Down-regulation of c-Fos/c-Jun AP-1 dimer activity by sumoylation - PubMed (original) (raw)
Down-regulation of c-Fos/c-Jun AP-1 dimer activity by sumoylation
Guillaume Bossis et al. Mol Cell Biol. 2005 Aug.
Abstract
The inducible transcriptional complex AP-1, composed of c-Fos and c-Jun proteins, is crucial for cell adaptation to many environmental changes. While its mechanisms of activation have been extensively studied, how its activity is restrained is poorly understood. We report here that lysine 265 of c-Fos is conjugated by the peptidic posttranslational modifiers SUMO-1, SUMO-2, and SUMO-3 and that c-Jun can be sumoylated on lysine 257 as well as on the previously described lysine 229. Sumoylation of c-Fos preferentially occurs in the context of c-Jun/c-Fos heterodimers. Using nonsumoylatable mutants of c-Fos and c-Jun as well as a chimeric protein mimicking sumoylated c-Fos, we show that sumoylation entails lower AP-1 transactivation activity. Interestingly, single sumoylation at any of the three acceptor sites of the c-Fos/c-Jun dimer is sufficient to substantially reduce transcription activation. The lower activity of sumoylated c-Fos is not due to inhibition of protein entry into the nucleus, accelerated turnover, and intrinsic inability to dimerize or to bind to DNA. Instead, cell fractionation experiments suggest that decreased transcriptional activity of sumoylated c-Fos is associated with specific intranuclear distribution. Interestingly, the phosphorylation of threonine 232 observed upon expression of oncogenically activated Ha-Ras is known to superactivate c-Fos transcriptional activity. We show here that it also inhibits c-Fos sumoylation, revealing a functional antagonism between two posttranslational modifications, each occurring within a different moiety of a bipartite transactivation domain of c-Fos. Finally we report that the sumoylation of c-Fos is a dynamic process that can be reversed via multiple mechanisms. This supports the idea that this modification does not constitute a final inactivation step that necessarily precedes protein degradation.
Figures
FIG. 1.
In vitro sumoylation of c-Fos and c-Jun. (A) Sumoylation of in vitro-translated c-Fos and c-FosK265R. Proteins were radioactively translated in the rabbit reticulocyte lysate and subjected to sumoylation in the presence of Sae1/Sae2, Ubc9, and either SUMO-1, SUMO-2, or SUMO-3 (see Materials and Methods). In vitro-translated c-Fos appears as a doublet (bracket) because of internal translation initiation. Sumoylated c-Fos is indicated. (B) Sumoylation of recombinant c-Fos. c-Fos and c-FosK265R were produced in E. coli and subjected to sumoylation as for panel A. Detection of unmodified and modified proteins was achieved by immunoblotting using the sc52 anti-c-Fos antibody.
FIG. 2.
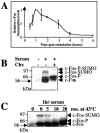
c-Fos modification by SUMO in vivo. (A) Expression kinetics of endogenous c-Fos in serum-stimulated HeLa cells. Cells were stimulated by addition of serum as described in Materials and Methods. They were lysed in a SDS-containing sample buffer at different time points poststimulation and proteins were subjected to immunoblotting analysis with the sc52 anti-c-Fos antibody. bb corresponds to a nonspecific background band detected by sc52 and comigrating with the fastest-migrating c-Fos species. Nonsumoylated c-Fos is indicated by a bracket. (B) c-Fos modification by the three SUMO forms. Asynchronous parental and His6-SUMO-1-, His6-SUMO-2-, or His6-SUMO-3-expressing HeLa cells were stimulated (+) or not (−) by serum for 1 hour to induce endogenous c-Fos expression. Cells were then lysed in a guanidinium HCl-containing buffer and His6-SUMO conjugates were affinity chromatography purified. Those were analyzed by immunoblotting with the sc52 antibody as well as a fraction (1%) of the total extract (Input) to verify comparable induction of c-Fos in the four cell lines. (C) K265 is the target for SUMO conjugation. Wild-type and K-to-R mutants were transfected in asynchronous HeLa cells together with a His6-SUMO-2 expression plasmid. SUMO conjugates and a fraction of the total extract were analyzed as for panel B. (D) SUMO conjugation to K265 during the G0/G1 phase transition. c-_fos_−/− f10 cells were stably transfected with vectors reproducing the transient expression of the natural c-fos gene upon serum stimulation and expressing c-Fos or c-FosK265R. Transient expression can be achieved owing to the presence of a minimal c-fos promoter containing the serum-responsive element (pSRE) and of the 3′ untranslated region, which carries the main mRNA instability determinants (3). Cells were arrested in G0 by serum deprivation for 36 h, stimulated with 20% serum, and treated as for panel A.
FIG. 3.
Regulation of c-Fos sumoylation. (A) Fraction of sumoylated c-Fos at various times post-serum stimulation. HeLa cells were serum deprived and stimulated as in Fig. 2A. Proteins samples were prepared at different times poststimulation and subjected to immunoblotting analysis using the sc52 anti-c-Fos antiserum. Luminograms exposed for different periods of time were analyzed by densitometry and the relative amounts of both c-Fos and sumoylated c-Fos were determined using the NIH Image software to plot the relative fractions of sumoylated c-Fos as a function of time; 1 was arbitrarily chosen as the maximal level of sumoylation of c-Fos found at time 1 h after addition of serum. The average of three independent experiments is presented. Similar results were obtained in experiments with fewer time points. The bars indicate the standard deviation. In the experiments conducted, 9% ± 1% of the c-Fos protein was found sumoylated 1 h poststimulation. (B) Inhibition of c-Fos sumoylation upon protein synthesis inhibition. HeLa cells were stimulated with 20% serum and 50 μg/ml cycloheximide was added 30 min post-serum addition; 30 min later, cells were lysed for immunoblotting analysis as for Fig. 2A. (C) Desumoylation of c-Fos upon heat shock. HeLa cells were stimulated with 20% serum for 1 hour, at which time they were heat shocked at 43°C for various periods of time. The immunoblotting analysis was conducted as for panel A. bb, background band detected by sc52. c-Fos-P, c-Fos-SUMO, and c-Fos-P-SUMO indicate phosphorylated, sumoylated, and phosphorylated/sumoylated c-Fos, respectively. The cell extracts used in panels B and C were also immunoblotted with anti-SUMO antibodies with no detectable variation in the bulk of sumoylated proteins between the various samples.
FIG. 4.
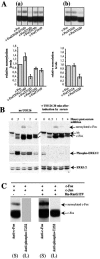
Influence of phosphorylation on c-Fos sumoylation. (A) Relative sumoylation levels of various c-Fos phosphorylation site mutants. Exponentially growing HeLa cells were transfected with plas-mids expressing the various mutants under standardized conditions to achieve similar expression levels in the presence of a c-Jun expression plasmid and analyzed by immunoblotting using the sc52 anti-Fos antiserum. Relative amounts were determined from densitometer analysis of luminograms. The data are the results of three independent experiments; 1 was arbitrarily chosen as the ratio of sumoylated c-Fos versus nonmodified c-Fos in reference c-Jun plus c-Fos transfections. In these experiments, the fraction of wild-type c-Fos that was sumoylated was approximately 8%. (a) Comparison of the various phosphomimetic mutants. (b) Comparison to that of threonine 232 alanine and aspartic acid mutants. (B) Effect of the inhibition of the Erk mitogen-activated protein kinase pathway on sumoylation of c-Fos. HeLa cells were stimulated by serum as for Fig. 2A. UO126 was added at a concentration of 10 μg/ml 20 min post-serum stimulation. Cell extracts were prepared at the indicated times for immunoblotting analysis. Immunoblots were probed with anti-Fos, anti-Erk, and anti-phospho-Erk1/2 antisera. The latter permitted visualization of the efficiency of Erk1/2 inactivation. Identical results were obtained when UO126 was added 10 or 30 min after the addition of serum. bb indicates a background band in the case of c-Fos, which is different from that seen in the experiments presented in the other figures due to a difference in antibody batch. (C) Detection of T232-phosphorylated c-Fos in HaRasG12V-expressing cells. HeLa cells were cotransfected with either c-Fos and c-Jun expression plasmids (also see Fig. 8) or the same plasmids plus a Ha-RasG12V-expressing construct. Immunoblots of total cell proteins were probed with either the sc52 antibody, to detect the bulk of c-Fos (posttranslationally modified or not), or the anti-phospho-T232 antiserum, which permits visualization of only the fraction of c-Fos modified on T232. Because only a fraction of c-Fos is modified on T232 and to a likely lower titer of the anti-phospho-T232 antiserum compared to the sc52 anti-c-Fos antibody, signal intensities were higher in the latter case than in the former. The luminograms presented are, therefore, not representative of the relative abundances of nonphosphorylated and T232-phosphorylated c-Fos proteins. Short-exposure-time luminograms (S) were selected to visualize the bulk of sumoylated and nonsumoylated c-Fos by the sc52 antiserum, whether Ha-RasG12V is expressed or not. In contrast, long exposure times (L) are presented in the case of detection with the anti-phospho-T232 antiserum to make it clear that no sumoylated c-Fos is detected with the anti-phospho-T232 antiserum.
FIG. 5.
Sumoylation of c-Jun on K229 and K257. Wild-type and mutant c-Jun in which either K229 or K257 or both were mutated in arginines were subjected to sumoylation in the presence or absence of recombinant PIASxβ under the same conditions as for Fig. 1A except that Ubc9 was used at a 10-fold lower concentration. c-Jun appears as multiple bands (bracket) when translated in the reticulocyte lysate. Sumoylated bands are indicated by arrows.
FIG. 6.
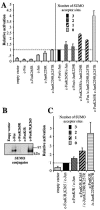
Inhibition of c-Fos and c-Jun sumoylation stimulates AP-1-dependent transcription activity. (A) Stimulation of AP-1 activity by non sumoylatable c-Fos and c-Jun. We transfected 0.4 × 106 asynchronous HeLa cells with (i) 200 ng of a luciferase reporter plasmid under the control of the collagenase promoter, (ii) 500 ng of an EGFP expression vector used for normalization, and (iii) 50 ng of expression plasmids for wild-type or mutant c-Fos and c-Jun as indicated. The different motifs of the bars indicate the number of SUMO acceptor sites present within c-Fos/c-Jun AP-1 complexes. In the initial calculation, fold activation values were calculated as the ratio between luciferase activity and EGFP fluorescence. The activity of the wild-type c-Fos/c-Jun dimer was used as an internal reference and arbitrarily set to 1. The results shown are the means of four independent experiments ± standard deviation. (B) Sumoylation of c-FosK/R and c-FosK/R,K265. HeLa cells were transfected with the indicated constructs together with a His6-SUMO-2 expression plasmid and SUMO conju-gates were purified and analyzed by immunoblotting with the sc52 anti c-Fos antibody as in Fig. 2B. (C) Transactivation properties of c-FosK/R and c-FosK/R,K265. This experiment was performed as for panel A with the indicated plasmids. The activity of the c-FosK/R,K265/c-Jun dimer, which is identical to that of the c-Fos/c-Jun dimer in panel A, was taken as the internal reference and arbitrarily set to 1. The results presented are the means of at least three independent experiments.
FIG. 7.
Inhibition of AP-1-dependent transactivation by chimeric c-Fos/SUMO proteins. (A) Structure of c-Fos(1-265)-SUMO chimeras. The C-terminal diglycine motifs of SUMO proteins was mutated into dialanines. In c-Fos(1-265)-SU-1 and -SU-2 as well as in c-Fos(1-265)-EGFP, the SUMO and EGFP moieties were separated from c-Fos(1-265) by a flexible (Ser-Gly3)3 linker. In the case of Su1-c-Fos, no linker was used between SUMO-1 and c-Fos. (B) Transactivation assays. We cotransfected 0.4 × 106 asynchronous HeLa cells with 200 ng of a luciferase reporter plasmid under the control of the collagenase promoter, 50 ng of the c-JunK229R,K257R, and appropriate concentrations of the other plasmids in order to express similar protein levels; 200 ng was used for the reference c-Fos(1-265) expression plasmid, and optimal concentrations for the other vectors were determined experimentally in preliminary transfection experiments in which Fos chimera protein levels were assayed by immunoblotting using an anti-c-Fos antiserum. Luciferase activity was measured 24 h posttransfection. Fold activation values correspond to the ratio between luciferase activity and EGFP fluorescence. The reference value obtained with the c-Fos(1-265)/c-JunK229/257R plasmids was arbitrarily set to 1. The results shown are the means of at least three independent experiments ± standard deviation. The intrinsic transactivation activity of c-Fos(1-265) was fivefold less than that of wild-type c-Fos in control experiments. (C) Structure of c-Fos-SUMO1 chimeras. Constructs were made as in panel A except that full-length c-Fos was used instead of c-Fos(1-265). (D) Transactivation assays. Experiments were conducted as for panel B.
FIG. 8.
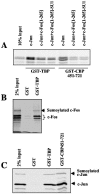
Heterodimerization of sumoylated c-Fos, binding to DNA, and interaction with CBP and TBP. (A) In vitro dimerization with c-Jun. In vitro-translated c-Fos and c-Jun were sumoylated using a Sae1/Sae2-, Ubc9-, and SUMO-1-containing reaction mix. Only a fraction of each protein was sumoylated, which allowed direct comparison of modified and unmodified proteins. A recombinant c-Jun protein was added to the c-Fos reaction (Aa), whereas a GST-c-Fos protein was added to the c-Jun one (Ab). In the former case, complexes were immunoprecipitated using the sc45 anti-c-Jun antibody, whereas in the latter, they were purified by affinity chromatography on glutathione beads. Bound proteins were then resolved by SDS-PAGE. (B) In vivo sumoylation of leucine zipper and DNA binding mutants. HeLa-SUMO-2 cells were transfected with equivalent amounts of plasmids coding for the the indicated proteins as described in Materials and Methods. c-Jun proteins were tagged with a Flag epitope. Cell extracts were prepared and subjected to either direct immunoblotting analysis to quantify the fraction of sumoylated c-Fos (Ba and Bb) or immunoprecipitation with an anti-Flag antibody to assay c-Fos and c-Jun mutant dimerization by immunoblotting (Bc). (Ba) Long- and short-exposure luminograms of an immunoblotting analysis of total cell extracts with the sc52 anti-cFos antiserum. (Bb) Fraction of sumoylated c-Fos determined as described for Fig. 3A. The values are the averages of two independent experiments. Relative sumoylation levels were calculated compared to the fraction of sumoylated c-Fos (8% ± 0.3) in the c-Fos plus c-Jun transfection. (Bc) Analysis of c-Fos and c-Jun mutant dimerization. Immunoprecipitations were carried out with the anti-Flag antiserum to pull down c-Jun-containing AP-1 complexes and three types of fractions, corresponding to the same initial volume of extract, were subjected to immunoblotting analysis using the anti-Flag and sc52 antisera to detect c-Jun and c-Fos proteins, respectively, in total extracts (T), immunoprecipitates (IP), and supernatants (S). Similar results were obtained with HeLa-SUMO-1 and HeLa-SUMO-3 cells (not shown). (C) Binding to DNA. The various proteins were produced by in vitro translation (right panel) and tested in electrophoretic mobility shift assays (left panel) using a synthetic 32P-labeled TRE probe. Competition was done with increasing quantities of an unlabeled TRE or a scrambled probe. The c-Jun/c-Jun homodimer, which binds less efficiently to DNA than c-Fos/c-Jun homodimers, is not visible in the figure.
FIG. 9.
Binding of c-Fos-containing AP-1 dimer to TBP and CBP. (A) Binding of AP-1 dimers containing c-Fos-SUMO chimeras to TBP and CBP. c-Jun was produced by in vitro translation in a radioactive form and the c-Fos proteins in a nonradioactive one. Heterodimers were formed in the presence of a twofold excess of c-Fos proteins. The homo- and heterodimers were pulled down with GST-TBP or GST-CBP451-721 using glutathione beads and analyzed by SDS-PAGE. c-Jun homodimers appear twice as intense as c-Fos/c-Jun heterodimers because they contained two radioactive molecule instead of one in the case of c-Fos/c-Jun heterodimers. (B) Binding of AP-1 dimers containing a sumoylated c-Fos to TBP. c-Fos was produced by in vitro translation in a radioactive form and was in vitro sumoylated as in Fig. 1A. It was then mixed with c-Jun translated in vitro in a nonradioactive form. The heterodimers were then pulled down with GST and GST-TBP as in panel A and analyzed by SDS-PAGE. The bound proteins were visualized by Phosphorimager analysis. (C) Binding of AP-1 dimers containing a sumoylated c-Jun to TBP and CBP. c-Jun was produced by in vitro translation in a radioactive form and was in vitro sumoylated. After mixing with c-Fos translated in vitro in a nonradioactive form, the heterodimers were then pulled down with GST, GST-TBP, and GST-CBP451-721 and analyzed by electrophoresis.
FIG. 10.
Intranuclear localization of sumoylated c-Fos. (A) Localization of the different constructs. HeLa cells were transiently transfected with plasmids encoding either c-Fos, c-Fos(1-265), or c-Fos(1-265)-SU-1. Cells were fixed 16 h after transfection for immunofluorescence analysis with the sc52 c-Fos antibody. (B) Nuclear fractionation experiments. Soluble and insoluble fractions were prepared as described in Materials and Methods and equal volumes of proteins were analyzed by immunoblotting using the sc52 anti-c-Fos antiserum. (a) HeLa cells were induced for 1 hour with 20% fresh serum. (b) Asynchronous HeLa cells were cotransfected with expression plasmids coding for c-Fos and c-Jun and cell fractionation was carried out 16 h later. (c and d) The same experiments as in b with c-Fos(1-265) and c-Fos(1-265)-SU-1 expression plasmids, respectively. Immunoblots were probed with anti-Phax and anti-topoisomerase I antisera to verify the quality of the fractionations.
Similar articles
- c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK.
Deng T, Karin M. Deng T, et al. Nature. 1994 Sep 8;371(6493):171-5. doi: 10.1038/371171a0. Nature. 1994. PMID: 8072547 - Menin uncouples Elk-1, JunD and c-Jun phosphorylation from MAP kinase activation.
Gallo A, Cuozzo C, Esposito I, Maggiolini M, Bonofiglio D, Vivacqua A, Garramone M, Weiss C, Bohmann D, Musti AM. Gallo A, et al. Oncogene. 2002 Sep 19;21(42):6434-45. doi: 10.1038/sj.onc.1205822. Oncogene. 2002. PMID: 12226747 - Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity.
Chinenov Y, Kerppola TK. Chinenov Y, et al. Oncogene. 2001 Apr 30;20(19):2438-52. doi: 10.1038/sj.onc.1204385. Oncogene. 2001. PMID: 11402339 Review. - Distinct roles of Jun : Fos and Jun : ATF dimers in oncogenesis.
van Dam H, Castellazzi M. van Dam H, et al. Oncogene. 2001 Apr 30;20(19):2453-64. doi: 10.1038/sj.onc.1204239. Oncogene. 2001. PMID: 11402340 Review.
Cited by
- The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition.
Gareau JR, Lima CD. Gareau JR, et al. Nat Rev Mol Cell Biol. 2010 Dec;11(12):861-71. doi: 10.1038/nrm3011. Nat Rev Mol Cell Biol. 2010. PMID: 21102611 Free PMC article. Review. - MicroRNA-29a promotes the proliferation of human nasal epithelial cells and inhibits their apoptosis and promotes the development of allergic rhinitis by down-regulating FOS expression.
Fan Y, Tang Z, Sun J, Zhao X, Li Z, Zheng Y, Zeng X, Feng J. Fan Y, et al. PLoS One. 2021 Aug 12;16(8):e0255480. doi: 10.1371/journal.pone.0255480. eCollection 2021. PLoS One. 2021. PMID: 34383804 Free PMC article. - Expression levels of transcription factors c-Fos and c-Jun and transmembrane protein HAb18G/CD147 in urothelial carcinoma of the bladder.
Huhe M, Liu S, Zhang Y, Zhang Z, Chen Z. Huhe M, et al. Mol Med Rep. 2017 May;15(5):2991-3000. doi: 10.3892/mmr.2017.6411. Epub 2017 Mar 30. Mol Med Rep. 2017. PMID: 28358415 Free PMC article. - Post-Translational Modifications of Kaposi's Sarcoma-Associated Herpesvirus Regulatory Proteins - SUMO and KSHV.
Campbell M, Izumiya Y. Campbell M, et al. Front Microbiol. 2012 Feb 14;3:31. doi: 10.3389/fmicb.2012.00031. eCollection 2012. Front Microbiol. 2012. PMID: 22347876 Free PMC article. - c-Jun downregulation by HDAC3-dependent transcriptional repression promotes osmotic stress-induced cell apoptosis.
Xia Y, Wang J, Liu TJ, Yung WK, Hunter T, Lu Z. Xia Y, et al. Mol Cell. 2007 Jan 26;25(2):219-32. doi: 10.1016/j.molcel.2007.01.005. Mol Cell. 2007. PMID: 17244530 Free PMC article.
References
- Abate, C., D. R. Marshak, and T. Curran. 1991. Fos is phosphorylated by p34cdc2, cAMP-dependent protein kinase and protein kinase C at multiple sites clustered within regulatory regions. Oncogene 6:2179-2185. - PubMed
- Acquaviva, C., F. Brockly, P. Ferrara, G. Bossis, C. Salvat, I. Jariel-Encontre, and M. Piechaczyk. 2001. Identification of a C-terminal tripeptide motif involved in the control of rapid proteasomal degradation of c-Fos proto-oncoprotein during the G(0)-to-S phase transition. Oncogene 20:7563-7572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous