Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation - PubMed (original) (raw)
Comparative Study
Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation
Ping Ling et al. Br J Pharmacol. 2004 May.
Erratum in
- Br J Pharmacol. 2004 Jul;142(6):1052
Abstract
1. During mast cell degranulation, histamine is released in large quantities. Human eosinophils were found to express histamine H(4) but not H(3) receptors. The possible effects of histamine on eosinophils and the receptor mediating these effects were investigated in our studies. 2. Histamine (0.01-30 microm) induced a rapid and transient cell shape change in human eosinophils, but had no effects on neutrophils. The maximal shape change was at 0.3 microm histamine with EC(50) at 19 nm. After 60 min incubation with 1 microm histamine, eosinophils were desensitized and were refractory to shape change response upon histamine restimulation. Histamine (0.01-1 microm) also enhanced the eosinophil shape change induced by other chemokines. 3. Histamine-induced eosinophil shape change was mediated by the H(4) receptor. This effect was completely inhibited by H(4) receptor-specific antagonist JNJ 7777120 (IC(50) 0.3 microm) and H(3)/H(4) receptor antagonist thioperamide (IC(50) 1.4 microm), but not by selective H(1), H(2) or H(3) receptor antagonists. H(4) receptor agonists imetit (EC(50) 25 nm) and clobenpropit (EC(50) 72 nm) could mimic histamine effect in inducing eosinophil shape change. 4. Histamine (0.01-100 microm) induced upregulation of adhesion molecules CD11b/CD18 (Mac-1) and CD54 (ICAM-1) on eosinophils. This effect was mediated by the H(4) receptor and could be blocked by H(4) receptor antagonists JNJ 7777120 and thioperamide. 5. Histamine (0.01-10 microm) induced eosinophil chemotaxis with an EC(50) of 83 nm. This effect was mediated by the H(4) receptor and could be blocked by H(4) receptor antagonists JNJ 7777120 (IC(50) 86 nm) and thioperamide (IC(50) 519 nm). Histamine (0.5 microm) also enhanced the eosinophil shape change induced by other chemokines. 6. In conclusion, we have demonstrated a new mechanism of eosinophil recruitment driven by mast cells via the release of histamine. Using specific histamine receptor ligands, we have provided a definitive proof that the H(4) receptor mediates eosinophil chemotaxis, cell shape change and upregulation of adhesion molecules. The effect of H(4) receptor antagonists in blocking eosinophil infiltration could be valuable for the treatment of allergic diseases. The histamine-induced shape change and upregulation of adhesion molecules on eosinophils can serve as biomarkers for clinical studies of H(4) receptor antagonists.
Figures
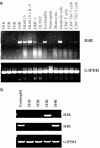
Figure 1
H4 receptor expression is restricted to eosinophils and dendritic cells. (a) RT–PCR detection of H4 receptor mRNA in different purified cell types and cell lines. Total RNA from different cell types were reversed transcribed and used as templates for PCR. (b) Human eosinophils express H4 but not H3 receptors. H3 or H4 receptor mRNA in human eosinophils was detected by RT–PCR using specific primers. For both (a) and (b), 25 PCR cycles were performed for the amplification of the H4 receptor. Human SK-N-MC cells transfected with the H1, H2, H3 or H4 receptor were used as controls for specificity of histamine receptor detection. G3PDH mRNA in RNA samples was amplified with specific primers as controls in PCR reactions.
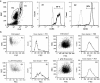
Figure 2
Histamine induces eosinophil shape change. (a) Eosinophils were distinguished from neutrophils in human PMNL by gating on cells with high levels of autoflourescence in flow cytometry analysis. The majority of the cell population with high autoflourescence, gated as R1 group, was CCR3+ eosinophils. The cell population with low autoflourescence, gated as R2 group, was CD16+ neutrophils. Histograms shown in solid lines are antibody-stained samples whereas those in broken lines are unstained controls. (b) Histamine induced cell shape change on eosinophils but not on neutrophils. Human PMNL were treated with 1 μ
M
histamine for 10 min and the change in cell shape was monitored by flow cytometry. Human eosinophils or neutrophils were gated in flow cytometry analysis based on their difference in autoflourescence. The cell size in histamine-treated samples was compared to that of the untreated control samples. The means of cell size in forward scattered signal (FSC) are shown.
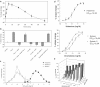
Figure 3
Kinetics and potency of histamine in triggering eosinophil shape change. (a) Kinetics of histamine induced eosinophil shape change. Human PMNL were treated with 1 μ
M
histamine and the change in eosinophil cell shape at different time points was studied by flow cytometry. The percentage of cell shape change was calculated based on the increase in FSC from those of untreated samples. Data are mean±s.d., _n_=3. (b) The shape change response of human eosinophils to histamine under different conditions was studied. Data are mean±s.d., _n_=3. (c) Titration of histamine effects on human eosinophil shape change and its comparison with chemokines. Human PMNL were treated with different concentrations of histamine or chemokines (eotaxin-2 or eotaxin) for 10 min. Eosinophil shape change was monitored by flow cytometry. Data are mean±s.d., _n_=3. (d) Determination of EC50 values of histamine and chemokines on eosinophil shape change. Human PMNL were treated with different concentrations of histamine or chemokines (eotaxin and eotaxin-2). Eosinophil shape change was monitored by flow cytometry. Data shown are a representative of six repeated experiments and each data point is mean±s.d., _n_=3. EC50 values were calculated with the GraphPad Prism program. (e) Histamine enhances eosinophil shape change when combined with chemokines MCP-3. Human PMNL were treated with histamine in combination with chemokines MCP-3 for 10 min. Eosinophil shape change was monitored by flow cytometry. Data shown are a representative of four repeated experiments.
Figure 4
Histamine-induced eosinophil shape change is mediated by the H4 receptor. (a) Histamine induced eosinophil shape change was blocked by the H4 receptor antagonist JNJ 7777120, the H3/H4 receptor antagonist thioperamide, but not by H1, H2 or H3 receptor antagonists. The H1, H2 and H3 receptor antagonists used in studies were diphenhydramine, ranitidine and JNJ 6379490, respectively. Human PMNL were pretreated with 10 μ
M
of different histamine receptor antagonists, followed by 10-min treatment with 1 μ
M
histamine. Eosinophil shape change was monitored by flow cytometry. The percentage of cell shape change was calculated based on the increase in FSC from those of untreated samples. Data shown are a representative of three repeated experiments. Data are mean±s.d. and _n_=3. Statistical significance (_P_-value) was determined by the Student's _t_-test. (b) Determination of IC50 values of JNJ 7777120, thioperamide and H3 receptor antagonist on histamine-induced eosinophil shape change. Human PMNL were pretreated with different antagonists for 10 min before inducing cell shape change with 1 μ
M
histamine. Eosinophil shape change was monitored by flow cytometry. The percentage of inhibition was calculated based on the decrease in shape change compared to samples treated with1 μ
M
histamine only. Data shown are a representative of four repeated experiments and each data point is a mean±s.d. and _n_=3. IC50 values were calculated with the GraphPad Prism program. (c) Concentration-dependent effects of histamine, H3/H4 receptor agonist imetit and H4 receptor agonist clobenpropit on human eosinophil shape change. Data are mean±s.d. and _n_=3. EC50 values were calculated with the GraphPad Prism program.
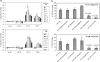
Figure 5
Histamine-induced adhesion molecule expression on eosinophils is mediated by the H4 receptor. (a) Cell surface expression of adhesion molecules CD11b/CD18 and CD54 on eosinophils was upregulated by histamine. Human PMNL were treated with different concentrations of histamine or chemokine eotaxin-2 for 10 min at 37°C. Cell samples were fixed with paraformaldehyde and stained with FITC-conjugated antibodies specific for CD11b, CD11a or CD54. Expression of adhesion molecules on eosinophils was monitored by flow cytometry. The percentage of upregulation was calculated based on the increase in expression levels from those of untreated samples. Data are mean±s.d. and _n_=3. (b) Histamine-induced adhesion molecule upregulation on eosinophils was blocked by the H4 receptor antagonist JNJ 7777120 and the H3/H4 receptor antagonist thioperamide, but not by H1, H2 or H3 receptor antagonists. The H1, H2 and H3 receptor antagonists used in studies were diphenhydramine, ranitidine and JNJ 6379490, respectively. Human PMNL were pretreated with 10 μ
M
of different histamine receptor antagonists for 10 min, followed by 10-min treatment with 1 μ
M
histamine at 37°C. Cell samples were fixed with paraformaldehyde and stained with FITC-conjugated antibodies specific for CD11b or CD54. Data are mean±s.d. and _n_=3. Statistical significance (_P_-value) was determined by the Student's _t_-test.
Figure 6
Histamine-induced human eosinophil chemotaxis is mediated by the H4 receptor. (a) Titration of histamine effects on human eosinophil chemotaxis. Chemotaxis of purified human eosinophils toward different concentration of histamine was studied in a Transwell system. Human eosinophils were placed in the transwell and histamine was added in the lower chamber. Eosinophils migrated into the lower chambers after 2 h incubation were counted for 1 min by flow cytometry. Data shown are a representative of three repeated experiments. Data are mean±s.d. and _n_=3. Statistical significance (_P_-value) was determined by the Student's _t_-test. (b) Determination of EC50 values of histamine and chemokines on eosinophil chemotaxis. Human eosinophil chemotaxis was studied with a titration of histamine or chemokines eotaxin-2 or MCP-3. Data shown are a representative of two repeated experiments. Data are mean±s.d. and _n_=3. EC50 values were calculated with the GraphPad Prizm program. (c) Histamine enhanced chemokine-induced eosinophil chemotaxis. The effects of histamine (0.5 μ
M
) on eosinophil chemotaxis induced by different concentrations of chemokine eotaxin-2 or MCP-3 were studied. Data shown are a representative from three repeated experiments. Data are mean±s.d. and _n_=3. Statistical significance (_P_-value) was determined by the Student's _t_-test. (d) Histamine-induced eosinophil chemotaxis was blocked by the H4 receptor antagonist JNJ 7777120 and the H3/H4 receptor antagonist thioperamide, but not by H1, H2 or H3 receptor antagonists. The H1, H2 and H3 receptor antagonists used in studies were diphenhydramine, ranitidine and JNJ 6379490, respectively. Histamine (10 μ
M
) was added in the lower chamber, while 10 μ
M
of different histamine receptor antagonists was added in both chambers. Data shown are a representative from four repeated experiments. Data are mean±s.d. and _n_=3. Statistical significance (_P_-value) was determined by the Student's _t_-test. (e) Determination of IC50 values of H4 receptor antagonists JNJ 7777120 and thioperamide in eosinophil chemotaxis assays. Histamine (1 μ
M
) was added in the lower chamber, while different concentrations of JNJ 7777120 or thioperamide were added in both chambers. The percentage of inhibition was calculated based on the decrease in migrated cell numbers compared to samples treated with 1 μ
M
of histamine only. Data shown are a representative from four repeated experiments. Data are mean±s.d. and _n_=3.
Comment in
- Histamine H4 antagonism: a therapy for chronic allergy?
Daugherty BL. Daugherty BL. Br J Pharmacol. 2004 May;142(1):5-7. doi: 10.1038/sj.bjp.0705730. Br J Pharmacol. 2004. PMID: 15130999 Free PMC article. Review. No abstract available.
Similar articles
- Histamine induces cytoskeletal changes in human eosinophils via the H(4) receptor.
Buckland KF, Williams TJ, Conroy DM. Buckland KF, et al. Br J Pharmacol. 2003 Nov;140(6):1117-27. doi: 10.1038/sj.bjp.0705530. Epub 2003 Oct 6. Br J Pharmacol. 2003. PMID: 14530216 Free PMC article. - Identification of a histamine H4 receptor on human eosinophils--role in eosinophil chemotaxis.
O'Reilly M, Alpert R, Jenkinson S, Gladue RP, Foo S, Trim S, Peter B, Trevethick M, Fidock M. O'Reilly M, et al. J Recept Signal Transduct Res. 2002 Feb-Nov;22(1-4):431-48. doi: 10.1081/rrs-120014612. J Recept Signal Transduct Res. 2002. PMID: 12503632 - Histamine H(2) receptors mediate the inhibitory effect of histamine on human eosinophil degranulation.
Ezeamuzie CI, Philips E. Ezeamuzie CI, et al. Br J Pharmacol. 2000 Oct;131(3):482-8. doi: 10.1038/sj.bjp.0703556. Br J Pharmacol. 2000. PMID: 11015298 Free PMC article. - Histamine H4 receptor antagonists: the new antihistamines?
Fung-Leung WP, Thurmond RL, Ling P, Karlsson L. Fung-Leung WP, et al. Curr Opin Investig Drugs. 2004 Nov;5(11):1174-83. Curr Opin Investig Drugs. 2004. PMID: 15573868 Review. - The role of histamine H4 receptor in immune and inflammatory disorders.
Zampeli E, Tiligada E. Zampeli E, et al. Br J Pharmacol. 2009 May;157(1):24-33. doi: 10.1111/j.1476-5381.2009.00151.x. Epub 2009 Mar 20. Br J Pharmacol. 2009. PMID: 19309354 Free PMC article. Review.
Cited by
- The role of the histamine H4 receptor in atopic dermatitis.
Mommert S, Gschwandtner M, Gutzmer R, Werfel T. Mommert S, et al. Curr Allergy Asthma Rep. 2011 Feb;11(1):21-8. doi: 10.1007/s11882-010-0162-7. Curr Allergy Asthma Rep. 2011. PMID: 21104170 Review. - Histamine H4 receptor antagonists as potent modulators of mammalian vestibular primary neuron excitability.
Desmadryl G, Gaboyard-Niay S, Brugeaud A, Travo C, Broussy A, Saleur A, Dyhrfjeld-Johnsen J, Wersinger E, Chabbert C. Desmadryl G, et al. Br J Pharmacol. 2012 Oct;167(4):905-16. doi: 10.1111/j.1476-5381.2012.02049.x. Br J Pharmacol. 2012. PMID: 22624822 Free PMC article. - Effect of alcaftadine 0.25% on ocular itch associated with seasonal or perennial allergic conjunctivitis: a pooled analysis of two multicenter randomized clinical trials.
Ciolino JB, McLaurin EB, Marsico NP, Ackerman SL, Williams JM, Villanueva L, Hollander DA. Ciolino JB, et al. Clin Ophthalmol. 2015 May 2;9:765-72. doi: 10.2147/OPTH.S80503. eCollection 2015. Clin Ophthalmol. 2015. PMID: 25999684 Free PMC article. - Modulation of tissue inflammatory response by histamine receptors in scorpion envenomation pathogenesis: involvement of H4 receptor.
Lamraoui A, Adi-Bessalem S, Laraba-Djebari F. Lamraoui A, et al. Inflammation. 2014 Oct;37(5):1689-704. doi: 10.1007/s10753-014-9898-x. Inflammation. 2014. PMID: 24858599 - Superior effect of MP-AzeFlu than azelastine or fluticasone propionate alone on reducing inflammatory markers.
Roca-Ferrer J, Pujols L, Pérez-González M, Alobid I, Callejas B, Vicens-Artés S, Fuentes M, Valero A, Picado C, Castor D, Nguyen D, Mullol J. Roca-Ferrer J, et al. Allergy Asthma Clin Immunol. 2018 Dec 18;14:86. doi: 10.1186/s13223-018-0311-4. eCollection 2018. Allergy Asthma Clin Immunol. 2018. PMID: 30574167 Free PMC article.
References
- BOUSQUET J., CHANEZ P., LACOSTE J.Y., BARNEON G., GHAVANIAN N., ENANDER I., VENGE P., AHLSTEDT S., SIMONY-LAFONTAINE J., GODARD P., MICHEL F.-B. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990;323:1033–1039. - PubMed
- CHURCH M.K., CAULFIELD J.P.Mast cell and basophil function Allergy 1993New York: Raven Press Ltd; ed. Holgate, S.T. & Church, M.K. Chapter 5.6
- FUNG-LEUG W.-P., THURMOND R.L., LING P., KARLSSON L.H4 receptor antagonists – the new antihistamines Curr Opin Investing Drugs 2004. in press - PubMed
- HILL S.J., GANELLIN C.R., TIMMERMAN H., SCHWARTZ J.C., SHANKLEY N.P., YOUNG J.M., SCHUNACK W., LEVI R., HAAS H.L. International union of pharmacology. XIII. Classification of histamine receptors. Pharmacol. Rev. 1997;49:253–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous