Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation - PubMed (original) (raw)
Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation
Shile Liang et al. Ann Biomed Eng. 2008 Apr.
Abstract
Adhesion to and subsequent extravasation through the endothelial lining of blood vessels is critical for tumor cells to establish metastases. Recent studies have indicated that polymorphonuclear neutrophils (PMNs) may enhance melanoma adhesion to the endothelium (EC) and subsequent extravasation under dynamic flow conditions. However, little is known about hydrodynamics involved in the tumor microenvironment within the microcirculation. In this study, effects of hydrodynamic flow on regulating melanoma cell adhesion to the EC have been investigated. Results indicate that under flow conditions, interactions between melanoma cells and the EC are distinctly different from PMN-EC interactions. Without expressions of surface integrins or sialylated molecules, most melanoma cells that express a high-level of intercellular adhesion molecule (ICAM-1) are not able to effectively adhere to the inflamed EC by themselves. Binding of melanoma cells and PMNs through ICAM-1 on melanoma cells and beta(2) integrins on PMNs has been shown to enhance melanoma cell arrest on the EC. Although PMN tethering on the EC is regulated by both the shear rate and shear stress, melanoma cell adhesion to the EC and subsequent extravasation via tethering PMN on the EC is predominantly regulated by shear rate, which partly is due to the shear-rate-dependent PMN-melanoma aggregation in shear flow. These findings provide a rationale and mechanistic basis for understanding of leukocyte-tumor cell interactions under flow conditions during tumor cell extravasation and metastasis.
Figures
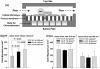
FIGURE 1
Effects of shear rate and shear stress on melanoma extravasation through EI monolayer. Shear rate and shear stress were isolated by varying viscosity with dextran-supplemented medium (0-4%). (a) Cross-section view of the flow-migration chamber shows the schematic of PMN-facilitated melanoma extravasation through EI monolayer in a shear flow. (b) Migration varies under constant shear stress but increasing shear rate. (c) Migration is unchanged over an order of magnitude of shear stress when shear rate is constant. All values are mean ± SEM for N ≥ 3.
FIGURE 2
Effects of shear rate and shear stress on melanoma adhesion to the EI monolayer. (a) PMN tethering frequency under flow conditions. (b) Melanoma cell adhesion to the EI monolayer under flow conditions. WM9 adhesion efficiency and data correction procedure are defined in “Materials and Methods”. All values are mean ± SEM for N ≥ 3.
FIGURE 3
The kinetics of PMN-WM9 aggregation under different shear conditions. (a) Detection of PMN-tumor cell aggregates by two-color flow cytometry. LDS-571-labeled PMNs (1 × 106/mL), TRITC-stained WM9 melanoma cells (1 × 106/mL), or both were sheared at 62.5 s-1 for 120 s in a cone-plate viscometer in the presence of 1 _μ_M fMLP. Upon termination of shear, aliquots were immediately fixed with 2% formaldehyde and subsequently analyzed in a GUAVA flow cytometer. Left panel shows PMN only using flow cytometry; middle panel shows WM9 only; and right panel shows WM9-PMN heterotypic aggregates. The population of WM9 cells was resolved into singlet and aggregates composed of a single WM9 cell bound to one, two, or more than two PMNs. The concentrations of these aggregates were represented by [WM9], [TP1], [TP2], and [TP3+], respectively. The gating was based on the specific fluorescence channel where each population fell in. (b) The percentage of melanoma cell in the heterotypic aggregations at different shear rates with a medium viscosity 1.0 cP. (c) The percentage of melanoma cells in the heterotypic aggregations at a fixed shear rate 62.5 s-1 while shear stress varied from 2 to 6.4 dyn/cm2. (d) The percentage of melanoma cells in the heterotypic aggregations under a fixed shear stress 2 dyn/cm2 while shear rate varied from 62.5 to 100 s-1. Values are mean ± S.E.M. for N ≥ 3. *p < 0.05.
FIGURE 4
Effects of _β_2 integrin-ICAM-1 binding on PMN-WM9 aggregation. Blocking of _β_2 integrin on PMNs significantly reduced PMN-WM9 aggregation compared with control. *p < 0.05 compared with the other two blocking cases. Values are mean ± SEM for N ≥ 3.
FIGURE 5
Effects of PMN tethering on melanoma cell adhesion to the EI monolayer. PMNs were treated with mAb to functionally block LFA-1 and Mac-1 (30 min, 4 °C); EI monolayer was treated with mAb against ICAM-1 (30 min, 4 °C); WM9 cells were treated with mAb against ICAM-1 (30 min, 4 °C). After each treatment, the excess of mAb was washed out by centrifuging cells down and re-suspending them in fresh medium. Cells were then injected into the parallel plate flow chamber for the adhesion assay. Blocking ICAM-1 on WM9 significantly reduced melanoma adhesion efficiency compared with both the control and anti-ICAM-1 on EI cases. *p < 0.05 compared with control samples. #p < 0.05 with respect to concurrent ICAM-1 blocking on the EI cells under the same shear condition. Values are mean ± SEM for N ≥ 3.
FIGURE 6
Population ratio effects on: (a) PMN-WM9 aggregation; (b) WM9 adhesion to the EI monolayer; and (c) C8161 extravasation under various shear conditions. Increase in ratio of PMN to WM9 significantly promotes PMN-WM9 aggregation, WM9 adhesion to the EI monolayer; and increase in ratio of PMN to C8161 increases subsequent extravasation through the EI monolayer. Values are mean ± SEM for N ≥ 3.
Similar articles
- Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation.
Slattery MJ, Liang S, Dong C. Slattery MJ, et al. Am J Physiol Cell Physiol. 2005 Apr;288(4):C831-9. doi: 10.1152/ajpcell.00439.2004. Epub 2004 Dec 15. Am J Physiol Cell Physiol. 2005. PMID: 15601752 Free PMC article. - Tumor cell extravasation mediated by leukocyte adhesion is shear rate dependent on IL-8 signaling.
Liang S, Hoskins M, Dong C. Liang S, et al. Mol Cell Biomech. 2010 Jun;7(2):77-91. Mol Cell Biomech. 2010. PMID: 20379392 Free PMC article. - Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion.
Liang S, Slattery MJ, Dong C. Liang S, et al. Exp Cell Res. 2005 Nov 1;310(2):282-92. doi: 10.1016/j.yexcr.2005.07.028. Epub 2005 Sep 9. Exp Cell Res. 2005. PMID: 16154563 Free PMC article. - Juxtacrine interactions of endothelial cells with leukocytes: tethering and signaling molecules.
Patel KD, Lorant E, Jones DA, Prescott M, McIntyre TM, Zimmerman GA. Patel KD, et al. Behring Inst Mitt. 1993 Aug;(92):144-64. Behring Inst Mitt. 1993. PMID: 8250808 Review. - Microfluidics for in vitro biomimetic shear stress-dependent leukocyte adhesion assays.
Bianchi E, Molteni R, Pardi R, Dubini G. Bianchi E, et al. J Biomech. 2013 Jan 18;46(2):276-83. doi: 10.1016/j.jbiomech.2012.10.024. Epub 2012 Nov 30. J Biomech. 2013. PMID: 23200903 Review.
Cited by
- Polymeric biomaterial-inspired cell surface modulation for the development of novel anticancer therapeutics.
Jangid AK, Kim S, Kim K. Jangid AK, et al. Biomater Res. 2023 Jun 21;27(1):59. doi: 10.1186/s40824-023-00404-8. Biomater Res. 2023. PMID: 37344853 Free PMC article. Review. - Changes in dynamics of tumor/endothelial cell adhesive interactions depending on endothelial cell growth state and elastic properties.
Xie L, Sun Z, Brown NJ, Glinskii OV, Meininger GA, Glinsky VV. Xie L, et al. PLoS One. 2022 Jun 6;17(6):e0269552. doi: 10.1371/journal.pone.0269552. eCollection 2022. PLoS One. 2022. PMID: 35666755 Free PMC article. - The 'Yin and Yang' of Cancer Cell Growth and Mechanosensing.
Amer M, Shi L, Wolfenson H. Amer M, et al. Cancers (Basel). 2021 Sep 23;13(19):4754. doi: 10.3390/cancers13194754. Cancers (Basel). 2021. PMID: 34638240 Free PMC article. Review. - Chimeric Antigen Receptor (CAR) T Cell Therapy for Metastatic Melanoma: Challenges and Road Ahead.
Soltantoyeh T, Akbari B, Karimi A, Mahmoodi Chalbatani G, Ghahri-Saremi N, Hadjati J, Hamblin MR, Mirzaei HR. Soltantoyeh T, et al. Cells. 2021 Jun 9;10(6):1450. doi: 10.3390/cells10061450. Cells. 2021. PMID: 34207884 Free PMC article. Review. - Cell Migration Guided by Cell-Cell Contacts in Innate Immunity.
Miskolci V, Klemm LC, Huttenlocher A. Miskolci V, et al. Trends Cell Biol. 2021 Feb;31(2):86-94. doi: 10.1016/j.tcb.2020.11.002. Epub 2020 Dec 3. Trends Cell Biol. 2021. PMID: 33281034 Free PMC article. Review.
References
- Bateman J, Parida S, Nash G. Neutrophil integrin assay for clinical studies. Cell Biochem. Funct. 1993;11:87–91. - PubMed
- Bell GI. Models for the specific adhesion of cells to cells. A theoretical framework for adhesion mediated by reversible bonds between cell surface molecules. Science. 1978;200:618–627. - PubMed
- Burdick M, McCarty O, Jadhav S, Konstantopoulos K. Cell-cell interactions in inflammation and cancer metastasis. IEEE Eng. Med. Biol. Mag. 2001;20:86–91. - PubMed
- Burdick M, McCaffery J, Kim Y, Bochner B, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am. J. Physiol. Cell Physiol. 2003;284:977–987. - PubMed
- Chambers A, MacDonald I, Schmidt E, Morris V, Groom A. Clinical targets for anti-metastasis therapy. Adv. Cancer Res. 2000;79:91–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR010732/RR/NCRR NIH HHS/United States
- R01 CA125707-02/CA/NCI NIH HHS/United States
- C06 RR016499/RR/NCRR NIH HHS/United States
- R01 AI047294/AI/NIAID NIH HHS/United States
- C06-RR-016499/RR/NCRR NIH HHS/United States
- R01 CA097306/CA/NCI NIH HHS/United States
- R01 CA125707-01A1/CA/NCI NIH HHS/United States
- M01-RR-010732/RR/NCRR NIH HHS/United States
- CA-97306/CA/NCI NIH HHS/United States
- R01 CA097306-04/CA/NCI NIH HHS/United States
- CA125707/CA/NCI NIH HHS/United States
- R01 CA125707/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous