Antitumor activity of targeting Src kinases in endothelial and myeloid cell compartments of the tumor microenvironment (original) (raw)
. Author manuscript; available in PMC: 2011 Feb 1.
Abstract
Purpose
Several Src family kinase (SFK) inhibitors have entered clinical trials based on their direct effects against tumor cells. Here we characterize the effects of targeting Src kinases on the tumor microenvironment and how these effects influence tumor growth.
Experimental Design
Human cancer cells grown in cell culture or in mice were treated with dasatinib, a small-molecule inhibitor of SFKs. Tumor cell, endothelial cell and myeloid cell compartments within the tumor microenvironment were analyzed. Primary human endothelial cells and freshly-isolated CD11b+/CD11c− myeloid cells from mice were treated with dasatinib in cell culture. Cellular functions and signaling pathways affected by dasatinib were evaluated.
Results
Dasatinib was not cytotoxic in cell culture against the human cancer cell lines investigated here. However, dasatinib administration in human tumor-bearing mice suppressed tumor growth associated with increased tumor cell apoptosis, decreased microvessel density and reduced intratumoral CD11b+ myeloid cells. Dasatinib directly inhibited motility and other functions of endothelial and myeloid cells, accompanied by inhibition of phosphorylation of SFKs and downstream signaling. Tumor-infiltrating myeloid cells were identified as the major source of MMP-9 in the tumor microenvironment. Dasatinib treatment reduced MMP-9 levels in the tumor microenvironment through simultaneous inhibition of recruitment of MMP9+ myeloid cells and MMP-9 gene expression in tumor-infiltrating myeloid cells.
Conclusions
These findings suggest that Src kinase inhibitors like dasatinib possess a previously unrecognized anti-cancer mechanism of action by targeting both host-derived endothelial and myeloid cell compartments within the tumor microenvironment.
Keywords: Src kinases, tumor microenvironment, endothelial cells, myeloid cells, cancer therapy
Introduction
Recent work has indicated that stromal cells within the tumor microenvironment undergo phenotypic and epigenetic changes during tumor initiation, progression and metastasis (1). The cross-talk between tumor cells and stromal cells, including endothelial cells, immune cells, fibroblasts and pericytes, leads to enhanced tumor growth, metastasis and altered response to anti-cancer therapy (2–4). These findings underscore the importance of developing therapeutics that target both tumor cells and the tumor microenvironment to treat cancer.
Angiogenesis, an important process in the tumor microenvironment and a valuable target for anti-cancer therapy, involves proliferation, migration and differentiation of vascular endothelial cells, modification of extracellular matrix (ECM) and recruitment of accessory cells (5–10). Multiple proangiogenic factors including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are usually found upregulated within the tumor microenvironment and associated with tumor progression (11). High levels of VEGF or bFGF sustain the activation of their cognate receptors and downstream effectors in endothelial cells, resulting in aberrant endothelial cell functions (7).
It has been well established that tumor-recruited immune cells participate in cancer development and mediate response to anti-cancer therapy (9, 12–14). Increased tumor infiltration by some immune cell subsets, such as macrophages, myeloid-derived suppressor cells (MDSCs) and neutrophils, correlates with increased angiogenesis and/or poor prognosis (13). Several lines of evidence have also indicated that tumor-infiltrating myeloid cells are important sources of proangiogenic factors such as VEGF, bFGF and MMP-9 within the tumor microenvironment (15–19).
Src family kinases (SFKs) comprise a subset of non-receptor protein tyrosine kinases that includes c-Src, Fyn, Yes, Lyn, Lck, Hck, Blk, Fgr and Yrk. It has been demonstrated that Src kinase activity and protein levels are significantly elevated in human cancers (20). Aberrant SFK function promotes tumor growth and metastasis by stimulating tumor cell proliferation, migration and invasion (21). Besides their roles in tumor cells, numerous studies have suggested that SFKs in the stromal cells can contribute to tumor growth (22–24). For example, SFKs, expressed in endothelial cells, coordinate multiple signaling pathways involved in regulating endothelial cell function (25), making SFKs attractive targets for antiangiogenic therapy.
Dasatinib (BMS-354825) was originally described as a dual kinase inhibitor of SFKs and BCR/ABL, and was later found to be a multiple kinase inhibitor of SFKs, BCR/ABL, c-kit, PDGFR and EphA2 (26–29). Dasatinib also has been shown to be an effective therapeutic for imatinib-resistant chronic myelogeneous leukemia (CML) (27). Furthermore, dasatinib has exhibited activities against some solid tumors by inducing tumor cell apoptosis, inhibiting tumor cell proliferation, and blocking tumor cell migration and invasion (30–32). Recently, it has been reported that dasatinib inhibits multiple myeloma-derived angiogenesis by inactivating the PDGFRβ and Src signaling pathway (33). However, the in vivo mechanism underlying the action of dasatinib and whether this finding can be applied to solid tumors remain to be determined. In the present study, we characterized the effects of targeting SFKs by dasatinib on distinct cellular compartments in the tumor microenvironment and how these effects influence tumor growth.
Materials and Methods
Animals and drug administration
_A_thymic nude mice (NCR-nu/nu) were obtained from Taconic (Germantown, NY). All animal studies were performed in accordance with the regulations of the Animal Resources Center of City of Hope Cancer Center. Mice were treated with dasatinib through oral gavage (p.o.) twice daily (B.I.D.). Control mice were given an equal volume of vehicle solution (propylene glycol: water, 1:1).
Tumor model
Nude mice were injected s.c. with 5×106 tumor cells. Once tumor became palpable, mice were randomly assigned to two groups and treated with vehicle solution or dasatinib for indicated days. Tumor volume was measured with a caliper every 3 or 4 d and calculated according to the formula: Tumor volume = length × (width)2 × 0.5
Immunohistology
Tumor samples were fixed in 10% formalin followed by paraffin embedding or were embedded in O.C.T. compound (Sakura Finetek) and frozen in liquid nitrogen. Paraffin-embedded tumors were sectioned and processed in COH Pathology Core. Sections were stained with anti-CD31 (1:50), anti-Ki67 (1:200), anti-p-Src (Tyr419) (1:250), anti-Src (1:250) and anti-MMP-9 (1:100) antibodies. To quantify tumor microvessel density, CD31+ vessels were counted manually from 10 randomly selected fields at x200 magnification by two observers who were blinded to the identity of the samples. The mitotic index of all cells in tumors was quantified by calculating the percentage of Ki67+ area relative to total field area using Image-J software.
Frozen tumor sections were fixed in 2% paraformaldehyde, permeabilized and stained with anti-CD31 (1:50), anti-F4/80 (1:50), anti-CD11b (1:50) and anti-MMP-9 (1:500) antibodies overnight at 4°C followed by incubation with Alexa Fluor 555 or 488 conjugated secondary antibodies (1:200; Invitrogen) for 30 min. After nuclear staining with Hoechst 33342, the slides were then examined under a Zeiss Upright LSM510 confocal microscope or a Nikon Eclipse TE2000-U fluorescence microscope. To determine apoptosis, TUNEL assay was performed using a DeadEnd™ Fluorometric TUNEL kit (Promega). The apoptotic index in tumor sections was quantified by calculating the number of apoptotic cells per field using Image-J software.
Statistical analysis
All the in vitro experiments were performed three times in duplicates or triplicates. In vivo mouse studies were repeated twice with similar results. Statistical significance of differences between control and drug treated groups was determined by a two-tailed t test. A value of p < 0.05 was considered statistically significant.
Details about cell isolation and culture conditions, reagents and antibodies, cell viability assay, cell apoptosis assay, cell migration assay, in vitro tube formation assay, cell detachment assay, chick aortic ring assay, in vivo Matrigel plug assay, siRNAs and transfection, immunoblotting and immunoprecipitation, flow cytometry analysis and real-time quantitative PCR are presented as supplementary information.
Results
Dasatinib inhibits endothelial cells but not tumor cells in culture
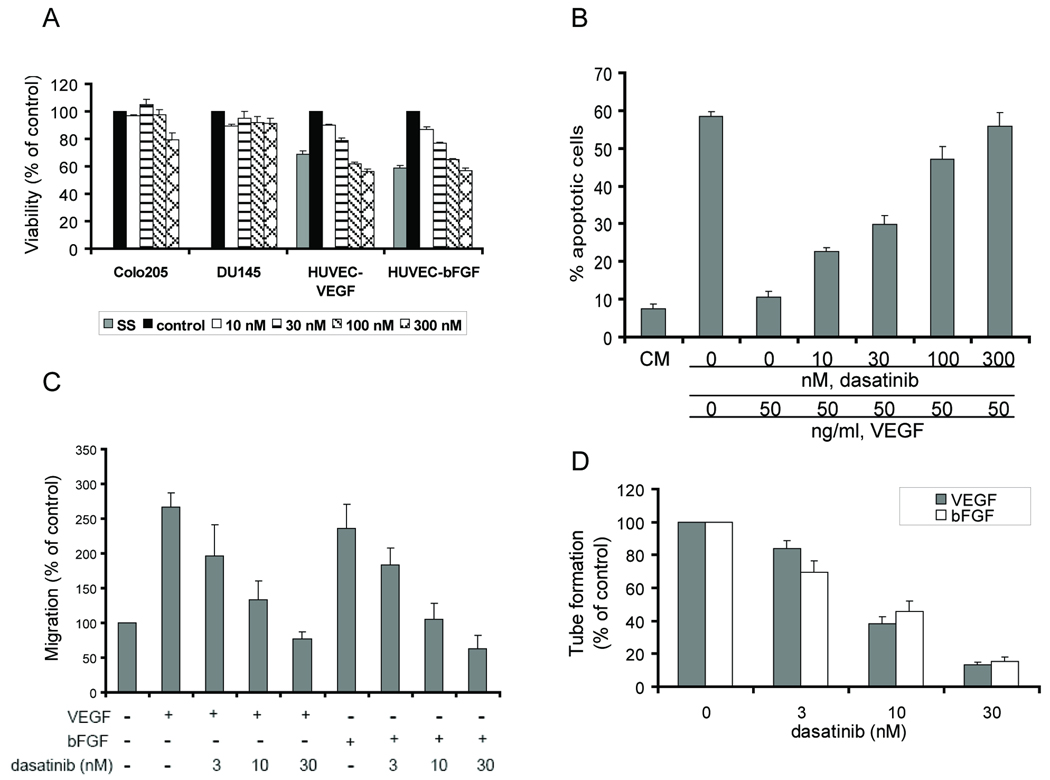
We first determined the effect of dasatinib on cell viability in vitro by using an MTS assay. After 48 h treatment, dasatinib inhibited VEGF- or bFGF-mediated HUVEC viability in a dose-dependent manner (Fig. 1_A_). In contrast, cell viability of two human cancer cell lines, DU145 and Colo205, was minimally affected at the same doses (Fig. 1_A_).
Fig. 1.
Dasatinib inhibited endothelial cell functions. A, DU145, Colo205 and HUVEC cells were treated with DMSO (control) or dasatinib for 48 h. HUVECs were serum-starved and treated with VEGF (HUVEC+VEGF) or bFGF (HUVEC+bFGF) to stimulate cell growth. Cell viability was measured by MTS assay. Average values in control (DMSO) wells were set as 100% viability. Results are expressed as mean ± SEM % of viability relative to control (n=3). SS: serum starvation. B, HUVECs were treated with DMSO or dasatinib for 48 h with or without VEGF. Cell apoptosis was measured by TUNEL assay. Results are shown as mean ± SEM % apoptotic cells (n=3). CM: complete medium. C, serum-starved HUVECs were treated with DMSO or dasatinib and allowed to migrate towards VEGF or bFGF for 6 h in a modified Boyden chamber assay. Average number of migrated cells in control (DMSO) wells was set as 100% migration. Results are expressed as mean ± SEM % of migration relative to control (n=4). D, Tube formation was quantified by counting the cord junctions of branches formed by endothelial cells. Average numbers of cord junctions in control wells (VEGF only or bFGF only) were set as 100% tube formation. Results are expressed as mean ± SEM % of tube formation relative to control (n=3).
Next, we evaluated whether dasatinib-mediated loss of endothelial cell viability was associated with induction of apoptosis. Prolonged incubation of HUVECs in low serum medium (0.1% FBS) induced profound (~60%) cell apoptosis, which could be reversed by VEGF or bFGF. Dasatinib treatment induced HUVEC apoptosis in the presence of VEGF or bFGF in a dose-dependent manner (Fig. 1_B_ and Supplementary Fig. 1_A_). At 300 nM, a clinically relevant concentration (34, 35), dasatinib treatment for 48 h inhibited more than 90% of VEGF-mediated or 50% of bFGF-mediated HUVEC survival. To confirm that dasatinib induced apoptosis, we examined the activation of caspases by immunoblot analyses. Dasatinib treatment increased the cleavage of caspase-3, caspase-7 and PARP, a substrate for both caspase-3 and -7, as early as 6 h (Supplementary Fig. 1_B_). The broad-spectrum caspase inhibitor Z-VAD-FMK, when cotreated with dasatinib, was able to reverse dasatinib-induced apoptosis, indicates that dasatinib-induced HUVEC apoptosis is caspase dependent (Supplementary Fig. 1_C_).
During dasatinib treatment, we observed concurrent loss of HUVEC adhesion along with induction of cell apoptosis. Therefore, we examined the effect of dasatinib on HUVEC adhesion to collagen I in a cell detachment assay. We found that dasatinib interfered with established HUVEC adhesion in a dose-and time-dependent manner (data not shown; Supplementary Fig. 1_D_). Cotreatment with 40 µM Z-VAD-FMK, which completely reversed dasatinib-induced apoptosis, did not prevent dasatinib-induced cell detachment (Supplementary Fig. 1_D_). Furthermore, DMSO- and dasatinib-treated HUVECs underwent cell death with the same kinetics when cells were maintained in suspension (Supplementary Fig. 1_E_). Together, these results suggest that the mechanism of dasatinib-induced apoptosis involves anoikis (36). However, the possibility that dasatinib-induced apoptosis and cell detachment were unrelated cannot be excluded based on these experiments.
The effect of dasatinib on HUVEC migration was measured by a modified Boyden chamber assay. Dasatinib inhibited HUVEC migration in response to VEGF or bFGF with IC50 values between 3–10 nM (Fig. 1_C_). Endothelial cells plated on growth factor-reduced Matrigel formed tubular structures upon stimulation with VEGF or bFGF. Our data indicate that dasatinib blocked tube formation of endothelial cells with IC50 values between 3–10 nM (Fig. 1_D_ and Supplementary Fig. 2).
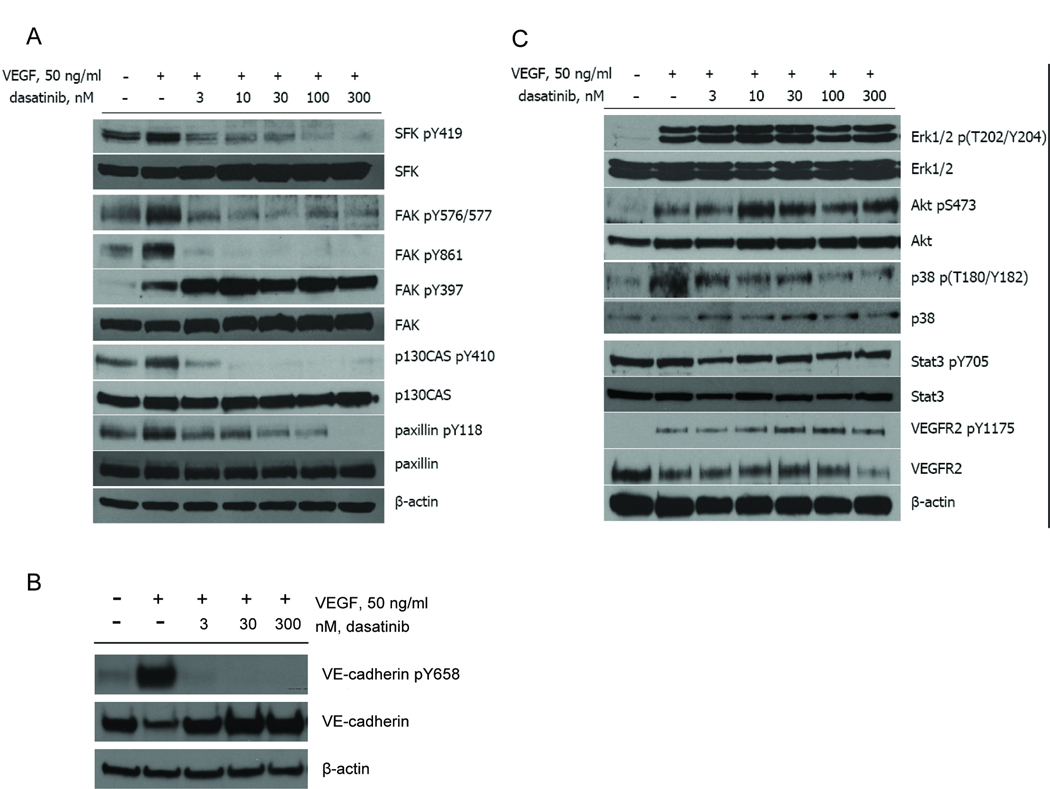
Dasatinib selectively blocks Src downstream signaling
Dasatinib was originally identified as a potent SFK inhibitor in an in vitro Src kinase assay (26). Autophosphorylation of Tyr419 in c-Src (or equivalent in other SFKs) in the kinase domain is required for catalytic activity (21). As expected, dasatinib blocked VEGF- (Fig. 2_A_) or bFGF- (Supplementary Fig. 3_A_) stimulated phosphorylation of SFKs on Tyr419 with an IC50 of ~3 nM. Next, we investigated the effect of dasatinib on known substrates of SFKs. In particular, SFKs have been implicated in regulating VEGF- or bFGF-mediated activation of focal adhesion kinase (FAK) and other focal adhesion proteins such as p130CAS and paxillin (21). Activation of the SFKs/FAK/p130CAS/paxillin signaling pathway has been suggested involved in regulation of cell adhesion, survival, proliferation and migration (21). Our data indicated that dasatinib inhibited VEGF- (Fig. 2_A_) or bFGF- (Supplementary Fig. 3_A_) induced Src-dependent phosphorylation of FAK (Tyr576/577, Tyr861), p130CAS (Tyr410) and paxillin (Tyr118) but not autophosphorylation of FAK (Tyr397). The IC50 values were in the low nM range and comparable with the IC50 values for inhibition of SFKs. These findings, along with other reports (reviewed in Ref 21), suggest a critical role of SFKs, downstream to FAK autophosphorylation and other stimuli, in regulating activation of focal adhesion proteins. The reason for increased expression of SFKs and autophosphorylation of FAK on Y397 following dasatinib treatment is not clear, but suggests a compensatory mechanism involving negative feedback regulation by SFK kinase activity.
Fig. 2.
Dasatinib selectively inhibited Src downstream signaling in HUVECs. A, B and C, Serum-starved Confluent HUVECs were pretreated with DMSO or dasatinib for 1 h prior to stimulation with 50 ng/ml VEGF for another 30 min (for SFKs, FAK, p130CAS, paxillin, VE-cadherin and Stat3) or 10 min (for VEGFR2, Erk1/2, Akt and p38). Cells were lysed and cell lysate was probed with indicated antibodies. β-actin was used to demonstrate protein loading.
We next examined the effect of dasatinib on individual family members of SFKs (c-Src, Fyn and Lyn) in HUVECs. Dasatinib inhibited phosphorylation of c-Src, Fyn and Lyn immunoprecipitated from HUVEC lysates at low nM concentrations (Supplementary Fig. 3_B_).
We also investigated other signaling pathways that could be associated with SFKs. VEGF induces VE-cadherin tyrosine phosphorylation in endothelial cells through Src kinases (37). Our data showed that Tyr658 phosphorylation of VE-cadherin was inhibited by dasatinib at IC50 values comparable with those for inhibition of SFKs (Fig. 2_B_). Furthermore, dasatinib, at higher concentrations, partially inhibited VEGF-induced phosphorylation of p38 MAPK in HUVECs (Fig. 2_C_). However, dasatinib treatment (up to 300 nM) had no effect on phosphorylation of VEGFR2 (Tyr1175), FGFRs (Tyr653/654), Stat3 (Tyr705 and Ser727), Erk1/2 (Thr202/Tyr204), Akt (Ser473), PLCγ1 (Tyr783) and eNOS (Ser1177) (Fig. 2_C_; data not shown).
Dasatinib inhibits angiogenesis ex vivo and in vivo
Endothelial cell growth supplement (ECGS) or VEGF stimulated vessel sprouting from chick aortic rings embedded in Matrigel. Treatment with 100 nM dasatinib for 72 h inhibited ECGS- or VEGF-induced vessel sprouting (Supplementary Fig. 4_A_). This inhibitory effect was dose- and time-dependent (data not shown). The effect of dasatinib on in vivo neovascularization was evaluated in a mouse Matrigel assay. The endothelial cell content in the Matrigel plugs was determined by immunostaining for CD31. Plugs containing VEGF and bFGF showed robust infiltration of endothelial cells; however, dasatinib treatment for 7 d led to a significant (p<0.001) reduction in the number of infiltrating endothelial cells (Supplementary Fig. 4_B–C_).
Dasatinib inhibits human tumor growth in mouse xenograft models
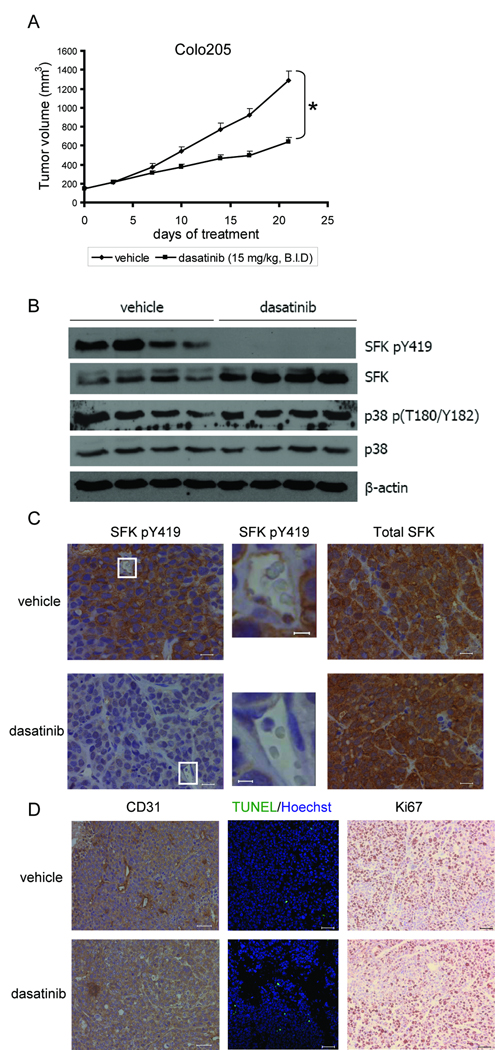
To test the hypothesis that dasatinib inhibits tumor growth through targeting the tumor microenvironment, Colo205 and DU145 human tumor cells were chosen for in vivo studies because their cell viability is relatively resistant to dasatinib in cell culture. In the Colo205 xenograft mouse model, dasatinib treatment (15 mg/kg, B.I.D.) for 21 d significantly (p<0.001) inhibited tumor growth by 50% as compared to that of vehicle-treated tumors (Fig. 3_A_). Dasatinib treatment showed no toxicity in these animals, as assessed by body weight loss as well as by histological evaluation of the mouse livers (data not shown). Western blot analyses of whole tumor lysates revealed that dasatinib potently inhibited phosphorylation of SFKs but not p38 (Fig. 3_B_). Immunostaining of tumor sections showed high levels of SFK phosphorylation in the endothelial cells and tumor cells of vehicle-treated tumors but not dasatinib-treated tumors (Fig. 3_C_). Total SFK protein expression was not inhibited by dasatinib treatment (Fig. 3_B–C_).
Fig. 3.
Dasatinib inhibited human tumor growth in mouse models. Mice bearing Colo205 tumors were treated with vehicle solution or dasatinib (15 mg/kg, B.I.D., p.o.) for 21 d. A, Tumor volume was shown as mean ± SEM. (*p<0.001, n=10) B, Whole tumor lysate was isolated from 4 vehicle- or dasatinib-treated tumors and immunoblots were probed with indicated antibodies. C, Tumor sections were immunostained for p-SFKs (left and middle) and total SFKs (right). Representative images were obtained with a x40 objective. Regions surrounded by white lines were further amplified to show p-SFKs staining in endothelial cells. (Scales bars, left and right: 20 µm; middle: 5 µm.) D, Tumor sections were immunostained for CD31, TUNEL (green)/Hoechst 33342 (blue) and Ki67. Scale bars, 50 µm.
Next, we investigated the mitotic and apoptosis index of vehicle- or dasatinib-treated tumors by immunostaining of tumor sections with anti-Ki67 and TUNEL assays. Ki67 content was not affected by dasatinib treatment (Fig. 3_D_). However, dasatinib treatment led to a significant (_p_=0.004) increase in tumor cell apoptosis (Fig. 3_D_). To determine whether the antitumor activity of dasatinib could be the result of angiogenesis inhibition, we performed immunostaining for CD31 and found that dasatinib significantly (p<0.001) decreased CD31+ tumor microvessel density (Fig. 3_D_). The quantitative analysis of Ki67, TUNEL and CD31 staining is summarized in Supplementary Table 1.
The antitumor and antiangiogenic activities of dasatinib were further confirmed in a DU145 xenograft mouse model. At a dose of 25 mg/kg (B.I.D., p.o.), dasatinib treatment for 22 d significantly (_p_=0.004) inhibited tumor growth, induced tumor cell apoptosis (_p_=0.019) and reduced tumor microvessel density (_p_=0.003) (Supplementary Fig. 5_A–B_). The quantitative analysis of TUNEL and CD31 staining is summarized in Supplementary Table 1. Consistently, dasatinib treatment potently blocked phosphorylation of SFKs/FAK/p130CAS/paxillin but not p38 in DU145 tumors (Supplementary Fig. 5_C_).
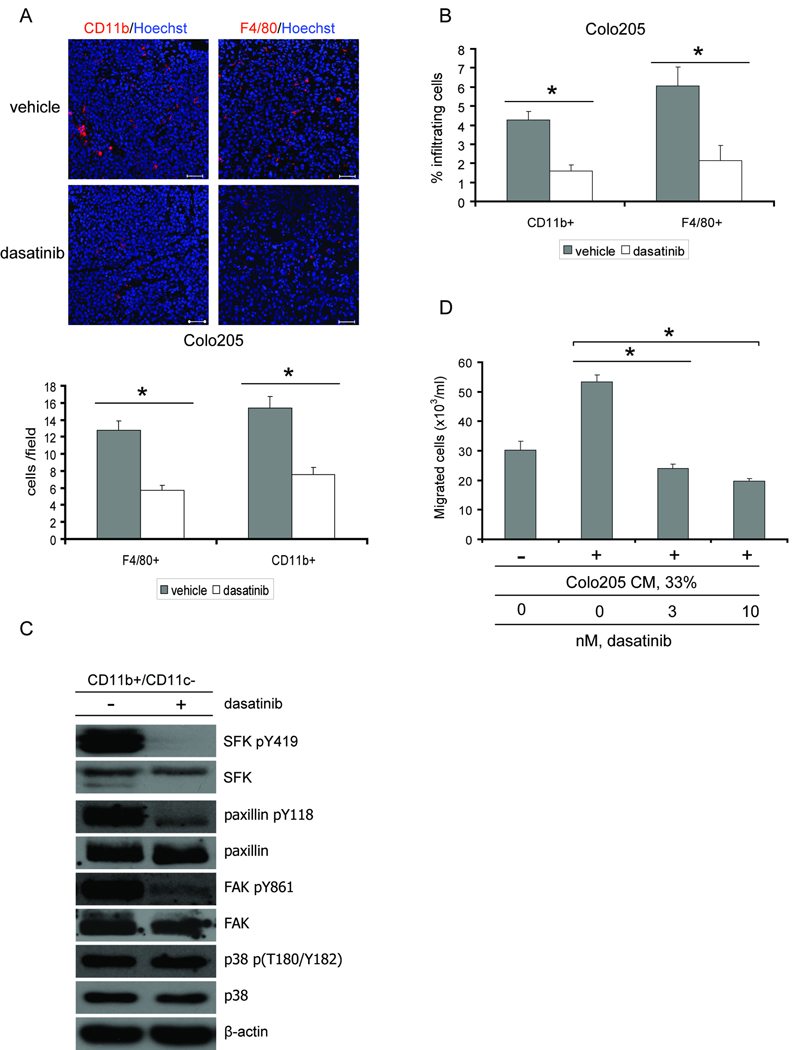
Dasatinib directly inhibits tumor-associated myeloid cells
The established role of tumor-associated myeloid cells in regulating tumor growth prompted us to investigate the effect of dasatinib on these cell populations. We performed immunostaining on tumor sections for F4/80, a macrophage-specific marker, and CD11b, a surface marker expressed by multiple myeloid cell subsets. We found that dasatinib administration significantly (p<0.01) reduced tumor infiltration of F4/80+ macrophages or CD11b+ myeloid cells (Fig. 4_A_ and Supplementary Fig. 6_A–B_). Suppressed recruitment of F4/80+ macrophages and CD11b+ myeloid cells by dasatinib was also confirmed by flow cytometry analyses (Fig. 4_B_). To verify inhibition of Src kinases, CD11b+/CD11c- myeloid cells were isolated from the spleens of tumor-bearing mice with or without dasatinib treatment, and subjected to Western blot analysis. Dasatinib treatment potently inhibited phosphorylation of SFKs, FAK and paxillin but not p38, Erk1/2, Akt and EphA2 in CD11b+/CD11c− myeloid cells (Fig. 4_C_ and data not shown). Phosphorylation of c-Kit (Y719) or PDGFRβ (Y1021, Y751) in lysates of isolated myeloid cells from either control or drug treated tumor-bearing mice was not detectable by Western blot analysis (data not shown).
Fig. 4.
Dasatinib directly inhibited tumor-associated myeloid cells. A, Upper, Colo205 tumor sections were immunostained for CD11b (red) or F4/80 (red). Representative images were obtained with a x20 objective. Scale bars, 50 µm. Lower, quantification of CD11b+ cells (*p<0.01, n=10) or F4/80+ cells per field (*p<0.01, n=10). Results are shown as mean ± SEM. B, Quantification of CD11b+ cells (*p<0.01, n=3) or F4/80+ cells (*p<0.05, n=3) by flow cytometry analyses. Results are shown as mean ± SEM % infiltrating cells. C, CD11b+/CD11c− cells were isolated from the spleens of Colo205 tumor-bearing mice treated with vehicle or dasatinib and subjected to Western blot analysis. Cell lysate was probed with indicated antibodies. D, CD11b+/CD11c− cells were isolated from the spleens of tumor-bearing mice prior to dasatinib treatment, treated with DMSO or dasatinib in vitro and allowed to migrate towards 33% tumor cell conditioned medium (CM) for 18 h in a modified Boyden Chamber assay. Results are shown as mean ± SEM × 103 migrated cells per ml medium (*p<0.001, n=8).
To determine whether myeloid cells were direct targets for dasatinib, we isolated CD11b+/CD11c− myeloid cells from the spleens of tumor-bearing mice prior to dasatinib treatment. Tumor-driven migration of myeloid cells was evaluated in a modified Boyden Chamber assay. As shown in Fig. 4_D_ and Supplementary Fig. 6_C_, tumor cell conditioned medium dramatically enhanced CD11b+/CD11c− myeloid cell migration, which was significantly (p<0.001) inhibited by dasatinib treatment at low nM concentrations. Taken together, these results suggest that dasatinib inhibits tumor infiltration of myeloid cells at least partially by directly impairing their migratory capacity.
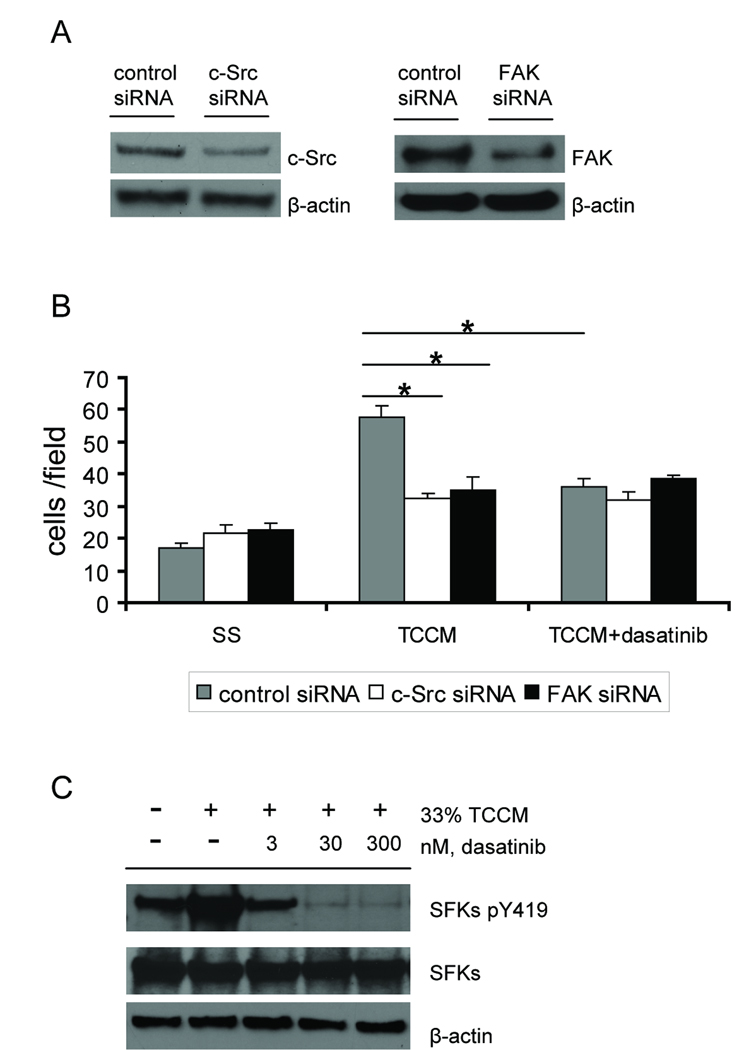
To delineate the mechanism underlying the migratory defects in dasatinib-treated myeloid cells, we transfected the RAW264.7 macrophage cell line with siRNAs that target c-Src or FAK. Expression of c-Src and FAK proteins were substantially inhibited by siRNA transfection (Fig. 5_A_). As shown in Fig. 5_B_, reduced expression of c-Src or FAK significantly (p<0.01) inhibited tumor cell conditioned medium-stimulated RAW264.7 cell migration. Treatment with 30 nM dasatinib inhibited migration of control siRNA-transfected RAW264.7 cells to a comparable level; however, dasatinib was not able to further inhibit migration in cells transfected with siRNAs against c-Src or FAK. Consistently, dasatinib blocked phosphorylation of SFKs in RAW264.7 cells stimulated with tumor cell conditioned medium (Fig. 5_C_). These results suggest that dasatinib and the siRNAs against c-Src or FAK act on the same signaling pathway that regulates macrophage migration, indicating that inhibition of Src and FAK by dasatinib is sufficient to block myeloid cell migration.
Fig. 5.
c-Src and FAK as targets for inhibition of macrophage migration. RAW264.7 cells were transiently transfected with control, c-Src or FAK siRNAs. A, lysate was prepared from RAW264.7 cells 72 h post-transfection and probed with indicated antibodies to measure the knock-down efficiency by siRNAs. B, RAW264.7 cells were harvested 72 h post-transfection, resuspended in basal medium and pretreated with DMSO or 30 nM dasatinib for 1 h before loading on the collagen I-coated inserts. Cell motility was evaluated by a modified Boyden chamber assay using 33% Colo205 tumor cell conditioned medium (TCCM) as a chemoattractant. Results were shown as mean ± SEM cells per field (x20) (*p<0.01, n=3). C, RAW264.7 cells were serum-starved for 6 h, pretreated with DMSO or dasatinib for 1 h prior to stimulation with or without 33% TCCM for another 2 h. Cell lysate was extracted and probed with indicated antibodies.
Dasatinib reduces MMP-9 levels in the tumor microenvironment
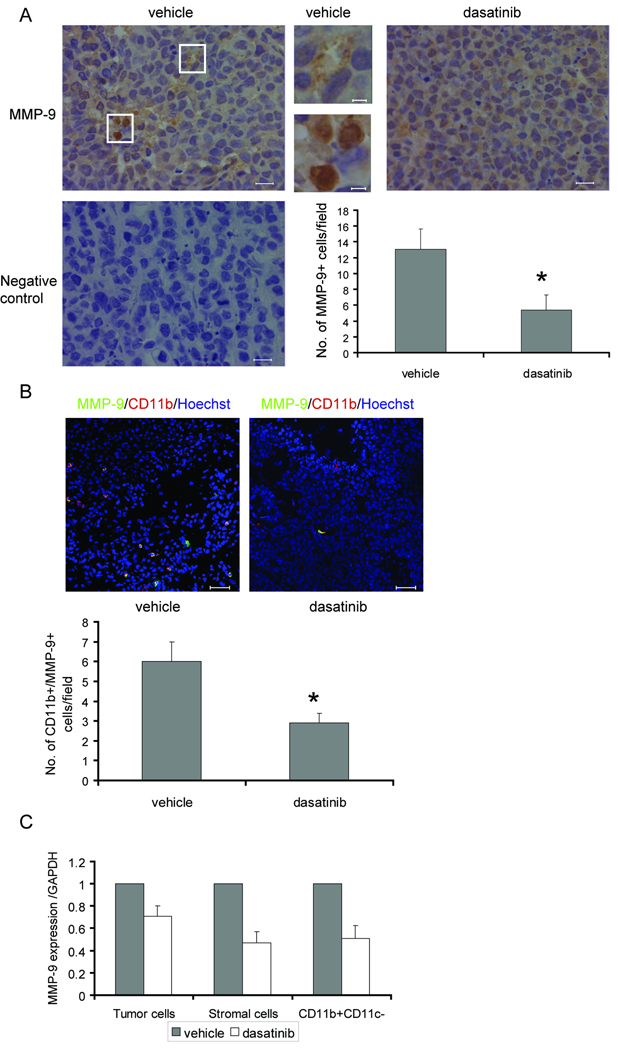
Previous studies have shown that expression of MMP-9 in tumor-infiltrating leukocytes is critical for tumor vasculature and tumor growth (15, 18, 19). Deletion of MMP-9 in myeloid immune suppressor cells completely abolishes their tumor promoting ability (16). To assess the effect of dasatinib on tumor MMP-9 levels, we first performed immunohistochemical analysis to identify the intratumoral expression pattern of MMP-9. In Colo205 xenograft tumors, we detected different expression patterns: while a majority of the cells displayed a weak and diffuse cytoplasmic staining of MMP-9, a small fraction of cells showed a stronger and granular staining (Fig. 6_A_).
Fig. 6.
Dasatinib reduced MMP-9 levels in the tumor microenvironment. A, Upper, MMP-9 immunostaining in Colo205 xenograft tumors treated with vehicle or dasatinib. Representative images were obtained with a x40 objective. Regions surrounded by white lines were further amplified to show different MMP-9 expression pattern. (Scales bars, left and right: 20 µm; middle: 5 µm.) Lower left, negative control for MMP-9 staining. Lower right, quantification of MMP-9+ cells per field (*_p_=0.025, n=10). Results are shown as mean ± SEM. B, Upper, immunostaining of tumor sections for CD11b (red) and MMP-9 (green). Confocal fluorescence images were obtained with a x20 objective. Scale bars, 50 µm. Lower, quantification of CD11b+/MMP-9+ cells per field (*_p_=0.032, n=10). Results are shown as mean ± SEM. C, Normalized MMP-9 mRNA expression in human tumor cells, mouse stromal cells and CD11b+/CD11c− tumor-associated myeloid cells. Results are shown as mean ± SEM (n=3).
To identify the MMP-9 expressing cells, Colo205 tumor sections were double-stained with MMP-9 and CD11b or F4/80. The majority of MMP-9+ cells were F4/80 negative (data not shown). In contrast, almost all the MMP-9+ cells are also CD11b positive (Fig. 6_B_). Since our results demonstrated that dasatinib inhibited tumor infiltration of CD11b+ cells, we investigated whether dasatinib influenced intratumoral MMP-9+ cells. Our data showed that dasatinib treatment led to a significant decrease of MMP-9+ cells (_p_=0.025) and MMP-9+/CD11b+ cells (_p_=0.032) in the tumor microenvironment (Fig. 6_A–B_).
We performed real-time PCR to investigate whether MMP-9 gene expression was affected by dasatinib in the tumor microenvironment. Primers designed specifically for human or mouse MMP-9 were used to identify MMP-9 mRNA levels in human Colo205 tumor cells or mouse stromal cells. Our results showed that dasatinib administration, which partially reduced MMP-9 mRNA in human tumor cells (~29%), reduced mouse MMP-9 mRNA to a greater extent in both host-derived stromal cells (~53%) and CD11b+/CD11c− tumor-infiltrating myeloid cells isolated from Colo205 xenografts (~48%) (Fig. 6_C_). Taken together, these results suggest that dasatinib reduces MMP-9 levels in the tumor microenvironment through simultaneous inhibition of recruitment of MMP-9 producing tumor-associated myeloid cells as well as their MMP-9 gene expression.
Discussion
In this study, we assessed the effects of targeting SFKs by dasatinib on distinct cell populations within the tumor microenvironment and how these effects influence tumor growth. Our studies using in vivo human tumor xenograft mouse models demonstrate that SFK inhibition by dasatinib suppresses tumor growth, associated with increased tumor cell apoptosis, decreased microvessel density and reduced intratumoral myeloid cells. It is notable that the viability of these tumor cell lines in culture is relatively resistant to dasatinib. By contrast, dasatinib displays potent activity against endothelial cell and myeloid cell functions that are essential for supporting tumor cell growth in vivo, suggesting that dasatinib inhibits tumor growth at least in part by directly targeting endothelial and myeloid cell compartments in the tumor microenvironment.
Another study recently reported that dasatinib, by targeting PDGFRβ and SFKs in both tumor cells and tumor-associated endothelial cells, inhibits multiple myeloma tumor growth (33). Although these data support our conclusion on the importance of SFKs in endothelial cells, we detected no expression of PDGFRβ in either HUVECs (data not shown) or the endothelial cell compartment of our tumor models (Supplementary Fig. 7). Furthermore, in our solid tumor models, SFK inhibition was not sufficient to directly induce cytotoxicity in tumor cells (Fig. 1_A_), which suggests the tumor microenvironment including endothelial cells and myeloid cells is an important target that mediates the anti-cancer activity of dasatinib in vivo.
Tumor-associated myeloid cells recently gained attention due to their important roles in supporting tumor growth and generating resistance to anticancer therapy. Our finding that dasatinib inhibits intratumoral infiltration of myeloid cells suggests a potential use of dasatinib to target myeloid cells in the tumor microenvironment. Dasatinib directly inhibited tumor cell conditioned medium-driven CD11b+/CD11c− myeloid cell migration, suggesting that the deficiency in myeloid cell migration is a consequence of a functional breakdown in the cell migration machinery, rather than a secondary effect due to tumor growth inhibition. We also demonstrated that siRNAs that specifically targeted c-Src or FAK reduced tumor cell conditioned medium-driven RAW264.7 cell migration. These results are consistent with previously published results showing that SFKs, together with their substrates FAK and paxillin, regulate motility of inflammatory cells (monocytes, macrophages, granulocytes, etc.) and their recruitment to inflammation sites (38–40). Our hypothesis that inhibition of Src and its downstream signaling by dasatinib is sufficient to block myeloid cell migration is supported by data showing that dasatinib selectively inhibited the SFKs/FAK/paxillin signaling pathway in CD11b+/CD11c− myeloid cells, and combination of siRNA against c-Src or FAK and dasatinib treatment did not achieve additional inhibition of RAW264.7 cell migration.
While our studies focus on the effect of dasatinib on cell motility, we cannot rule out the possibility that targeting SFKs may impact other properties of tumor-associated myeloid cells. A recent publication indicated that dasatinib inhibits cell proliferation of tumor-associated macrophages in vitro, although it is not clear whether this effect is Src dependent (41). Furthermore, it has been reported that decreased phosphorylation of VE-cadherin prevents transendothelial migration of leukocytes (42). It will be of interest to determine whether inhibition of VE-cadherin phosphorylation in endothelial cells by dasatinib contributes to reduced recruitment of myeloid cells.
Numerous studies have suggested an essential role of MMP-9 in myeloid cell-mediated oncogenic events (15–19, 43). The mechanism of action involves increased bioavailability of VEGF, mobilization of bone marrow-derived cells to the peripheral blood and recruitment of pericytes (43). We identified CD11b+ tumor-associated myeloid cells as the major source of MMP-9 within the tumor microenvironment. Our results further demonstrate that targeting SFKs by dasatinib reduces MMP-9 levels within the tumor microenvironment by simultaneous inhibition of tumor recruitment of CD11b+/MMP-9+ myeloid cells and also their MMP-9 gene expression. Previous work has shown that SFKs/FAK signaling regulates integrin-mediated MMP-9 expression in tumor cells and T lymphocytes (44, 45). It is possible that dasatinib treatment inactivates the SFKs/FAK signaling pathway and thereby inhibits MMP-9 gene expression in tumor-infiltrating myeloid cells.
Although the VEGF-VEGFR2 signaling axis is still the most recognized target for antiangiogenic therapy, refactory or progressive resistance to anti-VEGF agents has been widely reported (46, 47). One possible mechanism of action involves induction of other proangiogenic factors such as bFGF that compensate for the lack of VEGF (48). Our results show that dasatinib inhibits the angiogenic potential of endothelial cells in response to a wide variety of proangiogenic stimuli including VEGF, bFGF and ECGS, which further confirms SFKs as key downstream effectors of multiple angiogenic signaling pathways. These findings provide a rationale for combining dasatinib with anti-VEGF agents to test for potential synergistic antiangiogenic effects. Moreover, accumulation of MDSCs, a subset of myeloid cells defined by the expression of cell surface markers CD11b and Gr1, in tumors has been shown to suppress anti-tumor immunity and render tumors refractory to anti-VEGF agents (14). We show that dasatinib directly inhibits tumor-driven migration of CD11b+/CD11c− myeloid cells. It will be of interest to determine whether intratumoral recruitment of MDSCs is affected by dasatinib.
Several SFK inhibitors including dasatinib are currently in clinical trials to treat solid tumors based on their direct effects on tumor cells (49). Our results demonstrate another important property of dasatinib in that it can target both endothelial and myeloid cell compartments within the tumor microenvironment to inhibit tumor growth. Therefore, combining dasatinib treatment with other anticancer therapeutics may achieve additional clinical benefit.
Statement of Translational Relevance
Cross-talk between tumor cells and stromal cells within the tumor microenvironment mediates tumor initiation, progression and response to anticancer therapy. However, there are currently only a limited number of therapeutics that efficiently target the tumor microenvironment. Src kinases have been implicated to have important functions in tumor cell growth and metastasis. Their roles in the tumor microenvironment are yet not completely understood. Here we provided evidence that dasatinib, a Src kinase inhibitor, inhibits human tumor cell growth in mouse models but not in cell culture. Furthermore, dasatinib treatment directly inhibits endothelial cell and myeloid cell functions that are essential for sustaining tumor cell growth in vivo. These findings suggest that the tumor microenvironment is an important target for dasatinib. Our results have significant implications for treatment with dasatinib in combination with other therapeutics that directly target tumor cells.
Supplementary Material
1
8
9
10
11
2
3
4
5
6
7
Acknowledgements
We thank members of our laboratories for stimulating discussion. We also thank the Analytical Cytometry Core, the Pathology Core and the Microscopy Core at City of Hope Comprehensive Cancer Center. This work was partially supported by the National Cancer Institute (R01-CA115674) and Bristol-Myers Squibb.
References
- 1.Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Witz IP. Yin-yang activities and vicious cycles in the tumor microenvironment. Cancer Res. 2008;68:9–13. doi: 10.1158/0008-5472.CAN-07-2917. [DOI] [PubMed] [Google Scholar]
- 3.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 4.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18:27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 7.Munoz-Chapuli R, Quesada AR, Angel Medina M. Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci. 2004;61:2224–2243. doi: 10.1007/s00018-004-4070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takakura N. Role of hematopoietic lineage cells as accessory components in blood vessel formation. Cancer Sci. 2006;97:568–574. doi: 10.1111/j.1349-7006.2006.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 10.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relf M, LeJeune S, Scott PA, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 12.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19:247–258. doi: 10.1023/a:1015587423262. [DOI] [PubMed] [Google Scholar]
- 13.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 14.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 15.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 18.Jodele S, Chantrain CF, Blavier L, et al. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- 19.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 21.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- 23.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 24.Kilarski WW, Jura N, Gerwins P. Inactivation of Src family kinases inhibits angiogenesis in vivo: implications for a mechanism involving organization of the actin cytoskeleton. Exp Cell Res. 2003;291:70–82. doi: 10.1016/s0014-4827(03)00374-4. [DOI] [PubMed] [Google Scholar]
- 25.Kanda S, Miyata Y, Kanetake H, Smithgall TE. Non-receptor protein-tyrosine kinases as molecular targets for antiangiogenic therapy (Review) Int J Mol Med. 2007;20:113–121. [PubMed] [Google Scholar]
- 26.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:658–661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 27.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 28.Schittenhelm MM, Shiraga S, Schroeder A, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 29.Huang F, Reeves K, Han X, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 30.Nam S, Kim D, Cheng JQ, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 31.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 32.Trevino JG, Summy JM, Lesslie DP, et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962–972. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coluccia AM, Cirulli T, Neri P, et al. Validation of PDGFRbeta and c-Src tyrosine kinases as tumor/vessel targets in patients with multiple myeloma: preclinical efficacy of the novel, orally available inhibitor dasatinib. Blood. 2008;112:1346–1356. doi: 10.1182/blood-2007-10-116590. [DOI] [PubMed] [Google Scholar]
- 34.O'Hare T, Walters DK, Stoffregen EP, et al. Combined Abl inhibitor therapy for minimizing drug resistance in chronic myeloid leukemia: Src/Abl inhibitors are compatible with imatinib. Clin Cancer Res. 2005;11:6987–6993. doi: 10.1158/1078-0432.CCR-05-0622. [DOI] [PubMed] [Google Scholar]
- 35.Talpaz MKH, Shah NP, Donato N, et al. Hematologic and cytogenetic responses in Imatinib-resistant accelerated and blast phase chronic myeloid leukemia (CML) patients treated with the dual SRC/ABL kinase inhibitor BMS-354825: results from a phase I dose escalation study; ASH Annual Meeting; 2004. p. 10a. [Google Scholar]
- 36.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 37.Wallez Y, Cand F, Cruzalegui F, et al. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–1077. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 38.Baruzzi A, Caveggion E, Berton G. Regulation of phagocyte migration and recruitment by Src-family kinases. Cell Mol Life Sci. 2008;65:2175–2190. doi: 10.1007/s00018-008-8005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Owen KA, Pixley FJ, Thomas KS, et al. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brownlow N, Mol C, Hayford C, Ghaem-Maghami S, Dibb NJ. Dasatinib is a potent inhibitor of tumour-associated macrophages, osteoclasts and the FMS receptor. Leukemia. 2008 doi: 10.1038/leu.2008.237. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Alcaide P, Newton G, Auerbach S, et al. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25:35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- 44.Cortes-Reynosa P, Robledo T, Macias-Silva M, Wu SV, Salazar EP. Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008;27:220–231. doi: 10.1016/j.matbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Segarra M, Vilardell C, Matsumoto K, et al. Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. Faseb J. 2005;19:1875–1877. doi: 10.1096/fj.04-3574fje. [DOI] [PubMed] [Google Scholar]
- 46.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 48.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Johnson FM, Gallick GE. SRC family nonreceptor tyrosine kinases as molecular targets for cancer therapy. Anticancer Agents Med Chem. 2007;7:651–659. doi: 10.2174/187152007784111278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
8
9
10
11
2
3
4
5
6
7