceeded to endoreduplicate and accumulate 8N cells without preferential accumulation at 4N. Consistent with the interpretation of Deng et al., our results show that when a p53 checkpoint is triggered by DNA damage in G2/M, there is no evident hyperphosphorylation of p21, consistent with a negative regulatory function (Fig. 1). However, during cell cycle progression, in the absence of DNA damage, p21 appears to be promoting G2/M progression. In the presence of both DNA damage and spindle damage, cells appear to arrest early, at the S-G2 phase (data not shown), compared with microtubule damaged cells, which arrest at prometaphase, i.e., the nocodazole-treated cells (in the absence of DNA damage) proceed further, possibly due to the activity of phosphorylated p21. A potential contribution of hyperphosphorylated p21 in the mitotic spindle checkpoint needs to be explored further. It is clear that in a p53-deficient background in human cells, activation of the mitotic spindle checkpoint does not result in G2 arrest with 4N DNA content (7, 14), even though p21 becomes hyperphosphorylated (present studies). It has been demonstrated that p21 inhibition of cyclin E-CDK2 can prevent subsequent endoreduplication after mitotic disruption (67), although a role for later events in the cell cycle involving cyclin A-CDK2 and hyperphosphorylation of p21 has not been described. It is interesting, and perhaps relevant here, that deregulated expression of cyclin E but not cyclin A has been associated with chromosomal instability (66).
A number of open questions remain regarding the normal function of phosphorylated p21. The hyperphosphorylation of p21 may lead to a decrease in its half-life compared to the hypophosphorylated protein. However, it is possible that p21 might be dephosphorylated in a regulated manner during G2/M progression. Thus, it will be important in the future to examine the potential contribution of ubiquitination and degradation of p21 or dephosphorylation of p21 during G2/M progression or mitotic checkpoint activation. Similarly, further analysis is required to further understand the coordinated effects of p21 in S-G2 progression, particularly in between the late S-phase and early G2-phase events. Generating phospho-specific antibodies against Thr 57 of p21 and its analysis in tumor tissue might provide insight into tissue-specific or aberrant expression in tumors and may allow for colocalization with other mitotic markers. Further studies are required to better understand the coordinate regulation of p21 expression, subcellular localization, and function by various kinases that can phosphorylate p21 in normal or pathological states. Additionally, how p21 dephosphorylation or ubiquitination leads to G2/M exit and how this occurs relative to cyclin B1 and cyclin A degradation and function have to be established. Thus, while many questions are raised and suggested, the present studies provide important novel insights into the molecular events allowing positive control of G2/M cell cycle progression by p21 through specific phosphorylation at G2/M.
Supplementary Material
[Supplemental material]
Acknowledgments
We thank David T. Dicker for excellent assistance with flow cytometry.
This work was supported in part by funds from the Howard Hughes Medical Institute and NIH grants to W.S.E.-D.
Footnotes
REFERENCES
- 1.Adams, P. D., W. R. Sellers, S. K. Sharma, A. D. Wu, C. M. Nalin, and W. G. Kaelin, Jr. 1996. Identification of a cyclin-Cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol. 16:6623-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agami, R., and R. Bernards. 2000. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102**:**55-66. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama, T., T. Yoshida, T. Tsujita, M. Shimizu, T. Mizukami, M. Okabe, and S. Akinaga. 1997. G1 phase accumulation induced by UCN-01 is associated with dephosphorylation of Rb and CDK2 proteins as well as induction of CDK inhibitor p21/Cip1/Waf1/Sdi1 in p53-mutated human epidermoid carcinoma A431 cells. Cancer Res. 57**:**1495-1501. [PubMed] [Google Scholar]
- 4.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, and K. Fukumuro. 1999. Apoptosis inhibitory activity of cytoplasmic p21 Cip1/Waf1 in monocytic differentiation. EMBO J. 18**:**1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brugarolas, J., C. Chandrasekaran, J. I. Gordon, D. Beach, T. Jacks, and G. J. Hannon. 1995. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377**:**552-557. [DOI] [PubMed] [Google Scholar]
- 6.Bunz, F., A. A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy. K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282**:**1497-1501. [DOI] [PubMed] [Google Scholar]
- 7.Burns, T. F., P. Fei, K. A. Scata, D. T. Dicker, and W. S. El-Deiry. 2003. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (Taxol)-exposed cells. Mol. Cell. Biol. 16**:**5556-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayrol, C., and B. Ducommun. 1998. Interaction of cyclin-dependent kinases and PCNA modulates proteasome-dependent degradation of p21. Oncogene 17**:**2437-2444. [DOI] [PubMed] [Google Scholar]
- 9.Chan, T. A., H. Hermeking, C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1999. 14-3-3 sigma is required to prevent mitotic catastrophe after DNA damage. Nature 401**:**616-620. [DOI] [PubMed] [Google Scholar]
- 10.Chan, T. A., P. M. Hwang, H. Hermeking, K. W. Kinzler, and B. Vogelstein. 2000. Cooperative effects of genes controlling the G2/M checkpoint. Genes Dev. 14**:**1584-1588. [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, J., P. K. Jackson, M. W. Kirschner, and A. Dutta. 1995. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 374**:**386-388. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J., R. Peters, R. Saha, P. Lee, A. Theodoras, M. Pagano, G. Wagner, and A. Dutta. 1996. A 39 amino acid fragment of the cell cycle regulator p21 is sufficient to bind PCNA and partially inhibit DNA replication in vivo. Nucleic Acids Res. 24**:**1727-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21 Cip1 and p27 Kip1 CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18**:**1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng, C., P. Zhang, J. W. Harper, S. J. Elledge, and P. Leder. 1995. Mice lacking p21Cip1/Waf1 undergo normal development but are defective in G1 checkpoint control. Cell 82**:**675-684. [DOI] [PubMed] [Google Scholar]
- 15.Dulic, V., G. H. Stein, D. F. Far, and S. I. Reed. 1998. Nuclear accumulation of p21 Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol. Cell. Biol. 18**:**546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Deiry, W. S. 1998. p21/p53, cellular growth control and genomic integrity. Curr. Top. Microbiol. Immunol. 227**:**121-137. [DOI] [PubMed] [Google Scholar]
- 17.El-Deiry, W. S. 2001. Akt takes centre stage in cell-cycle deregulation. Nat. Cell Biol. 3**:**E71-E73. [DOI] [PubMed] [Google Scholar]
- 18.El-Deiry, W. S., T. Tokino, V. E. Velcutescu, D. B. Levy, R. Parsous, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. Waf1, a potential mediator of p53 tumor suppression. Cell 75**:**817-825. [DOI] [PubMed] [Google Scholar]
- 19.Erhardt, J. A., and R. N. Pittman. 1997. p21 Waf1 induces permanent growth arrest and enhances differentiation, but does not alter apoptosis in PC12 cells. Oncogene 16**:**443-451. [DOI] [PubMed] [Google Scholar]
- 20.Fiol, C. J., A. M. Mahrenholz, Y. Wang, R. W. Roeske, and P. J. Roach. 1987. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J. Biol. Chem. 262**:**14042-14048. [PubMed] [Google Scholar]
- 21.Fisk, H. A., and M. Winey. 2001. The mouse Mps1p-like kinase regulates centrosome duplication. Cell 106**:**95-104. [DOI] [PubMed] [Google Scholar]
- 22.Flores-Rozas, H., Z. Kelman, F. B. Dean, Z. Q. Pan, J. W. Harper, S. J. Elledge, M. O'Donnell, and J. Hurwitz. 1994. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzyme. Proc. Natl. Acad. Sci. USA 91:8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fotedar, R., P. Fitzgerald, T. Rousselle, D. Cannella, M. Doree, H. Messier, and A. Fotedar. 1996. p21 contains independent binding sites for cyclin and cdk2: both sites are required to inhibit cdk2 kinase activity. Oncogene 12**:**2155-2164. [PubMed] [Google Scholar]
- 24.Furstenthal, L., B. K. Kaiser, C. Swanson, and P. K. Jackson. 2001. Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication. J. Cell Biol. 152**:**1267-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glotzer, M., A. W. Murray, and M. W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature 349**:**32-138. [DOI] [PubMed] [Google Scholar]
- 26.Guadagno, T. M., and J. W. Newport. 1996. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell 84**:**73-82. [DOI] [PubMed] [Google Scholar]
- 27.Gulbis, J. M., Z. Kelman, J. Hurwitz, M. O'Donnell, and J. Kuriyan. 1996. Structure of the C-terminal region of p21 Waf1/Cip1 complexed with human PCNA. Cell 87:297-306. [DOI] [PubMed] [Google Scholar]
- 28.Hall, C., D. M. Nelson, X. F. Ye, K. Baker, J. A. DeCaprio, S. Seeholzer, M. Lipinski, and P. D. Adams. 2001. HIRA, the human homologue of yeast Hir1p and Hir2p, is a novel cyclin-cdk2 substrate whose expression blocks S-phase progression. Mol. Cell. Biol. 21**:**1854-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall-Jackson, C. A., D. A. E. Cross, N. Morrice, and C. Smythe. 1999. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene 18**:**6707-6713. [DOI] [PubMed] [Google Scholar]
- 30.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75**:**805-816. [DOI] [PubMed] [Google Scholar]
- 31.Harper, J. W., S. J. Elledge, K. Keyomarsi, B. Dyndacht, L. H. Tsai, P. Zhang, S. Dobrowolski, C. Bai, L. Connell-Crowley, E. Swindell, M. P. Fox, and N. Wei. 1995. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell. 6**:**387-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartley, R. S., R. E. Rempel, and J. L. Maller. 1996. In vivo regulation of the early embryonic cell cycle in Xenopus. Dev. Biol. 173**:**408-419. [DOI] [PubMed] [Google Scholar]
- 33.Hengst, L., U. Gopfert, H. A. Lashuel, and S. I. Reed. 1998. Complete inhibition of Cdk/cyclin by one molecule of p21 cip1. Genes Dev. 12**:**3882-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann, I., G. Draetta, and E. Karsenti. 1994. Activation of the phosphatase activity of human cdc25A by a CDK2 cyclin-E dependent phosphorylation at the G(1)/S transition. EMBO J. 13**:**4302-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu, B., J. Mitra, S. van den Heuvel, and G. H. Enders. 2001. S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol. Cell. Biol. 21**:**2755-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izumi, T., and J. L. Maller. 1995. Phosphorylation and activation of the Xenopus cdc25 phosphatase in the absence of cdc2 and CDK2 kinase-activity. Mol. Biol. Cell 6**:**215-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaumot, M., J. M. Estanol, O. Casanovas, X. Grana, N. Agell, and O. Bachs. 1997. The cell cycle inhibitor p21(CIP) is phosphorylated by cyclin A-CDK2 complexes. Biochem. Biophys. Res. Commun. 241**:**434-438. [DOI] [PubMed] [Google Scholar]
- 38.Jin, Z., D. T. Dicker, and W. S. El-Deiry. 2002. Enhanced sensitivity of G1 arrested human cancer cells suggests a novel therapeutic strategy using a combination of simvastatin and TRAIL. Cell cycle 1**:**82-89. [PubMed] [Google Scholar]
- 39.Kaneko, Y., N. Watanabe, H. Morisaki, H. Akita, A. Fujimoto, K. Tominaga, M. Terasawa, A. Tachibana, K. Ikeda, and M. Nakanishi. 1999. Cell cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene 18**:**3673-3681. [DOI] [PubMed] [Google Scholar]
- 40.King, R. W., R. J. Deshaies, J. M. Peters, and M. W. Kirschner. 1996. How proteolysis drives the cell cycle. Science 274**:**1652-1659. [DOI] [PubMed] [Google Scholar]
- 41.Kriwacki, R. W., L. Hengst, L. Tennant, S. I. Reed, and P. E. Wright. 1996. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc. Natl. Acad. Sci. USA 93**:**11504-11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11**:**847-862. [DOI] [PubMed] [Google Scholar]
- 43.Li, R., S. Waga, G. J. Hannon, D. Beach, and B. Stillman. 1994. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature 371**:**534-537. [DOI] [PubMed] [Google Scholar]
- 44.Li, R., G. J. Hannon, D. Beach, and B. Stillman. 1996. Subcellular distribution of p21 and PCNA in normal and repair-deficient cells following DNA damage. Curr. Biol. 6**:**189-199. [DOI] [PubMed] [Google Scholar]
- 45.Li, Y., C. W. Jenkins, M. A. Nichols, and Y. Xiong. 1994. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene 9**:**2261-2268. [PubMed] [Google Scholar]
- 46.Luo, Y., J. Hurwitz, and J. Massague. 1995. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21 cip1. Nature 375**:**159-161. [DOI] [PubMed] [Google Scholar]
- 47.Macleod, K. F., N. Sherry, G. Hannon, D. Beach, T. Tokino, K. Kinzler, B. Vogelstein, and T. Jacks. 1995. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 9**:**935-944. [DOI] [PubMed] [Google Scholar]
- 48.Morgan, D. O. 1995. Principles of CDK regulation. Nature 374**:**131-134. [DOI] [PubMed] [Google Scholar]
- 49.Morgan, D. O. 1997. Cyclin dependent kinases; engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13**:**261-291. [DOI] [PubMed] [Google Scholar]
- 50.Morgan, D. O. 1999. Regulation of the APC and the exit from mitosis. Nat. Cell Biol. 2**:**E47-E53. [DOI] [PubMed] [Google Scholar]
- 51.Nigg, E. A. 2001. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2**:**21-32. [DOI] [PubMed] [Google Scholar]
- 52.O'Conner, P. M., D. K. Ferris, M. Pagano, G. Draetta, J. Pines, T. Hunter, D. L. Longo, and K. W. Kohn. 1993. G2 delay induced by nitrogen mustard in human cells affects cyclin A/xsk2 and cyclin B1/cdc2-kinase complexes differently. J. Biol. Chem. 268**:**8298-8308. [PubMed] [Google Scholar]
- 53.Pandita, T. K., H. B. Lieberman, D.-S. Lim, S. Dhar, W. Zheng, Y. Taya, and M. B. Kastan. 2000. Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene 19**:**1386-1391. [DOI] [PubMed] [Google Scholar]
- 54.Parker, S. B., G. Eichele, P. Zhang, A. Rawls, A. T. Sands, A. Bradley, E. N. Olson, J. W. Harper, and S. J. Elledge. 1995. p53-independent expression of p21 Cip1 in mouse and other terminally differentiated cells. Science 267**:**1024-1027. [DOI] [PubMed] [Google Scholar]
- 55.Pines, J. 1999. Four-dimensional control of the cell cycle. Nat. Cell Biol. 1**:**E73—E79. [DOI] [PubMed] [Google Scholar]
- 56.Roberts, J. M. 1999. Evolving ideas about cyclins. Cell 98**:**129-132. [DOI] [PubMed] [Google Scholar]
- 57.Rosenblatt, J., Y. Gu, and D. O. Morgan,. 1992. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc. Natl. Acad. Sci. USA 89**:**2824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossig, L., C. Badorff, Y. Holzmann, A. M. Zeiher, and S. Dimmeler. 2002. Glycogen synthase kinase-3 couples Akt-dependent signaling to the regulation of p21Cip1 degradation. J. Biol. Chem. 277**:**9684-9689. [DOI] [PubMed] [Google Scholar]
- 59.Sarcevic, B., A. Mawson, R. T. Baker, and R. L. Sutherland. 2002. Regulation of the ubiquitin-conjugating enzyme hHR6A by CDK-mediated phosphorylation. EMBO J. 21**:**2009-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarkaria, J. N., S. T. Randal, E. C. Busby, A. P. Kennedy, D. E. Hill, and R. T. Abraham. 1998. Inhibition of phosphoinositides 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 58**:**4375-4382. [PubMed] [Google Scholar]
- 61.Schulman, B. A., D. L. Lindstrom, and E. Harlow. 1998. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc. Natl. Acad. Sci. USA 95**:**10453-10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott, M. T., N. Morrice, and K. L. Ball. 2000. Reversible phosphorylation at the C-terminal regulatory domain of p21 waf1/Cip1 modulates proliferating cell nuclear antigen binding. J. Biol. Chem. 275**:**11529-11537. [DOI] [PubMed] [Google Scholar]
- 63.Sherr, C. J. 1993. Mammalians G1 cyclins. Cell 73**:**1059-1065. [DOI] [PubMed] [Google Scholar]
- 64.Sherr, C. J. 1994. G1 phase progression: cycling on cue. Cell 79**:**551-555. [DOI] [PubMed] [Google Scholar]
- 65.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13**:**1501-1512. [DOI] [PubMed] [Google Scholar]
- 66.Spruck, C. H., K.-A. Won, and S. I. Reed. 1999. Deregulated cyclin E induces chromosome instability. Nature 401**:**297-300. [DOI] [PubMed] [Google Scholar]
- 67.Stewart, Z. A., S. D. Leach, and J. A. Pietenpol. 1999. p21 (Waf1/Cip1) inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol. Cell. Biol. 19**:**205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toyoshima-Morimoto, F., E. Taniguchi, N. Shinya, A. Iwamatsu, and E. Nishida. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410**:**215-220. [DOI] [PubMed] [Google Scholar]
- 69.Waga, S., G. J. Hannon, D. Beach, and B. Stillman. 1994. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369**:**574-578. [DOI] [PubMed] [Google Scholar]
- 70.Waga, S., and B. Stillman. 1998. Cyclin-dependent kinase inhibitor p21 modulates the DNA primer-template recognition complex. Mol. Cell. Biol. 18**:**4177-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waldmann, T., C. Lengauer, K. Kinzler, and B. Vogelstein. 1996. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 381**:**713-716. [DOI] [PubMed] [Google Scholar]
- 72.Warbrick, E. 1998. PCNA binding through a conserved motif. BioEssays 20**:**195-199. [DOI] [PubMed] [Google Scholar]
- 73.Warbrick, E., D. P. Lane, D. M. Glover, and L. S. Cox. 1995. A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21 waf1 and proliferating cell nuclear antigen. Curr. Biol. 5**:**275-282. [DOI] [PubMed] [Google Scholar]
- 74.Wei, Y., H. P. Jin, and J. W. Harper. 2003. The cyclin E/Cdk2 substrate and Cajal body component p220 (NPAT) activates histone transcription through a novel LisH-Like domain. Mol. Cell. Biol. 23**:**3669-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong, Y., G. J. Hannon, H. Zhang, D. Casso, R. Kobayashi, and D. Beach. 1993. p21 is a universal inhibitor of cyclin kinases. Nature 366**:**701-704. [DOI] [PubMed] [Google Scholar]
- 76.Xiong, Y., H. Zhang, and D. Beach. 1992. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 71**:**505-514. [DOI] [PubMed] [Google Scholar]
- 77.Ye, X., Y. Wei, G. Nalepa, and J. W. Harper. 2003. The cyclin E/Cdk2 substrate p220 (NPAT) is required for S-phase entry, histone gene expression, and Cajal body maintenance in human somatic cells. Mol. Cell. Biol. 23**:**8586-8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, H., Y. Xiong, and D. Beach. 1993. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol. Biol. Cell. 4**:**897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, H., G. J. Hannon, and D. Beach. 1994. p21-containing cyclin kinases exists in both active and inactive states. Genes Dev. 8**:**1750-1758. [DOI] [PubMed] [Google Scholar]
- 80.Zhang, H., G. J. Hannon, D. Casso, and D. Beach. 1994. p21 is a component of active cell cycle kinases. Cold Spring Harbor Symp. Quant. Biol. 59**:**21-29. [DOI] [PubMed] [Google Scholar]
- 81.Zhou, B.-B. S., P. Chaturvedi, K. Spring, K., S. P. Scott, R. A. Johnson, R. Mishra, M. R. Mattern, J. D. Winkler, and K. K. Khanna. 2000. Caffeine abolishes the mammalian G2/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J. Biol. Chem. 275**:**10342-10348. [DOI] [PubMed] [Google Scholar]
- 82.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M.-H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21 Cip1/Waf1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3**:**245-251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]