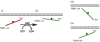A novel switch region regulates H-ras membrane orientation and signal output - PubMed (original) (raw)
A novel switch region regulates H-ras membrane orientation and signal output
Daniel Abankwa et al. EMBO J. 2008.
Abstract
The plasma membrane nanoscale distribution of H-ras is regulated by guanine nucleotide binding. To explore the structural basis of H-ras membrane organization, we combined molecular dynamic simulations and medium-throughput FRET measurements on live cells. We extracted a set of FRET values, termed a FRET vector, to describe the lateral segregation and orientation of H-ras with respect to a large set of nanodomain markers. We show that mutation of basic residues in helix alpha4 or the hypervariable region (HVR) selectively alter the FRET vectors of GTP- or GDP-loaded H-ras, demonstrating a critical role for these residues in stabilizing GTP- or GDP-H-ras interactions with the plasma membrane. By a similar analysis, we find that the beta2-beta3 loop and helix alpha5 are involved in a novel conformational switch that operates through helix alpha4 and the HVR to reorient the H-ras G-domain with respect to the plasma membrane. Perturbation of these switch elements enhances MAPK activation by stabilizing GTP-H-ras in a more productive signalling conformation. The results illustrate how the plasma membrane spatially constrains signalling conformations by acting as a semi-neutral interaction partner.
Figures
Figure 1
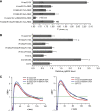
FRET vectors describe the lateral segregation of Ras proteins. (A) The extent of FRET between pairs of mCFP (blue squares) and mCit (yellow squares)-labelled Ras probes and complementary labelled nanodomain markers was measured. The nanodomain probing constructs were either Ras-derived membrane anchors or full-length H-Ras mutants (Supplementary Figures S1C and S4). High FRET values are expected for proteins that co-cluster, whereas a random distribution will result in no or very little FRET. In addition, markers of different lengths enable detection of conformational changes (Supplementary Figure S1B). The nanodomain markers C and D indicate that a set of nanodomain markers is used sequentially to characterize the lateral segregation of the Ras-derived probes by a set of FRET values. (B) Amino-acid sequences of Ras membrane anchors used for FRET experiments. (C) All mCFP/mCit-tagged proteins showed predominant localization to the plasma membrane and were homogeneously distributed when imaged by confocal microscopy (Supplementary Figure S4). We, therefore, increased the throughput of the analysis of >140 FRET pairs, by measuring fluorescence signals in a cytometer. The dependence of the FRET efficiency, E, on the normalized acceptor surface concentration, cA, at ∼1:1 donor–acceptor ratio was analysed using equation (1) (red curves), which yielded the characteristic FRET value, _E_max. The _E_max for most FRET pairs was higher than expected for randomly distributed donor and acceptor species (lower panel, calculated curve in blue), indicative of nanoclustering (Abankwa and Vogel, 2007). Data points are calculated from single cells, the example plots shown are mCFP-H-rasG12V/mCit-tH (upper panel) and mCFP-K-rasG12V/mCit-tH (lower panel). (D) The sample matrix shows _E_max values (±s.e.m. and number of independent experiments n) for Ras membrane anchor probe and marker FRET pairs. The lateral segregation of each Ras membrane anchor probe is described by the FRET vector, given in this example by the set of three _E_max values in each row. (E) FRET vectors can be plotted in a nanodomain marker 'space'. The direction of a vector describes the lateral segregation of a probe. Thus, the more similar the direction of FRET vectors, the more similar is the lateral segregation of the marker probes. The colouring matches that in (D).
Figure 2
Mutation of basic residues in helix α4 or the HVR has differential effects on the FRET vectors of GTP- and GDP-H-ras. MD simulations (Gorfe et al, 2007b) suggest GTP- and GDP-H-ras have different membrane interactions and orientations of the G-domain with respect to the plane of the membrane. (A) The GTP conformation is stabilized by membrane contacts of R128 and R135 on helix α4. (B) These contacts are lost in GDP-H-ras, which is stabilized by contacts of residues R169 and K170 in the HVR. Phosphorous atoms of lipid head groups of the inner membrane leaflet are shown in grey, the outer leaflet is shown as a grey line (not to scale) and H-ras lipid anchors are in light blue. Important basic residues in H-ras are shown in dark blue and acidic residues in red. (C, D) The matrices show eight-tuple FRET vectors for GTP- and GDP-H-ras with mutations at the indicated residues. Each FRET vector is the set of _E_max values of a mCFP-tagged H-ras mutant (blue) and eight mCit-tagged nanodomain markers (yellow). The matrix shows _E_max values±s.e.m. and number of independent experiments in brackets. _E_max values of each mutant–marker pair were compared with the corresponding value in the cognate wild-type background (grey FRET vectors) using _t_-tests; significant differences are shown by green shading. The matrices show different patterns of altered _E_max values in the GTP and GDP background.
Figure 3
A membrane-orientation switch region in H-ras. A combination of MD simulation and structural analysis (Supplementary Figure S2A) suggests operation of a membrane-orientation switch region in H-ras that comprises the β2–β3 loop, helix α5 and the HVR. (A, B) The models for GTP- and GDP-H-ras show networks of salt bridges between basic and acidic residues, which we propose are involved in the structural changes that reorient H-ras after GTP loading. Colouring is as in Figure 2A. Lipid molecules are represented as thin sticks. (C, D) The two matrices show FRET vectors of GTP- and GDP-H-ras with alanine substitutions at residues involved in the membrane-orientation switch. _E_max values are given±s.e.m., and number of independent experiments in brackets. _E_max values of each mutant–marker pair were compared with the corresponding value in the cognate wild-type background (grey FRET vectors in Figure 2C and D) using _t_-tests, significant differences are shown as green shading. (E, F) Correlation maps of all GTP- and GDP FRET vectors in C, D and Figure 2C and D. In these maps, mutated residues are arranged in a similar spatial layout as they appear in the H-ras structure, mutated residues are connected by lines if the correlation coefficient is >0.6, the thickness of the line is proportional to the correlation coefficient (given in Supplementary Figure S2B). Hence, mutants linked by thicker lines cause similar perturbations in the parent FRET vector. Changes in the correlation maps are suggestive of actual structural rearrangements, possibly linking regions of correlated motion. The network in E clearly shows that residues in the switch region stabilize a conformation that realizes the membrane contact of helix α4. The network in F reveals complex rearrangements of linkages in the switch region that correspond to a different stabilization of the GDP-H-ras conformation; the strongest participation is that between D47 and R169/K170.
Figure 4
The novel membrane-orientation switch regulates H-ras interaction with C-Raf and MAPK signaling. (A) HEK293 cells transiently expressing mGFP-tagged H-rasG12V mutants with or without an excess of mRFP-RBD were imaged using FLIM. The mean mGFP donor fluorescence lifetime (±s.e.m.) was determined for multiple ROIs and data pooled from three independent experiments. The number of ROIs analysed is given in brackets. Statistically significant differences from the lifetime of mGFP-H-rasG12V coexpressed with mRFP-RBD were assessed using _t_-tests (*P<0.05, **P<0.01, ***P<0.001). Whereas only the lifetime of mGFP-H-rasG12V in the absence of mRFP-RBD is shown, the lifetimes of all other mGFP-H-rasG12V mutants expressed alone were the same (Supplementary Figure S2C). (B) BHK cells transiently expressing fluorescently tagged H-rasG12V (GTP-H-ras) with the specified mutations were assayed for MAPK activation by quantitative immunoblotting for ppERK. The figure shows mean ppERK levels (±s.e.m.; _n_=3). *Significant (P<0.05) increase in MAPK activity compared with non-mutated H-rasG12V. (C) Intact 2D plasma membrane sheets prepared from BHK cells expressing mGFP-H-ras (GDP-H-ras) or mGFP-H-rasG12V proteins with the specified mutations were immunogold labelled and imaged by EM. The graphs show a statistical analysis of the resulting immunogold point patterns as weighted mean _K_-functions calculated from _n_=16–27 membrane sheets. The gold labelling density for these experiments was 281–478/μm2. Nanoclustering is quantified by the extent of the positive deflection of the L(r)–r curve out of the confidence interval for a random pattern (±1). The radius of the nanoclusters is correlated with the radius at which the L(r)–r deflection is maximum. Using parametric bootstrap tests, we detected no significant differences in either of these nanoclustering parameters from control GTP- or GDP-H-ras point patterns for any of the mutants that were evaluated.
Figure 5
A balance model identifies another level of regulating Ras signal output. The balance model introduces the orientation of the G-domain with respect to the plasma membrane as an additional determinant of signalling specificity among Ras isoforms. The balance is regulated by the membrane-orientation switch region, which comprises residues in the β2–β3 loop and helix α5, and is indicated by the black fulcrum. MD and FRET analysis of H-ras show that for inactive, GDP-bound Ras (red) contacts of residues in the hypervariable region (HVR) prevail (i) so the balance is shifted to the HVR. Upon GTP loading, Ras is reoriented by interactions of helix α4 with the membrane (ii) so the balance is shifted to helix α4. In the context of this balance model, different Ras isoforms of the Ras subfamily may adopt different preferred orientations with respect to the membrane depending on the precise combination of residues present in the HVR and helix α4 (Supplementary Figure S3). Schemes (iii) and (iv) show examples of the membrane-interaction balance being shifted more and less towards helix α4, respectively. We speculate that such submembrane orientations are an important determinant for the propensity of GTP-loaded Ras proteins to interact with other membrane-associated proteins, such as its activity modulators (e.g. galectins) and other effectors (e.g. PI3 kinase).
Similar articles
- GTP Binding and Oncogenic Mutations May Attenuate Hypervariable Region (HVR)-Catalytic Domain Interactions in Small GTPase K-Ras4B, Exposing the Effector Binding Site.
Lu S, Banerjee A, Jang H, Zhang J, Gaponenko V, Nussinov R. Lu S, et al. J Biol Chem. 2015 Nov 27;290(48):28887-900. doi: 10.1074/jbc.M115.664755. Epub 2015 Oct 9. J Biol Chem. 2015. PMID: 26453300 Free PMC article. - The higher level of complexity of K-Ras4B activation at the membrane.
Jang H, Banerjee A, Chavan TS, Lu S, Zhang J, Gaponenko V, Nussinov R. Jang H, et al. FASEB J. 2016 Apr;30(4):1643-55. doi: 10.1096/fj.15-279091. Epub 2015 Dec 30. FASEB J. 2016. PMID: 26718888 Free PMC article. - GTP-dependent segregation of H-ras from lipid rafts is required for biological activity.
Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. Prior IA, et al. Nat Cell Biol. 2001 Apr;3(4):368-75. doi: 10.1038/35070050. Nat Cell Biol. 2001. PMID: 11283610 - Structural requirements for the interaction of p21ras with GAP, exchange factors, and its biological effector target.
Polakis P, McCormick F. Polakis P, et al. J Biol Chem. 1993 May 5;268(13):9157-60. J Biol Chem. 1993. PMID: 8486615 Review. - Three-dimensional structure of p21 in the active conformation and analysis of an oncogenic mutant.
Wittinghofer F, Krengel U, John J, Kabsch W, Pai EF. Wittinghofer F, et al. Environ Health Perspect. 1991 Jun;93:11-5. doi: 10.1289/ehp.919311. Environ Health Perspect. 1991. PMID: 1773783 Free PMC article. Review.
Cited by
- Binding hotspots on K-ras: consensus ligand binding sites and other reactive regions from probe-based molecular dynamics analysis.
Prakash P, Hancock JF, Gorfe AA. Prakash P, et al. Proteins. 2015 May;83(5):898-909. doi: 10.1002/prot.24786. Epub 2015 Mar 25. Proteins. 2015. PMID: 25740554 Free PMC article. - SPRED1 Interferes with K-ras but Not H-ras Membrane Anchorage and Signaling.
Siljamäki E, Abankwa D. Siljamäki E, et al. Mol Cell Biol. 2016 Sep 26;36(20):2612-25. doi: 10.1128/MCB.00191-16. Print 2016 Oct 15. Mol Cell Biol. 2016. PMID: 27503857 Free PMC article. - Inhibition of RAS function through targeting an allosteric regulatory site.
Spencer-Smith R, Koide A, Zhou Y, Eguchi RR, Sha F, Gajwani P, Santana D, Gupta A, Jacobs M, Herrero-Garcia E, Cobbert J, Lavoie H, Smith M, Rajakulendran T, Dowdell E, Okur MN, Dementieva I, Sicheri F, Therrien M, Hancock JF, Ikura M, Koide S, O'Bryan JP. Spencer-Smith R, et al. Nat Chem Biol. 2017 Jan;13(1):62-68. doi: 10.1038/nchembio.2231. Epub 2016 Nov 7. Nat Chem Biol. 2017. PMID: 27820802 Free PMC article. - RAS nanoclusters are cell surface transducers that convert extracellular stimuli to intracellular signalling.
Zhou Y, Hancock JF. Zhou Y, et al. FEBS Lett. 2023 Mar;597(6):892-908. doi: 10.1002/1873-3468.14569. Epub 2023 Jan 18. FEBS Lett. 2023. PMID: 36595205 Free PMC article. Review. - Comparative structural dynamic analysis of GTPases.
Li H, Yao XQ, Grant BJ. Li H, et al. PLoS Comput Biol. 2018 Nov 9;14(11):e1006364. doi: 10.1371/journal.pcbi.1006364. eCollection 2018 Nov. PLoS Comput Biol. 2018. PMID: 30412578 Free PMC article.
References
- Abankwa D, Vogel H (2007) A FRET map of membrane anchors suggests distinct microdomains of heterotrimeric G proteins. J Cell Sci 120: 2953–2962 - PubMed
- Diggle PJ, Lange N, Benes FM (1991) Analysis of variance for replicated spatial point patterns in clinical neuroanatomy. J Am Stat Assoc 86: 618–625
- Diggle PJ, Mateu J, Clough HE (2000) A comparison between parametric and non-parametric approaches to the analysis of replicated spatial point patterns. Adv Appl Probab 32: 331–343
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous