Fine-tuning vascular fate during endothelial-mesenchymal transition (original) (raw)
. Author manuscript; available in PMC: 2018 Jan 1.
Published in final edited form as: J Pathol. 2016 Nov 10;241(1):25–35. doi: 10.1002/path.4814
Abstract
In the heart and other organs, endothelial-mesenchymal transition (EndMT) has emerged as an important developmental process that involves coordinated migration, differentiation, and proliferation of the endothelium. In multiple disease states including cancer angiogenesis and cardiovascular disease, the processes that regulate EndMT are recapitulated, albeit in an uncoordinated and dysregulated manner. Members of the transforming growth factor beta (TGFβ) super-family are well known to impart cellular plasticity during EndMT by the timely activation (or repression) of transcription factors and miRNAs in addition to epigenetic regulation of gene expression. On the other hand, fibroblast growth factors (FGFs) are reported to augment or oppose TGFβ-driven EndMT in specific contexts. Here, we have synthesized the currently understood roles of TGFβ and FGF signalling during EndMT and have provided a new, comprehensive paradigm that delineates how an autocrine and paracrine TGFβ/FGF axis coordinates endothelial cell specification and plasticity. We also provide new guidelines and nomenclature that considers factors such as endothelial cell heterogeneity to better define EndMT across different vascular beds. This perspective should therefore help to clarify why TGFβ and FGF can both cooperate with or oppose one another during the complex process of EndMT in both health and disease.
Keywords: Endothelial heterogeneity, endothelial plasticity, endothelial-to-mesenchymal transition, tumour microenvironment, TGFβ, bFGF, cardiovascular disease
EndMT: general characteristics and key signalling effectors
Endothelial-mesenchymal transition (EndMT), which is closely related to epithelial-mesenchymal transition (EMT), defines a process whereby endothelial cells (ECs) lose their endothelial specification and gain mesenchymal cell features [1]. During EndMT, individual transitioning ECs may detach from the endothelium, migrate into the sub-endothelial interstitia, and differentiate into a spectrum of mesenchymal-like cell types. The process of EndMT is perhaps most well-understood in the developing heart, where a subset of ECs lining the endocardium undergo EndMT to form the cardiac valves and septa between embryonic days 10.5 and 16.5 [2]. During the same embryonic stages, endocardial ECs also give rise to ~ 20% of cardiac perictyes and smooth muscle cells through EndMT [3]. Other studies have demonstrated the importance of EndMT in the progression of a number of pathological conditions including organ fibrosis, solid tumours, scleroderma, heterotopic ossification, pulmonary arterial hypertension, arteriosclerosis, and cerebral cavernous malformation [4–19]. Moreover, post-developmental EndMT disrupts EC homeostasis, leading to deterioration of vascular function and plaque formation observed in atherosclerosis and vein graft stenosis [20–22].
Multiple mesenchymal markers are used to characterize EndMT, including the myogenic proteins alpha smooth-muscle actin (SMA), transgelin (SM22) and calponin, and non-myogenic fibroblast markers such as vimentin, fibroblast-specific protein 1 (FSP1) and several collagens. Among the mesenchymal markers, SMA (a contractile protein also expressed by smooth muscle cells and pericytes) is a key factor often used to identify fully differentiated myofibroblasts, which are often termed “activated fibroblasts”. SMA not only exerts traction forces that are central in the alteration of tissue architecture during fibrosis, but it also plays an important role in myofibroblast differentiation and function [23]. Apart from SMA-mediated contractility, fully differentiated myofibroblasts can promote fibrosis through enhanced production of extracellular matrix (ECM) proteins and up-regulated expression of fibrogenic/inflammatory cytokines [24]. Due to the profibrotic characteristics of myofibroblasts in multiple disease states, there is major interest aimed at understanding how various cellular precursors, especially ECs, differentiate to form myofibroblasts.
In parallel to the gain in mesenchymal markers and functional properties, ECs also lose essential factors involved in endothelial specification and function. For example, suppression of EC signalling receptors such as vascular endothelial growth factor receptor 2 (VEGFR2) occurs during EndMT in solid tumours [6]. The loss of VEGFR2 may offer a mechanism for the ineffectiveness of anti-VEGF therapies in glioblastoma multiforme (GBM) [25]. Diminished expression of endothelial junctional proteins including vascular endothelial cadherin (VE-Cadherin) and CD31 may result in reduced cell-cell contact and increased motility, which are typical features of fibroblasts. Indeed, a phenotypic switch from a cobblestone monolayer towards spindly, disorganized fibroblast-like cells is a cardinal feature of ECs undergoing EndMT in vitro [26].
Many of the signallling networks that are commonly utilized during EMT are also co-opted during EndMT. The principal inducer of both EndMT and EMT is the powerful morphogen transforming growth factor beta (TGFβ), which consists of three isoforms TGFβ1, TGFβ2, and TGFβ3. All three TGFβ ligands bind to the type II receptor TGFBR2, which then recruits and activates the type I receptor TGFBR1 (or anaplastic lymphoma kinase 5 [ALK5]) to induce downstream signalling [27]. Activation of the TGFBR complex in turn stimulates EndMT through both SMAD-dependent and SMAD-independent pathways by regulating the activity of several “EndMT transcription factors” including Snail, Slug, and Twist [28–30]. These transcription factors ultimately reprogramme downstream gene expression leading to the phenotypic and functional changes in the endothelium that typify EndMT (as noted above). TGFβ signalling is strongly context- and cell type-dependent and is influenced by the microenvironment, interactions with auxiliary signalling pathways and transcription factors, and the epigenetic landscape [31,32]. Multiple signalling pathways merge with the TGFβ pathway to either augment or attenuate EndMT. For example, TGFβ enhances Notch1 activity which stimulates mesenchymal gene expression in cardiac ECs during cardiac valve development [33]. Induction of EndMT has been shown to require synergistic action of IL1β and TGFβ, although only TGFβ is necessary for sustained mesenchymal gene expression [34]. Depletion of endoglin, which indirectly inhibits ALK5-mediated TGFβ signalling, promotes EndMT in tumours, while a TGFβ superfamily member BMP7 was reported to antagonize TGFβ-induced EndMT or EMT both in vitro and in vivo [8,12,35].
In contrast to TGFβ, basic fibroblast growth factor (bFGF) has a more nuanced role during EndMT. On one hand, bFGF has a potent antagonistic effect on TGFβ-induced myofibroblast gene activation. In arteriosclerotic endothelium, for example, bFGF counteracts TGFβ signalling and reverses EndMT [36], while genetic ablation of endothelial bFGF signalling accelerates arteriosclerosis progression [7]. Similarly, in lymphatic ECs and freshly isolated tumour-specific ECs, bFGF blocks expression of TGFβ-induced myofibroblast markers and maintains endothelial specification and function [6,37]. This antagonistic effect of bFGF is achieved by disabling multiple “nodes” in the TGFβ signalling pathway [36–38]. On the other hand, previous studies suggest that bFGF signalling is crucial in promoting EndMT of corneal ECs, EMT during development, and myofibroblast differentiation in the lung (see discussion) [39–42]. These seemingly confusing data raise an obvious question: how does bFGF exert the exact opposite effect on the same process?
Given the emerging importance of EndMT in a variety of pathophysiological conditions, it is important to clarify the underlying molecular mechanisms that mediate the “tug of war” between TGFβ and bFGF during the regulation of EC fate and specification; specifically, we will delineate the current paradigm underlying both cooperative and non-cooperative roles of TGFβ and FGF signalling during EndMT/myofibroblast activation (Fig. 1). By integrating these two competing signalling pathways, new treatment strategies for cardiovascular diseases, or perhaps cancer, where EndMT is a driving force could be achieved.
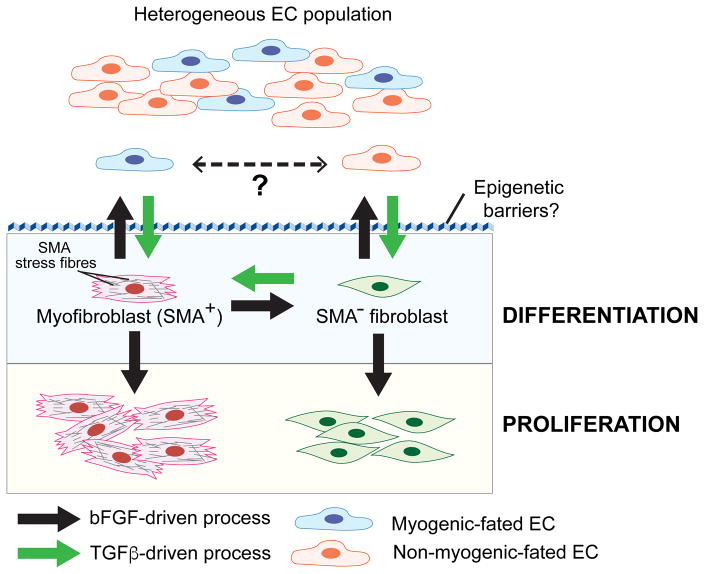
Fig. 1. bFGF and TGFβ signalling converge to regulate EndMT.
Heterogeneous EC populations consisting of at least two different subpopulations undergo distinct forms of TGFβ-stimulated EndMT. One EC subpopulation gives rise to myofibroblast-like cells (SMA+) while the other are SMA− “fibroblast-like” cells. It is unknown if the two EC subtypes interconvert in vivo. TGFβ stimulates EndMT and the conversion of SMA− fibroblasts to myofibroblasts (SMA+), whereas bFGF counteracts TGFβ signalling during these processes. Acquisition of a stable mesenchymal phenotype requires overcoming epigenetic barriers that maintain endothelial specification/identity. During EndMT, bFGF can also play a cooperative role by stimulating proliferation of differentiated myofibroblasts and fibroblasts.
bFGF is a critical endothelial cell mitogen
Fibroblast growth factors (FGFs) signal through four highly conserved receptor tyrosine kinases (RTKs) known as FGF receptors (FGFRs) 1 – 4. They regulate several major cellular processes during development, ranging from early embryonic mesoderm patterning to the formation of multiple organ systems [43–45]. In ECs, FGFR1 and FGFR2 are the most predominant isoforms, and upon ligand binding they dimerize and autophosphorylate to activate downstream signalling cascades. A total of 18 distinct mammalian FGFs have been discovered, among which the prototype FGFs, acidic FGF (aFGF) and bFGF, are particularly critical in early EC specification and vascular development [46].
aFGF and bFGF share 55% sequence homology and exert similar biological effects on many different cell types, although their signal strength depends on the expression of subtypes and splice variants of FGFRs and on ligand-receptor affinity [47]. In particular, bFGF is well-known for its potent pro-proliferative and pro-angiogenic effect on ECs. bFGF is also pivotal in the maintenance of endothelial VEGFR2 expression that is indispensable for VEGF-stimulated angiogenesis [48]. In the mature vasculature post-development, endothelial-specific FGF signalling is essential during injury response, as endothelial deletion of both FGFR1 and FGFR2 significantly delays wound healing of the skin and impairs hypoxia-induced angiogenic responses in the retina [49].
bFGF counteracts TGFβ signalling during EndMT
In addition to regulating EC proliferation, emerging evidence suggests that bFGF stimulation counteracts TGFβ-driven downstream cellular signalling during EndMT. Recently, high-throughput screening of 60 different combinations of ECM proteins identified bFGF as the strongest negative regulator of TGFβ-induced EndMT [50]. Other studies reported that bFGF decreased TGFβR1 levels and reversed the growth inhibitory effect of TGFβ1 on ECs [51]. Work by Chen et al. suggests that bFGF is essential in preventing EndMT in the normal endothelium [36]. Similarly, in lymphatic vessels, bFGF inhibits EndMT and maintains lymphatic EC identity by opposing TGFβ-activated SMAD2 via Ras/ERK MAPK signalling [37]. We recently uncovered at least two types of tumour-specific EC populations in mammary tumours that respond differently to TGFβ and undergo distinct forms of EndMT (Fig. 1) [6]. Despite displaying varying TGFβ responsiveness, bFGF was able to restore endothelial morphology and endothelial markers in both EC subtypes [6]. Gene expression analysis of these tumour-derived EC clones also revealed that bFGF reversed the expression of several TGFβ-stimulated genes, including the myofibroblast marker Acta2 (gene name for SMA) and Smad3, while other genes were synergistically activated by combinations of bFGF and TGFβ. Consistent with our findings, differential TGFβ reactivity of EC subpopulations derived from the same tissue has been reported by other groups, suggesting that EC heterogeneity dictates EndMT [52,53]. Thus, we may speculate that different vascular beds might show different proclivities to undergo EndMT in the adult. Especially in those tissues/organs where EndMT is known to occur during development (e.g. lung and heart), it is possible we will find the greatest capacity for EndMT to be recapitulated postnatally; especially following injury. Although it is unclear how bFGF might feed into this paradigm, we would anticipate that endothelial cells in the lung/feeding arteries would be most “receptive” to EndMT-modulating factors following tissue injury (hypoxia or fibrosis). Such “receptiveness” might underlie repair or remodeling during, for example, pulmonary arterial hypertension.
bFGF has also been reported to antagonize TGFβ signalling in other cell types. For instance, bFGF decreases TGFβ-stimulated SMA and mesenchymal gene expression in pericytes and airway smooth muscle cells [54]. aFGF was reported to function similarly to bFGF and counteract TGFβ signalling, although we found that aFGF was less potent than bFGF in blocking TGFβ-driven EndMT in tumour-derived ECs [6]. In one study, deprivation of aFGF or TGFβ stimulation induced expression of the mesenchymal genes SM22 and calponin in human umbilical vascular ECs (HUVECs), while treatment with aFGF blocked TGFβ-induced gene expression and diminished the acquired smooth muscle cell-like contractility [52]. This antagonistic effect of aFGF on TGFβ, reportedly mediated through the MEK/ERK pathway, was also observed in fibroblasts and in epithelial cells during EMT [55–57].
ECs secrete their own bFGF to counteract TGFβ-induced EndMT
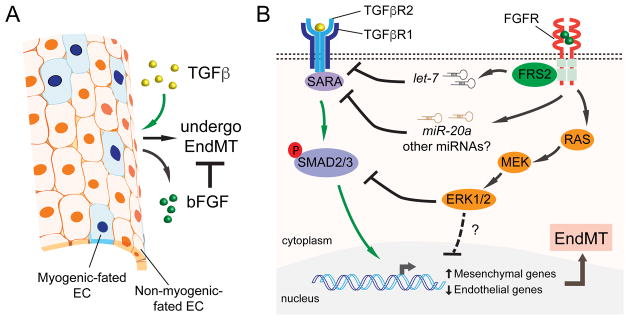
In ECs, TGFβ treatment induces bFGF production and secretion, which in turn inhibits TGFβ signalling in an autocrine feedback loop (Fig. 2A) [6]. For example, we found that conditioned media harvested from TGFβ-stimulated ECs contained high levels of bFGF, which repressed mesenchymal gene induction and preserved endothelial markers in secondary EC cultures. Capillary ECs were also shown to stimulate their own bFGF secretion which facilitated angiogenesis and vessel growth [58]. Furthermore, in human ECs, bFGF mRNA expression was induced by TGFβ as revealed by gene expression profiling [22]. Consistent with these findings, bFGF mRNA levels are significantly increased in lung tissues in transgenic mice with lung-specific TGFβ1 over-expression [59]. This observation is not only limited to ECs as studies of multiple mesenchymal cells, including human lung fibroblasts, prostate stromal cells, osteoblastic-like cells and corneal stromal fibroblasts have shown that bFGF expression is enhanced by TGFβ, and secreted bFGF activates the ERK pathway through autocrine activation of its own receptors [60–65]. It is possible that, in the face of TGFβ challenge during EndMT, ECs secrete bFGF as a protective factor to counteract TGFβ signalling thereby preserving EC function and specification. It is unknown, however, what downstream signalling intermediates are responsible for TGFβ-induced up-regulation and secretion of bFGF.
Fig. 2. Molecular mechanisms underlying bFGF’s antagonistic effect on TGFβ signalling during EndMT.
A) During EndMT, ECs up-regulate bFGF in response to TGFβ stimulation. bFGF secreted by these transitioning ECs counteracts TGFβ and maintains EC identity through autocrine/paracrine loops. B) TGFβ signals through a TGFβR1 and TGFβR2 receptor complex, which activates SMAD2/3 (and non-SMAD pathways) to induce EndMT. bFGF neutralizes TGFβ signalling by targeting multiple nodes of the TGFβ pathway. bFGF increases “anti-EndMT” microRNAs including let-7 and miR-20a leading to the down-regulation of TGFβ receptors and a receptor-associated protein SARA. Another pathway activated by bFGF is the Ras/MEK/ERK branch, which can oppose TGFβ signalling by suppressing SMAD2 phosphorylation. Additional auxiliary regulatory mechanisms through MEK/ERK may also contribute to bFGF’s antagonistic activities.
bFGF skews endothelial cells and fibroblasts towards a SMA− phenotype
It was recently proposed that cancer-associated fibroblasts (CAFs) are polarized into tumour-restricting (Type 1) versus tumour-promoting (Type 2) populations [66]. This dichotomy is reminiscent of differentially polarized macrophages and neutrophils that are also found in the microenvironment of solid tumours [67,68]. While Type 1 CAFs are SMA− and restrict tumour growth, Type 2 CAFs, albeit heterogeneous, are activated fibroblasts that up-regulate an array of mesenchymal markers including FSP1, vimentin, platelet-derived growth factor receptor beta (PDGFRβ), and the prototypical myofibroblast marker SMA. SMA+ CAFs are proposed enablers of tumour progression that interact with tumour cells and other stromal cells within the microenvironment to orchestrate tumour angiogenesis, inflammation, and metastasis [69]. Apart from tumours, SMA+ fibroblasts or myofibroblasts are frequently observed in wounds, fibrotic lesions and arteriosclerotic plaques. Although myofibroblasts are derived from diverse cellular precursors, once differentiated, all myofibroblasts appear to possess similar functions including exertion of traction force and ECM matrix deposition that are necessary for healing [70]. The accumulation of myofibroblasts is usually transient during acute injury; however, in chronic inflammatory conditions where myofibroblasts persist, excessive tissue contraction and ECM remodeling by these cells leads to destruction of normal tissue architecture and function.
In addition to its role during development, the process of EndMT generates myofibroblasts in tumours and other fibrotic conditions. Using a lineage-tracing model, Zeisberg and colleagues have found that at least 12% of SMA+ CAFs are derived from ECs in tumours [11]. Similar percentages of EC-originated myofibroblasts were also reported in renal and lung fibrosis [71,72]. Interestingly, using SMA and non-myogenic fibroblast markers such as FSP1, studies by our lab and others have revealed that distinct forms of EndMT give rise to SMA− and SMA+ CAF populations, suggesting that heterogeneous EC subpopulations undergo both myogenic (myofibroblast) and non-myogenic transitions (Fig. 1) [6,11,13,52,73]. Similarly, different forms and subtypes of EMT have also been identified. For example, the process whereby EMT progresses along a myogenic path to generate myofibroblasts is termed epithelial-myofibroblast transition (EMyT or EMyoT) [41,74].
Although it remains elusive how different SMA− and SMA+ cell populations interconvert, past studies have suggested that bFGF effectively directs fibroblasts towards the Type 1 phenotype. For instance, myofibroblast differentiation from adipose-derived mesenchymal stem cells or embryonic fibroblasts can be reversed by bFGF, which not only suppresses the expression of myofibroblast genes such as SMA, collagen 1 and fibronectin, but also preserves their Type 1 fibroblast-like morphology [75,76]. bFGF can also inhibit myofibroblast activation from mature proliferating fibroblasts or interstitial cells derived from various tissues [77–80]. In epithelial cells, bFGF prevents myofibroblast differentiation during EMyT while cooperating with TGFβ to drive the transition towards a more migratory and invasive mesenchymal-like cell type [41]. Remarkably, in the liver, transplantation of mesenchymal stem cells pre-conditioned by bFGF alleviates fibrosis likely through reduction of myofibroblast activation [81]. Similarly, in other mesenchymal cells such as pericytes, bFGF also skews differentiation towards a SMA− phenotype, while TGFβ initiates a SMA+ differentiation program [82].
Studies of hypertrophic scars and keloid tissue formation during wound healing have further established the anti-fibrotic effect of bFGF, revealed by decreased expression of SMA and ECM components such as collagen in activated fibroblasts [83]. In wound-healing models and in clinical studies, administration of bFGF reduced scar tissue and inhibited the pro-fibrogenic effect of TGFβ, indicating that the therapeutic benefits of bFGF are likely due to inhibition of TGFβ-induced phenotypic switching that would ordinarily functionalize myofibroblasts (Type 2) [84–87].
Molecular mechanisms of bFGF’s antagonistic action on TGFβ signalling during EndMT
The exact pathways that bFGF employs to oppose TGFβ signalling are not fully characterized. However, recent evidence suggests that bFGF stimulates several distinct pathways to modulate EndMT (Fig. 2B). One mechanism is regulation through induction of “anti-EndMT” microRNAs (miRNAs), such as let-7. In arteriosclerotic ECs, bFGF can signal through FGFR-FGFR substrate 2 (FRS2) to up-regulate let-7, which in turn down-regulates TGFβR1 to attenuate TGFβ-driven EndMT [36]. Compromised FGF signalling as a result of chronic inflammation, on the contrary, leads to a reduction of let-7 and enhanced TGFβ signalling which initiates EndMT. bFGF increases the expression of another miRNA, miR-20a, which simultaneously targets upstream TGFβ receptor components including ALK5 (TGFβR1), TGFβR2 and a receptor-associated protein called SMAD anchor for receptor activation (SARA) [38]. A second major signalling pathway that contributes to the antagonistic ability of bFGF is the MEK/ERK pathway. In ECs, activation of MEK/ERK by bFGF is critical for cell proliferation, migration, survival and VEGFR-dependent angiogenesis [88–90]. Studies by others, along with our own published data, have also shown that activation of the downstream MEK/ERK pathway is important in counteracting TGFβ-stimulated myofibroblast gene expression [37]. MEK/ERK activation can down-regulate TGFβ-stimulated SMAD2 phosphorylation, whereas overexpression of the Ras protein, an upstream effector of MEK/ERK signalling, can amplify bFGF-driven ERK signalling and prevent EndMT [37]. These effects are modulated specifically through FGFR1 in ECs, as depletion or loss of FGFR1 enhances SMAD2 phosphorylation and TGFβ-stimulated EndMT [91].
During TGFβ-stimulated EMT, activation of MEK/ERK by aFGF also strongly reduces SMAD2 phosphorylation thereby suppressing expression of the mesenchymal gene SMA and blocking the function of matrix metalloproteinases [56]. This effect is abolished specifically by a MEK inhibitor but not by inhibition of PI3K or p38. Identical signalling pathways are utilized during myofibroblast differentiation from fibroblast or mesenchymal stem cell precursors [75,76,79,92]. During such processes, TGFβ-stimulated myofibroblast differentiation from quiescent fibroblasts is inhibited by bFGF through MEK/ERK, which in turn suppresses SMAD-dependent signalling [76,79,92]. On the other hand, the integrin-activated focal adhesion kinase (FAK) pathway can enhance cell surface FGFR expression and FRS2-ERK activation, thereby strengthening FGF signalling [75]. Together, these data suggest that multiple mechanisms underlie bFGF’s EndMT-neutralizing ability and the downstream modulators activated by bFGF may vary in different cell types.
bFGF inhibits myofibroblast activation, but promotes proliferation and migration of myofibroblasts
Although bFGF can exert potent opposing effects on TGFβ signalling during myofibroblast generation from ECs and other cell types, the timing of bFGF signalling is critical. In fact, bFGF acts as a fibroblast mitogen, promoting myofibroblast proliferation after TGFβ-triggered mesenchymal transition. This temporal and dynamic interaction between bFGF and TGFβ has been demonstrated in studies of paraxial mesoderm myogenesis during chick embryonic development [93]. The investigators showed that a two-step mechanism likely occurred during myogenesis: pulse exposure of TGFβ was required to initiate myogenesis at the early stage whereas bFGF, albeit not necessary for myogenic commitment, was indispensable for maintaining long-term cell survival and proliferation. During EndMT, we have found that bFGF is most effective in inhibiting TGFβ-induced myofibroblast genes; however, in ECs exhibiting a partial EndMT phenotype where SMA is constitutively expressed, addition of bFGF was less effective at suppressing the myofibroblast genetic program [6]. Corroborating our data, Correia and colleagues also showed that bFGF curbs EndMT only at initiation (i.e. reduces the number of cells entering EndMT) through up-regulation of a negative EndMT regulator (miR-20a) but was unable to offset TGFβ signalling in cells that had already entered the EndMT program [38]. A similar observation was also reported in differentiated cardiac myofibroblasts where treatment with bFGF failed to suppress SMA expression [94]. These data suggest that the temporal expression pattern of bFGF is of critical importance during myofibroblast differentiation that is ultimately driven by TGFβ. It is proposed, therefore, that bFGF has a biphasic effect on EndMT: counteracting the pro-differentiation effect of TGFβ signalling at the initiation stage, but promoting fibroblast growth and survival once there is commitment towards the mesenchymal differentiation pathway. Additional mechanisms, perhaps epigenetic modifications, may also be at play in the regulation of stable and persistent myofibroblast gene expression in certain contexts [95,96].
Synergistic actions of bFGF and TGFβ control fibroblast differentiation during EndMT
Surprisingly, bFGF is also implicated in the promotion of EndMT in certain types of ECs, specifically corneal ECs [97,98], although in other cell types in the mammalian eye including corneal keratocytes and lens epithelial cells, bFGF suppresses TGFβ-induced myofibroblast gene induction [77,99]. Treatment of corneal ECs with ethylenediaminetetraacetic acid (EDTA), bFGF, and TGFβ lifts contact inhibition of the corneal EC monolayer, unlocks mitotic blockade, and induces EndMT [100,101]. While enhancing motility and proliferation of the corneal ECs, bFGF can also stabilize collagen 1 mRNA through post-transcriptional modifications, thereby promoting EndMT [102]. This mesenchymal re-programming is collectively achieved by activation of bFGF-mediated RhoA-ROCK and canonical Wnt/β-catenin signalling, in addition to augmentation of the TGFβ-mediated SMAD2/3 pathway [100,101].
Studies of EMT have also suggested a cooperative role for bFGF and TGFβ, especially during organogenesis. Both of these factors were shown, for example, to induce EMT in the epicardium during coronary vasculogenesis in avian heart development [103]. Isolated epicardial cells in response to the combination treatment of bFGF and TGFβ increased motility and proliferation, and penetrated the underlying matrix, which is a characteristic of EMT. In a different study where epicardial-lineage cells were generated from human pluripotent stem cells, addition of bFGF enhanced TGFβ-stimulated expression of mesenchymal markers including SMA and vimentin, although bFGF-induced proliferation and expression of the EMT transcription factor SNAI1 was counteracted by TGFβ [104]. During tooth development, bFGF induces EMT in tooth root enamel epithelial cells (Hertwig’s epithelial root sheath cells) to form cementoblast-like cells that are responsible for cementum formation, whereas TGFβ stimulates the same cells to transition to periodontal ligament fibroblast-like cells [107]. Here, the cooperative action of TGFβ and bFGF is also dependent, at least in part, on the coordinated modulation of the MEK/ERK pathway [41,107].
The profibrotic properties downstream of FGF signalling are additionally demonstrated during myofibroblast differentiation in alveolar development and regeneration. In transgenic mice that express a soluble dominant negative FGFR to attenuate FGF signalling, induction of SMA+ interstitial fibroblasts or alveolar myofibroblasts was suppressed during realveolarization post-injury [42]. However, FGF ligands other than bFGF and aFGF, including FGFs 8, 10 and 18, were shown to be more critical in the activation of myofibroblasts through FGFRs, suggestive of functional diversity of the FGF ligand family [105].
Targeting bFGF in fibrosis and vascular dysfunction
In light of the emerging evidence signifying EndMT as an underlying contributor to multiple disease states, especially those involving the vasculature, inhibition of pathophysiological EndMT has been proposed as a new therapeutic modality. For example, small-molecule inhibitors and antibodies targeting the TGFβ pathway have demonstrated beneficial effects in attenuating EndMT and slowing disease progression in multiple animal studies (for discussion see Sanchez-Duffhues et al.) [106]. Although no synthetic drugs that modulate bFGF signalling have been tested in EndMT models in vivo, clinical studies suggest that administration of bFGF reduces scar formation during wound healing [85]. As noted, TGFβ-driven EndMT is an important contributor to pulmonary fibrosis and pulmonary hypertension [17,19,72,107]; thus, it might be expected that local administration of bFGF or activation of bFGF signalling might reverse fibrosis in these conditions. Contrary to expectations, phase III clinical trials showed that inhibition of FGFRs, along with other tyrosine kinases including VEGFRs and PDGFRs, by the small-molecule inhibitor Nintedanib, delayed the progression of idiopathic pulmonary fibrosis, leading to the drug’s approval by the U.S. Food and Drug Administration in 2014 [108]. These studies demonstrate the complex role of TGFβ/FGF signalling in the pathogenesis of fibrotic organs/tissues and reinforce the concept that temporal inhibition, or activation, of different signalling effectors that control EndMT should be considered in the design of therapeutic strategies.
In summary, how FGF signalling interacts with the TGFβ pathway at the intracellular level is highly context-dependent and strongly influenced by intrinsic factors such as cell heterogeneity and extrinsic factors such as the cellular microenvironment (i.e. different cell types respond differently to FGF challenge or withdrawal). A good example are corneal ECs, which are derived from the neural crest [109] and behave opposite in their response to bFGF and TGFβ when compared with ECs derived from other vascular beds. In mouse mammary epithelial cells, it was shown that sensitivity to bFGF depends on expression of specific FGFR isoforms which are themselves mediated by TGFβ signalling [41]. Thus, differential expression of FGF receptors and the availability of redundant FGF ligands may also influence signal strength and hence the magnitude of EndMT.
Summary and perspective
bFGF is a crucial regulator of cellular differentiation and growth during development and disease. During EndMT, bFGF exerts a dual function depending on the cellular context and cell types involved: counteracting myofibroblast gene activation by TGFβ and restoring endothelial specification on one hand, while promoting proliferation and migration of differentiated fibroblasts on the other. The balance and timing of bFGF and TGFβ signalling is central during myofibroblast activation. ECs produce bFGF during EndMT perhaps to “fine-tune” the process and neutralize TGFβ stimulation. How TGFβ and bFGF crosstalk at the molecular level to regulate EndMT is not entirely clear, but it is possible that these two signalling networks converge at common signalling nodes such as MEK/ERK to modulate EC fate and differentiation. Thus, a delicate balance between these two signalling networks is required to maintain endothelial homeostasis in both quiescent and activated (e.g. injured or undergoing angiogenesis) vascular beds. bFGF maintains EC gene expression and morphology, whereas high levels of TGFβ (e.g. during inflammation) instigate EndMT, driving cells towards a mesenchymal phenotype (Fig. 3).
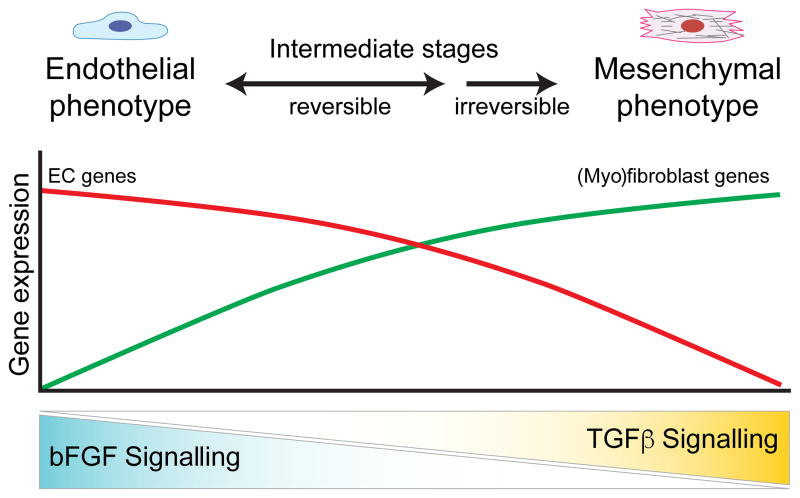
Fig. 3. Endothelial homeostasis and phenotype switching are coordinated by bFGF and TGFβ.
A balance of bFGF and TGFβ signalling is required for endothelial homeostasis and EndMT. High bFGF signalling alone preserves EC specification and gene expression, whereas high TGFβ and low bFGF signalling drives ECs to undergo EndMT. During EndMT, ECs transition through a spectrum of reversible intermediate stages before differentiating into a stable and committed (myo)fibroblast phenotype.
Multiple forms of EndMT can give rise to SMA+ and SMA− fibroblast-like populations, similar to the parallel process of EMT and EMyT. In addition, EndMT appears to be a gradual process whereby ECs undergo a spectrum of mutable and intermediate transitional stages before overcoming the epigenetic barriers to morph into fully differentiated and stable (myo)fibroblasts (Fig. 3) 1[1,11,110]. These intermediate cell types display characteristics of both endothelial and mesenchymal cells and their phenotypes are often reversible and likely controlled by multiple epigenetic effectors, although very little is known about how the epigenetic landscape coordinates EndMT and EC plasticity. Adding to this complexity, context-dependent bFGF signalling may elicit different responses during different forms and stages of EndMT. Moreover, different laboratories define EndMT using different criteria. For example, early studies concluding that bFGF was an EndMT-promoting factor defined EndMT as an acquisition of a spindly morphology and increased cell motility and proliferation, whereas more recent studies frequently use the expression of myofibroblast markers such as SMA, SM22 and collagen 1. These diverse characterization methods may lead to different interpretations and conclusions throughout the literature. It is recommended, therefore, to classify different forms of EndMT into different categories, such as endothelial-myofibroblast transition (EndMyoT), where definitive markers (e.g. SMA) can be used to distinguish this process. Such classification may facilitate future studies to elucidate the molecular mechanisms underlying the cooperative and non-cooperative roles of TGFβ and bFGF during myofibroblast differentiation. Dissecting and understanding how these pathways merge is essential for identifying novel molecular therapeutics for the treatment of, for example, cancer, arteriosclerosis and multiple fibrotic diseases.
Acknowledgments
A.C.D. is supported by a grant from the NIH (RO1-CA177874). Other funding support includes a pre-doctoral fellowship to L.X. (AHA 15PRE24470053).
Footnotes
No conflicts of interest were disclosed
Statement of author contributions: Both L.X. and A.C.D. wrote and revised the manuscript.
References
- 1.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garside VC, Chang AC, Karsan A, et al. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell Mol Life Sci. 2013;70:2899–917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q, Zhang H, Liu Y, et al. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat Commun. 2016;7:12422. doi: 10.1038/ncomms12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aisagbonhi O, Rai M, Ryzhov S, et al. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4:469–83. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley AC, Khan ZA, Shih S-C, et al. Calcification of multipotent prostate tumor endothelium. Cancer Cell. 2008;14:201–11. doi: 10.1016/j.ccr.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao L, Kim DJ, Davis CL, et al. Tumor endothelial cells with distinct patterns of TGFβ-driven endothelial-to-mesenchymal transition. Cancer Res. 2015;75:1244–54. doi: 10.1158/0008-5472.CAN-14-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P-Y, Qin L, Baeyens N, et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–28. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderberg C, Cunha SI, Zhai Z, et al. Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. J Exp Med. 2013;210:563–79. doi: 10.1084/jem.20120662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitao A, Sato Y, Sawada-Kitamura S, et al. Endothelial to mesenchymal transition via transforming growth factor-β1/Smad activation is associated with portal venous stenosis in idiopathic portal hypertension. Am J Pathol. 2009;175:616–26. doi: 10.2353/ajpath.2009.081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng B, Cao Y, Chen S, et al. mir-200b mediates endothelial-to-mesenchymal transition in diabetic cardiomyopathy. Diabetes. 2016;65:768–79. doi: 10.2337/db15-1033. [DOI] [PubMed] [Google Scholar]
- 11.Zeisberg EM, Potenta S, Xie L, et al. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 12.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 13.Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–7. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maddaluno L, Rudini N, Cuttano R, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–6. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 15.Kumarswamy R, Volkmann I, Jazbutyte V, et al. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361–9. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- 16.Choi S-H, Hong Z-Y, Nam J-K, et al. A hypoxia-induced vascular endothelial-to-mesenchymal transition in development of radiation-induced pulmonary fibrosis. Clin Cancer Res. 2015;21:3716–26. doi: 10.1158/1078-0432.CCR-14-3193. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez SA, Piera-Velazquez S. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of systemic sclerosis-associated pulmonary fibrosis and pulmonary arterial hypertension. Myth or reality? Matrix Biol. 2016;51:26–36. doi: 10.1016/j.matbio.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medici D, Shore EM, Lounev VY, et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–6. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranchoux B, Antigny F, Rucker-Martin C, et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006–18. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Duffhues G, de Vinuesa AG, Lindeman JH, et al. SLUG is expressed in endothelial cells lacking primary cilia to promote cellular calcification. Arterioscler Thromb Vasc Biol. 2015;35:616–27. doi: 10.1161/ATVBAHA.115.305268. [DOI] [PubMed] [Google Scholar]
- 21.Evrard SM, Lecce L, Michelis KC, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. 2016;7:11853. doi: 10.1038/ncomms11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooley BC, Nevado J, Mellad J, et al. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6:227ra34. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talele NP, Fradette J, Davies JE, et al. Expression of α-smooth muscle actin determines the fate of mesenchymal stromal cells. Stem Cell Rep. 2015;4:1016–30. doi: 10.1016/j.stemcr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2012;5:334–7. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang M, Liu T, Ma P, et al. c-Met–mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma. J Clin Invest. 2016;126:1801–14. doi: 10.1172/JCI84876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medici D, Kalluri R. Endothelial–mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin Cancer Biol. 2012;22:379–84. doi: 10.1016/j.semcancer.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 28.Franses JW, Baker AB, Chitalia VC, et al. Stromal endothelial cells directly influence cancer progression. Sci Transl Med. 2011;3:66ra5. doi: 10.1126/scitranslmed.3001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welch-Reardon KM, Wu N, Hughes CCW. A role for partial endothelial-mesenchymal transitions in angiogenesis? Arterioscler Thromb Vasc Biol. 2015;35:303–8. doi: 10.1161/ATVBAHA.114.303220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medici D, Potenta S, Kalluri R. Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J. 2011;437:515–20. doi: 10.1042/BJ20101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J-H, Wylie-Sears J, Bischoff J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem Biophys Res Commun. 2008;374:512–6. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maleszewska M, Moonen J-RAJ, Huijkman N, et al. IL-1β and TGFβ2 synergistically induce endothelial to mesenchymal transition in an NFκB-dependent manner. Immunobiology. 2013;218:443–54. doi: 10.1016/j.imbio.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Zeisberg M, Hanai J-I, Sugimoto H, et al. BMP-7 counteracts TGF-β1–induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–8. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 36.Chen P-Y, Qin L, Barnes C, et al. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012;2:1684–96. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichise T, Yoshida N, Ichise H. FGF2-induced Ras-MAPK signalling maintains lymphatic endothelial cell identity by upregulating endothelial-cell-specific gene expression and suppressing TGFβ signalling through Smad2. J Cell Sci. 2014;127:845–57. doi: 10.1242/jcs.137836. [DOI] [PubMed] [Google Scholar]
- 38.Correia ACP, Moonen J-RAJ, Brinker MGL, et al. FGF2 inhibits endothelial-mesenchymal transition through microRNA-20a-mediated repression of canonical TGF-beta signaling. J Cell Sci. 2016;129:569–79. doi: 10.1242/jcs.176248. [DOI] [PubMed] [Google Scholar]
- 39.Lee HT, Kay EP. FGF-2 induced reorganization and disruption of actin cytoskeleton through PI 3-kinase, Rho, and Cdc42 in corneal endothelial cells. Mol Vis. 2003;9:624–34. [PubMed] [Google Scholar]
- 40.Lee JG, Kay EP. Cross-talk among Rho GTPases acting downstream of PI 3-kinase induces mesenchymal transformation of corneal endothelial cells mediated by FGF-2. Invest Ophthalmol Vis Sci. 2006;47:2358–68. doi: 10.1167/iovs.05-1490. [DOI] [PubMed] [Google Scholar]
- 41.Shirakihara T, Horiguchi K, Miyazawa K, et al. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 2011;30:783–95. doi: 10.1038/emboj.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perl A-KT, Gale E. FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol. 2009;297:L299–308. doi: 10.1152/ajplung.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Moerlooze L, Spencer-Dene B, Revest J, et al. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 44.Kimelman D, Kirschner M. Synergistic induction of mesoderm by FGF and TGF-β and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987;51:869–77. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- 45.Slack JM, Darlington BG, Heath JK, et al. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987;326:197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- 46.Presta M, Dell’Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–97. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 48.Goldie LC, Nix MK, Hirschi KK. Embryonic vasculogenesis and hematopoietic specification. Organogenesis. 2008;4:257–63. doi: 10.4161/org.4.4.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oladipupo SS, Smith C, Santeford A, et al. Endothelial cell FGF signaling is required for injury response but not for vascular homeostasis. Proc Natl Acad Sci USA. 2014;111:13379–84. doi: 10.1073/pnas.1324235111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Calpe B, Zerdani J, et al. High-throughput investigation of endothelial-to-mesenchymal transformation (EndMT) with combinatorial cellular microarrays. Biotechnol Bioeng. 2015;113:1403–12. doi: 10.1002/bit.25905. [DOI] [PubMed] [Google Scholar]
- 51.Fafeur V, Terman BI, Blum J, et al. Basic FGF treatment of endothelial cells down-regulates the 85-KDa TGF beta receptor subtype and decreases the growth inhibitory response to TGF-beta 1. Growth Factors. 1990;3:237–45. doi: 10.3109/08977199009043908. [DOI] [PubMed] [Google Scholar]
- 52.Ishisaki A, Hayashi H, Li A-J, et al. Human umbilical vein endothelium-derived cells retain potential to differentiate into smooth muscle-like cells. J Biol Chem. 2003;278:1303–9. doi: 10.1074/jbc.M207329200. [DOI] [PubMed] [Google Scholar]
- 53.Paruchuri S, Yang J-H, Aikawa E, et al. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res. 2006;99:861–9. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papetti M, Shujath J, Riley KN, et al. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994–5005. doi: 10.1167/iovs.03-0291. [DOI] [PubMed] [Google Scholar]
- 55.Becerril C, Pardo A, Montano M, et al. Acidic fibroblast growth factor induces an antifibrogenic phenotype in human lung fibroblasts. Am J Respir Cell Mol Biol. 1999;20:1020–7. doi: 10.1165/ajrcmb.20.5.3288. [DOI] [PubMed] [Google Scholar]
- 56.Ramos C, Becerril C, Montaño M, et al. FGF-1 reverts epithelial-mesenchymal transition induced by TGF-{beta}1 through MAPK/ERK kinase pathway. Am J Physiol Lung Cell Mol Physiol. 2010;299:L222–31. doi: 10.1152/ajplung.00070.2010. [DOI] [PubMed] [Google Scholar]
- 57.Ramos C, Montaño M, Becerril C, et al. Acidic fibroblast growth factor decreases alpha-smooth muscle actin expression and induces apoptosis in human normal lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2006;291:L871–9. doi: 10.1152/ajplung.00019.2006. [DOI] [PubMed] [Google Scholar]
- 58.Schweigerer L, Neufeld G, Friedman J, et al. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–9. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 59.Kang H-R, Lee CG, Homer RJ, et al. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J Exp Med. 2007;204:1083–93. doi: 10.1084/jem.20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Story MT, Hopp KA, Meier DA, et al. Influence of transforming growth factor beta 1 and other growth factors on basic fibroblast growth factor level and proliferation of cultured human prostate-derived fibroblasts. Prostate. 1993;22:183–97. doi: 10.1002/pros.2990220302. [DOI] [PubMed] [Google Scholar]
- 61.Finlay GA, Thannickal VJ, Fanburg BL, et al. Transforming growth factor-beta 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem. 2000;275:27650–6. doi: 10.1074/jbc.M000893200. [DOI] [PubMed] [Google Scholar]
- 62.Goldsmith KT, Gammon RB, Garver RI. Modulation of bFGF in lung fibroblasts by TGF-beta and PDGF. Am J Physiol. 1991;261:L378–85. doi: 10.1152/ajplung.1991.261.6.L378. [DOI] [PubMed] [Google Scholar]
- 63.Hurley MM, Abreu C, Gronowicz G, et al. Expression and regulation of basic fibroblast growth factor mRNA levels in mouse osteoblastic MC3T3-E1 cells. J Biol Chem. 1994;269:9392–6. [PubMed] [Google Scholar]
- 64.Story MT, Hopp KA, Meier DA. Regulation of basic fibroblast growth factor expression by transforming growth factor beta in cultured human prostate stromal cells. Prostate. 1996;28:219–26. doi: 10.1002/(SICI)1097-0045(199604)28:4<219::AID-PROS2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 65.Kay EP, Lee MS, Seong GJ, et al. TGF-βs stimulate cell proliferation via an autocrine production of FGF-2 in corneal stromal fibroblasts. Curr Eye Res. 2009;17:286–93. doi: 10.1076/ceyr.17.3.286.5212. [DOI] [PubMed] [Google Scholar]
- 66.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Madar S, Goldstein I, Rotter V. “Cancer associated fibroblasts--”more than meets the eye. Trends Mol Med. 2013;19:447–53. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2010;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2010;175:1380–8. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hashimoto N, Phan SH, Imaizumi K. Endothelial–mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–72. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen P-Y, Simons M. When endothelial cells go rogue. EMBO Mol Med. 2015;8:1–2. doi: 10.15252/emmm.201505943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masszi A, Speight P, Charbonney E, et al. Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J Cell Biol. 2010;188:383–99. doi: 10.1083/jcb.200906155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greenberg RS, Bernstein AM, Benezra M, et al. FAK-dependent regulation of myofibroblast differentiation. FASEB J. 2006;20:1006–8. doi: 10.1096/fj.05-4838fje. [DOI] [PubMed] [Google Scholar]
- 76.Desai VD, Hsia HC, Schwarzbauer JE. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS ONE. 2014;9:e86865. doi: 10.1371/journal.pone.0086865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jester JV, Barry-Lane PA, Cavanagh HD, et al. Induction of [alpha]-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505. [PubMed] [Google Scholar]
- 78.Mattey DL, Dawes PT, Nixon NB, et al. Transforming growth factor beta 1 and interleukin 4 induced alpha smooth muscle actin expression and myofibroblast-like differentiation in human synovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann Rheum Dis. 1997;56:426–31. doi: 10.1136/ard.56.7.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cushing MC, Mariner PD, Liao J-T, et al. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J. 2008;22:1769–77. doi: 10.1096/fj.07-087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rønnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- 81.Kamada Y, Yoshida Y, Saji Y, et al. Transplantation of basic fibroblast growth factor-pretreated adipose tissue-derived stromal cells enhances regression of liver fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G157–67. doi: 10.1152/ajpgi.90463.2008. [DOI] [PubMed] [Google Scholar]
- 82.Thanabalasundaram G, Schneidewind J, Pieper C, et al. The impact of pericytes on the blood–brain barrier integrity depends critically on the pericyte differentiation stage. Int J Biochem Cell Biol. 2011;43:1284–93. doi: 10.1016/j.biocel.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Shi H-X, Lin C, Lin B-B, et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS ONE. 2013;8:e59966. doi: 10.1371/journal.pone.0059966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akita S, Akino K, Imaizumi T, et al. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen. 2008;16:635–41. doi: 10.1111/j.1524-475X.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 85.Ono I, Akasaka Y, Kikuchi R, et al. Basic fibroblast growth factor reduces scar formation in acute incisional wounds. Wound Repair Regen. 2007;15:617–23. doi: 10.1111/j.1524-475X.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 86.Eto H, Suga H, Aoi N, et al. Therapeutic potential of fibroblast growth factor-2 for hypertrophic scars: upregulation of MMP-1 and HGF expression. Lab Invest. 2012;92:214–23. doi: 10.1038/labinvest.2011.127. [DOI] [PubMed] [Google Scholar]
- 87.Xie JL, Bian HN, Qi SH, et al. Basic fibroblast growth factor (bFGF) alleviates the scar of the rabbit ear model in wound healing. Wound Repair Regen. 2008;16:576–81. doi: 10.1111/j.1524-475X.2008.00405.x. [DOI] [PubMed] [Google Scholar]
- 88.Pintucci G, Moscatelli D, Saponara F, et al. Lack of ERK activation and cell migration in FGF-2-deficient endothelial cells. FASEB J. 2002;16:108151–1111. doi: 10.1096/fj.01-0815fje. [DOI] [PubMed] [Google Scholar]
- 89.Nourse MB, Rolle MW, Pabon LM, et al. Selective control of endothelial cell proliferation with a synthetic dimerizer of FGF receptor-1. Lab Invest. 2007;87:828–35. doi: 10.1038/labinvest.3700597. [DOI] [PubMed] [Google Scholar]
- 90.Murphy DA, Makonnen S, Lassoued W, et al. Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006) Am J Pathol. 2006;169:1875–85. doi: 10.2353/ajpath.2006.050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen P-Y, Qin L, Tellides G, et al. Fibroblast growth factor receptor 1 is a key inhibitor of TGFβ signaling in the endothelium. Sci Signal. 2014;7:ra90–0. doi: 10.1126/scisignal.2005504. [DOI] [PubMed] [Google Scholar]
- 92.Khouw IMSL, van Wachem PB, Plantinga JA, et al. TGF-β and bFGF affect the differentiation of proliferating porcine fibroblasts into myofibroblasts in vitro. Biomaterials. 1999;20:1815–22. doi: 10.1016/s0142-9612(99)00077-0. [DOI] [PubMed] [Google Scholar]
- 93.Stern HM, Lin-Jones J, Hauschka SD. Synergistic interactions between bFGF and a TGF-beta family member may mediate myogenic signals from the neural tube. Development. 1997;124:3511–23. doi: 10.1242/dev.124.18.3511. [DOI] [PubMed] [Google Scholar]
- 94.Santiago JJ, Dangerfield AL, Rattan SG, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: Expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–84. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 95.Hu B, Gharaee-Kermani M, Wu Z, et al. Epigenetic regulation of myofibroblast differentiation by DNA methylation. Am J Pathol. 2010;177:21–8. doi: 10.2353/ajpath.2010.090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu M, Yao J, Cai L, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 97.Lee JG, Ko MK, Kay EP. Endothelial mesenchymal transformation mediated by IL-1β-induced FGF-2 in corneal endothelial cells. Exp Eye Res. 2012;95:35–9. doi: 10.1016/j.exer.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Lee JG, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp Eye Res. 2006;83:1309–16. doi: 10.1016/j.exer.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 99.Kurosaka D, Kato K, Nagamoto T. Growth factors influence contractility and alpha-smooth muscle actin expression in bovine lens epithelial cells. Invest Ophthalmol Vis Sci. 1995;36:1701–8. [PubMed] [Google Scholar]
- 100.Zhu Y-T, Chen H-C, Chen S-Y, et al. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J Cell Sci. 2012;125:3636–48. doi: 10.1242/jcs.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu Y-T, Li F, Han B, et al. Activation of RhoA-ROCK-BMP signaling reprograms adult human corneal endothelial cells. J Cell Biol. 2014;206:799–811. doi: 10.1083/jcb.201404032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ko MK, Kay EP. Regulatory role of FGF-2 on type I collagen expression during endothelial mesenchymal transformation. Invest Ophthalmol Vis Sci. 2005;46:4495–503. doi: 10.1167/iovs.05-0818. [DOI] [PubMed] [Google Scholar]
- 103.Morabito CJ, Dettman RW, Kattan J, et al. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev Biol. 2001;234:204–15. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 104.Witty AD, Mihic A, Tam RY, et al. Generation of the epicardial lineage from human pluripotent stem cells. Nat Biotechnol. 2014;32:1026–35. doi: 10.1038/nbt.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGowan SE, McCoy DM. Fibroblast growth factor signaling in myofibroblasts differs from lipofibroblasts during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol. 2015;309:L463–74. doi: 10.1152/ajplung.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanchez-Duffhues G, Orlova V, Dijke ten P. In Brief: Endothelial-to-mesenchymal transition. J Pathol. 2016;238:378–80. doi: 10.1002/path.4653. [DOI] [PubMed] [Google Scholar]
- 107.Choi S-H, Nam J-K, Kim B-Y, et al. HSPB1 inhibits the endothelial-to-mesenchymal transition to suppress pulmonary fibrosis and lung tumorigenesis. Cancer Res. 2016;76:1019–30. doi: 10.1158/0008-5472.CAN-15-0952. [DOI] [PubMed] [Google Scholar]
- 108.McCormack PL. Nintedanib: First global approval. Drugs. 2015;75:129–39. doi: 10.1007/s40265-014-0335-0. [DOI] [PubMed] [Google Scholar]
- 109.Chen Y, Huang K, Nakatsu MN, et al. Identification of novel molecular markers through transcriptomic analysis in human fetal and adult corneal endothelial cells. Hum Mol Gen. 2013;22:1271–9. doi: 10.1093/hmg/dds527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–49. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]