Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice - PubMed (original) (raw)
Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice
S D Dufresne et al. Mol Cell Biol. 2001 Jan.
Abstract
The p90 ribosomal S6 kinase (RSK), a cytosolic substrate for the extracellular signal-regulated kinase (ERK), is involved in transcriptional regulation, and one isoform (RSK2) has been implicated in the activation of glycogen synthase by insulin. To determine RSK2 function in vivo, mice lacking a functional rsk2 gene were generated and studied in response to insulin and exercise, two potent stimulators of the ERK cascade in skeletal muscle. RSK2 knockout (KO) mice weigh 10% less and are 14% shorter than wild-type (WT) mice. They also have impaired learning and coordination. Hindlimb skeletal muscles were obtained from mice 10, 15, or 30 min after insulin injection or immediately after strenuous treadmill exercise for 60 min. While insulin and exercise significantly increased ERK phosphorylation in skeletal muscle from both WT and KO mice, the increases were twofold greater in the KO animals. This occurred despite 27% lower ERK2 protein expression in skeletal muscle of KO mice. KO mice had 18% less muscle glycogen in the fasted basal state, and insulin increased glycogen synthase activity more in KO than WT mice. The enhanced insulin-stimulated increases in ERK and glycogen synthase activities in KO mice were not associated with higher insulin receptor or with IRS1 tyrosine phosphorylation or with IRS1 binding to phosphatidylinositol 3-kinase. However, insulin-stimulated serine phosphorylation of Akt was significantly higher in the KO animals. c-fos mRNA was increased similarly in muscle from WT and KO mice in response to insulin (2. 5-fold) and exercise (15-fold). In conclusion, RSK2 likely plays a major role in feedback inhibition of the ERK pathway in skeletal muscle. Furthermore, RSK2 is not required for activation of muscle glycogen synthase by insulin but may indirectly modulate muscle glycogen synthase activity and/or glycogen content by other mechanisms, possibly through regulation of Akt. RSK2 knockout mice may be a good animal model for the study of Coffin-Lowry syndrome.
Figures
FIG. 1
Gene-targeting strategy. (A) Construction of targeting vector comprised of a plasmid containing a disrupted fragment (neo insertion) of the RSK2 gene. This vector also comprises the herpes simplex virus thymidine kinase (HSV-TK) gene cassette. H, _Hin_dIII; X, _Xho_I; S, _Sph_I; E, _Eco_RI; K, _Kpn_I. (B) Products of _Hin_dIII restriction fragments used for identification WT and KO mice by Southern blotting. The WT genotype gives a 5.7-kb fragment and the targeted gene results in a 9.6-kb fragment. (C) Southern blot using _Hin_dIII-digested genomic DNA from WT and KO mice (both male and female). (D) Schematic drawing of the disrupted RSK2 protein showing the two kinase domains.
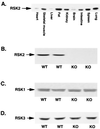
FIG. 2
(A) RSK2 tissue distribution in WT mice. Tissue lysates (100 μg of protein) were separated by SDS-PAGE and immunoblotted for RSK2. (B to D) RSK2, RSK1, and RSK3 content in WT and KO mice, respectively. Muscle lysates (100 μg of protein) were separated by SDS-PAGE and immunoblotted with antibody specific for either RSK1 (B), RSK2 (C), or RSK3 (D).
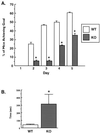
FIG. 3
Coordination and cognitive functioning of WT and KO mice. (A) Twenty-week-old WT (n = 22) and KO (n = 22) mice were subjected to a coordination test as described in the text. (B) Ten-week-old WT (n = 5) and KO (n = 5) mice were placed in a water-filled chamber and timed in their ability locate a submerged platform on the opposite side. Data are presented as mean ± standard error swim times from three separate experiments. ∗, P ≤ 0.05 versus WT.
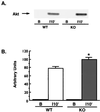
FIG. 4
Akt Ser473 phosphorylation. Muscle lysates (100 μg of protein) were separated by SDS-PAGE and immunoblotted using an antibody specific for Akt only when it is phosphorylated on Ser473, and bands were quantitated by densitometry. (A) Representative immunoblot of muscle lysates from KO (n = 4) and WT (n = 5) mice under basal conditions (lane B) and 10 min after insulin injection (lane 10′). No phosphorylated Akt was detected in basal samples from either group. (B) Quantitation of bands from mice following insulin treatment. A 28% greater insulin-stimulated Akt phosphorylation was observed in lysates from KO mice (∗, P < 0.02 versus WT). Unpaired t tests were used for comparisons between groups.
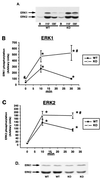
FIG. 5
Insulin-simulated ERK phosphorylation and ERK expression. Muscle lysates (100 μg of protein) were separated by SDS-PAGE and immunoblotted with an antibody specific for ERK1 and ERK2 phosphorylated on both tyrosine and threonine residues. (A) Representative immunoblot of muscle lysates from basal (lane B) and insulin-injected (10 and 30 min) KO and WT mice using phosphospecific ERK antibody. (B) Quantitation of multiple ERK1 bands by densitometry. (C) Quantitation of multiple ERK2 samples by densitometry. n = 5 to 16 per group. (D) Representative immunoblot demonstrating ERK expression. Muscle lysates were immunoblotted using an antibody that recognizes ERK regardless of its phosphorylation state. ∗, P < 0.001 versus basal; #, P < 0.05 versus WT. Unpaired t tests were used for comparisons between groups.
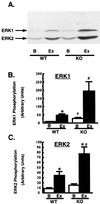
FIG. 6
Exercise-stimulated ERK phosphorylation. Muscle lysates (100 μg of protein) were immunoblotted using a phosphospecific antibody specific for ERK1 and ERK2 phosphorylated on both tyrosine and threonine residues. (A) Representative immunoblot of muscle lysates. Lanes: B, basal; Ex, after exercise. (B and C) Quantitation of multiple bands. n = 3 to 12 per group. ∗, P < 0.03 versus basal; #, P < 0.02 versus WT. Unpaired t tests were used for comparisons between groups.
Similar articles
- Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice.
Wojtaszewski JF, Higaki Y, Hirshman MF, Michael MD, Dufresne SD, Kahn CR, Goodyear LJ. Wojtaszewski JF, et al. J Clin Invest. 1999 Nov;104(9):1257-64. doi: 10.1172/JCI7961. J Clin Invest. 1999. PMID: 10545524 Free PMC article. - Jun N-terminal kinase mediates activation of skeletal muscle glycogen synthase by insulin in vivo.
Moxham CM, Tabrizchi A, Davis RJ, Malbon CC. Moxham CM, et al. J Biol Chem. 1996 Nov 29;271(48):30765-73. doi: 10.1074/jbc.271.48.30765. J Biol Chem. 1996. PMID: 8940056 - The role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes.
Kaidanovich O, Eldar-Finkelman H. Kaidanovich O, et al. Expert Opin Ther Targets. 2002 Oct;6(5):555-61. doi: 10.1517/14728222.6.5.555. Expert Opin Ther Targets. 2002. PMID: 12387679 Review.
Cited by
- Splicing of mouse p53 pre-mRNA does not always follow the "first come, first served" principle and may be influenced by cisplatin treatment and serum starvation.
Yang M, Wu J, Wu SH, Bi AD, Liao DJ. Yang M, et al. Mol Biol Rep. 2012 Sep;39(9):9247-56. doi: 10.1007/s11033-012-1798-2. Epub 2012 Jun 28. Mol Biol Rep. 2012. PMID: 22740133 - RSK2 signaling in medial habenula contributes to acute morphine analgesia.
Darcq E, Befort K, Koebel P, Pannetier S, Mahoney MK, Gaveriaux-Ruff C, Hanauer A, Kieffer BL. Darcq E, et al. Neuropsychopharmacology. 2012 Apr;37(5):1288-96. doi: 10.1038/npp.2011.316. Epub 2012 Jan 4. Neuropsychopharmacology. 2012. PMID: 22218090 Free PMC article. - Characterization of mouse Rsk4 as an inhibitor of fibroblast growth factor-RAS-extracellular signal-regulated kinase signaling.
Myers AP, Corson LB, Rossant J, Baker JC. Myers AP, et al. Mol Cell Biol. 2004 May;24(10):4255-66. doi: 10.1128/MCB.24.10.4255-4266.2004. Mol Cell Biol. 2004. PMID: 15121846 Free PMC article. - p90 RSK-1 associates with and inhibits neuronal nitric oxide synthase.
Song T, Sugimoto K, Ihara H, Mizutani A, Hatano N, Kume K, Kambe T, Yamaguchi F, Tokuda M, Watanabe Y. Song T, et al. Biochem J. 2007 Jan 15;401(2):391-8. doi: 10.1042/BJ20060580. Biochem J. 2007. PMID: 16984226 Free PMC article. - Metabolic Remodeling with Hepatosteatosis Induced Vascular Oxidative Stress in Hepatic ERK2 Deficiency Mice with High Fat Diets.
Kujiraoka T, Kagami K, Kimura T, Ishinoda Y, Shiraishi Y, Ido Y, Endo S, Satoh Y, Adachi T. Kujiraoka T, et al. Int J Mol Sci. 2022 Jul 31;23(15):8521. doi: 10.3390/ijms23158521. Int J Mol Sci. 2022. PMID: 35955653 Free PMC article.
References
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
- Aronson D, Boppart M D, Dufresne S D, Fielding R A, Goodyear L J. Exercise stimulates c-Jun NH2 kinase activity and c-Jun transcriptional activity in human skeletal muscle. Biochem Biophys Res Commun. 1998;251:106–110. - PubMed
- Bjorbaek C, Vik T A, Echwald S M, Yang P Y, Vestergaard H, Wang J P, Webb G C, Richmond K, Hansen T, Erikson R L. Cloning of a human insulin-stimulated protein kinase (ISPK-1) gene and analysis of coding regions and mRNA levels of the ISPK-1 and the protein phosphatase-1 genes in muscle from NIDDM patients. Diabetes. 1995;44:90–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous