Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts - PubMed (original) (raw)
Comparative Study
Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts
Q Wang et al. Mol Cell Biol. 1999 Jun.
Abstract
L6 myoblasts stably transfected with a GLUT4 cDNA harboring an exofacial myc epitope tag (L6-GLUT4myc myoblasts) were used to study the role of protein kinase B alpha (PKBalpha)/Akt1 in the insulin-induced translocation of GLUT4 to the cell surface. Surface GLUT4myc was detected by immunofluorescent labeling of the myc epitope in nonpermeabilized cells. Insulin induced a marked translocation of GLUT4myc to the plasma membrane within 20 min. This was prevented by transient transfection of a dominant inhibitory construct of phosphatidylinositol (PI) 3-kinase (Deltap85alpha). Transiently transfected cells were identified by cotransfection of green fluorescent protein. A constitutively active PKBalpha, created by fusion of a viral Gag protein at its N terminus (GagPKB), increased the cell surface density of GLUT4myc compared to that of neighboring nontransfected cells. A kinase-inactive, phosphorylation-deficient PKBalpha/Akt1 construct with the mutations K179A (substitution of alanine for the lysine at position 179), T308A, and S473A (AAA-PKB) behaved as a dominant-negative inhibitor of insulin-dependent activation of cotransfected wild-type hemagglutinin (HA)-tagged PKB. Furthermore, AAA-PKB markedly inhibited the insulin-induced phosphorylation of cotransfected BAD, demonstrating inhibition of the endogenous PKB/Akt. Under the same conditions, AAA-PKB almost entirely blocked the insulin-dependent increase in surface GLUT4myc. PKBalpha with alanine substitutions T308A and S473A (AA-PKB) or K179A (A-PKB) alone was a less potent inhibitor of insulin-dependent activation of wild-type HA-PKB or GLUT4myc translocation than was AAA-PKB. Cotransfection of AAA-PKB with a fourfold DNA excess of HA-PKB rescued insulin-stimulated GLUT4myc translocation. AAA-PKB did not prevent actin bundling (membrane ruffling), though this response was PI 3-kinase dependent. Therefore, it is unlikely that AAA-PKB acted by inhibiting PI 3-kinase signaling. These results outline an important role for PKBalpha/Akt1 in the stimulation of glucose transport by insulin in muscle cells in culture.
Figures
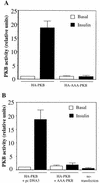
FIG. 1
AAA-PKB acts as a dominant-negative mutant of PKB. (A) L6-GLUT4myc myoblasts grown in six-well plates were transiently transfected with HA-tagged wild-type PKBα/Akt1 (HA-PKB; 0.4 μg per well) or with HA-tagged AAA-PKBα/Akt1 (HA-AAA-PKB; 0.4 μg per well) and incubated for 48 h in culture. Cells were serum deprived for 5 h and were left untreated (basal) or treated with 100 nM insulin for 10 min (insulin). Cell lysates were prepared, HA-tagged proteins were immunoprecipitated with anti-HA antibodies (3 μg), and PKB kinase activity in the immune complexes was measured as described in Materials and Methods. The basal activity of HA-PKB was assigned a value of 1.0; all other activities were expressed relative to this value. The results represent the means ± SE of data from three independent experiments. (B) Cells were transfected with HA-PKB (0.4 μg per well) in combination either with pcDNA3 vector alone (4 μg per well) or with untagged AAA-PKB in pcDNA3 (4 μg per well) as indicated. Cells were treated with insulin as indicated, and lysates were prepared and processed for the PKB/Akt kinase assay as described for panel A. The basal activity of HA-PKB cotransfected with pcDNA3 was assigned a value of 1.0; all other activities were expressed relative to this value. The results represent the means ± SE of data from five independent experiments.
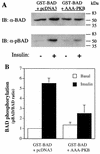
FIG. 2
Insulin-stimulated phosphorylation of GST-BAD is inhibited by coexpression of AAA-PKB. L6-GLUT4myc myoblasts grown in six-well plates were transiently transfected with pEBG-mBAD (0.4 μg per well) in combination with either pcDNA3 vector alone or HA-AAA-PKB (0.4 μg per well) and incubated for 48 h in culture. Cells were serum deprived for 5 h and were left untreated (−) or treated with 100 nM insulin (+) for 10 min. Cells were lysed in detergent-containing buffer as described in Materials and Methods and then immunoblotted (IB) with anti-BAD (α-BAD; 1:500) or anti-phospho(Ser136)-BAD (α-pBAD; 1:500) antibodies. (A) Representative immunoblots for GST-BAD protein (BAD, upper panel) or Ser136-phosphorylated GST-BAD (pBAD, lower panel). The positions of molecular mass markers are indicated on the right side of the gel in kilodaltons. (B) Autoradiographs of four experiments were densitometrically scanned, and the results were plotted as phosphorylated BAD (pBAD)/BAD protein ratios for each set of conditions (insulin-treated [insulin] or untreated [basal] cells) relative to the ratio calculated for basal cells transfected with pEG-mBAD and pcDNA3, with the latter being assigned a value of 1.
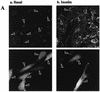
FIG. 3
Transient transfection of Δp85α PI 3-kinase inhibits, and GagPKB potentiates, insulin-stimulated translocation of GLUT4myc in muscle cells. L6-GLUT4myc myoblasts were transiently transfected with GFP (0.4 μg) (A) and cotransfected with HA-PKB (0.4 μg) (B), dominant-negative Δp85α (0.4 μg) (C), or GagPKB (0.4 μg) (D) and incubated for 48 h in culture. (A) Cells were left untreated (basal) or treated with 100 nM insulin for 20 min (insulin) and then processed for cell surface GLUT4myc detection with anti-myc antibody (1:100) followed by Cy3-conjugated goat anti-mouse antibody as indicated in Materials and Methods. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells (arrowheads) is shown. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of the transfected cells. Results shown are representative of at least five experiments. (B) Cells were fixed and processed for indirect immunofluorescence with anti-HA antibody (α-HA; 1:1,000) followed by detection with a Cy3-conjugated goat anti-mouse antibody. The panel on the left shows the number of cells in the field seen by phase-contrast microscopy. The middle and right panels show the GFP-positive cells and the HA-PKB-positive cells, respectively, in the same field. The results indicate complete overlap of GFP- and HA-PKB-positive cells. Results shown are representative of three experiments. (C) Cells were treated as described for panel A. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of cells transfected with Δp85α. Results shown are representative of three experiments. (D) Cells were treated as described for panel A. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of cells transfected with GagPKB. Results shown are representative of three experiments.
FIG. 3
Transient transfection of Δp85α PI 3-kinase inhibits, and GagPKB potentiates, insulin-stimulated translocation of GLUT4myc in muscle cells. L6-GLUT4myc myoblasts were transiently transfected with GFP (0.4 μg) (A) and cotransfected with HA-PKB (0.4 μg) (B), dominant-negative Δp85α (0.4 μg) (C), or GagPKB (0.4 μg) (D) and incubated for 48 h in culture. (A) Cells were left untreated (basal) or treated with 100 nM insulin for 20 min (insulin) and then processed for cell surface GLUT4myc detection with anti-myc antibody (1:100) followed by Cy3-conjugated goat anti-mouse antibody as indicated in Materials and Methods. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells (arrowheads) is shown. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of the transfected cells. Results shown are representative of at least five experiments. (B) Cells were fixed and processed for indirect immunofluorescence with anti-HA antibody (α-HA; 1:1,000) followed by detection with a Cy3-conjugated goat anti-mouse antibody. The panel on the left shows the number of cells in the field seen by phase-contrast microscopy. The middle and right panels show the GFP-positive cells and the HA-PKB-positive cells, respectively, in the same field. The results indicate complete overlap of GFP- and HA-PKB-positive cells. Results shown are representative of three experiments. (C) Cells were treated as described for panel A. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of cells transfected with Δp85α. Results shown are representative of three experiments. (D) Cells were treated as described for panel A. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of cells transfected with GagPKB. Results shown are representative of three experiments.
FIG. 3
Transient transfection of Δp85α PI 3-kinase inhibits, and GagPKB potentiates, insulin-stimulated translocation of GLUT4myc in muscle cells. L6-GLUT4myc myoblasts were transiently transfected with GFP (0.4 μg) (A) and cotransfected with HA-PKB (0.4 μg) (B), dominant-negative Δp85α (0.4 μg) (C), or GagPKB (0.4 μg) (D) and incubated for 48 h in culture. (A) Cells were left untreated (basal) or treated with 100 nM insulin for 20 min (insulin) and then processed for cell surface GLUT4myc detection with anti-myc antibody (1:100) followed by Cy3-conjugated goat anti-mouse antibody as indicated in Materials and Methods. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells (arrowheads) is shown. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of the transfected cells. Results shown are representative of at least five experiments. (B) Cells were fixed and processed for indirect immunofluorescence with anti-HA antibody (α-HA; 1:1,000) followed by detection with a Cy3-conjugated goat anti-mouse antibody. The panel on the left shows the number of cells in the field seen by phase-contrast microscopy. The middle and right panels show the GFP-positive cells and the HA-PKB-positive cells, respectively, in the same field. The results indicate complete overlap of GFP- and HA-PKB-positive cells. Results shown are representative of three experiments. (C) Cells were treated as described for panel A. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of cells transfected with Δp85α. Results shown are representative of three experiments. (D) Cells were treated as described for panel A. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of cells transfected with GagPKB. Results shown are representative of three experiments.
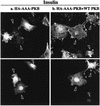
FIG. 4
AAA-PKB inhibits insulin-induced translocation of GLUT4myc in muscle cells. L6-GLUT4myc myoblasts were cotransfected with GFP and HA-AAA-PKB (0.4 μg each) and incubated for 48 h in culture. Cells were serum deprived for 5 h, left untreated or treated with 100 nM insulin for 20 min (insulin), and then processed for cell surface GLUT4myc detection with anti-myc (1:100) antibody followed by Cy3-conjugated goat anti-mouse antibody as indicated in Materials and Methods. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells is shown (arrowheads). The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b) for three separate experiments. Arrowheads indicate the positions of cells transfected with HA-AAA-PKB in the upper panels. Results shown are representative of at least five experiments.
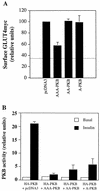
FIG. 5
Comparison of AAA-PKB, AA-PKB, and A-PKB with regard to insulin-stimulated GLUT4myc translocation and activation of HA-PKB by insulin. (A) L6-GLUT4myc myoblasts were cotransfected with GFP (0.4 μg) and HA-AAA-PKB, HA-AA-PKB, or HA-A-PKB (0.4 μg each) and incubated for 48 h in culture. Cells were serum deprived for 5 h, left untreated (basal) or treated with 100 nM insulin for 20 min (insulin), and then processed for detection of cell surface GLUT4myc with anti-myc antibody (1:100) followed by Cy3-conjugated goat anti-mouse antibody as indicated in Materials and Methods. The intensity of the fluorescent label of cell surface GLUT4myc was quantitated by using NIH Image soft ware. The pixel intensity of GLUT4myc staining per cell was measured in similar numbers of transfected and nontransfected cells. A value of 100% was assigned to nontransfected, insulin-stimulated cells within each field. The pixel intensity of the transfected cells in the same field was then calculated as a fraction of this value. The pixel intensity in parallel coverslips of unstimulated cells transfected with pcDNA3 and GFP only was also measured and is indicated by the dotted line. The results are means ± SE of data from at least three independent experiments under each set of conditions. (B) L6-GLUT4myc myoblasts were cotransfected with HA-PKB (0.4 μg) and empty pcDNA3 vector, untagged AAA-PKB, AA-PKB, or A-PKB (4.0 μg each) and incubated as described for panel A prior to analysis. Cells were left untreated (basal) or treated with 100 nM insulin for 10 min (insulin). Cell lysates were prepared, HA-tagged proteins were immunoprecipitated with anti-HA antibody (3 μg), and PKB kinase activity in the immune complexes was measured as described in Materials and Methods. The basal activity of HA-PKB cotransfected with pcDNA3 was assigned a value of 1.0. All other activities were expressed relative to this value. The results represent the means ± SE of data from three experiments under each set of conditions.
FIG. 6
Coexpression of wild-type PKB with AAA-PKB rescues the inhibition of insulin-stimulated GLUT4 translocation. L6-GLUT4myc myoblasts were cotransfected with GFP (0.4 μg), HA-AAA-PKB (0.4 μg), and pcDNA3 (1.6 μg) (a) or with GFP (0.4 μg), HA-AAA-PKB (0.4 μg), and HA-PKB (1.6 μg) (b) and incubated for 48 h in culture. Cells were serum deprived for 5 h, left untreated or treated with 100 nM insulin for 20 min, and then processed for cell surface GLUT4myc detection as indicated in Materials and Methods. Untreated cells are not shown. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells is shown (arrowheads). The upper panels show the cell surface GLUT4myc density for the transfection of AAA-PKB plus pcDNA3 vector alone (a) or the transfection of AAA-PKB with excess wild-type (WT) PKB (b). Arrowheads indicate the positions of transfected cells. Results shown are representative of at least three experiments.
FIG. 7
PI 3-kinase-dependent actin remodeling is not affected by AAA-PKB. L6-GLUT4myc myoblasts were cotransfected with GFP (0.4 μg) and Δp85α (0.4 μg) (a and b) or with GFP (0.4 μg) and AAA-PKB (0.4 μg) (c to f) and incubated for 48 h in culture. Cells were serum deprived for 5 h and left untreated (basal) or treated with 100 nM insulin for 5 min (insulin). Cells in panels e and f were pretreated with wortmannin for 15 min before treatment with insulin (or no further treatment). All cells were processed for detection of actin with rhodamine-phalloidin as indicated in Materials and Methods. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells is shown (arrowheads). The upper panels show filamentous actin in untreated cells (basal) (a and c), cells pretreated with wortmannin (Wort.) (e), insulin-treated cells (insulin) (b and d), or wortmannin pretreated, insulin-treated cells (Wort. + Ins.) (f). Arrowheads indicate the positions of transfected cells. Results shown are representative of at least three experiments.
Similar articles
- Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis.
Niu W, Huang C, Nawaz Z, Levy M, Somwar R, Li D, Bilan PJ, Klip A. Niu W, et al. J Biol Chem. 2003 May 16;278(20):17953-62. doi: 10.1074/jbc.M211136200. Epub 2003 Mar 11. J Biol Chem. 2003. PMID: 12637564 - Dependence of insulin-stimulated glucose transporter 4 translocation on 3-phosphoinositide-dependent protein kinase-1 and its target threonine-410 in the activation loop of protein kinase C-zeta.
Bandyopadhyay G, Standaert ML, Sajan MP, Karnitz LM, Cong L, Quon MJ, Farese RV. Bandyopadhyay G, et al. Mol Endocrinol. 1999 Oct;13(10):1766-72. doi: 10.1210/mend.13.10.0364. Mol Endocrinol. 1999. PMID: 10517677 - Insulin but not PDGF relies on actin remodeling and on VAMP2 for GLUT4 translocation in myoblasts.
Török D, Patel N, Jebailey L, Thong FS, Randhawa VK, Klip A, Rudich A. Török D, et al. J Cell Sci. 2004 Oct 15;117(Pt 22):5447-55. doi: 10.1242/jcs.01421. Epub 2004 Oct 5. J Cell Sci. 2004. PMID: 15466888 - The protein kinase B/Akt signalling pathway in human malignancy.
Nicholson KM, Anderson NG. Nicholson KM, et al. Cell Signal. 2002 May;14(5):381-95. doi: 10.1016/s0898-6568(01)00271-6. Cell Signal. 2002. PMID: 11882383 Review. - PKB/Akt: a key mediator of cell proliferation, survival and insulin responses?
Lawlor MA, Alessi DR. Lawlor MA, et al. J Cell Sci. 2001 Aug;114(Pt 16):2903-10. doi: 10.1242/jcs.114.16.2903. J Cell Sci. 2001. PMID: 11686294 Review.
Cited by
- Unveiling the Mechanism of Protective Effects of Tanshinone as a New Fighter Against Cardiovascular Diseases: A Systematic Review.
Dabbaghi MM, Soleimani Roudi H, Safaei R, Baradaran Rahimi V, Fadaei MR, Askari VR. Dabbaghi MM, et al. Cardiovasc Toxicol. 2024 Dec;24(12):1467-1509. doi: 10.1007/s12012-024-09921-x. Epub 2024 Sep 22. Cardiovasc Toxicol. 2024. PMID: 39306819 Review. - An antifibrotic compound that ameliorates hyperglycaemia and fat accumulation in cell and HFD mouse models.
Toma T, Miyakawa N, Arakaki Y, Watanabe T, Nakahara R, Ali TFS, Biswas T, Todaka M, Kondo T, Fujita M, Otsuka M, Araki E, Tateishi H. Toma T, et al. Diabetologia. 2024 Nov;67(11):2568-2584. doi: 10.1007/s00125-024-06260-y. Epub 2024 Sep 9. Diabetologia. 2024. PMID: 39251430 - Phosphorylation Codes in IRS-1 and IRS-2 Are Associated with the Activation/Inhibition of Insulin Canonical Signaling Pathways.
Martínez Báez A, Ayala G, Pedroza-Saavedra A, González-Sánchez HM, Chihu Amparan L. Martínez Báez A, et al. Curr Issues Mol Biol. 2024 Jan 9;46(1):634-649. doi: 10.3390/cimb46010041. Curr Issues Mol Biol. 2024. PMID: 38248343 Free PMC article. Review. - Effect of RONS-Induced Intracellular Redox Homeostasis in 6-NBDG/Glucose Uptake in C2C12 Myotubes and Single Isolated Skeletal Muscle Fibres.
Fernández-Puente E, Martín-Prieto E, Márquez CM, Palomero J. Fernández-Puente E, et al. Int J Mol Sci. 2023 Apr 29;24(9):8082. doi: 10.3390/ijms24098082. Int J Mol Sci. 2023. PMID: 37175789 Free PMC article. - Liver acts as a metabolic gate for the traumatic brain injury pathology: Protective action of thyroid hormone.
Khandelwal M, Krishna G, Ying Z, Gomez-Pinilla F. Khandelwal M, et al. Biochim Biophys Acta Mol Basis Dis. 2023 Aug;1869(6):166728. doi: 10.1016/j.bbadis.2023.166728. Epub 2023 May 1. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 37137432 Free PMC article.
References
- Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. - PubMed
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. - PubMed
- Bandyopadhyay G, Standaert M L, Galloway L, Moscat J, Farese R V. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. - PubMed
- Brown R E, Jarvis K L, Hyland K J. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989;180:136–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous