Direct interaction of resistance gene and avirulence gene products confers rice blast resistance - PubMed (original) (raw)
Direct interaction of resistance gene and avirulence gene products confers rice blast resistance
Y Jia et al. EMBO J. 2000.
Abstract
Rice expressing the Pi-ta gene is resistant to strains of the rice blast fungus, Magnaporthe grisea, expressing AVR-Pita in a gene-for-gene relationship. Pi-ta encodes a putative cytoplasmic receptor with a centrally localized nucleotide-binding site and leucine-rich domain (LRD) at the C-terminus. AVR-Pita is predicted to encode a metalloprotease with an N-terminal secretory signal and pro-protein sequences. AVR-Pita(176) lacks the secretory and pro-protein sequences. We report here that transient expression of AVR-Pita(176) inside plant cells results in a Pi-ta-dependent resistance response. AVR-Pita(176) protein is shown to bind specifically to the LRD of the Pi-ta protein, both in the yeast two-hybrid system and in an in vitro binding assay. Single amino acid substitutions in the Pi-ta LRD or in the AVR-Pita(176) protease motif that result in loss of resistance in the plant also disrupt the physical interaction, both in yeast and in vitro. These data suggest that the AVR-Pita(176) protein binds directly to the Pi-ta LRD region inside the plant cell to initiate a Pi-ta-mediated defense response.
Figures
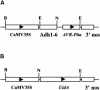
Fig. 1. Physical maps of the transgenes used for transient expression analysis. (A) AVR-Pita expression vector. The maize Adh1-6 intron was inserted after the CaMV 35S promoter resulting in a fusion promoter for expression of constructs encoding AVR-Pita223, AVR-Pita176 and AVR-Pita166. (B) GUS expression vector. Plasmid pML63 contained the uidA gene, encoding GUS, under control of the CaMV 35S promoter. The restriction endonucleases used for generating constructs are shown (B, _Bam_HI; E, _Eco_RI; N, _Nco_I). Both expression cassettes contain bacterial 3′ nos terminator sequences.
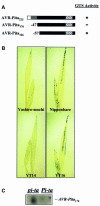
Fig. 2. Genotype-specific HR in rice seedlings induced by M.grisea carrying AVR-Pita. Sparse HR flecking is seen in _Pi-ta_-containing rice seedlings (A) Yashiro-mochi and (B) YT14, as expected. In contrast, typical symptoms of rice blast disease are seen in susceptible rice seedlings (C) Nipponbare and (D) YT16. Representative leaves are shown from rice seedlings germinated in plant nutrient medium and infected with avirulent M.grisea strain 4360-R-62 (see Materials and methods for details). Shown at 4 days after inoculation.
Fig. 3. AVR-Pita176 is an elicitor. (A) AVR-Pita polypeptides tested in the transient assay. The white region indicates the putative secretory signal sequence, the gray region indicates the putative pro-protein domain and the hatched region indicates the putative protease motif. The black region indicates the putative mature protein. The number of amino acids missing from the N-terminus is indicated. GUS activity is indicated by ‘+’, whereas decreased GUS activity is indicated by ‘–’. (B) Representative rice seedlings showing GUS activity. Two-leaf Pi-ta (Yashiro-mochi and YT14) and pi-ta (Nipponbare and YT16) seedlings were co-bombarded with _35S/Adh1-6::AVR-Pita_176 and 35S::uidA. Leaves were assayed histochemically for GUS activity and cleared in 70% ethanol to visualize GUS staining. (C) RNA gel blot analysis of AVR-Pita expression in the transient assay. YT14 (Pi-ta) and YT16 (pi-ta) were co-bombarded with the _35S/Adh1-6::AVR-Pita_176 and 35S::uidA plasmids. Leaf tissue was harvested 2 days after bombardment. Poly(A)+ mRNA was then extracted, blotted to Hybond-N and hybridized with a radiolabeled _AVR-Pita_176 probe. The AVR-Pita transcript is indicated. Similar loading was verified before blotting by visualizing mRNA in the gel stained with ethidium bromide.
Fig. 4. AVR-Pita176 interacts specifically with the Pi-ta LRD in the yeast two-hybrid system. (A) Mapping the interaction domain of the Pi-ta protein using the yeast two-hybrid system. The diagram (left) depicts the Pi-ta protein, the LRD region and a series of deletion constructs of Pi-ta. (1) YRG2 yeast cells expressing both bait and prey fusions were grown on yeast synthetic minimal (SD, Stratagene) liquid medium with omission of leucine and tryptophan (SD-LT), and (2) on yeast SD medium with omission of leucine, histidine and tryptophan for examination of HIS3 reporter gene (–LHT). (3) Yeast cells expressing both bait and prey fusions were grown on yeast SD-LT plates and assayed for lacZ activity as described in Materials and methods. Color development is shown after 24 h. _AVR-Pita_176 was cloned as an AD fusion (in pAD-GAL4) and each Pi-ta deletion construct was cloned as a BD fusion (in pBD-GAL4 Cam). The positive control was YRG2 cells containing p53 and pSV40 fusion constructs, which express proteins that interact in vivo, and the negative control was YRG2 cells containing pLamin C and pSV40 fusion constructs, which express proteins that do not interact in vivo (Stratagene). (B and C) Specificity of interaction of AVR-Pita176 with LRD polypeptide. Single amino acid substitutions that inactivate either LRD or AVR-Pita 176 in vivo eliminated growth in the absence of histidine and slowed color development with the lacZ reporter (referred to as an impaired physical interaction). LRD refers to the NΔ586 deletion of the Pi-ta protein from (A) above. LRDA918S refers to LRD containing S substituted for A at position 918 of the Pi-ta protein. avr-pita176E177D refers to the putative processed polypeptide that no longer confers avirulence due to an E to D substitution at residue 177 of AVR-Pita223. Similar expression of each BD fusion protein in yeast was verified by western blots.
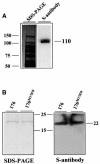
Fig. 5. The Pi-ta protein binds to AVR-Pita176 and its mutant in far-western analysis. (A) Expression of the Pi-ta protein in E.coli. A total bacterial soluble extract expressing S-tagged Pi-ta protein was subjected to SDS–PAGE, and proteins were electroblotted onto PVDF membrane and detected by chemiluminescent visualization using monoclonal S-antibody to detect the S tag (right). The duplicate Coomassie Blue-stained gel is shown on the left. Molecular weights (kDa) were estimated using Perfect S protein markers (Novagen). (B) Binding of the Pi-ta protein to membrane-blotted AVR-Pita176 proteins. Purified AVR-Pita176 and avr-pita176M178W proteins were subjected to SDS–PAGE, and stained by Coomassie Blue (left). A duplicate SDS–PAGE gel was electroblotted onto a nitrocellulose membrane (right), and the soluble extract expressing S-tagged-Pi-ta protein was added for binding. Bound Pi-ta protein was visualized using the monoclonal S-antibody as shown in (A). Perfect S protein markers were used.
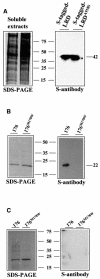
Fig. 6. AVR-Pita176 binds specifically to the Pi-ta LRD region in far-western analysis. (A) Expression of Pi-ta LRD polypeptides in E.coli. Total bacterial soluble extracts expressing S-tagged LRD and LRDA918S were subjected to SDS–PAGE, and then electroblotted onto PVDF membrane. LRD and LRDA918S were detected by chemiluminescent visualization using monoclonal S-antibody (right). The duplicate Coomassie Blue-stained SDS–PAGE gel is shown (left). The molecular weight (kDa) was estimated using the perfect S protein markers (Novagen). (B) Binding of the LRD polypeptide to membrane-bound AVR-Pita176. Purified AVR-Pita proteins were subjected to SDS–PAGE, and stained by Coomassie Blue (left). The duplicate SDS–PAGE gel was electroblotted onto a nitrocellulose membrane, and total soluble extract expressing S-tagged LRD was added for binding (see Materials and methods for details). The bound LRD was visualized by monoclonal S-antibody to detect S tag (right). Protein sizes (kDa) were determined as in (A). (C) In contrast, LRDA918S polypeptide failed to bind the membrane-blotted AVR-Pita176 proteins. Purified AVR-Pita proteins were subjected to SDS–PAGE, and stained by Coomassie Blue under identical conditions as in (B). The duplicate SDS–PAGE gel was electroblotted onto a nitrocellulose membrane, and the total soluble extract expressing S-tagged LRDA918S polypeptide was added for binding. Monoclonal S-antibody was added to detect bound LRDA918S (right). Protein sizes (kDa) were determined as in (A).
Similar articles
- A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta.
Orbach MJ, Farrall L, Sweigard JA, Chumley FG, Valent B. Orbach MJ, et al. Plant Cell. 2000 Nov;12(11):2019-32. doi: 10.1105/tpc.12.11.2019. Plant Cell. 2000. PMID: 11090206 Free PMC article. - Coevolutionary Dynamics of Rice Blast Resistance Gene Pi-ta and Magnaporthe oryzae Avirulence Gene AVR-Pita 1.
Jia Y, Zhou E, Lee S, Bianco T. Jia Y, et al. Phytopathology. 2016 Jul;106(7):676-83. doi: 10.1094/PHYTO-02-16-0057-RVW. Epub 2016 May 13. Phytopathology. 2016. PMID: 27070427 Review. - [Genetic diversity of AVR-pita alleles of rice blast fungus Magnaporthe grisea].
Ma BT, Qu GL, Shi J, Chen DX, Lin YF, Huang WJ, Li SG. Ma BT, et al. Fen Zi Xi Bao Sheng Wu Xue Bao. 2008 Dec;41(6):495-9. Fen Zi Xi Bao Sheng Wu Xue Bao. 2008. PMID: 19137822 Chinese. - Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence.
Zhou E, Jia Y, Singh P, Correll JC, Lee FN. Zhou E, et al. Fungal Genet Biol. 2007 Oct;44(10):1024-34. doi: 10.1016/j.fgb.2007.02.003. Epub 2007 Feb 21. Fungal Genet Biol. 2007. PMID: 17387027 - [The research progress on molecular genetics of pathogenicity of rice blast fungus.].
Huang JL, Wang GX. Huang JL, et al. Yi Chuan. 2005 May;27(3):492-8. Yi Chuan. 2005. PMID: 15985420 Review. Chinese.
Cited by
- Exploring the molecular mechanisms of rice blast resistance and advances in breeding for disease tolerance.
Younas MU, Qasim M, Ahmad I, Feng Z, Iqbal R, Jiang X, Zuo S. Younas MU, et al. Mol Biol Rep. 2024 Oct 26;51(1):1093. doi: 10.1007/s11033-024-10031-8. Mol Biol Rep. 2024. PMID: 39460780 Review. - Exploring Distribution and Evolution of Pi-ta Haplotypes in Rice Landraces across Different Rice Cultivation Regions in Yunnan.
Luo H, Lu L, Wang Q, Guo Z, Liu L, He C, Shi J, Dong C, Ma Q, Li J. Luo H, et al. Genes (Basel). 2024 Oct 15;15(10):1325. doi: 10.3390/genes15101325. Genes (Basel). 2024. PMID: 39457449 Free PMC article. - The roles of Magnaporthe oryzae avirulence effectors involved in blast resistance/susceptibility.
Liu X, Hu X, Tu Z, Sun Z, Qin P, Liu Y, Chen X, Li Z, Jiang N, Yang Y. Liu X, et al. Front Plant Sci. 2024 Oct 9;15:1478159. doi: 10.3389/fpls.2024.1478159. eCollection 2024. Front Plant Sci. 2024. PMID: 39445147 Free PMC article. Review. - Comparative Genomic Analyses of Colletotrichum lindemuthianum Pathotypes with Different Virulence Levels and Lifestyles.
Morelos-Martínez MI, Cano-Camacho H, Díaz-Tapia KM, Simpson J, López-Romero E, Zavala-Páramo MG. Morelos-Martínez MI, et al. J Fungi (Basel). 2024 Sep 13;10(9):651. doi: 10.3390/jof10090651. J Fungi (Basel). 2024. PMID: 39330411 Free PMC article. - Wheat Leaf Rust Fungus Effector Protein Pt1641 Is Avirulent to TcLr1.
Chang J, Mapuranga J, Li R, Zhang Y, Shi J, Yan H, Yang W. Chang J, et al. Plants (Basel). 2024 Aug 14;13(16):2255. doi: 10.3390/plants13162255. Plants (Basel). 2024. PMID: 39204691 Free PMC article.
References
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Smith,J.A., Seidman,J.G. and Struhl,K. (eds) (1987) Protocols in Molecular Biology. John Wiley and Sons, New York, NY.
- Bonas U. and van den Ackerveken,G. (1997) Recognition of bacterial avirulence proteins occurs inside the plant cell: a general phenomenon in resistance to bacterial disease? Plant J., 12, 1–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous