Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum - PubMed (original) (raw)
Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum
Nathalie Thiriet et al. J Neurosci. 2005.
Abstract
Methamphetamine (METH) is an illicit drug that causes neuronal apoptosis in the mouse striatum, in a manner similar to the neuronal loss observed in neurodegenerative diseases. In the present study, injections of METH to mice were found to cause the death of enkephalin-positive projection neurons but not the death of neuropeptide Y (NPY)/nitric oxide synthase-positive striatal interneurons. In addition, these METH injections were associated with increased expression of neuropeptide Y mRNA and changes in the expression of the NPY receptors Y1 and Y2. Administration of NPY in the cerebral ventricles blocked METH-induced apoptosis, an effect that was mediated mainly by stimulation of NPY Y2 receptors and, to a lesser extent, of NPY Y1 receptors. Finally, we also found that neuropeptide Y knock-out mice were more sensitive than wild-type mice to METH-induced neuronal apoptosis of both enkephalin- and nitric oxide synthase-containing neurons, suggesting that NPY plays a general neuroprotective role within the striatum. Together, our results demonstrate that neuropeptide Y belongs to the class of factors that maintain neuronal integrity during cellular stresses. Given the similarity between the cell death patterns induced by METH and by disorders such as Huntington's disease, our results suggest that NPY analogs might be useful therapeutic agents against some neurodegenerative processes.
Figures
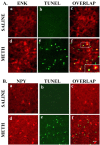
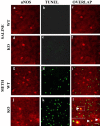
Figure 1.
METH causes selective neuronal death in the mouse striatum. Results of the immunohistochemical experiments are shown in red, and TUNEL-positive labels are shown in green. Immunopositive cells undergoing cell death are double labeled and appear in orange. A, ENK-positive cells are sensitive to METH-induced apoptosis. B, NPY-positive interneurons are spared from METH-induced apoptosis. No TUNEL-positive cells were observed in the saline-treated animals (Ab or Bb). The inset in Af shows an enlargement of an ENK-positive cell that was also TUNEL positive. Scale bar, 50 μm.
Figure 2.
METH administration significantly changes NPY and NPY receptor expression. Left, METH increased NPY expression significantly at 3 and 7 d. Middle, Y1 receptor level was progressively decreased at all time points after METH treatment. Right, Y2 receptor mRNA level was significantly decreased 12 h after METH injections and increased 1 d after METH injections. Statistical analysis was done by ANOVA, followed by Fisher's PLSD. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the saline-treated group. Rec., Receptor.
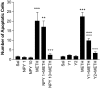
Figure 3.
NPY and NPY agonists inhibit METH-induced neuronal death in the mouse striatum. Quantitative data comparing the number of TUNEL-positive cells after METH administration alone or in combination with NPY at two dosages (1 or 10 μg) or with Y1 and Y2 receptor agonists are shown. The number of TUNEL-labeled cells induced by METH was reduced by NPY when given at 10 μg. Few cells were labeled after saline (Sal) or NPY administration (left). The Y1 agonist (Leu31-Pro34 NPY; 10 μg) partially blocked METH-induced apoptosis, whereas the Y2 receptor agonist [NPY (3-36); 10 μg] completely blocked the apoptotic effects of METH (right). Statistical analysis was done by ANOVA followed by Fisher's PLSD. **p < 0.01, *** p < 0.001, compared with saline-treated animals; ∘∘∘p < 0.001, compared with METH-treated animals.
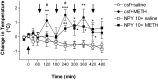
Figure 4.
Effects of NPY on METH-induced hyperthermia. Quantitative data represent the changes in temperature (in °C) after METH administration alone or in combination with 10 μg of NPY over 9.5 h of experimental session. Arrows indicate the times of injections: the first arrow (arrowhead up) indicates the intracerebroventricular injection of CSF or NPY (10 μg); the other arrows (arrowhead down) indicate intraperitoneal injections of either saline or METH. METH caused significant increases in body temperature that were attenuated by administration of NPY at early time points. Statistical analyses were done using ANOVA followed by Fisher's PLSD. *p < 0.05, **p < 0.01, *** p < 0.001, compared with saline-treated (csf+saline) animals; #p < 0.05, compared with CSF plus METH (csf+METH) animals.
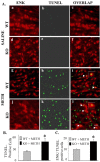
Figure 5.
METH-induced neuronal cell death is potentiated in NPY KO mice. A, Representative photomicrographs illustrating the effects of METH in the striatum of WT and NPY KO mice. No TUNEL-positive cells (green) were observed in the saline-treated animals for WT (Ab) or NPY KO (Ae) mice. After METH treatment, WT mice (Ah) show less TUNEL-positive cells than NPY KO animals (Ak). The number of ENK-positive neurons sensitive to METH-induced apoptosis (orange) is increased in the NPY KO animals (compare Ai with Al). The insets in Ai and Al show an enlargement of an ENK- and TUNEL-positive cell (arrowheads). Scale bar, 50 μm. B, Quantitative data comparing the two genotypes and their responses to METH. Significantly more neurons were killed by METH in NPY KO mice compared with WT animals (ANOVA, followed by Fisher's PLSD; *p < 0.05). C, Quantitative data comparing the response of ENK neurons to METH in the two genotypes. Significantly more ENK-positive neurons were killed by METH in NPY KO mice compared with WT animals (ANOVA, followed by Fisher's PLSD; *p < 0.05).
Figure 6.
nNOS neurons in NPY KO mice become sensitive to METH. nNOS-positive cells are shown in red, whereas TUNEL-positive cells are shown in green. The merged results, which show nNOS-positive cells that are also TUNEL positive, are shown in orange. No TUNEL-positive cells were observed in the saline-treated WT (b) or NPY KO (e) mice. After METH treatment, no nNOS-positive cells were TUNEL positive in the WT mice (i), whereas some nNOS neurons were also TUNEL positive in the NPY KO animals (l). The inset in l shows an enlargement of a nNOS- and TUNEL-positive cell (arrowheads). Scale bar, 50 μm.
Similar articles
- Modulation of methamphetamine-induced nitric oxide production by neuropeptide Y in the murine striatum.
Yarosh HL, Angulo JA. Yarosh HL, et al. Brain Res. 2012 Nov 5;1483:31-8. doi: 10.1016/j.brainres.2012.09.013. Epub 2012 Sep 13. Brain Res. 2012. PMID: 22982589 Free PMC article. - Protective role of neuropeptide Y Y₂ receptors in cell death and microglial response following methamphetamine injury.
Gonçalves J, Ribeiro CF, Malva JO, Silva AP. Gonçalves J, et al. Eur J Neurosci. 2012 Nov;36(9):3173-83. doi: 10.1111/j.1460-9568.2012.08232.x. Epub 2012 Jul 17. Eur J Neurosci. 2012. PMID: 22805317 - Distinct mechanisms mediating methamphetamine-induced neuronal apoptosis and dopamine terminal damage share the neuropeptide substance p in the striatum of mice.
Zhu JP, Xu W, Angulo JA. Zhu JP, et al. Ann N Y Acad Sci. 2006 Aug;1074:135-48. doi: 10.1196/annals.1369.013. Ann N Y Acad Sci. 2006. PMID: 17105911 Free PMC article. - Molecular analysis of central feeding regulation by neuropeptide Y (NPY) neurons with NPY receptor small interfering RNAs (siRNAs).
Higuchi H. Higuchi H. Neurochem Int. 2012 Nov;61(6):936-41. doi: 10.1016/j.neuint.2012.02.029. Epub 2012 Mar 5. Neurochem Int. 2012. PMID: 22414532 Review. - [Neuropeptide Y and autonomic nervous system].
Nozdrachev AD, Masliukov PM. Nozdrachev AD, et al. Zh Evol Biokhim Fiziol. 2011 Mar-Apr;47(2):105-12. Zh Evol Biokhim Fiziol. 2011. PMID: 21598694 Review. Russian.
Cited by
- Congenital abnormality effect of methamphetamine on histological, cellular and chromosomal defects in fetal mice.
Mirjalili T, Kalantar SM, Shams Lahijani M, Sheikhha MH, Talebi A. Mirjalili T, et al. Iran J Reprod Med. 2013 Jan;11(1):39-46. Iran J Reprod Med. 2013. PMID: 24639691 Free PMC article. - Methamphetamine-mediated endoplasmic reticulum (ER) stress induces type-1 programmed cell death in astrocytes via ATF6, IRE1α and PERK pathways.
Shah A, Kumar A. Shah A, et al. Oncotarget. 2016 Jul 19;7(29):46100-46119. doi: 10.18632/oncotarget.10025. Oncotarget. 2016. PMID: 27323860 Free PMC article. - Neuronal Stress and Injury Caused by HIV-1, cART and Drug Abuse: Converging Contributions to HAND.
Sanchez AB, Kaul M. Sanchez AB, et al. Brain Sci. 2017 Feb 23;7(3):25. doi: 10.3390/brainsci7030025. Brain Sci. 2017. PMID: 28241493 Free PMC article. Review. - Environmental enrichment during adolescence regulates gene expression in the striatum of mice.
Thiriet N, Amar L, Toussay X, Lardeux V, Ladenheim B, Becker KG, Cadet JL, Solinas M, Jaber M. Thiriet N, et al. Brain Res. 2008 Jul 30;1222:31-41. doi: 10.1016/j.brainres.2008.05.030. Epub 2008 May 20. Brain Res. 2008. PMID: 18585688 Free PMC article. - Methamphetamine toxicity and messengers of death.
Krasnova IN, Cadet JL. Krasnova IN, et al. Brain Res Rev. 2009 May;60(2):379-407. doi: 10.1016/j.brainresrev.2009.03.002. Epub 2009 Mar 25. Brain Res Rev. 2009. PMID: 19328213 Free PMC article. Review.
References
- Ali SF, Newport GD, Holson RR, Slikker Jr W, Bowyer JF (1994) Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res 658: 33-38. - PubMed
- Behrens MI, Koh JY, Muller MC, Choi DV (1996) NADPH diaphorase-containing striatal or cortical neurons are resistant to apoptosis. Neurobiol Dis 3: 72-75. - PubMed
- Bouali SM, Fournier A, St-Pierre S, Jolicoeur FB (1995) Influence of ambient temperature on the effects of NPY on body temperature and food intake. Pharmacol Biochem Behav 50: 473-475. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous