A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity - PubMed (original) (raw)
A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity
Orion D Weiner et al. Nat Cell Biol. 2002 Jul.
Abstract
When presented with a gradient of chemoattractant, many eukaryotic cells respond with polarized accumulation of the phospholipid PtdIns(3,4,5)P(3). This lipid asymmetry is one of the earliest readouts of polarity in neutrophils, Dictyostelium discoideum and fibroblasts. However, the mechanisms that regulate PtdInsP(3) polarization are not well understood. Using a cationic lipid shuttling system, we have delivered exogenous PtdInsP(3) to neutrophils. Exogenous PtdInsP(3) elicits accumulation of endogenous PtdInsP(3) in a positive feedback loop that requires endogenous phosphatidylinositol-3-OH kinases (PI(3)Ks) and Rho family GTPases. This feedback loop is important for establishing PtdInsP(3) polarity in response to both chemoattractant and to exogenous PtdInsP(3); it may function through a self-organizing pattern formation system. Emergent properties of positive and negative regulatory links between PtdInsP(3) and Rho family GTPases may constitute a broadly conserved module for the establishment of cell polarity during eukaryotic chemotaxis.
Conflict of interest statement
Competing Financial Interests: The authors declare that they have no competing financial interests.
Figures
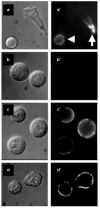
Figure 1. PtdlnsP3–histone induces translocation of PH-Akt–GFP
A spatial readout is shown for generation of PtdlnsP3 and Ptdlns(3,4)P2 at the plasma membrane in neutrophil-differentiated HL-60 cells. a–f, Time course of cells exposed to PtdlnsP3 (30 μM)–histone (10 μM). g, Unstimulated cells. h, Cells exposed to PtdlnsP3 (30 μM) without histone carrier. i, Cells exposed to Ptdlns(4,5)P2 (30 μM)–histone (10 μM).
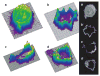
Figure 2. Surface plots of PH-Akt–GFP distribution in neutrophil-differentiated HL-60 cells
Height corresponds to fluorescence intensity of each pixel from confocal images of cells. a, An unstimulated cell. b, A cell stimulated with PtdlnsP3–histone for 30 s. c, A cell stimulated with PtdlnsP3–histone for 150 s. d, A cell expressing the chemokine receptor C5AR–GFP stimulated with 100 nM FMLP for 150 s. a′–d′, Confocal images from which the surface plots in a–d were generated.
Figure 3. PI(3)K and Rho GTPase inhibitors block PtdlnsP3–histone-induced PH-Akt–GFP translocation
a, Cells treated with LY 294002 (200 μM) for 20 min and then stimulated with PtdlnsP3 (30 μM)–histone (10 μM). Similar doses of LY 294002 were needed to block phosphorylation of Akt in response to chemoattractant (see Supplementary Information, Fig. S2). b,c, Cells pretreated with Clostridium difficile toxin B (90 μg ml−1) for 4–5 h and sequentially stimulated with PtdlnsP3–histone (b) and then insulin (c) to test for cell viability. In c, two of the three cells are viable, as judged by PH-Akt–GFP translocation in response to insulin (arrowheads)
Figure 4. Endogenous PI(3)K and Rho GTPases are not required for PtdlnsP3–histone uptake
a, Control cells treated with fluorescent NBD–PtdlnsP3 (30 μM)–histone (10 μM) for 5 min. b, Cells treated with NBD–PtdlnsP3 (30 μM) without histone carrier. c, Cells pretreated with LY 294002 (200 μM) for 20 min and then exposed to NBD–PtdlnsP3 (30 μM)–histone (10 μM). d, Cells were pretreated with toxB and then exposed to NBD–PtdlnsP3 (30 μM)–histone (10 μM). a–d, Nomarski images, a′–d′, immunofluorescence images.
Comment in
- Leading the way.
Meili R, Firtel RA. Meili R, et al. Nat Cell Biol. 2002 Jul;4(7):E171. doi: 10.1038/ncb0702-e171. Nat Cell Biol. 2002. PMID: 12105427 No abstract available.
References
- Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Science. 2000;287:1034–1036. - PubMed
- Niggli V, Keller H. Eur J Pharmacol. 1997;335:43–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM026875/GM/NIGMS NIH HHS/United States
- NS29632/NS/NINDS NIH HHS/United States
- R01 NS029632/NS/NINDS NIH HHS/United States
- R37 GM041890/GM/NIGMS NIH HHS/United States
- R01 GM041890/GM/NIGMS NIH HHS/United States
- GM26825/GM/NIGMS NIH HHS/United States
- GM62734-03/GM/NIGMS NIH HHS/United States
- GM41890/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous