Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities - PubMed (original) (raw)
Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities
Xiaolan Qian et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The three deleted in liver cancer genes (DLC1-3) encode Rho-GTPase-activating proteins (RhoGAPs) whose expression is frequently down-regulated or silenced in a variety of human malignancies. The RhoGAP activity is required for full DLC-dependent tumor suppressor activity. Here we report that DLC1 and DLC3 bind to human tensin1 and its chicken homolog. The binding has been mapped to the tensin Src homology 2 (SH2) and phosphotyrosine binding (PTB) domains at the C terminus of tensin proteins. Distinct DLC1 sequences are required for SH2 and PTB binding. DCL binding to both domains is constitutive under basal conditions. The SH2 binding depends on a tyrosine in DCL1 (Y442) but is phosphotyrosine-independent, a highly unusual feature for SH2 binding. DLC1 competed with the binding of other proteins to the tensin C terminus, including beta 3-integrin binding to the PTB domain. Point mutation of a critical tyrosine residue (Y442F) in DLC1 rendered the protein deficient for binding the tensin SH2 domain and binding full-length tensin. The Y442F protein was diffusely cytoplasmic, in contrast to the localization of wild-type DLC1 to focal adhesions, but it retained the ability to reduce the intracellular levels of Rho-GTP. The Y442F mutant displayed markedly reduced biological activity, as did a mutant that was RhoGAP-deficient. The results suggest that DLC1 is a multifunctional protein whose biological activity depends on cooperation between its tensin binding and RhoGAP activities, although neither activity depends on the other.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
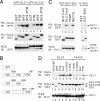
DLC1 and tensin form a complex in mammalian cells. (A) DLC1 was cotransfected with GFP or GFP-tensin into 293 cells, and specifically detected DLC1 was coimmunoprecipitated in the anti-GFP immunopellet (Left). Reciprocally, GFP-tensin transfected into H1299 cells was specifically detected by coimmunoprecipitation of endogenous DLC1 with a DLC1 antibody (Right). (B) Extracts from mel 1011 cells, which contain endogenous DLC1 and tensin, were immunoprecipitated with control IgG or a tensin antibody. Coprecipitated DLC1 was detected by a DLC1 antibody (Upper). The blot was reprobed with the tensin antibody (Lower).
Fig. 2.
DLC1 and DLC3α can be pulled down by tensin SH2 and PTB domains. (A) 293T cells were cotransfected with GFP-DLC1 or GFP-DLC3α and GST-tensin (chicken) fusion proteins as shown in B; amino acid numbers refer to chicken tensin. Cell extracts were pulled down by using glutathione Sepharose-4B (Gluta) and immunoblotted (IB) as indicated. The loading controls are also shown. (C) DLC1 and a RhoGAP-dead mutant R677A, but not a Y442F mutant, bind to the tensin SH2 domain in transfected 293T cells (Left). The endogenous DLC1 in H1299 cells was pulled down in association with the tensin SH2 domain (Right). (D) Characterization of tensin SH2 mutants for DLC1 binding. DLC1 and Y442F were cotransfected with GST and GST fusion proteins as indicated, and the pull-down was followed by immunoblotting.
Fig. 3.
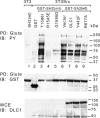
DLC1 interferes with proteins that bind the tensin SH2 domain in Src-transformed cells. v-Src-transformed 3T3 cells were cotransfected with the wild-type GST-SH2 domain with or without wild-type or mutant DLC1 as indicated. Transformed cells were also transfected with mutant GST-SH2 domains as indicated. Untransformed 3T3 cells were transfected with wild-type GST-SH2 domain. After Gluta pull-down, the proteins bound to the SH2 domain were detected by anti-pY blot (PY; Top). The expression of transfected GST fusion proteins and DLC1 is shown in Middle and Bottom, respectively.
Fig. 4.
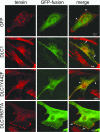
Colocalization of DLC1 with endogenous tensin. Human fibroblast line 1634 grown on coverslips was transfected with GFP or GFP-tagged DLC1 (wild type, Y442F, and R677A) as indicated. Endogenous tensin was stained with a tensin antibody and rhodamine-conjugated secondary antibody. Arrows (but not arrowheads) indicate some colocalization of DLC1 and tensin. Wild-type DLC1 and R667A contain peripheral spots that colocalize with tensin in the merged image, whereas Y442F and GFP have diffuse staining and rarely colocalize. By using vinculin as a marker, it was confirmed that these spots represent typical focal adhesions (see
SI Fig. 10
). At least 100 cells of each type were viewed. The images represent 85–90% of total viewed cells, except that ≈15% of cells expressing the Y442F mutant had a nuclear as well as a cytoplasmic localization. (Scale bars: 10 μm.) Expression of the transfected genes was confirmed by immunoblotting (data not shown).
Fig. 5.
The Y442F mutant reduces Rho-GTP but is deficient biologically. (A) Rho-GTP in H358 cells and stable clones expressing DLC1, Y442F, or R718A were analyzed by Rhotekin pull-down assay followed by anti-RhoA blotting (Top). The expression of DLC1 in the stable clones and total RhoA loading controls were confirmed by immunoblotting. These H358-derived cells were used for the biological experiments in the figure. (B) Transwell migration assay. After 3 days, migrated cells that had come through the transwell filter were photographed after staining and then solubilized and quantified colorimetrically. The data are the mean ± SD of triplicate well measurements from one representative experiment. (C) Anchorage-independent growth assay. Cells were grown in soft agar (0.4%) and photographed after 4 weeks. The quantitative data are the mean number of colonies (± SD) >400 μm in diameter from one representative experiment.
Fig. 6.
DLC1 can compete with β3-integrin for binding the tensin PTB domain. (A) Mapping the PTB domain of chicken tensin for DLC1 and β3-integrin binding. GST-PTB and premature termination mutants were cotransfected with equal amounts of DLC1 or β3-integrin in 293T cells. After GST-PTB pull-down, associated protein binding was assayed by anti-DLC1 and anti-β3 immunoblotting as indicated. Cell extracts were used as loading controls. (B) Competition assay. GST-PTB fragments and the combination of DLC1 vs. β3 were cotransfected at the indicated ratios in 293T cells. After Gluta pull-down, the DLC1 signal was reduced when using β3 as a competitor. The expression of transfected proteins and loading controls is shown.
Similar articles
- Deleted in liver cancer 1 (DLC1) utilizes a novel binding site for Tensin2 PTB domain interaction and is required for tumor-suppressive function.
Chan LK, Ko FC, Ng IO, Yam JW. Chan LK, et al. PLoS One. 2009;4(5):e5572. doi: 10.1371/journal.pone.0005572. Epub 2009 May 15. PLoS One. 2009. PMID: 19440389 Free PMC article. - Tensin1 positively regulates RhoA activity through its interaction with DLC1.
Shih YP, Sun P, Wang A, Lo SH. Shih YP, et al. Biochim Biophys Acta. 2015 Dec;1853(12):3258-65. doi: 10.1016/j.bbamcr.2015.09.028. Epub 2015 Sep 28. Biochim Biophys Acta. 2015. PMID: 26427649 Free PMC article. - Full activity of the deleted in liver cancer 1 (DLC1) tumor suppressor depends on an LD-like motif that binds talin and focal adhesion kinase (FAK).
Li G, Du X, Vass WC, Papageorge AG, Lowy DR, Qian X. Li G, et al. Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):17129-34. doi: 10.1073/pnas.1112122108. Epub 2011 Oct 3. Proc Natl Acad Sci U S A. 2011. PMID: 21969587 Free PMC article. - Tensin.
Lo SH. Lo SH. Int J Biochem Cell Biol. 2004 Jan;36(1):31-4. doi: 10.1016/s1357-2725(03)00171-7. Int J Biochem Cell Biol. 2004. PMID: 14592531 Review. - Regulation of deleted in liver cancer 1 tumor suppressor by protein-protein interactions and phosphorylation.
Ko FC, Ping Yam JW. Ko FC, et al. Int J Cancer. 2014 Jul 15;135(2):264-9. doi: 10.1002/ijc.28505. Epub 2013 Oct 17. Int J Cancer. 2014. PMID: 24114040 Review.
Cited by
- CDK5: an oncogene or an anti-oncogene: location location location.
Nikhil K, Shah K. Nikhil K, et al. Mol Cancer. 2023 Nov 23;22(1):186. doi: 10.1186/s12943-023-01895-8. Mol Cancer. 2023. PMID: 37993880 Free PMC article. Review. - Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in regulation of the cytoskeleton and cell motility.
Kim TY, Vigil D, Der CJ, Juliano RL. Kim TY, et al. Cancer Metastasis Rev. 2009 Jun;28(1-2):77-83. doi: 10.1007/s10555-008-9167-2. Cancer Metastasis Rev. 2009. PMID: 19221866 Free PMC article. Review. - Deleted in liver cancer-1 (DLC1): an emerging metastasis suppressor gene.
Popescu NC, Goodison S. Popescu NC, et al. Mol Diagn Ther. 2014 Jun;18(3):293-302. doi: 10.1007/s40291-014-0086-3. Mol Diagn Ther. 2014. PMID: 24519699 Free PMC article. Review. - Effects of DLC1 Deficiency on Endothelial Cell Contact Growth Inhibition and Angiosarcoma Progression.
Sánchez-Martín D, Otsuka A, Kabashima K, Ha T, Wang D, Qian X, Lowy DR, Tosato G. Sánchez-Martín D, et al. J Natl Cancer Inst. 2018 Apr 1;110(4):390-399. doi: 10.1093/jnci/djx219. J Natl Cancer Inst. 2018. PMID: 29202196 Free PMC article. - SRC substrate surprise.
Martin GS. Martin GS. Cancer Cell. 2009 Sep 8;16(3):176-8. doi: 10.1016/j.ccr.2009.08.017. Cancer Cell. 2009. PMID: 19732716 Free PMC article.
References
- Vogelstein B, Kinzler KW. Nat Med. 2004;10:789–799. - PubMed
- Karnoub AE, Symons M, Campbell SL, Der CJ. Breast Cancer Res Treat. 2004;84:61–71. - PubMed
- Ridley AJ. Breast Cancer Res Treat. 2004;84:13–19. - PubMed
- Gomez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC. BioEssays. 2005;27:602–613. - PubMed
- Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, Popescu NC. Cancer Res. 1998;58:2196–2199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous