Partial penetrance facilitates developmental evolution in bacteria (original) (raw)
. Author manuscript; available in PMC: 2010 Jan 23.
Published in final edited form as: Nature. 2009 Jul 5;460(7254):510–514. doi: 10.1038/nature08150
Abstract
Development normally occurs similarly in all individuals within an isogenic population, but mutations often affect the fate of individual organisms differently1-4. This phenomenon, known as partial penetrance, has been observed in diverse developmental systems. However, it remains unclear how the underlying genetic network specifies the set of possible alternative fates and how the relative frequencies of these fates evolve5-8. Here, we identify a stochastic cell fate determination process that operates in Bacillus subtilis sporulation mutants and show how it allows genetic control of the penetrance of multiple fates. Mutations in an inter-compartmental signaling process generate a set of discrete alternative fates not observed in wild-type cells, including rare formation of two viable “twin” spores, rather than one within a single cell. By genetically modulating chromosome replication and septation, we could systematically tune the penetrance of each mutant fate. Furthermore, signaling and replication perturbations synergize to dramatically increase the penetrance of twin sporulation. These results suggest a potential pathway for developmental evolution between monosporulation and twin sporulation through states of intermediate twin penetrance. Furthermore, time-lapse microscopy of twin sporulation in wild-type Clostridium oceanicum showed a strong resemblance to twin sporulation in these B. subtilis mutants9,10. Together the results suggest that noise can facilitate developmental evolution by enabling the initial expression of discrete morphological traits at low penetrance, and allowing their stabilization by gradual adjustment of genetic parameters.
Under nutrient limited conditions, an individual B. subtilis cell can develop into a resilient dormant spore11. Many sporulation mutations reduce the fraction of cells that sporulate successfully (Fig. 1a,b).4,12-14 This makes sporulation an ideal model system to study the origins and impact of partial penetrance.
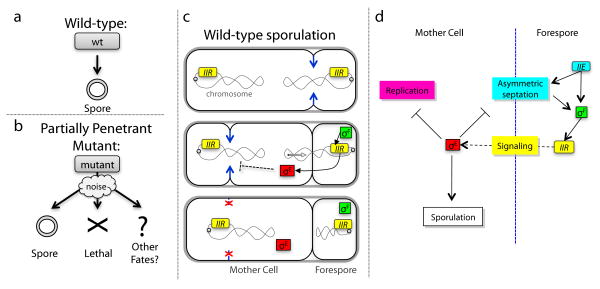
Figure 1. Partial penetrance in the developmental process of sporulation.
(a) In wild-type sporulation each sporulating cell produces a single spore. (b) Partially penetrant mutants exhibit a mixture of normal sporulation, lethal failures (‘X’) and alternative viable fates (‘?’) due to cellular fluctuations (cloud). (c,d) Schematic illustrations of events (c) and genetic interactions (d) leading to differentiation of the mother cell and forespore compartments (see text).
At the onset of sporulation, B. subtilis cells divide asymmetrically into smaller (forespore) and larger (mother-cell) compartments. Septation leads to the forespore-specific activation of the transcriptional regulator σF (Fig. 1c,d)11. σF in turn activates expression of spoIIR, which initiates an inter-compartmental signaling cascade that activates the mother cell-specific regulator σE, causing mother cell differentiation11,15. Deleting σE allows a second asymmetric septum to form, resulting in ‘abortively disporic’ cells with two DNA-containing immature forespores, and a mother cell devoid of DNA16,17. Attenuation of spoIIR expression results in a partially penetrant mixture of successfully sporulating and abortively disporic cells12,13.
To explore the effects of spoIIR mutations on sporulation penetrance, we constructed a set of strains, collectively denoted as spoIIRPP mutants, where the rate and/or the time of onset of spoIIR expression is specifically perturbed (supplementary methods and Fig. S1-S4). We characterized spoIIRPP mutants by time-lapse imaging of cells expressing fluorescent reporters for σF and σE activity (Methods, Fig. S1 and supp. Mov. 1). These movies revealed a diverse set of discrete cell fates (Fig. 2), whose relative frequencies depended on the type and severity of the perturbation to spoIIR expression. In all of these mutants, after an initial asymmetric septation and activation of σF in the forespore, mother cells exhibited one of three ‘primary’ fates (Fig. 2a-c). One population of cells activated σE and continued to sporulate normally (Fig. 2a). Another population formed the abortively disporic morphology (Fig. 2b). In the third population, neither σE activation nor asymmetric septation was observed (Fig. S5). In these cells, the activated forespores did not develop further, but the mother cells continued to grow in a process we term sporulation ‘escape’ (Figs. 2c, S5 and methods)11,18.
Figure 2. Time-lapse movies reveal alternative developmental pathways in spoIIRPP signaling mutants.
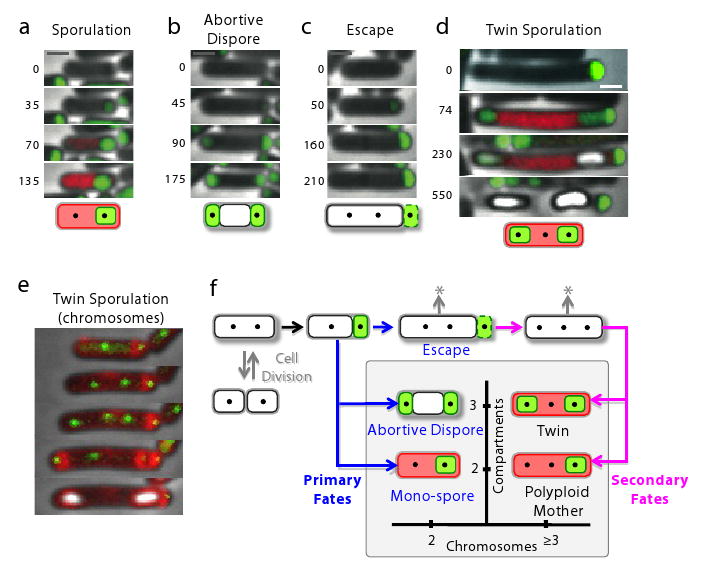
(a-d) Green and red represent fluorescent protein expression from σF and σE-dependent promoters, respectively, overlaid on phase contrast images (gray). Developing forespores appear white at late times. Times indicated in minutes from σF activation. (a) Normal sporulation. (b) Abortively disporic cells. (c) Escaping cells activate σF but continue to elongate without activating σE (Fig. S5). Note that the activated forespore (right) does not develop further. (d) Twin sporulation occurs after escape. Green fluorescence at the initial time-point is a remnant of escape from the previous sporulation attempt. (e) Chromosome over-replication occurs prior to the formation of twin forespores. TetR-GFP-tagged chromosomal loci appear as green “dots”. Membrane staining (red) shows septation events. The rightmost dot is the remnant from a previous escape. (f) Schematic diagram showing the temporal sequence of events leading to observed terminal fates, which are classified by the numbers of chromosomes (x-axis) and compartments (y-axis). * indicates potential for return to vegetative division and/or additional sporulation attempt. Scale bar, 1μm.
Escape enabled the formation of an additional cell type. About ∼25% of the time, escaping cells immediately re-initiated sporulation. In ∼5% of such cases two new forespores developed sequentially (∼20 min apart) within the same mother cell (Figs. 2d, S6). Unlike abortively disporic cells, these “twin” sporulating cells completed sporulation, producing two mature viable spores in a single mother cell compartment (Fig. S7). Twins exhibited the transcriptional and morphological hallmarks of proper sporulation (Figs. 2d and S8). Twin spores germinated properly, and were UV-resistant (Fig. S9). Similar morphologies (called ‘bipolar’) appear to have evolved independently many times in the class Clostridia9,10, whose sporulation pathway is homologous to Bacillus19, but have not been observed in Bacillus itself (Fig. S10). Twin sporulation may be adaptive under some conditions, including when vegetative growth is inhibited and proliferation occurs principally by sporulation10,20.
The ability of cells to form twins is surprising because only two chromosome copies are present during normal sporulation11, while twins require at least three. Therefore, we tracked the number and cellular compartment of chromosomes tagged with TetR-GFP “dots” bound to a cassette of chromosomally integrated tetO operators (Figs. 2e, S11)21,22. We found that 30% of ‘escaping’ spoIIR mutant cells over-replicated to produce 3 or more chromosomal dots within a single cell. 15% of these cells then underwent two consecutive asymmetric septation events without additional replication, producing twins. Because spoIIRPP mutations cannot affect chromosome number prior to the initial septation event (when spoIIR is first expressed), this result explains why twins only formed as secondary fates in spoIIRPP mutants. Furthermore, some of these over-replicating (polyploid) cells sporulated normally (to produce a single spore) despite the presence of extra chromosomal copies in the mother-cell (see Figs. 2f, S11).
What determines the fate of an individual cell within a clonal mutant population? Because spoIIR mutations reveal alternative fates, and because sporulation penetrance is directly linked to the strength of spoIIR mutations12,13 (Fig S3,S4), fluctuations in spoIIR expression represent the most direct candidate for fate determination. To test this hypothesis, we constructed a specific spoIIRPP strain, called spoIIRPP-CY (Fig S2), where spoIIR expression is reduced and delayed by ∼10 min, and its expression can be monitored using a co-transcribed yfp reporter. The onset of spoIIR expression can be compared in the same cell to that of another, non-delayed, σF-dependent promoter controlling cfp expression (Figs. 3a,S2). We observed variation of 5 minutes in the timing and ∼56% in the rate of spoIIR expression (n=148 cells, inset, Fig. 3b). However, spoIIR expression rate fluctuations explained only ∼15% of the decision between sporulation and other fates (based on Relative Mutual Information, p<10-4). Variation in the expression delay had weaker explanatory power. Thus, fluctuations directly related to the genetic perturbation in spoIIR only partially account for cell fate. Much of the fate decision is apparently determined by other fluctuations, whose effects are revealed when spoIIR expression is attenuated.
Figure 3. Noise and gene expression control cell fate in a hierarchical fashion.
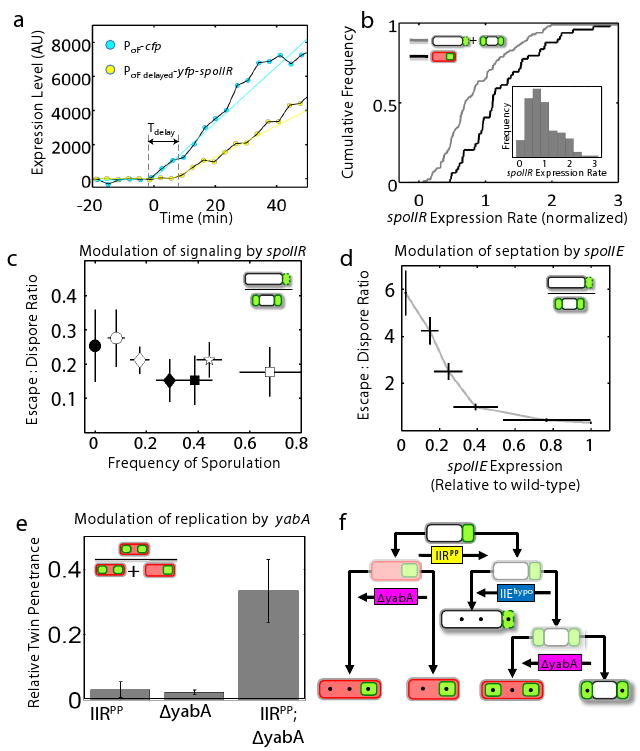
(a) Time traces indicating delay (arrow) and reduction (slope of yellow line compared to cyan line) in spoIIR expression rate of a typical spoIIRPP-CY cell. (b) Cumulative histograms of spoIIR expression rate are shown for two sub-populations of a single spoIIRPP strain in the same microcolony (_n_=150 cells). Sporulating cells show a systematically higher level of spoIIR expression. Inset: cell-cell variability in spoIIR expression rate. (c-e) Systematic genetic manipulation of fate penetrance. Error bars (s.e) are based on three replicate experiments. (c) spoIIR expression controls the overall frequency of sporulation (x-axis) but does not systematically affect the ratio of escape cells to abortive disporics (y-axis). Points represent spoIIRPP strains differing in spoIIR expression level and delay (supplementary methods). (d) spoIIE expression level tunes the penetrance ratio of escape to abortively disporic fates (methods, Fig. S12). (e) Deletion of yabA interacts synergistically with spoIIRPP mutants to increase twin penetrance (see also Fig. S11). (f) Fate determination can be controlled hierarchically—different genes affect different decision points.
Next, we analyzed the genetic control of fate penetrance in the spoIIRPP mutants. Sporulation frequency varied from 0% in a Δ_spoIIR_ mutant to approximately 75% across the set of spoIIRPP strains. Strikingly, however, the ratio of escape and abortively disporic frequencies remained approximately constant across this range (Fig. 3c, supp. methods). This behavior suggests that two independent levels of primary cell fate determination can be distinguished: The decision between sporulating and non-sporulating fates depends on how spoIIR is perturbed, while the decision between escape and abortively disporic fates is independent of this perturbation (Fig. 3f).
Can the ratio of escape and abortively disporic frequencies also be controlled? The membrane protein SpoIIE promotes asymmetric septum formation23, and hence could promote a second asymmetric septation and thus an abortively disporic fate, over escape (Fig. 1d). To test this hypothesis, we constructed a strain, spoIIEhypo, where the level of spoIIE expression could be modulated by the inducer IPTG without affecting its temporal dynamics (Methods and Fig. S12). This strain included a Δ_spoIIR_ mutation to make the measurement independent of the additional indirect function of SpoIIE in spoIIR activation11 (Fig. 1d). As expected, the ratio of escape and abortively disporic frequencies increased as the level of spoIIE expression was reduced (Figs. 3d,S12).
To similarly increase the frequency of twin sporulation would require increasing both the number of chromosomes and the number of compartments generated during sporulation (Fig. 2f). Perturbations of spoIIR expression primarily affect compartment number, but have only a modest effect on chromosome number (through the escape state). We reasoned that mutations that increase chromosome copy number could synergize with spoIIRPP mutations, increasing the frequency of twins at the expense of abortively disporic cells. To test this possibility, we used two mutations that increase chromosome replication: a null mutation of the chromosome replication inhibitor YabA (ref. 24), and hypomorphic fusion of chromosome replication regulator Spo0J with GFP (Ref. 22) (Fig. S11). Intriguingly, these over-replication mutations generated a low penetrance of twins in the wild-type background (Fig. 3e), suggesting that they may affect inter-compartmental signaling. Combining these mutations with spoIIRPP mutants permitted twins, as well as polyploid mother cells, to occur as a primary fate, because some cells now possess additional chromosomes prior to the first asymmetric septation event (Fig S11). Finally, the over-replication mutations synergized with signaling mutations, increasing the penetrance of the twin fate to >30% of all viable sporangia, a level comparable to those observed in some natural twin-forming species25 (Fig. 3e, S11). Together, these results indicate, first, that all four terminal fates can be ‘primary’ (i.e. occur without a round of escape), and second, that the penetrance of all fates can be manipulated by specific genetic perturbations affecting the processes signaling, septation, and replication (Fig. 3f).
At the evolutionary level, partial penetrance could facilitate transitions between discrete phenotypes by enabling gradual changes in their frequencies, rather than an all-or-none switch from one phenotype to the other, which might require simultaneous changes in multiple processes. As described above, mutations that increase the probability of either additional septation or replication increase the penetrance of the maladaptive abortive dispore state or the polyploid monospore state, respectively (Figs. 2f and 3). Two conditions would thus facilitate the gradual evolution of twin sporulation: First, a relatively high sporulation efficiency for the polyploid monospore state would allow sequential accumulation of mutations that favor over-replication to be followed by additional mutations that affect septation. In fact, we found that polyploid cells carry a fitness cost far smaller than abortive dispores, completing sporulation successfully about 75% as often as normal sporulating cells (Fig. S11). Thus, there does not appear to be any fundamental obstacle to high fitness for this state. Second, mutations that cause correlation between replication and septation would favor the monospore and twin fates over the polyploid and abortive dispore states. Analysis of fate frequencies revealed evidence for these correlations and their genetic control: In spoIIRPP spo0J-gfp mutants, the frequency of re-septation (forming 3 compartments) differs by ∼3-fold depending on the number of chromosomes observed prior to asymmetric septation (p<10-3 Fig. 4A). This correlation enhances the penetrance of twins and reduces the penetrance of the maladaptive abortive dispore state. Neither over-replication nor re-septation is known to occur in wild-type strains; as a result, this correlation may not be under selection, and could therefore vary between backgrounds. Consistent with this, the measured interaction between spoIIRPP and yabA mutations differed significantly between two closely related strain backgrounds (Fig. 4b). Together, these results suggest that under appropriate selection for twins, B. subtilis could acquire mutations that affect septation, replication, and their correlation, and thereby enable gradual evolution of twin sporulation.
Figure 4. Evolution of twin sporulation.
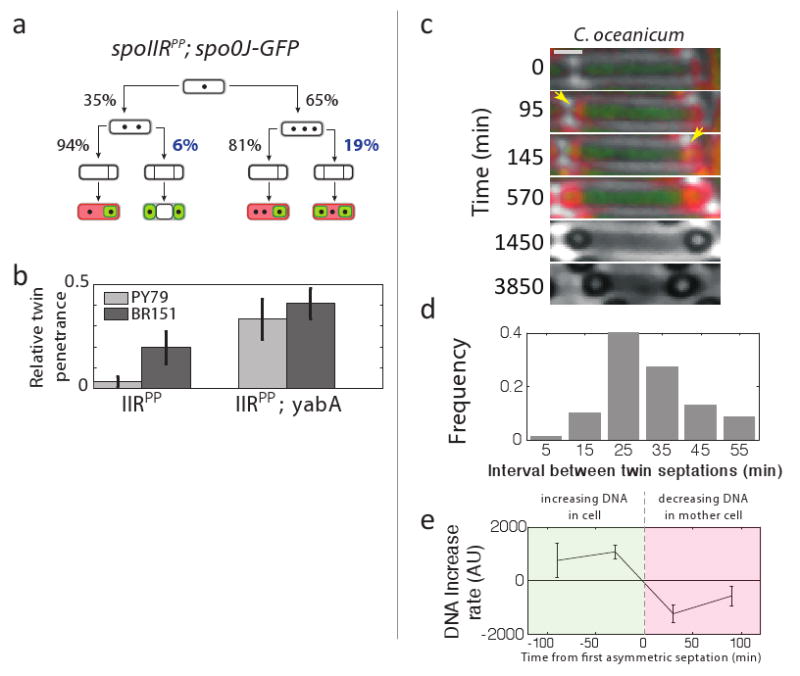
(a) Fate tree showing relative frequencies of over-replication (second row) and additional septation (third row) inferred from analysis of terminal fates (bottom row) of _n=_285 individual cells. Note that the probability of having three compartments depends on chromosome number (blue percentages). Day to day variation was ≤ 2% across all measurements. (b) Strain backgrounds PY79 (used throughout the paper) and BR151 differ in twin penetrance with the same spoIIRPP mutation (error bars, s.e., based on multiple experiments). yabA mutations reduce this difference. (c-e) Twin sporulation in C. oceanicum resembles that in B. subtilis mutants. (c) Filmstrip shows typical events during C. oceanicum sporulation (times in minutes from first frame). Shown are DNA (green), membrane staining (red), and phase contrast (gray). Yellow arrowheads mark first appearance of asymmetric septa. (d) The distribution of time intervals between two septation events during twin sporulation (_n_=70). (e) The rate of change of DNA staining was quantified in individual cells. Staining increases prior to septation (green area), consistent with chromosome replication, and decreases after septation (red area), consistent with transport of DNA into forespores. Data were averaged over _n_=30 cells due to cell-cell variability (error bars, s.e.m.).
Finally, we sought to determine whether natural twin sporulation occurs in a manner similar to that observed in B. subtilis mutants. We acquired time-lapse movies of C. oceanicum, a marine anaerobe which exhibits twin sprorulation, with fluorescent membrane and DNA probes (Movie 2)9. We observed partially penetrant mixture of fates including twins and mono-spores, but not the abortively disporic fate. Analysis of DNA content in monospores was consistent with a subpopulation of polyploid mother cells, as in B. subtilis (Fig. S13). We analyzed the temporal sequence of septation and chromosome replication events in individual cells (Fig. 4c, supp. Movie 2 and Methods). DNA staining was observed to increase prior to the first septation event, and subsequently decrease in the mother cell (Fig. 4e), consistent with replication before the first septation, and subsequent translocation of DNA into the forespores, as occurs in B. subtilis mutants. The two forespore compartments were formed by consecutive septation events separated by an interval of 30±10 minutes, slightly longer than observed in B. subtilis (Figs. 4d,S6). Together, these results suggest that the natural process of twin development is similar to that observed in the B. subtilis mutants.
The concept of developmental canalization was introduced to explain the reproducibility of wild-type development and its contrast with developmental variability in mutants26,27. However, it has been difficult to understand how canalization arises at the molecular level in specific genetic networks and, conversely, how its disruption facilitates the evolution of novel developmental programs. In this case, competition among the core processes of septation, replication, and signaling is crucial for generating discrete alternative morphologies (Fig. 3f). Mutations that increase the penetrance of twin sporulation and reduce its dependence on noise provide a gradual mechanism for the stabilization of a discrete change in developmental morphology. The ability to combine single-cell, genetic, and evolutionary analysis in bacterial developmental systems like B. subtilis sporulation may help to identify basic principles underlying the evolution of developmental mechanisms.
Methods Summary
Strains and conditions
All B. subtilis strains, unless otherwise stated, were derived from parental strain PY79 (ref 28) using standard protocols29. Details of plasmid and strain construction are described in Methods and SI Methods section. Sporulation in liquid culture was performed using the exhaustion method in MSSM12. C. oceanicum ATCC 25647 (ref 9), was obtained from the American Type Culture Collection.
Experimental procedure
B. subtilis cells were placed on agarose pads containing sporulation-inducing re-suspension medium. Time-lapse microscopy protocol is described in Methods. Exposure times were minimized to prevent photo-damage. For membrane staining 0.2-1 μg/ml FM4-64 (Invitrogen) was added to the agarose pad before adding cells, or mitotracker green (Invitrogen) was added at a concentration of 5 μg/ml. Regulated promoters of the strains spoIIRhypo and spoIIEhypo were controlled by addition of appropriate levels of IPTG to the agarose pad. C. oceanicum microscopy was similar except anaerobic conditions were maintained by a custom nitrogen flow chamber. Chromosomes were stained with Vybrant DyeCycle Green (Invitrogen).
Quantitative analysis
Quantitative movie analysis used custom image analysis code in Matlab (Mathworks Inc.), similar to that previously described30. Briefly, phase contrast or fluorescence images were segmented by edge detection to identify individual cells. Segmented cells were tracked semi-automatically from frame to frame based on position and orientation. Fluorescence was defined as the sum of pixel intensities within the area of the cell. When the same structure appears at both poles of the cell (e.g. in abortively disporic cells), fluorescence was separately calculated for the two halves of the cell. Fate frequencies were manually scored based on criteria detailed in methods section.
Supplementary Material
1
Acknowledgments
We thank Jared Leadbetter and Eric Matson for their help with the anaerobic species. We thank R. Losick, A. Grossman, M. Fujita and A. Arkin for strains and advice. We thank R. Kishony, D. Jones, Wolfgang Schwarz, G. Suel, J.-G. Ojalvo, B. Shraiman, J. Levine, J. C. W. Locke, D. Sprinzak, L. Cai, and other members of MBE and PJP labs for helpful discussions. Work in P.J.P.'s laboratory was supported by Public Health Service Grant GM43577 from the National Institutes of Health. Work in M.B.E.'s lab was supported by NIH grants R01GM079771, P50 GM068763, NSF CAREER Award 0644463 and the Packard Foundation. A.E. was supported by the International Human Frontier Science Organization and European Molecular Biology Organization.
References
- 1.Horvitz HR, Sulston JE. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics. 1980;96:435–54. doi: 10.1093/genetics/96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–24. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- 3.Sangster TA, et al. HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coote JG. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J Gen Microbiol. 1972;71:1–15. doi: 10.1099/00221287-71-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford SL, Henikoff S. Quantitative epigenetics. Nat Genet. 2003;33:6–8. doi: 10.1038/ng0103-6. [DOI] [PubMed] [Google Scholar]
- 6.Felix MA, Wagner A. Robustness and evolution: concepts, insights and challenges from a developmental model system. Heredity. 2008;100:132–40. doi: 10.1038/sj.hdy.6800915. [DOI] [PubMed] [Google Scholar]
- 7.West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci U S A. 2005;102(1):6543–9. doi: 10.1073/pnas.0501844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirschner M, Gerhart J. The Plausibility of Life: Resolving Darwin's Dilemma. Yale University Press; 2005. [Google Scholar]
- 9.Smith LD. Clostridium oceanicum, sp. n., a sporeforming anaerobe isolated from marine sediments. J Bacteriol. 1970;103:811–3. doi: 10.1128/jb.103.3.811-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angert ER. Alternatives to binary fission in bacteria. Nat Rev Microbiol. 2005;3:214–24. doi: 10.1038/nrmicro1096. [DOI] [PubMed] [Google Scholar]
- 11.Hilbert DW, Piggot PJ. Compartmentalization of Gene Expression during Bacillus subtilis Spore Formation. Microbiol Mol Biol Rev. 2004;68:234–262. doi: 10.1128/MMBR.68.2.234-262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khvorova A, Chary VK, Hilbert DW, Piggot PJ. The chromosomal location of the Bacillus subtilis sporulation gene spoIIR is important for its function. J Bacteriol. 2000;182:4425–9. doi: 10.1128/jb.182.16.4425-4429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zupancic ML, Tran H, Hofmeister AE. Chromosomal organization governs the timing of cell type-specific gene expression required for spore formation in Bacillus subtilis. Mol Microbiol. 2001;39:1471–81. doi: 10.1046/j.1365-2958.2001.02331.x. [DOI] [PubMed] [Google Scholar]
- 14.Piggot PJ, Coote JG. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–62. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karow ML, Glaser P, Piggot PJ. Identification of a gene, spoIIR, that links the activation of sigma E to the transcriptional activity of sigma F during sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1995;92:2012–6. doi: 10.1073/pnas.92.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichenberger P, Fawcett P, Losick R. A three-protein inhibitor of polar septation during sporulation in Bacillus subtilis. Mol Microbiol. 2001;42:1147–1162. doi: 10.1046/j.1365-2958.2001.02660.x. [DOI] [PubMed] [Google Scholar]
- 17.Pogliano J, et al. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol Microbiol. 1999;31:1149–59. doi: 10.1046/j.1365-2958.1999.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dworkin J, Losick R. Developmental commitment in a bacterium. Cell. 2005;121:401–9. doi: 10.1016/j.cell.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Paredes CJ, Alsaker KV, Papoutsakis ET. A comparative genomic view of clostridial sporulation and physiology. Nat Rev Micro. 2005;3:969–978. doi: 10.1038/nrmicro1288. [DOI] [PubMed] [Google Scholar]
- 20.Angert ER, Losick RM. Propagation by sporulation in the guinea pig symbiont Metabacterium polyspora. Proc Natl Acad Sci U S A. 1998;95:10218–23. doi: 10.1073/pnas.95.17.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dworkin J, Losick R. Does RNA polymerase help drive chromosome segregation in bacteria? Proc Natl Acad Sci U S A. 2002;99:14089–94. doi: 10.1073/pnas.182539899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PS, Lin DC, Moriya S, Grossman AD. Effects of the chromosome partitioning protein Spo0J (ParB) on oriC positioning and replication initiation in Bacillus subtilis. J Bacteriol. 2003;185:1326–37. doi: 10.1128/JB.185.4.1326-1337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Yehuda S, Losick R. Asymmetric cell division in B. subtilis involves a spiral-like intermediate of the cytokinetic protein FtsZ. Cell. 2002;109:257–66. doi: 10.1016/s0092-8674(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 24.Noirot-Gros MF, et al. Functional dissection of YabA, a negative regulator of DNA replication initiation in Bacillus subtilis. Proc Natl Acad Sci U S A. 2006;103:2368–73. doi: 10.1073/pnas.0506914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keis S, Shaheen R, Jones DT. Emended descriptions of Clostridium acetobutylicum and Clostridium beijerinckii, and descriptions of Clostridium saccharoperbutylacetonicum sp. nov. and Clostridium saccharobutylicum sp. nov. Int J Syst Evol Microbiol. 2001;51:2095–103. doi: 10.1099/00207713-51-6-2095. [DOI] [PubMed] [Google Scholar]
- 26.Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 27.Rutherford S, Hirate Y, Swalla BJ. The Hsp90 capacitor, developmental remodeling, and evolution: the robustness of gene networks and the curious evolvability of metamorphosis. Crit Rev Biochem Mol Biol. 2007;42:355–72. doi: 10.1080/10409230701597782. [DOI] [PubMed] [Google Scholar]
- 28.Youngman P, Perkins JB, Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]
- 29.Harwood CR, Cutting SM. Molecular biological methods for Bacillus. Chichester; New York: Wiley; 1990. [Google Scholar]
- 30.Rosenfeld N, Young JW, Alon U, Swain PS, Elowitz MB. Gene regulation at the single-cell level. Science. 2005;307:1962–5. doi: 10.1126/science.1106914. [DOI] [PubMed] [Google Scholar]
- 31.Suel GM, Garcia-Ojalvo J, Liberman LM, Elowitz MB. An excitable gene regulatory circuit induces transient cellular differentiation. Nature. 2006;440:545–50. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- 32.Suel GM, Kulkarni RP, Dworkin J, Garcia-Ojalvo J, Elowitz MB. Tunability and noise dependence in differentiation dynamics. Science. 2007;315:1716–9. doi: 10.1126/science.1137455. [DOI] [PubMed] [Google Scholar]
- 33.Becker EC, Pogliano K. Cell-specific SpoIIIE assembly and DNA translocation polarity are dictated by chromosome orientation. Mol Microbiol. 2007;66:1066–79. doi: 10.1111/j.1365-2958.2007.05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeigler DR, et al. The origins of 168, W23, and other Bacillus subtilis legacy strains. J Bacteriol. 2008;190:6983–95. doi: 10.1128/JB.00722-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1