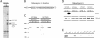The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms - PubMed (original) (raw)
The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms
Carsten Schnatwinkel et al. PLoS Biol. 2004 Sep.
Abstract
The small GTPase Rab5 is a key regulator of clathrin-mediated endocytosis. On early endosomes, within a spatially restricted domain enriched in phosphatydilinositol-3-phosphate [PI(3)P], Rab5 coordinates a complex network of effectors that functionally cooperate in membrane tethering, fusion, and organelle motility. Here we discovered a novel PI(3)P-binding Rab5 effector, Rabankyrin-5, which localises to early endosomes and stimulates their fusion activity. In addition to early endosomes, however, Rabankyrin-5 localises to large vacuolar structures that correspond to macropinosomes in epithelial cells and fibroblasts. Overexpression of Rabankyrin-5 increases the number of macropinosomes and stimulates fluid-phase uptake, whereas its downregulation inhibits these processes. In polarised epithelial cells, this function is primarily restricted to the apical membrane. Rabankyrin-5 localises to large pinocytic structures underneath the apical surface of kidney proximal tubule cells, and its overexpression in polarised Madin-Darby canine kidney cells stimulates apical but not basolateral, non-clathrin-mediated pinocytosis. In demonstrating a regulatory role in endosome fusion and (macro)pinocytosis, our studies suggest that Rab5 regulates and coordinates different endocytic mechanisms through its effector Rabankyrin-5. Furthermore, its active role in apical pinocytosis in epithelial cells suggests an important function of Rabankyrin-5 in the physiology of polarised cells.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
Figure 1. A Protein of 130 kDa Is a New Rab5 Effector
(A) GST-Rab5-GDP and GST-Rab-GTPγS were loaded on beads and incubated with bovine brain cytosol. Bound proteins were eluted and analysed by SDS-PAGE followed by Coommasie Blue staining. The positions of the already known Rab5 effectors (EEA1, Rabaptin-5, hVps34, p110β, and Rabenosyn-5) and of the new Rab5 effector are indicated. (B) Schematic representation of the domain organisation in Rabankyrin-5. ANK, ankyrin repeats. (C) Bovine brain cytosol or HeLa cell cytosol was incubated with GST-Rab5-GDP– or GST-Rab5-GTPγS–loaded beads. Subsequently the beads were washed, and bound proteins were eluted and analysed by Western blotting using anti–Rabankyrin-5 antibodies. (D) GST-Rab4, -5, -7, and -11 fusion proteins were preloaded with GDP or GTPγS and incubated with in vitro-translated 35S-methionine–labelled Rabankyrin-5 full-length protein. As a control, bound and unbound material was analysed by SDS-PAGE followed by phosphoimager analysis. (E) Rabankyrin-5 binds most strongly to PI(3)P. Recombinant full-length Rabankyrin-5 was incubated with liposomes containing 2% of the indicated phosphoinositide. Bound Rabankyrin-5 was detected by Western blotting.
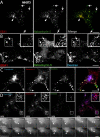
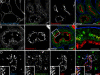
Figure 3. Rabankyrin-5 Associates with Two Types of Rab5-Containing Vesicles in A431 Cells, Early Endosomes, and Macropinosomes
(A) A431 cells stably transfected for GFP-Rab5wt were immunostained for endogenous Rabankyrin-5 and EEA1. While there is perinuclear overlap between Rab5, Rabankyrin-5, and EEA1 in nontransfected cells (arrowheads), some smaller peripheral structures are devoid of EEA1 (arrows). (B) Overexpression of Rabankyrin-5 in A431 by using a recombinant adenovirus construct of Rabankyrin-5 causes an accumulation of peripheral, enlarged Rab5-positive structures, costained mainly by Rabankyrin-5 (arrows) but not detectable for EEA1. (C) Rabankyrin-5 localises on EGF-induced and -enriched macropinosomes. Serum-starved A431 cells (16 h) were incubated for 7 min with 100 ng/ml rhodamine-conjugated EGF to induce macropinocytosis and 1 μg/ml Cy5-labelled transferrin. Endogenous Rabankyrin-5 localises to enlarged EGF-containing macropinosomes, indicated by the lack of transferrin labelling (arrows), but also to EGF- and transferrin-containing endosomes (arrowheads). (D) Rabankyrin-5 structures contain tyrosine-phosphorylated proteins. A431 cells, stably transfected for GFP-Rab5, were stimulated with 50 ng/ml EGF for 7 min and immediately processed for immunofluorescence. Costaining of Rabankyrin-5 and tyrosine-phosphorylated proteins (α-4G10) reveal the localisation of Rabankyrin-5 to plasma membrane ruffles. Scale bars represent 10 μm.
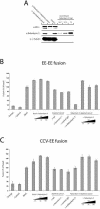
Figure 2. Rabankyrin-5 Stimulates Homotypic Fusion between Early Endosomes and Heterotypic Fusion between Early Endosomes and CCVs In Vitro
(A) Rabankyrin-5 was efficiently immunodepleted from HeLa cytosol without affecting the EEA1 concentration. Twenty micrograms of HeLa cell cytosol, either treated with control IgGs and immunodepleted for Rabankyrin-5 or nontreated, was applied to SDS-PAGE and probed for the indicated antigens by Western blotting. Anti-γ tubulin was used as a loading control. (B and C) Addition of recombinant Rabankyrin-5 stimulates homotypic and heterotypic fusion. Fusion (B) between early endosomes (EE-EE fusion) and (C) of early endosomes to CCVs (CCV-EE fusion) in vitro was performed in the presence of 3 mg/ml HeLa cytosol and an ATP-regenerating system (basal) and in the absence or presence of the indicated reagents. For some conditions, cytosol was immunodepleted for Rabankyrin-5 (columns 8–13) and in addition supplemented with anti–Rabankyrin-5 antibodies to neutralise the function of membrane-associated proteins (column 10). Background fusion activity is demonstrated by ATP (−Energy) or cytosol (−Cytosol) omission.
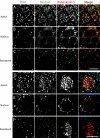
Figure 4. Rabankyrin-5 Localises to Macropinosomes in NIH3T3 Cells
(A) NIH3T3 cells directly fixed and immunostained for endogenous Rabankyrin-5 and EEA1 reveal segregation of Rabankyrin-5 from EEA1-containing structures (arrows) in the cell periphery, while there is colocalisation in the cell centre (arrowheads). (B) Overexpression of Rabankyrin-5 increases the number of peripheral enlarged structures devoid of EEA1. NIH3T3 cells were infected with recombinant adenovirus for Rabankyrin-5 for 18 h. Dextran (2,5 mg/ml) uptake was performed for 3 min at 37 °C, fixed, and immunostained for the indicated antigens. (C and D) Formation of enlarged Rabankyrin-5 structures requires PI3-K activity. NIH3T3 cells either (C) DMSO treated or (D) pretreated for 20 min with wortmannin (WM; 100 nM) were incubated for 8 min with 0,5 μg/ml rhodamine-labelled transferrin and 2,5 mg/ml FITC-labelled dextran (MW, 10.000), fixed, processed for immunofluorescence, and analysed by confocal scanning microscopy. (E) NIH3T3 cells transiently transfected for YFP-Rabankyrin-5 and CFP-actin were imaged using time-lapse video microscopy to visualise the formation of macropinosomes by actin-driven membrane ruffles. Images were taken for the indicated time points. The arrowhead points towards Rabankyrin-5 association to an enlarged vesicle driven by actin dynamics over time. Scale bars represent 10 μm.
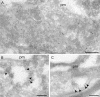
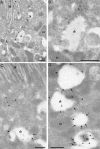
Figure 5. Immunolocalisation of Endogenous and Overexpressed Rabankyrin-5 in NIH3T3 Cells
Untransfected NIH3T3 cells or cells transfected with Rabankyrin-5 were labelled with antibodies to Rabankyrin-5 followed by 10 nm protein A gold. (A) Transfected cell showing labelling of a group of vesicular structures underlying the plasma membrane (pm). (B and C) In control (untransfected) cells, low but specific labelling for Rabankyrin-5 (arrowheads) is associated with compartments close to the pm. Scale bars represent 200 nm.
Figure 6. Inhibition of Rab5 Activity Decreases Fluid-Phase Uptake
NIH3T3 cells transiently transfected for either (A) GFP-Rab5wt or (B) RN-tre were subjected to a 30-min uptake of rhodamine-conjugated transferrin (1 μg/ml) or dextran (MW, 70.000; 3 mg/ml) at 37 °C and further processed for confocal imaging with indicated antibodies. (A) Rab5wt transfected cells show colocalisation of Rabankyrin-5–labelled macropinocytic structures, indicated by the lack of transferrin accumulation, with Rab5. (B) Cells transiently transfected for RN-tre (asterisk) show a significant reduction of fluid-phase uptake compared to nontransfected cells. (C) Fluid-phase dextran quantification of single cells transfected for RN-tre by measuring internalised fluorescence intensity (p > 0,001). Values shown are means ± standard deviation of at least 15 cells. Scale bars represent 10 μm.
Figure 7. Rabankyrin-5 Overexpression Increases, whereas Knock-Down Decreases Fluid-Phase Uptake, Specifically
(A–C) NIH3T3 cells were either mock-infected or infected with recombinant adenovirus encoding Rabankyrin-5. Simultaneous uptake of HRP (5 mg/ml) and biotinylated transferrin (2 μg/ml) was performed at 37 °C for the indicated time points. (C) NIH3T3 cells were pulsed with HRP (10 mg/ml) for 10 min. Recycled HRP into the medium was determined after the indicated time points. (D–F) A431 cells were treated with esiRNA against Rabankyrin-5 or control treated for 4 d. (D) Western blot analysis revealed a 50% reduction of Rabankyrin-5 in whole-cell lysate. (E and F) Serum-starved cells (1 h) were stimulated with 50 ng/ml EGF in complete medium for the indicated time points in the presence of 5 mg/ml HRP and 2 μg/ml biotinylated transferrin. Values shown are means ± standard deviation and were performed in duplicates. The results are representatives of at least two independent experiments.
Figure 8. Rabankyrin-5 Localises on Vesicular Structures Underlying the Apical Brush Border in Mouse Kidney Proximal Tubules
Mice were perfused either (A–C) directly with 4% PFA or (D) 5 min after injection of HRP into the femoral vein. Immunofluorescence was performed on semi-thin cryosections (500 nm). (C) Higher magnification of the inset in (B). Scale bars represent 10 μm.
Figure 9. Immunolocalisation of Rabankyrin-5 in the Mouse Kidney
Mouse kidney cortex was processed for frozen section immunoelectron microscopy. Sections were (A and B) single labelled for Rabankyrin-5 (arrowheads, 10 nm) or (C and D) double labelled (arrows, 5 nm) for Rabankyrin-5 and LAMP-1. (A) Low-magnification view of the apical region of two proximal tubule cells demonstrates low labelling for Rabankyrin-5 on apical microvilli (M) but stronger labelling (arrowheads) of large subapical electron-lucent vesicular structures (asterisks). One of these structures is shown at higher magnification in (B). L, lateral membrane. (C) Rabankyrin-5 labels LAMP-1–negative subapical structures as well as compartments showing low LAMP-1 labelling (arrows and asterisk). (D) Rabankyrin-5 (arrowheads) associates with compartments, which show no or weak labelling for LAMP-1 (asterisks). In addition, low Rabankyrin-5 labelling is associated with more strongly labelled LAMP-1–positive compartments. Note that there is some nonspecific labelling of mitochondria (m). Scale bars represent 500 nm.
Figure 10. Rabankyrin-5 Colocalises to Apically Labelled Structures in Filter-Grown MDCK Cells
MDCK cells were seeded on polycarbonate filters for 4 d and either (A–C) mock-infected or (D–F) infected with an adenovirus construct expressing full-length Rabankyrin-5 for another 18 h. Cells were then incubated for 13 min with 5 mg/ml Alexa 488 dextran, added to the apical medium, fixed, and stained for the indicated antigens. Images represent confocal scans just beneath (A and D) the apical plasma membrane, (B and E) the nuclear area, and (C and F) the basal plasma membrane. Endogenous Rabankyrin-5 and EEA1 localise to several dextran-labelled structures, often with ring-shaped appearance in the apical region. Overexpression of Rabankyrin-5 seems to increase the number of enlarged and dextran-labelled structures, some of which are depleted of EEA1 (arrows) (B). Scale bars represent 10 μm.
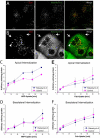
Figure 11. Overexpression of Rabankyrin-5 Stimulates Fluid-Phase Uptake from the Apical Plasma Membrane
(A and B) MDCK cells plated on coverslips were (A) mock-infected or (B) infected with Rabankyrin-5 and processed for immunofluorescence. Under both conditions EEA1 colocalised almost completely with Rabankyrin-5, while there were some Rabankyrin-5 structures devoid of EEA1. Upon Rabankyrin-5 overexpression these EEA1-lacking structures were enlarged macropinocytic-like vesicles localised predominantly in the periphery of the cell, while other enlarged compartments containing EEA1 were centripetally located. (C–F) Filter-grown MDCK cells were either mock-infected or infected with recombinant adenovirus for full-length Rabankyrin-5. HRP (5 mg/ml) or biotinylated Fab fragments (50 μg/ml) were internalised from the indicated chambers for various time points. Whereas Rabankyrin-5 overexpression (C) specifically increased HRP uptake from the apical domain, (D) basolateral HRP uptake and (E and F) Fab uptake at both domains were unaffected (p < 0,01). Values shown are means ± standard deviation of at least three independent experiments. Scale bars represent 10 μm.
Similar articles
- Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells.
Bucci C, Wandinger-Ness A, Lütcke A, Chiariello M, Bruni CB, Zerial M. Bucci C, et al. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5061-5. doi: 10.1073/pnas.91.11.5061. Proc Natl Acad Sci U S A. 1994. PMID: 8197185 Free PMC article. - Rab5 regulates motility of early endosomes on microtubules.
Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Nielsen E, et al. Nat Cell Biol. 1999 Oct;1(6):376-82. doi: 10.1038/14075. Nat Cell Biol. 1999. PMID: 10559966 - EEA1 links PI(3)K function to Rab5 regulation of endosome fusion.
Simonsen A, Lippé R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. Simonsen A, et al. Nature. 1998 Jul 30;394(6692):494-8. doi: 10.1038/28879. Nature. 1998. PMID: 9697774 - [Effectors of GTPase Rab5 in endocytosis and signal transduction].
Olchowik M, Miaczyńska M. Olchowik M, et al. Postepy Biochem. 2009;55(2):171-80. Postepy Biochem. 2009. PMID: 19824473 Review. Polish. - Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons.
Carney DS, Davies BA, Horazdovsky BF. Carney DS, et al. Trends Cell Biol. 2006 Jan;16(1):27-35. doi: 10.1016/j.tcb.2005.11.001. Epub 2005 Dec 2. Trends Cell Biol. 2006. PMID: 16330212 Review.
Cited by
- Rab5 and Rab4 regulate axon elongation in the Xenopus visual system.
Falk J, Konopacki FA, Zivraj KH, Holt CE. Falk J, et al. J Neurosci. 2014 Jan 8;34(2):373-91. doi: 10.1523/JNEUROSCI.0876-13.2014. J Neurosci. 2014. PMID: 24403139 Free PMC article. - Endosomal compartment contributes to the propagation of CD95/Fas-mediated signals in type II cells.
Matarrese P, Manganelli V, Garofalo T, Tinari A, Gambardella L, Ndebele K, Khosravi-Far R, Sorice M, Esposti MD, Malorni W. Matarrese P, et al. Biochem J. 2008 Aug 1;413(3):467-78. doi: 10.1042/BJ20071704. Biochem J. 2008. PMID: 18442358 Free PMC article. - Varroa destructor parasitism has a greater effect on proteome changes than the deformed wing virus and activates TGF-β signaling pathways.
Erban T, Sopko B, Kadlikova K, Talacko P, Harant K. Erban T, et al. Sci Rep. 2019 Jun 28;9(1):9400. doi: 10.1038/s41598-019-45764-1. Sci Rep. 2019. PMID: 31253851 Free PMC article. - Ameobal pathogen mimivirus infects macrophages through phagocytosis.
Ghigo E, Kartenbeck J, Lien P, Pelkmans L, Capo C, Mege JL, Raoult D. Ghigo E, et al. PLoS Pathog. 2008 Jun 13;4(6):e1000087. doi: 10.1371/journal.ppat.1000087. PLoS Pathog. 2008. PMID: 18551172 Free PMC article. - Biorecognition and subcellular trafficking of HPMA copolymer-anti-PSMA antibody conjugates by prostate cancer cells.
Liu J, Kopecková P, Bühler P, Wolf P, Pan H, Bauer H, Elsässer-Beile U, Kopecek J. Liu J, et al. Mol Pharm. 2009 May-Jun;6(3):959-70. doi: 10.1021/mp8002682. Mol Pharm. 2009. PMID: 19344119 Free PMC article.
References
- Allen LA, Aderem A. Mechanisms of phagocytosis. Curr Opin Immunol. 1996;8:36–40. - PubMed
- Amyere M, Mettlen M, Van D, Platek A, Payrastre B, et al. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous