Prevention of fat-induced insulin resistance by salicylate - PubMed (original) (raw)
. 2001 Aug;108(3):437-46.
doi: 10.1172/JCI11559.
Y J Kim, J J Fillmore, Y Chen, I Moore, J Lee, M Yuan, Z W Li, M Karin, P Perret, S E Shoelson, G I Shulman
Affiliations
- PMID: 11489937
- PMCID: PMC209353
- DOI: 10.1172/JCI11559
Prevention of fat-induced insulin resistance by salicylate
J K Kim et al. J Clin Invest. 2001 Aug.
Abstract
Insulin resistance is a major factor in the pathogenesis of type 2 diabetes and may involve fat-induced activation of a serine kinase cascade involving IKK-beta. To test this hypothesis, we first examined insulin action and signaling in awake rats during hyperinsulinemic-euglycemic clamps after a lipid infusion with or without pretreatment with salicylate, a known inhibitor of IKK-beta. Whole-body glucose uptake and metabolism were estimated using [3-(3)H]glucose infusion, and glucose uptake in individual tissues was estimated using [1-(14)C]2-deoxyglucose injection during the clamp. Here we show that lipid infusion decreased insulin-stimulated glucose uptake and activation of IRS-1-associated PI 3-kinase in skeletal muscle but that salicylate pretreatment prevented these lipid-induced effects. To examine the mechanism of salicylate action, we studied the effects of lipid infusion on insulin action and signaling during the clamp in awake mice lacking IKK-beta. Unlike the response in wild-type mice, IKK-beta knockout mice did not exhibit altered skeletal muscle insulin signaling and action following lipid infusion. In summary, high-dose salicylate and inactivation of IKK-beta prevent fat-induced insulin resistance in skeletal muscle by blocking fat-induced defects in insulin signaling and action and represent a potentially novel class of therapeutic agents for type 2 diabetes.
Figures
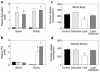
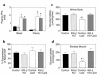
Figure 1
Metabolic parameters and insulin-stimulated whole-body and skeletal muscle (soleus) glucose uptake in awake control (black bars), salicylate (light gray bars), lipid (open bars), and lipid-salicylate (dark gray bars) rats. (a) Plasma salicylate concentrations during basal and hyperinsulinemic-euglycemic clamp. (b) Plasma FFA concentrations during basal and hyperinsulinemic-euglycemic clamp. (c) Insulin-stimulated whole-body glucose uptake in vivo. (d) Insulin-stimulated skeletal muscle glucose uptake in vivo. Values are means ± SE for six to seven experiments. *P < 0.05 versus control group.
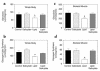
Figure 2
Insulin-stimulated whole-body and skeletal muscle (soleus) glucose metabolic flux in awake control (black bars), salicylate (light gray bars), lipid (open bars), and lipid-salicylate (dark gray bars) rats. (a) Insulin-stimulated whole-body glycolysis in vivo. (b) Insulin-stimulated whole-body glycogen/lipid synthesis in vivo. (c) Insulin-stimulated skeletal muscle glycolysis in vivo. (d) Insulin-stimulated skeletal muscle glycogen synthesis in vivo. Values are means ± SE for six to seven experiments. *P < 0.05 versus control group.
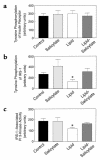
Figure 3
Insulin signaling in skeletal muscle (gastrocnemius) of the control (black bars), salicylate (light gray bars), lipid (open bars), and lipid-salicylate (dark gray bars) rats. (a) Insulin-stimulated tyrosine phosphorylation of insulin receptor. (b) Insulin-stimulated tyrosine phosphorylation of IRS-1. (c) Insulin-stimulated IRS-1–associated PI 3-kinase activity. Values are means ± SE for six to seven experiments. *P < 0.05 versus control group.
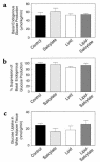
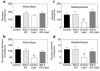
Figure 4
Insulin action in liver and epididymal white adipose tissue in the control (black bars), salicylate (light gray bars), lipid (open bars), and lipid-salicylate rats (dark gray bars). (a) Basal rates of endogenous glucose production. (b) Percent suppression of basal endogenous glucose production during insulin-stimulated state. (c) Insulin-stimulated glucose uptake in epididymal white adipose tissue. Values are means ± SE for six to seven experiments.
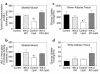
Figure 5
Metabolic parameters and insulin-stimulated whole-body and skeletal muscle (gastrocnemius) glucose uptake in awake control (black bars), IKK-β KO (light gray bars), control-lipid (open bars), and IKK-β KO-lipid (dark gray bars) mice. (a) Plasma FFA concentrations during basal and hyperinsulinemic-euglycemic clamp. (b) Insulin-stimulated percent suppression of basal EGP. (c) Insulin-stimulated whole-body glucose uptake in vivo. (d) Insulin-stimulated skeletal muscle glucose uptake in vivo. Values are means ± SE for three to five experiments. *P < 0.05 versus control group; †P < 0.05 versus IKK-β KO-lipid group.
Figure 6
Insulin-stimulated whole-body and skeletal muscle (gastrocnemius) glucose metabolic flux in awake control (black bars), IKK-β KO (light gray bars), control-lipid (open bars), and IKK-β KO-lipid (dark gray bars) mice. (a) Insulin-stimulated whole-body glycolysis in vivo. (b) Insulin-stimulated whole-body glycogen/lipid synthesis in vivo. (c) Insulin-stimulated skeletal muscle glycolysis in vivo. (d) Insulin-stimulated skeletal muscle glycogen synthesis in vivo. Values are means ± SE for three to five experiments. *P < 0.05 versus control group; †P < 0.05 versus IKK-β KO-lipid group.
Figure 7
Insulin signaling in skeletal muscle (gastrocnemius) and insulin action in fat of the control (black bars), IKK-β KO (light gray bars), control-lipid (open bars), and IKK-β KO-lipid (dark gray bars) mice. (a) Insulin-stimulated tyrosine phosphorylation of IRS-1 in skeletal muscle. (b) Insulin-stimulated IRS-1–associated PI 3-kinase activity in skeletal muscle. (c) Insulin-stimulated glucose uptake in intrascapular brown adipose tissue. (d) Insulin-stimulated glucose uptake in epididymal white adipose tissue. Values are means ± SE for three to five experiments. *P < 0.05 versus control group; †P < 0.05 versus IKK-β KO-lipid group.
Comment in
- Turning down insulin signaling.
Birnbaum MJ. Birnbaum MJ. J Clin Invest. 2001 Sep;108(5):655-9. doi: 10.1172/JCI13714. J Clin Invest. 2001. PMID: 11544268 Free PMC article. No abstract available. - The effect of salicylates on insulin sensitivity.
Netea MG, Tack CJ, Netten PM, Lutterman JA, Van der Meer JW. Netea MG, et al. J Clin Invest. 2001 Dec;108(11):1723-4. doi: 10.1172/JCI14455. J Clin Invest. 2001. PMID: 11733568 Free PMC article. No abstract available.
References
- DeFronzo RA. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. - PubMed
- Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. - PubMed
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. - PubMed
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK051729/DK/NIDDK NIH HHS/United States
- R01 DK-45493/DK/NIDDK NIH HHS/United States
- R01 DK-59635/DK/NIDDK NIH HHS/United States
- U24 DK-59635/DK/NIDDK NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- P30 DK-45735/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- DK-51729/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous