Phenotype and Genotype of Pancreatic Cancer Cell Lines (original) (raw)
. Author manuscript; available in PMC: 2011 May 1.
Abstract
The dismal prognosis of pancreatic adenocarcinoma (PA) is due in part due to a lack of molecular information regarding disease development. Established cell lines remain a useful tool for investigating these molecular events. Here we present a review of available information on commonly used PA cell lines as a resource to help investigators select the cell lines most appropriate for their particular research needs. Information on clinical history, in vitro and in vivo growth characteristics, phenotypic characteristics, such as adhesion, invasion, migration and tumorigenesis, and genotypic status of commonly altered genes (KRAS, p53, p16, and SMAD4) was evaluated. Identification of both consensus and discrepant information in the literature suggests careful evaluation before selection of cell lines and attention be given to cell line authentication.
Keywords: adhesion, angiogenic potential, invasion, migration, pancreatic adenocarcinoma cell lines, tumorigenicity
INTRODUCTION
Pancreatic adenocarcinoma (PA) is an aggressive disease that develops in a relatively symptom-free manner and is usually advanced at the time of diagnosis. As is common in epithelial tumors, carcinogenesis develops through accumulation of mutations and genetic lesions leading to activation of oncogenes and inactivation of tumor suppressor genes. Since multiple combinations of mutations can lead to the development of PA 1, disease sub-classes may present different survival strategies requiring multiple targeted intervention strategies. A thorough understanding of the specific cellular and molecular mechanisms of PA development and progression is required in order to identify early detection strategies, preventative measures, and effective interventions.
Both in vitro and in vivo experimentation involving cancer cell lines remains a convenient starting point for discovery and proof-of-concept studies. Investigations in colon and breast cancer indicate that cell lines recapitulate the genomic events leading to neoplastic changes seen in patient samples 2, 3. It is likely that a similar situation occurs in PA, which is supported by the fact that the four most common mutations occurring in PA tumors are found in cell lines at similar percentages (see below) and PA cell lines demonstrate disparate phenotypes and genotypes that are representative of PA sub-classes. This diversity facilitates mechanistic inferences and aids in proving causality through gain- and loss-of-function experiments. Examples of studies that capitalized on phenotypic differences in PA cell lines have provided mechanistic insight through linkage of differential expression of specific proteins to tumor growth, invasion and metastasis 4, 5 and chemotherapeutic drug resistance 6. Choosing cell lines for specific phenotypic characteristics is challenging due to the lack of comprehensive comparative studies. Furthermore, there are many apparent contradictory reports concerning both phenotype and genotype of PA cell lines. Here we present a review of the current information characterizing the eleven most commonly referenced pancreas cancer cell lines. Our goal was to identify consensus in the literature regarding phenotype and genotype as well as provide a compendium of PA cell line information that can be used as a reference and starting point for researchers.
CLINICAL PICTURE AND CELL LINE DERIVATION
Information concerning the clinical course of the donor patient and the site of derivation are important in defining the biologic and pathologic characteristics of the tumor cell line and should be considered in designing in vitro experiments. General characteristic of the donor subject, disease course, and cell line as well as the relevant references describing the original cell line derivation are shown in Table 1. More extensive descriptions of the histological appearance and differentiation of the tumor cell lines are summarized below. All donor patients were Caucasian between the ages of 26–65.
Table 1.
Donor patient information and cell line characteristics.
| Cell Line | Age | Gender | Derivationa | Metastasis | Proliferationb | Differentiation | Ref. |
|---|---|---|---|---|---|---|---|
| AsPC-1 | 62 | Female | Ascites | Yes | 38–40 hrs | Poor | 7 |
| BxPC-3 | 61 | Female | Primary tumor | No | 48–60 hrs | Moderate to poor | 8 |
| Capan-1 | 40 | Male | Liver metastasis | Yes | ndc | Well | 9 |
| Capan-2 | 56 | Male | Primary tumor | No | 96 hrs | Well | 11 |
| CFPAC-1 | 26 | Male | Liver metastasis | Yes | 31 hrs | Well | 12 |
| HPAC | 64 | Female | Primary tumor | ndc | 41 hrs | Moderate | 16 |
| HPAF-II | 44 | Male | Ascites | Yes | 42 hrs | Well | 17 |
| Hs 766T | 46 | Male | Lymph node metastasis | Yes | 6–7 days | ndc | 19 |
| MIA PaCa-2 | 65 | Male | Primary tumor | ndc | 40 hrs | Poor | 20 |
| PANC-1 | 56 | Female | Primary tumor | Yes | 52 hrs | Poor | 21 |
| SU.86.86 | 57 | Female | Liver metastasis | Yes | 77 hrs | Moderate to poor | 22 |
AsPC-1 was obtained from a 62-year-old woman with adenocarcinoma of the head of the pancreas and metastases to several abdominal organs. The patient received radiation and chemotherapy but eventually developed ascites and died two weeks later. The ascitic cell culture was noted to produce abundant mucin as well as carcinoembryonic antigen 7.
BxPC-3 was cultured from a 61-year-old woman’s adenocarcinoma of the body of the pancreas. The patient died 6 months later despite radiation and chemotherapy. No evidence of metastasis was found. Tumors grown in nude mice resemble the primary tumor of the patient and produced carcinoembryonic antigen, human pancreas cancer-associated antigen, human pancreas-specific antigen, and traces of mucin 8.
Capan-1 was obtained from a liver metastasis of a 40-year-old male with a pancreas adenocarcinoma in the head of the pancreas. Metastases were present in regional lymph nodes. In athymic mice, Capan-1 derived tumor produced mucin and was morphologically and biochemically similar to the tumor of origin 9. Although not reported in the original publication, a doubling time of 41 hours was subsequently determined for Capan-110.
Capan-2 originated from a 56-year-old male with pancreatic adenocarcinoma. The primary tumor involved the head of the pancreas and infiltrated the duodenal wall distal to the ampulla. The patient underwent pancreatectomy, cholecystectomy, partial gastrectomy, large and small bowel omentectomy and splenectomy. The patient received postoperative chemotherapy and died 6.75 years later 11.
CFPAC-1 was obtained from a liver metastasis of a 26-year old male with cystic fibrosis. Laparotomy revealed a well-differentiated adenocarcinoma in the head of the pancreas and multiple liver metastases 12. Carriers of mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) commonly exhibit an idiopathic pancreatitis, which is a risk factor for pancreas cancer. In addition, it has been proposed that CFTR carrier status is a direct risk factor for young onset (before age 60) pancreas cancer 13. On the contrary, other reports suggest there is no connection between cystic fibrosis and pancreas cancer 14, 15. Exactly what role, if any, CFTR carrier status plays in the pathogenesis of pancreas cancer remains unresolved. It is likely that the CFPAC-1 pancreas cancer cell line may have resulted from unique molecular events that may not be representative of the basic pathogenesis which characterizes the majority of pancreas cancer cases.
HPAC was derived from an adenocarcinoma found in the head of the pancreas in a 64-year-old female. The tumor was histologically described as moderately well-differentiated and of ductal origin 16.
HPAF-II was obtained from the ascites of a 44-year-old male with pancreas adenocarcinoma and metastases to the liver, diaphragm and lymph nodes 17.
Hs 766T was derived from the lymph node metastasis of a 64-year-old male with pancreas carcinoma 18, 19.
MIA PaCa-2 was derived from the pancreas adenocarcinoma of a 65-year-old man who presented with abdominal pain for 6 months and a palpable upper abdominal mass. The tumor involved the body and tail of the pancreas and had infiltrated the periaortic area. The tumor did not express measurable amounts of carcinoembryonic antigen and an alkaline phosphatase stain was negative 20.
PANC-1 was cultured from a 56-year-old male with an adenocarcinoma in the head of the pancreas which invaded the duodenal wall. Metastases in one peripancreatic lymph node were discovered during a pancreaticoduodenectomy. In culture, the cell line was not found to secrete significant carcinoembryonic antigen 21.
SU.86.86 was obtained from a 57-year-old woman with an adenocarcinoma of the head of the pancreas. There was extensive metastasis to the liver and the tumor specimen was obtained from a liver metastasis. Histological evaluation showed a moderate-to-poorly differentiated adenocarcinoma 22.
Table 1 lists doubling times as reported in the publication that originally described the cell line. Since differences in proliferation rates may arise due to variations in culture conditions, these doubling times might best be used as a relative guide. Subsequent studies report substantial differences from the original publications. For example, McIntyre and Kim report doubling times of 26 hours for MIA PaCa-2 and 28 hours for PANC-1, whereas the original doubling times were reported as 40 and 52 hours respectively 10.
PHENOTYPE
For this review we focused on commonly used experimental systems involving PA cell line behavior in tissue culture and mouse xenograft models (adhesion, migration, invasion, angiogenic potential, and tumorigenicity). Our goal was to identify consensus differences in studies that compared multiple cell lines. Many of the studies were qualitative for which we report relative phenotypic differences. Where possible, we report quantitative, statistically significant differences.
Adhesion
The high metastatic potential of pancreas cancer underscores the importance of understanding the properties of cancer cell adhesion, which influences tumor growth and largely determines metastatic potential. Cell adhesion is mediated by the interaction of extracellular matrix components with cell-surface molecules. Many of the studies in pancreas cancer have focused on alterations in adhesive properties by the addition of cytokines or anti-cancer drugs in tissue culture, although several studies document adhesion to various substrates in two or more PA cell lines allowing the comparison of relative affinities (Table 2). Commonly studied extracellular matrix components mediating cell attachment were fibronectin, a glycoprotein found in basement membranes and connective tissues, collagens I and IV, found in tissue stroma and basement membranes, respectively 23, and laminin, the main noncollagenous glycoprotein in the basement membrane 24.
Table 2.
Comparison of the adhesive ability of pancreas cancer cell lines to various extra cellular matrix proteins.
| Substrate | Relative Affinity | Ref. |
|---|---|---|
| Collagen I | ||
| Capan-1 > PANC-1 > MIA PaCa-2 | 10 | |
| AsPC-1 > BxPC-3 = PANC-1 > MIA PaCa-2 | 30 | |
| Capan-1 > MIA PaCa-2 | 27 | |
| Capan-1 > PANC-1, MIA PaCa-2 = 0 | 26 | |
| Capan-1 > MIA PaCa-2 | 28 | |
| PANC-1 = BxPC-3 > SU.86.86 > AsPC-1 | 29 | |
| Capan-1 = BxPC-3 = PANC-1 > > MIA PaCa-2 | 25 | |
| Collagen IV | ||
| PANC-1 > Capan-1 > MIA PaCa-2 | 10 | |
| AsPC-1 > PANC-1 > MIA PaCa-2 > BxPC-3 | 30 | |
| AsPC-1 > BxPC-3 > Capan-2 | 122 | |
| Fibronectin | ||
| PANC-1 > BxPC-3 > AsPC-1 > SU.86.86 | 29 | |
| BxPC-3 = MIA PaCa-2 > AsPC-1 = PANC-1 | 30 | |
| BxPC-3 > PANC-1 | 31 | |
| MIA PaCa-2 = Capan-1 = PANC-1 | 26 | |
| MIA PaCa-2 = Capan-1 | 28 | |
| Capan-1 = BxPC-3 = PANC-1 > MIA PaCa-2 | 25 | |
| Laminin | ||
| BxPC-3 = PANC-1 > SU.86.86 > AsPC-1 | 29 | |
| AsPC-1 > BxPC-3 = MIA PaCa-2 = PANC-1 | 30 | |
| BxPC-3 > PANC-1 | 31 | |
| Capan-1 = MIA PaCa-2 = PANC-1 | 26 | |
| Capan-1 > MIA PaCa-2 | 28 | |
| BxPC-3 > Capan-1 = MIA PaCa-2 = PANC-1 | 25 |
Agreement among reports of adhesive propensities exists for several cell lines. Specifically, Capan-1 bound more avidly to type I collagen compared with MIA PaCa-2 10, 25–28. Additionally, BxPC-3 and PANC-1 appeared to bind with equivalent affinity to type I collagen 25, 29, 30. However, consensus in binding affinity with one medium does not necessarily translate to other types of binding material. For example, when Capan-1 and MIA PaCa-2 were plated on laminin, several groups observed equivalent binding affinity and one group reported that Capan-1 bound less avidly than MIA PaCa-2 25, 26, 28. Also, when BxPC-3 and PANC-1 were plated on laminin two groups reported that they bound equivalently and two groups reported that BxPC-3 bound more avidly 25, 29–31.
The type of extracellular matrix component utilized is an important variable to consider when comparing and or designing an experiment. For, example when PANC-1 and Capan-1 were plated on a mixture of type I, II, and IV collagen they had close to complete adherence by nine hours, but only roughly 10% and 25% adherence respectively on type II collagen alone at the same time point 24. This experiment also illustrates that the length of time allowed for adherence can alter the outcome. Temporal differences between studies may at least partially explain some of the reported discrepancies, but the pervasive lack of consensus suggests other variables may be important. Cell quantification techniques differ between experimental groups with spectrophotometry and light microscopy being the two most common methodologies. Other variables that could influence experimental outcomes include cell culture conditions and extracellular matrix handling. All of these variables make it difficult to compare the results of different experiments, and individual investigators should verify the differential adhesive properties of the specific cell lines used in a given study.
Cell Migration and Invasion
Just as tumor cell adhesion is a key mediator in the process of metastasis and invasion, cell migration is also an important component in the spread of pancreas cancer 5. In early stages of the disease, cancer spread is thought to occur after tumor cells infiltrate the peritoneal cavity and gain access to blood vessels 32. Thus, the study of the migratory abilities of pancreas cancer cells is necessary to provide insight into the biological processes that mediate metastasis. Several techniques have proven useful for assessing cell migration. With the traditional Boyden chamber, cells migrate in a chemotactic gradient through pores of a filter separating two chambers 33. A modification of this technique using transwell inserts in 24 and 96 well culture plates allows for replicate parallel experiments under identical culture conditions. Another common technique is the wound-healing cell migration assay 34, which quantifies the time or distance for cells to repopulate an artificial wound scratched into a near confluent cell culture.
Reports of migration assay data that directly compare two or more PA cell lines are limited. In a transwell migration assay, Stahle and coworkers demonstrated that PANC-1 cells had 5-fold greater motility than BxPC-3 cells 35. PANC-1 cells also appeared to migrate predominantly as single cells whereas BxPC-3 cells migrated as a tightly packed sheet in a wound healing assay 35. Migration of PANC-1 cells was also greater than BxPC-3 cells on transwell filters coated with collagen 1 36. Cell motility can also be assessed by measuring the area of phagokinetic tracks of cells moving through colloid-plated substrates. Using this technique, Lin and colleagues showed that HPAF-II cells had greater motility than BxPC-3 cells37.
Another important phenotype of PA cells is its invasive properties, as pancreas cancer is highly aggressive and invasive by nature, with almost all patients presenting with metastasis at the time of diagnosis. Indeed, the presence of metastases is thought to be responsible for the poor prognosis of this disease 38. Over 80% of pancreas cancer patients have tumor extension into the peripancreatic tissues and metastases to local lymph nodes 5. Invasion is a unique characteristic of malignant cells and is a key step in the series of events which lead to metastasis 39. Whereas migration assays monitor cell movement, invasion assays monitor cell movement through a specific matrix, usually measured in transwell filters coated with reconstituted extracellular matrix, with Matrigel (a mixture of laminin, type IV collagen, entactin and heparin sulfate) being the most commonly used extracellular matrix40.
The current literature contains a number of studies that have analyzed the invasive propensities of PA cell lines through various substrates (Table 3). There does appear to be agreement that Capan-1 and MIA PaCa-2 cells have similar invasive properties in Matrigel 25, 27,41. In contrast, there are discrepancies when BxPC-3 is compared to MIA PaCa-2. Funahashi 42 reported that BxPC-3 was more invasive in Matrigel, collagen, and laminin than MIA PaCa-2, however, several other investigators observed similar invasive properties for BxPC-3 and MIA PaCa-2 cells in Matrigel 25, 39, 43. Another controversy lies in the relative invasive ability of MIA PaCa-2 and PANC-1 cells. Specifically, Duxbury reported that PANC-1 exhibited more invasion through Matrigel than MIA PaCa-2 44, but two other groups claimed that MIA PaCa-2 was more invasive than PANC-1 39, 41. Some of the above studies utilized commercially available kits for the measurement of invasion 39, 43, 45, 46, while others made their own 25, 27, 37, 41, 42. Thus, subtle differences in methodology may explain the varied results. Other important variables to consider are cell culture conditions, time, and cell quantification techniques. The variability in invasion properties between experiments again suggests that each investigator characterize their own cell lines when drawing conclusions from differential invasive capabilities.
Table 3.
Comparison of invasive abilities of PA cell lines. Data abstraction was limited to those studies that directly compared multiple cell lines. Measured invasiveness is given in parentheses (mean invaded cells per high-powered field, unless otherwise noted) after each cell line.
| Substrate | Relative Effect | Time (Hrs) | Ref. |
|---|---|---|---|
| Matrigel | |||
| BxPC-3 (41 ± 4.2) > MIA PaCa-2 (14.7 ± 3.7) | 24 | 42 | |
| BxPC-3 (73.3) = MIA PaCa-2 (90.3) | 24 | 43 | |
| BxPC-3 (~45) = MIA PaCa-2 (~47) = PANC-1 (~40) | 12 | 39 | |
| HPAF-II (~310)a > BxPC-3 (~25) | 24 | 37 | |
| Capan-1 (~420) = MIA PaCa-2 (~480)b > PANC-1 (~310) | 48 | 41 | |
| Capan-1 (~35)c = MIA PaCa-2 (~32) | 48 | 27 | |
| Capan-1 (~190) > AsPC-1 (~95) | 96 | 45 | |
| Capan-2 (44.3 ± 4.3) > AsPC-1 (23.3 ± 5) | 24 | 46 | |
| BxPC-3 (~6) = Capan-1 (~6.5) = MIA PaCa-2 (~11) = PANC-1 (~8) | 48d | 25 | |
| Fibronectin | |||
| BxPC-3 (42.7 ± 2.2) > MIA PaCa-2 (24 ± 2.9) | 24 | 42 | |
| Collagen IV | |||
| BxPC-3 (~25) > AsPC-1 (~20) = Capan-2 (~17) | 24 | 122 | |
| Laminin | |||
| BxPC-3 (66.7 ± 11.9) > MIA PaCa-2 (7.3 ± 0.7) | 24 | 42 |
Angiogenic Potential
Angiogenesis, the process by which cancerous cells induce proliferation of endothelial cells leading to subsequent formation of new blood vessels, is quintessential to tumor growth and metastasis 47. Tumor microvessel density (MVD) is generally considered a valid predictor of tumor progression and patient survival, predominantly in tumors that induce significant neovascularization such as breast and prostate carcinomas 48. The prognostic capacity of MVD in PA is ambiguous with some reports indicating a significant correlation 49, 50 while others showed none 51–53. Tumor proliferation is associated with an angiogenic switch in which the presence of pro-angiogenic factors outweigh anti-angiogenic factors 54–57 and expression of pro-angiogenic factors, particularly vascular endothelial growth factor (VEGF), correlates with MVD in PA 49, 50, 53. The aggressiveness of PA may be linked to angiogenesis in that many pro-angiogenic factors over expressed in PA are also mitogenic 58.
Expression of pro-angiogenic cytokines, chemokines, enzymes and their products have been used to assess the angiogenic potential of PA cell lines. Recent attention has focused on the inducible enzyme cyclooxygenase-2 (COX-2), also known as prostaglandin-endoperoxide synthase 2 (PTGS2), which is over expressed in multiple malignancies, including pancreatic cancer 59, 60. One mechanism through which COX-2 promotes angiogenesis is by converting arachidonic acid into bioactive molecules, such as prostaglandin E2 (PGE2), which act as activating factors in angiogenesis 61. Furthermore, selective COX-2 inhibitors potentiate the growth inhibitory effects of chemotherapeutic agents used in pancreatic cancer treatment 62, 63. In addition to their direct stimulatory role in angiogenesis 64, prostaglandins activate Nuclear Factor-κB, which in turn up-regulates the expression of COX-2 65 contributing to angiogenic signal propagation. Expression in PA cell lines of the cytokines IL-1α and IL-8 have also been used as surrogate markers of angiogenesis. IL-8 has is known to induce proliferation and chemotaxis of vascular endothelial cells 66–68 and promotes growth of pancreatic tumors 69
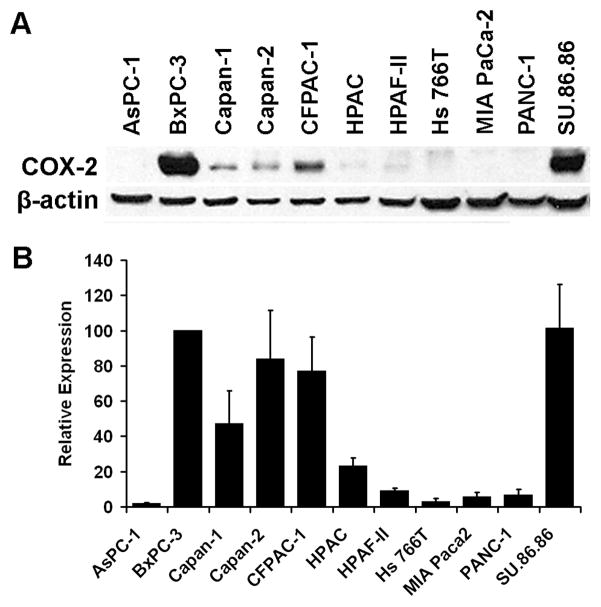
Although several groups have reported differential COX-2 expression in PA cell lines 70–73, very little quantitative information is available. In our laboratory, we used relative quantitation to measure basal COX-2 protein expression in the 11 cell lines (Figure 1, Table 4) by densitometric analysis of Western blots. These data are consistent with qualitative reports that showed that COX-2 protein expression was detectable in BxPC-3, Capan-1, Capan-2 and HPAF-II, but not AsPC-1, MIA PaCa-2 or PANC-1 cell lines 70–73. Furthermore, with the exception of Capan-2, the measured expression of COX-2 protein was remarkably similar to the reported differential levels of PGE2 74, 75 suggesting that expression corresponds to function of COX-2 in PA cell lines. In our study, Capan-2 showed highly variable COX-2 expression in the four replicate experiments (Figure 1) indicating that COX-2 expression was highly dependent in experimental conditions in this cell line. Capan-2, and PANC-1, also showed variable relative expression of other pro-angiogenic factors (Table 4). However, for those cell lines examined in three or more studies, consensus was achieved for several cell lines. BxPC-3 and Capan-1 both showed consistently high levels of pro-angiogenic factors suggesting a high angiogenic potential for these cell lines, whereas AsPC-1 and MIA PaCa-2 showed consistently low levels of pro-angiogenic factors.
Figure 1.
Relative expression of COX-2 in PA cell lines. Basal expression of COX-2 was determined by Western blot analysis in PA cell line lysates and quantified by densitometry. A: Representative Western blot. All cell lines were acquired from the American Type Culture Collection (ATCC, Manassas, VA) and propagated in ATCC recommended media. Cells were grown to 80% confluence before preparation of cell lysates. Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes using standard protocols. The membranes were probed with antibodies to COX-2 (mouse monoclonal, Cayman Chemical, Ann Arbor, MI) and β-actin (rabbit monoclonal, Cell Signaling Technology, Danvers, MA) or GAPDH (mouse monoclonal, Novus Biologicals, Littleton, CO) as total protein loading controls. B: Densitometric analyses. Autoradiographs of Western blots were quantified using ImageJ software (National Institutes of Health, http://rsb.info.nih.gov/ij/). COX-2 intensity was first normalized to the corresponding loading control (β-actin or GAPDH) and then normalized to the BxPC-3 ratio for each blot. Data represents the combined results of four independent experiments (mean ± SEM).
Table 4.
Quantitative measurements of pro-angiogenic factors in pancreatic cancer cell lines. The quantitative measurement in the specified units is reported after each cell line (parentheses). Data abstraction was limited to those studies that directly compared multiple cell lines.
| Factor | Relative Effect | Units | Ref. |
|---|---|---|---|
| COX-2 | Capan-2 (200) > SU 86.86 (102) > BxPC-3 (94)> CFPAC-1 (87) > Capan-1 (61) > Hs 766T (26) > HPAC (23) > HPAF-II | Relative | This |
| (7) > MIA PaCa-2 (3) > PANC-1 (3) > AsPC-1 (1) | Intensity | Study | |
| PGE2 | |||
| BxPC-3 (92.3) > CFPAC-1 (26.2) > MIA PaCa-2 (undetectable) | pg/ml | 74 | |
| BxPC-3 (48) > Capan-1 (18) = Capan-2 (18) > HPAF-II (15) > Hs 766T (3) > AsPC-1 (2) > PANC-1 (1) | pg/μg protein | 75 | |
| VEGF | |||
| BxPC-3 (2000) > AsPC-1 (250) | pg/mg protein | 59 | |
| HPAF-II (8120) > AsPC-1 (4200) | pg/106 cells | 87 | |
| BxPC-3 (2383) > PANC-1 (1415) > Capan-1 (981) > MIA PaCa-2 (453) > AsPC-1 (179) | pg/106 cells | 123 | |
| Capan-1 (1900) > PANC-1 (1700) > MIA PaCa-2 (850) | pg/ml | 124 | |
| IL-8 | |||
| BxPC-3 (3.4) > MIA PaCa-2 (0.5) > Capan-2 (0.4) | ng/ml/106 cells | 76 | |
| Capan-1 (1700) > Capan-2 (220) | pg/ml | 125 |
A question remains as to the value of measuring pro-angiogenic factors as a surrogate for angiogenic potential rather than direct measurement of differential angiogenesis in PA cell lines. Although several studies report direct measurement of angiogenesis for individual cell lines, there is a paucity of information directly comparing multiple cell lines. Matsuo et al. showed that BxPC-3 cells secreted high levels of IL-1α and IL-8 whereas Capan-2 and MIA PaCa-2 cells secreted much lower levels of IL-8 and undetectable levels of IL-1α 76. Consistent with a role for these cytokines in angiogenesis, tube formation by HUVEC cells was significantly enhanced by co-culture with BxPC-3, but not MIA PaCa-2 cell lines and enhanced tube formation was attenuated in the presence of IL-1α or IL-8 blocking antibodies 76. These data are consistent with the consensus conclusion drawn above, that BxPC-3 and Capan-1 have high pro-angiogenic potential and ASPC-1 and MIA PaCa-2 have low pro-angiogenic potential, and suggest that levels of pro-angiogenic factor may be a useful surrogate for angiogenic potential.
Tumorigenicity
Tumorigenicity describes a cancer cell line’s ability or propensity to produce tumors in vivo. Tumor volume, tumor mass, frequency to develop and/or rate of growth have been used to estimate tumorigenicity. These parameters have been commonly measured after injecting a suspension of pancreas cancer cells into the subcutaneous tissue of an immunocompromised mouse and allowing a tumor to form. Tumor volume and/or mass are then measured at autopsy. Other methods to determine tumorigenicity include intraperitoneal or intravenous injection of tumor cells, orthotopic transplantation of tumor tissue obtained from a donor nude mouse with a subcutaneous tumor, or direct orthotopic injection of human PA cells into the pancreas of nude mice. Individually, these models recapitulate some, but not all, aspects of the natural course of clinical tumor progression. Furthermore, research has shown that the site of growth influences genetic signaling and thus could be a confounding variable when comparing studies 77. Although our review yielded no overall consensus for tumorgenicity in PA cell lines, it is clear that differential tumorgenicity is highly dependent on the specific characteristic measured.
Subcutaneous injections of tumor cells is technically convenient in that the injection site is readily accessible, multiple tumors can be grown in the same mouse, and repeated measurements of tumor size can be easily made lending power to statistical comparisons. Of the few studies that had quantitative measures of tumor size in the subcutaneous injection model, little consensus was apparent (Table 5). In two studies, BxPC-3 tumors were consistently larger than PANC-1 tumors 78, 79 whereas a third showed the opposite 80. The time for tumor development appears to be a critical factor in the subcutaneous injection model and may contribute to the reported discrepancies in tumor size. Diaz and coworkers 81 injected mice with suspensions of either BxPC-3, Capan-1 or PANC-1 cells and found that after 14 weeks only the Capan-1-injected mice had developed tumors. It reportedly took more than 4 months for the BxPC-3 and PANC-1 mice to develop tumors. Elevated latency for BxPC-3 tumor development was confirmed in another study in which BxPC-3 tumors did not begin growing until 40 days after injection 82. In the same study, Capan-1 tumors had measurable growth increase ten days after injection. Another study showed a 4 week latency for PANC-1 tumor formation and a 3 week latency period for MIA PaCa-2 tumors 83. Taken together, the results of these studies suggest a consensus in which Capan-1 cells rapidly form tumors whereas BxPC-3 and PANC-1 cells have high latency periods before tumor development after subcutaneous injection.
Table 5.
Differential tumorigenicity of PA cell lines. In all experiments, mice received subcutaneous injections of PA cell lines. The reported tumor volume measurement (mm3) is given in parentheses after each cell line.
| # Cells Injected | Relative Effect (Tumor Size, mm3) | Time (Days) | Ref. |
|---|---|---|---|
| 1 × 106 | BxPC-3 (~2000) > PANC-1 (~500) | 54 | 78 |
| 1 × 106 | PANC-1 (~570) > BxPC-3 (~155) | 42 | 80 |
| 1 × 106 | BxPC-3 (~2000) > AsPC-1 (~1450) > PANC-1 (~750) > MIA PaCa-2 (~525) | 21 | 79 |
| 3 × 106 | AsPC-1 (~2250) > HPAC (~1300) | 24 | 126 |
When Severe Combined Immunodeficiency (SCID) mice received intraperitoneal injections of 5 × 106 cells 100% of the Capan-1, 86% of the Panc-1, and 66% of the MIA PaCa-2 developed primary tumors after one week. Thus it appears that Capan-1 can consistently produce subcutaneous and intraperitoneal tumors, but there is variability in the ability of MIA PaCa-2 and Panc-1 to produce tumors. In terms of intraperitoneal tumor size after 30 days the MIA PaCa-2 tumors were the largest. Capan-1 tumors were second largest and PANC-1 produced the smallest tumors 84.
An alternative method for determining tumorigenicity parameters involves using donor nude mice to grow tumors, which are then removed, sliced into small fragments and then placed directly into the pancreas of a recipient nude mouse. The subsequent tumors in the recipient mouse are then used to measure tumor volume, tumor mass, or growth rate. Since latency is less of a problem, this method often yields robust tumor growth. Eibl et al. 85 reported that nude mice that received orthotopic implants of 1 mm3 Capan-2 and MIA PaCa-2 tumor fragments into the tail of the pancreas developed significantly smaller Capan-2 tumors than MIA PaCa-2 tumors. They also report that 100% of the recipient mice developed tumors. In another study, nude mice were subjected to orthotopic transplants of 1mm3 tumor fragments into the tail of the pancreas, and the results showed that tumor volumes were largest for MIA PaCa-2, PANC-1 tumors were second largest, and finally AsPC-1 yielded the smallest tumors 86. A similar study resulted in HPAF-II tumors that were twice as large as tumors in the AsPC-1 group 87. Thus it appears that MIA PaCa-2 tumor implants consistently produced larger tumors whereas AsPC-1 tumor implants produced smaller tumors.
Orthotopic implantation of tumor fragments may not fully recapitulate the early events of clinical tumor development since angiogenic signals and tumor microenvironment are established in the subcutaneous setting. Orthotopic injection of tumor cells requires de novo tumor development in the context of the pancreas and thus should better mimic the clinical course of the disease. Few studies have been reported that compare multiple cell lines although one comprehensive study in which SCID mice received injections of 106 PA cells directly into the pancreas yielded the following data concerning the percentage of mice that developed tumors after 100 days: AsPC-1: 100% (10/10), CFPAC-1: 100% (10/10), HPAF-II: 100% (8/8), Capan-2: 90% (9/10), Hs 766T: 90% (9/10), HPAC: 88% (7/8), PANC-1: 80% (8/10), and BxPC-3: 67% (6/9) 88. Katayama et al. 89 observed that tumor masses were consistently higher for orthotopically injected MIA PaCa-2 than for HPAC cells when measured between 2 and 5 weeks. Another study showed that MIA PaCa-2 and AsPC-1 cells had similar growth characteristics at 2 and 5 weeks post orthotopic injection 90.
One study of interest compared tumors developed from implanted tumor fragments to tumors developed from direct injection of tumor cells into the pancreas of nude mice 91. Tumors developed in 100% of mice subjected to implantation, however, tumor development varied in mice subjected to orthotopic injection. Specifically, AsPC-1 had 100% tumor development compared to 92% for HPAF-II, 83% for MIA PaCa-2, and 33% for Capan-1. At necropsy (14 weeks) tumor volume was largest to smallest in the following order: HPAF-II > MIA PaCa-2 > AsPC-1 Capan-1 for implanted tumors 91. Tumor volumes showed a similar magnitude and propensity using injected cell lines, although Capan-1 tumors were significantly smaller than seen for implanted tumors 91. This study demonstrates that, at least for some cell lines, tumorigenicity may vary depending on the methodology used.
GENOTYPE
Pancreatic cancers accumulate multiple genetic alterations, including frequent mutations in the KRAS (v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog), TP53 (encoding the p53 protein), CDKN2A (also known as p16 or p16INK4a), and SMAD4 (SMAD family member 4, also known as DPC4; deleted in pancreatic carcinoma locus 4) genes 92–94. A summary of these four mutations in PA cell lines is presented in Table 6. Information on the genotype of these cell lines provide a background for understanding how alterations in these pathways contribute to the growth characteristics, tumorigenicity, and chemosensitivity. The relationship of genotype to phenotype is still unclear, as there are few studies available that directly assess the effect of these mutations on cell behavior. There is some evidence that the mutational status of KRAS, TP53, CDKN2A/p16, and SMAD4/DPC4 do not correlate with either the grade of differentiation 95 or the biological behavior 96 of pancreatic cancer cell lines. However, one group found that in vivo metastatic behavior was associated with p53 status, suggesting that genotype and phenotype may be related 88.
Table 6.
Genotype. The four most common mutations in pancreas cancer.
| Cell Line | KRASa | TP53 | CDKN2A/p16 | SMAD4/DPC4 |
|---|---|---|---|---|
| AsPC-1 | 12 Asp88, 101, 107, 110, 114, 127, 128 | 135 Δ1 bp88, 101, 107, 108Intron 4 Δ200 bp splice site101HD exon 5110 | WT88Δ2 bp107–109HD110 | WT88, 107, 114HD110100Thr113 |
| BxPC-3 | WT88, 101, 110, 114 | 220 Cys88, 101, 108, 110 | WT88bHD108–110 | HD88, 110, 112, 114 |
| Capan-1 | 12 Val88, 101, 127, 128 | 159 Val88, 101, 108 | HD88, 108, 109 | 577 Leu88343 STOP113 |
| Capan-2 | 12 Val88, 101, 114 | WT88, 108Intron 4 Δ200 bp splice site101 | WT886 bp ins1087 bp ins109 | WT88 |
| CFPAC-1 | 12 Val88, 107 | 242 Arg88, 107, 108 | WT108, 109WT88bPromoter methylation107 | HD88, 107, 112 |
| HPAC | 12 Asp88 | WT88 | 112 amber STOP88 | WT88 |
| HPAF-II | 12 Asp88, 101, 107 | 151 Ser88, 101, 107 | Δ20–2588Δ26–27109Δ29–34107 | WT88, 101, 107 |
| Hs 766T | WT88, 101, 11461 His102d | WT88, 108Mut 225–282101cΔexons 2–4e | WT88Intron 2 splice site108, 109 | HD88, 112, 114 |
| MIA PaCa-2 | 12 Cys101, 107, 110, 128 | 248 Trp101, 107, 108, 110 | HD107–110 | WT107, 110 |
| PANC-1 | 12 Asp88, 101, 107, 110, 127, 128 | 273 His88, 107, 108, 110, 127273 Cys101 | HD88, 107–110 | WT88, 107, 110 |
| SU.86.86 | 12 Asp110 | 245 Ser108, 110 | HD108–110 | WT110 |
KRAS mutations are very common in pancreatic cancer, occurring in almost all primary tumors, and are present early in the progression of the disease 97–99. The RAS family members (H-, K- and N-RAS) are guanine nucleotide binding proteins that transmit signals from cell surface receptors by cycling from an inactive GDP-bound state to an active GTP-bound state. Mutations in codons 12, 13, or 61 inhibit the GTPase activity of RAS, leading to oncogenic RAS protein that is constitutively activated in its GTP-bound state, inducing multiple signaling pathways 100. Of clinical significance are the findings that activating mutations of KRAS activate the Raf/mitogen-activated protein kinase pathway and the Akt/protein kinase B pathway, resulting in the up-regulation of COX-2 transcription and stabilization of its mRNA, respectively63. Loukopoulos et al. directly measured the four most common mutations using sequence analysis in ten pancreatic cancer cell lines 88. Mutations were found in the second base of codon 12 of KRAS in all cell lines but two (Hs 766T, BxPC-3) 88. In a similar study by Berrozpe and co-workers, KRAS codon 12 mutations were found in 14 of 17 pancreatic cancer cell lines analyzed while Hs 766T, BxPC-3, and SW979 were determined to be wild-type 101. In these and other studies which looked exclusively at exon 1 of KRAS, no mutations were found for Hs 766T. In a subsequent assay assessing the activation state of RAS by measuring the percentage of RAS bound to GTP, Hs 766T was found to contain a high level of activated RAS, similar to cell lines containing a codon 12 mutation. Sequencing of KRAS exon 2 revealed an activating mutation in codon 61 of Hs 766T 102. There is a consensus that BxPC-3 cells contain wild-type RAS and are not RAS-activated. Consequently, although BxPC-3 is one of the most commonly used PA cell lines, it is probably not representative of the majority of pancreatic cancers.
Inactivation of the CDKN2A/p16 tumor suppressor gene is thought to be an early event in the progression of pancreas cancers, since CDKN2A/p16 inactivation can be found in up to 40% of precursor PanIN (Pancreatic Intraepithelial Neoplasia) lesions 103, 104. The p16 pathway is disrupted, either by mutation, homozygous deletion, or promoter methylation, in up to 98% of all pancreatic carcinomas 105. In a recent examination of 25 primary ductal adenocarcinomas, p16 was inactivated or mutated in 80% of tumors 106. The most common cause of p16 inactivation was aberrant promoter methylation, seen in 52% of cases. Sequence mutations (16%) and homozygous deletions (12%) were also found. Correspondingly, p16 is also inactivated in many pancreas cancer cell lines. Using PCR and direct sequencing of exons 1 and 2, Loukopoulos found alterations of CDKN2A/p16 in 7 of 10 cell lines 88. Capan-1 and PANC-1 contained homozygous deletions, while HPAF-II had an in-frame deletion and HPAC had a mutation in exon 2. In each case, sequence analysis detected only the mutated allele, indicating a loss of the normal allele which is important for loss of tumor suppressor function. CFPAC-1 contained wild-type sequence but did not express protein, as shown by western blotting. This is in agreement with previous reports that the CDKN2A/p16 promoter is methylated in CFPAC-1 cells 107. Similarly, BxPC-3 showed a wild-type sequence but undetectable protein product. This may be explained by the presence of a homozygous deletion in exons 2–3 108–110. AsPC-1, Capan-2 and Hs 766T were reported to be wild-type for the CDKN2A/p16 sequence. However, AsPC-1 has also been shown in other reports to have either a homozygous deletion of CDKN2A/p16 exons 2–3110 and/or a frameshift mutation 107–109. Capan-2 does express p16 protein, however this protein was shown by other groups to be inactivated by an insertion in codons 11 and 12 108, 109. There is also disagreement on the status of Hs 766T, which was shown to be wild-type for CDKN2A/p16 but has also been found to contain possible mutations 108, 109. Considering these discrepancies, it is possible that all of these cell lines are lacking functional CDKN2A/p16. Additionally, taking into account epigenetic changes such as methylation, CDKN2A/p16 deficiency may be the most common occurrence in the development of pancreas cancer.
Mutations in the TP53 tumor suppressor gene are common in many types of human tumors, including more than 50% of pancreatic adenocarcinomas, where they occur late in the tumorigenesis process 94. Berrozpe et al. 101 reported TP53 mutations in 26% of primary pancreas cancers and metastases. Interestingly, mutations were much more common in cell lines, with 15 of 17 pancreatic cancer cell lines showing mutations. Moore found TP53 mutations in 95% of the cell lines tested 107. In the Loukopoulos study, TP53 mutations were missense in eight of ten cell lines, with Capan-2 and HPAC being wild-type 88. As seen with CDKN2A/p16, only the mutant p53 allele was detected, indicating loss of the wild-type allele. Capan-2 has been reported by several groups to be wild-type for TP53, but it should be noted that Berrozpe found a 200-bp deletion 101. In support of Capan-2 possessing wild-type TP53 is a study showing that radiation was able to induce elevated TP53 and p21WAF1/CIP1 protein expression in Capan-2 cells, suggesting the presence of a functional TP53 response. This response to radiation was not seen in PANC-1 or MIA PaCa-2 cells, which contain TP53 mutations 111. There is also some discrepancy on the TP53 status of Hs 766T, with some groups finding mutations while others report wild-type sequence. In one case, the presence of a mutation was detected between codons 225–282, but the actual mutation was not sequenced 101. Overall, mutations in TP53 were very common in PA cell lines. TP53 and CDKN2A/p16 both play significant roles in G1/S cell cycle checkpoint control and maintenance of genome integrity after DNA damage. The high frequency of loss of CDKN2A/p16 and TP53 underscores the importance of abrogation of the G1/S cell cycle checkpoint in the progression of pancreatic cancer.
SMAD4/DPC4, a member of the transforming growth factor β family and also a tumor suppressor, is inactivated in approximately 48 – 55% of invasive pancreatic adenocarcinomas 112, 113. Accordingly, SMAD4/DPC4 inactivation has been found at a similar rate in PA cell lines. BxPC-3, CFPAC-1, and Hs 766T have all been shown to lack SMAD4/DPC4 protein due to homozygous deletions 88, 110, 112, 114, 115. Capan-1 cells possess a point mutation in SMAD4/DPC4 that result in loss of expression 88, 113. However, no SMAD4/DPC4 alterations have been found in Capan-2, MIA PaCa-2, PANC-1, or SU.86.86 88, 107, 110. Divergent results have been seen for AsPC-1, with some groups finding wild-type sequence 88, 107, 114 while others have reported a non-conservative point mutation 113 or homozygous deletion 110. In a comprehensive analysis by Moore, using direct sequencing as well as methylation-specific PCR to test for the four mutations in 22 pancreatic cancer cell lines, inactivation of SMAD4/DPC4 was always found along with alterations in the three other genes 107. This supports data from a study on the molecular pathogenesis of pancreas adenocarcinoma which shows that loss of SMAD4/DPC4 occurs late in the progression towards invasive cancers 97.
To summarize, there is a consensus that KRAS is activated in 10 of 11 cell lines, with BxPC-3 being the wild-type exception. SMAD4/DPC4 is clearly inactivated in 4 of the 11 cell lines. AsPC-1 was the only cell line with divergent results for SMAD4/DPC4. The status of the tumor suppressor genes TP53 and CDKN2A/p16 are more inconsistent. The three cell lines AsPC-1, Capan-2, and Hs 766T showed variable alterations in these genes. It is possible that these cells have acquired additional alterations during routine culturing. It is also been suggested that heterogeneous populations in the original tumor could provide a source of different genetic variants 110. With this in mind, researchers should be aware of the potential for discrepancies in the mutational status of cell lines currently being used in each individual laboratory versus that reported in the literature.
CONCLUSIONS
Sufficient discrepancies exist in the literature for both phenotype and genotype of PA cell lines to warrant careful scrutiny during cell line selection and thorough application of appropriate controls during experimental design. Although some discrepancies might be explained by technical differences in the application of specific assays, it is likely that differential selection of a heterogeneous tumor cell population inherent in the individual cell lines and differential accumulation of genetic changes (“drift”) contribute to the reported disparities. Phenotypic differences due to differential propagation of cell lines have been well documented with long-term sub-culturing resulting in divergent effects on morphology, development, and gene expression 116–121. Long-term propagation may introduce selective pressures leading to overgrowth of faster growing or more adherent sub-types. Cross-contamination, mycoplasma contamination, and miss-identification have long plagued the study of cell lines and are also likely contributing factors to the observed discrepancies. These findings suggest that investigators authenticate cell lines and limit the number of passages.
Several points of consensus were identified in our review; however, it is clear that reproduction of prior results may be problematic and investigators should limit interpretations to internally consistent data. Although it may be convenient to consider PA cell lines as representing a homogeneous population of cells, the potential for genetic drift and the presence of tumor cell sub-types suggests that it may be expedient and more reflective of the clinical situation to consider PA cell lines as a heterogeneous population of cells.
Acknowledgments
This work was supported in part by a Ruth L. Kirschstein National Research Service Award from the National Institutes of Health (T35HL07744) to E.L.D.
Contributor Information
Emily L. Deer, Department of Surgery, University of Utah School of Medicine, Salt Lake City, Utah.
Jessica Gonzalez-Hernandez, Department of Surgery, University of Utah School of Medicine, Salt Lake City, Utah.
Jill D. Coursen, Department of Surgery, University of Utah School of Medicine, Salt Lake City, Utah.
Jill E. Shea, Department of Surgery, University of Utah School of Medicine, Salt Lake City, Utah.
Josephat Ngatia, Department of Surgery, University of Utah School of Medicine, Salt Lake City, Utah.
Courtney L. Scaife, Department of Surgery, University of Utah School of Medicine, Salt Lake City, Utah.
Matthew A. Firpo, Department of Surgery, University of Utah School of Medicine, Salt Lake City, Utah.
Sean J. Mulvihill, Department of Surgery, University of Utah School of Medicine, Salt Lake City, Utah.
References
- 1.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas EJ, Fiegler H, Rowan A, et al. Array comparative genomic hybridization analysis of colorectal cancer cell lines and primary carcinomas. Cancer Res. 2004;64:4817–4825. doi: 10.1158/0008-5472.CAN-04-0328. [DOI] [PubMed] [Google Scholar]
- 3.Larramendy ML, Lushnikova T, Bjorkqvist AM, et al. Comparative genomic hybridization reveals complex genetic changes in primary breast cancer tumors and their cell lines. Cancer Genet Cytogenet. 2000;119:132–138. doi: 10.1016/s0165-4608(99)00226-5. [DOI] [PubMed] [Google Scholar]
- 4.Arumugam T, Simeone DM, Van Golen K, et al. S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res. 2005;11:5356–5364. doi: 10.1158/1078-0432.CCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 5.Marchesi F, Monti P, Leone BE, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–8427. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 6.Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen WH, Horoszewicz JS, Leong SS, et al. Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites. In Vitro. 1982;18:24–34. doi: 10.1007/BF02796382. [DOI] [PubMed] [Google Scholar]
- 8.Tan MH, Nowak NJ, Loor R, et al. Characterization of a new primary human pancreatic tumor line. Cancer Invest. 1986;4:15–23. doi: 10.3109/07357908609039823. [DOI] [PubMed] [Google Scholar]
- 9.Kyriazis AP, Kyriazis AA, Scarpelli DG, et al. Human pancreatic adenocarcinoma line Capan-1 in tissue culture and the nude mouse: morphologic, biologic, and biochemical characteristics. Am J Pathol. 1982;106:250–260. [PMC free article] [PubMed] [Google Scholar]
- 10.McIntyre LJ, Kim YS. Effects of sodium butyrate and dimethylsulfoxide on human pancreatic tumor cell lines. Eur J Cancer Clin Oncol. 1984;20:265–271. doi: 10.1016/0277-5379(84)90194-9. [DOI] [PubMed] [Google Scholar]
- 11.Kyriazis AA, Kyriazis AP, Sternberg CN, et al. Morphological, biological, biochemical, and karyotypic characteristics of human pancreatic ductal adenocarcinoma Capan-2 in tissue culture and the nude mouse. Cancer Res. 1986;46:5810–5815. [PubMed] [Google Scholar]
- 12.Schoumacher RA, Ram J, Iannuzzi MC, et al. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc Natl Acad Sci U S A. 1990;87:4012–4016. doi: 10.1073/pnas.87.10.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McWilliams R, Highsmith WE, Rabe KG, et al. Cystic fibrosis transmembrane regulator gene carrier status is a risk factor for young onset pancreatic adenocarcinoma. Gut. 2005;54:1661–1662. doi: 10.1136/gut.2005.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezzilli R, Morselli-Labate AM, Mantovani V, et al. Mutations of the CFTR gene in pancreatic disease. Pancreas. 2003;27:332–336. doi: 10.1097/00006676-200311000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Malats N, Casals T, Porta M, et al. Cystic fibrosis transmembrane regulator (CFTR) DeltaF508 mutation and 5T allele in patients with chronic pancreatitis and exocrine pancreatic cancer. PANKRAS II Study Group. Gut. 2001;48:70–74. doi: 10.1136/gut.48.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gower WRRR, Jr, Godellas CV, Fabri PJ. HPAC, a new human glucocorticoid-sensitive pancreatic ductal adenocarcinoma cell line. In Vitro Cell Dev Biol. 1994;30A:151–161. doi: 10.1007/BF02631438. [DOI] [PubMed] [Google Scholar]
- 17.Metzgar RS, Gaillard MT, Levine SJ, et al. Antigens of human pancreatic adenocarcinoma cells defined by murine monoclonal antibodies. Cancer Res. 1982;42:601–608. [PubMed] [Google Scholar]
- 18.Owens RB, Smith HS, Nelson-Rees WA, et al. Epithelial cell cultures from normal and cancerous human tissues. J Natl Cancer Inst. 1976;56:843–849. doi: 10.1093/jnci/56.4.843. [DOI] [PubMed] [Google Scholar]
- 19.Smith HS. In vitro properties of epithelial cell lines established from human carcinomas and nonmalignant tissue. J Natl Cancer Inst. 1979;62:225–230. [PubMed] [Google Scholar]
- 20.Yunis AA, Arimura GK, Russin DJ. Human pancreatic carcinoma (MIA PaCa-2) in continuous culture: sensitivity to asparaginase. Int J Cancer. 1977;19:218–235. doi: 10.1002/ijc.2910190118. [DOI] [PubMed] [Google Scholar]
- 21.Lieber M, Mazzetta J, Nelson-Rees W, et al. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int J Cancer. 1975;15:741–747. doi: 10.1002/ijc.2910150505. [DOI] [PubMed] [Google Scholar]
- 22.Drucker BJ, Marincola FM, Siao DY, et al. A new human pancreatic carcinoma cell line developed for adoptive immunotherapy studies with lymphokine-activated killer cells in nude mice. In Vitro Cell Dev Biol. 1988;24:1179–1187. doi: 10.1007/BF02624187. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre LJ, Kleinman HK, Martin GR, et al. Attachment of human pancreatic tumor cell lines to collagen in vitro. Cancer Res. 1981;41:3296–3299. [PubMed] [Google Scholar]
- 24.Givant-Horwitz V, Davidson B, Reich R. Laminin-induced signaling in tumor cells: the role of the M(r) 67,000 laminin receptor. Cancer Res. 2004;64:3572–3579. doi: 10.1158/0008-5472.CAN-03-3424. [DOI] [PubMed] [Google Scholar]
- 25.Greco E, Basso D, Fogar P, et al. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1beta not by transforming growth factor-beta1. Int J Biol Markers. 2005;20:235–241. doi: 10.1177/172460080502000406. [DOI] [PubMed] [Google Scholar]
- 26.Navaglia F, Fogar P, Greco E, et al. CD44v10: an antimetastatic membrane glycoprotein for pancreatic cancer. Int J Biol Markers. 2003;18:130–138. doi: 10.1177/172460080301800206. [DOI] [PubMed] [Google Scholar]
- 27.Shirk AJ, Kuver R. Epidermal growth factor mediates detachment from and invasion through collagen I and Matrigel in Capan-1 pancreatic cancer cells. BMC Gastroenterol. 2005;5:12. doi: 10.1186/1471-230X-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefani AL, Basso D, Panozzo MP, et al. Cytokines modulate MIA PaCa 2 and CAPAN-1 adhesion to extracellular matrix proteins. Pancreas. 1999;19:362–369. doi: 10.1097/00006676-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Arao S, Masumoto A, Otsuki M. Beta1 integrins play an essential role in adhesion and invasion of pancreatic carcinoma cells. Pancreas. 2000;20:129–137. doi: 10.1097/00006676-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Sawai H, Yamamoto M, Okada Y, et al. Alteration of integrins by interleukin-1alpha in human pancreatic cancer cells. Pancreas. 2001;23:399–405. doi: 10.1097/00006676-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Lohr M, Trautmann B, Gottler M, et al. Expression and function of receptors for extracellular matrix proteins in human ductal adenocarcinomas of the pancreas. Pancreas. 1996;12:248–259. doi: 10.1097/00006676-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Kleeff J, Friess H, Berberat PO, et al. Pancreatic cancer--new aspects of molecular biology research. Swiss Surg. 2000;6:231–234. doi: 10.1024/1023-9332.6.5.231. [DOI] [PubMed] [Google Scholar]
- 33.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai AQ, Landman KA, Hughes BD. Multi-scale modeling of a wound-healing cell migration assay. J Theor Biol. 2007;245:576–594. doi: 10.1016/j.jtbi.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Stahle M, Veit C, Bachfischer U, et al. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci. 2003;116:3835–3846. doi: 10.1242/jcs.00679. [DOI] [PubMed] [Google Scholar]
- 36.Menke A, Philippi C, Vogelmann R, et al. Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res. 2001;61:3508–3517. [PubMed] [Google Scholar]
- 37.Lin M, DiVito MM, Merajver SD, et al. Regulation of pancreatic cancer cell migration and invasion by RhoC GTPase and caveolin-1. Mol Cancer. 2005;4:21. doi: 10.1186/1476-4598-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrell TJ, Barbot DJ, Rosato FE. Pancreatic resection combined with intraoperative radiation therapy for pancreatic cancer. Ann Surg. 1997;226:66–69. doi: 10.1097/00000658-199707000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takada M, Nakamura Y, Koizumi T, et al. Suppression of human pancreatic carcinoma cell growth and invasion by epigallocatechin-3-gallate. Pancreas. 2002;25:45–48. doi: 10.1097/00006676-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Albini A, Iwamoto Y, Kleinman HK, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 41.Ellenrieder V, Hendler SF, Ruhland C, et al. TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer. 2001;93:204–211. doi: 10.1002/ijc.1330. [DOI] [PubMed] [Google Scholar]
- 42.Funahashi H, Takeyama H, Sawai H, et al. Alteration of integrin expression by glial cell line-derived neurotrophic factor (GDNF) in human pancreatic cancer cells. Pancreas. 2003;27:190–196. doi: 10.1097/00006676-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Nio Y, Ohmori H, Minari Y, et al. A quinolinone derivative, vesnarinone (OPC-8212), significantly inhibits the in vitro and in vivo growth of human pancreatic cancer cell lines. Anticancer Drugs. 1997;8:686–695. doi: 10.1097/00001813-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Duxbury MS, Ito H, Zinner MJ, et al. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–1456. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 45.Jimenez RE, Hartwig W, Antoniu BA, et al. Effect of matrix metalloproteinase inhibition on pancreatic cancer invasion and metastasis: an additive strategy for cancer control. Ann Surg. 2000;231:644–654. doi: 10.1097/00000658-200005000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okada Y, Takeyama H, Sato M, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto-oncogene with reference to glial-cell-line-derived neurotrophic factor (GDNF) Int J Cancer. 1999;81:67–73. doi: 10.1002/(sici)1097-0215(19990331)81:1<67::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 47.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005;46:481–489. doi: 10.1111/j.1365-2559.2005.02142.x. [DOI] [PubMed] [Google Scholar]
- 49.Ikeda N, Adachi M, Taki T, et al. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo Y, Baba H, Fukuda T, et al. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 51.Ellis LM, Takahashi Y, Fenoglio CJ, et al. Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur J Cancer. 1998;34:337–340. doi: 10.1016/s0959-8049(97)10068-5. [DOI] [PubMed] [Google Scholar]
- 52.Fujimoto K, Hosotani R, Wada M, et al. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34:1439–1447. doi: 10.1016/s0959-8049(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 53.Itakura J, Ishiwata T, Friess H, et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3:1309–1316. [PubMed] [Google Scholar]
- 54.Fang J, Yan L, Shing Y, et al. HIF-1alpha-mediated up-regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res. 2001;61:5731–5735. [PubMed] [Google Scholar]
- 55.Giordano FJ, Johnson RS. Angiogenesis: the role of the microenvironment in flipping the switch. Curr Opin Genet Dev. 2001;11:35–40. doi: 10.1016/s0959-437x(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 56.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 57.Udagawa T, Fernandez A, Achilles EG, et al. Persistence of microscopic human cancers in mice: alterations in the angiogenic balance accompanies loss of tumor dormancy. Faseb J. 2002;16:1361–1370. doi: 10.1096/fj.01-0813com. [DOI] [PubMed] [Google Scholar]
- 58.Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer. 2003;2:8. doi: 10.1186/1476-4598-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu J, Lloyd FL, Trifan OC, et al. Potential involvement of the cyclooxygenase-2 pathway in the regulation of tumor-associated angiogenesis and growth in pancreatic cancer. Mol Cancer Ther. 2003;2:1–7. [PubMed] [Google Scholar]
- 60.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 62.Ding XZ, Hennig R, Adrian TE. Lipoxygenase and cyclooxygenase metabolism: new insights in treatment and chemoprevention of pancreatic cancer. Mol Cancer. 2003;2:10. doi: 10.1186/1476-4598-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Rayes BF, Ali S, Sarkar FH, et al. Cyclooxygenase-2-dependent and -independent effects of celecoxib in pancreatic cancer cell lines. Mol Cancer Ther. 2004;3:1421–1426. [PubMed] [Google Scholar]
- 64.Form DM, Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983;172:214–218. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- 65.Poligone B, Baldwin AS. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J Biol Chem. 2001;276:38658–38664. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 66.Arenberg DA, Kunkel SL, Polverini PJ, et al. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97:2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 68.Miller LJ, Kurtzman SH, Wang Y, et al. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998;18:77–81. [PubMed] [Google Scholar]
- 69.Trevino JG, Summy JM, Gray MJ, et al. Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res. 2005;65:7214–7222. doi: 10.1158/0008-5472.CAN-04-3858. [DOI] [PubMed] [Google Scholar]
- 70.Eibl G, Bruemmer D, Okada Y, et al. PGE(2) is generated by specific COX-2 activity and increases VEGF production in COX-2-expressing human pancreatic cancer cells. Biochem Biophys Res Commun. 2003;306:887–897. doi: 10.1016/s0006-291x(03)01079-9. [DOI] [PubMed] [Google Scholar]
- 71.Eibl G, Reber HA, Wente MN, et al. The selective cyclooxygenase-2 inhibitor nimesulide induces apoptosis in pancreatic cancer cells independent of COX-2. Pancreas. 2003;26:33–41. doi: 10.1097/00006676-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Molina MA, Sitja-Arnau M, Lemoine MG, et al. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356–4362. [PubMed] [Google Scholar]
- 73.Yip-Schneider MT, Sweeney CJ, Jung SH, et al. Cell cycle effects of nonsteroidal anti-inflammatory drugs and enhanced growth inhibition in combination with gemcitabine in pancreatic carcinoma cells. J Pharmacol Exp Ther. 2001;298:976–985. [PubMed] [Google Scholar]
- 74.Raut CP, Nawrocki S, Lashinger LM, et al. Celecoxib inhibits angiogenesis by inducing endothelial cell apoptosis in human pancreatic tumor xenografts. Cancer Biol Ther. 2004;3:1217–1224. doi: 10.4161/cbt.3.12.1221. [DOI] [PubMed] [Google Scholar]
- 75.Yip-Schneider MT, Barnard DS, Billings SD, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139–146. doi: 10.1093/carcin/21.2.139. [DOI] [PubMed] [Google Scholar]
- 76.Matsuo Y, Sawai H, Funahashi H, et al. Enhanced angiogenesis due to inflammatory cytokines from pancreatic cancer cell lines and relation to metastatic potential. Pancreas. 2004;28:344–352. doi: 10.1097/00006676-200404000-00025. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura T, Fidler IJ, Coombes KR. Gene expression profile of metastatic human pancreatic cancer cells depends on the organ microenvironment. Cancer Res. 2007;67:139–148. doi: 10.1158/0008-5472.CAN-06-2563. [DOI] [PubMed] [Google Scholar]
- 78.Aubert M, Crotte C, Bernard JP, et al. Decrease of human pancreatic cancer cell tumorigenicity by alpha1,3galactosyltransferase gene transfer. Int J Cancer. 2003;107:910–918. doi: 10.1002/ijc.11470. [DOI] [PubMed] [Google Scholar]
- 79.Miknyoczki SJ, Chang H, Klein-Szanto A, et al. The Trk tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits significant antitumor efficacy in preclinical xenograft models of human pancreatic ductal adenocarcinoma. Clin Cancer Res. 1999;5:2205–2212. [PubMed] [Google Scholar]
- 80.Fukasawa M, Korc M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 2004;10:3327–3332. doi: 10.1158/1078-0432.CCR-03-0820. [DOI] [PubMed] [Google Scholar]
- 81.Diaz VM, Planaguma J, Thomson TM, et al. Tissue plasminogen activator is required for the growth, invasion, and angiogenesis of pancreatic tumor cells. Gastroenterology. 2002;122:806–819. doi: 10.1053/gast.2002.31885. [DOI] [PubMed] [Google Scholar]
- 82.Delesque N, Buscail L, Esteve JP, et al. sst2 somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Res. 1997;57:956–962. [PubMed] [Google Scholar]
- 83.Freeman JW, Mattingly CA, Strodel WE. Increased tumorigenicity in the human pancreatic cell line MIA PaCa-2 is associated with an aberrant regulation of an IGF-1 autocrine loop and lack of expression of the TGF-beta type RII receptor. J Cell Physiol. 1995;165:155–163. doi: 10.1002/jcp.1041650118. [DOI] [PubMed] [Google Scholar]
- 84.Fogar P, Greco E, Basso D, et al. Suicide gene therapy with HSV-TK in pancreatic cancer has no effect in vivo in a mouse model. Eur J Surg Oncol. 2003;29:721–730. doi: 10.1016/j.ejso.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258–262. doi: 10.1097/01.mpa.0000175176.40045.0f. [DOI] [PubMed] [Google Scholar]
- 86.Bhargava S, Stummeyer T, Hotz B, et al. Selective inhibition of endothelin receptor A as an anti-angiogenic and anti-proliferative strategy for human pancreatic cancer. J Gastrointest Surg. 2005;9:703–709. doi: 10.1016/j.gassur.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 87.Hotz HG, Hines OJ, Masood R, et al. VEGF antisense therapy inhibits tumor growth and improves survival in experimental pancreatic cancer. Surgery. 2005;137:192–199. doi: 10.1016/j.surg.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 88.Loukopoulos P, Kanetaka K, Takamura M, et al. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas. 2004;29:193–203. doi: 10.1097/00006676-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 89.Katayama M, Sanzen N, Funakoshi A, et al. Laminin gamma2-chain fragment in the circulation: a prognostic indicator of epithelial tumor invasion. Cancer Res. 2003;63:222–229. [PubMed] [Google Scholar]
- 90.Torgenson MJ, Shea JE, Firpo MA, et al. Natural History of Pancreatic Cancer Recurrence Following “Curative” Resection in Athymic Mice. J Surg Res. 2007 doi: 10.1016/j.jss.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 91.Hotz HG, Reber HA, Hotz B, et al. An orthotopic nude mouse model for evaluating pathophysiology and therapy of pancreatic cancer. Pancreas. 2003;26:e89–98. doi: 10.1097/00006676-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 92.Cowgill SM, Muscarella P. The genetics of pancreatic cancer. Am J Surg. 2003;186:279–286. doi: 10.1016/s0002-9610(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 93.Schneider G, Schmid RM. Genetic alterations in pancreatic carcinoma. Mol Cancer. 2003;2:15. doi: 10.1186/1476-4598-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moore PS, Beghelli S, Zamboni G, et al. Genetic abnormalities in pancreatic cancer. Mol Cancer. 2003;2:7. doi: 10.1186/1476-4598-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sipos B, Moser S, Kalthoff H, et al. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch. 2003;442:444–452. doi: 10.1007/s00428-003-0784-4. [DOI] [PubMed] [Google Scholar]
- 96.Monti P, Marchesi F, Reni M, et al. A comprehensive in vitro characterization of pancreatic ductal carcinoma cell line biological behavior and its correlation with the structural and genetic profile. Virchows Arch. 2004;445:236–247. doi: 10.1007/s00428-004-1053-x. [DOI] [PubMed] [Google Scholar]
- 97.Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–912. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 98.Mahlamaki EH, Kauraniemi P, Monni O, et al. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 2004;6:432–439. doi: 10.1593/neo.04130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luttges J, Reinecke-Luthge A, Mollmann B, et al. Duct changes and K-ras mutations in the disease-free pancreas: analysis of type, age relation and spatial distribution. Virchows Arch. 1999;435:461–468. doi: 10.1007/s004280050428. [DOI] [PubMed] [Google Scholar]
- 100.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 101.Berrozpe G, Schaeffer J, Peinado MA, et al. Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer. Int J Cancer. 1994;58:185–191. doi: 10.1002/ijc.2910580207. [DOI] [PubMed] [Google Scholar]
- 102.Yip-Schneider MT, Lin A, Barnard D, et al. Lack of elevated MAP kinase (Erk) activity in pancreatic carcinomas despite oncogenic K-ras expression. Int J Oncol. 1999;15:271–279. doi: 10.3892/ijo.15.2.271. [DOI] [PubMed] [Google Scholar]
- 103.Fukushima N, Sato N, Ueki T, et al. Aberrant methylation of preproenkephalin and p16 genes in pancreatic intraepithelial neoplasia and pancreatic ductal adenocarcinoma. Am J Pathol. 2002;160:1573–1581. doi: 10.1016/S0002-9440(10)61104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gerdes B, Ramaswamy A, Kersting M, et al. p16(INK4a) alterations in chronic pancreatitis-indicator for high-risk lesions for pancreatic cancer. Surgery. 2001;129:490–497. doi: 10.1067/msy.2001.112071. [DOI] [PubMed] [Google Scholar]
- 105.Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126–3130. [PubMed] [Google Scholar]
- 106.Attri J, Srinivasan R, Majumdar S, et al. Alterations of tumor suppressor gene p16INK4a in pancreatic ductal carcinoma. BMC Gastroenterol. 2005;5:22. doi: 10.1186/1471-230X-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moore PS, Sipos B, Orlandini S, et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 108.Caldas C, Hahn SA, da Costa LT, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994;8:27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- 109.Huang L, Goodrow TL, Zhang SY, et al. Deletion and mutation analyses of the P16/MTS-1 tumor suppressor gene in human ductal pancreatic cancer reveals a higher frequency of abnormalities in tumor-derived cell lines than in primary ductal adenocarcinomas. Cancer Res. 1996;56:1137–1141. [PubMed] [Google Scholar]
- 110.Sun C, Yamato T, Furukawa T, et al. Characterization of the mutations of the K-ras, p53, p16, and SMAD4 genes in 15 human pancreatic cancer cell lines. Oncol Rep. 2001;8:89–92. doi: 10.3892/or.8.1.89. [DOI] [PubMed] [Google Scholar]
- 111.Mohiuddin M, Chendil D, Dey S, et al. Influence of p53 status on radiation and 5-flourouracil synergy in pancreatic cancer cells. Anticancer Res. 2002;22:825–830. [PubMed] [Google Scholar]
- 112.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 113.Schutte M, Hruban RH, Hedrick L, et al. DPC4 gene in various tumor types. Cancer Res. 1996;56:2527–2530. [PubMed] [Google Scholar]
- 114.Aoki Y, Hosaka S, Tachibana N, et al. Reassessment of K-ras mutations at codon 12 by direct PCR and sequencing from tissue microdissection in human pancreatic adenocarcinomas. Pancreas. 2000;21:152–157. doi: 10.1097/00006676-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 115.Chen WB, Lenschow W, Tiede K, et al. Smad4/DPC4-dependent regulation of biglycan gene expression by transforming growth factor-beta in pancreatic tumor cells. J Biol Chem. 2002;277:36118–36128. doi: 10.1074/jbc.M203709200. [DOI] [PubMed] [Google Scholar]
- 116.Briske-Anderson MJ, Finley JW, Newman SM. The influence of culture time and passage number on the morphological and physiological development of Caco-2 cells. Proc Soc Exp Biol Med. 1997;214:248–257. doi: 10.3181/00379727-214-44093. [DOI] [PubMed] [Google Scholar]
- 117.Chang-Liu CM, Woloschak GE. Effect of passage number on cellular response to DNA-damaging agents: cell survival and gene expression. Cancer Lett. 1997;113:77–86. doi: 10.1016/s0304-3835(97)04599-0. [DOI] [PubMed] [Google Scholar]
- 118.Esquenet M, Swinnen JV, Heyns W, et al. LNCaP prostatic adenocarcinoma cells derived from low and high passage numbers display divergent responses not only to androgens but also to retinoids. J Steroid Biochem Mol Biol. 1997;62:391–399. doi: 10.1016/s0960-0760(97)00054-x. [DOI] [PubMed] [Google Scholar]
- 119.Langeler EG, van Uffelen CJ, Blankenstein MA, et al. Effect of culture conditions on androgen sensitivity of the human prostatic cancer cell line LNCaP. Prostate. 1993;23:213–223. doi: 10.1002/pros.2990230304. [DOI] [PubMed] [Google Scholar]
- 120.Sambuy Y, De Angelis I, Ranaldi G, et al. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 121.Yu H, Cook TJ, Sinko PJ. Evidence for diminished functional expression of intestinal transporters in Caco-2 cell monolayers at high passages. Pharm Res. 1997;14:757–762. doi: 10.1023/a:1012150405949. [DOI] [PubMed] [Google Scholar]
- 122.Sawai H, Okada Y, Funahashi H, et al. Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol Cancer. 2005;4:37. doi: 10.1186/1476-4598-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luo J, Guo P, Matsuda K, et al. Pancreatic cancer cell-derived vascular endothelial growth factor is biologically active in vitro and enhances tumorigenicity in vivo. Int J Cancer. 2001;92:361–369. doi: 10.1002/ijc.1202. [DOI] [PubMed] [Google Scholar]
- 124.Holloway SE, Beck AW, Shivakumar L, et al. Selective Blockade of Vascular Endothelial Growth Factor Receptor 2 With an Antibody Against Tumor-Derived Vascular Endothelial Growth Factor Controls the Growth of Human Pancreatic Adenocarcinoma Xenografts. Ann Surg Oncol. 2006 doi: 10.1245/ASO.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 125.Blanchard JA, 2nd, Barve S, Joshi-Barve S, et al. Antioxidants inhibit cytokine production and suppress NF-kappaB activation in CAPAN-1 and CAPAN-2 cell lines. Dig Dis Sci. 2001;46:2768–2772. doi: 10.1023/a:1012795900871. [DOI] [PubMed] [Google Scholar]
- 126.Tong WG, Ding XZ, Hennig R, et al. Leukotriene B4 receptor antagonist LY293111 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2002;8:3232–3242. [PubMed] [Google Scholar]
- 127.Butz J, Wickstrom E, Edwards J. Characterization of mutations and loss of heterozygosity of p53 and K-ras2 in pancreatic cancer cell lines by immobilized polymerase chain reaction. BMC Biotechnol. 2003:3, 11. doi: 10.1186/1472-6750-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kita K, Saito S, Morioka CY, et al. Growth inhibition of human pancreatic cancer cell lines by anti-sense oligonucleotides specific to mutated K-ras genes. Int J Cancer. 1999;80:553–558. doi: 10.1002/(sici)1097-0215(19990209)80:4<553::aid-ijc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]