GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism - PubMed (original) (raw)
GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism
T Laux et al. J Cell Biol. 2000.
Abstract
The dynamic properties of the cell cortex and its actin cytoskeleton determine important aspects of cell behavior and are a major target of cell regulation. GAP43, myristoylated alanine-rich C kinase substrate (MARCKS), and CAP23 (GMC) are locally abundant, plasmalemma-associated PKC substrates that affect actin cytoskeleton. Their expression correlates with morphogenic processes and cell motility, but their role in cortex regulation has been difficult to define mechanistically. We now show that the three proteins accumulate at rafts, where they codistribute with PI(4,5)P(2), and promote its retention and clustering. Binding and modulation of PI(4, 5)P(2) depended on the basic effector domain (ED) of these proteins, and constructs lacking the ED functioned as dominant inhibitors of plasmalemmal PI(4,5)P(2) modulation. In the neuron-like cell line, PC12, NGF- and substrate-induced peripheral actin structures, and neurite outgrowth were greatly augmented by any of the three proteins, and suppressed by DeltaED mutants. Agents that globally mask PI(4,5)P(2) mimicked the effects of GMC on peripheral actin recruitment and cell spreading, but interfered with polarization and process formation. Dominant negative GAP43(DeltaED) also interfered with peripheral nerve regeneration, stimulus-induced nerve sprouting and control of anatomical plasticity at the neuromuscular junction of transgenic mice. These results suggest that GMC are functionally and mechanistically related PI(4,5)P(2) modulating proteins, upstream of actin and cell cortex dynamics regulation.
Figures
Figure 1
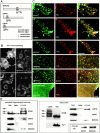
GAP43, MARCKS, and CAP23 accumulate at plasmalemmal rafts, where they codistribute with PI(4,5)P2. (A) Schematic representation of GMC, with the relative position of the EDs. (B) Plasmalemmal immunoreactivity detected with the PI(4,5)P2 antibody kt3g is suppressed by treatments that mask or reduce cell membrane PI(4,5)P2. Treatment times are as follows: neomycin and LiCl, 24 h; and calcimycin, 15 min. (C) Codistribution of PI(4,5)P2 immunoreactivity with endogenous GMC in hippocampal neuron growth cones and transiently transfected COS-7 cells. Note the absence of clustered labeling pattern for PI(4,5)P2 and MARCKS in cells fixed with glutaraldehyde. (D) GMC accumulate in raft fractions from hippocampal neuron cultures, adult brain homogenate, and stably transfected PC12B clones. The samples are fractions from the sucrose density gradient used to isolate rafts; equal amounts of total protein were loaded on each lane. Lysate: brain homogenate. Bars: (B) 25 μm; (C) 3 μm.
Figure 2
The distributions of PI(4,5)P2 and GMC proteins at the cell membrane are interdependent. (A) Hippocampal neurons (left, nontransgenic; middle, transgenic overexpressing GAP43), COS cells (right) and PC12B cells overexpressing GMC proteins exhibit larger PI(4,5)P2 clusters. Quantitative analysis: COS cells, one 20 × 20 μm2 bin analyzed per cell (n = 20). (B) GAP43 and MARCKS bind to PI(4,5)P2-containing (PIPs) but not to PC lipid vesicles; GAP43(Ser42Asp) binds more weakly; and GAP43(ΔED) does not bind. Quantitative analysis of GAP43 binding (immunoblot signal intensities, AU) to PIPs is shown in the graph (n = 4). (C) Cyclodextrin disperses plasmalemmal GMC-PI(4,5)P2 microdomains; GMC protect partially against dispersion by cyclodextrin. Raft fractions: PC12B-GAP43 cells, immunoblot. Photographs: COS cells treated with cyclodextrin (5 mM, 30 min); left, nontransfected (compare to nontreated patterns in A). Quantitative analysis: COS cells, with and without cyclodextrin; one 20 × 20 μm2 bin per cell (n = 40). Bars: 10 μm.
Figure 3
GMC modulate plasmalemmal PI(4,5)P2 clusters independent of actin cytoskeleton integrity. Transiently transfected COS cells; (insets) details at 4×. Disruption of the actin cytoskeleton with cytochalasin D (20 min; A), or constitutively active (LIM)K1 (B) did not affect PI(4,5)P2 clusters, nor the effect of GMC proteins (bottom panels in A, and bottom panels in B) on cluster size. (B, top panels) Actin cytoskeleton clumping in cells overexpressing (LIM)K1. (B, central and bottom panels) Triple labelings of cells overexpressing (LIM)K1 (clumped actin cytoskeleton), with or without MARCKS. Bars: 5 μm.
Figure 4
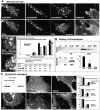
Mutants of MARCKS and GAP43 lacking the ED accumulate at GMC-PI(4,5)P2 microdomains and interfere with PI(4,5)P2 accumulation at the microdomains. (A, top three rows) Hippocampal neuron growth cones from wild-type (top) and Thy1-GAP43(ΔED) mice; bottom rows: single and double (bottom) transfections of COS cells, as indicated. (B) Quantitative analysis of transfection experiments; COS cells had up to 200–300 PI(4,5)P2 clusters; cells with <10 clusters were scored as negative. n = 200. Bars: 5 μm.
Figure 5
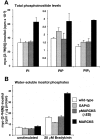
MARCKS, but not GAP43, nor pMARCKS(ΔED) affects bulk phosphoinositide contents in PC12B clones. (A) Bulk contents of PI, PIP, and PIP2 (n = 4). (B) Bradykinin-induced (20 μM, 15 min) hydrolysis of PI(4,5)P2 by PLC (n = 4).
Figure 6
Roles of GMC and PI(4,5)P2 in actin regulation at the cell periphery. (A) RITC-phalloidin patterns of PC12B cells 3 h after plating. The figure shows representative examples obtained with a clone expressing MARCKS; comparable results were obtained with GAP43 and CAP23 clones. (B) Quantitative analysis of phalloidin labeling profiles (see Materials and Methods for details). The schematic on the left shows how rectangular bins were placed (six cell profiles are superimposed for each plot on the right). Bar, 25 μm.
Figure 7
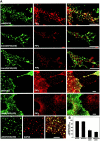
Opposite effects of GMC and their ΔED mutants on plasmalemmal actin dynamics. RITC-phalloidin labelings of PC12B clones. (A) Cells grown in the presence of NGF for 4 d. Rectangles show the position of the details shown in the bottom rows. (B) Cells grown for 1 d in the presence of NGF and drugs, as indicated. Note the induction of symmetrical actin structures in the presence of neomycin, partial rescue of polarization by MARCKS, and opposite effects of the PLC inhibitor U-73122. Also note that neomycin partially rescued peripheral actin accumulation in GAP43(ΔED) cells. Bar: 10 μm (A, top rows); 25 μm (B).
Figure 8
Role of GMC proteins in neurite outgrowth in PC12B cells. (A) NGF-induced neurite outgrowth (4-d cultures). Quantitative analysis: 150 neurons per 4-d culture (N = 4). (B) Spreading and process outgrowth in the absence of NGF; cells 3 h after plating on a collagen-coated substrate. Neomycin and LiCl promoted symmetrical cell spreading and suppressed process outgrowth. Quantitative analysis of the data: all cells (total ∼500) from randomly selected fields. (C) Transgene expression levels (immunoblots) in the PC12B clones shown in Fig. 6 Fig. 7 Fig. 8. Equal amounts of total protein per lane. (D) Induction of PIP3 synthesis in NGF-treated PC12B clones. Note indistinguishable induction of this PI3-kinase product in the clones (n = 6). Bars: 25 μm.
Figure 9
Role of GMC-mediated regulation in anatomical plasticity and peripheral nerve regeneration. (A) Expression of GAP43(ΔED) at the adult neuromuscular junction (medial gastrocnemius muscle). (insets) Same synapse visualized with RITC–α-bungarotoxin. (B) Neuromuscular junction configurations at medial gastrocnemius of wild-type and Thy1-GAP43(ΔED) mice (line-13). Note circular shapes and enlargements of nerve terminal branches in the mutants (arrows). (C) Synaptic sprouting 7 d after BotA treatment. The photographs show sprouts in medial gastrocnemius (or soleus, where indicated) of Thy1-GAP43(ΔED) mice (or wild-type mice (wt), where indicated)). The two panels on the right show details of sprouts. (arrows) Unusual features of sprouts in the presence of GAP43(ΔED) (curved, lamellae, and side branches); and (asterisk) neuromuscular junctions. (E) Reinnervation of triceps surae, 14 d after nerve crush in wild-type and Thy1 transgenic mice, as indicated. Neuromuscular junction transgene immunoreactivity in line-13 was about threefold higher than in line-19, and comparable to that of the other transgenic lines included in the analysis (n = 4). (F) Representative camera lucida of triceps surae reinnervation 14 d after sciatic nerve crush. The synaptic band (acetylcholine esterase reaction product) is in blue, and nerve profiles (silver stain) are in red. Bars: 25 μm.
Figure 10
Proposed model of PI(4,5)P2 modulation by GMC proteins at plasmalemmal microdomains, and its effects on actin cytoskeleton dynamics. Filled circles in the PI(4,5)P2 symbols indicate masking by GMC; the bold segment in the GMC symbol indicates the basic domain; the bold lines at the cell periphery represent actin structures.
Similar articles
- Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity.
Frey D, Laux T, Xu L, Schneider C, Caroni P. Frey D, et al. J Cell Biol. 2000 Jun 26;149(7):1443-54. doi: 10.1083/jcb.149.7.1443. J Cell Biol. 2000. PMID: 10871284 Free PMC article. - The motility-associated proteins GAP-43, MARCKS, and CAP-23 share unique targeting and surface activity-inducing properties.
Wiederkehr A, Staple J, Caroni P. Wiederkehr A, et al. Exp Cell Res. 1997 Oct 10;236(1):103-16. doi: 10.1006/excr.1997.3709. Exp Cell Res. 1997. PMID: 9344590 - New EMBO members' review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts.
Caroni P. Caroni P. EMBO J. 2001 Aug 15;20(16):4332-6. doi: 10.1093/emboj/20.16.4332. EMBO J. 2001. PMID: 11500359 Free PMC article. Review. - Actin cytoskeleton: thinking globally, actin' locally.
Lanier LM, Gertler FB. Lanier LM, et al. Curr Biol. 2000 Sep 21;10(18):R655-7. doi: 10.1016/s0960-9822(00)00685-0. Curr Biol. 2000. PMID: 10996803 - Spatial control of actin-based motility through plasmalemmal PtdIns(4,5)P2-rich raft assemblies.
Golub T, Pico C. Golub T, et al. Biochem Soc Symp. 2005;(72):119-27. doi: 10.1042/bss0720119. Biochem Soc Symp. 2005. PMID: 15649136 Review.
Cited by
- Bilateral regulation of EGFR activity and local PI(4,5)P2 dynamics in mammalian cells observed with superresolution microscopy.
Abe M, Yanagawa M, Hiroshima M, Kobayashi T, Sako Y. Abe M, et al. Elife. 2024 Nov 8;13:e101652. doi: 10.7554/eLife.101652. Elife. 2024. PMID: 39513999 Free PMC article. - A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate promotes neuronal differentiation in SH-SY5Y human neuroblastoma cells.
Ferdous J, Naitou K, Shiraishi M. Ferdous J, et al. J Vet Med Sci. 2024 Nov 1;86(11):1136-1144. doi: 10.1292/jvms.24-0276. Epub 2024 Sep 27. J Vet Med Sci. 2024. PMID: 39343539 Free PMC article. - Temporal Quantitative Proteomic and Phosphoproteomic Profiling of SH-SY5Y and IMR-32 Neuroblastoma Cells during All-_Trans_-Retinoic Acid-Induced Neuronal Differentiation.
Leung TCN, Lu SN, Chu CN, Lee J, Liu X, Ngai SM. Leung TCN, et al. Int J Mol Sci. 2024 Jan 15;25(2):1047. doi: 10.3390/ijms25021047. Int J Mol Sci. 2024. PMID: 38256121 Free PMC article. - Targeting N-Myristoylation Through NMT2 Prevents Cardiac Hypertrophy and Heart Failure.
Tomita Y, Anzai F, Misaka T, Ogawara R, Ichimura S, Wada K, Kimishima Y, Yokokawa T, Ishida T, Takeishi Y. Tomita Y, et al. JACC Basic Transl Sci. 2023 Sep 6;8(10):1263-1282. doi: 10.1016/j.jacbts.2023.06.006. eCollection 2023 Oct. JACC Basic Transl Sci. 2023. PMID: 38094695 Free PMC article. - MARCKS and PI(4,5)P2 reciprocally regulate actin-based dendritic spine morphology.
Calabrese B, Halpain S. Calabrese B, et al. Mol Biol Cell. 2024 Feb 1;35(2):ar23. doi: 10.1091/mbc.E23-09-0370. Epub 2023 Dec 13. Mol Biol Cell. 2024. PMID: 38088877 Free PMC article.
References
- Aderem A. The MARCKS family of protein kinase-C substrates. Biochem. Soc. Trans. 1995;23:587–591. - PubMed
- Aigner L., Arber S., Kapfhammer J.P., Laux T., Schneider C., Botteri F., Brenner H.-R., Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
- Arber S., Barbayannis T.A., Hanser H, Schneider C., Stanyon C.A., Bernard O., Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. - PubMed
- Baetge E.E., Hammang J.P. Neurite outgrowth in PC12 cells deficient in GAP-43. Neuron. 1991;6:21–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous