Comparison of the Rta/Orf50 transactivator proteins of gamma-2-herpesviruses - PubMed (original) (raw)
Comparative Study
Comparison of the Rta/Orf50 transactivator proteins of gamma-2-herpesviruses
Blossom Damania et al. J Virol. 2004 May.
Abstract
The viral immediate-early transactivator Rta/Orf50 is necessary and sufficient to initiate Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) reactivation from latently infected cells. Since Rta/Orf50 is conserved among all known gamma-2-herpesviruses, we investigated whether the murine gamma-68-herpesvirus (MHV-68) and rhesus monkey rhadinovirus (RRV) homologs can functionally substitute for KSHV Rta/Orf50. (i) Our comparison of 12 KSHV promoters showed that most responded to all three Rta/Orf50proteins, but three promoters (vGPCR, K8, and gB) responded only to the KSHV Rta/Orf50 transactivator. Overall, the activation of KSHV promoters was higher with KSHV Rta than with the RRV and MHV-68 Rta. (ii) Only the primate Rta/Orf50 homologs were able to interfere with human p53-depedent transcriptional activation. (iii) Transcriptional profiling showed that the KSHV Rta/Orf50 was more efficient than it's homologs in inducing KSHV lytic transcription from the latent state. These results suggest that the core functionality of Rta/Orf50 is conserved and independent of its host, but the human protein has evolved additional, human-specific capabilities.
Figures
FIG. 1.
Three Rta/Orf50 proteins of the gamma-2-herpesvirus family. (A) ClustalW alignment of the three Rta/Orf50 proteins of KSHV, RRV, and MHV-68. Conserved amino acids are indicated by stars, and similarly charged residues are indicated by a semicolon. (B) Western blot of Rta/Orf50 protein expression after transient transfection into 293 cells. KSHV Rta/Orf50 was detected with an antibody against Rta/Orf50, and RRV and MHV-68 Rta/Orf50 proteins were epitope tagged and detected with antibodies against either an AU1 or Flag tag, respectively. (C) Western blot of the three Rta/Orf50 proteins in the four different cell lines. Lanes: 1 and 5, BJAB B cells; 2 and 6, BCBL-1 B cells; 3 and 7, SLK endothelial cells; 4 and 8, 293 epithelial cells. One hundred-fifty micrograms of total cell extract from the BJAB, BCBL, and SLK cells and 80 μg of cell extract from the 293 cells were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blots were performed with either an AU1 antibody to detect RRV Orf50, Flag antibody to detect MHV-68 Orf50, or Rta antibody (K. Ueda) to detect KSHV Orf50.
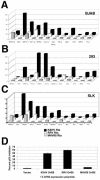
FIG. 2.
Transactivation functions of KSHV, RRV, and MHV-68 Rta/Orf50. Panels A, B, and C depict the results of the transfection data from Table 1 for BJAB, 293, and SLK cells, respectively. (D) Inhibition of human p53 transactivation of a p53-responsive promoter/luciferase construct in p53-negative (10)1 cells. Promoter activity was measured as relative light units normalized to a cotransfected β-galactosidase promoter. The ability of all three Rta/Orf50 proteins to inhibit p53 activity is quantified as a percentage of inhibition.
FIG.3.
Ability of the three Rta/Orf50 proteins to reactivate KSHV from latently infected BCBL-1 cells. (A) “Heatmap” representation of KSHV mRNA after transfection with KSHV, RRV, or MHV-68 Rta/Orf50 as determined by real-time quantitative RT-PCR (16). The three leftmost panels represent mock-treated cells and two replicate experiments of sorted, Rta/Orf50-transfected cells after 48 h. (B) KSHV mRNA levels in the total culture 72 h after transfection with KSHV, RRV, or MHV-68 Rta/Orf50. Darker shades of gray correspond to higher mRNA levels on a log 2 scale. (C) Plots of three individual relative mRNA (Δ CT) levels selected from panel A for KSHV v-cyclin (CYC), Mta/Orf57, and vGPCR on the vertical axis for each of the experiments on the horizontal axis. (D) mRNA levels (Δ CT) after electroporation with KSHV, RRV, or MHV68 Rta/Orf50 on the horizontal axis relative to the mRNA levels after electroporation with KSHV Rta/Orf50.
Similar articles
- The Rta/Orf50 transactivator proteins of the gamma-herpesviridae.
Staudt MR, Dittmer DP. Staudt MR, et al. Curr Top Microbiol Immunol. 2007;312:71-100. doi: 10.1007/978-3-540-34344-8_3. Curr Top Microbiol Immunol. 2007. PMID: 17089794 Review. - Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50/Rta lytic switch protein functions as a tetramer.
Bu W, Carroll KD, Palmeri D, Lukac DM. Bu W, et al. J Virol. 2007 Jun;81(11):5788-806. doi: 10.1128/JVI.00140-07. Epub 2007 Mar 28. J Virol. 2007. PMID: 17392367 Free PMC article. - Identification of Novel Kaposi's Sarcoma-Associated Herpesvirus Orf50 Transcripts: Discovery of New RTA Isoforms with Variable Transactivation Potential.
Wakeman BS, Izumiya Y, Speck SH. Wakeman BS, et al. J Virol. 2016 Dec 16;91(1):e01434-16. doi: 10.1128/JVI.01434-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795414 Free PMC article. - Rhesus monkey rhadinovirus: a model for the study of KSHV.
O'Connor CM, Kedes DH. O'Connor CM, et al. Curr Top Microbiol Immunol. 2007;312:43-69. doi: 10.1007/978-3-540-34344-8_2. Curr Top Microbiol Immunol. 2007. PMID: 17089793 Review.
Cited by
- KSHV RTA antagonizes SMC5/6 complex-induced viral chromatin compaction by hijacking the ubiquitin-proteasome system.
Han C, Zhang D, Gui C, Huang L, Chang S, Dong L, Bai L, Wu S, Lan K. Han C, et al. PLoS Pathog. 2022 Aug 1;18(8):e1010744. doi: 10.1371/journal.ppat.1010744. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35914008 Free PMC article. - KSHV RTA Induces Degradation of the Host Transcription Repressor ID2 To Promote the Viral Lytic Cycle.
Combs LR, Spires LM, Alonso JD, Papp B, Toth Z. Combs LR, et al. J Virol. 2022 Jun 22;96(12):e0010122. doi: 10.1128/jvi.00101-22. Epub 2022 May 23. J Virol. 2022. PMID: 35604218 Free PMC article. - Regulation of Ov2 by virus encoded microRNAs.
Nightingale K, Dry I, Hopkins J, Dalziel R. Nightingale K, et al. Vet Res Commun. 2019 May;43(2):99-104. doi: 10.1007/s11259-019-09749-9. Epub 2019 Mar 19. Vet Res Commun. 2019. PMID: 30888610 Free PMC article. - RTA Occupancy of the Origin of Lytic Replication during Murine Gammaherpesvirus 68 Reactivation from B Cell Latency.
Santana AL, Oldenburg DG, Kirillov V, Malik L, Dong Q, Sinayev R, Marcu KB, White DW, Krug LT. Santana AL, et al. Pathogens. 2017 Feb 16;6(1):9. doi: 10.3390/pathogens6010009. Pathogens. 2017. PMID: 28212352 Free PMC article. - Molecular biology of KSHV in relation to AIDS-associated oncogenesis.
Greene W, Kuhne K, Ye F, Chen J, Zhou F, Lei X, Gao SJ. Greene W, et al. Cancer Treat Res. 2007;133:69-127. doi: 10.1007/978-0-387-46816-7_3. Cancer Treat Res. 2007. PMID: 17672038 Free PMC article. Review.
References
- Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. - PMC - PubMed
- Chang, J., and D. Ganem. 2000. On the control of late gene expression in Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8). J. Gen. Virol. 81:2039-2047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA109232/CA/NCI NIH HHS/United States
- CA 096500/CA/NCI NIH HHS/United States
- CA 109232/CA/NCI NIH HHS/United States
- RR 1555777/RR/NCRR NIH HHS/United States
- R01 CA096500/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous