The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells - PubMed (original) (raw)
The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells
Monica L Guzman et al. Blood. 2005.
Abstract
Recent studies have described malignant stem cells as central to the initiation, growth, and potential relapse of acute and chronic myelogenous leukemia (AML and CML). Because of their important role in pathogenesis, rare and biologically distinct leukemia stem cells (LSCs) represent a critical target for therapeutic intervention. However, to date, very few agents have been shown to directly target the LSC population. The present studies demonstrate that parthenolide (PTL), a naturally occurring small molecule, induces robust apoptosis in primary human AML cells and blast crisis CML (bcCML) cells while sparing normal hematopoietic cells. Furthermore, analysis of progenitor cells using in vitro colony assays, as well as stem cells using the nonobese diabetic/severe combined immunodeficient (NOD/SCID) xenograft model, show that PTL also preferentially targets AML progenitor and stem cell populations. Notably, in comparison to the standard chemotherapy drug cytosine arabinoside (Ara-C), PTL is much more specific to leukemia cells. The molecular mechanism of PTL-mediated apoptosis is strongly associated with inhibition of nuclear factor kappa B (NF-kappaB), proapoptotic activation of p53, and increased reactive oxygen species (ROS). On the basis of these findings, we propose that the activity of PTL triggers LSC-specific apoptosis and as such represents a potentially important new class of drugs for LSC-targeted therapy.
Figures
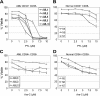
Figure 1.
PTL induces apoptosis in CD34+CD38- AML cells but not in normal cells in a dose-dependent manner. In vitro cultures were maintained for 18 hours followed by analysis of viability using annexin-V labeling. Each plot shows the average percent cell viability for CD34+CD38- AML (A,C) and normal (N) cells (B,D) treated with increasing concentrations of PTL (A-B) or Ara-C (C-D). Each error bar represents the SD. All assays were performed in triplicate.
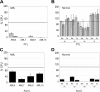
Figure 2.
In vitro colony assays for AML and normal cells treated with PTL and Ara-C. AML versus normal cells in panels A and B were treated with 5 μM ( ) or 7.5 μM PTL (
) or 7.5 μM PTL ( ). AML versus normal cells in panels C and D were treated with 5 μM Ara-C (▪). All treatments were performed for 18 hours in suspension culture, followed by plating in methylcellulose culture. Error bars represent the SD. Average percent of colony-forming units (CFU) are normalized to untreated control (horizontal bar). All assays were performed in triplicate. Mye represents myeloid; Ery, erythroid.
). AML versus normal cells in panels C and D were treated with 5 μM Ara-C (▪). All treatments were performed for 18 hours in suspension culture, followed by plating in methylcellulose culture. Error bars represent the SD. Average percent of colony-forming units (CFU) are normalized to untreated control (horizontal bar). All assays were performed in triplicate. Mye represents myeloid; Ery, erythroid.
Figure 3.
PTL inhibits NOD/SCID repopulating ability for AML but not normal cells. Percentage of engraftment for NOD/SCID mice that received a transplant with AML (A) or normal CB (B) cells after 18 hours of culture with or without 7.5 μM PTL. Each • or ▴ represents a single animal analyzed at 6 to 8 weeks after transplantation. Each plot represents an AML/CB specimen. Mean engraftment is indicated by the horizontal bars.
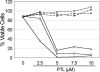
Figure 4.
NAC treatment abolishes PTL apoptosis induction in AML cells. Percent viability of CD34+ cells from 3 different AML specimens treated with increasing concentrations of PTL. Cells were precultured with 800 μM NAC (- - -) versus untreated controls (—) for 1 hour and immediately washed and treated with PTL for 18 hours. Specimens shown correspond to AML5 (□), AML10 (⋄) and AML15 (▵).
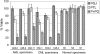
Figure 5.
PGJ2 increases the sensitivity of leukemia cells to PTL. Average percent viability for CD34+CD38- cells normalized to untreated controls. Three AML, 3 bcCML, and 3 normal specimens were treated for 18 hours with 0.5 μM PGJ2 ( ), 2.5 μM PTL (□), or both (
), 2.5 μM PTL (□), or both ( ). Each error bar represents the SD. All assays were performed in triplicate.
). Each error bar represents the SD. All assays were performed in triplicate.
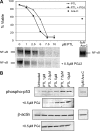
Figure 6.
Apoptosis induction by PTL or PTL/PGJ2 correlates to inhibition of NF-κB and increased phosphorylation of p53(ser15). (A) Percent viability and NF-κB electrophoretic mobility shift assay (EMSA) of a representative AML specimen treated with increasing concentrations of PTL alone (•) or in combination with 0.5 μM PGJ2 (▪). The viability is compared with and Ara-C (5μM; ♦) treatments. (B) Immunoblot analysis of phospho-p53(ser15) and actin for the same representative AML specimen treated with increasing dose of PTL 0.5 μM PGJ2.
Similar articles
- Myeloperoxidase expression as a potential determinant of parthenolide-induced apoptosis in leukemia bulk and leukemia stem cells.
Kim YR, Eom JI, Kim SJ, Jeung HK, Cheong JW, Kim JS, Min YH. Kim YR, et al. J Pharmacol Exp Ther. 2010 Nov;335(2):389-400. doi: 10.1124/jpet.110.169367. Epub 2010 Aug 10. J Pharmacol Exp Ther. 2010. PMID: 20699435 - Iron Oxide Nanoparticles Combined with Cytosine Arabinoside Show Anti-Leukemia Stem Cell Effects on Acute Myeloid Leukemia by Regulating Reactive Oxygen Species.
Dou J, Li L, Guo M, Mei F, Zheng D, Xu H, Xue R, Bao X, Zhao F, Zhang Y. Dou J, et al. Int J Nanomedicine. 2021 Feb 17;16:1231-1244. doi: 10.2147/IJN.S278885. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33633448 Free PMC article. - An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells.
Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, Vaughan A, Sweeney CJ, Matthews W, Carroll M, Liesveld JL, Crooks PA, Jordan CT. Guzman ML, et al. Blood. 2007 Dec 15;110(13):4427-35. doi: 10.1182/blood-2007-05-090621. Epub 2007 Sep 5. Blood. 2007. PMID: 17804695 Free PMC article. - TIM-3 as a novel therapeutic target for eradicating acute myelogenous leukemia stem cells.
Kikushige Y, Miyamoto T. Kikushige Y, et al. Int J Hematol. 2013 Dec;98(6):627-33. doi: 10.1007/s12185-013-1433-6. Epub 2013 Sep 18. Int J Hematol. 2013. PMID: 24046178 Review. - The Emerging Potential of Parthenolide Nanoformulations in Tumor Therapy.
An T, Yin H, Lu Y, Liu F. An T, et al. Drug Des Devel Ther. 2022 Apr 29;16:1255-1272. doi: 10.2147/DDDT.S355059. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35517982 Free PMC article. Review.
Cited by
- The leukemic stem cell.
Jordan CT. Jordan CT. Best Pract Res Clin Haematol. 2007 Mar;20(1):13-8. doi: 10.1016/j.beha.2006.10.005. Best Pract Res Clin Haematol. 2007. PMID: 17336250 Free PMC article. Review. - Enhancement of parthenolide-induced apoptosis by a PKC-alpha inhibition through heme oxygenase-1 blockage in cholangiocarcinoma cells.
Yun BR, Lee MJ, Kim JH, Kim IH, Yu GR, Kim DG. Yun BR, et al. Exp Mol Med. 2010 Nov 30;42(11):787-97. doi: 10.3858/emm.2010.42.11.082. Exp Mol Med. 2010. PMID: 20938215 Free PMC article. - Blastic phase of chronic myelogenous leukemia.
Karbasian Esfahani M, Morris EL, Dutcher JP, Wiernik PH. Karbasian Esfahani M, et al. Curr Treat Options Oncol. 2006 May;7(3):189-99. doi: 10.1007/s11864-006-0012-y. Curr Treat Options Oncol. 2006. PMID: 16615875 Review. - NCI first International Workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on the biological considerations of hematological relapse following allogeneic stem cell transplantation unrelated to graft-versus-tumor effects: state of the science.
Cairo MS, Jordan CT, Maley CC, Chao C, Melnick A, Armstrong SA, Shlomchik W, Molldrem J, Ferrone S, Mackall C, Zitvogel L, Bishop MR, Giralt SA, June CH. Cairo MS, et al. Biol Blood Marrow Transplant. 2010 Jun;16(6):709-28. doi: 10.1016/j.bbmt.2010.03.002. Epub 2010 Mar 12. Biol Blood Marrow Transplant. 2010. PMID: 20227509 Free PMC article. - Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals.
Fong D, Yeh A, Naftalovich R, Choi TH, Chan MM. Fong D, et al. Cancer Lett. 2010 Jul 1;293(1):65-72. doi: 10.1016/j.canlet.2009.12.018. Epub 2010 Jan 20. Cancer Lett. 2010. PMID: 20089354 Free PMC article.
References
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3: 730-737. - PubMed
- Blair A, Hogge DE, Sutherland HJ. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34(+)/CD71(-)/HLA-DR. Blood. 1998;92: 4325-4335. - PubMed
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367: 645-648. - PubMed
- Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89: 3104-3112. - PubMed
- Blair A, Sutherland HJ. Primitive acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo lack surface expression of c-kit (CD117). Exp Hematol. 2000;28: 660-671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous